Abstract
Background and purpose
Many different factors have been hypothesized to modulate cognition in an aging population according to their functioning at baseline.
Methods
This retrospective study quantifies the relative contribution of age and sex as demographic factors, comorbidity, education and occupation (classified with the International Standard Classification of Occupation 2008) as cognitive reserve proxies in accounting for cognitive aging. All participants (3081) were evaluated at baseline with a complete neuropsychological test battery (T1) and those with unimpaired profiles were classified as subjective cognitive decline, those mildly impaired as mild neurocognitive decline and those severely impaired as major neurocognitive decline. From the first assessment 543 individuals were assessed a second time (T2), and 125 a third time (T3). Depending on whether they maintained or worsened their profile, based on their initial performance, participants were then classified as resistant or declining.
Results
At baseline, all individuals showed education and occupation as the best predictors of performance, in addition to age. Furthermore, across assessments, the resistant had higher levels of education and occupation than the declining. In particular, the education and occupation predicted cognitive performance in all groups considered, from the subjective cognitive decline to the one with the most severely impaired participants.
Conclusions
This study highlights the role of working activity in protecting from cognitive decline across all fragile elderly groups and even more so the individuals who are at very high risk of decline.
Keywords: cognitive decline, cognitive reserve, dementia, education, occupation
This retrospective study highlights the role of education and occupation in protecting cognitive decline in the elderly. In two follow‐ups, cognitively resistant participants showed higher education and more complex occupation than declining participants.

INTRODUCTION
Cognitive reserve (CR) is a construct that has been operationalized using several proxies such as education, occupation, indices of quality of life and physical activity. High values of such proxies have been found to have a positive influence on cognitive health and to significantly discriminate between healthy and pathological aging. Education has been repeatedly associated with lower risk of developing dementia and with improved cognitive functioning [1, 2]. Indeed, individual differences in cognitive decline have often been observed in association with educational level [3], with highly educated patients maintaining their functioning longer. In line with previous longitudinal cohort studies [4], a recent longitudinal study involving 28,417 individuals confirmed the association between education and cognitive performance at baseline and between education and delayed onset of cognitive decline [1].
As mentioned above, other factors, such as occupation or leisure activities [5, 6, 7], have been hypothesized to contribute to CR in addition to formal education. Social, mental and physical activities have beneficial effects on cognition in the elderly and have a protective role against dementia (see a review of longitudinal studies published between 1996 and 2004 by Fratiglioni et al. [8]). A satisfactory social life is considered of great importance in decreasing the risk of morbidity and death in old age [9], as well as in people with dementia [10]. An active daily social life involves a series of tasks that rely on cognition, and indeed older adults who have taken part in leisure activities show a lower risk of dementia than those with lesser involvement [11].
The cognitive processes required for job duties may also contribute to CR [12]. Early evidence suggested that occupation, together with education, plays a crucial role against the development of dementia [13]. This has been replicated in subsequent studies with large groups [12, 14]. One critical aspect that has been poorly investigated in previous studies is when and how the different CR proxies have an effect. In fact, most studies adopted a cross‐sectional approach [15], which does not allow to clearly verify the change of cognitive profiles over time.
In this retrospective study, the effect of demographic factors (age and sex), the presence of comorbidities and CR proxies (education and occupation) on a continuum from healthy to pathological aging and their persistence over time were investigated. In the baseline study, 3018 individuals underwent a first neuropsychological assessment (T1) for suspected pathological aging. It was predicted that, the younger the age and the higher the CR proxies, the better the cognitive outcomes. In follow‐up studies, 543 participants underwent a second (T2) and 125 a third (T3) neuropsychological assessment. In addition, to replicate the same pattern found in the baseline study, a slower cognitive decline over time was expected in participants with higher levels of CR.
The novelty of the present study consists in the inclusion of individuals with an unimpaired cognitive profile (e.g., subjective cognitive decline, SCD). There are several reasons why individuals with SCD may be of particular interest for studying the effects of CR. They have been less investigated than major neurocognitive disorder (MajorCD) or mild neurocognitive disorder (MildCD), although they represent a midway condition along the continuum between healthy and pathological aging [20]. Individuals with SCD have a high risk of developing dementia [21, 22], although their global cognitive performance is largely unimpaired.
METHODS AND RESULTS
Baseline study
From a cohort of 4638 individuals, 3081 (2010 females) entered the study (mean age 78.2 years, SD = 7.79, range 45–99; mean education 6.99 years, SD = 3.85, range 0–25 years). Predictors of cognitive outcomes were age and sex as sociodemographic variables, comorbidity (number of concomitant relevant pathologies) as the clinical variable, and education and occupation as proxies of CR. Occupation was classified using the International Standard Classification of Occupations 2008 (ISCO‐08) code [16] which is fully supported by the international community as an accepted standard for international labour statistics. This system classifies each working activity along a continuous numeric value where, the higher the numerical code is, the lower is the complexity of the occupation in terms of skills and cognitive resources involved (e.g., 9629 corresponds to ‘elementary workers not elsewhere classified’, whilst 0110 corresponds to ‘commissioned armed forces officers’).
At each assessment, participants underwent a complete neuropsychological evaluation, but only six tests routinely used in our clinical unit, and known to be sensitive to detecting cognitive decline in the elderly, were selected for the present analysis: (1) Mini‐Mental State Examination [17], Italian version [18] for a global measure of cognition; (2) famous face naming test, to measure proper name retrieval [19]; (3) memory test, to measure episodic memory; (4) fluency test, to measure lexical access and executive function; (5) Trail Making Test A (TMT), to assess selective and spatial attention and psychomotor speed; and (6) clock test, to measure visuo‐spatial abilities, planning and praxis. The last three tests are all taken from the Esame Neuropsicologico Breve 2 (ENB‐2) battery [21]. Three diagnostic groups were identified based on psychometric data and anamnestic and clinical information: (1) SCD [23] with 507 participants (17%); (2) MildND with 584 participants (19%); and (3) MajorND due to Alzheimer's disease with 1980 participants (64%). The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Ethical Committee of the School of Psychology of the University of Padua.
Results
Generalized linear model (GLM) analyses were carried out to verify whether and how age, sex, education, occupation and comorbidities could predict participants' cognitive performance in the six tests. Models with best fit (after entering one predictor at a time, based on R 2) were those with all five predictors and all these models were significant (see model fit measure in Table 1). A consistent effect of age and education was expected but, interestingly, occupation was also found to be predictive on all tests except for clock and TMT. Sex and comorbidity played a minor role and predicted only two tests each. In particular, females outperformed males in naming faces, whilst males outperformed females in the clock. A similar verbal/visuo‐spatial gender difference had been observed in a previous study on the effect of CR on aging [3], whilst Tappen [24] found that in an older adult population men were better than women at the clock test.
TABLE 1.
Generalized linear models
| Models | Predictors (beta and p) | Model fit measure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Education | Occupation | Comorbidity | ||||||||
| Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | R 2 | p | |
| MMSE | −0.250 | <0.001 | 0.030 | 0.135 | 0.259 | <0.001 | −0.048 | 0.037 | −0.030 | 0.111 | 0.164 | <0.001 |
| Memory | −0.312 | <0.001 | 0.003 | 0.952 | 0.246 | <0.001 | −0.067 | 0.017 | −0.020 | 0.367 | 0.201 | <0.001 |
| Faces | −0.300 | <0.001 | −0.140 | 0.013 | 0.212 | <0.001 | −0.066 | 0.042 | −0.039 | 0.141 | 0.175 | <0.001 |
| Fluency | −0.213 | <0.001 | 0.021 | 0.065 | 0.268 | <0.001 | −0.067 | 0.016 | −0.077 | <0.001 | 0.166 | <0.001 |
| TMT | 0.250 | <0.001 | −0.035 | 0.497 | −0.271 | <0.001 | 0.052 | 0.083 | 0.083 | <0.001 | 0.192 | <0.001 |
| Clock | −0.193 | <0.001 | 0.187 | <0.001 | 0.247 | <0.001 | −0.017 | 0.568 | −0.006 | 0.803 | 0.134 | <0.001 |
The contribution of each predictor to the model. Model fit measures indicate the significance of models with all covaried predictors.
Significant probability for each test is indicated in bold.
Abbreviations: MMSE, Mini‐Mental State Examination; TMT, Trail Making Test A.
The effect of age and education persisted within each of the three diagnostic groups (SCD, MildND, MajorND) and, although occupation did not play a role within each group, it differed significantly between them (F(2, 900) = 31.0, p < 0.001): SCD had the lowest score (i.e., more complex jobs), whilst MajorND had the highest score (i.e., less complex jobs) (see Figure 1).
FIGURE 1.
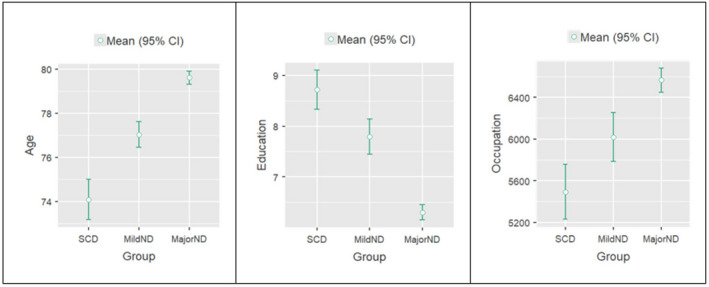
Predictors in the groups. The graphs show the mean differences in age (years), education (years) and occupation (ISCO‐08 codes: lower scores indicate more complex occupations) resulting from one‐way ANOVA between the three groups (SCD, MildND and MajorND)
Follow‐up studies
In all, 543 individuals from the baseline study underwent a second assessment (T2) and 125 underwent a third assessment (T3). In a series of GLM analyses, age, sex, education, occupation and comorbidity were introduced as predictors and participants' performance in the six cognitive tests as the dependent variable. At T2 all GLM analyses were significant in predicting the outcomes in the cognitive tests. The effect of education was confirmed in all six tests, and age had an effect only in two. No other effect was found. At T3, education was the only significant predictor in three tests.
Participants were then sorted into two groups based on clinical evidence and on their cognitive performance in the tests. At T2 and T3, individuals who performed worse than in the previous assessment were put in a single group and named declining. This group also included MajorND participants who maintained the same clinical diagnosis but had a worsened cognitive performance (no MajorND patient improved on cognitive tests). Participants who at T2 (or T3) did not worsen their profile were put in a single group and named resistant (see Table S1 for more details about participants' changing cognitive profiles). At each assessment, the declining group showed lower average scores at T2 than at T1 and at T3 than at T2 on four out of six tests. Instead, the average scores on all six tests of the resistant group did not change at either T2 or T3 (see Table 2).
TABLE 2.
Resistant and declining participants
| From T1 to T2 | From T2 to T3 | |||||
|---|---|---|---|---|---|---|
| t (df) | Mean diff. | p value | t (df) | Mean diff. | p value | |
| Declining | ||||||
| MMSE | 11.4 (399) | 2.80 | <0.001 | 5.92 (92) | 2.47 | <0.001 |
| Memory | 4.15 (220) | 0.64 | 0.005 | 5.35 (51) | 4.75 | <0.001 |
| Faces | 3.92 (83) | 0.99 | <0.001 | 3.17 (17) | 1.33 | 0.006 |
| Fluency | 1.18 (176) | 0.24 | 0.240 | 1.62 (52) | 0.63 | 0.111 |
| TMT | −3.08 (182) | −14.25 | 0.002 | −1.97 (54) | −15.38 | 0.054 |
| Clock | 1.75 (225) | 0.37 | 0.082 | 2.26 (64) | 0.79 | 0.027 |
| Resistant | ||||||
| MMSE | 0.32 (81) | 0.11 | 0.710 | 1.20 (15) | 1.00 | 0.248 |
| Memory | 1.29 (87) | 1.06 | 0.200 | 0.87 (17) | 1.28 | 0.398 |
| Faces | 1.58 (29) | 0.73 | 0.125 | 2.61 (3) | 1.25 | 0.080 |
| Fluency | 0.32 (83) | 0.13 | 0.751 | −1.99 (17) | −1.52 | 0.066 |
| TMT | −0.42 (29) | −1.56 | 0.672 | −1.46 (15) | −6.63 | 0.165 |
| Clock | −1.04 (60) | −0.34 | 0.301 | 0–38 (13) | 0.18 | 0.707 |
Paired sample t tests which show the mean difference of the six tests between T1 and T2 and between T2 and T3 in the two groups separately (declining and resistant).
Significant probability for each test is indicated in bold.
Abbreviations: MMSE, Mini‐Mental State Examination; TMT, Trail Making Test A.
Results
The time interval between assessments (from T1 to T2) was different amongst participants: short (1 year; 242 participants), medium (2–3 years; 207 participants) and long (more than 3 years; 94 participants). Assuming that the longer the time the higher the possible cognitive decline, the aim of this analysis was to verify if the effect of CR proxies changed depending on the time elapsed between assessments.
At T2, as expected, the resistant group had higher education than the declining group (t = 8.04, df = 1064, mean difference 2.43, p < 0.001) at all time intervals: short (t = 5.67, df = 480, mean difference 2.77, p < 0.001), medium (t = 3.02, df = 398, mean difference 1.34, p = 0.003), long (t = 5.43, df = 182, mean difference 3.77, p < 0.001). However, more interestingly, the resistant showed higher occupation than the declining (t = −4.80, df = 924, mean difference −979, p < 0.001) across the three time intervals: short (t = −2.69, df = 424, mean difference −819, p = 0.007); medium (t = −2.60, df = 342, mean difference −858, p = 0.01); long (t = −3.30, df = 154, mean difference −1565, p = 0.001). Noteworthy, the resistant had the highest education and also more complex occupations when the interval was the longest (see Figure 2).
FIGURE 2.
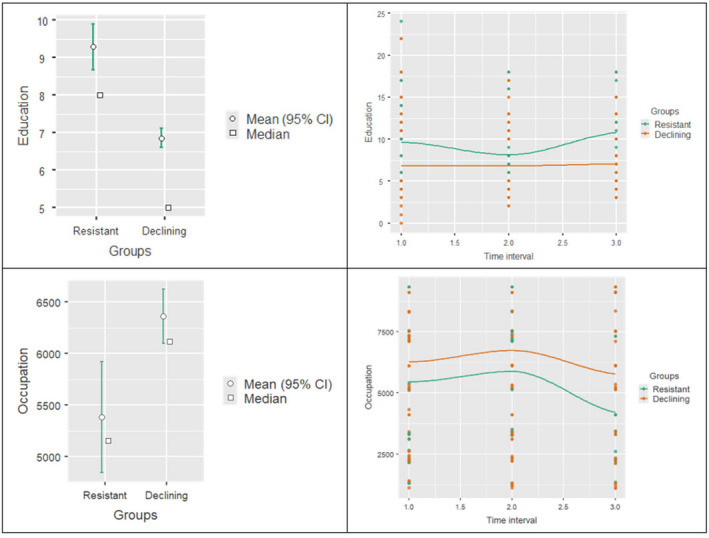
Education and occupation effects on resistant and declining individuals at the second assessment, at different time intervals. The two graphs on the left‐hand side show the mean difference of education and occupation between resistant and declining, whilst the two graphs on the right‐hand side show the mean difference of education and occupation between resistant and declining at the three time intervals
Of the 125 participants who underwent a third assessment (T3), 104 were declining (83.9%) and only 21 (16.1%) were resistant. Once again, the resistant were not only more educated (t = 3.86, df = 361, mean difference 2.49, p < 0.001) but held a more complex occupation than the declining (t = 2.25, df = 319, mean difference −941, p = 0.025) (see Figure 3). The time intervals between assessments were not considered in this analysis.
FIGURE 3.
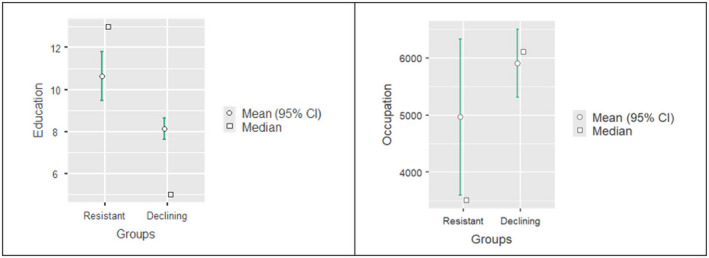
Education and occupation effects on resistant and declining individuals at the third assessment. The graphs represent the mean difference of education (on the left‐hand side) and occupation (on the right‐hand side) between resistant and declining at the third assessment (T3)
DISCUSSION
This study aimed at identifying which factors may have a modulating effect on cognition in aging and how such an effect may change across time. In the baseline study, the higher the education, the better the performance, an effect already well known and reported in the literature. However, with this study a new contribution is also made to the field by demonstrating the role of occupation as a good predictor of participants' performance. The younger participants, the more educated ones and those with more complex jobs showed better cognitive performance. Whilst it is known that cognition deteriorates with aging and in particular in less educated people [3, 25], the clear‐cut effect of occupation found in this study points to the relevance of occupation in operationalizing CR. Different from education, which is mostly although not exclusively acquired during the first part of life, occupation is built on activities carried out during adulthood and demonstrates the benefits of life‐long learning mechanisms. Adult learning seems to be quite effective in preserving cognition in the elderly [15, 26].
Age and education were confirmed as predictors of performance in all tests for all three diagnostic groups (SCD, MildND and MajorND). It was found that education does protect people potentially at risk of developing cognitive decline (SCD group), but more interestingly these same individuals also held more complex jobs than the other two groups. Thus, it seems that less impaired individuals had CR proxies to the highest degree.
Across assessments, the explanatory power of predictors seems progressively attenuated. In fact, as age increases, there are a multitude of factors that affect the elderly's cognition and give rise to huge heterogeneity [27]. Education was the only variable that continued to predict performance over time, although its effect decreased at T2 and T3.
When considering all participants, progressive worsening was found from one assessment to the next in most tests. However, when they were sorted into resistant (i.e., those who maintained their profile) or declining (i.e., those whose profile worsened), the former showed significantly higher education and more complex occupation than the latter at both T2 and T3. This result suggests a critical role of CR proxies in characterizing the evolution of dementia.
There are a number of limitations of this retrospective study. First, the patients, in our sample, could have been misdiagnosed because of the lack of precise biomarkers. However, structural and functional neuroimaging information was used to obtain the clinical diagnosis at baseline. It is noted that the number of participants between assessments decreased significantly, as is usual in the majority of studies that consider an elderly population with a percentage of pathological individuals. Another limitation may be that the concept of CR in this study was confined to education and occupation, even though CR is likely to be more than that. As this is a retrospective study, further useful data and information (e.g., lifestyle, place of residence etc.) to better understand how our participants aged could not be obtained. Although SCD individuals within the continuum between normal and pathological aging are considered, in future studies it would be appropriate to include also non‐clinical participants.
To our knowledge, this is one of the few studies including the category of SCD individuals when CR is used as a potential modulator of age‐related trajectories. Moreover, the importance of working activities carried out during adulthood was demonstrated to be a critical factor of CR and cognitive performance in SCD individuals [28, 29].
In conclusion, our longitudinal study on healthy and pathological participants maintains that, together with social connectedness, ongoing sense of purpose and ability to function independently, higher levels of CR contribute to mental health and general wellbeing along the trajectories of aging.
CONSENT TO PARTICIPATE
All patients gave their written informed consent for the use of their data for research purposes at the moment of their consultation.
CONSENT FOR PUBLICATION
Patients signed informed consent regarding publishing their data under the guarantee of anonymity.
CONFLICT OF INTEREST
None reported.
AUTHOR CONTRIBUTIONS
Sara Mondini: Conceptualization (lead); data curation (lead); formal analysis (equal); investigation (lead); methodology (equal); project administration (equal); writing—original draft (equal); writing—review and editing (equal). Veronica Pucci: Data curation (equal); formal analysis (equal); methodology (equal); software (equal); writing—original draft (equal); writing—review and editing (equal). Sonia Montemurro: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); writing—original draft (equal); writing—review and editing (equal). Raffaella Ida Rumiati: Conceptualization (lead); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (supporting); software (equal); writing—original draft (equal); writing—review and editing (equal).
ETHICAL APPROVAL
The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Ethical Committee of the School of Psychology of the University of Padua.
Supporting information
ACKNOWLEDGEMENTS
All participants who took part in this research are thanked. Open Access funding provided by Universita degli Studi di Padova within the CRUI‐CARE Agreement. [Correction added 20 May 2022, after first online publication: CRUI funding statement has been added.
Mondini S, Pucci V, Montemurro S, Rumiati RI. Protective factors for subjective cognitive decline individuals: trajectories and changes in a longitudinal study with Italian elderly. Eur J Neurol.2022;29:691–697. doi: 10.1111/ene.15183
Funding information
None reported
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are not publicly available due to information that could compromise participant privacy but are available from the corresponding author on reasonable request.
REFERENCES
- 1. Clouston SAP, Smith DM, Mukherjee S, et al. Education and cognitive decline: an integrative analysis of global longitudinal studies of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):e151‐e160. doi: 10.1093/geronb/gbz053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stern Y, Arenaza‐Urquijo EM, Bartrés‐Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's Dement. 2020;16(9):1305‐1311. doi: 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Carret N, Lafont S, Letenneur L, Dartigues JF, Mayo W, Fabrigoule C. The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Dev Neuropsychol. 2003;23(3):317‐337. doi: 10.1207/S15326942DN2303_1 [DOI] [PubMed] [Google Scholar]
- 4. Zahodne LB, Manly JJ, Brickman AM, et al. Is residual memory variance a valid method for quantifying cognitive reserve? A longitudinal application. Neuropsychologia. 2015;77:260‐266. doi: 10.1016/j.neuropsychologia.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stern Y, Alexander GE, Prohovnik I, et al. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology. 1995;45(1):55‐60. [DOI] [PubMed] [Google Scholar]
- 6. Pettigrew C, Shao Y, Zhu Y, et al. Self‐reported lifestyle activities in relation to longitudinal cognitive trajectories. Alzheimer Dis Assoc Disord. 2019;33(1):21‐28. doi: 10.1097/WAD.0000000000000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rusmaully J, Dugravot A, Moatti JP, et al. Contribution of cognitive performance and cognitive decline to associations between socioeconomic factors and dementia: a cohort study. PLoS Medicine. 2017;14(6):1‐17. doi: 10.1371/journal.pmed.1002334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fratiglioni L, Paillard‐Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343‐353. doi: 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- 9. Iwasaki M, Otani T, Sunaga R, et al. Social networks and mortality based on the Komo‐Ise cohort study in Japan. Int J Epidemiol. 2002;31(6):1208‐1218. doi: 10.1093/ije/31.6.1208 [DOI] [PubMed] [Google Scholar]
- 10. Balouch S, Rifaat E, Chen HL, Tabet N. Social networks and loneliness in people with Alzheimer's dementia. Int J Geriatr Psychiatry. 2019;34(5):666‐673. doi: 10.1002/gps.5065 [DOI] [PubMed] [Google Scholar]
- 11. Seeman TE, Crimmins E. Social environment effects on health and aging: integrating epidemiologic and demographic approaches and perspectives. Ann N Y Acad Sci. 2001;954:88‐117. doi: 10.1111/j.1749-6632.2001.tb02749.x [DOI] [PubMed] [Google Scholar]
- 12. Pool LR, Weuve J, Wilson RS, Bültmann U, Evans DA, Mendes de Leon CF. Occupational cognitive requirements and late‐life cognitive aging. Neurology. 2016;86(15):1386‐1392. doi: 10.1212/WNL.0000000000002569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White L, Katzman R, Losonczy K, et al. Association of education with incidence of cognitive impairment in three established populations for epidemiologic studies of the elderly. J Clin Epidemiol. 1994;47(4):363‐374. doi: 10.1016/0895-4356(94)90157-0 [DOI] [PubMed] [Google Scholar]
- 14. Andel R, Dávila‐Roman AL, Grotz C, Small BJ, Markides KS, Crowe M. Complexity of work and incident cognitive impairment in Puerto Rican older adults. J Gerontol B Psychol Sci Soc Sci. 2019;74(5):785‐795. doi: 10.1093/geronb/gbx127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clare L, Wu YT, Teale JC, et al. Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: a cross‐sectional study. PLoS Medicine. 2017;14(3):1‐14. doi: 10.1371/journal.pmed.1002259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. International Labour Office . International standard classification of Occupations 2008 (ISCO‐08): Structure, Group Definitions and Correspondence Tables; Geneva, Switzerland: ILO; 2012. [Google Scholar]
- 17. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 18. Magni E, Binetti G, Bianchetti A, Rozzini R, Trabucchi M. Mini‐Mental State Examination: a normative study in Italian elderly population. Eur J Neurol. 1996;3(3):198‐202. doi: 10.1111/j.1468-1331.1996.tb00423.x [DOI] [PubMed] [Google Scholar]
- 19. Semenza C, Mondini S, Borgo F, Pasini M, Sgaramella MT. Proper names in patients with early Alzheimer's disease. Neurocase. 2003;9(1):63‐69. doi: 10.1076/neur.9.1.63.14370 [DOI] [PubMed] [Google Scholar]
- 20. Slot RE, Sikkes SA, Berkhof J, et al. Subjective cognitive decline and rates of incident Alzheimer’s disease and non–Alzheimer’s disease dementia. Alzheimer’s & Dementia. 2019;15(3):465‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mondini S, Mapelli D, Vestri A, Arcara G & Bisiacchi P (Eds.). Esame Neuropsicologico Breve 2. Milan, Italy: Raffaello Cortina, Publisher; 2011. [Google Scholar]
- 22. Rabin LA, Smart CM & Amariglio RQ. Subjective cognitive decline in preclinical Alzheimer’s disease. Annual review of clinical psychology. 2017;13:369‐396. [DOI] [PubMed] [Google Scholar]
- 23. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844‐852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tappen R. The clock drawing test. Res Gerontol Nurs. 2019;12(1):2‐4. doi: 10.2307/1130982 [DOI] [PubMed] [Google Scholar]
- 25. Chan D, Shafto M, Kievit R, et al. Lifestyle activities in mid‐life contribute to cognitive reserve in late‐life, independent of education, occupation, and late‐life activities. Neurobiol Aging. 2018;70:180‐183. doi: 10.1016/j.neurobiolaging.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36(4):441‐454. doi: 10.1017/S0033291705006264 [DOI] [PubMed] [Google Scholar]
- 27. Netz Y, Lidor R, Ziv G. Small samples and increased variability—discussing the need for restricted types of randomization in exercise interventions in old age. Eur Rev Aging Phys Act. 2019;16:17. doi: 10.1186/s11556-019-0224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bessi V, Mazzeo S, Padiglioni S, et al. From subjective cognitive decline to Alzheimer's disease: the predictive role of neuropsychological assessment, personality traits, and cognitive reserve. A 7‐year follow‐up study. J Alzheimer's Dis. 2018;63:1523‐1535. doi: 10.3233/JAD-171180 [DOI] [PubMed] [Google Scholar]
- 29. Lojo‐Seoane C, Facal D, Guàrdia‐Olmos J, Pereiro AX, Juncos‐Rabadán O. Effects of cognitive reserve on cognitive performance in a follow‐up study in older adults with subjective cognitive complaints. The role of working memory. Front Aging Neurosci. 2018;10:189. 10.3389/fnagi.2018.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to information that could compromise participant privacy but are available from the corresponding author on reasonable request.


