Abstract
For many cancers, adolescents and young adults (AYAs) have a poorer prognosis than pediatric patients. Our study evaluates survival outcomes of children (0‐17 years) and AYAs (18‐39 years) diagnosed with acute myeloid leukemia (AML) in the Netherlands between 1990 and 2015 (N = 2058) utilizing the population‐based Netherlands Cancer Registry, which includes information on therapy and site of primary treatment. Five‐ and 10‐year relative (disease‐specific) survival were estimated for all patients, children and AYAs. Multivariable analyses were performed using generalized linear models (excess mortality) and logistic regression (early mortality). AYAs with AML had a substantially lower 5‐ and 10‐year relative survival than children (5‐year: 43% vs 58%; 10‐year: 37% vs 51%). The gap in 5‐year relative survival was largest (nearly 20 percent‐points) in 2010 to 2015, despite survival improvements over time across all ages. The multivariable‐adjusted excess risk of dying was 60% higher in AYAs (95% CI: 37%‐86%). Early mortality (death within 30 days of diagnosis) declined over time, and did not differ between children and AYAs. In conclusion, AYAs diagnosed with AML in the Netherlands had a worse prognosis than pediatric patients. The survival gap seemed most pronounced in recent years, suggesting that improvements in care resulting in better outcome for children have not led to equal benefits for AYAs.
Keywords: acute myeloid leukemia, adolescents and young adults, children, early mortality, relative survival
What's new?
For many cancers, children under 18 have better survival outcomes than adolescents and young adults, age 18‐39. Here, the authors evaluated long‐term survival of young people diagnosed with acute myeloid leukemia in the Netherlands. When they compared outcomes, they found that adolescents and young adults had a worse prognosis than children. Although survival has improved across all age groups, the disparity between children and adolescents and young adults has widened. Treatments that improved outcomes for children, therefore, do not appear to have benefited adolescents and young adults to the same degree.

Abbreviations
- ALL
acute lymphoblastic leukemia
- alloSCT
allogeneic SCT
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- ATO
arsenic trioxide
- ATRA
all‐trans retinoic acid
- autoSCT
autologous SCT
- AYA
adolescent and young adult
- BRP
Personal Records Database
- CBF
core‐binding factor
- CBS
Statistics Netherlands
- CCMO
Central Committee on Research Involving Human Subjects
- CI
confidence interval
- CR1
first complete remission
- DCOG
Dutch Childhood Oncology Group
- FLT3‐ITD
fms‐related tyrosine kinase 3
- GLMs
generalized linear models
- HOVON
Dutch‐Belgian Hemato‐Oncology Cooperative Group
- HRs
hazard ratios
- ICCC‐3
International Classification of Childhood Cancer
third edition
- ICD‐O‐3
International Classification of Diseases for Oncology, third edition
- IKNL
Netherlands Comprehensive Cancer Organization
- ML‐DS
myeloid leukemia associated with Down syndrome
- NCI
National Cancer Institute
- NCR
Netherlands Cancer Registry
- NUP98
nucleoporin 98kD
- OR
odds ratio
- PALGA
Nationwide Network and Registry of Histopathology and Cytopathology
- SAKK
Swiss Group for Clinical Cancer Research
- SCT
stem cell transplantation
- WHO
World Health Organization
1. INTRODUCTION
Acute myeloid leukemia (AML) comprises 15% to 20% of all pediatric leukemias. AML is a life‐threatening disease and accounts disproportionately for about one‐third of the deaths from childhood leukemia. After a peak in infants, the incidence of AML declines, reaching a minimum in 1 to 9 year old children, which is followed by a gradual increase through adolescence and (young) adulthood. 1 , 2 The prognosis of pediatric AML patients has improved in the past decades with 5‐year survival rates currently about 70% to 75% in Europe and the United States. 2 , 3 , 4 , 5 , 6 The favorable trend in survival of pediatric AML has been attributed to better supportive care, optimization of risk stratification, intensification of chemotherapy, improvements in stem cell transplantation (SCT) and higher salvage rates at relapse. 2 , 7 , 8
Adolescent and young adult (AYA) cancer patients are often reported to have an inferior prognosis compared to children with the same disease. Although the higher rate of survival improvement among AYAs since the early 1990s narrowed the survival gap in Europe, AYAs with cancer still had a worse prognosis than pediatric patients in the mid‐2000s. In the same period, survival of AYAs with cancer in the United States increased at a similar rate as that of children and remained to lag behind. 9 Age at diagnosis is an important prognostic factor for AML and is inversely associated with the probability of survival. 1
So far, population‐based survival of children and AYAs diagnosed with AML has been investigated in a limited number of studies from Europe and the United States. 10 , 11 , 12 , 13 , 14 In general, AYAs experienced lower long‐term survival compared to pediatric patients; however, the observed differences did not always reach statistical significance, perhaps because of small sample sizes. Moreover, results were generally not specified by cytogenetic risk group, therapy or site of treatment.
Long‐term survival of children and AYAs with AML in the Netherlands has not been compared yet, despite the availability of high‐quality data collected by the Netherlands Cancer Registry (NCR). According to a recent study focusing on Dutch pediatric AML patients, 5‐year overall survival for the period 2010 to 2015 was 84% for younger children (1‐9 years) and 66% for older children and adolescents (10‐17 years). 15 So, increasing age was already associated with worse survival outcome within the pediatric AML population.
In the present study, we investigated long‐term survival of children and AYAs (0‐39 years) diagnosed with AML in the Netherlands between 1990 and 2015 using population‐based, nationwide data from the NCR including information on therapy and site of primary treatment. Our primary objective was to determine whether AYA AML patients (18‐39 years) also have a worse prognosis than pediatric AML patients (0‐17 years) in the Netherlands and if differences in prognosis have changed over time due to improved survival.
2. PATIENTS AND METHODS
2.1. Data collection
All patients aged 0 to 39 years who were diagnosed with AML in the Netherlands between 1990 and 2015 were identified using the NCR. The NCR is a population‐based cancer registry with national coverage since 1989 and has an estimated completeness of at least 96%. 16 , 17 The NCR receives information concerning all incident cancers diagnosed in the Netherlands from the Nationwide Network and Registry of Histopathology and Cytopathology (PALGA) and the National Registry of Hospital Discharges. After notification, trained registration clerks of the NCR collect data on patient, tumor and treatment characteristics from medical records. Annual linkage with the nationwide Personal Records Database (BRP) is performed to check vital status (last linkage: 1 February 2020).
AML was defined based on International Classification of Childhood Cancer, third edition (ICCC‐3), diagnostic group Ib “Acute myeloid leukemias”. AML diagnoses were selected from the NCR using International Classification of Diseases for Oncology, third edition (ICD‐O‐3), morphology codes 9840, 9841 (ICD‐O‐2), 9861, 9864 (ICD‐O‐2), 9865 to 9867, 9869 to 9874, 9877 to 9879, 9891, 9895 to 9898, 9910 to 9912, 9920, 9930, 9931 and 9932 (ICD‐O‐2). Since 2001, core‐binding factor (CBF) leukemia (t[8;21][q22;q22], inv[16][p13.1;q22] or t[16;16][p13.1;q22]) has consistently been registered in the NCR based on morphology code. To obtain information regarding CBF leukemia for children diagnosed during the entire study period, linkage was performed with the database of the Dutch Childhood Oncology Group (DCOG), which includes all patients treated at pediatric oncology centers in the Netherlands. 15 Cytogenetic testing for CBF leukemia has been performed more structurally since 2001. Acute promyelocytic leukemia (APL, ICD‐O‐3 code 9866) and myeloid leukemia associated with Down syndrome (ML‐DS, ICD‐O‐3 code 9898) have a much better prognosis than the other AML subtypes and are treated differently. Therefore, APL patients (N = 202) were analyzed separately. ML‐DS patients (N = 28) were excluded from the relative survival, excess mortality and early mortality analyses, because ML‐DS was not diagnosed in AYAs. Depending on their age at diagnosis, patients were classified as either children (0‐17 years) or AYAs (18‐39 years). Patients were followed from their date of diagnosis until death, emigration, loss to follow‐up or 1 February 2020 (end of follow‐up), whichever occurred first.
2.2. Statistical analyses
Descriptive statistics were used to assess characteristics of the study population. Statistical significance of differences between children and AYAs was tested by Pearson's Χ 2 tests or Fisher's exact tests (N ≤ 5 in one or more categories) for categorical variables.
Five‐ and 10‐year relative survival rates were estimated utilizing the traditional cohort approach by applying the strs procedure in Stata 18 using 6‐month intervals during the first year of follow‐up and annual intervals thereafter. Relative survival estimates disease‐specific survival by correcting for mortality due to competing causes and is calculated by dividing the observed patient survival by the expected survival of a comparable cohort from the general population. 18 Expected probabilities of survival were determined by the Ederer II method 19 using Dutch population life tables stratified by age, sex and calendar year, which were obtained from Statistics Netherlands (CBS). Linear trends in relative survival over the diagnostic periods (1990‐1999, 2000‐2009 and 2010‐2015) were assessed by generalized linear models (GLMs) using a Poisson assumption for the observed number of deaths. 18 GLMs were also utilized to investigate the relation between age at diagnosis and excess mortality, which is the mortality analogue of relative survival. To adjust for potential confounding, multivariable models were run including sex, diagnostic period, SCT (only for AML) and site of primary treatment. Patients were classified as being treated at an academic hospital when they had received chemotherapy or a SCT at such type of hospital. All GLMs were adjusted for follow‐up time in years. The 1 to 9 year age group was used as reference when modeling excess mortality for more detailed age categories, because infants (0 years) with AML have a clearly worse prognosis than older children and the main aim of our study was to compare survival outcomes of children and AYAs. Patients who died on the day of diagnosis were included in the survival models with a follow‐up time of 1 day.
Early mortality (death within 30 days of diagnosis) was examined as a secondary outcome to evaluate whether the potentially worse long‐term survival of AYAs compared to children could be the result of higher mortality among AYAs shortly after diagnosis. Patients who died on the day of diagnosis were therefore included in the early mortality analyses. Logistic regression analyses were performed to investigate univariable and multivariable associations of age at diagnosis with early mortality. Multivariable analyses were adjusted for sex, diagnostic period and site of primary treatment. Furthermore, diagnostic period was entered as a continuous term into logistic regression models to obtain P‐values for linear trends in early mortality over time. Patients diagnosed by autopsy were excluded from the relative survival, excess mortality and early mortality analyses (AML: N = 7, 0.4%; APL: N = 0). Death certificate only (DCO) cases are not included in the NCR as there is no linkage with the cause‐of‐death registry at an individual basis due to legislation.
CBF leukemia has more consistently been tested and registered since 2001. To evaluate the potentially confounding effect of this favorable risk subtype on the excess mortality estimates, GLMs with and without adjustment for CBF leukemia (no vs yes) were compared for the period 2001 to 2015. Furthermore, relative survival was estimated in children and AYAs diagnosed with CBF leukemia after 2001.
Statistical analyses were executed using Stata 16 (StataCorp LLC, College Station, Texas). Two‐sided P‐values <.05 were considered statistically significant.
3. RESULTS
3.1. Characteristics study population
In the period 1990 to 2015, 2058 patients aged below 40 years were diagnosed with AML in the Netherlands, of whom 675 were children (0‐17 years) and 1383 were AYAs (18‐39 years). Patient characteristics are presented in Table 1. The median age at diagnosis was 6 years in children and 31 years in AYAs. Pediatric AML patients were more commonly boys compared to girls, whereas both sexes were fairly equally represented among AYAs. With respect to the AML subtypes, APL occurred more frequently in AYAs (12% vs 5%), whereas the opposite pattern was observed for ML‐DS (0% vs 4%) and CBF leukemia (6% vs 14%). The proportion of patients treated at a nonacademic hospital was almost three times higher among AYAs compared to children (21% vs 8%). Of the patients who were treated at a nonacademic hospital, 82% received primary treatment at a teaching hospital. Furthermore, AYAs were nearly twice more likely to receive SCT than children (43% vs 23%). Patients with AML (excluding APL and ML‐DS) and APL were further analyzed separately. After exclusion of diagnoses based on autopsy, 1821 AML patients and 202 APL patients could be included in the analyses. ML‐DS patients (N = 28) were excluded.
TABLE 1.
Characteristics of children (0‐17 years) and AYAs (18‐39 years) diagnosed with AML in the Netherlands between 1990 and 2015
| Characteristics | Total | Children (0‐17 years) | AYAs (18‐39 years) | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P (χ 2) a | |
| Overall | 2058 | 675 | 1383 | ||||
| Median age at diagnosis in years, IQR | 26 | 21 | 6 | 12 | 31 | 11 | |
| Sex | .001 | ||||||
| Male | 1040 | 50.5 | 377 | 55.9 | 663 | 47.9 | |
| Female | 1018 | 49.5 | 298 | 44.2 | 720 | 52.1 | |
| Period of diagnosis | .04 | ||||||
| 1990‐1999 | 833 | 40.5 | 247 | 36.6 | 586 | 42.4 | |
| 2000‐2009 | 769 | 37.4 | 270 | 40.0 | 499 | 36.1 | |
| 2010‐2015 | 456 | 22.2 | 158 | 23.4 | 298 | 21.6 | |
| Subtype | <.001 | ||||||
| APL | 202 | 9.8 | 34 | 5.0 | 168 | 12.2 | |
| ML‐DS | 28 | 1.4 | 28 | 4.2 | 0 | 0.0 | |
| Myeloid sarcoma | 21 | 1.0 | 9 | 1.3 | 12 | 0.9 | |
| CBF leukemia: t(8;21) or inv(16)/t(16;16) b | 174 | 8.5 | 95 | 14.1 | 79 | 5.7 | |
| AML other | 1633 | 79.4 | 509 | 75.4 | 1124 | 81.3 | |
| Site of primary treatment | <.001 | ||||||
| Nonacademic hospital | 346 | 16.8 | 51 | 7.6 | 295 | 21.4 | |
| Academic hospital | 1711 | 83.2 | 624 | 92.4 | 1087 | 78.7 | |
| Therapy | |||||||
| Chemo | 1946 | 94.7 | 645 | 95.8 | 1301 | 94.1 | .11 |
| SCT | 744 | 36.2 | 155 | 23.0 | 589 | 42.6 | <.001 |
Notes: The % missing values was <1% for all variables included in this table. Statistically significant P values (P < .05) are displayed in bold.
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; AYAs, adolescents and young adults; CBF, core‐binding factor; IQR, interquartile range; ML‐DS, myeloid leukemia associated with Down syndrome; SCT, stem cell transplantation.
Fisher's Exact test was used instead of Pearson's Χ 2 test when N ≤ 5 in one or more categories.
CBF leukemia was more consistently tested and registered as from 2001.
3.2. Acute myeloid leukemia
3.2.1. Relative survival
The 5‐year relative survival of AML patients younger than 40 years in the Netherlands increased by 22 percent‐points during 1990 to 2015 from 40% to 62% (P trend < .001, Table 2). Similarly, the 10‐year relative survival improved from 37% to 47% between 1990 and 2009. Children (N = 612) had a better prognosis than AYAs (N = 1209, Table 2; Figure 1). Overall, 5‐ and 10‐year relative survival were approximately 15 percent‐points higher in children compared to AYAs. The rise in 5‐year relative survival between 1990 and 2015 was more pronounced among children (+25%, P trend < .001) than AYAs (+19%, P trend < .001), causing the survival gap to be largest in the latest period. In 2010 to 2015, the 5‐year relative survival of children was 74%, which was nearly 20 percent‐points higher than the corresponding estimate for AYAs (55%). Furthermore, the survival advantage of children over AYAs generally remained present when considering subgroups based on sex, site of primary treatment and therapy (Table 2). Regarding the AML subtypes, CBF leukemia was more structurally tested and registered as from 2001. Within the group of patients diagnosed with CBF leukemia since 2001 (children: N = 68, AYAs: N = 68), children also had a better 5‐year relative survival than AYAs (84% vs 75%), though this difference did not reach statistical significance.
TABLE 2.
Five‐year and 10‐year relative survival a of children (0‐17 years) and AYAs (18‐39 years) diagnosed with AML (excl. APL and ML‐DS) in the Netherlands between 1990 and 2015, overall and by subgroup
| 5‐year relative survival (1990‐2015) | 10‐year relative survival (1990‐2009) b | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Children (0‐17 years) | AYAs (18‐39 years) | Total | Children (0‐17 years) | AYAs (18‐39 years) | |||||||||||||
| N | % | SE | N | % | SE | N | % | SE | N | % | SE | N | % | SE | N | % | SE | |
| Overall | 1821 | 47.8 | 1.2 | 612 | 57.5 | 2.0 | 1209 | 42.9 | 1.4 | 1435 | 41.8 | 1.3 | 473 | 51.2 | 2.3 | 962 | 37.1 | 1.6 |
| Sex | ||||||||||||||||||
| Male | 935 | 46.9 | 1.6 | 341 | 55.5 | 2.7 | 594 | 42.0 | 2.0 | 738 | 40.4 | 1.8 | 270 | 48.3 | 3.0 | 468 | 35.7 | 2.2 |
| Female | 886 | 48.8 | 1.7 | 271 | 60.0 | 3.0 | 615 | 43.8 | 2.0 | 697 | 43.3 | 1.9 | 203 | 55.1 | 3.5 | 494 | 38.5 | 2.2 |
| Period of diagnosis | ||||||||||||||||||
| 1990‐1999 | 751 | 39.5 | 1.8 | 229 | 48.8 | 3.3 | 522 | 35.4 | 2.1 | 751 | 36.9 | 1.8 | 229 | 47.1 | 3.3 | 522 | 32.4 | 2.1 |
| 2000‐2009 | 684 | 49.3 | 1.9 | 244 | 56.2 | 3.2 | 440 | 45.4 | 2.4 | 684 | 47.1 | 1.9 | 244 | 55.0 | 3.2 | 440 | 42.7 | 2.4 |
| 2010‐2015 | 386 | 61.6 | 2.5 | 139 | 74.1 | 3.7 | 247 | 54.5 | 3.2 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| P trend | <.001 | <.001 | <.001 | <.001 | .04 | .001 | ||||||||||||
| Site of primary treatment | ||||||||||||||||||
| Nonacademic hospital | 287 | 35.0 | 2.8 | 44 | 36.4 | 7.3 | 243 | 34.7 | 3.1 | 249 | 29.4 | 2.9 | 43 | 37.3 | 7.4 | 206 | 27.7 | 3.1 |
| Academic hospital | 1534 | 50.2 | 1.3 | 568 | 59.1 | 2.1 | 966 | 45.0 | 1.6 | 1186 | 44.4 | 1.5 | 430 | 52.6 | 2.4 | 756 | 39.7 | 1.8 |
| Therapy | ||||||||||||||||||
| Chemo | 1731 | 49.7 | 1.2 | 587 | 59.3 | 2.0 | 1144 | 44.8 | 1.5 | 1357 | 43.6 | 1.4 | 451 | 53.0 | 2.4 | 906 | 38.9 | 1.6 |
| SCT | 732 | 53.3 | 1.9 | 150 | 53.4 | 4.1 | 582 | 53.2 | 2.1 | 551 | 48.0 | 2.1 | 130 | 51.7 | 4.4 | 421 | 46.8 | 2.5 |
Note: Statistically significant P values (P < .05) are displayed in bold.
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; AYAs, adolescents and young adults; ML‐DS, myeloid leukemia associated with Down syndrome; SCT, stem cell transplantation; SE, standard error.
Expected probabilities of survival were estimated using the Ederer II method.
10‐year relative survival could not be estimated for the most recent period 2010 to 2015 due to incomplete follow‐up.
FIGURE 1.
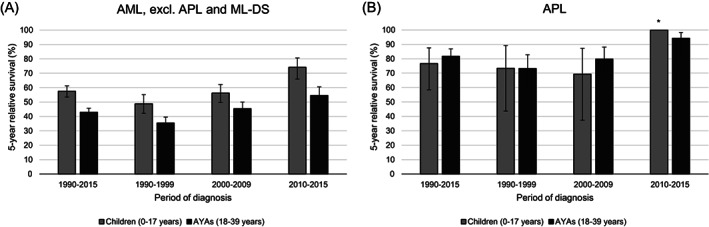
Five‐year relative survival of children (0‐17 years) and AYAs (18‐39 years) diagnosed with AML (excl. APL and ML‐DS, A) and APL (B) in the Netherlands between 1990 and 2015, overall and by diagnostic period. The error bars depict 95% CI of the survival estimates. *N at risk <10. AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; AYAs, adolescents and young adults; ML‐DS, myeloid leukemia associated with Down syndrome
Age‐specific 5‐year relative survival of AML is displayed in Figure 2. In the most recent time period, young children (1‐9 year olds) had the best prognosis (5‐year relative survival 84%). After a decline to 65% to 70% in 10 to 17 year olds, the 5‐year relative survival continued to drop to just over 50% in 18 to 24 year olds. Thereafter, 5‐year relative survival remained stable at 50% to 60% in older patients up to 39 years. Table S1 contains 5‐year relative survival estimates of AML for some alternative categories of age.
FIGURE 2.
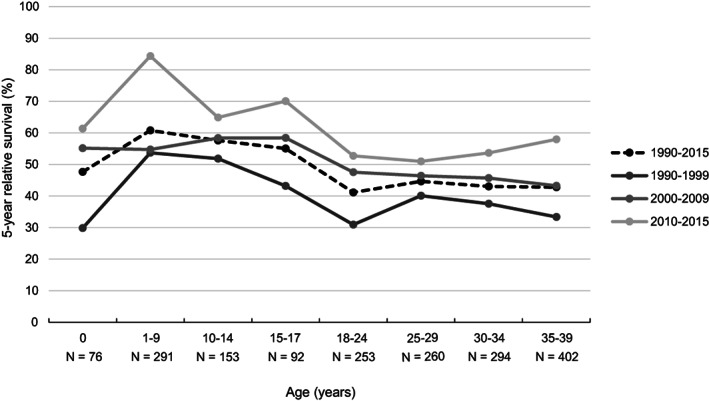
Age‐specific 5‐year relative survival of children and AYAs (0‐39 years) diagnosed with AML (excl. APL and ML‐DS) in the Netherlands between 1990 and 2015, overall and by diagnostic period. For each age category, the number of patients at risk in the entire period 1990 to 2015 is displayed. AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; AYAs, adolescents and young adults; ML‐DS, myeloid leukemia associated with Down syndrome
3.2.2. Regression excess mortality due to AML
Table 3 shows associations of age with excess mortality due to AML until 5 years of follow‐up. After adjustment for follow‐up time, sex, diagnostic period, SCT and site of primary treatment, AYAs experienced a 60% (95% confidence interval [CI]: 37%‐86%) higher excess mortality compared to children. Using the 1 to 9 year age category as reference, excess hazard ratios (HRs) of mortality were almost two times increased in 18 to 29 and 30 to 39 year olds (both P < .001). Patients aged 10 to 17 years did not have a significantly higher excess risk of dying than 1 to 9 year olds (excess HR = 1.2). Although being present in all diagnostic periods, the higher excess mortality associated with adolescence and young adulthood was most evident in the latest period (Table S2). In 2010 to 2015, the excess risk of dying was almost 2.5 times increased in 10 to 17 year olds (P = .02) and 4 to 5 times in 18 to 29 and 30 to 39 year olds (both P < .001), when compared to 1 to 9 year olds. Excess HR estimates obtained using alternative cut‐offs for age are shown in Table S3. A sensitivity analysis including patients diagnosed after 2001 showed that additional adjustment for CBF leukemia did not relevantly affect the effect estimates of age (data not shown). Finally, diagnosis in 2000 to 2009 and 2010 to 2015 was associated with a significantly decreased excess mortality when compared to diagnosis in 1990 to 1999 in multivariable analyses (data not shown).
TABLE 3.
Univariable and multivariable associations a of age with excess mortality due to AML (excl. APL and ML‐DS) in children and AYAs (0‐39 years) until 5 years of follow‐up, the Netherlands, 1990‐2015
| Variable | Nat risk | Until 5 years of follow‐up (1990‐2015) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Adjusted 1 b | Adjusted 2 c | ||||||||
| Excess HR | 95% CI | P‐value | Excess HR | 95% CI | P‐value | Excess HR | 95% CI | P‐value | ||
| Age | ||||||||||
| Children (0‐17 years) | 612 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||||
| AYAs (18‐39 years) | 1209 | 1.50 | (1.30‐1.73) | <.001 | 1.47 | (1.28‐1.70) | <.001 | 1.60 | (1.37‐1.86) | <.001 |
| Age (years) | ||||||||||
| 0 | 76 | 1.72 | (1.20‐2.47) | .003 | 1.83 | (1.27‐2.63) | .001 | 1.81 | (1.26‐2.60) | .001 |
| 1‐9 | 291 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||||
| 10‐17 | 245 | 1.17 | (0.90‐1.53) | .24 | 1.20 | (0.92‐1.56) | .18 | 1.24 | (0.95‐1.62) | .11 |
| 18‐29 | 513 | 1.69 | (1.36‐2.10) | <.001 | 1.68 | (1.35‐2.08) | <.001 | 1.82 | (1.45‐2.28) | <.001 |
| 30‐39 | 696 | 1.73 | (1.40‐2.13) | <.001 | 1.73 | (1.40‐2.13) | <.001 | 1.93 | (1.55‐2.40) | <.001 |
Note: Statistically significant P values (P < .05) are displayed in bold.
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; AYAs, adolescents and young adults; CI, confidence interval; excess HR, excess hazard ratio; ML‐DS, myeloid leukemia associated with Down syndrome; SCT, stem cell transplantation.
All models were adjusted for follow‐up time (years).
Additionally adjusted for sex (male, female) and period of diagnosis (1990‐1999, 2000‐2009, 2010‐2015).
Additionally adjusted for sex (male, female), period of diagnosis (1990‐1999, 2000‐2009, 2010‐2015), SCT (no, yes) and site of primary treatment (nonacademic hospital, academic hospital).
3.2.3. Early mortality
One patient was censored alive at a follow‐up of less than 30 days and therefore excluded from the early mortality analyses. In total, 114 patients (6%) died within 30 days of diagnosis between 1990 and 2015. During the study period, early mortality decreased by 5 percent‐points reaching 4% in 2010 to 2015 (P trend = .001), which was largely the result of the marked decline from 9% to 5% that occurred between 1990 to 1999 and 2000 to 2009. The frequency of early death was comparable among children (N = 39, 6%) and AYAs (N = 75, 6%, Figure 3). Figure 4 reveals that early mortality was lowest among 1 to 9 year olds, of whom 3% (N = 9) experienced early death between 1990 and 2015, and increased to 6% to 8% in older age categories.
FIGURE 3.
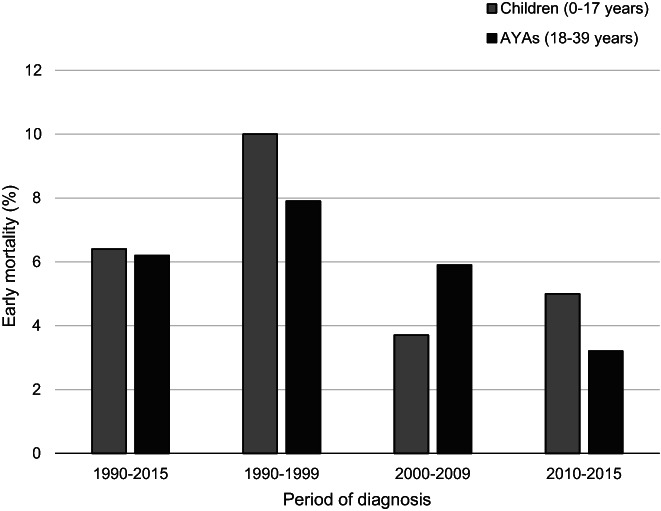
Early mortality (death within 30 days of diagnosis) in children (0‐17 years) and AYAs (18‐39 years) diagnosed with AML (excl. APL and ML‐DS) in the Netherlands between 1990 and 2015, overall and by diagnostic period. AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; AYAs, adolescents and young adults; ML‐DS, myeloid leukemia associated with Down syndrome
FIGURE 4.
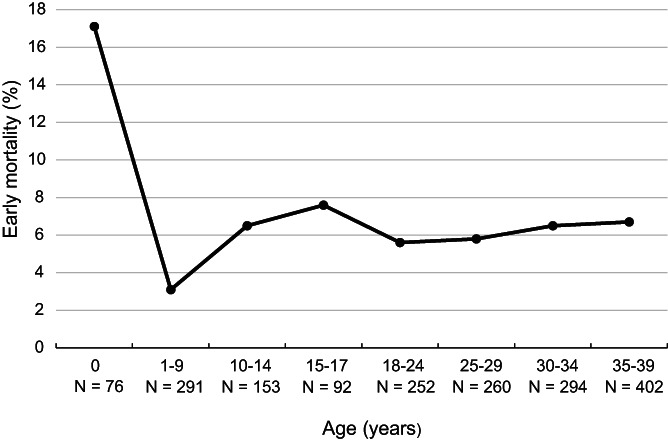
Age‐specific early mortality (death within 30 days of diagnosis) in children and AYAs (0‐39 years) diagnosed with AML (excl. APL and ML‐DS) in the Netherlands between 1990 and 2015. For each age category, the number of patients at risk in the entire period 1990 to 2015 is displayed. AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; AYAs, adolescents and young adults; ML‐DS, myeloid leukemia associated with Down syndrome
Multivariable‐adjusted associations of age with early mortality after AML diagnosis are presented in Table 4. The risk of early mortality did not significantly differ between children and AYAs (odds ratio [OR] AYAs vs children, 95% CI: 0.7, 0.4‐1.0) after adjustment for sex, diagnostic period and site of primary treatment. When compared to 1990 to 1999, significantly reduced ORs of early death were observed for the two more recent time periods (ie, 2000‐2009 and 2010‐2015, data not shown).
TABLE 4.
Univariable and multivariable associations of age with early mortality a due to AML (excl. APL and ML‐DS) in children and AYAs (0‐39 years), the Netherlands, 1990‐2015
| Univariable | Adjusted 1 b | Adjusted 2 c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Nearly death | OR | 95% CI | P‐value | OR | 95% CI | P‐value | OR | 95% CI | P‐value |
| Age | ||||||||||
| Children (0‐17 years) | 39 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||||
| AYAs (18‐39 years) | 75 | 0.97 | (0.65‐1.45) | .89 | 0.95 | (0.63‐1.42) | .80 | 0.68 | (0.44‐1.04) | .07 |
| Age (years) | ||||||||||
| 0 | 13 | 6.47 | (2.65‐15.79) | <.001 | 7.23 | (2.93‐17.86) | <.001 | 6.74 | (2.67‐17.00) | <.001 |
| 1‐9 | 9 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||||
| 10‐17 | 17 | 2.34 | (1.02‐5.34) | .04 | 2.40 | (1.05‐5.51) | .04 | 2.12 | (0.91‐4.93) | .08 |
| 18‐29 | 29 | 1.88 | (0.88‐4.03) | .10 | 1.86 | (0.86‐3.99) | .11 | 1.19 | (0.54‐2.63) | .66 |
| 30‐39 | 46 | 2.22 | (1.07‐4.59) | .03 | 2.26 | (1.09‐4.70) | .03 | 1.57 | (0.74‐3.34) | .24 |
Note: Statistically significant P values (P < .05) are displayed in bold.
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; AYAs, adolescents and young adults; CI, confidence interval; ML‐DS, myeloid leukemia associated with Down syndrome; OR, odds ratio.
Early mortality was defined as death within 30 days of diagnosis.
Adjusted for sex (male, female) and period of diagnosis (1990‐1999, 2000‐2009, 2010‐2015).
Adjusted for sex (male, female), period of diagnosis (1990‐1999, 2000‐2009, 2010‐2015) and site of primary treatment (nonacademic hospital, academic hospital).
3.3. Acute promyelocytic leukemia
Children and AYAs diagnosed with APL in the Netherlands had a 5‐ and 10‐year relative survival of 81% (1990‐2015) and 74% (1990‐2009), respectively (Table S4). A striking rise in 5‐year relative survival from 78% to 95% (+17 percent‐points) was observed between 2000 to 2009 and 2010 to 2015. The prognosis of children (N = 34) and AYAs (N = 168) with APL seemed roughly comparable with 5‐year relative survival estimates of 77% and 82%, respectively (Figure 1; Table S4). Moreover, the excess risk of mortality until 5 years of follow‐up did not significantly differ between the groups after adjustment for follow‐up time, sex, diagnostic period and site of primary treatment (excess HR AYAs vs children, 95% CI: 0.7, 0.3‐1.5). In total, 19 APL patients (9%) died within 30 days of diagnosis among which were 4 children (12%) and 15 AYAs (9%). Early mortality markedly decreased between 2000 to 2009 and 2010 to 2015 from 13% to 4%. These results should, however, be interpreted with caution as the number of early deaths among APL patients was small.
4. DISCUSSION
In this nationwide population‐based study among Dutch AML patients, we showed that AYAs (18‐39 years) had a worse 5‐year relative survival compared to children (0‐17 years) in the period 1990 to 2015. The survival gap was consistently present across the calendar periods of diagnosis, but was most pronounced in the latest period 2010 to 2015. These findings were supported by multivariable analyses in which we showed that excess mortality due to AML in AYAs was 60% higher in the total study period and more than 160% higher in 2010 to 2015. Early mortality did not differ between children and AYAs. Overall, favorable trends over time were observed for both 5‐year relative survival (increase) and early mortality (decline).
In contrast to AML, 5‐year relative survival did not seem to differ between children and AYAs diagnosed with APL. The prognosis of APL patients improved considerably between 2000 to 2009 and 2010 to 2015 reaching a 5‐year relative survival of 95%.
Similar to our findings for the Netherlands, several population‐based studies from Europe have consistently reported lower long‐term survival for AYAs with AML compared to pediatric patients. 11 , 13 , 14 According to the EUROCARE‐5 study (2000‐2007), 5‐year relative survival after AML diagnosis was 61% in European children (0‐14 years), which was 11 percent‐points higher than the corresponding estimate for AYAs (15‐39 years, P < .001). 11 In the Nordic countries, 5‐year relative survival estimates (2000‐2013) were 74% for 0 to 14 year olds, 69% for 15 to 24 year olds and 62% for 25 to 34 year olds. 14 The 5‐year relative survival of children (0‐17 years) in our study was 74% in 2010 to 2015, which was almost 20 percent‐points higher than the 55% observed among AYAs. Besides the European studies, two US studies have evaluated the prognosis of children and AYAs with AML in the general population between the 1970s/1980s and early 2010s. 10 , 12 Five‐year overall survival was 35% to 40% among AYAs compared to 45% to 52% among children. In addition to the lower survival, AYAs also experienced higher early mortality.
Currently, the outcome of young Dutch APL patients is excellent. We estimated a 5‐year relative survival of 95% in the most recent time period. The prognosis of APL strongly improved between 2000 to 2009 and 2010 to 2015, most likely as the result of the combined use of all‐trans retinoic acid (ATRA), initially with chemotherapy and later with arsenic trioxide (ATO), which has made APL the most curable form of AML in children and adults. 20 , 21 , 22 , 23 In line with the present results, previous publications have also reported similar population‐based survival in pediatric and AYA patients with APL. 12 , 24
Several factors have been proposed in the literature that could potentially contribute to the inferior prognosis of AYAs with cancer compared to pediatric patients, including differences in cancer biology, the lack of standardized treatment (not valid for AML), increased treatment‐related toxicity or transplant‐procedure related mortality with older age, longer delays in diagnosis and treatment, problems with adherence to treatment plans, lower participation rates in clinical trials and insurance barriers. 14 , 25 , 26 , 27
Additional research efforts are essential in the endeavor to improve the management of AYAs with AML and to narrow the currently existing survival gap. Future studies should, for instance, focus on disease biology and treatment characteristics. Differences in disease biology and disease‐specific therapies are suggested to be more important contributors to the survival disparity between pediatric and AYA AML patients than social issues. 12 Moreover, the inclusion of information concerning cause of death (eg, refractory disease, relapse, infection, treatment‐related toxicity, etc) could add valuable information as contributions of the various causes of death may differ depending on age at diagnosis.
AYAs with AML are thought to have different biological disease characteristics compared to children. Pediatric AML seems to differ cytogenetically and molecularly from adult AML. 10 , 28 , 29 , 30 For instance, the favorable risk subtype CBF AML has a higher relative frequency among children and adolescents. On the other hand, adult AML patients present more often with abnormalities of chromosomes 5 and 7, and internal tandem duplication mutations of fms‐related tyrosine kinase 3 (FLT3‐ITD), which are associated with a worse prognosis. Contrastingly, adverse risk translocations involving the gene nucleoporin 98 kD (NUP98) are almost exclusive to those of younger age and are more prevalent in children. 10 , 28 , 29 , 30 In children, single gene abnormalities are almost absent. Furthermore, Creutzig et al observed a gradual decline in the relative frequency of favorable cytogenetics from the age of 2 years, while the opposite seemed to apply to poor risk features. 31 Studies specifically focusing on cytogenetic and molecular abnormalities occurring in childhood and adolescence/young adulthood AML are, however, rather scarce.
The subtype information available in our study primarily related to morphology, which is of little prognostic value. Since 2001, CBF leukemia has consistently been registered in the NCR and patients have more structurally been tested for this subtype. 15 To investigate the role of CBF leukemia in the survival disparity that we observed between pediatric and AYA AML, we performed several sensitivity analyses in patients diagnosed after 2001. First, these analyses showed that adjustment for CBF leukemia in the multivariable models did not substantially alter the HR estimates of age. Second, within the subgroup of CBF leukemia patients, the survival gap remained present. The abovementioned findings indicate that the worse prognosis of AYA AML patients cannot (fully) be explained by differences in the relative frequency of CBF leukemia. Therefore, differences in other disease biological characteristics, diagnostic‐ and treatment‐related factors or, for example, retrieval at relapse, are likely to be involved as well.
For acute lymphoblastic leukemia (ALL), most AYAs fare better by adherence to pediatric(‐inspired) treatment regimens instead of adult ALL protocols. 26 , 32 , 33 , 34 Only a few studies have investigated the potential benefits of a similar approach in AYA AML with somewhat contradictory findings. 26 Generally, pediatric AML protocols include higher‐intensity chemotherapy cycles compared to adult protocols and SCT is given more restrictively 26 , 35 ; however, differences may be subtle in some settings. According to Woods et al, overall survival was significantly higher in 16 to 21 year old AML patients treated on pediatric trials (Children's Oncology Group) compared to adult trials (Cancer and Leukemia Group B and Southwest Oncology Group) 36 ; however, participants of the pediatric trials were significantly younger. An Australian study has reported 5‐year overall survival of 84% and 64% among AML patients aged 15 to 24 years treated on pediatric and adult protocols, respectively (P log‐rank = .08). 37 In the Nordic countries, 5‐year overall survival did not significantly differ between 15 to 18 year old AML patients treated in pediatric (51%) and adult (70%) settings, 38 although the inclusion of patients in the pediatric cohort started earlier. Two additional studies also reported comparable outcomes for pediatric and adult AML protocols, but each of these studies had its limitations (ie, large timespan of the data and large variety of institutional protocols included, and homogeneity of the pediatric and adult treatment regimens, respectively). 26 , 39 , 40
During our study period, pediatric AML patients in the Netherlands were treated according to one of six consecutive DCOG treatment protocols (ANLL‐87, ANLL‐92/94, ANLL‐97, AML 15, DB AML‐01 and NOPHO‐DBH AML 2012), 15 which generally consisted of four to five chemotherapy courses. The induction phase usually encompassed two cycles of an anthracycline, cytarabine and a third drug, which was followed by consolidation using high‐dose cytarabine as a single agent or in combination with, for example, mitoxantrone or etoposide. In the 1990s, it was standard of care to proceed with allogeneic SCT (alloSCT) after attaining first complete remission (CR1). Subsequently, use of upfront alloSCT became restricted to specific risk groups and was even completely omitted from the DB AML‐01 protocol. In the current NOPHO‐DBH AML 2012 protocol, alloSCT in CR1 has been reintroduced for poor risk patients. 15 For a nice schematic overview of the active DCOG AML protocols, we refer to Figure S1 of a previously published article by Reedijk et al. 15 AYAs with AML were treated according to protocols designed by the Dutch‐Belgian Hemato‐Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) (HOVON‐4(A), ‐29, ‐42(A), ‐92, ‐102 and ‐132). The remission‐induction regimens consisted of two chemotherapy cycles including an anthracycline, cytarabine and generally a third drug if the patient was included in a clinical trial and randomized into the treatment arm. Patients achieving CR were subsequently treated with alloSCT, autologous SCT (autoSCT) or a third chemotherapy cycle, depending on their prognostic risk profile. 41 , 42 , 43 , 44 , 45 , 46 , 47
AYA AML patients in the present study were almost twice more likely to receive SCT in CR1 compared to children, whereas only subtle differences in chemotherapy use were observed. In AML treatment, SCT is generally reserved for high‐risk patients and was used with a lot of restriction in pediatric patients in the given time period because of the associated long‐term toxicity. Pediatricians therefore often only transplanted as salvage therapy in CR2 rather than in CR1. Consequently, the relative frequency of SCT could possibly be a proxy for the proportion of high‐risk patients, but also reflects specific concerns among pediatricians. 48 In our multivariable analysis, the excess HR of dying for AYAs vs children did not attenuate after adjustment for SCT, suggesting that differences in the appliance of SCT are not responsible for the lower relative survival of AYA AML patients in the Netherlands; however, it should be noted that the available treatment information was very general and possibly not sufficiently detailed to reveal meaningful differences between children and AYAs. We had, for example, no details concerning type of SCT (autologous/allogeneic) or salvage therapy. In contrast to alloSCT, which is standard in higher risk AML, autoSCT is used in AYA patients with a more favorable prognosis.
Similar to other countries, AYAs diagnosed with AML in the Netherlands are less likely to enroll in clinical trials than pediatric patients (AYAs, 18‐40 years: 68% vs children: 87%). 15 , 49 Higher participation rates of AYAs in and better compliance with clinical trials may advance the development of successful treatments for this specific patient group. Health insurance is obligatory in the Netherlands ensuring equal access to health care for all inhabitants. Therefore, insurance issues cannot have played a role in the worse outcome of AYAs in the present study.
Our study evaluated population‐based survival of children and AYAs diagnosed with AML in the Netherlands using nationwide, high‐quality data of the NCR, which has no restrictions regarding age or hospital of treatment. The relatively large number of patients is a major advantage of our investigation that allowed separate analysis for specific subgroups and the evaluation of survival trends over time. An additional strength includes the estimation of relative survival, instead of observed survival, to take the higher background mortality among AYAs into account. A weakness is the lack of detailed information regarding (cyto)genetic abnormalities and treatment. Moreover, the classification and registration of AML by the NCR has changed during the investigated period. The ICD‐O‐3 is based on the World Health Organization (WHO) classification of hematological malignancies and has been used for case ascertainment since 2001, whereas the ICD‐O‐2 (applied from 1993 to 2000) used the French‐American‐British classification of AML. 49
In cancer research, no international consensus has been reached on the definition of AYAs yet, resulting in considerable variability in age ranges used to delineate AYAs across studies. 11 , 14 An age range of 15 to 39 years has been proposed by the US National Cancer Institute (NCI) and has been accepted by the European Network for Cancer in Children and Adolescents. 11 , 50 In the present study, we slightly deviated from the NCI definition by considering AYAs as those aged 18 to 39 years at diagnosis, because 18 years is the upper age limit for treatment at a pediatric oncology center in the Netherlands and almost all AML patients below this age (>95%) are treated at such center. 51 In addition to the comparison of children vs AYAs, we also estimated relative survival for smaller age intervals to gain more insight into the specific age at which the survival of young AML patients starts to decline.
In conclusion, AYAs diagnosed with AML in the Netherlands between 1990 and 2015 had a worse prognosis than pediatric patients. The survival disparity between children and AYAs seemed most pronounced in the latest period, suggesting that improvements in care leading to better outcome for younger children have not resulted in equal benefits for AYAs yet.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
According to the Central Committee on Research Involving Human Subjects (CCMO), this observational study does not require approval from an ethics committee in the Netherlands. Use of the anonymous data for our study was approved by the Privacy Review Board of the Netherlands Cancer Registry and the Biobank and Data Access Committee of the Princess Máxima Center for pediatric oncology, following the principles of the Code of Good Conduct of the Federa (https://www.federa.org/codes-conduct).
Supporting information
Table S1 Age‐specific 5‐year relative survival of children and AYAs (0‐39 years) diagnosed with AML (excl. APL and ML‐DS) in the Netherlands between 1990 and 2015, overall and by period of diagnosis.
Table S2 Multivariable associations of age with excess mortality due to AML (excl. APL and ML‐DS) in children and AYAs (0‐39 years) until 5 years of follow‐up stratified by period of diagnosis, the Netherlands, 1990‐2015.
Table S3 Univariable and multivariable associations of alternative categories of age with excess mortality due to AML (excl. APL and ML‐DS) in children and AYAs (0‐39 years) until 5 years of follow‐up, the Netherlands, 1990‐2015.
Table S4 Five‐year and 10‐year relative survival of children (0‐17 years) and AYAs (18‐39 years) diagnosed with APL in the Netherlands between 1990 and 2015, overall and by subgroup.
ACKNOWLEDGMENTS
The authors would like to thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the NCR. Moreover, DCOG and HOVON/SAKK are acknowledged for the design of new treatment protocols and the recruitment of patients into AML trials. In addition, all oncologists who have treated young AML patients and have entered their data are thanked. The authors also thank Dr. Avinash Dinmohamed (IKNL) for sharing his knowledge on statistical analyses, the NCR data and registration practices. The study was performed with financial support from Erasmus MC‐Sophia Children's Hospital, Rotterdam, the Netherlands. The funder had no role in the study design, data collection, analyses and interpretation of the results, nor in the writing of this manuscript.
Schulpen M, Goemans BF, Kaspers GJL, Raaijmakers MHGP, Zwaan CM, Karim‐Kos HE. Increased survival disparities among children and adolescents & young adults with acute myeloid leukemia: A Dutch population‐based study. Int. J. Cancer. 2022;150(7):1101‐1112. doi: 10.1002/ijc.33878
The data in our manuscript have been presented at the ASH “American Society of Hematology” Annual Meeting, held in 11 to 14 December 2021.
Funding information Erasmus MC‐Sophia Children's Hospital, Rotterdam, The Netherlands
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available on request from the Netherlands Cancer Registry. To obtain data of children diagnosed with cancer in the Netherlands since 2014, an additional permission from the Biobank and Data Access Committee of the Princess Máxima Center for pediatric oncology is required. Further information is available from the corresponding author upon request.
REFERENCES
- 1. Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Hematol Oncol Clin North Am. 2010;24(1):35‐63. [DOI] [PubMed] [Google Scholar]
- 2. de Rooij JD, Zwaan CM, van den Heuvel‐Eibrink M. Pediatric AML: from biology to clinical management. J Clin Med. 2015;4(1):127‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE‐5—a population‐based study. Lancet Oncol. 2014;15(1):35‐47. [DOI] [PubMed] [Google Scholar]
- 4. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83‐103. [DOI] [PubMed] [Google Scholar]
- 5. Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33(27):2949‐2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elgarten CW, Aplenc R. Pediatric acute myeloid leukemia: updates on biology, risk stratification, and therapy. Curr Opin Pediatr. 2020;32(1):57‐66. [DOI] [PubMed] [Google Scholar]
- 7. Rasche M, Zimmermann M, Borschel L, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML‐BFM trials from 1987 to 2012. Leukemia. 2018;32(10):2167‐2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasche M, Zimmermann M, Steidel E, et al. Survival following relapse in children with acute myeloid leukemia: a report from AML‐BFM and COG. Cancers (Basel). 2021;13(10):2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trama A, Bernasconi A, McCabe MG, et al. Is the cancer survival improvement in European and American adolescent and young adults still lagging behind that in children? Pediatr Blood Cancer. 2019;66(1):e27407. [DOI] [PubMed] [Google Scholar]
- 10. Abrahao R, Keogh RH, Lichtensztajn DY, et al. Predictors of early death and survival among children, adolescents and young adults with acute myeloid leukaemia in California, 1988‐2011: a population‐based study. Br J Haematol. 2016;173(2):292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trama A, Botta L, Foschi R, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000‐07: population‐based data from EUROCARE‐5. Lancet Oncol. 2016;17(7):896‐906. [DOI] [PubMed] [Google Scholar]
- 12. Nasir SS, Giri S, Nunnery S, Martin MG. Outcome of adolescents and young adults compared with pediatric patients with acute myeloid and promyelocytic leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(2):126‐132. [DOI] [PubMed] [Google Scholar]
- 13. Xie S, Hossain MJ. Survival differences in childhood and young adult acute myeloid leukemia: a cross‐national study using US and England data. Cancer Epidemiol. 2018;54:19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rostgaard K, Hjalgrim H, Madanat‐Harjuoja L, Johannesen TB, Collin S, Hjalgrim LL. Survival after cancer in children, adolescents and young adults in the Nordic countries from 1980 to 2013. Br J Cancer. 2019;121(12):1079‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reedijk AMJ, Klein K, Coebergh JWW, et al. Improved survival for children and young adolescents with acute myeloid leukemia: a Dutch study on incidence, survival and mortality. Leukemia. 2019;33(6):1349‐1359. [DOI] [PubMed] [Google Scholar]
- 16. Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol. 1993;22(3):369‐376. [DOI] [PubMed] [Google Scholar]
- 17. van der Sanden GA, Coebergh JW, Schouten LJ, Visser O, van Leeuwen FE. Cancer incidence in The Netherlands in 1989 and 1990: first results of the nationwide Netherlands cancer registry. Coordinating Committee for Regional Cancer Registries. Eur J Cancer. 1995;31A(11):1822‐1829. [DOI] [PubMed] [Google Scholar]
- 18. Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. 2015;15(1):186‐215. [Google Scholar]
- 19. Ederer F, Heise H. Instructions to IBM 650 Programmers in Processing Survival Computations. Methodological Note no.10. End Results Evaluation Section. Bethesda MD: National Cancer Institute; 1959. [Google Scholar]
- 20. Testi AM, Pession A, Diverio D, et al. Risk‐adapted treatment of acute promyelocytic leukemia: results from the International Consortium for Childhood APL. Blood. 2018;132(4):405‐412. [DOI] [PubMed] [Google Scholar]
- 21. Thomas X. Acute promyelocytic leukemia: a history over 60 years‐from the most malignant to the most curable form of acute leukemia. Oncol Ther. 2019;7(1):33‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conneely SE, Stevens AM. Advances in pediatric acute promyelocytic leukemia. Children (Basel). 2020;7(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu ZL, Huang XJ. Therapeutic approaches for acute promyelocytic leukaemia: moving towards an orally chemotherapy‐free era. Front Oncol. 2020;10:586004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abrahao R, Ribeiro RC, Medeiros BC, Keogh RH, Keegan TH. Disparities in early death and survival in children, adolescents, and young adults with acute promyelocytic leukemia in California. Cancer. 2015;121(22):3990‐3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bleyer A, Barr R. Cancer in young adults 20 to 39 years of age: overview. Semin Oncol. 2009;36(3):194‐206. [DOI] [PubMed] [Google Scholar]
- 26. Wood WA, Lee SJ. Malignant hematologic diseases in adolescents and young adults. Blood. 2011;117(22):5803‐5815. [DOI] [PubMed] [Google Scholar]
- 27. Rubnitz JE, Pounds S, Cao X, et al. Treatment outcome in older patients with childhood acute myeloid leukemia. Cancer. 2012;118(24):6253‐6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ofran Y, Rowe JM. Acute myeloid leukemia in adolescents and young adults: challenging aspects. Acta Haematol. 2014;132(3–4):292‐297. [DOI] [PubMed] [Google Scholar]
- 29. Tarlock K, Meshinchi S. Pediatric acute myeloid leukemia: biology and therapeutic implications of genomic variants. Pediatr Clin North Am. 2015;62(1):75‐93. [DOI] [PubMed] [Google Scholar]
- 30. Conneely SE, Rau RE. The genomics of acute myeloid leukemia in children. Cancer Metastasis Rev. 2020;39(1):189‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821‐3830. [DOI] [PubMed] [Google Scholar]
- 32. Boissel N, Auclerc MF, Lheritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE‐93 and LALA‐94 trials. J Clin Oncol. 2003;21(5):774‐780. [DOI] [PubMed] [Google Scholar]
- 33. de Bont JM, Holt B, Dekker AW, van den Berg A, Sonneveld P, Pieters R. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in The Netherlands. Leukemia. 2004;18(12):2032‐2035. [DOI] [PubMed] [Google Scholar]
- 34. Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Creutzig U, Kutny MA, Barr R, Schlenk RF, Ribeiro RC. Acute myelogenous leukemia in adolescents and young adults. Pediatr Blood Cancer. 2018;65(9):e27089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woods WG, Franklin AR, Alonzo TA, et al. Outcome of adolescents and young adults with acute myeloid leukemia treated on COG trials compared to CALGB and SWOG trials. Cancer. 2013;119(23):4170‐4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White VM, Skaczkowski G, Pinkerton R, et al. Clinical management of Australian adolescents and young adults with acute lymphoblastic and myeloid leukemias: a national population‐based study. Pediatr Blood Cancer. 2018;65(11):e27349. [DOI] [PubMed] [Google Scholar]
- 38. Wennstrom L, Edslev PW, Abrahamsson J, et al. Acute myeloid leukemia in adolescents and young adults treated in pediatric and adult departments in the Nordic countries. Pediatr Blood Cancer. 2016;63(1):83‐92. [DOI] [PubMed] [Google Scholar]
- 39. Razzouk BI, Estey E, Pounds S, et al. Impact of age on outcome of pediatric acute myeloid leukemia: a report from 2 institutions. Cancer. 2006;106(11):2495‐2502. [DOI] [PubMed] [Google Scholar]
- 40. Creutzig U, Buchner T, Sauerland MC, et al. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML‐BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer. 2008;112(3):562‐571. [DOI] [PubMed] [Google Scholar]
- 41. Lowenberg B, Boogaerts MA, Daenen SM, et al. Value of different modalities of granulocyte‐macrophage colony‐stimulating factor applied during or after induction therapy of acute myeloid leukemia. J Clin Oncol. 1997;15(12):3496‐3506. [DOI] [PubMed] [Google Scholar]
- 42. Lowenberg B, van Putten W, Theobald M, et al. Effect of priming with granulocyte colony‐stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349(8):743‐752. [DOI] [PubMed] [Google Scholar]
- 43. Ossenkoppele GJ, Löwenberg B. HOVON 92: toevoeging van laromustine (Cloretazine) aan standaard remissieinductie chemotherapie bij patiënten jonger dan 65 jaar met AML en MDS (RAEB met IPSS>1,0). Ned Tijdschr Hematol. 2009;6(1):32‐33. (in Dutch). [Google Scholar]
- 44. Lowenberg B, Pabst T, Vellenga E, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364(11):1027‐1036. [DOI] [PubMed] [Google Scholar]
- 45. Pabst T, Vellenga E, van Putten W, et al. Favorable effect of priming with granulocyte colony‐stimulating factor in remission induction of acute myeloid leukemia restricted to dose escalation of cytarabine. Blood. 2012;119(23):5367‐5373. [DOI] [PubMed] [Google Scholar]
- 46. Lowenberg B, Pabst T, Maertens J, et al. Therapeutic value of clofarabine in younger and middle‐aged (18‐65 years) adults with newly diagnosed AML. Blood. 2017;129(12):1636‐1645. [DOI] [PubMed] [Google Scholar]
- 47. Lowenberg B, Pabst T, Maertens J, et al. Addition of lenalidomide to intensive treatment in younger and middle‐aged adults with newly diagnosed AML: the HOVON‐SAKK‐132 trial. Blood Adv. 2021;5(4):1110‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116(13):2205‐2214. [DOI] [PubMed] [Google Scholar]
- 49. Dinmohamed AG, Visser O, van Norden Y, et al. Treatment, trial participation and survival in adult acute myeloid leukemia: a population‐based study in The Netherlands, 1989‐2012. Leukemia. 2016;30(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 50. US Department of Health and Human Services, US National Institutes of Health, US National Cancer Institute, Adolescent and Young Adult Oncology Progress Review Group . Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. US Department of Health and Human Services; 2006. [Google Scholar]
- 51. Reedijk AMJ, van der Loo M, Visser O, et al. Site of childhood cancer care in The Netherlands. Eur J Cancer. 2017;87:38‐46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Age‐specific 5‐year relative survival of children and AYAs (0‐39 years) diagnosed with AML (excl. APL and ML‐DS) in the Netherlands between 1990 and 2015, overall and by period of diagnosis.
Table S2 Multivariable associations of age with excess mortality due to AML (excl. APL and ML‐DS) in children and AYAs (0‐39 years) until 5 years of follow‐up stratified by period of diagnosis, the Netherlands, 1990‐2015.
Table S3 Univariable and multivariable associations of alternative categories of age with excess mortality due to AML (excl. APL and ML‐DS) in children and AYAs (0‐39 years) until 5 years of follow‐up, the Netherlands, 1990‐2015.
Table S4 Five‐year and 10‐year relative survival of children (0‐17 years) and AYAs (18‐39 years) diagnosed with APL in the Netherlands between 1990 and 2015, overall and by subgroup.
Data Availability Statement
The data that support the findings of our study are available on request from the Netherlands Cancer Registry. To obtain data of children diagnosed with cancer in the Netherlands since 2014, an additional permission from the Biobank and Data Access Committee of the Princess Máxima Center for pediatric oncology is required. Further information is available from the corresponding author upon request.


