Abstract
Introduction
Pain is the most common symptom in acute pancreatitis (AP) and is among the diagnostic criteria. Therefore, we aimed to characterize acute abdominal pain in AP.
Methods
The Hungarian Pancreatic Study Group prospectively collected multicentre clinical data on 1435 adult AP patients between 2012 and 2017. Pain was characterized by its intensity (mild or intense), duration prior to admission (hours), localization (nine regions of the abdomen) and type (sharp, dull or cramping).
Results
97.3% of patients (n = 1394) had pain on admission. Of the initial population with acute abdominal pain, 727 patients answered questions about pain intensity, 1148 about pain type, 1134 about pain localization and 1202 about pain duration. Pain was mostly intense (70%, n = 511/727), characterized by cramping (61%, n = 705/1148), mostly starting less than 24 h prior to admission (56.7%, n = 682/1202). Interestingly, 50.9% of the patients (n = 577/1134) had atypical pain, which means pain other than epigastric or belt‐like upper abdominal pain. We observed a higher proportion of peripancreatic fluid collection (19.5% vs. 11.0%; p = 0.009) and oedematous pancreas (8.4% vs. 3.1%; p = 0.016) with intense pain. Sharp pain was associated with AP severity (OR = 2.481 95% CI: 1.550–3.969) and increased mortality (OR = 2.263, 95% CI: 1.199–4.059) compared to other types. Longstanding pain (>72 h) on admission was not associated with outcomes. Pain characteristics showed little association with the patient's baseline characteristics.
Conclusion
A comprehensive patient interview should include questions about pain characteristics, including pain type. Patients with sharp and intense pain might need special monitoring and tailored pain management.
Significance
Acute abdominal pain is the leading presenting symptom in acute pancreatitis; however, we currently lack specific guidelines for pain assessment and management. In our cohort analysis, intense and sharp pain on admission was associated with higher odds for severe AP and several systemic and local complications. Therefore, a comprehensive patient interview should include questions about pain characteristics and patients with intense and sharp pain might need closer monitoring.
1. INTRODUCTION
Acute pancreatitis (AP) is one of the most common acute gastrointestinal diseases to result in hospital admission (Roberts et al., 2017). Acute abdominal pain is the leading presenting symptom in acute pancreatitis, and it is included among the diagnostic criteria (Banks et al., 2013). Since the pain can be excruciating, adequate pain management is of the utmost importance. However, we currently lack specific guidelines for pain management in AP; instead, general perioperative strategies are recommended (Stigliano et al., 2017). This approach in AP is not based on robust scientific data, since our knowledge is insufficient in both basic science and clinical settings (Barreto & Saccone, 2012; Pezzilli et al., 2010). We also lack studies that evaluate pain characteristics and pain management in everyday practice (Pezzilli et al., 2007; Phillip et al., 2013). Nor are sufficient data available on the relation between patients’ clinical parameters and pain characteristics.
Nevertheless, understanding these factors could help to identify risk groups that require special attention as regards pain management and to choose or even expand the available analgesics for them, thus providing personalized medicine. Obviously, therapy should be tailored to the intensity of pain. The significance of pain type (quality descriptors) in other diseases has also been researched extensively, mostly for chronic pain (Asthana et al., 2020; Dworkin et al., 2007; Erdogan et al., 2019; Galli et al., 2019; Rau et al., 2018; Sharma et al., 2016). Recommendations for acute and chronic pain also suggest pain type‐based phenotyping of patients, since pain is a complex phenomenon and pain type may influence the efficacy of certain drug classes (Chou et al., 2016; Dworkin et al., 2005; Edwards et al., 2016). Clarification of these issues could also help to discover new targets for both basic and clinical research.
Early identification of patients at a higher risk of severe AP and mortality is important for proper monitoring and management. The most frequently used prognostic scores, such as the Ranson score and APACHE‐II, are difficult to follow, can be evaluated only after 72 h of hospitalization, and are not sufficiently accurate, according to the limited evidence in the literature. These prognostic scores do not address questions concerning pain or other clinical symptoms (Hagjer & Kumar, 2018; Harshit Kumar & Singh Griwan, 2018; Tan et al., 2017). Indeed, the role of pain characteristics in AP prognosis has been suggested by a few studies but without strong supporting evidence (Kapoor et al., 2013; Phillip et al., 2013).
Here, we aimed to elucidate the relationship between the characteristics of pain on admission and the main outcomes of AP and to investigate the possible prognostic role of pain. We also intended to identify clinical parameters that potentially influence pain intensity, type, localization and duration prior to admission in AP and to describe pain management in everyday practice.
2. METHODS
2.1. Study design, setting and population
This study is a post hoc cohort analysis of an international prospective registry conducted by the Hungarian Pancreatic Study Group, which collected data on consecutive acute pancreatitis cases between 2012 and 2017. There were 1435 adult (>18 years) patients enrolled from 19 Hungarian and eleven foreign institutions (Figure S1).
Acute pancreatitis was diagnosed when two out of the three criteria were met (typical abdominal pain for acute pancreatitis, pancreas enzymes at least three times greater than the normal upper limit, and abnormal findings on abdominal imaging; Banks et al., 2013; Hritz et al., 2015).
Data on demographics, alcohol consumption, smoking, family and personal medical history and symptoms were collected by physicians and trained clinical administrators through predefined patient questionnaires on admission and each day during the entire hospital stay. Relevant clinical data on diagnostic and therapeutic approaches and main outcomes (severity, mortality, complications, length of hospital stay (LOS) and necessity of analgesia) were also collected during physical examinations and from medical records into standardized forms. The process was approved through a four‐level quality check system. As regards on‐admission abdominal pain, we had information on 1432 cases. The quality of the data is shown in detail in Table S1.
2.2. Pain assessment (groups)
Patients were classified into subgroups based on pain assessment.
To our knowledge, there are no specific recommendations for pain assessment in acute pancreatitis; hence, we evaluated pain based on categories commonly used in clinical practice.
Our analysis involved four on‐admission pain characteristics: pain intensity, pain type, pain localization and pain duration. Pain‐free cases according to a Visual Analog Scale (VAS 0) were considered a separate category. Patients were interviewed on admission to the ward, but they had to recall their pain characteristics in the period immediately before hospital admission. Clinicians were responsible for interviewing patients within a relatively short time on admission. Failure to do so might result in missing data.
All these variables were patient‐reported. Of the initial population with acute abdominal pain, 727 patients answered questions on pain intensity (this question was only included in 2015), 1148 on pain type, 1134 on pain localization and 1202 on pain duration, resulting in four different sample sizes for the analyses (Figure 1).
FIGURE 1.
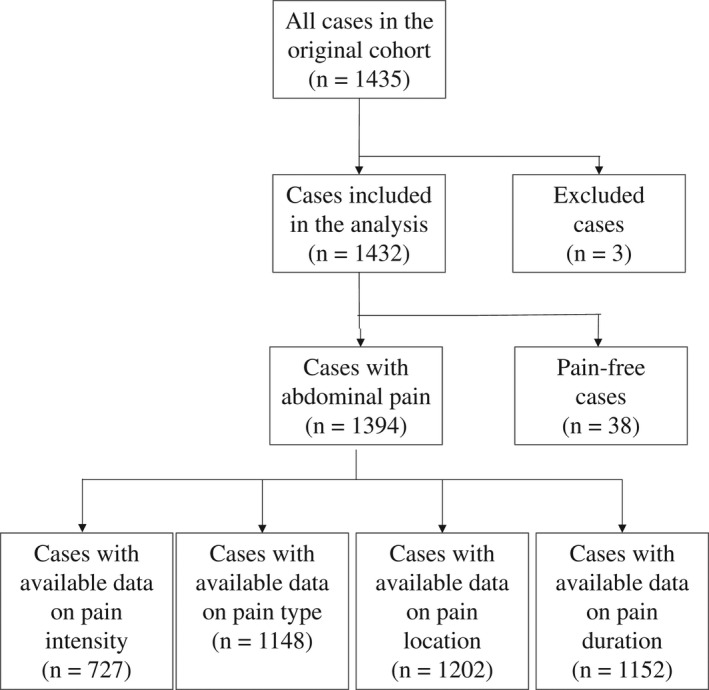
Flowchart of included patients
For each pain characteristic, we used the highest possible case numbers where the data investigated were available. The representativeness of the groups can be seen in Table S2.
Pain intensity was measured by the VAS on a scale from 1 (one) to 10 (ten). One indicated ‘very mild pain’ and ten ‘the worst pain imaginable” (Phillip et al., 2013). We categorized pain intensity into the following subgroups: VAS 1–6 (mild or moderate) and VAS 7–10 (intense; Figure 2).
FIGURE 2.
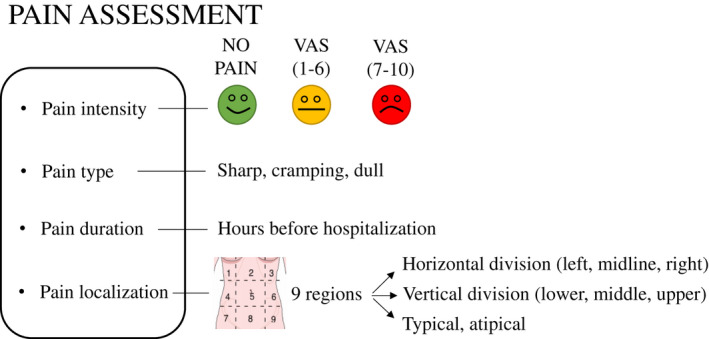
Pain characteristics groups (VAS; Visual Analog Scale)
Patients assessed pain type by three different predefined categories (cramping, dull, or sharp pain; Figure 2). We used these descriptors as collective concepts, sharp pain for ‘incisive pressure’, cramping pain for ‘constructive pressure’, and dull pain for dullness categories with multiple possible vocabularies. The original Hungarian version of the questionnaire was then translated into the various relevant languages. Other questionnaires frequently used for different disorders, including the Short‐Form McGill Pain Questionnaire (SF MPQ‐2) and the Pain Quality Assessment Scale (PQAS; Drewes et al., 2017; Teo et al., 2017) in chronic pancreatitis, contain similar categories.
Pain localization was established by routine physical examination according to the nine abdominal regions (1: right hypochondrium; 2: epigastrium; 3: left hypochondrium; 4: right flank; 5: umbilical; 6: left flank; 7: right groin; 8: pubic; 9: left groin). The localization of pain was analysed according to three divisions (Figure 2).
typical/atypical: typical pain means pain in the epigastrium or in the upper abdomen in a belt‐like fashion;
horizontal division with upper, middle and lower abdominal pain and
vertical division with left‐sided, midline and right‐sided abdominal pain.
Data on pain duration prior to hospitalization were primarily collected in the database in terms of hours. We used a division by days (0–24, 25–48, 49–72, >72 h) in the analyses (Figure 2).
2.3. Other confounding factors
A history of smoking and alcohol consumption was described based on predefined questionnaires, from which we later calculated pack year and daily alcohol consumption in grams. The patients were also asked whether they had a history of acute or chronic pancreatitis.
Weight and height were measured by study nurses or trained clinical administrators, then body mass index (BMI) was calculated. BMI ≥30 kg/m2 was defined as obesity according to the WHO classification (Obesity: preventing & managing the global epidemic. Report of a WHO consultation, 2000). The presence of abdominal tenderness and guarding was determined by the examining physician.
We considered hypertension if blood pressure was above 140/90 mmHg or the patient was on anti‐hypertensive medication. Diabetes mellitus was defined according to the American Diabetes Association Criteria (Chamberlain et al., 2016). The Charlson Comorbidity Index (CCI) was defined by reviewing electronic discharge files as described by Szakács et al. (Charlson et al., 1987; Szakacs et al., 2018).
2.4. Outcomes
2.4.1. Primary outcomes
The severity of AP and complications were defined based on the revised Atlanta classification (Banks et al., 2013). The revised classification differentiates between mild (no local or systemic complications), moderate (local complication or organ failure persisting no more than 48 h) and severe AP (organ failure persisting more than 48 h). The definition of each local (acute peripancreatic fluid collections, pancreatic necrosis or pseudocysts) and systemic (respiratory failure, heart failure or renal failure), complication can be found in Table S3. We studied other outcome measures, such as hospital mortality, LOS and new‐onset diabetes.
2.4.2. Secondary outcomes
To assess on‐admission imaging findings, we reviewed the radiological description of ultrasound (US) imaging, computerized tomography (CT) and chest X‐rays. We evaluated the following imaging findings: pleural fluid, hypo‐ or hyperechogenicity of the pancreas, oedematous pancreas, enlarged pancreas, pancreatic duct dilatation, pancreatic calcification, acute peripancreatic fluid collection, on‐admission necrosis or on‐admission fluid collection.
In‐hospital opioid use was defined when there was evidence of opioid administration at least once during hospitalization. We also calculated the number of days with analgesics (non‐steroid anti‐inflammatory drugs (NSAIDs), paracetamol or opioids) if the details of pain management were available for the whole hospital stay. Where possible, the number of days with opioids was also calculated.
2.5. Statistical analyses
The analysis was performed with descriptive statistics—median with 25% and 75% quartiles (Q1 and Q3 respectively), and relative frequency—a goodness‐of‐fit χ 2 test (for categorical data in the representativeness analysis), binominal (for dichotomous data in the representativeness analysis) and one‐sample median tests (for continuous data in the representativeness analysis), odds ratio with 95% CI (for dichotomous data in the main analysis), χ 2 test with the Z test (for categorical data in the main analysis), the Mann–Whitney test, the Kruskal–Wallis test with the Mann–Whitney test as a post hoc test and the Bonferroni correction to adjust Spearman's rank correlation (for continuous data in the main analysis). A two‐sided p‐value of <0.05 was considered statistically significant. The available‐case analysis was used for missing data. Statistical analyses were performed with SPSS 25.0 software (IBM Corporation).
2.6. Ethical approval
The operation of the AP Registry was approved by the Scientific and Research Ethics Committee of the Medical Research Council, Hungary (22254‐1/2012/EKU, 17787‐8/2020/EÜIG). Informed consent forms were obtained from all participants before enrolment. The study was conducted in accordance with the Helsinki Declaration.
3. RESULTS
3.1. Characteristics of the overall cohort
In total, 1432 cases with acute pancreatitis were included in the analysis. All the patients were monitored until discharge. The clinical characteristics for the whole sample are shown in Table 1.
TABLE 1.
General characteristics of the study population
| Overall (n = 1432) | |
|---|---|
| Age, years, median (Q1–Q3) | 57 (43–69) |
| Gender | |
| Male, n (%) | 817 (56.9) |
| Female, n (%) | 618 (43.1) |
| Medication taken regularly a | |
| NSAIDs or paracetamol, n (%) | 31 (2.9) |
| Opioid, n (%) | 5 (0.5) |
| Benzodiazepines, n (%) | 96 (9.0) |
| Antidepressants, n (%) | 30 (2.8) |
| Anticonvulsant, n (%) | 20 (1.9) |
| Aetiology (pure) | |
| Biliary, n (%) | 564 (39.4) |
| Alcoholic, n (%) | 305 (21.3) |
| Hypertriglyceridaemic, n (%) | 83 (5.8) |
| Post‐ERCP, n (%) | 41 (2.9) |
| Idiopathic, n (%) | 300 (20.9) |
| Other, n (%) | 139 (9.7) |
| Length of hospital stay, median (Q1–Q3) | 9 (6–13) |
| Mortality, n (%) | 36 (2.5) |
| Severity of pancreatitis | |
| Mild, n (%) | 987 (68.9) |
| Moderate, n (%) | 368 (25.7) |
| Severe, n (%) | 77 (5.38) |
| Local complications, n (%) | 435 (30.5) |
| Fluid collection, n (%) | 373 (26.2) |
| Pseudocyst, n (%) | 126 (8.8) |
| Necrosis, n (%) | 132 (9.3) |
| Systemic complication, n (%) | 115 (8.1) |
| Respiratory failure, n (%) | 68 (4.8) |
| Heart failure, n (%) | 26 (1.8) |
| Renal failure, n (%) | 43 (3.0) |
Data on medication taken regularly were available in 1069 cases.
More males were affected than females in our cohort (n = 817, 56.9% vs. n = 618, 43.1%). A biliary aetiology (n = 564, 39.4%) was the most common, followed by an alcoholic aetiology (n = 305, 21.3%). Most of the patients had a mild, non‐fatal disease; mild AP was observed in 68.9% of the cases (n = 987), moderate AP in 25.7% (n = 368) and severe AP in 5.4% (n = 77), while in‐hospital mortality occurred in 2.5% (n = 36).
3.2. Diagnosis of AP
Of the 1432 patients, 1394 (97.3%) had abdominal pain on admission.
Abnormal pancreas structure was detected in 646 cases by imaging on admission (52.1%). 1149 USs, 235 CTs and 450 chest X‐rays were performed on admission. The most common imaging findings were enlarged pancreas (n = 231; 18.7%) and peripancreatic fluid collection (n = 207, 16.7%). Other abnormalities can be seen in Table S4.
Amylase levels were at least three times greater than the normal upper limit in 996 cases (69.6%), while lipase levels were diagnostic in 752 cases (52.4%).
3.3. Patients without pain
Thirty‐six patients reported no pain on admission, of whom 72.2% (n = 26) had mild AP, 19.4% (n = 7) had moderate AP and 8.3% (n = 3) had severe AP. One patient (2.8%) without on‐admission pain died. The proportion of systemic (8.3%, n = 3) and local complications (25%, n = 9) did not differ from that of the overall cohort. About one‐fifth of the no‐pain cases were post‐ERCP pancreatitis (19.4%, n = 7). The proportion of other aetiologies (alcoholic, biliary, hypertriglyceridaemic, idiopathic, etc.) was similar to that of the overall cohort.
3.4. Pain management
Analgesic data were complete for the total LOS in 882 (61.6%) cases.
In summary, 745/882 (85.5%) patients were administered analgesics at least once during the hospital stay, out of whom 678/882 (76.6%) received them on the day of admission. Opioids were administered at least once during the hospital stay in 155 cases (17.6%).
The median duration of pain management was 3 days (IQR 2–6). In the patient group requiring analgesics, the median LOS was 8 days (IQR 6–12) compared to patients without pain management, where LOS was 7 days (IQR 5–11; p < 0.001).
The median length of opioid therapy was 2 days (IQR 1–4). In the patient group requiring opioids, the median LOS was 9 days (IQR 5–14) compared to patients without opioid therapy, where LOS was 8 days (IQR 6–13; p < 0.001).
To our knowledge, 5 of the patients received epidural analgesia.
3.5. Individual effect analysis of pain characteristics
Relations between the four pain characteristics and demographic and clinical outcomes were analysed.
Most of the patients described their pain as VAS 7–10 (n = 511; 70.3%), characterized as cramping (n = 705; 61.4%), localized in the upper abdomen (n = 525; 46.4%) and starting within 24 h prior to admission (n = 682; 56.7%).
3.5.1. Pain intensity
We found no statistically significant difference in age, gender, BMI, alcohol consumption, smoking habit, history of pancreatic diseases, other examined comorbidities, aetiology and findings on physical examination in comparing patients with VAS 1–6 and 7–10 (Table S5).
Main outcomes
Pain intensity as an ordinal variable was associated with the disease severity (p < 0.021). However, we found no statistically significant difference between the VAS 1–6 and VAS 7–10 groups as regards the main outcomes (severity, mortality, complications and LOS), although we detected a tendency towards a higher proportion of severe AP among patients with VAS 7–10. The AP severity distribution of individuals with VAS 1–6 and VAS 7–10 was as follows: mild AP = 74.5%/74.2%, moderate AP = 23.1%/21.3% and severe AP = 2.3%/4.5%.
Unexpectedly, VAS 1–6 was associated with a longer hospital stay (median 8 days IQR (6–13) in VAS 1–6 vs. median 7.5 days IQR (5–10) in VAS 7–10, p = 0.001; Table S5).
Patients with VAS 7–10 pain on admission were more likely to require opioids during their hospital stay (OR = 2.561, 95% CI: 1.573–4.169) than patients with VAS 1–6. Higher pain intensity on admission was also associated with the duration of the analgesic treatment (median 2 days IQR (1–5) in VAS 1–6 vs. median 3 days IQR (2–5) in VAS 7–10, p = 0.009), but not with the duration of opioid treatment (Table S5).
On‐admission imaging
We observed a significantly increased number of acute peripancreatic fluid collection (OR = 1.587, 95% CI: 1.133–2.224) and oedematous pancreas (OR = 1.955 95% CI: 1.178–3.246) via imaging on admission with VAS 7–10 compared to VAS 1–6 (Table S4).
3.5.2. Pain type
Comparing patients with different types of pain, we found no difference in age, gender, BMI, smoking habit, history of pancreatic diseases, diabetes mellitus or other metabolic diseases or findings on the physical examination (Table S6).
Patients with cramping pain tended to have a biliary aetiology, and they were less likely to have an alcoholic aetiology compared to dull or sharp pain (p < 0.05).
Abdominal guarding was more frequent when sharp pain was present compared to cramping and dull pain (26.2% vs. 16.5% and 26.2%, p < 0.05).
Main outcomes
Sharp pain was associated with a 2.6‐fold increase in mortality odds (OR = 2.632, 95% CI: 1.063–6.514) compared to other types of pain (dull + cramping pain). Sharp pain might also be a risk factor for severe disease (OR = 2.206, 95% CI: 1.199–4.059), especially for systemic complications (OR = 2.481, 95% CI: 1.550–3.969), including new‐onset diabetes (OR = 2.561, 95% CI: 1.472–4.456) and respiratory (OR = 3.220, 95% CI: 1.806–5.740) and heart failure (OR = 3.222, 95% CI: 1.319–7.869). There were also increased odds for necrosis development with sharp pain (OR = 1.653, 95% CI: 1.060–2.580). Cramping pain was associated with a longer LOS (p < 0.05; Figure 3).
FIGURE 3.
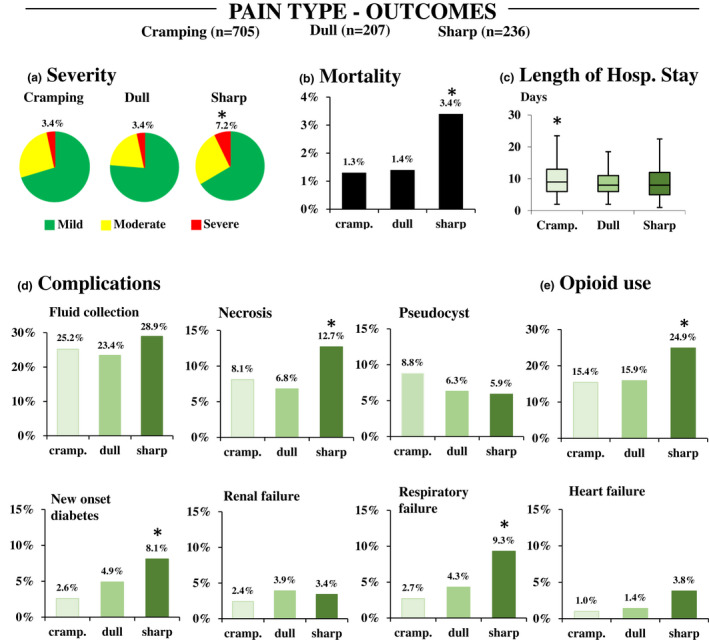
Main outcomes of AP in pain type groups (cramping and dull vs. sharp). (a) Severity (*OR = 2.206 95%: 1.199–4.059); (b) Mortality (*OR = 2.632 95% CI: 1.063–6.514) Length of hospital stay (*p < 0.05); (d) Complications: fluid collection; necrosis (*OR = 1.653 95% CI: 1.060–2.580); pseudocysts; new‐onset diabetes (*OR = 2.561 95% CI: 1.472–4.456); respiratory failure (OR = 3.220 95% CI: 1.806–5.740); renal failure; heart failure (*OR = 3.222 95% CI: 1.329–7.869); (e) Opioid use (*OR = 3.250 95% CI: 1.585–3.194)
Sharp pain was associated with a higher proportion of opioid administration compared to cramping and dull pain (OR = 2.250 95% CI: 1.585–3.194). Cramping and sharp pain were associated with longer analgesic requirement compared to dull pain (median 4 days IQR (2–6) and median 4 days IQR (2–7) vs. median 2 days IQR (2–6), respectively, p = 0.005). Pain type was not associated with the length of opioid administration (p = 0.938).
On‐admission imaging
There was no difference between pain type categories in the presence of on‐admission abnormalities on imaging (Table S4).
3.5.3. Pain localization
An unexpectedly high percentage of patients (n = 557, 50.8%) had atypical pain on admission, mostly presenting with umbilical or right rib pain. In addition, we found a greater chance of atypical pain with obesity (OR = 1.320 95% CI: 1.036–1.681), hypertension (OR = 1.303 95% CI: 1.016–1.669) and hyperlipidaemia (OR = 1.889 95% CI: 1.302–2.741; Table S7). Also, pain typical of acute pancreatitis was associated with a higher proportion of peripancreatic fluid collection (Table S4).
There were only a few notable differences as regards pain location in main outcomes.
We were unable to support it statistically, but, apparently, left, lower abdominal pain was associated with a worse prognosis (Table S8). At the same localization, the proportion of idiopathic cases seemed to be higher compared to other localizations. Although these localizations were considered rare in the cohort.
3.5.4. Pain duration
Median pain duration on admission was 24 h (IQR 10–72 h). Pain duration on admission was not associated with age, gender, smoking habit, history of pancreatic diseases or metabolic diseases, CCI, or findings on physical examination (Table S9).
Main outcomes
Surprisingly, pain duration prior to hospitalization was not associated with severity, mortality, LOS or different systemic or local complications. Patients with pain duration of fewer than 24 h prior to hospitalization required opioid administration more frequently compared to patients with longstanding pain (≥72 h; 22.9% vs. 9.2%, p < 0.001).
On‐admission imaging
Findings from on‐admission imaging were independent of pain duration on admission.
3.6. Relation between pain characteristics
There was a weak negative correlation between pain intensity and pain duration (r = −0.168, p < 0.001). In addition, patients with sharp pain had a significantly shorter duration of pain on admission compared to cramping (p < 0.001) or dull pain (p = 0.003). Less intense pain was characterized by dull pain rather than by sharp or cramping pain (p < 0.001), while sharp pain was more typical of more intense pain (p < 0.001).
Further results are described in detail in the Supporting information.
4. DISCUSSION
This prospective, multicentre, international cohort study characterizes acute abdominal pain in AP.
4.1. Generalizability of the registry data
The mortality and severity rates for AP in our study are consistent with the more favourable international data (Brindise et al., 2019; Zhu et al., 2017). This could be explained by the high rates of adherence to international and national guidelines among most of the participating centres, including timely intervention with fluid replacement and early enteral feeding (Hritz et al., 2015; Parniczky et al., 2016; Working Group, 2013).
Our results on pain characteristics were also comparable with previous findings. Patients experienced mostly VAS 7–10 pain intensity starting within 24 h prior to hospitalization (Pai et al., 2017; PanWessex Study et al., 2019; Phillip et al., 2013). Importantly, this is the first study to investigate the aspect of pain quality descriptors, in other words, pain type in AP.
4.2. Pain characteristics
Interestingly, we discovered an unexpected feature of sharp pain: it was associated with unfavourable disease outcomes, such as higher systemic complications and mortality rates.
Previous data suggest that pain type (quality descriptors) might be related to the mechanism of pain. Understanding the pain mechanism can aid in choosing the optimal therapeutic approach (Asthana et al., 2020; Erdogan et al., 2019). Sharp pain is sometimes interpreted as a sign of neuropathic pain (Mackey et al., 2012). The presence of neuropathic pain is a known phenomenon in pancreas cancer and a novelty in chronic pancreatitis (Demir et al., 2015, 2019). Unfortunately, the currently available data in AP do not allow us to elucidate the mechanisms behind sharp pain. To determine whether neuropathy is present, specifically validated questionnaires, such as the DN4 questionnaire, could be used in the future (VanDenKerkhof et al., 2018).
Nevertheless, it may entail a different mechanism of not only pain but also inflammatory processes in the pancreas since sharp pain was associated with a worse disease course in our study. A different inflammatory process could be further supported by the higher proportion of abdominal guarding among patients with sharp pain, which is usually considered a sign of stronger inflammation in the abdomen. However, we need further investigations to explore this topic through an examination of laboratory samples as well as histological and imaging findings and an assessment of pain type in more detail with validated questionnaires.
In our cohort, patients with sharp pain experienced the strongest pain, which was comparable to findings by Dworkin et al. in both neuropathic and non‐neuropathic pain (Dworkin et al., 2007). Furthermore, pain intensity correlated with the severity of AP as an ordinal variable, and certain abnormal imaging findings on admission, such as enlarged pancreas and peripancreatic fluid collection, were more common in the case of more intense pain. In chronic pancreatitis, a similar association between pain intensity and morphological changes could not be identified (Madzak et al., 2017). However, VAS 1–6 and VAS 7–10 groups did not differ significantly concerning disease outcomes although some tendency could be observed with an apparently higher rate of severe AP in VAS 7–10 groups. Surprisingly, despite the apparently milder disease, VAS 1–6 was associated with a significantly longer LOS. Since LOS can be influenced by various variables, it is hard to explain this conflicting result. The difference between the two groups is clinically not significant since it is less than 1 day. Nevertheless, after examining the longest hospitalizations, we came to the conclusion that a significant proportion of them is linked to pancreatitis unrelated causes or only indirectly related causes such as nosocomial infections (Clostridium difficile infection, pneumonia, urinary tract infection) or iatrogenic disorders (e.g. bleeding after ERCP, drug side effects). In VAS 1–6 group, investigations to rule out malignancies appear to be more common since a few patients presented with pain lasting for weeks. Moreover, extremely long LOS could be explained mostly by decompensation of comorbidities. While in the VAS 7–10 group, recurrent pain due to local complications and the antibiotic or surgical treatment of coexistent cholecystitis seems to be more prevalent. Also, extremely long LOS was mainly because of AP‐related complications.
The possible prognostic role of on‐admission pain should be further characterized, including adjustment to potential confounding factors of both pain and disease outcomes.
Typically, the patients with more intense and sharp abdominal pain turned to doctors earlier in our study. Still, the duration of pain before hospitalization was not influenced by any other factors under examination, such as age, gender or positive personal or family history with AP, as shown in an earlier study as well. Nor did the authors of the mentioned article noted a link between pain duration and in‐hospital outcomes, a finding which is consistent with our own (Phillip et al., 2013).
Earlier studies have suggested that pain assessment in different diseases might depend on the patients’ gender because women and men describe and process pain differently (Fillingim et al., 2009; Rau et al., 2018; Unruh, 1996). However, data are not consistent throughout the abdominal pain literature (Fillingim et al., 2009), and we were also unable to confirm a gender difference in patients with AP. As with gender, there are contradictions in the literature about the effect of age on pain perception and analgesic consumption (Banks et al., 2013; Galli et al., 2019). We were also unable to detect an age‐dependent pattern of pain in AP. According to our results, components of metabolic syndrome—which can make people more vulnerable to complications of AP (Mosztbacher et al., 2020; Szentesi et al., 2019)—show links to atypical pain of currently unknown significance. We may assume that this is due to diabetic neuropathy. However, this is contradicted by the fact that among the metabolic components, diabetes was the one that was not associated with atypical pain. Unfortunately, the currently available data do not allow us to interpret our findings in more detail. Further studies with a larger sample size could confirm or reject altered pain perception in patients with metabolic syndrome.
Atypical pain was relatively common in our cohort. The most accepted diagnostic guideline, the revised Atlanta classification, defines acute pancreatic pain as ‘abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back)’. When forming our groups in pain localization, we were also only able to rely on this phrase and other phrases that appear frequently in the AP literature and in clinical practice. Nevertheless, seeing the high number of patients with atypical pain in our cohort, it would be reasonable to reconsider what typical pain in AP means. The localization of pain in AP could be more diverse than we think. Reconsideration of the diagnostic criterion may facilitate early recognition of AP in centres where imaging and laboratory examinations are not easily accessible.
Lower and left abdominal pain probably caused diagnostic difficulty, suggested by the high proportion of idiopathic cases. However, even in these cases, an effort should be made to determine disease causality (Zadori et al., 2020).
4.3. Pain management
Pain management in AP is, without a doubt, of particular importance. Unfortunately, according to previous systematic reviews (Basurto Ona et al., 2013; Meng et al., 2013), only a few randomized clinical trials have investigated this topic. In addition, we need further descriptions of analgesic strategy in a real‐world setting (Pezzilli et al., 2007). Therefore, we briefly reviewed the use of opioid and non‐opioid pain medications in our registry.
Adequate analgesia may improve disease outcome and patient satisfaction by enabling early feeding and mobilization. Non‐opioid analgesics may be particularly recommended, since morphine may worsen the severity of AP because of known and hypothesized side effects (Barlass et al., 2018), while NSAIDs can relieve inflammation according to a systematic review of animal and clinical studies (Wu et al., 2020). However, a systematic review found that patients administered with opioids might need fewer supplementary analgesics, but the pain intensity of these patients was similar to that of the controls (including NSAID treatment), pointing to the ongoing debate in this field. Moreover, a recent study comparing pentazocine, an opioid and diclofenac, has found a significantly longer pain‐free period, less rescue analgesia, similar side effect profile and disease course in the pentazocine group. Nevertheless, patients in both groups had very fast recovery. The authors have explained it with the proper pain management, resulting in decrease in sympathetic activity and neuroimmune inflammation (Mahapatra et al., 2019).
Contrary to these results, in our cohort, cases administered with painkiller, especially with opioid had longer LOS compared to patients without painkiller and without opioids respectively. However, patients were not treated with rigorous, predetermined pain management strategies in our registry, it was rather based on the preferences of physicians. Besides pain intensity, pain management was likely dependent from the disease severity. For example patients with more severe disease tended to be treated with opioids. Of course, a direct toxic effect of opioids cannot be ruled out in this case either.
So, the question whether opioids or NSAIDs are better has not been decided. Since this is a registry‐based analysis, a definitive conclusion on this topic cannot be drawn from our data without the possibility of selection bias. The high percentage of missing data in these parameters should be also considered as limitation. The high proportion of missing data is primarily explained by the temporary or permanent transfer of patients to another ward or department, on which days the paper‐based documentation became inaccessible for research personnel.
Nevertheless, it should be highlighted that the percentage of opioid use in our cohort is relatively low. Despite the steady rise in opioid consumption, Central and Eastern Europe, from where most of our data originate, has a more restrictive opioid policy. In fact, according to the analysis of worldwide pain management strategies, only North America had a very high rate of opioid administration (93% vs. 27% in other regions). This extremely high difference might be explained by the shortcomings of AP guidelines on pain management (Matta et al., 2020). To fully elucidate this question and to compare pain relief achieved by opioids and non‐opioids, carefully designed randomized controlled trials (RCT) are needed in AP. The already existing RCTs provide limited data with relatively low patient numbers (16 to 50); therefore, more evidence is warranted.
In essence, the use of an enhanced recovery strategy applied in postoperative care may also be recommended in AP (Dong et al., 2019). Tailored therapy (besides pain intensity, therapy that is also tailored to pain type) would facilitate the development of enhanced recovery strategies (Wu et al., 2020). Nevertheless, proper pain assessment must precede pain management (Vivian et al., 2019).
In our cohort, the characteristics of on‐admission pain were associated with the frequency of opioid administration and the duration of analgesic requirement, possibly suggesting that these pain characteristics may persist.
Unfortunately, any association with analgesics is highly dubious since analgesic administration might depend on several factors, including pain intensity, patient's age, comorbidities and the severity of AP. Moreover, we were not able to perform analyses on active substances and dosages because of poorly reported data.
4.4. Strength and limitations
This study examines the role of abdominal pain in AP in a unique and detailed fashion. The data came from an international, multicentre collaboration with 1432 consecutive patients with AP, thus improving its external validity. The similar mortality and severity rates to those of published international data serve as confirmation. We took several variables into account collected and validated in four steps by trained research staff, including clinical research administrators and clinicians.
This study also has limitations. First, a high percentage of missing data in some variables can lead to selection bias. To evaluate the influential power of missing data, we compared the whole cohort to the analysed cohorts, where complete data on a given pain characteristic was available. We found differences when we compared the pain intensity and pain type cohort to the whole cohort. Namely, a lower proportion of severe AP in the pain intensity and pain type cohort was found. Since the question about pain intensity was only included in 2015, improved management of patients over time may explain this phenomenon. Moreover, complete documentation on pain management was only available in 61.6% of the cases. Second, much of our data were based on questionnaires; thus, the role of recall bias may arise. Third, we collected data on pain at a single point in time on the day of hospital admission, which does not consider changes in pre‐admission pain, pain trajectories during hospitalization, and the effect of therapy.
4.5. Implications for practice
Our research pointed out that a comprehensive patient interview should include questions about pain characteristics. However, there is a pressing need for validated pain quality assessment tools in AP translated into various languages to improve clinical trials and practice.
Patients with sharp and intense pain might require special monitoring and tailored pain management.
4.6. Implications for research
Since pain in AP can be very severe and difficult to manage, it is essential to explore the mechanism of pain and to understand its relationship with the disease course and patients’ characteristics to optimize pain management.
The pathophysiology of pain should be further investigated, for example to explore the possibility of neuropathy. Studies should also focus on the association between pain characteristics and inflammatory parameters. Furthermore, future studies should investigate pain trajectories in AP as well as the transition from acute to chronic pain and the influence of pain trajectories on long‐term quality of life.
5. CONCLUSION
Intense and sharp pain on admission was associated with higher odds for severe AP and several systemic and local complications. VAS 7–10 was linked to peripancreatic fluid and oedematous pancreas. Therefore, the question arises whether patients with more intense pain require closer monitoring and whether pain relief could improve AP outcome.
Sharp pain was associated with the highest pain intensity. The mechanism of pain type is currently unknown but should be further investigated to clarify whether these patients require different pain management strategies besides closer monitoring due to a more severe disease course.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHORS’ CONTRIBUTIONS
All the authors critically revised the final version of the manuscript and approved it. FM, AS, SK, AP and PH participated in drafting the concept, interpreting the data and writing the manuscript. FM also participated in patient enrollment and data collection. Besides reviewing the manuscript, the following authors also provided substantial assistance in patient enrollment and study design: ÁV, JB, IS, ZS, IF, JG, JH, ZV, EF, SC, VS, ERM, ÁM, PV, GP, DS, NF, AM, TN, ZM, AV and PJH. GN performed the statistical analysis and wrote the relevant parts of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors are very grateful to those patient enrolling centres that has not achieved the contribution necessary to earn authorship: Polyclinic of Hospitaler Brothers of Saint John of God, Budapest, Hungary; Dr. Réthy Pál Hospital, Békéscsaba, Hungary; Dr. Bugyi István Hospital, Szentes, Hungary; Borsod‐Abaúj‐Zemplén County Hospital and University Teaching Hospital, Miskolc, Hungary; Bács‐Kiskun County Hospital, Kecskemét, Hungary; Healthcare Center of Csongrád County, Makó, Hungary; Markusovszky University Teaching Hospital, Szombathely, Hungary; Mures County Emergency Hospital, Targu Mures, Romania; Vilnius University Hospital Santariskiu Klinikos, Lithuania, Hungary; Hospital of Bezmialem Vakif University, Istanbul, Turkey; Saint Luke Clinical Hospital, St. Petersburg, Russia; Vítkovická Hospital a.s., Ostrava, Czech Republic; Gomel Regional Clinical Hospital, Gomel, Belarus; Pauls Stradius Clinical University Hospital, Riga, Latvia; Bogomolets National Medical University, Kiev, Ukraine; Keio University, Tokyo, Japan
Földi, M. , Gede, N. , Kiss, S. , Vincze, Á. , Bajor, J. , Szabó, I. , Szepes, Z. , Izbéki, F. , Gervain, J. , Hamvas, J. , Vitális, Z. , Fehér, E. , Crai, S. , Sallinen, V. , Ramirez‐Maldonado, E. , Meczker, Á. , Varjú, P. , Poropat, G. , Stimac, D. , … Szentesi, A. ; on behalf of the Hungarian Pancreatic Study Group . (2022). The characteristics and prognostic role of acute abdominal on‐admission pain in acute pancreatitis: A prospective cohort analysis of 1432 cases. European Journal of Pain, 26, 610–623. 10.1002/ejp.1885
Péter Hegyi and Andrea Szentesi contributed equally to this study.
Funding information
This study was funded by Economic Development and Innovation Operative Programme Grants ‘GINOP‐2.3.2‐15‐2016‐00048 ‐ STAY ALIVE’ and ‘GINOP‐2.3.2‐15‐2016‐00015 – I‐KOM’ co‐financed by the European Union within the framework of Programme Széchenyi 2020 (PH). It was also funded by the ÚNKP‐20‐3, a New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development, and Innovation Fund (MF) and the Research Fund (300909) at Medical School, University of Pécs (AS). The funders had no effect on the concept, data collection and analysis, or writing of the manuscript.
REFERENCES
- Asthana, R. , Zhang, L. , Wan, B. A. , Gallo‐Hershberg, D. , Giotis, A. , Pasetka, M. , van Draanen, J. , Goodall, S. , Diaz, P. L. , Drost, L. , Chow, E. , & De Angelis, C. (2020). Pain descriptors of taxane acute pain syndrome (TAPS) in breast cancer patients‐a prospective clinical study. Supportive Care in Cancer, 28(2), 589–598. 10.1007/s00520-019-04845-7 [DOI] [PubMed] [Google Scholar]
- Banks, P. A. , Bollen, T. L. , Dervenis, C. , Gooszen, H. G. , Johnson, C. D. , Sarr, M. G. , Tsiotos, G. G. , & Vege, S. S. ; Acute Pancreatitis Classification Working, G . (2013). Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut, 62(1), 102–111. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- Barlass, U. D. R. , Cheema, H. , George, J. , Sareen, A. , Dixit, A. , Yuan, Z. , Giri, B. , Meng, J. , Banerjee, S. , Dudeja, V. , Dawra, R. K. , Roy, S. , & Saluja, A. K. (2018). Morphine worsens the severity and prevents pancreatic regeneration in mouse models of acute pancreatitis. Gut, 67(4), 600–602. 10.1136/gutjnl-2017-313717 [DOI] [PubMed] [Google Scholar]
- Barreto, S. G. , & Saccone, G. T. (2012). Pancreatic nociception–revisiting the physiology and pathophysiology. Pancreatology, 12(2), 104–112. 10.1016/j.pan.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Basurto Ona, X. , Rigau Comas, D. , & Urrútia, G. (2013). Opioids for acute pancreatitis pain. Cochrane Database of Systematic Reviews. 10.1002/14651858.CD009179.pub2 [DOI] [PubMed] [Google Scholar]
- Brindise, E. , Elkhatib, I. , Kuruvilla, A. , & Silva, R. (2019). Temporal trends in incidence and outcomes of acute pancreatitis in hospitalized patients in the United States from 2002 to 2013. Pancreas, 48(2), 169–175. 10.1097/MPA.0000000000001228 [DOI] [PubMed] [Google Scholar]
- Chamberlain, J. J. , Rhinehart, A. S. , Shaefer, C. F. , & Neuman, A. (2016). Diagnosis and management of diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Annals of Internal Medicine, 164(8), 542–552. 10.7326/M15-3016 [DOI] [PubMed] [Google Scholar]
- Charlson, M. E. , Pompei, P. , Ales, K. L. , & MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40, 373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- Chou, R. , Gordon, D. B. , de Leon‐Casasola, O. A. , Rosenberg, J. M. , Bickler, S. , Brennan, T. , Carter, T. , Cassidy, C. L. , Chittenden, E. H. , Degenhardt, E. , Griffith, S. , Manworren, R. , McCarberg, B. , Montgomery, R. , Murphy, J. , Perkal, M. F. , Suresh, S. , Sluka, K. , Strassels, S. , … Wu, C. L. (2016). Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. The Journal of Pain, 17(2), 131–157. 10.1016/j.jpain.2015.12.008 [DOI] [PubMed] [Google Scholar]
- Demir, I. E. , Friess, H. , & Ceyhan, G. O. (2015). Neural plasticity in pancreatitis and pancreatic cancer. Nature Reviews Gastroenterology & Hepatology, 12(11), 649–659. 10.1038/nrgastro.2015.166 [DOI] [PubMed] [Google Scholar]
- Demir, I. E. , Heinrich, T. , Carty, D. G. , Saricaoglu, Ö. C. , Klauss, S. , Teller, S. , Kehl, T. , Mota Reyes, C. , Tieftrunk, E. , Lazarou, M. , Bahceci, D. H. , Gökcek, B. , Ucurum, B. E. , Maak, M. , Diakopoulos, K. N. , Lesina, M. , Schemann, M. , Erkan, M. , Krüger, A. , … Ceyhan, G. O. (2019). Targeting nNOS ameliorates the severe neuropathic pain due to chronic pancreatitis. EBioMedicine, 46, 431–443. 10.1016/j.ebiom.2019.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, E. , Chang, J. I. , Verma, D. , Butler, R. K. , Villarin, C. K. , Kwok, K. K. , Chen, W. , & Wu, B. U. (2019). Enhanced recovery in mild acute pancreatitis: A randomized controlled trial. Pancreas, 48(2), 176–181. 10.1097/MPA.0000000000001225 [DOI] [PubMed] [Google Scholar]
- Drewes, A. M. , Bouwense, S. A. W. , Campbell, C. M. , Ceyhan, G. O. , Delhaye, M. , Demir, I. E. , Garg, P. K. , van Goor, H. , Halloran, C. , Isaji, S. , Neoptolemos, J. P. , Olesen, S. S. , Palermo, T. , Pasricha, P. J. , Sheel, A. , Shimosegawa, T. , Szigethy, E. , Whitcomb, D. C. , & Yadav, D. ; Working group for the International Consensus Guidelines for Chronic, P . (2017). Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology, 17(5), 720–731. 10.1016/j.pan.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Dworkin, R. H. , Jensen, M. P. , Gammaitoni, A. R. , Olaleye, D. O. , & Galer, B. S. (2007). Symptom profiles differ in patients with neuropathic versus non‐neuropathic pain. The Journal of Pain, 8(2), 118–126. 10.1016/j.jpain.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Dworkin, R. H. , Turk, D. C. , Farrar, J. T. , Haythornthwaite, J. A. , Jensen, M. P. , Katz, N. P. , Kerns, R. D. , Stucki, G. , Allen, R. R. , Bellamy, N. , Carr, D. B. , Chandler, J. , Cowan, P. , Dionne, R. , Galer, B. S. , Hertz, S. , Jadad, A. R. , Kramer, L. D. , Manning, D. C. , … Witter, J. (2005). Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain, 113(1–2), 9–19. 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Edwards, R. R. , Dworkin, R. H. , Turk, D. C. , Angst, M. S. , Dionne, R. , Freeman, R. , Hansson, P. , Haroutounian, S. , Arendt‐Nielsen, L. , Attal, N. , Baron, R. , Brell, J. , Bujanover, S. , Burke, L. B. , Carr, D. , Chappell, A. S. , Cowan, P. , Etropolski, M. , Fillingim, R. B. , … Yarnitsky, D. (2016). Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain, 157(9), 1851–1871. 10.1097/j.pain.0000000000000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan, O. , Malek, M. , Janal, M. N. , & Gibbs, J. L. (2019). Sensory testing associates with pain quality descriptors during acute dental pain. European Journal of Pain, 23(9), 1701–1711. 10.1002/ejp.1447 [DOI] [PubMed] [Google Scholar]
- Fillingim, R. B. , King, C. D. , Ribeiro‐Dasilva, M. C. , Rahim‐Williams, B. , & Riley, 3rd, J. L. (2009). Sex, gender, and pain: A review of recent clinical and experimental findings. The Journal of Pain, 10(5), 447–485. 10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, G. , Lenggenhager, B. , Scivoletto, G. , Giannini, A. M. , & Pazzaglia, M. (2019). "My friend, the pain": Does altered body awareness affect the valence of pain descriptors? Journal of Pain Research, 12, 1721–1732. 10.2147/JPR.S191548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagjer, S. , & Kumar, N. (2018). Evaluation of the BISAP scoring system in prognostication of acute pancreatitis—A prospective observational study. International Journal of Surgery, 54(Pt A), 76–81. 10.1016/j.ijsu.2018.04.026 [DOI] [PubMed] [Google Scholar]
- Harshit Kumar, A. , & Singh Griwan, M. (2018). A comparison of APACHE II, BISAP, Ranson's score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterology Report, 6(2), 127–131. 10.1093/gastro/gox029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hritz, I. , Czako, L. , Dubravcsik, Z. , Farkas, G. , Kelemen, D. , Lasztity, N. , Morvay, Z. , Oláh, A. , Pap, Á. , Párniczky, A. , Sahin‐Tóth, M. , Szentkereszti, Z. , Szmola, R. , Szücs, Á. , Takács, T. , Tiszlavicz, L. , Hegyi, P. , & Munkacsoport, M. H. , Hungarian Pancreatic Study Group . (2015). Acute pancreatitis. Evidence‐based practice guidelines, prepared by the Hungarian Pancreatic Study Group. Orvosi Hetilap, 156(7), 244–261. 10.1556/OH.2015.30059 [DOI] [PubMed] [Google Scholar]
- Kapoor, K. , Repas, K. , Singh, V. K. , Conwell, D. L. , Mortele, K. J. , Wu, B. U. , & Banks, P. A. (2013). Does the duration of abdominal pain prior to admission influence the severity of acute pancreatitis? Journal of the Pancreas, 14(2), 171–175. 10.6092/1590-8577/1283 [DOI] [PubMed] [Google Scholar]
- Mackey, S. , Carroll, I. , Emir, B. , Murphy, T. K. , Whalen, E. , & Dumenci, L. (2012). Sensory pain qualities in neuropathic pain. The Journal of Pain, 13(1), 58–63. 10.1016/j.jpain.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madzak, A. , Olesen, S. S. , Lykke Poulsen, J. , Bolvig Mark, E. , Mohr Drewes, A. , & Frokjaer, J. B. (2017). MRI assessed pancreatic morphology and exocrine function are associated with disease burden in chronic pancreatitis. European Journal of Gastroenterology and Hepatology, 29(11), 1269–1275. 10.1097/MEG.0000000000000955 [DOI] [PubMed] [Google Scholar]
- Mahapatra, S. J. , Jain, S. , Bopanna, S. , Gupta, S. , Singh, P. , Trikha, A. , Sreenivas, V. , Shalimar, & Garg, P. K. (2019). Pentazocine, a kappa‐opioid agonist, is better than diclofenac for analgesia in acute pancreatitis: A randomized controlled trial. American Journal of Gastroenterology, 114(5), 813–821. 10.14309/ajg.0000000000000224 [DOI] [PubMed] [Google Scholar]
- Matta, B. , Gougol, A. , Gao, X. , Reddy, N. , Talukdar, R. , Kochhar, R. , Goenka, M. K. , Gulla, A. , Gonzalez, J. A. , Singh, V. K. , Ferreira, M. , Stevens, T. , Barbu, S. T. , Nawaz, H. , Gutierrez, S. C. , Zarnescu, N. O. , Capurso, G. , Easler, J. , Triantafyllou, K. , … Papachristou, G. I. (2020). Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clinical Gastroenterology and Hepatology, 18(7), 1567–1575. 10.1016/j.cgh.2019.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, W. , Yuan, J. , Zhang, C. , Bai, Z. , Zhou, W. , Yan, J. , & Li, X. (2013). Parenteral analgesics for pain relief in acute pancreatitis: A systematic review. Pancreatology, 13(3), 201–206. 10.1016/j.pan.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Mosztbacher, D. , Hanák, L. , Farkas, N. , Szentesi, A. , Mikó, A. , Bajor, J. , Sarlós, P. , Czimmer, J. , Vincze, Á. , Hegyi, P. J. , Erőss, B. , Takács, T. , Czakó, L. , Németh, B. C. , Izbéki, F. , Halász, A. , Gajdán, L. , Hamvas, J. , Papp, M. , … Hungarian Pancreatic Study, G . (2020). Hypertriglyceridemia‐induced acute pancreatitis: A prospective, multicenter, international cohort analysis of 716 acute pancreatitis cases. Pancreatology, 20(4), 608–616. 10.1016/j.pan.2020.03.018 [DOI] [PubMed] [Google Scholar]
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation . (2000). World Health Organ Tech Rep Ser (Vol. 894(i‐xii), pp. 1‐253). [PubMed] [Google Scholar]
- Pai, C. G. , Kamath, M. G. , Shetty, M. V. , & Kurien, A. (2017). Continuing episodes of pain in recurrent acute pancreatitis: Prospective follow up on a standardised protocol with drugs and pancreatic endotherapy. World Journal of Gastroenterology, 23(19), 3538–3545. 10.3748/wjg.v23.i19.3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PanWessex Study Group and on behalf of the Wessex Surgical Trainee Research Collaborative , Mirnezami, A. , Knight, B. , Moran, B. , Noble, F. , Branagan, G. , Primrose, J. , Pearson, K. , West, M. , Curtis, N. , Pucher, P. , Cuttress, R. , Pugh, S. , & Underwood, T. (2019). Population‐based observational study of acute pancreatitis in southern England. Annals of the Royal College of Surgeons of England, 101(7), 487–494. 10.1308/rcsann.2019.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Párniczky, A. , Kui, B. , Szentesi, A. , Balázs, A. , Szűcs, Á. , Mosztbacher, D. , Czimmer, J. , Sarlós, P. , Bajor, J. , Gódi, S. , Vincze, Á. , Illés, A. , Szabó, I. , Pár, G. , Takács, T. , Czakó, L. , Szepes, Z. , Rakonczay, Z. , Izbéki, F. , … Hungarian Pancreatic Study, G . (2016). Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS One, 11(10), e0165309. 10.1371/journal.pone.0165309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzilli, R. , Morselli‐Labate, A. M. , & Corinaldesi, R. (2010). NSAIDs and acute pancreatitis: A systematic review. Pharmaceuticals, 3(3), 558–571. 10.3390/ph3030558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzilli, R. , Uomo, G. , Gabbrielli, A. , Zerbi, A. , Frulloni, L. , De Rai, P. , Castoldi, L. , Cavallini, G. , & Di Carlo, V. (2007). A prospective multicentre survey on the treatment of acute pancreatitis in Italy. Digestive and Liver Disease, 39(9), 838–846. 10.1016/j.dld.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Phillip, V. , Schuster, T. , Hagemes, F. , Lorenz, S. , Matheis, U. , Preinfalk, S. , Lippl, F. , Saugel, B. , Schmid, R. M. , & Huber, W. (2013). Time period from onset of pain to hospital admission and patients’ awareness in acute pancreatitis. Pancreas, 42(4), 647–654. 10.1097/MPA.0b013e3182714565 [DOI] [PubMed] [Google Scholar]
- Rau, C. L. , Yang, J. L. , Lin, J. J. , Wu, P. C. , Hou, C. Y. , Song, C. Y. , & Hsieh, C. L. (2018). Pain quality descriptors and sex‐related differences in patients with shoulder pain. Journal of Pain Research, 11, 1803–1809. 10.2147/JPR.S169006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, S. E. , Morrison‐Rees, S. , John, A. , Williams, J. G. , Brown, T. H. , & Samuel, D. G. (2017). The incidence and aetiology of acute pancreatitis across Europe. Pancreatology, 17(2), 155–165. 10.1016/j.pan.2017.01.005 [DOI] [PubMed] [Google Scholar]
- Sharma, S. , Pathak, A. , & Jensen, M. P. (2016). Words that describe chronic musculoskeletal pain: Implications for assessing pain quality across cultures. Journal of Pain Research, 9, 1057–1066. 10.2147/JPR.S119212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigliano, S. , Sternby, H. , de Madaria, E. , Capurso, G. , & Petrov, M. S. (2017). Early management of acute pancreatitis: A review of the best evidence. Digestive and Liver Disease, 49(6), 585–594. 10.1016/j.dld.2017.01.168 [DOI] [PubMed] [Google Scholar]
- Szakács, Z. , Gede, N. , Pécsi, D. , Izbéki, F. , Papp, M. , Kovács, G. , Fehér, E. , Dobszai, D. , Kui, B. , Márta, K. , Kónya, K. , Szabó, I. , Török, I. , Gajdán, L. , Takács, T. , Sarlós, P. , Gódi, S. , Varga, M. , Hamvas, J. , … Hegyi, P. (2018). Aging and comorbidities in acute pancreatitis II: A cohort‐analysis of 1203 prospectively collected cases. Frontiers in Physiology, 9, 1776. 10.3389/fphys.2018.01776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi, A. , Párniczky, A. , Vincze, Á. , Bajor, J. , Gódi, S. , Sarlós, P. , Gede, N. , Izbéki, F. , Halász, A. , Márta, K. , Dobszai, D. , Török, I. , Farkas, H. , Papp, M. , Varga, M. , Hamvas, J. , Novák, J. , Mickevicius, A. , Maldonado, E. R. , … Hegyi, P. (2019). Multiple hits in acute pancreatitis: Components of metabolic syndrome synergize each other's deteriorating effects. Frontiers in Physiology, 10, 1202. 10.3389/fphys.2019.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Y. H. A. , Rafi, S. , Tyebally Fang, M. , Hwang, S. , Lim, E. W. , Ngu, J. , & Tan, S. M. (2017). Validation of the modified Ranson versus Glasgow score for pancreatitis in a Singaporean population. ANZ Journal of Surgery, 87(9), 700–703. 10.1111/ans.13139 [DOI] [PubMed] [Google Scholar]
- Teo, K. , Johnson, M. H. , Drewes, A. M. , & Windsor, J. A. (2017). A comprehensive pain assessment tool (COMPAT) for chronic pancreatitis: Development, face validation and pilot evaluation. Pancreatology, 17(5), 706–719. 10.1016/j.pan.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Unruh, A. M. (1996). Gender variations in clinical pain experience. Pain, 65(2–3), 123–167. [DOI] [PubMed] [Google Scholar]
- VanDenKerkhof, E. G. , Stitt, L. , Clark, A. J. , Gordon, A. , Lynch, M. , Morley‐Forster, P. K. , Nathan, H. J. , Smyth, C. , Toth, C. , Ware, M. A. , & Moulin, D. E. (2018). Sensitivity of the DN4 in screening for neuropathic pain syndromes. Clinical Journal of Pain, 34(1), 30–36. 10.1097/AJP.0000000000000512 [DOI] [PubMed] [Google Scholar]
- Vivian, E. , Cler, L. , Conwell, D. , Coté, G. A. , Dickerman, R. , Freeman, M. , Gardner, T. B. , Hawes, R. H. , Kedia, P. , Krishnamoorthi, R. , Oduor, H. , Pandol, S. J. , Papachristou, G. I. , Ross, A. , Sethi, A. , Varadarajulu, S. , Vege, S. S. , Wassef, W. , Wilcox, C. M. , … Tarnasky, P. (2019). Acute pancreatitis task force on quality: Development of quality indicators for acute pancreatitis management. American Journal of Gastroenterology, 114(8), 1322–1342. 10.14309/ajg.0000000000000264 [DOI] [PubMed] [Google Scholar]
- Working Group IAP/APA Acute Pancreatitis Guidelines . (2013). IAP/APA evidence‐based guidelines for the management of acute pancreatitis. Pancreatology, 13(4 Suppl 2), e1–e15. 10.1016/j.pan.2013.07.063 [DOI] [PubMed] [Google Scholar]
- Wu, D. , Bai, X. , Lee, P. , Yang, Y. , Windsor, J. , & Qian, J. (2020). A systematic review of NSAIDs treatment for acute pancreatitis in animal studies and clinical trials. Clinics and Research in Hepatology and Gastroenterology, 44S, 100002. 10.1016/j.clirex.2019.100002 [DOI] [PubMed] [Google Scholar]
- Zadori, N. , Parniczky, A. , Szentesi, A. , & Hegyi, P. (2020). Insufficient implementation of the IAP/APA guidelines on aetiology in acute pancreatitis: Is there a need for implementation managers in pancreatology? United European Gastroenterology Journal, 8(3), 246–248. 10.1177/2050640620918695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Pan, X. , Zeng, H. , He, W. , Xia, L. , Liu, P. I. , Zhu, Y. , Chen, Y. , & Lv, N. (2017). A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised Atlanta classification in Jiangxi, China over an 8‐year period. Pancreas, 46(4), 504–509. 10.1097/MPA.0000000000000776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


