Abstract
BACKGROUND
Phytosanitary irradiation is used to control insect pests of quarantine concern on exported fresh horticultural products. Generic irradiation doses of 150 and 400 Gy are approved for tephritid fruit flies and all other insects, respectively. Other invertebrates such as gastropods (snails and slugs) may be classified as quarantine pests and require a disinfestation treatment. Parmarion martensi Simroth (Stylommatophora: Ariophantidae) is a semi‐slug quarantine pest sometimes found on fresh sweet potatoes and other fruits and vegetables exported from Hawai'i to the continental USA. Also, P. martensi is a host of the parasitic nematode Angiostrongylus cantonensis (Rhabditida: Angiostrongylidae), the causative agent of neuroangiostrongyliasis or rat lungworm disease in humans. We conducted a study to determine if phytosanitary irradiation could control P. martensi and thereby reduce the risk of transmitting A. cantonensis in the USA.
RESULTS
Two‐, 12‐, and 21‐week‐old P. martensi were treated with X‐ray radiation at a dose of 150 or 400 Gy or left untreated as controls then held in the laboratory for up to 250 days. Survivorship and reproduction were recorded every 2–3 days and individual weights were measured biweekly. Irradiation at 150 and 400 Gy reduced growth and increased the mortality rate compared to untreated controls and prevented reproduction.
CONCLUSION
Phytosanitary irradiation treatment at doses ≥150 Gy will prevent the establishment of viable populations of P. martensi. The literature on radiation tolerance in gastropods suggests that the internationally approved generic dose for tephritid fruit flies of 150 Gy may be effective against many slug and snail pest species.
Keywords: X‐ray irradiation, quarantine pest, phytosanitary, gastropod, rat lungworm disease
Phytosanitary Irradiation at 150 and 400 Gy reduced growth, increased the mortality rate and prevented reproduction in Parmarion martensi, a semi‐slug quarantine pest of fresh produce exported from Hawaii.

© 2021 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
1. INTRODUCTION
Fresh produce exported from Hawai'i to the continental USA may harbor quarantine insect pests and thus the application of a postharvest quarantine treatment such as irradiation is required by law for many fruits and vegetables. 1 , 2 Other invertebrates besides insects can be quarantine pests, and may also require a disinfestation treatment, including snails and slugs (Class Gastropoda). 3 , 4 Recently, the semi‐slug Parmarion martensi Simroth (Stylommatophora: Ariophantidae) has been found on fruit, vegetable, nursery, and floriculture crops exported from Hawai'i to California, especially sweet potatoes. P. martensi is considered an actionable quarantine pest (i.e. action required: return to port of origin, fumigate, or destroy) because it is not known to occur in California or elsewhere in the continental USA. 4 This semi‐slug is of special concern in Hawai'i and elsewhere because of its invasion history and high infection prevalence and parasite load of a zoonotic nematode Angiostrongylus cantonensis (Chen) (Rhabditida: Angiostrongylidae). 5 , 6 , 7 , 8 A. cantonensis is the etiological agent of neuroangiostrongyliasis or rat lungworm disease, a potentially debilitating form of meningitis in humans. 9 , 10 , 11
P. martensi was discovered in the state of Hawai'i on the island of O'ahu in 1996, 12 but spread to east Hawai'i Island possibly as early as 1999, though it was not officially recorded there until 2004. 6 This invasive semi‐slug is now common in the area where most of the state's documented cases of human neuroangiostrongyliasis have occurred. 13 P. martensi is considered a high‐risk carrier of A. cantonensis because of its climbing behavior, abundance around human dwellings, high infection prevalence, and high worm burdens. 6 , 7 , 8 , 14 , 15 Outbreaks of human infection appear to be associated with the invasion of P. martensi on Hawai'i Island, Maui, and Okinawa, Japan. 5 , 6 , 16 , 17 P. martensi is a called a semi‐slug because it has a visible soft shell on the dorsum of the body, whereas in a true slug the shell is completely internal and not visible.
The life cycle of the parasitic nematode A. cantonensis is particularly complex and there are numerous pathways for the movement or spread of this parasite to new locations. Rattus rats are definitive hosts, intermediate hosts include many terrestrial and aquatic gastropods (snails and slugs), and paratenic hosts include frogs, toads, some crustaceans, centipedes, and flatworms. 10 , 18 Rats and paratenic hosts acquire the nematode by eating a gastropod intermediate host or possibly through contaminated water. 19 , 20 Humans are dead‐end hosts and only third‐stage larvae (L3) found in gastropods and paratenic hosts can cause an infection, most commonly through the accidental ingestion of L3 larvae in infected gastropods. 21 Hence there is special concern about exported produce infested with P. martensi not only because this species is invasive and may harbor high loads of A. cantonensis, but also because P. martensi is an important vector of transmission to other hosts, and therefore poses significant environmental, economic, and human health risks.
Phytosanitary irradiation doses applied to exported Hawaiian fresh fruit and vegetables include 150 Gy for tephritid fruit flies and 400 Gy for other insect pests. 1 , 22 The term ‘phytosanitary’ refers to preserving the health of plants, and phytosanitary measures ensure that plant pests that could harm the health of plant resources or the economy of importing countries are not present on the exported produce. Phytosanitary irradiation protocols are designed to prevent reproduction through the prevention of adult emergence or through sterility of the adult. 1 Absorbed dose is measured in grays (Gy), which is a unit of measure of radiation energy. The source of ionizing radiation can be gamma rays produced by radionuclides (e.g. from cobalt‐60), or electrons or X‐rays generated from machine sources. 1 , 2 The 150 Gy generic treatment for tephritid fruit flies is an internationally approved protocol, 23 and the 400 Gy generic treatment for all other insects (except pupae and adults of Lepidoptera which may require higher doses) is an approved protocol in the USA, Australia, and New Zealand. 24 Sweet potatoes exported from Hawai'i to the continental USA are irradiated at 150 or 400 Gy for control of several regulated insect pests that feed internally and therefore cannot be detected by inspection. 25 P. martensi has been found on Hawai'i sweet potatoes during inspection before and after export (Fig. 1), causing rejection at significant cost to the farmer or exporter. The question is whether the irradiation doses that are used to control the regulatory target insects in fresh produce like sweet potatoes are also effective against the nontarget semi‐slug P. martensi. Phytosanitary irradiation treatments do not cause immediate mortality and therefore the desired response is to prevent development to adulthood or induce sterility in reproductive adults rather than mortality. 1 , 22 We studied radiotolerance in P. martensi to determine if phytosanitary irradiation at 150 or 400 Gy could reduce survival and stop reproduction, thus reducing the risk of establishment of this semi‐slug in the USA mainland and thereby limiting human exposure to and transmission of the pathogenic nematode A. cantonensis.
Figure 1.

This Parmarion martensi semi‐slug was found on ‘Okinawan’ sweet potato during inspection in Hawai'i before irradiation and export to the continental USA. The presence of P. martensi during pre‐departure inspection of sweet potatoes may result in rejection and return to the exporter, whereas the detection of P. martensi after shipment may result in a decision to return to the port of origin, fumigate, or destroy at significant cost to the exporter.
2. MATERIALS AND METHODS
2.1. Rearing
P. martensi were collected from the wild and reared on a diet of dry dog food and fresh fruits and vegetables in temperature‐controlled cabinets at 21 °C, 98% relative humidity, and 12 h:12 h light:dark cycle following the methods described by Hamilton et al. 26 Under these rearing conditions, time from egg to first reproduction averaged 165 days, adults laid approximately 35 eggs, and egg hatch was about 53%. Survivorship was >90% for up to a year. P. martensi is hermaphroditic, so any individual of reproductive age may lay eggs.
Offspring from the wild‐collected adults were used in the irradiation tests. Three P. martensi age classes were irradiated: 2, 12 and 25 weeks old. At 2 and 12 weeks old, semi‐slugs were immature (juvenile) and actively feeding and growing, whereas at 25 weeks semi‐slugs were full size adults and actively reproducing (reproductive). Juvenile or reproductive adult P. martensi were reared with siblings from a common egg cluster until irradiation treatment. After irradiation, individuals from families were randomly assigned to rearing containers in cohorts of 10 individuals of the same age class. All P. martensi used in tests were raised from eggs and therefore were not infected with A. cantonensis.
2.2. Irradiation treatment
For treatment with irradiation, individual semi‐slugs in 50 mL plastic Solo cups (Lake Forest, Illinois, USA) were transported to a commercial irradiation facility (RPH Hawaii Pride, Keaau, Hawai'i) for treatment with X‐rays using an electron linear accelerator (5 MeV, model TB‐5/15; Titan Corp., San Diego, CA, USA). This facility was designed to apply low‐dose irradiation for phytosanitation of fresh agricultural produce. The target doses were 0 (nonirradiated control), 150, and 400 Gy. At each dose, three optichromic dosimeters (FWT‐70−83M; Far West Technology, Goleta, CA, USA) were placed in individual Solo containers but without semi‐slugs. To control dose uniformity (the ratio of the maximum/minimum dose), the Solo cups holding semi‐slugs or dosimeters were placed perpendicular to the x‐ray beam and elevated by placement on a fibreboard box and positioned in the center of the carrier for treatment at each dose. After irradiation treatment, dosimeters were read with an FWT‐200 reader (Far West Technology) at 620 nm absorbance to measure dose accuracy and variation. Average measured doses (and dose range) for the target treatments of 150 and 400 Gy were 158 (143–184) and 393 (353–422) Gy.
2.3. Data collection
After irradiation treatment, semi‐slugs were returned to the laboratory, transferred to rearing containers in groups of 10 individuals, and held under standard rearing conditions. 26 Individual weights were recorded biweekly and survivorship and reproduction were recorded every 2–3 days until all irradiated semi‐slugs in a cohort had perished or until reproduction in nonirradiated control semi‐slugs had stopped (~217–259 days post treatment). Irradiation treatments at 150 and 400 Gy were replicated 7–11 times each for a total of 70–110 semi‐slugs per treatment depending on availability of semi‐slugs in each age class (Table 1). Untreated controls were replicated 4‐6 times for a total of 40–60 semi‐slugs depending on the age class.
Table 1.
Summary of Parmarion martensi weight change, egg production, and survival post‐irradiation treatment
| Age (weeks) | Treatment (Gy) | Total no. slugs | Start mean wt (g) a | 4‐week mean wt (g) | Percentage wt change at 4‐weeks | Mean eggs/adult b | Total eggs | Total hatch number (%) | Survival post‐irradiation (days) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | |||||||||
| 2 | 0 | 60 | 0.02 | 0.95 | +4650% | 13.3* | 795 | 517 (65) | 199.3 | 9 | 217 |
| 150 | 110 | 0.02 | 0.05 | +150% | 0.00 | 0 | 0 | 28.3 | 6 | 217 | |
| 400 | 110 | 0.02 | 0.03 | +50% | 0.00 | 0 | 0 | 29.3 | 2 | 68 | |
| 12 | 0 | 40 | 2.18 | 3.65 | +67% | 115.6* | 4625 | 2930 (63) | 217.9 | 105 | 259 |
| 150 | 70 | 2.29 | 3.06 | +34% | 0.00 | 0 | 0 | 117.8 | 14 | 259 | |
| 400 | 70 | 2.26 | 2.69 | +19% | 0.00 b | 0 | 0 | 77.8 | 2 | 120 | |
| 21 | 0 | 50 | 3.91 | 4.22 | +8% | 68.5* | 3423 | 1873 (55) | 81.8 | 62 | 96 |
| 150 | 80 | 3.93 | 4.00 | +0.1% | 1.31 | 105 | 0 | 47.6 | 19 | 96 | |
| 400 | 80 | 3.93 | 3.61 | ‐8% | 0.19 | 15 | 0 | 36.1 | 6 | 68 | |
Results are from replicated groups of 10 individual semi‐slugs.
Weight at the time of irradiation.
* denotes significant effect.
2.4. Statistical analysis
Survivorship in the irradiation treatments for the duration of the experiment was plotted and analyzed for differences using the survival package in R v. 3.6.3. For this analysis, each semi‐slug was scored as alive or dead at the end of each time interval. Survival is the proportion surviving as determined using a Kaplan–Meier product‐limit estimate. A log‐rank test was applied to the data using a chi‐squared statistic. Because of high survival in the nonirradiated control groups (>90%), median (50%) survival and confidence intervals could not be calculated. Data on lifetime reproduction (number of eggs per adult) were subjected to statistical analysis using generalized linear models (GLM) assuming a Poisson distribution and using a log link function. 27 Weight gain data were plotted as means for each cohort for the duration of the experiment and data at 4 weeks were used as an indicator of treatment effects and analyzed by age group (2‐, 12‐, 21‐week‐old semi‐slugs) using linear regression (response at 0, 150, 400 Gy). The 4‐week snapshot was selected after reviewing survivorship data to ensure that many P. martensi were still alive in all age classes in both irradiation treatments at this time.
3. RESULTS
3.1. Survivorship
In total, 670 P. martensi were used across the three age groups (Table 1). Irradiation significantly reduced survivorship compared with nonirradiated controls in all age groups. Survival analysis showed that P. martensi irradiated at 150 and 400 Gy died at approximately the same rate in 2‐week‐old P. martensi (chi‐square = 3.0, df = 1, P = 0.08), whereas P. martensi irradiated at 400 Gy died significantly faster than those irradiated at 150 Gy in the 12‐week‐old (chi‐square = 38.2, df = 1, P < 0.001) and 21‐week‐old groups (chi‐square = 17.6, df = 1, P < 0.001) (Fig. 2). Mean survival time post irradiation treatment varied by age group and radiation dose (Table 1). Semi‐slugs in the 21‐week‐old group had mean survival times of 47.6 and 36.1 days in the 150 and 400 Gy treatments, respectively. Semi‐slugs in the 12‐week‐old group had mean survival times of 117.9 and 77.8 days at 150 and 400 Gy, respectively. These longer survival times in 12‐week‐old versus 21‐week‐old semi‐slugs suggests inherently higher radiation tolerance but nonirradiated 12‐week‐old semi‐slugs also had longer survival times than 21‐week‐old semi‐slugs (Table 1). All irradiated semi‐slugs in the 21‐week‐old group died within 96 days, whereas one semi‐slug in the 2‐week‐old group and four semi‐slugs in the 12‐week‐old group irradiated at 150 Gy survived well past their normal reproductive period without laying eggs up to the point at which the experiment was terminated at 217 and 259 days post‐treatment, respectively (Table 1).
Figure 2.
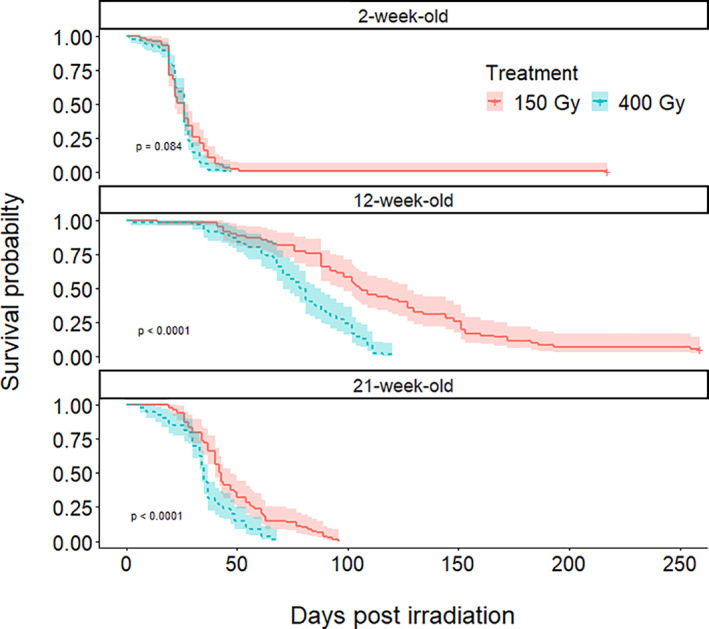
Parmarion martensi survival probability (with 2 standard error cloud) over time post irradiation treatment in each age class. Survivorship was reduced more in the 400 Gy treatment than in the 150 Gy treatment in the 12‐week‐old and 21‐week‐old groups.
3.2. Weight gain
In all age groups, P. martensi showed lower mean weight gain per group after irradiation compared with nonirradiated controls and the effects increased with increasing dose (Table 1 and Fig. 3). At 4 weeks post‐irradiation, the regression lines describing weight gain were y = 0.04–0.001*dose (R 2 = 0.44) for 2‐week‐olds, y = 3.5–0.002*dose for 12‐week‐olds (R 2 = 0.41), and y = 4.2–0.0015*dose (R 2 = 0.44) for 21‐week‐olds, and all showed a significant negative slope. Compared to their weight at the time of irradiation, weight gain was greatly reduced in 2‐week‐old P. martensi (50–150% increase) compared to the untreated controls (4650% increase) (Table 1). The upward trending weight gain line in the 2‐week graph (Fig. 3) represents the one surviving semi‐slug at 150 Gy that continued to feed and grow for the duration of the experiment, but without laying eggs. In the 21‐week‐old group, where many P. martensi had reached full size and started reproducing at the time of irradiation, individuals treated at 400 Gy in fact lost weight, probably because of reduced feeding. Immediately after treatment, irradiated semi‐slugs displayed unusual behavior by remaining still and out in the open rather than immediately moving to a protected hiding place as expected. However, within 1 day they had moved to a protected hiding spot. Some irradiated semi‐slugs lived for many weeks after irradiation and appeared to move normally but feed less.
Figure 3.
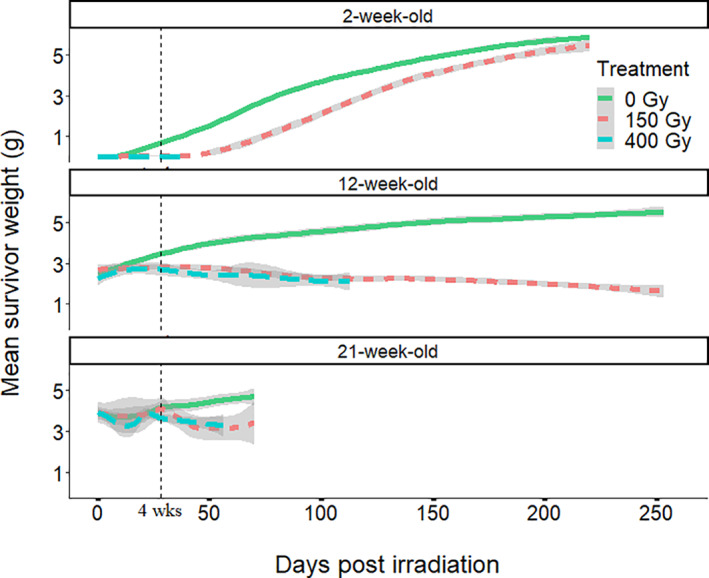
Parmarion martensi mean weight change of survivors in each cohort (with 2 standard error cloud) over time post‐irradiation treatment in each age class. Weight gain was reduced in all age groups after irradiation. The upward trending weight gain line in the 2‐week‐old graph represents the one surviving semi‐slug at 150 Gy which continued to feed and grow for the duration of the experiment, but without laying eggs.
3.3. Reproduction
Reproduction was greatly reduced or prevented by irradiation at both 150 and 400 Gy, with significant effects of age (chi‐square = 4.4, df = 1, P = 0.035) and irradiation treatment (chi‐square = 1401, df = 1, P < 0.0001) (Table 1). Two‐week‐old and 12‐week‐old immature P. martensi did not produce any eggs after irradiation at either dose. Actively reproducing semi‐slugs in the 21‐week‐old group produced a total of 105 eggs after radiation treatment at 150 Gy and 15 eggs at 400 Gy. All eggs were produced within the first week following irradiation and none of them hatched. In total, 8843 eggs were collected from semi‐slugs in control groups (Table 1), and on average 48.7% of the eggs in individual egg clusters hatched. Thus, irradiation at 150 and 400 Gy prevented reproduction in immature P. martensi (2‐ and 12‐week‐old semi‐slugs) and caused sterility in reproductive P. martensi (21‐week‐old semi‐slugs).
4. DISCUSSION
In our study, irradiation had broad deleterious effects on P. martensi growth and reproduction. Irradiation of P. martensi at phytosanitary doses of 150 and 400 Gy caused reduced weight gain in all age classes because of reduced feeding and possibly lower assimilation of nutrients, and shortened survivorship significantly. Irradiation arrested egg development in immature stages and prevented egg hatching in actively reproducing adults, which demonstrated its efficacy as a quarantine treatment. No post‐irradiation recovery was observed at the doses tested. Therefore, irradiation treatment at 150 or 400 Gy will provide quarantine security because any P. martensi on exported irradiated fresh produce will be unable to reproduce.
Whereas irradiation at phytosanitary doses can stop reproduction in P. martensi, it is unknown how irradiation might affect the parasitic nematode A. cantonensis inside them. Nematodes can be highly tolerant of irradiation, with doses to prevent reproduction ranging from 100 to 2000 Gy. 28 However, two previous studies suggested that irradiation treatment at doses like those used for phytosanitary irradiation, i.e. 150–400 Gy, may be able to limit or prevent development and reproduction in A. cantonensis. Pai et al. 29 found no nematodes or pathologic findings in the heart, lung, or brain tissue of rats infected with A. cantonensis larvae collected from snails that had been irradiated at 500 Gy and concluded that this dose was sufficient to prevent successful infection; in their study, larvae irradiated at 250 Gy that succeeded in maturing to the adult stage also failed to reproduce. In immunization studies, Lee 30 reported that an irradiation dose of 400 Gy significantly reduced development of third‐stage larvae of A. cantonensis, and that third‐stage larvae irradiated at 200 Gy which succeeded in maturing to the adult stage did not reproduce. We are planning additional studies to examine the effects phytosanitary irradiation at 150 and 400 Gy on A. cantonensis survival, infectivity, and development in rats.
Phytosanitary irradiation treatments for pests requiring a high degree of quarantine security are approved after large‐scale confirmatory testing of the most tolerant life stage at a dose predicted to cause essentially 100% mortality or sterility. 1 The USDA has used response at the probit 9 level (99.9968% mortality or sterility at the 95% confidence level) as the basis for approving treatments against tephritid fruit flies, which requires testing of 93 613 individuals with no survivors. 31 Other countries accept quarantine treatment efficacy at a 99.99% response level which requires testing 29 956 individuals with no survivors. This level of testing is feasible for tephritid fruit flies because of their short generation time, high fecundity, and availability of artificial diets which allow for rearing of large numbers of individuals. Schortemeyer et al. 32 and Follett et al. 33 have pointed out that large‐scale testing may be impractical for many quarantine pests, such as wood boring insects and ants, because of their long generation time, difficulties rearing, and longevity, and this may also be the case for many gastropods. Low replication studies with gastropod pests may still be useful to predict an irradiation dose for control. Ideally, testing should include multiple doses causing >90% mortality as well as several doses causing 100% mortality to help pinpoint an effective dose with statistical rigor. 22
In our study, a radiation dose of 150 Gy prevented reproduction in P. martensi, but lower doses were not tested and a dose less than 150 Gy may have been effective for control. Radiation tolerance information is now available for five species of gastropods in five families. Hollingsworth et al. 34 showed that reproduction in the orchid snail Zonitoides arboreus (Say) (Stylommatophora: Gastrodontidae) could be prevented by irradiation at 70 Gy, suggesting gastropods may be controlled with relatively low radiation doses. Hallman 35 showed that eggs laid by adults of the brown garden snail, Cornu aspersum (Müller) (Stylommatophora: Helicidae), did not hatch at radiation doses ≥75 Gy. Several radiotolerance studies have been conducted with the freshwater snail, Biomphalaria glabrata (Say) (Siphonariida: Planorbidae), an intermediate host of the trematode Schistosoma mansoni Sambon (Strigeidida: Schistosomatidae), a causative agent of schistosomiasis in humans. Perlowagora‐Szumlewicz 36 observed limited egg hatch in B. glabrata at 84 Gy but no egg hatch at 112 Gy, Cantinha et al. 37 reported no egg hatch in B. glabrata at 90 Gy, and Laird et al. 38 found no egg hatch in B. glabrata at radiation doses of 160, 320 and 640 Gy but reported recovery of egg fertility at 2 months post treatment at 80 Gy. Fujita and Egami 39 showed a reduction and subsequent recovery of egg laying in the pond snail Physella acuta (Draparnaud) (Siphonariida: Physidae) irradiated at 100 Gy. Our results with P. martensi are consistent with radiation tolerance research of other snails which suggests that doses in the range of 70–160 Gy can prevent reproduction. Post‐irradiation recovery may occur at certain substerilizing doses for some species. Although radiation tolerance information is available for only a small subset of gastropod species, consistent results suggest that the internationally approved and widely used generic dose for tephritid fruit flies of 150 Gy may be effective in providing quarantine security against many gastropods. Irradiation doses well below those causing full sterility may have other significant negative effects on reproductive performance. For example, in B. glabrata, 160 Gy completely prevented egg hatch, but a dose of 20 Gy reduced egg hatch to 5.9% compared with 98.8% in nonirradiated controls. 38 In addition to physiological and genetic changes, irradiation may change gastropod behavior. For example, irradiation at 15 and 35 Gy decreased photo‐response to light gradients and reduced aversion to salt in adults of the grey garden slug, Deroceras reticulatum (Müller) (Stylommatophora: Agriolimacidae). 40
The Class Gastropoda includes an estimated 65 000 to 80 000 species. 41 A 2009 risk assessment identified 46 snail species from 18 families as priority quarantine pests in the USA and included P. martensi. 4 The US Department of Agriculture, Animal Plant Health Inspection Service (APHIS), Plant Protection and Quarantine (PPQ) Branch maintains a database of intercepted gastropods and other mollusks, and many species are designated as quarantine pests, whether actionable or not. 3 P. martensi is an actionable quarantine pest for the continental USA, US Virgin Islands, American Samoa, and Guam. It is established across south‐east and east Asia and the Hawaiian Islands, and most recently was discovered in Puerto Rico (where the congener Parmarion intermedium is also established and is also a vector of A. cantonensis) (D Robinson, pers. comm., 2021). In Hawai'i, P. martensi is a polyphagous agricultural pest and has been found attacking fruit and vegetable crops such as lettuce and papaya in the field. 42 The California Department of Agriculture intercepted P. martensi 37 times between 2009 and 2016 and is now using dogs to detect this quarantine species on nursery stock and plant parts from Hawai'i 43 ; P. martensi was intercepted mainly on potted nursery crops such as Dracaena spp., but also on floriculture crops, ginger, taro stems, and sweet potatoes. Our results show that phytosanitary irradiation can prevent movement of viable P. martensi in treated agricultural products such as sweet potatoes, but ginger, taro stems, and floriculture crops do not typically receive any type of postharvest disinfestation treatment and rely instead on field control, farm certification, and inspection. Field control methods for slugs and snails include sanitation (e.g. removal of objects that serve as hiding places) and the use of poison food baits, such as those containing iron phosphate or metaldehyde. 42 Hence, nursery and floriculture crops provide a significant pathway for movement of viable P. martensi, some of which may be infected with A. cantonensis.
5. CONCLUSION
Phytosanitary irradiation treatments have been developed for quarantine insect pests including the generic radiation doses of 150 Gy for tephritid fruit flies and 400 Gy for other insects. Other invertebrates besides insects may be classified as quarantine pests and require a disinfestation treatment, including snails and slugs (Class Gastropoda). Irradiation at 150 and 400 Gy reduced growth, increased the mortality rate, and prevented reproduction in the high priority semi‐slug pest P. martensi, a vector of the human pathogenic nematode A. cantonensis. Phytosanitary irradiation treatment at doses ≥150 Gy will prevent the establishment of reproductive populations of P. martensi and thereby reduce the risk of transmission of A. cantonensis. Although radiation tolerance information is available for only a small subset of gastropod species, consistent results suggest that the internationally approved and widely used generic dose for tephritid fruit flies of 150 Gy may be effective in providing quarantine security against many gastropods.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
We are grateful to Dorothy Alontaga, Julie Clapp, and David Robinson (U.S. Department of Agriculture, Animal and Plant Health Inspection Service (USDA)‐APHIS) for providing information on gastropod pest interceptions. Melissa Postler (USDA‐U.S. Department of Agriculture, Agricultural Research Service (ARS)) assisted with radiation dosimetry. Funding for this work was provided by the US Department of Agriculture, Agricultural Research Service. the University of Hawai'i at Hilo, Daniel K. Inouye College of Pharmacy. and the Hawai'i State Legislature. This article reports the results of research only. Mention of a proprietary product does not constitute an endorsement or a recommendation by USDA for its use. USDA is an equal opportunity employer.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Follett PA, Phytosanitary irradiation for fresh horticultural commodities: generic treatments, current issues and next steps. Stewart Postharvest Rev 3:7 (2014). [Google Scholar]
- 2. Barkai‐Golan R and Follett PA, Irradiation for Quality Improvement, Microbial Safety and Phytosanitation of Fresh Produce. Academic Press, San Diego, CA, p. 302 (2017). [Google Scholar]
- 3. Robinson DG, Alien invasions: the effects of the global economy on non‐marine gastropod introductions into the United States. Malacologia 41:413–438 (1999). [Google Scholar]
- 4. Cowie RH, Dillon RT, Robinson DG and Smith JW, Alien non‐marine snails and slugs of priority quarantine importance in the United States: a preliminary risk assessment. Am Malacol Bull 27:113–132 (2009). [Google Scholar]
- 5. Asato R, Taira K, Nakamura M, Kudaka J, Itokazu K and Kawanaka M, Changing epidemiology of Angiostrongyliasis cantonensis in Okinawa prefecture Japan. Jpn J Infect Dis 57:184–186 (2004). [PubMed] [Google Scholar]
- 6. Hollingsworth RG, Kaneta R, Sullivan JJ, Bishop HS, Qvarnstrom Y, da Silva AJ et al., Distribution of Parmarion cf. martensi (Pulmonata: Helicarionidae), a new semi‐slug pest on Hawaii Island, and it's potential as a vector for human angiostrongyliasis. Pac Sci 61:457–467 (2007). [Google Scholar]
- 7. Qvarnstrom Y, Bishop HS and da Silva AJ, Detection of rat lungworm in intermediate, definitive, and paratenic hosts obtained from environmental sources. Hawai'i J Med Public Health 72:63–69 (2013). [PMC free article] [PubMed] [Google Scholar]
- 8. Medeiros MC, Rollins RL, Echaluse MV and Cowie RH, Species identity and size are associated with rat lungworm infection in gastropods. Ecohealth 17:183–193 (2020). [DOI] [PubMed] [Google Scholar]
- 9. Hochberg NS, Park SY, Blackburn BG, Sejvar JJ, Gaynor K, Chung H et al., Distribution of eosinophilic meningitis cases attributable to Angiostrongylus cantonensis, Hawaii. Emerg Infect Dis 13:1675–1680 (2007). [DOI] [PubMed] [Google Scholar]
- 10. Cowie RH, Biology, Systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawai'i J Med Public Health 72:6–9 (2013). [PMC free article] [PubMed] [Google Scholar]
- 11. Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R et al., Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143:1087–1118 (2016). [DOI] [PubMed] [Google Scholar]
- 12. Cowie RH, Catalog and bibliography of the nonindigenous nonmarine snails and slugs of the Hawaiian islands, Bishop Museum Occasional Papers, 50: 1–66 (1997). [Google Scholar]
- 13. Johnston DI, Dixon MC, Elm JL Jr, Calimlim PS, Sciulli RH and Park SY, Review of cases of angiostrongyliasis in Hawaii, 2007–2017. Am J Trop Med Hyg 101:608–616 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JR, Hayes KA, Yeung NW and Cowie RH, Diverse gastropod hosts of Angiostrongylus cantonensis, the rat lungworm, globally and with a focus on the Hawaiian islands. PLoS One 9:e94969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jarvi SI, Howe K and Macomber P, Angiostrongyliasis or rat lungworm disease: a perspective from Hawai'i. Curr Trop Med Rep 5:59–66 (2018). [Google Scholar]
- 16. Cowie R, Hayes K, Kim J, Bustamente K and Yeung N, Parmarion martensi Simroth, 1893 (Gastropoda: Ariophantidae), an intermediate host of Angiostrongylus cantonensis (rat lungworm), on Maui. Bishop Museum Occasional Papers, 123: 7–10 (2018). [Google Scholar]
- 17. Jarvi SI, Eamsobhana P, Quarta S, Howe K, Jacquier S, Hanlon A et al., Estimating human exposure to rat lungworm (Angiostrongylus cantonensis) on Hawai'I Island: a pilot study. Am J Trop Med Hyg 102:69–77 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niebuhr CN, Jarvi SI, Kaluna L, Fletcher BLT, Deane AR, Leinbach IL et al., Occurrence of rat lungworm (Angiostrongylus cantonensis) in invasive coqui frogs (Eleutherodactylus coqui) and other hosts in Hawaii. J Wildl Dis 56:203–207 (2020). [PubMed] [Google Scholar]
- 19. Richards CS and Merritt JW, Studies on Angiostrongylus cantonensis in molluscan intermediate hosts. J Parasitol 53:382–388 (1967). [PubMed] [Google Scholar]
- 20. Crook JR, Fulton SE and Supanwong K, The infectivity of third stage Angiostrongylus cantonensis larvae shed from drowned Achatina fulica snails and the effect of chemical agents on infectivity. Trans R Soc Trop Med Hyg 65:602–605 (1971). [DOI] [PubMed] [Google Scholar]
- 21. Wang QP, Lai DH, Zhu XQ, Chen XG and Lun ZR, Human angiostrongyliasis. Lancet Infect Dis 8:621–630 (2008). [DOI] [PubMed] [Google Scholar]
- 22. Follett PA, Generic radiation quarantine treatments: the next steps. J Econ Entomol 102:1399–1406 (2009). [DOI] [PubMed] [Google Scholar]
- 23. IPPC , (International Plant Protection Convention), Irradiation Treatment for Fruit Flies of the Family Tephritidae (Generic), ISPM 28, Annex 7. IPPC/Food and Agriculture Organization of the United Nations, Rome: (2009). [Google Scholar]
- 24. Roberts P and Follett PA, Food irradiation for phytosanitary and quarantine purposes, in Food Irradiation Technologies: Concepts, Applications and Outcomes, ed. by Ferreira ICFR, Antonio AL and Verde SC. Royal Society of Chemistry, Cambridge, UK, pp. 169, 454–182 (2017). [Google Scholar]
- 25. Follett PA, Irradiation as a methyl bromide alternative for postharvest control of Omphisa anastomosalis (Lepidoptera: Pyralidae) and Euscepes postfasciatus and Cylas formicarius elegantulus (Coleoptera: Curculionidae) in sweet potatoes. J Econ Entomol 99:32–37 (2006). [DOI] [PubMed] [Google Scholar]
- 26. Hamilton LJ, Tagami Y, Kaluna L, Jacob J, Jarvi SI and Follett PA, Demographics of the semi‐slug Parmarion martensi, an intermediate host for Angiostrongylus cantonensis in Hawaii, during laboratory rearing. Parasitology 148:153–158 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. SAS , JMP User's Guide. SAS Inc, Carey, NC, USA: (2015). [Google Scholar]
- 28. Myers RY, Follett PA, Mello CL and Snook KA, Effects of irradiation on the reproduction of Rotylenchulus reniformis . Nematology 20:265–269 (2018). [Google Scholar]
- 29. Pai H‐H, Ko Y‐C and Chen E‐R, Killing effect of gamma irradiation on Angiostrongylus cantonensis in snails. Am J Trop Med Hyg 48:827–830 (1993). [DOI] [PubMed] [Google Scholar]
- 30. Lee SH, The use of irradiated third‐stage larvae of Angiostrongylus cantonensis as antigen to immunize albino rats against homologous infection. Proc Helminthol Soc Wash 36:95–97 (1969). [Google Scholar]
- 31. Couey HM and Chew V, Confidence limits and sample size in quarantine research. J Econ Entomol 79:887–890 (1986). [Google Scholar]
- 32. Schortemeyer M, Thomas K, Haack RA, Uzunovic A, Hoover K, Simpson JA et al., Appropriateness of probit‐9 in the development of quarantine treatments for timber and timber commodities. J Econ Entomol 104:717–731 (2011). [DOI] [PubMed] [Google Scholar]
- 33. Follett PA, Porcel S and Calcaterra LC, Effects of irradiation on queen survivorship and reproduction in the invasive fire ant Solenopsis invicta (hymenoptera: Formicidae) and a proposed phytosanitary irradiation treatment for ants. J Econ Entomol 109:2348–2354 (2016). [DOI] [PubMed] [Google Scholar]
- 34. Hollingsworth RG, Follett PA and Armstrong JA, Effects of irradiation on the reproductive ability of Zonitoides arboreus, a snail pest of orchids. Ann Appl Biol 143:395–399 (2003). [Google Scholar]
- 35. Hallman GJ, Phytosanitary irradiation of the invasive herbivorous terrestrial snail Cornu aspersum (Stylommatophora: Helicidae). Fla Entomol 99:156–158 (2016). [Google Scholar]
- 36. Perlowagora‐Szumlewicz A, Effect of ionizing radiation on the population kinetics of the snail Australorbis glabratus: age at exposure and the effects on reproduction. Radiat Res 23:392–404 (1964). [PubMed] [Google Scholar]
- 37. Cantinha RS, Amaral A, Borrely SI, Nakano E, Silva LRS and Melo AMMA, Effects of high dose rate gamma radiation on survival and reproduction of Biomphalaria glabrata , in Proceedings of the International Nuclear Atlantic Conference, Vol. 7. Associação Brasileira de Energia Nuclear, Rio de Janeiro, p. 245 (2009). [Google Scholar]
- 38. Laird F, Chiriboga J, Pellegrino J, Colón JI and Martínez Silva R, Effect of radiation on the reproductive potential of Biomphalaria glabrata . Rev Bras Pesqui Med Biol 1:157–162 (1968). [Google Scholar]
- 39. Fujita S and Egami N, Effect of gamma irradiation on the reproductive system of the pond snail Physa acuta . Radiat Res 98:362–369 (1984). [PubMed] [Google Scholar]
- 40. Kaufman BZ, Ripatti PO and Markova LV, Effect of gamma irradiation on the preference behavior and lipid metabolism in grey garden slug Deroceras reticulatum Mull. Biology . Bull Russ Acad Sci 31:476–479 (2004). [PubMed] [Google Scholar]
- 41. Bouchet P, Rocroi J‐P, Frýda J, Hausdorf B, Ponder W, Valdes A et al., Classification and nomenclator of gastropod families. Malacologia 47:1–368. ConchBooks: Hackenheim, Germany. ISBN 3‐925919‐72‐5, ISBN 3‐925919‐72‐4. 397 pp (2005). [Google Scholar]
- 42. Hollingsworth RG, Howe K and Jarvi SI, Control measures for slug and snail hosts of Angiostrongylus cantonensis, with special reference to the semi‐slug Parmarion martensi. Hawai'i journal of Medicine & Public . Health 72:75–80 (2013). [PMC free article] [PubMed] [Google Scholar]
- 43. Leathers J, California Pest Rating for a Semi‐SLug, Parmarion martensi (Simroth), Gastropoda: Helicarionidae, pest rating: A. Retrieved from the California Department of Food and Agriculture website (2016). Available: https://blogs.cdfa.ca.gov/Section3162/?p=1708 [26 May 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


