Abstract
Objective
Prior to the Continuous Monitoring and Control of Hypoglycaemia (COACH) study described herein, no study had been powered to evaluate the impact of non‐adjunctive RT‐CGM use on the rate of debilitating moderate or severe hypoglycaemic events.
Research Design and Methods
In this 12‐month observational study, adults with insulin‐requiring diabetes who were new to RT‐CGM participated in a 6‐month control phase where insulin dosing decisions were based on self monitoring of blood glucose values, followed by a 6‐month phase where decisions were based on RT‐CGM data (i.e. non‐adjunctive RT‐CGM use); recommendations for RT‐CGM use were made according to sites' usual care. The primary outcome was change in debilitating moderate (requiring second‐party assistance) and severe (resulting in seizures or loss of consciousness) hypoglycaemic event frequency. Secondary outcomes included changes in HbA1c and diabetic ketoacidosis (DKA) frequency.
Results
A total of 519 participants with mean (SD) age 50.3 (16.1) years and baseline HbA1c 8.0% (1.4%) completed the study, of whom 32.8% had impaired hypoglycaemia awareness and 33.5% had type 2 diabetes (T2D). The mean (SE) per‐patient frequency of hypoglycaemic events decreased by 63% from 0.08 (0.016) during the SMBG phase to 0.03 (0.010) during the RT‐CGM phase (p = 0.005). HbA1c decreased during the RT‐CGM phase both for participants with type 1 diabetes (T1D) and T2D and there was a trend towards larger reductions among individuals with higher baseline HbA1c.
Conclusions
Among adults with insulin‐requiring diabetes, non‐adjunctive use of RT‐CGM data is safe, resulting in significantly fewer debilitating hypoglycaemic events than management using SMBG.
Keywords: COACH, continuous glucose monitoring, severe hypoglycaemia
What is already known?
Although some studies have shown that real‐time continuous glucose monitoring (RT‐CGM) use reduces debilitating severe hypoglycaemic events, none have been powered accordingly, they included protocol‐specific structured RT‐CGM training, and some had narrow inclusion criteria, limiting generalizability of results.
What this study has found?
The COACH study was appropriately powered and demonstrates that among adults with insulin‐requiring T1D or T2D treated with site‐specific usual care, using RT‐CGM as a replacement for fingersticks (i.e. non‐adjunctive use) for diabetes management results in significantly fewer debilitating hypoglycaemic events than blood glucose‐based management.
What are the implications of the study?
Non‐adjunctive RT‐CGM use is associated with favourably reducing severe hypoglycaemia among patients with T1D and insulin‐treated T2D.
1. INTRODUCTION
Intensive diabetes treatment via self monitoring of blood glucose (SMBG) and multiple daily insulin injections (MDI) has been shown to improve glycaemic control and reduce the progression of vascular complications in participant with diabetes. 1 , 2 However, insulin users are at increased risk for severe hypoglycaemia, which can be devastating. 3 , 4 Furthermore, while frequent glucose monitoring with SMBG is correlated with lower HbA1c, its episodic and volitional nature often leaves patients unaware of their glucose levels or trends; many patients have persistent and prolonged hypoglycaemia even with frequent SMBG testing. 5
Although limited by self report, recent studies of the prevalence of severe hypoglycaemic events in participant with diabetes have reported high event frequencies. An analysis of the T1D Exchange Clinic Registry found that 3–11% of study participants experienced at least one severe hypoglycaemic event within the past 3 months, with higher prevalence reported by those who were not using continuous glucose monitoring (CGM) systems. 6 Using a retrospective questionnaire, the DIALOG study reported that 31.5% of participants with T1D and 21.7% of participants with insulin‐treated T2D experienced a severe hypoglycaemic event during the previous year. 7 A separate survey in the United States reported annual severe hypoglycaemia incidences of 51.4% (T1D) and 33.4% (insulin‐ or secretagogue‐treated T2D). 8
Real‐time continuous glucose monitoring (RT‐CGM) systems offer an advance over SMBG, providing glucose values and trends frequently and automatically to a receiver or smart device. RT‐CGM systems include optional alerts for existing or impending hypoglycaemia and hyperglycaemia. Early‐generation RT‐CGM systems were characterized by short wear times, frequent nuisance alarms, painful insertion and obtrusive transmitters; required daily calibration; and necessitated confirmatory fingersticks to make diabetes treatment decisions (i.e. were labelled only for adjunctive use). However, modern RT‐CGM systems have overcome these early limitations. Some are sufficiently accurate to be used as a replacement for fingersticks (i.e. labelled for non‐adjunctive use) and most are increasingly easy to use and easy to wear; altogether these advances have helped drive the overwhelming positive clinical outcomes observed with RT‐CGM. 9 , 10 , 11 In recognition of these benefits, RT‐CGM use has become a standard of care, 12 , 13 strongly recommended for all participants with diabetes treated with intensive insulin therapy. 13
Numerous randomized controlled trials have demonstrated that RT‐CGM use reduces frequency and duration of CGM‐measured hypoglycaemia in populations with T1D, independent of non‐adjunctive or adjunctive labelling. 14 , 15 , 16 , 17 , 18 , 19 , 20 Although some studies have shown that RT‐CGM use also reduces severe hypoglycaemic events, 16 , 17 , 18 , 19 , 21 none were powered accordingly and some had narrow inclusion criteria (adults with T1D with history of severe hypoglycaemia or impaired awareness of hypoglycaemia), 16 , 21 limiting generalizability of results. In addition, diabetes management was prescriptive and participants and clinicians had structured RT‐CGM training. Consequently, there is a need to determine if non‐adjunctive use of RT‐CGM can reduce debilitating hypoglycaemia in a more heterogeneous population of insulin users (including both individuals with T1D or T2D) in the absence of protocol‐specific RT‐CGM training.
2. METHODS
2.1. Study design and participants
The COACH study was a prospective, observational non‐inferiority study conducted at 19 sites across the United States designed to assess if non‐adjunctive and routine use of RT‐CGM for clinical decisions impacts safety of diabetes management across a broad population of users. This study was recommended by an FDA advisory panel during their deliberations of non‐adjunctive use of the Dexcom G5 RT‐CGM System (Dexcom, Inc., San Diego, CA). 22 Based in part on these deliberations, this study defined moderate hypoglycaemic events as those requiring assistance of a second participant to resolve and severe hypoglycaemic events as those resulting in seizures or loss of consciousness, even though the customary definition of severe hypoglycaemia includes the requirement of a second party to treat. The protocol and consent forms were approved by an Institutional Review Board (Advarra IRB, Approval ID: Pro00020506). Written informed consent was obtained from each participant.
Adult participants with any baseline HbA1c were eligible if they were new to RT‐CGM, had insulin‐treated T1D or T2D (diagnosis based on clinical history); with established treatment ≥3 months), had access to a smartphone and/or computer, and were able to participate in the study for 12 months. Exclusion criteria included use of RT‐CGM within the past 12 months, pregnancy, and dialysis. Centres were not given additional guidance on participant selection beyond the inclusion/exclusion criteria.
2.2. Procedures
Participants first completed a 6‐month control phase during which they based treatment decisions on SMBG values using their personal blood glucose metre, followed by a 6‐month intervention phase during which they based treatment decisions on RT‐CGM values from the G5 System (twice daily calibration required via personal blood glucose metre). During both study phases, device training and diabetes management were per the sites’ usual approach. This included establishing glycaemic targets and recommendations about SMBG frequency, dosing rules and suggestions, hypoglycaemia and hyperglycaemia management and prevention, and RT‐CGM alert settings.
The protocol included three clinic visits at Months 0, 6 and 12. At Month 0, demographic data, baseline HbA1c (obtained by point of care or local laboratory), clinical history, diabetes history (including history of mild/severe hypoglycaemia or episodes of diabetic ketoacidosis [DKA] in the past 6 months) and SMBG testing frequency data were collected. Moderate hypoglycaemic events were defined as those requiring assistance of a second participant to resolve; severe hypoglycaemic events were defined as those resulting in seizure or loss of consciousness. DKA required treatment at a healthcare facility. Monthly between‐visit phone calls (10 total) during each study phase assessed if any hypoglycaemic or DKA events occurred and the details surrounding an event. The calls only focused on collecting data on hypoglycaemia and DKA and did not include diabetes management discussions. During the Month 6 and Month 12 clinic visits, HbA1c levels were obtained. At the Month 6 clinic visit, training was provided on RT‐CGM use and patients were given instructions to upload RT‐CGM data on a monthly basis. RT‐CGM‐based metrics of glycaemic control will be reported separately.
2.3. Outcomes
The primary endpoint was the change in moderate or severe hypoglycaemic event frequency. The mean number of overall hypoglycaemic events per participant, as well as the number of participants with ≥1 hypoglycaemic event during the SMBG and RT‐CGM phases, were calculated. Analysis was also performed to calculate the change in hypoglycaemic events by subgroup (e.g. by diabetes type, insulin delivery modality, diabetes duration, history of hypoglycaemia/DKA, age and hypoglycaemia awareness [Gold score ≥ 4]). Additional analyses were performed to exclude hypoglycaemic events that occurred when participants were not wearing the RT‐CGM system, as these events were unrelated to non‐adjunctive RT‐CGM use.
Secondary outcomes were the change in self‐reported episodes of DKA that included treatment at a healthcare facility or emergency room, as well as changes in HbA1c levels after 6 months of RT‐CGM use. For HbA1c analysis, participants were grouped into categories based on their baseline HbA1c level at the initial clinic visit (Month 0). The change in HbA1c associated with RT‐CGM use, as well as the change in hypoglycaemic event frequency, was compared across the various starting HbA1c groups.
2.4. Statistical analysis and considerations
Participants who enrolled and met eligibility criteria were included in the intent‐to‐treat (ITT) population. Participants who completed the 12‐month follow‐up were included in the per‐protocol (PP) population. Analysis of hypoglycaemic/DKA events and change in HbA1c were based on the PP dataset. Hypoglycaemic events were analysed in aggregate and separately by moderate and severe hypoglycaemic events. The non‐inferiority of RT‐CGM to SMBG in terms of hypoglycaemic events was tested in a repeated‐measures Poisson regression model, where the non‐inferiority margin was 10%. Superiority was also subsequently tested. The change in HbA1c from Month 6 to Month 12 was analysed for 517 participants in the PP group who provided values at both time points.
The study was designed to enroll a total of 1100 participants with a single interim analysis to yield 80% power and demonstrate non‐inferiority of RT‐CGM to SMBG using a 2.5% significance level; a triangular (Whitehead) alpha spending function was used to determine the interim and final stopping rules. The sample size calculation assumed a RT‐CGM effect of a 10% decrease in the hypoglycaemia event rate, a standard baseline hypoglycaemia event rate of 1.2 events per patient per 6 months, 7 an over‐dispersion factor of 3 and an intra‐patient correlation of 0.2. A single interim analysis was conducted at completion of 50% of the total sample size. Based on the interim analysis results, the trial could either be stopped for efficacy (p‐value < 0.014) or for futility (p‐value > 0.138).
3. RESULTS
Adult enrollment was stopped due to demonstrated efficacy from the planned interim analysis and results for the adult population are described here. Paediatric enrollment is ongoing. Between 20 October 2017 and 9 October 2019, 624 adults were enrolled and assessed for eligibility; 4 did not meet inclusion/exclusion criteria and the remaining 620 formed the intent‐to‐treat population (Figure 1). Of the 624 adults, 519 completed the 12‐month follow‐up visit and formed the per‐protocol (PP) population (Table 1). Approximately 1/3 (n = 174) had T2D and 29.7% used pumps. During the control phase, mean (SD) daily SMBG frequency was 4.1 (1.8); this fell to 2.5 (1.0) per day during the intervention phase for a mean (95% CI) difference of −1.6 (−1.7 to −1.4) tests per day, consistent with twice daily calibrations.
FIGURE 1.
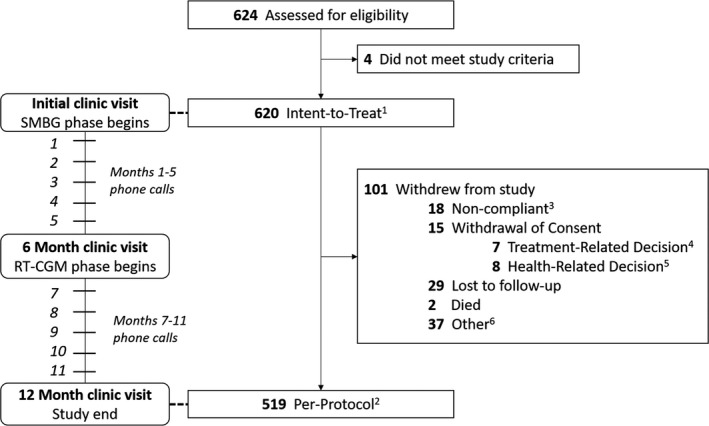
Flowchart of COACH study completion. [1] Intent‐to‐treat (ITT) population includes all enrolled patients that meet all inclusion criteria and none of the exclusion criteria. [2] Per‐protocol (PP) population includes all ITT patients that completed the 12‐month follow‐up. [3] Non‐compliant includes: does not want study treatment, poor outcome, visit too lengthy, travel difficulty. [4] Treatment‐related decisions includes: changed doctor, PCP prescribed Dexcom G6 CGM, PCP prescribed other CGM, and other treatment requested. [5] Health‐related decisions includes: AE, developed end‐stage renal disease, poor health. [6] Other includes: moved, finances, other
TABLE 1.
Baseline characteristics of COACH participants
|
Per‐protocol (n = 519) |
Type 1 (n = 345) |
Type 2 (n = 174) |
|
|---|---|---|---|
| Age | |||
| Mean (SD) | 50.3 (16.1) | 45.2 (15.9) | 60.5 (11.0) |
| Median | 52.0 | 43.0 | 62.0 |
| Min, Max | 18, 86 | 18, 83 | 20, 86 |
| Gender, n (%) | |||
| Male | 281 (54.1%) | 181 (52.5%) | 100 (57.5%) |
| Female | 238 (45.9%) | 164 (47.5%) | 74 (42.5%) |
| Race, n (%) | |||
| American Indian or Alaska Native | 2 (0.4%) | 0 (0.0%) | 2 (1.1%) |
| Asian | 8 (1.5%) | 3 (0.9%) | 5 (2.9%) |
| Black or African American | 27 (5.2%) | 12 (3.5%) | 15 (8.6%) |
| Native Hawaiian or Other Pacific Islander | 1 (0.2%) | 1 (0.3%) | 0 (0.0%) |
| White | 468 (90.2%) | 326 (94.5%) | 142 (81.6%) |
| Multiple races/other | 13 (2.5%) | 3 (0.9%) | 10 (5.7%) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 56 (10.8%) | 26 (7.5%) | 30 (17.2%) |
| Not Hispanic or Latino | 459 (88.4%) | 316 (91.6%) | 143 (82.2%) |
| Not reported | 4 (0.8%) | 3 (0.9%) | 1 (0.6%) |
| Duration of diabetes (years) | |||
| Mean (SD) | 22.1 (13.4) | 24.0 (14.8) | 18.4 (8.8) |
| Median | 20.0 | 23.0 | 17.0 |
| Min, Max | 0, 71 | 0, 71 | 1, 50 |
| Type of diabetes | |||
| Type 1 | 345 (66.5%) | 100% | 0% |
| Type 2 | 174 (33.5%) | 0% | 100% |
| Number of years on insulin | |||
| Mean (SD) | 19.4 (14.4) | 23.9 (15.0) | 10.5 (7.5) |
| Median | 16.0 | 23.0 | 9.3 |
| Min, Max | 0.4, 71.0 | 0.6, 71.0 | 0.4, 33.0 |
| SMBG frequency (per day) | |||
| Mean (SD) | 4.0 (2.3) | 4.5 (2.5) | 3.0 (1.6) |
| Median | 4.0 | 4.0 | 3.0 |
| Min, Max | 0, 24 | 0, 24 | 0, 8 |
| Primary insulin delivery method | |||
| CSII | 154 (29.7%) | 140 (40.6%) | 14 (8.0%) |
| MDI | 365 (70.3%) | 205 (59.4%) | 160 (92.0%) |
| HbA1c mmol/mol | |||
| Mean (SD) | 64 (15.3) | 64 (15.3) | 64 (17.5) |
| Median (IQR) | 62 | 62 | 61 |
| Min, Max | 28, 143 | 30, 114 | 28, 143 |
| HbA1c % | |||
| Mean (SD) | 8.0 (1.4) | 8.0 (1.4) | 8.0 (1.6) |
| Median (IQR) | 7.8 | 7.8 | 7.7 |
| Min, Max | 4.7, 15.2 | 4.9, 12.6 | 4.7, 15.2 |
| Gold score ≥ 4 | 169/516 (32.8%) | 114/343 (33.2%) | 55/173 (31.8%) |
| Recent hypoglycaemic event | 31/519 (6.0%) | 25/345 (7.2%) | 6/174 (3.4%) |
| Recent DKA event | 9/519 (1.7%) | 7/345 (2.0%) | 2/174 (1.1%) |
3.1. Primary outcome (hypoglycaemic events)
A total of 42 hypoglycaemic events (35 from participants with T1D and 7 from participants with T2D) occurred during the SMBG phase, compared to 16 events (T1D: 13, T2D: 3) during the RT‐CGM phase; 2 of these occurred while participants were not wearing the RT‐CGM system at the time of the event. Figure 2a summarizes the 6‐month event prevalence for overall, moderate, severe, daytime and nighttime hypoglycaemic events. Twenty‐nine participants (25 with T1D and 4 with T2D) reported at least one hypoglycaemic event during the SMBG phase, compared to 12 participants (T1D: 10, T2D: 2) who reported at least one hypoglycaemic event during the RT‐CGM phase; 2 participants, both with T1D, reported at least 1 hypoglycaemic event while not wearing the RT‐CGM during the RT‐CGM period.
FIGURE 2.
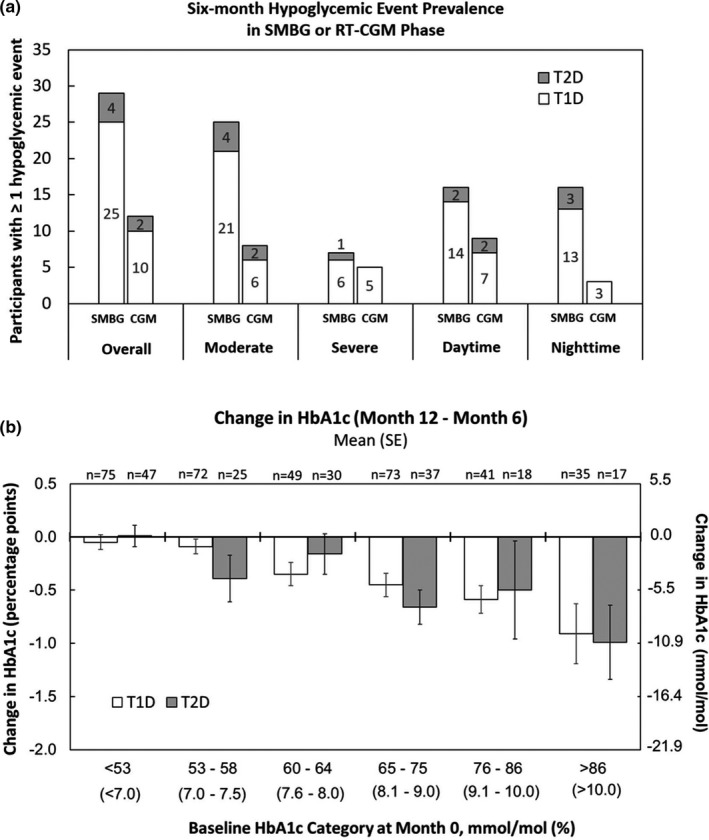
(a) Six‐month prevalences of hypoglycaemic events during periods of SMBG and RT‐CGM use in the PP population for participants with T2D (grey bars) or T1D (white bars). (b) Change in HbA1c levels between Month 6 (end of SMBG phase) and Month 12 (end of CGM phase) for participants with T1D (white bars) and T2D (grey bars). Participants were grouped by baseline (Month 0) HbA1c levels
Table 2 summarizes the mean (SE) hypoglycaemic events per participant during the SMBG and RT‐CGM phases for various subgroups based on disease type, method of insulin delivery, hypoglycaemia awareness, diabetes duration and age. In almost all subgroups examined, hypoglycaemic event frequency decreased. The largest reduction was observed among participants with starting HbA1c levels <53 mmol/mol (<7.0%).
TABLE 2.
Mean (SE) and proportional change in hypoglycaemic event rates (per participant per 6‐month period) for subgroups of the per‐protocol population (n = 519) a
| Subgroup | No. of subjects | Hypoglycaemic event rate (per participant per 6‐month period) | Rate ratio (95% CI) | |
|---|---|---|---|---|
| SMBG phase (Months 1–6) | CGM phase (Months 7–12) | |||
| Overall b | 519 | 0.081 (0.016) | 0.031 (0.001) c | 0.38 (0.19–0.78) |
| Type of diabetes | ||||
| T1D | 345 | 0.101 (0.022) | 0.038 (0.014) | 0.37 (0.17–0.81) |
| T2D | 174 | 0.040 (0.022) | 0.017 (0.013) | 0.43 (0.07–2.64) |
| Method of insulin delivery | ||||
| CSII | 154 | 0.091 (0.028) | 0.071 (0.032) | 0.79 (0.30–2.07) |
| MDI | 365 | 0.077 (0.020) | 0.014 (0.006) | 0.18 (0.07–0.44) |
| Baseline HbA1c | ||||
| <53 mmol/mol (<7.0%) | 122 | 0.090 (0.035) | 0.008 (0.008) | 0.09 (0.02–0.42) |
| 53–58 mmol/mol (7.0%–7.5%) | 97 | 0.062 (0.029) | 0.031 (0.023) | 0.50 (0.10–2.48) |
| 60–64 mmol/mol (7.6%–8.0%) | 79 | 0.051 (0.040) | 0.063 (0.052) | 1.25 (0.19–8.07) |
| 65–75 mmol/mol (8.1%–9.0%) | 110 | 0.109 (0.039) | 0.027 (0.016) | 0.25 (0.07–0.96) |
| 76–86 mmol/mol (9.1%–10.0%) | 59 | 0.136 (0.066) | 0.051 (0.029) | 0.38 (0.09–1.50) |
| >86 mmol/mol (>10%) | 52 | 0.019 (0.019) | 0.019 (0.019) | 1.00 (0.06–15.99) |
| History of hypoglycaemia and/or DKA in the 6 months prior to enrolment | ||||
| Yes | 38 | 0.368 (0.127) | 0.079 (0.044) | 0.21 (0.08–0.61) |
| No | 481 | 0.058 (0.014) | 0.027 (0.011) | 0.46 (0.20–1.09) |
| Age (years) | ||||
| 18–25 | 40 | 0.050 (0.035) | 0.025 (0.025) | 0.50 (0.13–2.00) |
| 26–64 | 352 | 0.065 (0.018) | 0.023 (0.009) | 0.35 (0.13–0.90) |
| >64 | 127 | 0.134 (0.041) | 0.055 (0.034) | 0.41 (0.13–1.32) |
| Hypoglycaemia awareness d | ||||
| Impaired (Gold ≥ 4) | 169 | 0.107 (0.037) | 0.036 (0.017) | 0.33 (0.12–0.96) |
| Intact (Gold < 4) | 347 | 0.069 (0.016) | 0.029 (0.013) | 0.42 (0.16–1.06) |
Includes all events, whether RT‐CGM was worn or not.
The overall event rate while participants wore the RT‐CGM was 0.027 (0.010).
p = 0.005 for superiority versus SMBG phase.
Gold scores were not available from three participants.
3.2. Secondary outcomes (DKA events, HbA1c changes)
Two participants (both with T2D) reported one DKA event each during the SMBG phase; none were reported during the RT‐CGM phase. The mean (SE) HbA1c at Month 0 and Month 6 were 64 (0.7) mmol/mol and 64 (0.7) mmol/mol (8.0% [0.1%]) and (8.0% [0.1%]) respectively (n.s.). The overall mean (SE) difference in HbA1c levels between Month 6 and Month 12 was −0.35 (0.04) percentage points; it was −0.34 (0.05) percentage points for participants with T1D (N = 344) and −0.37 (0.09) percentage points for participants with T2D (N = 173). All changes from Month 6 to Month 12 were statistically significant (paired t‐test p‐values < 0.0001). The change in HbA1c between Month 12 and 6 was further broken down into subgroups based on baseline HbA1c levels at Month 0. As shown in Figure 2b, participants with higher baseline HbA1c levels experienced larger HbA1c decreases during the RT‐CGM phase, regardless of diabetes type. Regression to the mean effects were minimized in this analysis by categorizing patients into baseline HbA1c levels and measuring the change between Month 12 and Month 6 within these groups. Although the mean HbA1c was unchanged (Month 6 to Month 12) for those with baseline HbA1c values <53 mmol/mol (<7.0%) (Figure 2b), in this group, the mean frequency of hypoglycaemic events during the RT‐CGM phase was 0.09 times that of what was observed during the SMBG phase (Table 2), approximately a 91% reduction.
3.3. Device‐related adverse events
There was one mild AE reported during the SMBG phase that was due to skin irritation. During the RT‐CGM phase, there were 15 participants who experienced device‐related AEs (11 mild, 4 moderate and 0 severe). Of the 11 participants with mild AEs, 1 was due to hypoglycaemia and 10 were insertion‐site related (2 from bleeding, 3 from haematoma and 5 from skin irritation). Of the four participants with moderate AEs, four were due to hypoglycaemia and two were due to local infection at the insertion site.
4. DISCUSSION
Numerous well‐designed studies have demonstrated that RT‐CGM use reduces frequency and duration of CGM‐measured hypoglycaemia, independent of non‐adjunctive or adjunctive labelling. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 23 , 24 , 25 Although some studies have shown that RT‐CGM use also reduces severe hypoglycaemic events, 16 , 17 , 18 , 19 , 21 none were powered accordingly and some had narrow inclusion criteria (adults with T1D with history of severe hypoglycaemia or impaired awareness of hypoglycaemia), 16 , 21 limiting generalizability of results. In addition, diabetes management may have been prescriptive and participants and clinicians may have had structured RT‐CGM training.
To our knowledge, the COACH study is the first study specifically powered to detect changes in debilitating hypoglycaemic events from non‐adjunctive use of RT‐CGM. To demonstrate non‐inferiority and considering the low hypoglycaemic event frequencies, the COACH study aimed to enrol a large number of participants and was stopped at the planned interim analysis due to efficacy. In this year‐long study, adults new to RT‐CGM managed their diabetes using SMBG for 6 months before transitioning to the Dexcom G5 for 6 months. The study relied on monthly phone calls to document episodes of symptomatic hypoglycaemia requiring the assistance of another participant.
More participants reported at least one debilitating hypoglycaemic event during the SMBG phase compared to the RT‐CGM phase, and the mean frequency of overall hypoglycaemic events per participant was significantly lower during the RT‐CGM phase. This trend towards reduced frequency was observed across all categories of hypoglycaemic events (Figure 2a) and almost all population subgroups (Table 2). Change in HbA1c levels following RT‐CGM adoption was also analysed by comparing HbA1c levels from the Month 6 clinic visit to the Month 12 clinic visit. The changes in HbA1c associated with RT‐CGM use were similar for participant with T1D and T2D and were related to starting HbA1c levels, with the greatest benefit observed for participants who had higher starting HbA1c values (e.g. baseline HbA1c >86 mmol/mol [>10%]). This agrees with DIAMOND 26 and GOLD 27 studies.
Surprisingly, the rates of moderate and severe hypoglycaemia observed during the COACH study were lower than anticipated and far less than previously reported by studies such as the T1D Exchange Clinic Registry 6 or DIALOG. 7 The rates of hypoglycaemic events in earlier studies may have been overestimated due to patient recall over periods of many months. However, despite the low rates of hypoglycaemia observed in COACH study participants, there was still a marked decrease in frequency of clinically significant events during the non‐adjunctive RT‐CGM phase.
Unlike the previous studies that focused on participant with T1D with problematic or high risk of hypoglycaemia and that provided protocol‐specific RT‐CGM training across multiple sessions, 16 the COACH study included a broad population of insulin users with T1D and T2D whose device training and diabetes management during the SMBG and RT‐CGM phases were per the site's usual practice. Regardless of participant's type of diabetes or hypoglycaemia awareness or history, a reduction in consequential hypoglycaemic events occurred during the RT‐CGM phase (Table 2).
Study strengths were the large number of participants and study sites from across the United States, the broad inclusion criteria, and the study design that allowed each participant to act as their own control. Limitations include is that we did not capture actual SMBG behaviours during the initial 6‐month study period, potentially biasing the results. However, baseline rates of severe hypoglycaemia were so low that SMBG behaviours were likely not confounding. Additionally, specifics of RT‐CGM use (during the CGM phase) including threshold alert settings, feature‐set use, and how or if CGM was used as a behaviour modification tool were not collected. A second limitation is that the usual care afforded by participating sites may not be representative of the care typically provided to insulin‐requiring patients by primary care physicians. In addition, it remains possible that the impact of RT‐CGM on hypoglycaemic event frequency may have been even more pronounced with current‐generation, factory‐calibrated RT‐CGM systems. For instance, previous work has reported that transitioning from the G5 to the G6 system is associated with a decrease in the proportion of time spent in hypoglycaemia. 11
RT‐CGM is an important tool in diabetes management that can guide insulin dosing decisions and help improve glycaemic control. The COACH study evaluated the safety and efficacy of non‐adjunctive use of the Dexcom G5 system for diabetes management and was powered to assess the impact of RT‐CGM on clinically significant moderate and severe hypoglycaemic events. This study is important for understanding the risks of these clinically meaningful events in participant with insulin‐requiring T1D or T2D. Compared to SMBG, non‐adjunctive RT‐CGM use reduced rates of hypoglycaemic events in participants receiving care according to sites’ usual practice. Further analysis will be performed to evaluate the paediatric population, CGM‐based outcomes, patient reported outcomes and economic implications.
PRIOR PUBLICATION
Parts of this work were presented in abstract form at the 81st Scientific Sessions of the American Diabetes Association, June 25–29, 2021 (69‐LB and 76‐LB).
CONFLICT OF INTEREST
Drs. Beck and Price are employees and shareholders of Dexcom, Inc. Dr. Kelly has received consulting fees from Dexcom, Inc.
AUTHOR CONTRIBUTIONS
All authors participated in the research and preparation of the manuscript. SEB was involved in conceptualization, methodology and supervision. CK was involved in statistical analysis, data curation and validation. DAP was involved in funding acquisition, conceptualization, methodology, and writing—reviewing and editing. DAP is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis.
ACKNOWLEDGEMENTS
The authors thank Jeffrey Bowers, Jeremy Gorelick, Nelly Njeru, John Welsh, Christy Chao, Sarah Puhr and Andrew Balo of Dexcom, and Diana Le of Kelly Statistical Consulting for assistance with conducting and reporting the study. Dexcom is a registered trademark of Dexcom, Inc., in the United States and/or other countries.
APPENDIX 1.
Sites and principal investigators of the COACH study group are as follows:
Research Institute of Dallas: Stephen Aronoff, MD; Vanderbilt Diabetes and Obesity Clinical Trials Center: Shichun Bao, MD, PhD; Iowa Diabetes & Endocrinology Research Center: Anuj Bhargava, MD; Amarillo Medical Specialists, LLP: William Biggs, MD, PhD; Northshore University Health System: Liana Billings, MD; Texas Diabetes and Endocrinology: Thomas Blevins, MD; Atlanta Diabetes Associates: Bruce WBode, MD; International Diabetes Center Research: Anders Carlson, MD; William Sansum Diabetes Center: Kristin Castorino, DO; Diabetes and Endocrine Associates: Raymond Fink, MD; Texas Diabetes and Endocrinology: Lindsay Harrison, MD; Mills‐Peninsula Medical Center, Dorothy L. and James E. Frank Diabetes Research Institute: David Klonoff, MD; Diabetes & Endocrine Associates: Sarah Konigsberg, MD; Mountain Diabetes and Endocrine Center: Wendy Lane, MD; Rocky Mountain Diabetes & Osteoporosis Center: David Liljenquist, MD; Texas Diabetes and Endocrinology: Kerem Ozer, MD; Center of Excellence in Diabetes and Endocrinology: Gnanagurudasan Prakasam, MD, MRCP, MHA; Whittier Institute for Diabetes: Athena Tsimikas, MD; Advanced Research Institute: Jack Wahlen, MD.
Beck SE, Kelly C, Price DA; for the COACH Study Group . Non‐adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): Results of a post‐approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
Funding information
Dexcom, Inc. provided funding and devices for the study.
Contributor Information
Stayce E. Beck, Email: stayce.beck@dexcom.com.
for the COACH Study Group:
Stephen Aronoff, Shichun Bao, Anuj Bhargava, William Biggs, Liana Billings, Thomas Blevins, Bruce W Bode, Anders Carlson, Kristin Castorino, Raymond Fink, Lindsay Harrison, David Klonoff, Sarah Konigsberg, Wendy Lane, David Liljenquist, Kerem Ozer, Gnanagurudasan Prakasam, Athena Tsimikas, and Jack Wahlen
REFERENCES
- 1. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract. 1995;28(2):103‐117. [DOI] [PubMed] [Google Scholar]
- 2. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 3. DCCT Research Group . Hypoglycemia in the diabetes control and complications trial. The diabetes control and complications trial research group. Diabetes. 1997;46(2):271‐286. [PubMed] [Google Scholar]
- 4. Mathieu C. Minimising hypoglycaemia in the real world: the challenge of insulin. Diabetologia. 2021;64(5):978‐984. [DOI] [PubMed] [Google Scholar]
- 5. Bode BW, Schwartz S, Stubbs HA, Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care. 2005;28(10):2361‐2366. [DOI] [PubMed] [Google Scholar]
- 6. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cariou B, Fontaine P, Eschwege E, et al. Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin‐treated type 2 diabetes mellitus patients in a real‐life setting: results from the DIALOG study. Diabetes Metab. 2015;41(2):116‐125. [DOI] [PubMed] [Google Scholar]
- 8. Ratzki‐Leewing A, Harris S, Zou G, Ryan B. Real‐world estimates of severe hypoglycemia and associated healthcare utilization in the US: baseline results of the iNPHORM study. Diabetes Technol Ther. 2021;23(S2):A‐140. [Google Scholar]
- 9. Acciaroli G, Welsh JB, Akturk HK. Mitigation of rebound hyperglycemia with real‐time continuous glucose monitoring data and predictive alerts. J Diabetes Sci Technol. 2021;1932296820982584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akturk HK, Dowd R, Shankar K, Derdzinski M. Real‐world evidence and glycemic improvement using Dexcom G6 features. Diabetes Technol Ther. 2021;23(S1):S21‐S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Puhr S, Derdzinski M, Welsh JB, Parker AS, Walker T, Price DA. Real‐world hypoglycemia avoidance with a continuous glucose monitoring system's predictive low glucose alert. Diabetes Technol Ther. 2019;21(4):155‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association . 7. Diabetes technology: standards of medical care in diabetes‐2021. Diabetes Care. 2021;44(Suppl 1):S85‐S99. [DOI] [PubMed] [Google Scholar]
- 13. Grunberger G, Sherr J, Allende M, et al. American association of clinical endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27(6):505‐537. [DOI] [PubMed] [Google Scholar]
- 14. Riddlesworth T, Price D, Cohen N, Beck RW. Hypoglycemic event frequency and the effect of continuous glucose monitoring in adults with type 1 diabetes using multiple daily insulin injections. Diabetes Ther. 2017;8(4):947‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olafsdottir AF, Polonsky W, Bolinder J, et al. A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD‐3). Diabetes Technol Ther. 2018;20(4):274‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heinemann L, Freckmann G, Ehrmann D, et al. Real‐time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367‐1377. [DOI] [PubMed] [Google Scholar]
- 17. Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388‐2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group . A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care. 2021;44(2):464‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visser MM, Charleer S, Fieuws S, et al. Comparing real‐time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6‐month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397(10291):2275‐2283. [DOI] [PubMed] [Google Scholar]
- 21. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open‐label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893‐902. [DOI] [PubMed] [Google Scholar]
- 22. FDA advisory panel votes to recommend non‐adjunctive use of Dexcom G5 mobile CGM. Diabetes Technol Ther. 2016;18(8):512‐516. [DOI] [PubMed] [Google Scholar]
- 23. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371‐378. [DOI] [PubMed] [Google Scholar]
- 24. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379‐387. [DOI] [PubMed] [Google Scholar]
- 25. Šoupal J, Petruželková J, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow‐up from the COMISAIR study. Diabetes Care. 2020;43:37‐43. [DOI] [PubMed] [Google Scholar]
- 26. Billings LK, Parkin CG, Price D. Baseline glycated hemoglobin values predict the magnitude of glycemic improvement in patients with type 1 and type 2 diabetes: subgroup analyses from the DIAMOND study program. Diabetes Technol Ther. 2018;20(8):561‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olafsdottir AF, Bolinder J, Heise T, et al. The majority of people with type 1 diabetes and multiple daily insulin injections benefit from using continuous glucose monitoring: an analysis based on the GOLD randomized trial (GOLD‐5). Diabetes Obes Metab. 2021;23(2):619‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]


