Abstract
A rapid and simple most-probable-number (MPN) procedure for the enumeration of dissimilatory arsenic-reducing bacteria (DARB) is presented. The method is based on the specific detection of arsenite, the end product of anaerobic arsenate respiration, by a precipitation reaction with sulfide. After 4 weeks of incubation, the medium for the MPN method is acidified to pH 6 and sulfide is added to a final concentration of about 1 mM. The brightly yellow arsenic trisulfide precipitates immediately and can easily be scored at arsenite concentrations as low as 0.05 mM. Abiotic reduction of arsenate upon sulfide addition, which could yield false positives, apparently produces a soluble As-S intermediate, which does not precipitate until about 1 h after sulfide addition. Using the new MPN method, population estimates of pure cultures of DARB were similar to direct cell counts. MPNs of environmental water and sediment samples yielded DARB numbers between 101 and 105 cells per ml or gram (dry weight), respectively. Poisoned and sterilized controls showed that potential abiotic reductants in environmental samples did not interfere with the MPN estimates. A major advantage is that the assay can be easily scaled to a microtiter plate format, enabling analysis of large numbers of samples by use of multichannel pipettors. Overall, the MPN method provides a rapid and simple means for estimating population sizes of DARB, a diverse group of organisms for which no comprehensive molecular markers have been developed yet.
Dissimilatory arsenic-reducing bacteria (DARB) may play a central role in determining the fate and transport of arsenic in the environment (2, 11). Capable of utilizing this toxic metalloid as a terminal electron acceptor in anaerobic respiration, these bacteria mediate the reduction of arsenate [As(V)] to arsenite [As(III)] (17). This is environmentally significant because at circumneutral pH, arsenite occurs as uncharged H3AsO3, which is thought to be both more toxic and mobile than arsenate, its oxidized counterpart. Indeed, arsenate displays high affinity for iron and manganese (oxy)hydroxides and has a tendency to coprecipitate with these solids. DARB may be involved in the solubilization of arsenic by reducing arsenate either while sorbed (2, 9, 11) or while in solution after liberation through reductive dissolution of the sorbent (oxy)hydroxides (7). In either case, their activity may ultimately lead to increased arsenic exposure of animals and plants and to increased mobilization of arsenic into water supply systems.
Despite the potential environmental significance of DARB, the assessment of their population size and activity has remained scarce. This is at least partly due to the relatively recent discovery of this type of metabolism, with only seven bacterial strains capable of dissimilatory arsenic reduction described in the literature (1, 14–16, 19, 23). Nonetheless, it is already apparent that these bacteria stem from phylogenetically and metabolically diverse groups, including sulfate-reducing bacteria (SRB) and iron-reducing bacteria from the gram-positive phylum, the epsilon and delta Proteobacteria phyla, and a to-date-unique branch within the Bacteria. This presumably large and still poorly explored diversity makes the design of phylogenetically based probes or primers for DARB identification and quantification currently impossible. Furthermore, the enzymes and genes involved in arsenic respiration are only beginning to be elucidated (13), so that no functional gene probes for the identification of bacteria which possess the capability to reduce arsenic are available yet. Thus, culture-based approaches, despite their potential limitations and biases, are still invaluable in estimating population sizes of DARB in environmental samples.
Here, we report the development of a rapid and simple most-probable-number (MPN) enumeration protocol for bacteria with arsenic-reducing capabilities. The procedure is based on detection of arsenic reduction in the MPN tubes; however, it replaces lengthy and cumbersome analytical techniques that detect arsenic reduction via spectroscopic techniques (11) with a simple selective precipitation of arsenite as the brightly yellow arsenic trisulfide (As2S3). The method is thus analogous to the commonly used iron sulfide precipitation in MPN assays of SRB (24).
MATERIALS AND METHODS
Environmental samples.
Water, sediment, and soil samples were collected from representative arsenic-contaminated environments in Massachusetts. Anoxic water and sediment were obtained from Spy Pond, Arlington. Wetland soil cores were collected from the arsenic-contaminated Wells G & H area, Woburn, Mass.
Strain.
A dissimilatory arsenic-reducing bacterium, strain L27, was used as a control organism to evaluate the effectiveness of the MPN method. The strain was isolated in August 1999 from Spy Pond by plating dilutions of anoxic water on lactate-arsenate minimal medium (Kuai et al., unpublished data).
MPN medium and anaerobic technique.
A freshwater minimal medium containing 10 mM acetate and 5 mM lactate as carbon sources, and 5 mM sodium arsenate as the electron acceptor (referred to hereafter as MPN medium) was used for the MPN procedure. It was buffered to pH 6.8 by adding NaHCO3 (1.9 g/liter). Salts used (in grams per liter) were KH2PO4 (0.14), NH4Cl (0.25), KCl (0.5), CaCl2 · 2H2O (0.15), NaCl (1.0), MgCl2 · 6H2O (0.62). Vitamins used (in milligrams per liter) were p-aminobenzoic acid (0.05), thiamine-HCl (0.02), pyridoxine-HCl (B6) (0.1), and cyanocobalamin (B12) (0.001). Trace minerals added from a 1,000-fold-concentrated mixture were (in milligrams per liter) MnCl2 · 4H2O (0.1), CoCl2 · 6H2O (0.12), ZnCl2 (0.07), H3BO3 (0.06), NiCl2 · 6H2O (0.025), CuCl2 · 2H2O (0.015), Na2MoO4 · 2H2O (0.025), and FeCl2 · 4H2O (1.5). Nine milliliters of medium was dispensed to tubes used for the MPN method (referred to hereafter as MPN tubes), autoclaved, and transferred to an anaerobic glove box (Coy Laboratories), where it was equilibrated with the N2-CO2-H2 (80:15:5) atmosphere for several days before inoculation.
MPN procedure.
Cultures and environmental samples were homogenized by vortexing and were 10-fold serially diluted to 10−9 in anaerobic water. One-mililiter aliquots of each dilution of the homogenate were transferred to anaerobic culture tubes containing 9 ml of growth medium. For each sample, 10 replicate MPN assays were set up with five replicate MPN tubes for each dilution step, and the tubes were incubated in the anaerobic hood at room temperature. The presence of arsenite was evaluated after 0, 7, 14, 21, and 28 days by evaluating arsenic accumulation in two of the MPN dilution series for chemical and microbiological analyses. Arsenite in the MPN tubes was first determined by subsampling each tube for spectrophotometric measurements, after which the remainder of the sample was used for evaluation of the precipitation of arsenic trisulfide (As2S3). For the latter, the tubes were first acidified by addition of 100 μl of 1 N HCl followed by addition of sulfide from a concentrated stock to a 1 to 1.5 mM final concentration. The sulfide stock was prepared by dissolving Na2S · 9H2O crystals in Milli-Q water (Millipore), which was deoxygenated by boiling and bubbling with N2 gas. The solution was filtered (0.2-μm-pore-size filter), transferred to a sterile serum vial under an N2 stream, capped with a rubber stopper, and stored in an anaerobic glove box. The sulfide concentration in the stock solution was checked each time before use. The effectiveness of the MPN assay was also evaluated in a 96-well microtiter plate format by scaling the volumes to 200 μl. Each well contained 180 μl of medium to which 20 μl of diluted environmental samples was added. No drying out of the plates was observed during the incubation period when plates were covered with their standard lids. MPN tables were constructed according to standard methods (3).
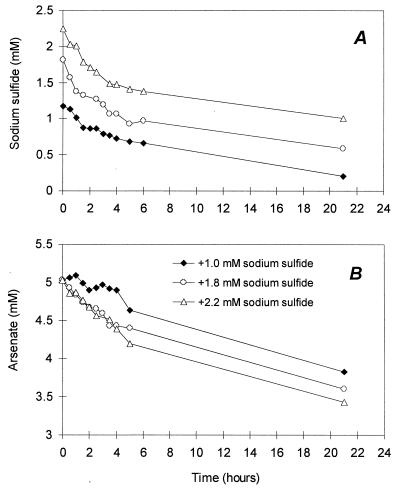
The potential for false positives in the MPN assay due to arsenite production by abiotic reduction of arsenate by the added sulfide was evaluated in two control experiments. The amounts of arsenate reduced and sulfide oxidized were measured over time in replicate tubes containing 5 mM arsenate and approximately 1.2, 1.8, and 2.3 mM sulfide in acidified, anaerobic medium. Tubes were incubated outside the glove box as in the MPN procedure. The experiment served also to score the time of appearance of yellow precipitate upon sulfide addition. Abiotic arsenate reduction by substrates in the environmental samples was tested by autoclaving, filtering, and poisoning (with 15 mg of HgCl2 per liter) the original samples before inoculation of the MPN tubes.
Chemical analyses and cell counts.
The molybdenum blue spectrophotometric assay was used to determine arsenite concentration (12). Arsenite was calculated from the difference of total arsenic minus arsenate and background phosphate. The sulfide concentration was assayed by the methylene blue method (5). Ferrous iron [Fe(II)] was determined using the ferrozine spectrophotometric assay (22). Total cell numbers in the cultures and environmental samples were determined by DAPI staining and counting using a Zeiss Axioscope epifluorescence microscope. The total number of arsenic-reducing bacteria in the different dilutions was also evaluated by CFU counts, by spreading a 0.1-ml subsample of all dilutions on agar plates containing the same medium as the MPN tubes and by counting colonies after 4 weeks of incubation.
RESULTS
Control experiments.
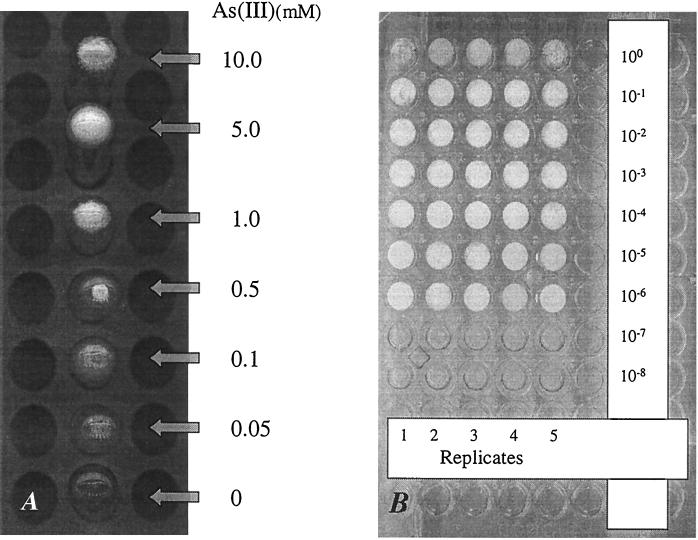
The sensitivity and specificity of the arsenite-sulfide precipitation were evaluated in preliminary experiments. The addition of sulfide to a final concentration between 1 and 1.5 mM to the acidified minimal medium led to the formation of a bright yellow precipitate within seconds. An easily visible precipitate is formed over a large concentration range, with as little as 0.05 mM arsenite forming detectable yellow particles (Fig. 1A). The precipitation reaction has, thus, a similar detection limit as the spectrophotometric method used to check for the presence of arsenite. Although sulfide addition to the acidified medium led to an immediate abiotic reduction of arsenate at a high rate (Fig. 2), yellow precipitate only started to form after about 1 h in samples containing 1.5 and 2 mM sulfide. When samples were not shaken immediately after sulfide addition, the precipitate formed after about 40 min upon addition of 1.5 and 2.0 mM sulfide, possibly due to locally elevated sulfide concentrations (data not shown).
FIG. 1.
Black and white photograph of yellow arsenic trisulfide precipitation in MPN tubes and microtiter plates after addition of sulfide to 1 mM. Photographs show underside of MPN tubes containing a dilution of arsenite (0.05 to 10 mM) in buffer (A) and a microtiter plate containing an MPN assay of arsenate-reducing populations in Wells G&H area wetland sediment yielding a population estimate of 9.2 × 104 cells g−1 (dry weight) (B). A 10-fold dilution series of sediment was aliquoted into growth medium in the microtiter wells and was incubated for 4 weeks prior to sulfide addition.
FIG. 2.
Time course of arsenate reduction (A) and sulfide oxidation (B) in MPN medium acidified to pH 6.
The utility of the arsenic trisulfide precipitation as an indicator for arsenite in MPN determination of arsenic-reducing bacteria population size was first tested using a pure culture of the DARB strain L27 (Table 1). All tubes that scored positive for the presence of arsenite by spectrophotometric measurement also produced the bright yellow precipitate upon acidification and sulfide addition, leading to identical MPN estimates. The MPN estimates increased about 10-fold from 0.4 × 107 and 0.8 × 107 to 7.9 × 107 over the 4-week incubation period and were similar to DAPI counts of the inoculum from 3 weeks onwards (Table 1). Cell estimates by CFU counts determined on identical medium and carbon sources yielded only 1.8 × 103 cells despite the same length of incubation.
TABLE 1.
Estimation of total cell numbers by DAPI direct counts and of DARB populations by MPN and CFU in a pure culture of strain L27 and diverse environmental samples
| Inoculum and method | Cell no. estimate at incubation time (wk):
|
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| As-reducing strain L27a | |||||
| DAPIb | (6.3 ± 0.3) × 107 | ||||
| MPNc | (0.4, 0.8) × 107 | (2.2, 2.8) × 107 | (4.4, 7.9) × 107 | (7.9, 7.9) × 107 | |
| CFUd | NDe | ND | ND | (1.8 ± 0.5) × 103 | |
| Spy Pond wateraf | |||||
| DAPI | (2.1 ± 0.9) × 108 | ||||
| MPN | (0.2, 0.5) × 103 | (0.8, 1.1) × 103 | (1.3, 2.4) × 103 | (2.4, 2.4) × 103 | |
| CFU | ND | ND | ND | (3.6 ± 1.2) × 102 | |
| Spy Pond waterag | |||||
| DAPI | (2.5 ± 1.2) × 108 | ||||
| MPN | 4, 4 | 9, 4 | 16, 8 | 13, 11 | |
| CFU | ND | (2.8 ± 0.3) × 102 | (2.8 ± 0.3) × 102 | (2.8 ± 0.3) × 102 | |
| Spy Pond sedimenth | |||||
| DAPI | (2.9 ± 0.9) × 1011 | ||||
| MPN | (7.6, 11) × 103 | (7.6, 7.6) × 104 | (7.6, 1.4) × 104 | (7.6, 1.4) × 104 | |
| CFU | ND | (7.3 ± 2) × 104 | (7.3 ± 2) × 104 | (7.3 ± 2) × 104 | |
| Wetland sedimenth | |||||
| DAPI | (5.4 ± 0.9) × 1011 | ||||
| MPN | (4.2, 3.1) × 104 | (13.1, 8.6) × 104 | (6.2, 9.2) × 104 | (6.2, 9.2) × 104 | |
| CFU | ND | (7.0 ± 1) × 105 | (7.0 ± 1) × 105 | (7.0 ± 1) × 105 | |
Results in cells per milliliter.
DAPI counts are averages of results from three replicate filters that were counted at 10 fields each.
MPN tests were carried out in duplicate for each weekly time point; both estimates are shown.
CFU estimates are averages of counts obtained for six replicate plates.
ND, not determined.
Sample collected on 3 August 2000.
Sample collected on 25 September 2000.
Cells g−1 dry weight.
Environmental DARB MPN estimates.
Sulfide precipitation and spectrophotometric determination of arsenite also yielded identical MPN estimates of DARB population sizes for diverse freshwater environments. Samples collected from the water column of Spy Pond had the lowest counts, with about 10 cells ml−1. Arsenic-contaminated sediment samples from Spy Pond and the Wells G&H area both had comparable counts, with a final maximum of 9.2 × 104 cells gram dry weight−1. CFU counts were in all cases comparable or even higher than MPN estimates. Overall, the DARB populations made up only a minor component of the total community as determined by comparison of MPN estimates and DAPI direct counts (Table 1). Control MPN tubes inoculated with autoclaved, HgCl2 poisoned or filtered environmental samples showed no arsenite formation, indicating that abiotic reactions were not skewing the estimates.
Microtiter plate MPN assay.
The arsenic trisulfide MPN procedure can readily be scaled to volumes appropriate for microtiter plates (Fig. 1B; Table 2). A dilution of a pure culture of strain L27 was used as inoculum for 10 replicate tube and microtiter assays, respectively (Table 2). For the latter, the sample was serially diluted and 20 μl was added to 180 μl of growth medium in the wells. Acidification (5 μl) and sulfide addition to 1 to 1.5 mM were carried out by using a multichannel pipettor. Comparison of the MPN values over a 4-week incubation period showed no significant difference (t test, P = 0.005) between the average cell number estimates of the two assays, and the final estimate after 4 weeks was in good agreement with DAPI counts of the original culture (Table 2).
TABLE 2.
Comparison of MPN estimation of cell numbers of strain L27a by tube and microtiter plate assay
| MPN assay typeb | DAPI count at wk:
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Tube | (0.5 ± 0.7) × 108 | (1.1 ± 1.2) × 108 | (2.7 ± 3.5) × 108 | (3.2 ± 1.9) × 108 |
| Microtiter plate | (0.4 ± 0.3) × 108 | (1.0 ± 1.0) × 108 | (2.2 ± 2.4) × 108 | (2.8 ± 1.7) × 108 |
DAPI counts of the density of strain L27 in the culture used for comparison of the tube and microtiter assay yielded 3.1 × 108 cells ml−1.
Assays were carried out in 10 replicates for each week and assay type.
Environmental samples from the Wells G&H sediment were also compared by the two assays and yielded similar results for the microtiter plate and the tube-based assay. Yellow precipitate was clearly visible in all positive microtiter wells (Fig. 1B).
DISCUSSION
The MPN assay described here gives a rapid estimation of arsenic-reducing bacteria in diverse environmental samples. Arsenite, as a specific end product of DARB metabolism, is detected as the brightly yellow arsenic trisulfide precipitate. This allows easy visual scoring of positive tubes in the MPN assay and is analogous to the formation of black iron sulfide precipitate upon Fe(II) addition in the widely used MPN assays of SRB. Our results suggest that the arsenite precipitation may also be used for assessment of reductive detoxification under aerobic conditions, provided that sufficient arsenite accumulates. We observed no abiotic arsenite oxidation in fully aerated medium over 5 days, and acidification and sulfide addition also triggered the formation of the yellow precipitate (data not shown). The kinetics of formation of the precipitate at slightly acidic pH is extremely rapid, ensuring no interference from potential abiotic reduction of arsenate. Thus, in the absence of comprehensive and facile tests allowing the enumeration of DARB, this new MPN procedure may prove useful in obtaining a more detailed picture of the ecology of these relatively recently described organisms. Furthermore, the method may also provide a rapid means for isolation of DARB from high-dilution MPN tubes.
The use of the arsenic trisulfide precipitate as an indicator of As(III) production was based on a recent demonstration of the potential involvement of DARB in production of this mineral (18). A stability diagram constructed in this study showed that As2S3 formation is robust over a wide range of concentrations of the reactants, provided the pH is lower than 6.5 (18). Consequently, an acidification step is included in the MPN assay prior to addition of sulfide to ensure precipitation over a wide range of arsenite concentrations. Indeed, a clearly visible precipitate forms in the As(III) concentration range between 0.05 and 10 mM when a final concentration of sulfide between 1 to 1.5 mM is used (Fig. 1A). At pH 6, the kinetics of precipitation is also extremely rapid, with clearly visible As2S3 formation taking place within seconds of addition of sulfide in both MPN tubes and microtiter plate wells. This is in stark contrast to the abiotic reduction of arsenate by sulfide, which, despite being rapid (Fig. 2), does not produce visible precipitate under the given conditions until about 1 h after sulfide addition. This confirms a recently reported experiment in which neither soluble arsenite nor arsenic trisulfide precipitate was observed for several days after arsenate and sulfide were reacted at pH 4 (20). This was attributed to the formation of a soluble arsenic-sulfide complex, which displays considerable stability at acidic pH (20). Thus, a similar intermediate, which is, however, slightly less stable due to the higher pH of the MPN medium, may form in the MPN tubes upon abiotic arsenate reduction.
The choice of the type of carbon sources and electron acceptor concentration in the MPN medium was based on a literature survey of dissimilatory metal-reducing bacteria, including DARB (for a partial summary see http://parsons-19.mit.edu/cgi-bin/database.pl). Acetate and lactate emerged as the common electron donors in anaerobic metal respiration. Of 92 strains examined for utilization of various carbon sources, 44 and 48 grew on acetate and lactate, respectively. Of 41 strains tested with both substrates, only 15 were capable of growing on both. It was thus decided to utilize a minimal medium with acetate and lactate as the carbon sources for the MPN procedure. While acetate is a nonfermentable carbon source, lactate can be fermented by some microorganisms. The inclusion of lactate may thus lead to the positive scoring of fermenters, which possess the capability of reductively detoxifying arsenate. This type of detoxification, which is encoded by the ars genes and affords no energetic gain for the organisms, appears to be widespread among aerobic and anaerobic microorganisms (16, 21). Hence, if exclusively DARB numbers are to be estimated, two control MPNs might be included: (i) a lactate medium with no electron acceptors for scoring growth of lactate fermenters, and (ii) a glucose-arsenate medium to identify fermenters with reductive detoxification capabilities. Glucose is a readily fermentable substrate that has, despite extensive testing, to date only been found to serve as a carbon source for a single iron-reducing bacterium (6). The electron acceptor concentration in the MPN medium was set at 5 mM arsenate to allow high growth yield yet avoid excessive toxicity. This was based on absence of inhibition in DARB cultures for arsenate at concentrations up to 10 mM (15, 16, 19). Similar media have yielded phylogenetically diverse isolates of DARB from a variety of environments. In our laboratory, the same medium was used to assess the diversity of DARB from a single environmental sample, which has to date led to the identification of 12 different species as assessed by 16S ribosomal DNA differences (Kuai et al., unpublished data). Thus, the method may prove useful for the rapid identification and isolation of DARB from high-dilution MPN tubes, which is an approach increasingly used to avoid isolation bias observed in enrichment culture from low-dilution environmental samples (10).
The magnitude of DARB populations detected in the sediment and wetland samples by the arsenic trisulfide precipitation method was comparable to MPN estimates, which were obtained for contaminated Lake Coeur d'Alene, Idaho, sediments using hydride generation inductively coupled plasma mass spectrometry detection of arsenite (11). In both cases, MPN estimates were on the order of 104 cells g sediment−1. These numbers are somewhat low compared to those typically obtained for SRB or iron- or manganese-reducing bacteria, which are often between 105 to 106 cells g sediment−1. Such difference in population sizes is, however, not surprising considering the large difference in concentration between these electron acceptors even in contaminated environments (4, 8, 11). More surprising was the discrepancy between MPN and CFU estimates in our study. While CFU counts in the pure culture test were much lower (Table 1) they were comparable to or even exceeded the MPN estimates in the environmental samples (Table 1). Although we do not have a complete explanation for this phenomenon, the agreement of direct counts and MPNs for the pure culture indicates that the strain used to establish the method grows poorly on plates (Table 1). Furthermore, when arsenate minimal medium plates are inoculated with environmental samples, we have observed that a large percentage of the colonies cannot be replated on the same medium. This may be caused by carryover of organic material from the environmental samples, since a low dilution of the samples—typically 10−1—has to be plated to obtain statistically significant numbers of CFU. This organic material may encourage the growth of non-DARB organisms on the plates.
A major advantage of the DARB MPN method presented here is the potential for scaling of the assay to a microtiter plate format since the precipitate is easily visible even in small volume (Fig. 1B). This allows the simultaneous processing of a large number of samples due to the use the plates in conjunction with multichannel pipettors. Using this approach, we have observed good agreement in MPN estimates for the same sample when comparing tubes and plates. A further advantage of the miniaturization is that inocula can be drawn from sample volumes, which are small enough to represent relevant spatial heterogeneity in the environment. Thus, the new arsenic trisulfide MPN protocol presented here should considerably simplify the estimation and isolation of DARB populations in a variety of environments.
ACKNOWLEDGMENTS
This work was partially supported by a grant from the Edgerly Foundation.
We also thank Dianne Newman (Caltech) for many helpful discussions and Vanja Klepac for help with analytical procedures.
REFERENCES
- 1.Ahmann D, Krumholz L R, Hemond H, Lovely D R, Morel F M M. Microbe grows by reducing arsenic. Nature. 1994;371:750. doi: 10.1038/371750a0. [DOI] [PubMed] [Google Scholar]
- 2.Ahmann D, Krumholz L R, Hemond H F, Lovely D R, Morel F M M. Microbial mobilization of arsenic from sediments of the Aberjona Watershed. Environ Sci Technol. 1997;31:2923–2930. [Google Scholar]
- 3.American Public, Health Association . 9221 C. Estimation of bacterial density. 1992. pp. 9–45. –9–52. In A. E. Greedberg, L. S. Clesceri, and A. D. Eaton (ed.), Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, Washington, D.C. [Google Scholar]
- 4.Aurillio A C, Mason R P, Hemond H F. Speciation and fate of arsenic in three lakes of the Aberjona Watershed. Environ Sci Technol. 1994;28:577–585. doi: 10.1021/es00053a008. [DOI] [PubMed] [Google Scholar]
- 5.Cline J D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–458. [Google Scholar]
- 6.Coates J D, Councell T, Ellis D J, Lovley D R. Carbohydrate oxidation coupled to Fe(III) reduction, a novel form of anaerobic metabolism. Anaerobe. 1998;4:277–282. doi: 10.1006/anae.1998.0172. [DOI] [PubMed] [Google Scholar]
- 7.Cummings D E, Caccavo F J, Fendorf S, Rosenzweig R F. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ Sci Technol. 1999;33:723–729. [Google Scholar]
- 8.Cummings D E, March A W, Bostick B, Spring S, Caccavo F J, Fendorf S, Rosenzweig R F. Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d'Alene, Idaho) Appl Environ Microbiol. 2000;66:154–162. doi: 10.1128/aem.66.1.154-162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowdle P R, Laverman A M, Oremland R S. Bacterial dissimilatory reduction of arsenic(V) to arsenic(III) in anoxic sediments. Appl Environ Microbiol. 1996;62:1664–1669. doi: 10.1128/aem.62.5.1664-1669.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunbar J, White S, Forney L. Genetic diversity through the looking glass: effect of enrichment bias. Appl Environ Microbiol. 1997;63:1326–1331. doi: 10.1128/aem.63.4.1326-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington J M, Fendorf S E, Rosenzweig R F. Biotic generation of arsenic(III) in metal(loid)-contaminated freshwater lake sediments. Environ Sci Technol. 1998;32:2425–2430. [Google Scholar]
- 12.Johnson D L, Pilson M E Q. Spectrophotometric determination of arsenite, arsenate, and phosphate in natural waters. Anal Chem Acta. 1972;58:289–299. [Google Scholar]
- 13.Krafft T, Macy J M. Purification and characterization of the respiratory arsenate reductase of Chrysiogenes arsenatis. Eur J Biochem. 1998;255:647–653. doi: 10.1046/j.1432-1327.1998.2550647.x. [DOI] [PubMed] [Google Scholar]
- 14.Laverman A M, Swizer-Blum J, Schaefer J K, Philips E J P, Lovley D R, Oremland R S. Growth of strain SES-3 with arsenate and diverse electron acceptors. Appl Environ Microbiol. 1995;61:3556–3561. doi: 10.1128/aem.61.10.3556-3561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macy J M, Nunan K, Hagen K D, Dixon D R, Harbour P J, Cahill M, Sly L I. Chrysiogenes arsenatis gen. nov., sp. nov., a new arsenate-respiring bacterium isolated from gold mine wastewater. Int J Syst Bacteriol. 1996;46:1153–1157. doi: 10.1099/00207713-46-4-1153. [DOI] [PubMed] [Google Scholar]
- 16.Macy J M, Santini J M, Pauling B V, O'Neill A H, Sly L I. Two new arsenate/sulfate-reducing bacteria: mechanism of arsenate reduction. Arch Microbiol. 2000;173:49–57. doi: 10.1007/s002030050007. [DOI] [PubMed] [Google Scholar]
- 17.Newman D K, Ahmann D, Morel F M M. A brief review of microbial arsenate respiration. Geomicrobiology. 1998;15:255–268. [Google Scholar]
- 18.Newman D K, Beveridge T J, Morel F M M. Precipitation of As2S3 by Desulfotomaculum aripigmentum. Appl Environ Microbiol. 1997;63:2022–2028. doi: 10.1128/aem.63.5.2022-2028.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman D K, Kennedy E K, Coates J D, Ahmann D, Ellis D J, Lovley D R, Morel F M M. Dissimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum sp. nov. Arch Microbiol. 1997;63:380–388. doi: 10.1007/s002030050512. [DOI] [PubMed] [Google Scholar]
- 20.Rochette E A, Bostick B C, Li G, Fendorf S. Kinetics of arsenate reduction by dissolved sulfide. Environ Sci Technol. 2000;34:4714–4720. [Google Scholar]
- 21.Silver S. Bacterial resistance to toxic metal ions—a review. Gene. 1996;179:9–19. doi: 10.1016/s0378-1119(96)00323-x. [DOI] [PubMed] [Google Scholar]
- 22.Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982;43:319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swizer-Blum J, Bindi A B, Buzzelli J F, Stolz J F, Oremland R S. Bacillus arsenicoselenatis, sp. nov., and Bacillus selenititreducens, sp. nov.: two haloakiliphiles from Mono Lake, CA that respire oxyanions of selenium and arsenic. Arch Microbiol. 1998;171:19–30. doi: 10.1007/s002030050673. [DOI] [PubMed] [Google Scholar]
- 24.Vester F, Ingvorsen K. Improved most-probable-number method to detect sulfate-reducing bacteria with natural media and a radiotracer. Appl Environ Microbiol. 1998;65:1700–1707. doi: 10.1128/aem.64.5.1700-1707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]