Abstract
We designed a systematic literature review to identify available evidence on adherence to and persistence with antidiabetic medication in people with type 2 diabetes (T2D). Electronic screening and congress searches identified real‐world noninterventional studies (published between 2010 and October 2020) reporting estimates of adherence to and persistence with antidiabetic medication in adults with T2D, and associations with glycaemic control, microvascular and/or macrovascular complications, hospitalizations and healthcare costs. Ninety‐two relevant studies were identified, the majority of which were retrospective and reported US data. The proportions of patients considered adherent (median [range] 51.2% [9.4%‐84.3%]) or persistent (median [range] 47.7% [16.9%‐94.0%]) varied widely across studies. Multiple studies reported an association between greater adherence/persistence and greater reductions in glycated haemoglobin levels. Better adherence/persistence was associated with fewer microvascular and/or macrovascular outcomes, although there was little consistency across studies in terms of which outcomes were improved. More adherent and more persistent patients were typically less likely to be hospitalized or to have emergency department visits/admissions and spent fewer days in hospital annually than less adherent/persistent patients. Greater adherence and persistence were generally associated with lower hospitalization costs, higher pharmacy costs and lower or budget‐neutral total healthcare costs compared with lower adherence/persistence. In conclusion, better adherence and persistence in people with T2D is associated with lower rates of microvascular and/or macrovascular outcomes and inpatient hospitalization, and lower or budget‐neutral total healthcare expenditure. Education and treatment strategies to address suboptimal adherence and persistence are needed to improve clinical and economic outcomes.
Keywords: adherence, GLP‐1RAs, healthcare costs, insulin, oral antidiabetic medications, persistence, resource utilization, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is a chronic, progressive disease that has a substantial clinical impact on patients as well as imposing an economic burden on healthcare systems. 1 T2D is associated with cardiovascular, renal, retinal and neurological complications, and it has been estimated that 50% of people with T2D have early signs of these conditions at diagnosis. 2 Complications account for a considerable proportion of the lifetime costs of treating diabetes, 3 and are also linked to reduced health‐related quality of life 4 and increased indirect costs from lost workplace productivity. 5 The risk of T2D complications is higher in patients with poor glycaemic control. 6 , 7 , 8 , 9 , 10 , 11 , 12 Various demographic, social, and patient‐ and physician‐related factors contribute to the likelihood of people with T2D achieving glycaemic control, 13 , 14 including the extent to which patients are adherent to and persistent with antidiabetic medication. 15 , 16 Although there is evidence that adherence and persistence are associated with improved outcomes, medication‐taking behaviour is not usually considered by decision‐makers and payers alongside clinical benefits and health utility gains when evaluating T2D treatments.
Patients are closely monitored in clinical trials, therefore adherence and persistence during these trials is not representative of medication‐taking behaviour in real‐world settings. 17 Observational studies must thus be used to estimate adherence and persistence rates and to evaluate the link between medication‐taking behaviour and clinical or economic outcomes. As there is a considerable volume of real‐world evidence in T2D, a systematic review of the literature is a robust way to identify such studies and collate their results. An earlier systematic literature review (SLR) of articles published from 2007 to 2014 found that higher rates of adherence to antidiabetic medication were associated with not only better glycaemic control and fewer hospitalizations but also lower healthcare costs. 18 During the past few years, however, the use of newer drug classes such as dipeptidyl peptidase‐4 inhibitors, glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) and sodium‐glucose cotransporter‐2 inhibitors has increased. 19 Consequently, a new review of the literature is warranted to examine medication‐taking behaviour and its link to outcomes across the current spectrum of available antidiabetic medications.
The present SLR was designed to identify relevant evidence on the patterns of adherence to and persistence with antidiabetic medication in people with T2D, as well as clinical and economic outcomes linked to adherence and persistence, over the period of 2010 to 2020.
2. MATERIALS AND METHODS
2.1. Systematic literature review
Electronic searches were designed to identify real‐world noninterventional studies reporting estimates of adherence to and persistence with antidiabetic medications in people with T2D and associations with clinical and economic outcomes. Journal publications from January 2010 to October 2020 were included in electronic searches, and relevant congress publications from January 2018 to October 2020 were also identified. Systematic searches were conducted in October 2020 in the Medical Literature Analysis and Retrieval System Online (MEDLINE), Embase, Evidence‐Based Medicine Reviews and EconLit. Any databases that were not up to date were also searched via the University of York Centres for Reviews and Dissemination website. The search strategy for MEDLINE is shown in Table S1. The congresses searched are listed in Table S2.
Titles and abstracts were screened in a double‐blind manner by two independent reviewers to determine whether they met the eligibility criteria for inclusion (Table 1). Any disagreements between reviewers were referred to a third reviewer and resolved by consensus. The reference lists of included studies and relevant reviews/editorials were reviewed to identify any further eligible publications that had not been detected in the database searches. All publications meeting the criteria were obtained as full articles and reassessed, and relevant data from publications included after full‐text review were entered into a data extraction table. Quality assessment was carried out on the studies using the critical appraisal tools from the Joanna Briggs Institute. 20
TABLE 1.
Eligibility criteria
| Criteria | Include | Exclude |
|---|---|---|
| Population | People with T2D, regardless of age or disease severity |
|
|
||
|
||
|
||
| Intervention | Pharmacological antidiabetic medications | Nonpharmacological interventions (eg, diet‐based interventions, lifestyle changes, guidelines, digital apps) |
| Outcomes |
|
Outcomes not listed in “include” column |
|
||
|
||
|
||
|
||
| Study design | Studies which have utilized real‐world data to investigate outcomes of interest, including: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Geography | No restriction | ‐ |
| Publication date |
Full publications: 2010 onwards (last 10 years) Conference abstracts: 2017 onwards (last 3 years) |
Full publications: pre‐2010 Conference abstracts: pre‐2017 |
| Language | English‐language publications or non‐English‐language publications with an English abstract | Non‐English‐language publications without an English abstract |
Abbreviations: HbA1c, glycated haemoglobin; RCT, randomized controlled trial; T1D, type 1 diabetes; T2D, type 2 diabetes.
2.2. Outcomes
Relevant outcomes in the SLR were estimates of adherence to and persistence with antidiabetic medications and their associations with glycaemic control, microvascular and macrovascular outcomes, hospitalizations and healthcare costs. We defined adherence as the extent to which a person's antidiabetic medication‐taking behaviour corresponds with recommendations from their healthcare provider. In the studies identified, it was most often measured as proportion of days covered (PDC) by medication, or medication possession ratio (MPR) as detailed below. Persistence, the duration of antidiabetic medication use by a patient, was usually measured as the proportion of patients who remained on treatment for a specified period or as the mean number of days to treatment discontinuation within the observation period.
3. RESULTS
3.1. Search results
In total, 3227 references were included for screening, of which 508 were determined to be relevant for full‐text review (see Figure S1 for the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses [PRISMA] diagram). In total, 255 publications met the inclusion criteria at full‐text review, and an additional eight publications were identified by hand searches.
Only full publications reporting data on the associations between adherence/persistence and the clinical/economic outcomes of interest were prioritized for data extraction and are the focus of this manuscript (n = 92). 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 The remaining 171 publications (conference abstracts [n = 47] and full publications reporting estimates of adherence/persistence but not associations with clinical/economic outcomes [n = 124]) were excluded.
3.2. Study characteristics
Of the 92 full publications included, 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 39 studies were published from 2010 to 2015 and 53 from 2016 to 2020. Most of the studies were retrospective observational cohort studies or database analyses; only 13 of the 92 studies (14%) were prospective. 21 , 23 , 33 , 45 , 59 , 67 , 70 , 87 , 92 , 93 , 97 , 102 , 111 Sixty of the 92 studies (65%) were from the United States. The remaining studies were from Europe (Germany, 24 , 93 Italy, 35 , 81 Spain, 47 Sweden 112 and Switzerland 57 ; 7% of studies in total), Asia (China, 29 India, 97 Iran, 45 Israel, 74 , 75 Pakistan 92 and Taiwan 1 , 33 ; 9% of studies in total), Canada (2%) 49 , 111 and Australia (1%). 64 Three studies were multinational (3%). 70 , 102 , 106 Some studies had additional patient inclusion criteria such as a focus on older 28 , 77 , 85 , 96 or younger patients 27 or US veterans. 41 , 42 , 43 , 62 , 78 Table S3 shows the number of studies reporting each outcome.
3.3. Estimates of adherence
In total, 71 studies included estimates of adherence (cited in Table S3). There was variation in how adherence was defined: 30 studies used PDC 22 , 28 , 29 , 30 , 31 , 34 , 36 , 37 , 38 , 39 , 48 , 50 , 56 , 57 , 60 , 63 , 71 , 75 , 76 , 78 , 79 , 80 , 82 , 83 , 85 , 86 , 88 , 98 , 100 , 110 and 26 studies used the MPR. 23 , 29 , 32 , 33 , 35 , 41 , 42 , 44 , 49 , 51 , 53 , 54 , 55 , 64 , 68 , 73 , 77 , 90 , 91 , 95 , 101 , 103 , 104 , 106 , 108 , 109 MPR is calculated as the total days' supply of treatment in the defined period, divided by the total number of days in the defined period. It is easy to estimate but can lead to overestimation of adherence if patients refill prescriptions early. 113 PDC is the most commonly used indirect measure of adherence for chronic diseases. 113 It is a more conservative measure than MPR as it accounts for overlapping days between prescriptions, so it cannot exceed 100% and therefore avoids falsely inflating mean population adherence. A PDC or MPR >80% was the threshold most frequently used to define adherence. Sixteen studies used other methods to define adherence, including self‐reported measures and pill counts. 21 , 26 , 45 , 46 , 47 , 59 , 65 , 67 , 70 , 84 , 87 , 92 , 93 , 94 , 97 , 111
Study follow‐up duration ranged from 3 months to 10 years; 22 of the 71 studies had a 12‐month follow‐up period. Overall, 28 studies examined adherence to oral antidiabetic medications (OADs), 23 , 29 , 33 , 44 , 45 , 48 , 49 , 51 , 53 , 54 , 55 , 56 , 57 , 59 , 60 , 63 , 71 , 78 , 82 , 84 , 85 , 86 , 90 , 91 , 92 , 94 , 98 , 110 eight studies examined insulin, 26 , 32 , 39 , 70 , 77 , 88 , 104 , 109 seven studies examined injectable GLP‐1RAs 30 , 37 , 38 , 79 , 80 , 83 , 106 and one study examined combination therapies. 75 Twenty studies reported adherence estimates for multiple classes (OADs, insulins and/or GLP‐1RAs). 22 , 28 , 31 , 34 , 35 , 36 , 41 , 42 , 50 , 64 , 68 , 73 , 76 , 95 , 101 , 103 , 108 Seven studies did not report which antidiabetic medication(s) were included. 21 , 46 , 67 , 87 , 93 , 97 , 100
Estimates of adherence varied considerably across studies, from 9.4% to 84.3%, across all medication classes. In general, reported rates of adherence to antidiabetic medications were relatively low: the median adherence across all studies was 51.2%. There was no clear consensus across studies regarding which medication classes were associated with higher adherence.
3.4. Estimates of persistence
Estimates of persistence were reported in 31 studies (Table S3). In most studies, persistence was estimated based on the fill time between prescriptions or medication insurance claims. A gap in medication of ≥90 days was used to define discontinuation of medication (non‐persistence) in nine studies, 24 , 29 , 30 , 40 , 58 , 69 , 96 , 106 , 109 whereas thresholds of ≥30, 25 , 31 , 52 , 89 45, 79 , 80 60 60 , 61 , 90 , 107 or 120 days 74 were used in other studies. Thirteen studies used other definitions for treatment gaps indicating discontinuation, such as a gap exceeding the 90th percentile of the mean duration of prescription fills. 77 , 104
All 31 studies were retrospective. Study duration ranged from 6 months 75 , 107 to 3 years, 94 and was 12 months in 17 studies. 24 , 25 , 29 , 30 , 31 , 40 , 52 , 58 , 60 , 62 , 69 , 77 , 80 , 90 , 96 , 104 Overall, six studies examined persistence with OADs, 29 , 58 , 60 , 62 , 90 , 94 11 studies with insulin, 24 , 25 , 40 , 52 , 61 , 77 , 89 , 96 , 104 , 107 , 109 seven studies with injectable GLP‐1RAs 30 , 38 , 74 , 79 , 80 , 81 , 106 and four studies with combination therapies. 69 , 75 , 105 , 112 Three studies reported persistence estimates for multiple classes of antidiabetic medication. 27 , 31 , 66
As was the case for adherence, persistence estimates varied widely among studies. The proportion of persistent patients ranged from 16.9% to 94.0% (median: 47.7%) in the studies reporting persistence with the most frequently studied classes of antidiabetic medication (OADs, insulins and GLP‐1RAs).
3.5. Associations between adherence/persistence and clinical and economic outcomes
3.5.1. Glycaemic control
The specific glycaemic outcomes assessed in the studies examining adherence and persistence were overall change in glycated haemoglobin (HbA1c) level, expressed as a percentage, the proportion of patients achieving a target HbA1c or the incidence of hypoglycaemia.
An association between medication adherence and glycaemic control was reported in 42 studies (Table S3), 30 of which investigated OADs or multiple classes of antidiabetic medications. Better adherence to antidiabetic medication was generally associated with improved glycaemic control. A significantly greater decrease in HbA1c, or a lower HbA1c at follow‐up, in more adherent versus less adherent patients was reported by most studies investigating this outcome. 22 , 29 , 30 , 44 , 47 , 73 , 79 , 85 In the remaining studies, the HbA1c reduction was nonsignificantly lower in the more adherent patients 94 or similar in the two groups. 78 The studies also consistently reported a significantly higher likelihood of more adherent patients achieving specific HbA1c targets, such as a ≥1.0% reduction 29 or reduction to <7%, 45 than less adherent patients. 29 , 45 , 56 , 78 , 82 , 84 , 85 , 90 , 94
Four studies investigated the association between adherence to antidiabetic medication and risk of hypoglycaemia. Two of these studies investigated OADs (including sulphonylureas) and found no significant association, 78 , 91 and two studies investigating multiple antidiabetic medication classes found significantly lower rates of acute complications, including hypoglycaemia, in more versus less adherent patients. 28 , 34
Fewer studies (n = 20) investigated the association between persistence and glycaemic control, including six studies examining injectable GLP‐1RAs, 30 , 38 , 74 , 79 , 81 , 106 six examining combination therapies 27 , 66 , 69 , 75 , 105 , 112 and four each examining insulin 40 , 96 , 107 , 109 and OADs. 58 , 60 , 62 , 94 Persistence with medication was also generally associated with better glycaemic control. All but one 106 of the 14 studies examining GLP‐1RAs, insulin or OADs reported a greater reduction in HbA1c, a greater proportion of patients achieving a target HbA1c or a trend for better outcomes in persistent than in nonpersistent patients. 30 , 38 , 40 , 58 , 60 , 62 , 74 , 79 , 81 , 94 , 96 , 107 , 109 However, the results were more heterogeneous in the studies investigating combination therapies, 27 , 66 , 69 , 75 , 105 , 112 with only two studies clearly demonstrating superior glycaemic control in persistent compared with nonpersistent patients. 69 , 112 Lin et al, 69 in a study of patients receiving GLP‐1RAs and basal insulin, also reported that medication persistence was linked to lower rates of hypoglycaemia. Figure 1 summarizes the findings from studies investigating the association between adherence or persistence and change in HbA1c.
FIGURE 1.

Change in HbA1c level from baseline by A, adherence to and B, persistence with antidiabetic medication in studies reporting this outcome. Bars with an asterisk indicate statistically significant results for adherent/persistent versus nonadherent/nonpersistent patients (P < 0.05). Min et al 78 and Reynolds et al 94 did not report P values for adherent versus nonadherent patients. Eliasson et al 112 and Melzer‐Cohen et al 75 did not report P values for persistent versus nonpersistent patients. For adherence, studies were included if they reported change in HbA1c (follow‐up times varied, as indicated for each study); for persistence, studies were included if they reported change in HbA1c over 6, 12 or 24 months. aData shown are means, except for Min et al 78 which are median. HbA1c, glycated haemoglobin
3.5.2. Microvascular and macrovascular outcomes
Nine studies investigated the associations between adherence to antidiabetic medications and microvascular and/or macrovascular outcomes. 22 , 46 , 48 , 50 , 63 , 97 , 98 , 99 , 108 Five of these examined OADs 48 , 50 , 63 , 98 , 99 ; medication class was mixed or not specified in the remaining studies. 22 , 46 , 97 , 108 Most studies were retrospective, and four of these studies had >54 000 participants. 48 , 50 , 63 , 99 The two prospective studies were small, with <250 patients each. 97 , 98 Follow‐up ranged from 3 months 97 to 10 years, 63 and was ≥5 years in four studies. 46 , 48 , 63 , 108
The microvascular outcomes examined included peripheral vascular disease, retinopathy, nephropathy, renal events, neuropathy and amputations/ulcers. The macrovascular outcomes included cerebrovascular disease, stroke, transient ischaemic attack, ischaemic heart disease, myocardial infarction and angina (Table 2). Six studies examined both microvascular and macrovascular outcomes. 22 , 46 , 48 , 50 , 98 , 99
TABLE 2.
Findings from studies reporting associations between adherence or persistence and microvascular and/or macrovascular outcomes a
| Statistically significant associations | No significant associations found | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Country, design | Treatment | Number of patients | Follow‐up | Microvascular outcomes | Macrovascular outcomes | Microvascular outcomes | Macrovascular outcomes | |
| Adherence | |||||||||
| An and Nichol 22 | United States, retrospective | OADs (biguanides, SUs, TZDs and/or insulin‐sensitizing agents) and hypertension medication | 2334 | 33 months | Any microvascular outcome (from renal failure and diabetic retinopathy) | Any macrovascular outcome (from MI and stroke) | |||
| Fukuda and Mizobe 46 | Japan, retrospective | NR | 11 331 | 96 months |
Retinopathy Nephropathy Neuropathy |
IHD Cerebrovascular disease Chronic arterial occlusion |
|||
| Gatwood et al 48 | United States, retrospective | OADs (not specified) | 159 032 | 5 years | Retinopathy |
Stroke MI |
Neuropathy |
TIA Angina |
|
| Gibson et al 50 | United States, retrospective | SUs, meglitinides, biguanides, TZDs or AGIs | 55 356 (OADs only) | 18 months |
Amputations/ulcers Renal events Neuropathy Retinopathy |
MI | PVD | Cerebrovascular disease | |
| SUs, meglitinides, biguanides, TZDs or AGIs ± insulin | 96 734 (OADs ± insulin) |
Amputations/ulcers Renal events Neuropathy Retinopathy |
MI Cerebrovascular disease |
PVD | |||||
| Kim et al 63 | Korea, retrospective | Biguanide, SUs and others | 65 067 | 10 years | NR | Cerebrovascular disease | NR | MI | |
| Samu et al 97 | India, prospective | NR | 86 | 3 months | Neuropathy (diabetic foot) | NR | |||
| Sattler et al 98 | United States, prospective | OADs (not specified) | 243 | 12 months | Rate of “no (microvascular or macrovascular) complication” was higher in adherent than nonadherent patients |
PVD Nephropathy Neuropathy |
Cerebrovascular complications Cardiovascular complications |
||
| Simpson et al 99 | United States, retrospective | OADs (not specified) | 54 505 | 2.3 years (mean) | Any macrovascular outcome (from MI, stroke, heart failure, angina, CABG and angioplasty) | Any microvascular outcome (nephropathy, neuropathy, PVD or retinopathy) | |||
| Yu et al 108 | United States, retrospective | Insulin and/or OADs | 4708 | 12‐90 months | Any microvascular outcome (from diabetic foot, neuropathy, retinopathy and nephropathy) | NR | NR | NR | |
| Persistence | |||||||||
| Iglay et al 58 | United States, retrospective | SUs (first, second and third generation) | 104 082 | 12 months | PVD |
Stroke TIA CHF MI IHD |
|||
| Kalirai et al 61 | United States, retrospective | Insulin detemir or insulin glargine | 23 645 | 24 months |
Nephropathy Neuropathy |
Retinopathy |
CVD Cerebrovascular disease |
||
Abbreviations: AGI, alpha glucosidase inhibitor; CABG, coronary artery bypass graft; CHF, chronic heart failure; CVD, cardiovascular disease; IHD, ischaemic heart disease; MI, myocardial infarction; NR, not reported; OAD, oral antidiabetic drug; PVD, peripheral vascular disease; SU, sulphonylurea; TIA, transient ischaemic attack; TZD, thiazolidinedione.
Where there are discrepancies reported int results in the same article depending on the statistical model, the key findings as reported by authors in the article abstract are shown.
In general, adherence to antidiabetic medication was associated with lower rates of both microvascular and macrovascular complications compared with nonadherence, but there was substantial heterogeneity across the study results (Table 2). For example, the four largest studies, all of which assessed adherence to OADs, found that adherent patients have significantly lower rates of some outcomes but not others, with no consensus on which outcome category (microvascular or macrovascular) was significantly linked to adherence. 48 , 50 , 63 , 99 The medications that patients received were not fully reported in each study, and therefore no inferences between outcomes and medication class can be made using these data.
Two large US studies that examined the association between persistence and microvascular and macrovascular outcomes were identified (Table 2). Iglay et al 58 included 104 082 patients followed up for 1 year and found lower rates in persistent versus nonpersistent patients for all cardiovascular, cerebrovascular and peripheral vascular outcomes examined. Kalirai et al 61 studied 23 645 patients and reported significantly lower rates of nephropathy and neuropathy after insulin initiation in persistent versus nonpersistent patients, but no significant difference in the rates of retinopathy, cardiovascular disease (CVD) or cerebrovascular disease. 61
3.5.3. Hospitalizations
The association between medication adherence and rates of hospitalization was reported in 18 studies, for patients receiving insulin, 26 , 88 OADs, 33 , 48 , 54 , 55 , 57 , 71 , 94 injectable GLP‐1RAs 83 or multiple treatment classes, 28 , 34 , 36 , 47 , 50 , 101 or with treatment not specified. 93 , 100 The specific outcomes investigated included hospital inpatient admissions, emergency department (ED) visits and admissions, and outpatient visits. Follow‐up duration ranged from 6 months to 7 years, with a median of 3 years. Most studies included >5000 patients; six studies had >90 000 patients (Table S4). 28 , 34 , 48 , 50 , 100 , 101
Patients who were more adherent to antidiabetic medications were generally less likely to be hospitalized (and/or were less likely to have ED visits or admissions, or spent fewer days in hospital annually) than less adherent patients in the majority of studies across all treatment classes (Table S4). 26 , 28 , 33 , 34 , 48 , 50 , 54 , 57 , 71 , 83 , 88 , 94 , 100 , 101 The association between adherence and outpatient visits was more complex. Some studies found no significant association between adherence and outpatient visits, 47 , 93 whereas others found that adherence was linked to having more outpatient visits (Table S4). 36 , 50 , 55 Among the latter studies, two were designed to investigate whether the rate of outpatient visits influenced adherence, and concluded that more frequent outpatient visits led to better adherence to antidiabetic medication; however, these studies did not report rates of hospitalization or other interactions with the healthcare system. 36 , 55 The third study reported that greater adherence was linked to more outpatient visits, but fewer ED visits and hospitalizations. 50
Eleven studies reported data on the association between persistence and the rate of hospitalization (including ED visits and/or ED admissions) in patients receiving insulin, 40 , 52 , 61 , 77 , 89 , 104 , 107 injectable GLP‐1RAs, 74 , 106 OADs 94 or combination therapy. 69 Follow‐up duration ranged from 6 months to 3 years, and sample sizes ranged from 534 to 23 645 patients (Table S5). Interruption or discontinuation of therapy was associated with an increased rate of hospitalization and longer hospital stays in most studies, 40 , 52 , 69 , 77 , 89 , 94 , 104 , 107 but not in all studies. 74 , 106 However, four 52 , 61 , 69 , 77 of the five studies that reported data on outpatient visits found no significant association with persistence (Table S5).
3.5.4. Healthcare costs
Associations between adherence and healthcare costs in people with T2D were reported in 20 studies that investigated OADs, 23 , 29 , 33 , 49 , 53 , 54 insulin, 26 , 32 , 39 , 88 injectable GLP‐1RAs 30 or multiple treatment classes, 28 , 34 , 35 , 41 , 50 , 68 , 72 , 76 or that did not specify the treatment. 46 Thirteen studies were from the United States, with the remaining studies from Canada, 49 Italy, 35 Japan, 46 Korea 23 , 54 and Taiwan. 33 , 68 Seven studies had a follow‐up duration of ≤1 year and nine studies had ≥3 years' follow‐up. Sample size varied widely, from 301 to 0.74 million patients, with a median of 17 982 patients.
Despite substantial heterogeneity across studies, greater adherence was generally associated with lower inpatient admission costs but higher pharmacy costs. 26 , 28 , 29 , 30 , 33 , 34 , 39 , 41 , 49 , 72 , 76 , 88 Most studies found that total healthcare expenditure in adherent patients was lower than 28 , 32 , 39 , 49 , 53 , 54 , 72 , 88 or similar to 23 , 26 , 34 , 76 that in nonadherent patients (Figure 2), but in two studies adherence was associated with higher total costs than nonadherence. 30 , 33
FIGURE 2.
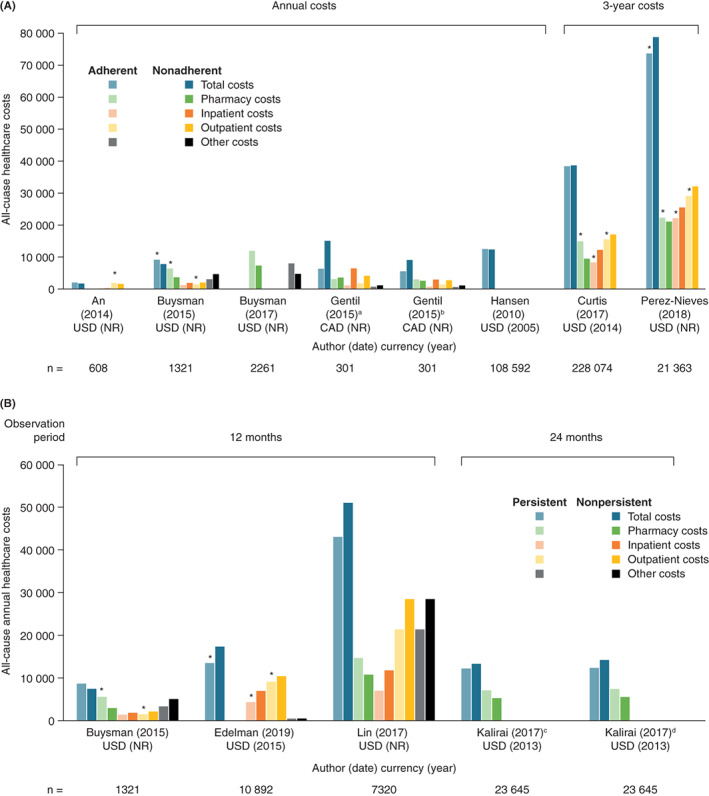
Healthcare costs by A, adherence to and B, persistence with antidiabetic medication in selected relevant studies. Bars with an asterisk indicate statistically significant results for adherent/persistent versus nonadherent/nonpersistent patients (P < 0.05). Buysman et al, 29 Gentil et al 49 and Hansen et al 53 did not report P values for adherent versus nonadherent patients. Kalirai et al 61 and Lin et al 69 did not report P values for persistent versus nonpersistent patients. aData from the subset of patients with depression/anxiety. bData from the subset of patients without depression/anxiety. cData from the first year of treatment. dData from the second year of treatment. CAD, Canadian dollar; ED, emergency department; FU, follow‐up; NR, not reported; USD, United States dollars
In total, 11 studies reported data on healthcare costs associated with persistence on insulin, 24 , 25 , 40 , 52 , 61 , 77 , 89 , 96 , 107 injectable GLP‐1RAs 30 and/or combination therapies. 69 The study duration was relatively short for studying persistence: 6 months 107 or 12 months 24 , 25 , 30 , 40 , 52 , 69 , 77 , 96 in most studies and 24 months 61 , 89 in two studies. Persistence was typically associated with higher pharmacy costs 24 , 25 , 29 , 30 , 40 , 52 , 61 , 69 , 77 , 89 , 107 but lower healthcare costs, including acute care costs, inpatient and outpatient visits and ED visits. 24 , 25 , 52 , 61 , 69 , 89 Overall, total healthcare expenditure for persistent patients across studies was typically lower than or similar to that for nonpersistent patients (Figure 2).
4. DISCUSSION
Observational studies are recognized by payers and other stakeholders as an important means of obtaining data on medication‐taking behaviour, which cannot be assessed in clinical trials. 17 We reviewed the available evidence from observational studies on adherence to and persistence with antidiabetic medication in people with T2D, and how these relate to clinical and economic outcomes. Despite heterogeneity across studies in terms of antidiabetic medications used, length of follow‐up, geography, patients' clinical and demographic characteristics and the specific outcomes examined, some findings were consistent. Overall rates of adherence and persistence in people with T2D are suboptimal, as previously reported, 114 but better adherence and persistence are associated with clinical benefits, including improved glycaemic control, fewer hospitalizations and ED visits, and lower incidences of microvascular and macrovascular complications. Adherence and persistence were linked to lower rates of some microvascular and/or macrovascular outcomes but not others, which may be attributable in part to disparities in medications used, study setting and design. Overall, several outcomes that predict disability and absenteeism in people with T2D, including myocardial infarction, stroke, peripheral neuropathy, retinopathy and diabetic foot, 115 were associated with worse adherence and/or persistence in at least some of the studies identified in this review, highlighting the relevance of these outcomes in treatment decision‐making.
As a chronic condition affecting multiple organ systems, T2D is associated with substantial and rising healthcare costs. The International Diabetes Federation estimates that the worldwide health expenditure due to diabetes in adults has increased threefold in the past 15 years, from $232 billion in 2007 to $760 billion in 2019, of which 50% is attributable to managing diabetes complications 115 ; therefore, the influence of adherence and persistence on healthcare costs in T2D is a pertinent area for study. In the present SLR, we found an association between better adherence and persistence and either lower or similar total healthcare costs, compared with worse adherence and persistence. Cost estimates varied widely across the studies identified, which was likely to be owing to disparities in study variables and location. Generally, both adherence and persistence were associated with higher pharmacy costs that were offset by lower hospitalization costs, resulting in lower or budget‐neutral total healthcare expenditure for adherent/persistent patients. An association between adherence and reduced total health expenditure has been reported for several other chronic conditions. 116 Notably, more than two‐thirds of the studies reporting cost data were from North America, and most of the remainder were from Asia, with only one study from a European country (Italy). Further evidence is therefore needed, in particular from Europe, on the associations between adherence and persistence and healthcare costs.
This was a large SLR, including studies from all geographical regions and examining a broad range of outcomes. Although the outcomes examined are not independent from each other—for example, reduced rates of complications result in lower healthcare costs—this is nonetheless a comprehensive overview of the impact of adherence and persistence. Further SLRs could be used to capture additional outcomes linked to suboptimal adherence and persistence: previous studies have reported increased absenteeism, 117 more days of short‐term disability 117 and greater mortality. 63 , 118 Although most studies reported the antidiabetic medication class used, discrepancies across studies in patient characteristics, study methodology and duration did not enable direct comparisons to be made between medication classes for any of the outcomes. Approximately half of the studies identified in the SLR were published between 2010 and 2015, meaning that more recently approved antidiabetic medications were not included in most of these studies. Further good‐quality observational studies are needed to systematically compare adherence and persistence across different drug classes and drugs with different modes and frequency of administration and different treatment benefits for complications such as CVD.
Several limitations of the studies identified in this review should be noted, particularly the fact that many were retrospective analyses using healthcare and claims databases. Such studies are inherently vulnerable to the effects of confounding, whereby certain factors, such as education, lifestyle and other sociodemographic variables, could influence both adherence to medication and health outcomes. Inertia in treatment decision‐making may also confound the relationship between medication‐taking behaviour and outcomes: adherence to or persistence with antidiabetic medication that has not been optimized is unlikely to be reflected in clinical benefit. Furthermore, PDC and MPR are useful proxies for adherence, but may not always accurately reflect actual medication‐taking behaviour. Finally, although comparing different antidiabetic medication classes is of great clinical interest, the substantial inter‐study heterogeneity in patient characteristics and methods used to estimate adherence/persistence in this SLR did not enable meaningful comparisons across drug classes.
The present SLR highlights the benefits that can be achieved by using therapeutic approaches that improve adherence and persistence as well as clinical outcomes. Across various diseases, higher compliance, a close correlate of adherence, has been reported for dosing regimens that require less frequent administration. 119 To illustrate, a study included in this SLR examining two populations receiving GLP‐1RAs in Germany and the United Kingdom found that twice‐daily exenatide was associated with a 30% to 40% greater likelihood of treatment discontinuation than once‐daily liraglutide. 106 Furthermore, a recent meta‐analysis of seven studies investigating 75 159 people with T2D reported an 11% lower risk of nonadherence with once‐weekly versus once‐daily injectable GLP‐1RAs. 120 Persistence is also favourably influenced by less frequent dosing regimens: in a real‐world, United States‐based study, the use of once‐weekly injectable GLP‐1RAs was associated with better persistence and adherence than daily regimens in propensity score‐matched cohorts. 121 Evidence from populations with other chronic diseases such as osteoporosis 122 and CVD 123 also indicates that lower dosing frequency predicts better adherence and/or persistence. The use of medications early in the treatment pathway that are linked to symptomatic benefit in addition to adherence and persistence may provide an ongoing positive effect on health outcomes; however, to achieve sustained improvements, the use of treatment regimens that enhance adherence and persistence should also be considered as part of wider, holistic treatment strategies for people with T2D. As indicated by the studies identified in this review, many of which were carried out in primary care databases, routine management of patients with T2D is increasingly delivered in a primary care setting. 124 Consequently, it is vital that primary care physicians receive education in strategies to maximize adherence and persistence, in communicating the benefits of this to patients, and in understanding and addressing reasons for poor adherence or persistence. Other approaches to maximizing the likelihood of adherence and persistence that may be applicable in primary care include the use of personalized digital technologies. 125
In this SLR of studies published between 2010 and 2020, greater adherence to and persistence with antidiabetic medication in adults with T2D was typically associated with better clinical and economic outcomes. These findings suggest that the clinical benefits of adherence and persistence for patients are likely to be reflected in positive impacts for payers, healthcare systems and society. Further investigation of the factors that determine medication‐taking behaviour should be used to identify barriers to optimal adherence and persistence in people with T2D.
CONFLICT OF INTEREST
M.E. has received honoraria from AstraZeneca, Boehringer Ingelheim and Novo Nordisk. S.E. and M.F. are employees of Novo Nordisk A/S. J.F. was an employee of Novo Nordisk A/S at the time of the review, and is now an employee of Ferring Pharmaceuticals A/S. P.H. is an employee of Mtech Access, funded by Novo Nordisk A/S to carry out the SLR. W.P. has served as a consultant for Eli Lilly, Novo Nordisk and Sanofi.
AUTHOR CONTRIBUTIONS
Mads Faurby, João Diogo Da Rocha Fernandes and Pollyanna Hudson: designed the SLR. All authors contributed to the interpretation of data and critical review of the manuscript and approved the final version for submission.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14603.
Supporting information
Table S1. Search strategy for MEDLINE
Table S2. List of congresses searched
Table S3. Number of studies reporting each outcome
Table S4. Summary of studies reporting the associations between adherence to antidiabetic medication and inpatient admissions, ED visits/admissions and outpatient claims in patients with T2D
Table S5. Summary of studies reporting the associations between persistence with antidiabetic medication and inpatient admissions, ED visits/admissions and outpatient claims in patients with T2D
Figure S1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) diagram.
ACKNOWLEDGMENTS
The development of this SLR was carried out by Mtech Access, Bicester, UK, funded by Novo Nordisk A/S. The authors acknowledge the medical writing support of Oxford PharmaGenesis, Oxford, UK, funded by Novo Nordisk A/S.
Evans M, Engberg S, Faurby M, Fernandes JDDR, Hudson P, Polonsky W. Adherence to and persistence with antidiabetic medications and associations with clinical and economic outcomes in people with type 2 diabetes mellitus: A systematic literature review. Diabetes Obes Metab. 2022;24(3):377‐390. doi: 10.1111/dom.14603
Funding information Novo Nordisk A/S
DATA AVAILABILITY STATEMENT
‘All data analyzed during this systematic literature review are published elsewhere, and relevant collated data are included in this article [and/or] its supplementary material files. Further enquiries can be directed to the corresponding author’
REFERENCES
- 1. Alzaid A, Ladron de Guevara P, Beillat M, Lehner Martin V, Atanasov P. Burden of disease and costs associated with type 2 diabetes in emerging and established markets: systematic review analyses. Expert Rev Pharmacoecon Outcomes Res. 2021;21(4):785‐798. [DOI] [PubMed] [Google Scholar]
- 2. Diabetes.co.uk. Type 2 Diabetes. 15 January 2019. https://www.diabetes.co.uk/type2-diabetes.html. Accessed August 5, 2021.
- 3. Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med. 2013;45(3):253‐261. [DOI] [PubMed] [Google Scholar]
- 4. Shao H, Yang S, Fonseca V, Stoecker C, Shi L. Estimating quality of life decrements due to diabetes complications in the United States: the health utility index (HUI) diabetes complication equation. Pharmacoeconomics. 2019;37(7):921‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersson E, Persson S, Hallen N, et al. Costs of diabetes complications: hospital‐based care and absence from work for 392,200 people with type 2 diabetes and matched control participants in Sweden. Diabetologia. 2020;63(12):2582‐2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kontopantelis E, Springate DA, Reeves D, et al. Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study. Diabetologia. 2015;58(3):505‐518. [DOI] [PubMed] [Google Scholar]
- 7. Rawshani A, Svensson AM, Gudbjörnsdottir S. Glycaemic control and incidence of dementia in 363,573 patients with type 2 diabetes: an observational study. Diabetologia. 2015;58(1):S5. [Google Scholar]
- 8. Svensson E, Baggesen LM, Johnsen SP, et al. Early glycemic control and magnitude of HbA1c reduction predict cardiovascular events and mortality: population‐based cohort study of 24,752 metformin initiators. Diabetes Care. 2017;40(6):800‐807. [DOI] [PubMed] [Google Scholar]
- 9. Tijera FHDL, Servin‐Caamano A, Navarro‐Estrada A, Soto‐Martinez K, Alexanderson‐Rosas EG, Cruz‐Estrada A. Uncontrolled glycaemia is the strongest predictive factor for liver‐related and cardiovascular death, in cirrhotic patients with type 2 diabetes. Gastroenterology. 2019;156(6):S‐1367. [Google Scholar]
- 10. Turgeon RD, Koshman SL, Youngson E, Pearson GJ. Association between hemoglobin A1c and major adverse coronary events in patients with diabetes following coronary artery bypass surgery. Pharmacotherapy. 2020;40(2):116‐124. [DOI] [PubMed] [Google Scholar]
- 11. van Wijngaarden RPT, Overbeek JA, Heintjes EM, et al. Relation between different measures of glycemic exposure and microvascular and macrovascular complications in patients with type 2 diabetes mellitus: an observational cohort study. Diabetes Ther. 2017;8(5):1097‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitsios JP, Ekinci EI, Mitsios GP, Churilov L, Thijs V. Relationship between glycated hemoglobin and stroke risk: a systematic review and meta‐analysis. J Am Heart Assoc. 2018;7(11):e007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng LJ, Wang W, Lim ST, Wu VX. Factors associated with glycaemic control in patients with diabetes mellitus: a systematic literature review. J Clin Nurs. 2019;28(9‐10):1433‐1450. [DOI] [PubMed] [Google Scholar]
- 14. de Pablos‐Velasco P, Parhofer KG, Bradley C, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf). 2014;80(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 15. Rhee MK, Slocum W, Ziemer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240‐250. [DOI] [PubMed] [Google Scholar]
- 16. Guerci B, Chanan N, Kaur S, Jasso‐Mosqueda JG, Lew E. Lack of treatment persistence and treatment nonadherence as barriers to glycaemic control in patients with type 2 diabetes. Diabetes Ther. 2019;10(2):437‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts MH, Ferguson GT. Real‐world evidence: bridging gaps in evidence to guide payer decisions. Pharmacoecon Open. 2021;5(1):3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capoccia K, Odegard PS, Letassy N. Medication adherence with diabetes medication: a systematic review of the literature. Diabetes Educ. 2016;42(1):34‐71. [DOI] [PubMed] [Google Scholar]
- 19. Engler C, Leo M, Pfeifer B, et al. Long‐term trends in the prescription of antidiabetic drugs: real‐world evidence from the Diabetes Registry Tyrol 2012‐2018. BMJ Open Diabetes Res Care. 2020;8(1):e001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joanna Briggs Institute (JBI) . Critical Appraisal Tools [online]. https://joannabriggs.org/critical-appraisal-tools. Accessed September 9, 2021.
- 21. Aikens JE, Piette JD. Longitudinal association between medication adherence and glycaemic control in type 2 diabetes. Diabet Med. 2013;30(3):338‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. An J, Nichol MB. Multiple medication adherence and its effect on clinical outcomes among patients with comorbid type 2 diabetes and hypertension. Med Care. 2013;51(10):879‐887. [DOI] [PubMed] [Google Scholar]
- 23. An SY, Kim HJ, Chun KH, et al. Clinical and economic outcomes in medication‐adherent and ‐nonadherent patients with type 2 diabetes mellitus in the Republic of Korea. Clin Ther. 2014;36(2):245‐254. [DOI] [PubMed] [Google Scholar]
- 24. Anderten H, Dippel FW, Kostev K. Early discontinuation and related treatment costs after initiation of basal insulin in type 2 diabetes patients: a German primary care database analysis. J Diabetes Sci Technol. 2015;9(3):644‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ascher‐Svanum H, Lage MJ, Perez‐Nieves M, et al. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5(1):225‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ayyagari R, Wei W, Cheng D, Pan C, Signorovitch J, Wu EQ. Effect of adherence and insulin delivery system on clinical and economic outcomes among patients with type 2 diabetes initiating insulin treatment. Value Health. 2015;18(2):198‐205. [DOI] [PubMed] [Google Scholar]
- 27. Barnes NS, White PC, Hutchison MR. Time to failure of oral therapy in children with type 2 diabetes: a single center retrospective chart review. Pediatr Diabetes. 2012;13(7):578‐582. [DOI] [PubMed] [Google Scholar]
- 28. Boye KS, Curtis SE, Lage MJ, Garcia‐Perez LE. Associations between adherence and outcomes among older, type 2 diabetes patients: evidence from a medicare supplemental database. Patient Prefer Adherence. 2016;10:1573‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buysman EK, Anderson A, Bacchus S, Ingham M. Retrospective study on the impact of adherence in achieving glycemic goals in type 2 diabetes mellitus patients receiving canagliflozin. Adv Ther. 2017;34(4):937‐953. [DOI] [PubMed] [Google Scholar]
- 30. Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;02(32):341‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carls GS, Tuttle E, Tan RD, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real‐world use of GLP‐1 RA and DPP‐4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40(11):1469‐1478. [DOI] [PubMed] [Google Scholar]
- 32. Chandran A, Bonafede MK, Nigam S, Saltiel‐Berzin R, Hirsch LJ, Lahue BJ. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8(3):148‐158. [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng SH, Chen CC, Tseng CH. Does medication adherence lead to lower healthcare expenses for patients with diabetes? Am J Manag Care. 2013;19(8):662‐670. [PubMed] [Google Scholar]
- 34. Curtis SE, Boye KS, Lage MJ, Garcia‐Perez LE. Medication adherence and improved outcomes among patients with type 2 diabetes. Am J Manag Care. 2017;23(7):e208‐e214. [PubMed] [Google Scholar]
- 35. Degli Esposti L, Saragoni S, Buda S, Sturani A, Degli EE. Glycemic control and diabetes‐related health care costs in type 2 diabetes; retrospective analysis based on clinical and administrative databases. Clinicoecon Outcomes Res. 2013;5(1):193‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dobbins JM, Elliott SW, Cordier T, et al. Primary care provider encounter cadence and hba1c control in older patients with diabetes. Am J Prev Med. 2019;57(4):e95‐e101. [DOI] [PubMed] [Google Scholar]
- 37. Durden E, Lenhart G, Lopez‐Gonzalez L, Hammer M, Langer J. Predictors of glycemic control and diabetes‐related costs among type 2 diabetes patients initiating therapy with liraglutide in the United States. J Med Econ. 2016;19(4):403‐413. [DOI] [PubMed] [Google Scholar]
- 38. Durden E, Liang M, Fowler R, Panton UH, Mocevic E. The effect of early response to GLP‐1 RA therapy on long‐term adherence and persistence among type 2 diabetes patients in the United States. J Manag Care Spec Pharm. 2019;25(6):669‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eby EL, Bajpai S, Faries DE, Haynes VS, Lage MJ. The association between adherence to insulin therapy and health care costs for adults with type 2 diabetes: evidence from a U.S. retrospective claims database. J Manag Care Spec Pharm. 2020;26(9):1081‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edelman SV, Ermakova A, Xiong Y, Sieradzan R, Taylor SD. Persistence with basal‐bolus insulin therapy in patients with type 2 diabetes mellitus and effect on clinical and economic outcomes: a retrospective claims database study. J Manag Care Spec Pharm. 2019;25(12):1420‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Egede LE, Gebregziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012;35(12):2533‐2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Egede LE, Gebregziabher M, Echols C, Lynch CP. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother. 2014;48(5):562‐570. [DOI] [PubMed] [Google Scholar]
- 43. Egede LE, Gebregziabher M, Hunt KJ, et al. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care. 2011;34(4):938‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farmer AJ, Rodgers LR, Lonergan M, et al. Adherence to oral glucose‐lowering therapies and associations with 1‐year HbA1c: a retrospective cohort analysis in a large primary care database. Diabetes Care. 2016;39(2):258‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farsaei S, Sabzghabaee AM, Zargarzadeh AH, Amini M. Adherence to glyburide and metformin and associated factors in type 2 diabetes in Isfahan, Iran. Iran J Pharm Sci. 2011;10(4):933‐939. [PMC free article] [PubMed] [Google Scholar]
- 46. Fukuda H, Mizobe M. Impact of nonadherence on complication risks and healthcare costs in patients newly‐diagnosed with diabetes. Diabetes Res Clin Pract. 2017;123:55‐62. [DOI] [PubMed] [Google Scholar]
- 47. García Díaz E, Ramírez Medina D, García López A, Morera Porras ÓM. Determinants of adherence to hypoglycemic agents and medical visits in patients with type 2 diabetes mellitus. Endocrinol Diabetes Nutr. 2017;64(10):531‐538. [DOI] [PubMed] [Google Scholar]
- 48. Gatwood JD, Chisholm‐Burns M, Davis R, et al. Differences in health outcomes associated with initial adherence to oral antidiabetes medications among veterans with uncomplicated type 2 diabetes: a 5‐year survival analysis. Diabet Med. 2018;35(11):1571‐1579. [DOI] [PubMed] [Google Scholar]
- 49. Gentil L, Vasiliadis HM, Préville M, Berbiche D. Adherence to oral antihyperglycemic agents among older adults with mental disorders and its effect on health care costs, Quebec, Canada, 2005‐2008. Prev Chronic Dis. 2015;12:E230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gibson TB, Song X, Alemayehu B, et al. Cost sharing, adherence, and health outcomes in patients with diabetes. Am J Manag Care. 2010;16(8):589‐600. [PubMed] [Google Scholar]
- 51. Gordon J, McEwan P, Idris I, Evans M, Puelles J. Treatment choice, medication adherence and glycemic efficacy in people with type 2 diabetes: a UK clinical practice database study. BMJ Open Diabetes Res Care. 2018;6:e000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hadjiyianni I, Desai U, Suzuki S, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation: a retrospective database study among people with type 2 diabetes mellitus in Japan. Diabetes Ther. 2017;8(1):149‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hansen RA, Farley JF, Droege M, Maciejewski ML. A retrospective cohort study of economic outcomes and adherence to monotherapy with metformin, pioglitazone, or a sulfonylurea among patients with type 2 diabetes mellitus in the United States from 2003 to 2005. Clin Ther. 2010;32(7):1308‐1319. [DOI] [PubMed] [Google Scholar]
- 54. Hong JS, Kang HC. Relationship between oral antihyperglycemic medication adherence and hospitalization, mortality, and healthcare costs in adult ambulatory care patients with type 2 diabetes in South Korea. Med Care. 2011;49(4):378‐384. [DOI] [PubMed] [Google Scholar]
- 55. Hong JS, Kang HC. Relationship between continuity of ambulatory care and medication adherence in adult patients with type 2 diabetes in Korea: a longitudinal analysis. Med Care. 2014;52(5):446‐453. [DOI] [PubMed] [Google Scholar]
- 56. Horii T, Momo K, Yasu T, Kabeya Y, Atsuda K. Determination of factors affecting medication adherence in type 2 diabetes mellitus patients using a nationwide claim‐based database in Japan. PLoS One. 2019;14:e0223431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huber CA, Rapold R, Brüngger B, Reich O, Rosemann T. One‐year adherence to oral antihyperglycemic medication and risk prediction of patient outcomes for adults with diabetes mellitus: an observational study. Medicine. 2016;95(26):e3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Iglay K, Qiu Y, Steve Fan CP, Li Z, Tang J, Laires P. Risk factors associated with treatment discontinuation and down‐titration in type 2 diabetes patients treated with sulfonylureas. Curr Med Res Opin. 2016;32(9):1567‐1575. [DOI] [PubMed] [Google Scholar]
- 59. Ito H, Ando S, Tsugami E, et al. Changes in medication adherence and unused drugs after switching from daily dipeptidyl peptidase‐4 inhibitors to once‐weekly trelagliptin in patients with type 2 diabetes. Diabetes Res Clin Pract. 2019;153:41‐48. [DOI] [PubMed] [Google Scholar]
- 60. Jude EB, O'Leary C, Myland M, et al. Evaluating glycaemic control in patients poorly controlled on oral antidiabetic drugs in real‐world setting: results from assessing the Appropriate Timing of Type 2 diAbetes INtensification (ATTAIN). Endocrinol Diabetes Metab. 2020;3:e00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kalirai S, Duan R, Liu D, Reed BL. Economic impact of treatment duration and persistence with basal insulin in previously insulin‐naive users. J Manag Care Spec Pharm. 2017;23(3):327‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim N, Agostini JV, Justice AC. Refill adherence to oral hypoglycemic agents and glycemic control in veterans. Ann Pharmacother. 2010;44(5):800‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim YY, Lee JS, Kang HJ, Park SM. Effect of medication adherence on long‐term all‐cause‐mortality and hospitalization for cardiovascular disease in 65,067 newly diagnosed type 2 diabetes patients. Sci Rep. 2018;8(1):12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Krass I, Hebing R, Mitchell B, et al. Diabetes management in an Australian primary care population. J Clin Pharm Ther. 2011;36(6):664‐672. [DOI] [PubMed] [Google Scholar]
- 65. Kreyenbuhl J, Leith J, Medoff DR, et al. A comparison of adherence to hypoglycemic medications between type 2 diabetes patients with and without serious mental illness. Psychiatry Res. 2011;188(1):109‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Levin PA, Wei W, Zhou S, Xie L, Baser O. Outcomes and treatment patterns of adding a third agent to 2 OADS in patients with type 2 diabetes. J Manag Care Spec Pharm. 2014;20(5):501‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li X, Gao M, Zhang S, et al. Medication adherence mediates the association between type d personality and high HbA1c level in Chinese patients with type 2 diabetes mellitus: a six‐month follow‐up study. J Diabetes Res. 2017;2017:7589184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lin CS, Khan H, Chang RY, et al. A study on the impact of poor medication adherence on health status and medical expense for diabetes mellitus patients in Taiwan: a longitudinal panel data analysis. Medicine (Baltimore). 2020;99(26):e20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lin J, Lingohr‐Smith M, Fan T. Real‐world medication persistence and outcomes associated with basal insulin and glucagon‐like peptide 1 receptor agonist free‐dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res. 2017;9:19‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Linetzky B, Jiang D, Funnell MM, Curtis BH, Polonsky WH. Exploring the role of the patient‐physician relationship on insulin adherence and clinical outcomes in type 2 diabetes: insights from the MOSAIc study. J Diabetes. 2017;9(6):596‐605. [DOI] [PubMed] [Google Scholar]
- 71. Lo‐Ciganic WH, Donohue JM, Thorpe JM, et al. Using machine learning to examine medication adherence thresholds and risk of hospitalization. Med Care. 2015;53(8):720‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. MacEwan JP, Sheehan JJ, Yin W, et al. The relationship between adherence and total spending among medicare beneficiaries with type 2 diabetes. Am J Manag Care. 2017;23(4):248‐252. [PubMed] [Google Scholar]
- 73. McAdam‐Marx C, Bellows BK, Unni S, et al. Impact of adherence and weight loss on glycemic control in patients with type 2 diabetes: cohort analyses of integrated medical record, pharmacy claims, and patient‐reported data. J Manag Care Spec Pharm. 2014;20(7):691‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Melzer‐Cohen C, Chodick G, Husemoen LLN, Rhee N, Shalev V, Karasik A. A retrospective database study of liraglutide persistence associated with glycemic and body weight control in patients with type 2 diabetes. Diabetes Ther. 2019;10(2):683‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Melzer‐Cohen C, Chodick G, Naftelberg S, Shehadeh N, Karasik A. Metabolic control and adherence to therapy in type 2 diabetes mellitus patients using IDegLira in a real‐world setting. Diabetes Ther. 2020;11(1):185‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meng J, Casciano R, Lee YC, et al. Effect of diabetes treatment‐related attributes on costs to type 2 diabetes patients in a real‐world population. J Manag Care Spec Pharm. 2017;23(4):446‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Miao R, Wei W, Lin J, Xie L, Baser O. Does device make any difference? A real‐world retrospective study of insulin treatment among elderly patients with type 2 diabetes. J Diabetes Sci Technol. 2014;8(1):150‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Min JY, Griffin MR, Chipman J, et al. Recent metformin adherence and the risk of hypoglycaemia in the year following intensification with a sulfonylurea. Diabet Med. 2019;36(4):482‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mody R, Grabner M, Yu M, et al. Real‐world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin. 2018;34(6):995‐1003. [DOI] [PubMed] [Google Scholar]
- 80. Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12‐month follow‐up in a real‐world setting in the United States. Diabetes Obes Metab. 2019;21(4):920‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Morieri ML, Frison V, Rigato M, et al. Effectiveness of dulaglutide in the real world and in special populations of type 2 diabetic patients. J Clin Endocrinol Metab. 2020;105(7):dgaa204. [DOI] [PubMed] [Google Scholar]
- 82. Mosen DM, Glauber H, Stoneburner AB, Feldstein AC. Assessing the association between medication adherence and glycemic control. Am J Pharm Benefits. 2017;9(3):82‐88. [Google Scholar]
- 83. Nguyen H, Dufour R, Caldwell‐Tarr A. Glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) therapy adherence for patients with type 2 diabetes in a Medicare population. Adv Ther. 2017;34(3):658‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nichols GA, Conner C, Brown JB. Initial nonadherence, primary failure and therapeutic success of metformin monotherapy in clinical practice. Curr Med Res Opin. 2010;26(9):2127‐2135. [DOI] [PubMed] [Google Scholar]
- 85. Nichols GA, Raebel MA, Dyer W, Schmittdiel JA. The effect of age and comorbidities on the association between the medicare STAR oral antihyperglycemic adherence metric and glycemic control. J Manag Care Spec Pharm. 2018;24(9):856‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nichols GA, Rosales AG, Kimes TM, Tunceli K, Kurtyka K, Mavros P. The change in HbA1c associated with initial adherence and subsequent change in adherence among diabetes patients newly initiating metformin therapy. J Diabetes Res. 2016;2016(9687815):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Perez‐Nieves M, Boye KS, Kiljanski J, Cao D, Lage MJ. Adherence to basal insulin therapy among people with type 2 diabetes: a retrospective cohort study of costs and patient outcomes. Diabetes Ther. 2018;9(3):1099‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Perez‐Nieves M, Kabul S, Desai U, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in the US. Curr Med Res Opin. 2016;32(4):669‐680. [DOI] [PubMed] [Google Scholar]
- 90. Pollack M, Chastek B, Williams SA, Moran J. Impact of treatment complexity on adherence and glycemic control: an analysis of oral antidiabetic agents. J Clin Outcomes Manag. 2010;17(6):20‐30. [Google Scholar]
- 91. Quilliam BJ, Ozbay AB, Sill BE, Kogut SJ. The association between adherence to oral anti‐diabetic drugs and hypoglycaemia in persons with type 2 diabetes. Diabet Med. 2013;30(11):1305‐1313. [DOI] [PubMed] [Google Scholar]
- 92. Rana B, Bukhsh A, Khan TM, Sarwar A, Omer MO, Jamshed SQ. Evaluation of therapeutic effectiveness of prescribed medications in patients with type 2 diabetes mellitus: findings from a tertiary care hospital, Lahore, Pakistan. J Pharm Bioallied Sci. 2017;9(2):121‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Raum E, Krämer HU, Rüter G, et al. Medication non‐adherence and poor glycaemic control in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2012;97(3):377‐384. [DOI] [PubMed] [Google Scholar]
- 94. Reynolds K, An J, Wu J, et al. Treatment discontinuation of oral hypoglycemic agents and healthcare utilization among patients with diabetes. J Diabetes Complications. 2016;30(8):1443‐1451. [DOI] [PubMed] [Google Scholar]
- 95. Rodgers LR, Weedon MN, Henley WE, Hattersley AT, Shields BM. Cohort profile for the MASTERMIND study: using the clinical practice research Datalink (CPRD) to investigate stratification of response to treatment in patients with type 2 diabetes. BMJ Open. 2017;7:e017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sambamoorthi U, Garg R, Deb A, Fan T, Boss A. Persistence with rapid‐acting insulin and its association with A1C level and severe hypoglycemia among elderly patients with type 2 diabetes. Curr Med Res Opin. 2017;33(7):1309‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Samu AM, Amirthalingam PS, Mohammed OS. Assessment of patient medication adherence among the type 2 diabetes mellitus population with peripheral diabetic neuropathy in South India. J Taibah Univ Med Sci. 2017;12(2):164‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sattler EL, Lee JS, Bhargava V. Food insecurity and medication adherence in low‐income older Medicare beneficiaries with type 2 diabetes. J Nutr Gerontol Geriatr. 2014;33(4):401‐417. [DOI] [PubMed] [Google Scholar]
- 99. Simpson SH, Lin M, Eurich DT. Medication adherence affects risk of new diabetes complications: a cohort study. Ann Pharmacother. 2016;50(9):741‐746. [DOI] [PubMed] [Google Scholar]
- 100. Snider JT, Seabury S, Lopez J, McKenzie S, Wu Y, Goldman DP. Impact of type 2 diabetes medication cost sharing on patient outcomes and health plan costs. Am J Manag Care. 2016;22(6):433‐440. [PubMed] [Google Scholar]
- 101. Sun P, Lian J. Treatment adherence in newly diagnosed type 2 diabetes: patient characteristics and long‐term impact of adherence on inpatient care utilization. Postgrad Med. 2016;128(4):338‐345. [DOI] [PubMed] [Google Scholar]
- 102. Vichayanrat A, Matawaran BJ, Wibudi A, et al. Assessment of baseline characteristics, glycemic control and oral antidiabetic treatment in Asian patients with diabetes: the registry for assessing OAD usage in diabetes management (REASON) Asia study. J Diabetes. 2013;5(3):309‐318. [DOI] [PubMed] [Google Scholar]
- 103. Virdi N, Daskiran M, Nigam S, Kozma C, Raja P. The association of self‐monitoring of blood glucose use with medication adherence and glycemic control in patients with type 2 diabetes initiating non‐insulin treatment. Diabetes Technol Ther. 2012;14(9):790‐798. [DOI] [PubMed] [Google Scholar]
- 104. Wang L, Wei W, Miao R, Xie L, Baser O. Real‐world outcomes of US employees with type 2 diabetes mellitus treated with insulin glargine or neutral protamine Hagedorn insulin: a comparative retrospective database study. BMJ Open. 2013;3:e002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wilding J, Godec T, Khunti K, et al. Changes in HbA1c and weight, and treatment persistence, over the 18 months following initiation of second‐line therapy in patients with type 2 diabetes: results from the United Kingdom clinical practice research Datalink. BMC Med. 2018;16(116):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wilke T, Mueller S, Groth A, et al. Non‐persistence and non‐adherence of patients with type 2 diabetes mellitus in therapy with glp‐1 receptor agonists: a retrospective analysis. Diabetes Ther. 2016;7(1):105‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wu EQ, Zhou S, Yu A, et al. Outcomes associated with insulin therapy disruption after hospital discharge among patients with type 2 diabetes mellitus who had used insulin before and during hospitalization. Endocr Pract. 2012;18(5):651‐659. [DOI] [PubMed] [Google Scholar]
- 108. Yu AP, Yu YF, Nichol MB. Estimating the effect of medication adherence on health outcomes among patients with type 2 diabetes—an application of marginal structural models. Value Health. 2010;13(8):1038‐1045. [DOI] [PubMed] [Google Scholar]
- 109. Zhou FL, Xie L, Pan C, et al. Relationship between treatment persistence and A1C trends among patients with type 2 diabetes newly initiating basal insulin. Diabetes Obes Metab. 2018;20(5):1298‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhu VJ, Tu W, Rosenman MB, Overhage JM. Nonadherence to oral antihyperglycemic agents: subsequent hospitalization and mortality among patients with type 2 diabetes in clinical practice. Stud Health Technol Inform. 2015;216:60‐63. [PubMed] [Google Scholar]
- 111. Zongo A, Guénette L, Moisan J, Grégoire JP. Predictive validity of self‐reported measures of adherence to noninsulin antidiabetes medication against control of glycated hemoglobin levels. Can J Diabetes. 2016;40(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 112. Eliasson B, Ekelund J, Miftaraj M, et al. Persistence with ideglira in patients in clinical practice: a nationwide observational study in Sweden. Diabetes Ther. 2020;11(8):1807‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Forbes CA, Deshpande S, Sorio‐Vilela F, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin. 2018;34(9):1613‐1625. [DOI] [PubMed] [Google Scholar]
- 114. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. International Diabetes Federation . IDF Diabetes Atlas, 9th Edition 2019. https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf#page=62&zoom=auto. Accessed August 3, 2021.
- 116. Roebuck MC, Liberman JN, Gemmill‐Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood). 2011;30(1):91‐99. [DOI] [PubMed] [Google Scholar]
- 117. Carls GS, Roebuck MC, Brennan TA, Slezak JA, Matlin OS, Gibson TB. Impact of medication adherence on absenteeism and short‐term disability for five chronic diseases. J Occup Environ Med. 2012;54(7):792‐805. [DOI] [PubMed] [Google Scholar]
- 118. Yen FS, Wei JC, Liu JS, Hsu CC, Hwu CM. Persons with type 2 diabetes and high insulin persistence were associated with a lower risk of mortality: a nationwide retrospective cohort study. J Diabetes Investig. 2021;12(2):146‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296‐1310. [DOI] [PubMed] [Google Scholar]
- 120. Weeda ER, Muraoka AK, Brock MD, Cannon JM. Medication adherence to injectable glucagon‐like peptide‐1 (GLP‐1) receptor agonists dosed once weekly vs once daily in patients with type 2 diabetes: a meta‐analysis. Int J Clin Pract. 2021;75(9):e14060. [DOI] [PubMed] [Google Scholar]
- 121. Liebl A, Arora R, Fernandes J, Polonsky W. Adherence and persistence in patients with type 2 diabetes initiating once‐weekly versus daily injectable GLP‐1 RAs in US clinical practice (STAY study). American Diabetes Association 81st Scientific Session 2021; 83‐LB. https://eventpilot.us/web/page.php?page=IntHtml&project=ADA21&id=1397. Accessed August 16, 2021.
- 122. Fatoye F, Smith P, Gebrye T, Yeowell G. Real‐world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open. 2019;9(4):e027049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Weeda ER, Coleman CI, McHorney CA, Crivera C, Schein JR, Sobieraj DM. Impact of once‐ or twice‐daily dosing frequency on adherence to chronic cardiovascular disease medications: a meta‐regression analysis. Int J Cardiol. 2016;216:104‐109. [DOI] [PubMed] [Google Scholar]
- 124. Riordan F, McHugh SM, O'Donovan C, Mtshede MN, Kearney PM. The role of physician and practice characteristics in the quality of diabetes management in primary care: systematic review and meta‐analysis. J Gen Intern Med. 2020;35(6):1836‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wu IXY, Kee JCY, Threapleton DE, et al. Effectiveness of smartphone technologies on glycaemic control in patients with type 2 diabetes: systematic review with meta‐analysis of 17 trials. Obes Rev. 2018;19(6):825‐838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategy for MEDLINE
Table S2. List of congresses searched
Table S3. Number of studies reporting each outcome
Table S4. Summary of studies reporting the associations between adherence to antidiabetic medication and inpatient admissions, ED visits/admissions and outpatient claims in patients with T2D
Table S5. Summary of studies reporting the associations between persistence with antidiabetic medication and inpatient admissions, ED visits/admissions and outpatient claims in patients with T2D
Figure S1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) diagram.
Data Availability Statement
‘All data analyzed during this systematic literature review are published elsewhere, and relevant collated data are included in this article [and/or] its supplementary material files. Further enquiries can be directed to the corresponding author’


