Abstract
Oxidized halogen antimicrobials, such as hypochlorous and hypobromous acids, have been used extensively for microbial control in industrial systems. Recent discoveries have shown that acylated homoserine lactone cell-to-cell signaling molecules are important for biofilm formation in Pseudomonas aeruginosa, suggesting that biofouling can be controlled by interfering with bacterial cell-to-cell communication. This study was conducted to investigate the potential for oxidized halogens to react with acylated homoserine lactone-based signaling molecules. Acylated homoserine lactones containing a 3-oxo group were found to rapidly react with oxidized halogens, while acylated homoserine lactones lacking the 3-oxo functionality did not react. The Chromobacterium violaceum CV026 bioassay was used to determine the effects of such reactions on acylated homoserine lactone activity. The results demonstrated that 3-oxo acyl homoserine lactone activity was rapidly lost upon exposure to oxidized halogens; however, acylated homoserine lactones lacking the 3-oxo group retained activity. Experiments with the marine alga Laminaria digitata demonstrated that natural haloperoxidase systems are capable of mediating the deactivation of acylated homoserine lactones. This may illustrate a natural defense mechanism to prevent biofouling on the surface of this marine alga. The Chromobacterium violaceum activity assay illustrates that reactions between 3-oxo acylated homoserine lactone molecules and oxidized halogens do occur despite the presence of biofilm components at much greater concentrations. This work suggests that oxidized halogens may control biofilm not only via a cidal mechanism, but also by possibly interfering with 3-oxo acylated homoserine lactone-based cell signaling.
Bacteria in nature are most frequently encountered not as free-swimming organisms but as surface-attached communities known as biofilms (5). Organisms residing within biofilms possess a number of advantages over their free-swimming or planktonic counterparts, including increased resistance to adverse environmental conditions and antibacterial agents. The ubiquity of biofilm development can cause significant problems in the areas of public health (30, 37), medicine (11, 18, 22), and industry (4, 23). Accordingly, there has been a great deal of research to better understand biofilm development and to identify improved strategies for biofouling control.
Recent work in this area has focused on the role of cell-to-cell signaling within biofilm populations. It is now believed that many different types of bacteria are able to produce and respond to various hormone-like signal molecules (8). A particular subset of these molecules, the acylated homoserine lactones (acyl HSLs), have been shown to be involved in biofilm formation and dispersal with Pseudomonas aeruginosa (7). This finding suggests that interference with acyl HSL-based signaling may provide a novel mechanism for biofilm control.
In fact, natural systems that exploit this biofouling control strategy already exist. The marine alga Delisea pulchra produces halogenated furanone compounds that interfere with acyl HSL-based signaling systems and thus discourage biofilm formation on the seaweed surface (12, 13). These furanones are analogs to naturally occurring acyl HSL signal molecules and appear to act as competitive inhibitors to acyl HSL signal receptor proteins (21). Other marine organisms must also find ways to deal with biofilm formation on their surfaces (32). A number of seaweeds appear to use haloperoxidases for this purpose (40, 41). Haloperoxidases catalyze the oxidation of bromide and/or chloride with hydrogen peroxide to produce the microbicidal compound hypobromous acid (HOBr) or hypochlorous acid (HOCl), respectively. Haloperoxidase-based antimicrobial systems have also been found elsewhere in nature (2, 15, 28, 31, 38), including in the eosinophil population of mammalian leukocytes (10, 17, 19, 35, 39).
Oxidized halogen compounds are also widely used to control biofouling in industrial and potable water systems. In fact, the strategy of using stabilized halogen antimicrobials has recently been imitated for industrial microbial fouling control applications (6, 24, 25). Stabilized halogen antimicrobials have been shown to more effectively penetrate and disinfect biofilms than free halogen (14).
Since HOCl and HOBr are highly toxic to a wide variety of microorganisms, it is generally accepted that these compounds control biofilm through a biocidal mechanism or by oxidation of biofilm components. To date, no reports have been published that acyl HSL molecules are susceptible to attack by oxidized hypohalite and stabilized hypohalite antimicrobials. This study was conducted to investigate the possibility of oxidized halogens to react with acyl HSL-based signaling molecules, potentially disrupting bacterial cell-to-cell communication. Experiments were also conducted to determine if haloperoxidase systems from the brown alga Laminaria digitata was capable of mediating this reaction.
MATERIALS AND METHODS
Reagents.
Acyl HSLs were obtained from Quorum Sciences (Coralville, Iowa). Stock solutions (2.3 mM) were prepared by dissolving the appropriate amount of acyl HSL in ethanol and diluting it with deionized water to a final ethanol concentration of 10% for hexanoyl and 3-oxo-hexanoyl HSL and 50% for dodecanoyl and 3-oxo-dodecanoyl HSL. All experiments were conducted with equivalent levels of ethanol (including control samples) to compensate for any effect resulting from the ethanol alone. Clorox bleach (Clorox, Oakland, Calif.) was used as a source of sodium hypochlorite. STABREX biocide (ONDEO Nalco, Naperville, Ill.) was used as a source of stabilized sodium hypobromite. Oxidized halogen concentrations of these solutions were determined by iodometric titration (1). Stock solutions were prepared in distilled water to a final concentration of 14.1 mM (1,000 ppm as Cl2). Reagents for the DPD (N,N-diethylphenylenediamine) test were obtained from Hach Company (Loveland, Colo.). This test is frequently used to determine the level of halogen antimicrobials in industrial and potable water systems.
Reaction of oxidized halogens with acyl HSL signal compounds.
To study potential reactions between oxidized halogens and acyl HSLs, a solution of approximately 0.14 mM (10 ppm as Cl2) oxidized halogen (sodium hypochlorite or stabilized sodium hypobromite) was prepared in 10 ml of 0.1 M sodium phosphate buffer at pH 6 or in synthetic cooling water (1.5 mM CaCl2, 0.8 mM MgSO4, 2.2 mM NaHCO3) at pH 8.3. Precise initial halogen concentrations were determined by removing a 1-ml aliquot, diluting it with 9 ml of deionized water, and assaying for total halogen by the standard DPD colorimetric method (1). The oxidant concentrations were chosen to approximate the conditions that might be found in an actual industrial or natural system while still allowing for accurate measurement of the halogen (6). The reaction was initiated by adding acyl HSL to a final concentration of 0.05 mM. The reaction was followed by removal of 1-ml aliquots over time, dilution of them 5- or 10-fold in deionized water, and measurement of the total halogen level with the DPD test.
To examine the effect of halogen-mediated reactions on acyl HSL activity, either sodium hypochlorite or stabilized sodium hypobromite was added to 10 ml of a buffered solution (0.1 M sodium phosphate [pH 6] or synthetic cooling water [pH 8.3]) of acyl HSL (either hexanoyl HSL or 3-oxo-hexanoyl HSL). Final concentrations were 0.05 mM for the acyl HSL compound and 10 ppm as Cl2 for the oxidized halogen. At various time points, 90-μl aliquots were removed from this reaction mixture and added to 10 μl of 10% sodium bisulfite in order to quench the reaction by inactivating unreacted oxidized halogen compounds. The quenched solution was then assayed for acyl HSL activity.
Acyl HSL activity assays.
Acyl HSL activity was monitored with the Chromobacterium violaceum CV026 assay (3). Expression of the purple pigment violacein by C. violaceum is regulated by N-hexanoyl-l-HSL. The C. violaceum CV026 strain is a double mini-Tn5 mutant that does not independently produce the pigment. However, in the presence of extracellular HSLs (i.e., from other organisms or in growth medium), the production of the pigment can be restored. To monitor the degradation of HSL, C. violaceum CV026 was streaked on Luria-Bertani (LB) agar plates, and 15 μl of the test solution was dropped next to the streak. Plates were incubated at 30°C for 24 to 48 h and examined for pigment production. Pigment production was ranked (versus that of a positive control) from 4 (much pigment—same as control) to 0 (no pigment).
Acyl HSL activity was also monitored by using the Agrobacterium tumefaciens cross-feeding assay (33). A. tumefaciens A136 (33) was streaked on LB agar overlaid with 40 μl (20 mg/ml) of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and 15 μl of the test solution was dropped next to the streak. Plates were incubated at 30°C for 24 to 48 h and examined for pigment production.
L. digitata oxidation deactivation of 3-oxo-hexanoyl HSL.
Fresh samples of L. digitata were shipped on ice from Newfoundland, Canada (All Material Products, Inc., Isle Aux Morts, Newfoundland, Canada). Samples of this marine alga were maintained and testing was conducted in synthetic seawater (Instant Ocean, Aquarium Systems, Mentor, Ohio) at pH 8.3. Testing was conducted immediately after the algal samples were received. The production of HOBr was monitored by the procedure outlined by Wever et al. (40). Thirty grams of L. digitata frond were placed in 100 ml of synthetic seawater supplemented with 2 mM H2O2, 100 mM KBr, and 20 μM phenol red (or combinations thereof). The formation of HOBr was monitored by the oxidation of the phenol red to bromophenol blue. The bromophenol blue concentration was measured at 592 nm at 30, 60, 120, and 180 min.
Separate experiments were conducted to determine whether the haloperoxidase system within L. digitata is capable of mediating the deactivation of acyl HSLs. These experiments were conducted in synthetic seawater (100 ml) supplemented with 100 mM KBr and containing 5 ppm of 3-oxo-hexanoyl HSL. The three test conditions were (i) 2 mM H2O2, (ii) 30 g of L. digitata frond, and (iii) 2 mM H2O2 with 30 g of L. digitata frond. A control containing no additions was also included. Acyl HSL activity was monitored over time (0, 30, 60, 120, and 180 min) by removing 15-μl aliquots of the test solutions for use in the previously described C. violaceum activity assay.
Reactions between acyl HSLs and oxidized halogens in the presence of biofilm components.
Biofilms were grown on glass slides suspended in a flask filled with 250 ml of synthetic cooling water that was inoculated with Pseudomonas aeruginosa. The flask was dosed with 0.5 ml of tryptic soy broth daily, and following 7 days of growth, the biofilm was scraped from two glass slides into 5 ml of sterile synthetic cooling water. The 3-oxo-hexanoyl HSL was added to this solution to a final concentration of 0.05 mM, and potential reactions with halogen were initiated by adding stabilized sodium hypobromite to a final concentration of 10 ppm as Cl2. A control was taken prior to addition of stabilized hypobromite, and the reaction was sampled at 5 min. Solutions were assayed for the halogenated acyl HSL reaction product by using a previously published high-performance liquid chromatography (HPLC) methodology (27). The liquid chromatograph consisted of a Waters (Milford, Mass.) 600E pump, 717 Plus autosampler, and 996 diode array detector; a PE Nelson (Cupertino, Calif.) Turbochrom Client/Server data system; and Agilent (Wilmington, Del.) Zorbax SB-CN guard (5 cm by 4.6 mm) and analytical (25 cm by 4.6 mm) columns (5-μm-diameter particles). The mobile phase was prepared with HPLC-grade acetonitrile, reagent-grade concentrated phosphoric acid (EM, Gibbstown, N.J.), and water purified with a Millipore (Bedford, Mass.) cartridge system. The exact running conditions involved a premixed mobile phase of 10% (vol/vol) acetonitrile acidified with 5 mM phosphoric acid, an injection volume of 100 μl, and an UV detection wavelength of 200 nm. Prior to analysis, samples were filtered with 0.45-μm-pore-diameter Millipore Millex filters.
RESULTS
Reaction of oxidized halogens with acyl HSL signal compounds.
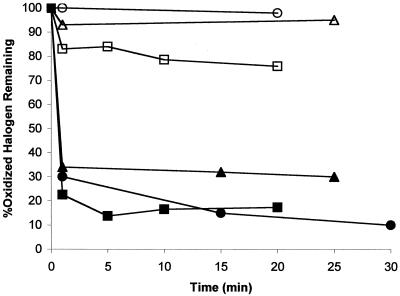
The potential for halogen biocides to react with acyl HSL signaling molecules was first tested with the DPD colorimetric assay. The acyl HSL molecules tested included hexanoyl HSL, 3-oxo-hexanoyl HSL, dodecanoyl HSL, and 3-oxo-dodecanoyl HSL (43). Previous work has shown that 3-oxo-dodecanoyl HSL is important in P. aeruginosa biofilm formation (7). Oxidized halogen consumption over time as a result of added acyl HSL is shown in Fig. 1. This experiment was conducted in synthetic cooling water at pH 8.3. When the experiment was conducted in phosphate buffer at pH 6, the reaction occurred at a slightly slower rate. It was found that acyl HSL compounds containing a 3-oxo moiety reacted very rapidly with both HOCl and stabilized HOBr. The amount of oxidized halogen lost was about two times the amount of 3-oxo-acyl HSL on a molar basis. Accordingly, in Fig. 1, the oxidant level does not fall to zero because of an excess of oxidant in the assay. However, in a separate experiment with excess 3-oxo acyl HSL, all of the oxidant was consumed (data not shown). Acyl HSLs lacking the 3-oxo group consumed very little oxidized halogen. The small amount of consumption observed was found to be due to the ethanol present in acyl HSL stock solutions. The same amount of consumption was noted in control reactions with a blank ethanol solution containing no added acyl HSL.
FIG. 1.
Consumption of oxidized halogen by acyl HSL compounds. ○, HOCl plus hexanoyl HSL; ●, HOCl plus 3-oxo-hexanoyl HSL; ▵, stabilized HOBr plus hexanoyl HSL; ▴, stabilized HOBr plus 3-oxo-hexanoyl HSL; □, HOCl plus dodecanoyl HSL; ▪, HOCl plus 3-oxo-dodecanoyl HSL .
Effect of oxidized halogen antimicrobials on acylated HSL activity.
The preceding experiments demonstrated that a reaction occurs between oxidized halogen antimicrobials and 3-oxo-acyl HSL molecules (indicated by consumption of the oxidized halogen). Further experiments were necessary to determine how the activity of the signal molecules was affected by this reaction. The C. violaceum bioassay was used to study acyl HSL activity. Since HSLs with acyl chains of greater than nine carbons are not detected by this assay (3), it was not possible to test for inactivation of dodecanoyl and 3-oxo-dodecanoyl HSLs by this method.
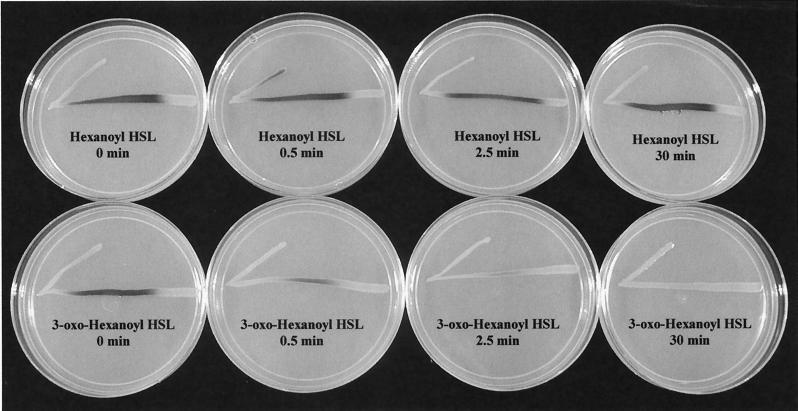
The results of the C. violaceum CV026 activity assay for reaction of HOCl with hexanoyl and 3-oxo-hexanoyl HSL are shown in Fig. 2. This assay was conducted in phosphate buffer at pH 6. The slower reaction time at pH 6 (versus that at pH 8.3) allows for the loss of activity to be illustrated over time. When the same reaction is conducted at pH 8.3, the C. violaceum CV026 activity assay shows a complete loss of activity within 1 min. The pigment production by C. violaceum CV026 indicates the presence of the acyl HSL; therefore, the lack of pigment production would be an indication of acyl HSL deactivation. In agreement with the oxidized halogen measurement (Fig. 1), these results illustrated that 3-oxo-hexanoyl HSL activity was rapidly destroyed, while hexanoyl HSL activity was unaffected. Similar results were observed for the stabilized HOBr reaction with these acyl HSLs. The same results were also observed with the Agrobacterium tumefaciens acyl HSL reporter bioassay (data not shown) (33).
FIG. 2.
Effect of HOCl on activity of hexanoyl and 3-oxo-hexanoyl HSL as shown by the C. violaceum CV026 bioassay.
L. digitata oxidation deactivation of 3-oxo-hexanoyl HSL.
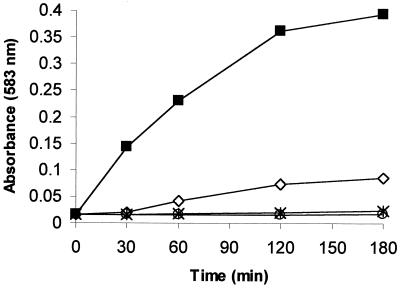
As described in previous studies (40), bromoperoxidase from the brown alga L. digitata can catalyze the oxidation reduction reaction of bromide to HOBr in the presence of H2O2. L. digitata and H2O2 must both be present for a significant level of HOBr to form (measured as the oxidation of phenol red to bromophenol blue by the produced HOBr). These results are clearly shown in Fig. 3. In the control sample and under test conditions with H2O2 alone, no oxidation of phenol red is observed. In test samples containing the brown algae alone, only a very slight oxidation of phenol red to bromophenol blue is observed. This may be an indication of H2O2 production by the algae resulting in a slight conversion of the phenol red to bromophenol blue. Finally, under test conditions with samples containing both L. digitata and H2O2, a significant oxidation of the phenol red to bromophenol blue occurs.
FIG. 3.
Formation of bromophenol blue by bromination of phenol red. ○, sewater control; ∗, H2O2 control; ◊, L. digitata; ■, L. digitata plus H2O2 and KBr.
A separate set of experiments were conducted to determine if naturally produced HOBr would deactivate acyl HSLs. The experimental setup for these studies was similar to that described above, with the exception that 5 ppm 3-oxo-hexanoyl HSL was added to the test solutions in place of phenol red. The loss of activity of the 3-oxo hexanoyl HSL (determined with the C. violaceum CV026 activity bioassay) by reaction with the oxidized halogen produced by L. digitata is summarized in Table 1. Degradation of 3-oxo hexanoyl HSL in the control sample was very slow. The degradation was also slow in samples containing only algae or only H2O2. This was not surprising, because, as the experiments above (Fig. 3) demonstrated, there was little to no HOBr produced under such conditions. As previously stated, the acyl HSL molecule itself will break down slowly in solution, especially under alkaline conditions (as seen in the control). This was found to be due to hydrolysis of the lactone ring (27). However, the sample that contained both the brown algae and the H2O2 demonstrated a significant increase in the degradation of the 3-oxo hexanoyl HSL as monitored by the C. violaceum CV026 bioassay. As noted in Fig. 3, significant levels of HOBr would be produced under such conditions. Therefore, the alga-produced HOBr did deactivate the 3-oxo-hexanoyl HSL (Table 1).
TABLE 1.
Deactivation of 3-oxo-hexanoyl-HSL by bromoperoxidase-catalyzed formation of HOBr
| Sample | HSL activity at mina
|
||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | 180 | |
| Control | ++++ | ++++ | ++++ | ++ | + |
| L. digitata | NAb | +++ | +++ | ++ | + |
| H2O2 | NA | +++ | +++ | + | + |
| L. digitata + H2O2 | NA | + | + | − | − |
++++, strong activity; − no activity.
NA, not applicable.
Reactions between acyl HSLs and oxidized halogens in the presence of biofilm components.
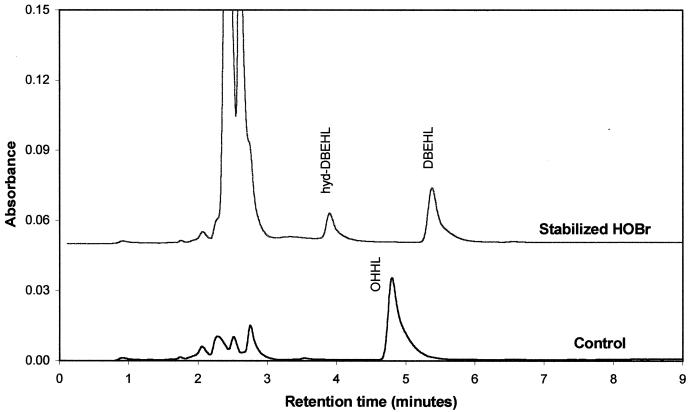
It could be argued that a reaction between oxidized halogen antimicrobials and trace levels of 3-oxo-HSL would not be expected to occur in a biofilm matrix with high levels of biofilm components. If biofilm components are the preferred reactant for oxidized halogens, little or no loss of a signal molecule would be observed. However, when the 3-oxo acyl HSL reaction was carried out in the presence of biofilm, the halogenated acyl HSL reaction products were detected. Figure 4 presents overlaid liquid chromatograms of a 0.05 mM 3-oxo-hexanoyl homoserine lactone pre-reaction control and the reaction with stabilized hypobromite in a P. aeruginosa biofilm medium buffered at pH 8.3. The chromatographic traces illustrate how the 3-oxo-HSL is completely converted to the dibrominated products DBEHL and hyd-DBEHL (27). Peaks eluting at or near the void volume of the chromatographic system at 2.5 min are biofilm components and antimicrobial. Therefore, the reaction between the 3-oxo-HSLs can occur even under conditions in which biofilm components are present at much higher concentrations than the acyl HSL levels.
FIG. 4.
Liquid chromatogram showing formation of the halogenation products DBEHL and hyd-DBEHL from 3-oxo-hexanoyl HSL (OHHL) reacted with stabilized HOBr in the presence of biofilm.
DISCUSSION
Oxidized halogens are used extensively for microbial control in both natural and industrial systems. It is generally believed that the efficacy of these compounds is entirely a result of their biocidal activity and reactivity with biofilm components, such as extracellular polymeric substances. However, experience has shown that biocidal activity does not necessarily correlate with biofilm dispersal. It is well known that many biocides can kill bacteria within biofilms without causing any significant biofilm removal (9). Hypohalous acids, however, have been observed to induce dispersal of biofilm in addition to their cidal effects (14, 20, 36). It is hypothesized here that the reason for this phenomenon is that these antimicrobials are capable of attacking acyl HSL signal compounds.
The results presented in this study demonstrate that oxidized halogen antimicrobials rapidly react with 3-oxo-acyl HSLs at dilute concentrations. Upon reaction, measurement of oxidized halogen reveals that two molecules of hypohalous acid are consumed for every molecule of 3-oxo-acyl HSL. Furthermore, such reactions were shown to eliminate the ability of the signal molecule to function properly as a cell-to-cell signal. Acyl HSLs not possessing the β-keto functionality were not affected by oxidized halogens under the conditions employed in this study.
Further work has shown that 3-oxo-acyl HSLs, as well as other β-diketone-like compounds, are susceptible to attack by halogen oxidants at the α-carbon position between the two carbonyls. The α-carbon is rapidly dihalogenated and is subsequently hydrolyzed at the pH values tested. The ultimate products of the reaction are a dihalogenated acyl HSL and a carboxylic acid (27).
The deactivation of acyl HSL signaling molecules by halogen antimicrobials may be a natural method for biofilm control. Previous work by Wever et al. (40), partly repeated in this study, demonstrates that the brown alga L. digitata produces HOBr acid. The formation of this oxidized halogen antimicrobial is catalyzed by bromoperoxidase in a redox reaction that occurs in the presence of bromide and H2O2. In the natural environment, bromide is found in seawater and H2O2 is produced by L. digitata. The function of the HOBr produced by the brown algae is not completely understood. One hypothesis is that H2O2 is produced by the algal cells during photosynthesis and is removed as waste via peroxidase activity (29). Another, more likely hypothesis is that the HOBr produced by the seaweed may act as a defense mechanism against microorganisms that would colonize the surface of the plant (29). HOBr is a strong antimicrobial agent that can inhibit biofilm formation through a cidal mechanism. The authors of this paper hypothesize that deactivation of acyl HSL cell-to-cell signaling molecules by halogen antimicrobials may be an alternate defense mechanism. Work reported here demonstrates that the HOBr produced by the algae can deactivate 3-oxo-acyl HSL molecules.
Hypohalous acids are known to react with a variety of biopolymers that are present in biofilms (16, 42). Therefore, one might predict that the reaction of oxidized halogen with biofilm components would predominate over reactions with acyl HSL signal molecules. However, preliminary investigations have indicated that oxidized halogens react much more rapidly with 3-oxo-acyl HSLs than with typical polysaccharide biofilm components such as alginate (data not shown). Furthermore, when reactions between acyl HSL and stabilized HOBr are carried out in the presence of biofilm components, the halogenated acyl HSL reaction products are detected (Fig. 4). Therefore, such reactions proceed despite the much higher concentration of biofilm components compared to the acyl HSL levels.
This study demonstrates that oxidized halogen compounds can react with and inactivate 3-oxo-acyl HSL signal compounds. Is this a significant mechanism by which biofilm can be controlled, or is it merely a reaction that happens to occur under experimental conditions? The rapidity of the reaction at dilute concentrations along with the increasing number of organisms found to employ acyl HSL-based signaling systems suggests that this could be a relevant reaction in natural and industrial microbial control. As stated earlier, the 3-oxo-acyl HSL produced by P. aeruginosa (3-oxo-dodecanoyl HSL) is believed to be responsible for biofilm differentiation (7). Recently it has been suggested that the other HSL produced by P. aeruginosa, butyryl HSL (which lacks the 3-oxo functionally), may be important in biofilm dispersal (D. Davies, Symposium 192/Q, 99th Gen. Meet. Am. Soc. Microbiol., 1999). If this is indeed true, then the results presented here demonstrate that oxidized halogens are able to influence biofilm development by selectively inactivating 3-oxo-dodecanoyl HSL, creating an excess of butyryl HSL, which could encourage biofilm dispersal. This hypothesis requires further investigations, which are currently in progress.
Natural biofilms, however, are most frequently comprised of a consortium of microbial species; therefore, the usefulness of such reactions toward inhibiting biofilm development may be dependent on the extent to which microorganisms in addition to P. aeruginosa rely on acyl HSLs for cell-to-cell communication. Recent literature suggests that acyl HSL-based signaling systems are widespread in gram-negative bacteria (34). Furthermore, acyl HSLs have been detected in naturally occurring biofilms (26). Considering this and the fact that higher organisms such as D. pulchra are known to inhibit biofouling by interfering with acyl HSL-based signaling (21), it is suggested that halogen-mediated deactivation of 3-oxo acyl HSLs may represent an alternate biofouling control strategy.
ACKNOWLEDGMENTS
We thank Simon Swift for the C. violaceum CV026 reporter strain. We thank Clay Fuqua for the A. tumefaciens reporter bioassay and for assistance in obtaining the acyl HSLs.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. Washington, D.C.: American Public Health Association; 1992. [Google Scholar]
- 2.Barnett P, Hemrika W, Dekker H L, Muijsers A O, Renirie R, Wever R. Isolation, characterization, and primary structure of the vanadium chloroperoxidase from the fungus Embellisia didymospora. J Biol Chem. 1998;273:23381–23387. doi: 10.1074/jbc.273.36.23381. [DOI] [PubMed] [Google Scholar]
- 3.Camara M, Daykin M, Chhabra S R. Detection, purification, and synthesis of N-acylhomoserine lactone quorum sensing signal molecules. Methods Microbiol. 1998;27:319–330. [Google Scholar]
- 4.Chaudhary A, Gupta L K, Gupta J K, Banerjee U C. Studies on slime-forming organisms of a paper mill: slime production and its control. J Ind Microbiol Biotechnol. 1997;18:348–352. [Google Scholar]
- 5.Costerton W J, Lewandowski Z. Microbial biofilms. Annu Rev Biochem. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 6.Dallmier A W, Martens J D, McCoy W F. Performance of stabilized halogen biocides in cooling water. Technical paper 398. Houston, Tex: National Association of Corrosion Engineers; 1997. [Google Scholar]
- 7.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 8.Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: ASM Press; 1999. [Google Scholar]
- 9.Exner M, Tuschewitzki G J, Scharnagel J. Influence of biofilms on chemical disinfectants and mechanical cleaning. Zentralbl Bakteriol Mikrobiol Hyg Ser B. 1987;183:549–563. [PubMed] [Google Scholar]
- 10.Foote C S, Goyne T E, Lehrer R I. Assessment of chlorination by human neutrophils. Nature. 1983;301:715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- 11.Gill J F, Chakrabarty A M. Alginate production by the mucoid Pseudomonas aeruginosa associated with cystic fibrosis. Microbiol Sci. 1987;4:296–299. [PubMed] [Google Scholar]
- 12.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P D, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gram L, de Nys R, Maximilien R, Givskov M, Steinberg P, Kjelleberg S. Inhibitory effects of secondary metabolites from the red alga Delisea pulchra on swarming motility of Proteus mirabilis. Appl Environ Microbiol. 1996;62:4284–4287. doi: 10.1128/aem.62.11.4284-4287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griebe T, Chen C-I, Srinivasan R, Geesey G G, Stewart P S. Proceedings of the Center for Biofilm Engineering. Bozeman: Montana State University; 1994. Analysis of biofilm disinfection by monochloramine and free chlorine; pp. 151–61. [Google Scholar]
- 15.Hallenberg P F, Hager L P. Purification of chloroperoxidase from Caldariomyces fumago. Methods Enzymol. 1978;52:521–529. doi: 10.1016/s0076-6879(78)52057-0. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins C L, Davies M J. Degradation of hyaluronic acid, poly- and monosaccharides, and model compounds by hypochlorite: evidence for radical intermediates and fragmentation. Free Radic Biol Med. 1998;24:1396–1410. doi: 10.1016/s0891-5849(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 17.Jong E C, Henderson W R, Klebanoff S J. Bactericidal activity of eosinophil peroxidase. J Immunol. 1980;124:1378–1382. [PubMed] [Google Scholar]
- 18.Khoury A E, Lam K, Ellis B, Costerton J W. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 1991;38:M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Klebanoff S J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968;95:2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeChevallier M W, Lowry C D, Lee R G. Disinfecting biofilm in a model distribution system. J Am Water Works Assoc. 1990;82:87–99. [Google Scholar]
- 21.Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:283–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 22.Marsh P D, Bradshaw D J. Dental plaque as a biofilm. J Ind Microbiol. 1995;15:169–175. doi: 10.1007/BF01569822. [DOI] [PubMed] [Google Scholar]
- 23.Mattila-Sandholm T, Wirtanen G. Biofilm formation in the industry: a review. Food Rev Int. 1992;8:573–603. [Google Scholar]
- 24.McCoy W F. Imitating natural microbial fouling control. Mater Performance. 1998;37:45–48. [Google Scholar]
- 25.McCoy W F, Allain E J, Yang S, Dallmier A W. Strategies used in nature for microbial fouling control: applications for industrial water treatment. Technical paper 520. Houston, Tex: National Association of Corrosion Engineers; 1998. [Google Scholar]
- 26.McLean R J C, Whiteley M, Stickler D J, Fuqua W C. Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol Lett. 1997;154:259–263. doi: 10.1111/j.1574-6968.1997.tb12653.x. [DOI] [PubMed] [Google Scholar]
- 27.Michels J J, Allain E J, Borchardt S A, Hu P, McCoy W F. Degradation pathway of homoserine lactone bacterial signal molecules by halogen antimicrobials identified by liquid chromatography with photodiode array and mass spectrometric detection. J Chromatogr. 2000;898:153–165. doi: 10.1016/s0021-9673(00)00849-9. [DOI] [PubMed] [Google Scholar]
- 28.Moore R M, Webb M, Tokarczyk R, Wever R. Bromoperoxidase and iodoperoxidase enzymes and production of halogenated methanes in marine diatom cultures. J Geophys Res. 1996;101:20899–20908. [Google Scholar]
- 29.Pedersen M, Collen J, Abrahamsson K, Ekdahl A. Production of halocarbons from seaweeds: an oxidative stress reaction? Sci Mar. 1996;60:257–263. [Google Scholar]
- 30.Sjöberg A M, Wirtanen G, Mattila-Sandholm T. Biofilm and residue investigations of detergents on surfaces of food processing equipment. Food Bioprod Proc. 1995;73:17–21. [Google Scholar]
- 31.Small A L, McFall-Ngai M J. Halide peroxidase in tissues that interact with bacteria in the host squid Euprymna scolopes. J Cell Biochem. 1999;72:445–457. [PubMed] [Google Scholar]
- 32.Steinberg P D, Schneider R, Kjelleberg S. Chemical defenses of seaweeds against microbial colonization. Biodegradation. 1997;8:211–220. [Google Scholar]
- 33.Stickler D J, Morris N S, McLean R J C, Fuqua C. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl Environ Microbiol. 1998;64:3486–3490. doi: 10.1128/aem.64.9.3486-3490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swift S, Williams P, Stewart G S A B. N-Acylhomoserine lactones and quorum sensing are widespread in the proteobacteria. In: Winans S, Dunny G, editors. Quorum sensing. Washington, D.C.: ASM Press; 1999. pp. 291–314. [Google Scholar]
- 35.Thomas E L, Bozeman P M, Jefferson M M, King C C. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. J Biol Chem. 1995;270:2906–2913. doi: 10.1074/jbc.270.7.2906. [DOI] [PubMed] [Google Scholar]
- 36.van der Wende E. Biocide action of chlorine on Pseudomonas aeruginosa biofilm. Ph.D. dissertation. Bozeman: Montana State University; 1991. [Google Scholar]
- 37.van der Wende E, Characklis W G, Smith D B. Biofilms and bacterial drinking water quality. Wat Res. 1989;23:1313–1322. [Google Scholar]
- 38.Van Schijndel J W P M, Vollenbroek E G M, Wever R. The chloroperoxidase from the fungus Curvularia inaequalis: a novel vanadium enzyme. Biochim Biophys Acta. 1993;1161:249–256. doi: 10.1016/0167-4838(93)90221-c. [DOI] [PubMed] [Google Scholar]
- 39.Weiss S J, Test S T, Eckmann C M, Roos D, Regiani S. Brominating oxidants generated by human eosinophils. Science. 1986;234:200–203. doi: 10.1126/science.3018933. [DOI] [PubMed] [Google Scholar]
- 40.Wever R, Tromp M G M, Krenn B E, Marjani A, Van Tol M. Brominating activity of the seaweed Ascophyllum nodosum: impact on the biosphere. Environ Sci Technol. 1991;25:446–449. [Google Scholar]
- 41.Wever R, Tromp M G M, van Schijndel J W P M, Vollenbroek E, Olsen R L, Fogelqvist E. Bromoperoxidases: their role in the formation of HOBr and bromoform by seaweeds. In: Oremland R S, editor. Proceedings of the Biotechnology Center, E.C. Amsterdam, The Netherlands: Slater Institute for Biochemical Research; 1993. pp. 811–824. [Google Scholar]
- 42.Xu X, Stewart P S, Chen X. Transport limitation of chlorine disinfection of Pseudomonas aeruginosa entrapped in alginate beads. Biotechnol Bioeng. 1996;49:93–100. doi: 10.1002/(SICI)1097-0290(19960105)49:1<93::AID-BIT12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Beaber J W, Moré M I, Fuqua C, Eberhard A, Winans S C. Analogs of autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibits activity of the TraR protein of Agrobacterium tumefaciens. J Bacteriol. 1998;180:5398–5405. doi: 10.1128/jb.180.20.5398-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]