Conflicts of interest
CR has received compensation as a speaker, consultant, or advisor for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Dermira, Dr Reddys, Janssen, LEO Pharma, Eli Lilly and Company, Novartis, Regeneron‐Sanofi, and UCB. LG has been a consultant, investigator, and speaker for AbbVie, Amgen, Celgene, Eli Lilly and Company, Janssen, LEO Pharma, Merck, and Pfizer; has been a speaker and consultant for Valeant and Tribute; and has been an investigator for UCB and Sun Pharmaceuticals. PF has served as an investigator, speaker, advisor, or received travel/grant/research support from 3M/iNova/Valeant, Abbott/AbbVie, Amgen, Arcutis, Ascent, Aslan, Aspen, Australian Ultraviolet Services, Biogen Idec, Boehringer Ingelheim, Botanix, Bristol‐Myers Squibb, Celgene, Celtaxsys, Clinuvel, Cutanea, Dermira, CSL, Eli Lilly and Company, Galderma, Genentech, GlaxoSmithKline/Stiefel, Hexima, Janssen‐Cilag, LEO Pharma/Peplin, Merck Serono, Novartis, Regeneron, Reistone, Roche, Sanofi, Schering‐Plough/MSD, Sun Pharma, UCB Pharma, Valeant, and Wyeth/Pfizer. JW has been an investigator for AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Eli Lilly and Company, GlaxoSmithKline, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Stiefel, and Valeant; has been a speaker and served on an advisory board for AbbVie, Eli Lilly and Company, and Janssen. RTB, GG, KS, MM‐M, and CCB are full‐time employees and shareholders of Eli Lilly and Company. JFM is a consultant and/or investigator for Merck, Bristol‐Myers Squibb, AbbVie, Dermavant, Eli Lilly and Company, Novartis, Janssen, UCB, Sanofi, Regeneron, Arena, Sun Pharma, Biogen, Pfizer, EMD Sorono, Avotres, and LEO Pharma.
Funding source
None.
Editor
Genital psoriasis (GenPs) has been reported in up to 63% of patients with psoriasis. It is often associated with significant impairment of health‐related quality of life (HRQoL), 1 , 2 including a significantly greater impact on sexual health than psoriasis with no genital involvement. 1 , 3 Despite its prevalence and burden, GenPs is often underdiagnosed and undertreated. 4 , 5
Ixekizumab (IXE), a high‐affinity monoclonal antibody selectively targeting interleukin‐17A (IL‐17A), is the only United States Food and Drug Administration‐approved treatment for patients with moderate‐to‐severe plaque psoriasis that includes labelling information about successful treatment of patients with genital involvement. 6 In the IXORA‐Q trial, IXE provided significant improvements in HRQoL and in the sexual impact of GenPs following 12 weeks of treatment. 7 , 8 Here, we report the persistence of these improvements through 52 weeks.
Patient eligibility criteria have been previously published 6 , 7 , 8 ; 149 patients were enrolled in the induction period (Weeks 0–12); patients randomised to either placebo (PBO) or Ixekizumab (IXEQ2W); most had GPSIS‐Impact scores of ≥3 (moderate‐to‐very high), nearly one‐third of patients had GPSIS‐Avoidance score of 5 (always avoid sexual activity due to GenPs). At Week 12, 93% of patients (PBO: n = 65; IXE Q2W: n = 74) entered the open‐label treatment period wherein all patients received IXE 80 mg every 4 weeks (Q4W) until Week 52, with initial PBO randomised patients receiving a 160 mg IXE starting dose. Dosing could be increased if necessary to achieve or maintain satisfactory disease control. 6
Patients treated with IXE achieved persistent improvements in HRQoL, as measured by DLQI (0,1) and multiple domains of SF‐36, and in the sexual impact of GenPs, at Week 12; these improvements persisted through Week 52. In the group that received IXE in both periods (IXE/IXE), the proportion of patients with GPSIS‐Avoidance (1,2) response persisted through Week 52, with 80% (n = 24/30) achieving GPSIS‐Avoidance (1,2). Upon switching from PBO to IXE, GPSIS‐Avoidance (1,2) responses rapidly increased from 26% (n = 9/35) (Week 12) to 72% (n = 21/29) (Week 16) and 79% (n = 23/29) (Week 52) (Fig. 1a). Upon switching from PBO to IXE, GPSIS‐Impact (1,2) response rapidly increased from 53% (n = 9/171) (Week 12) to 94% (15/16) (Week 16) and persisted through Week 52 (93%, n = 13/14). GPSIS‐Impact (1,2) responses were numerically higher in the PBO/IXE group during the open‐label treatment period (Fig. 1b).
Figure 1.
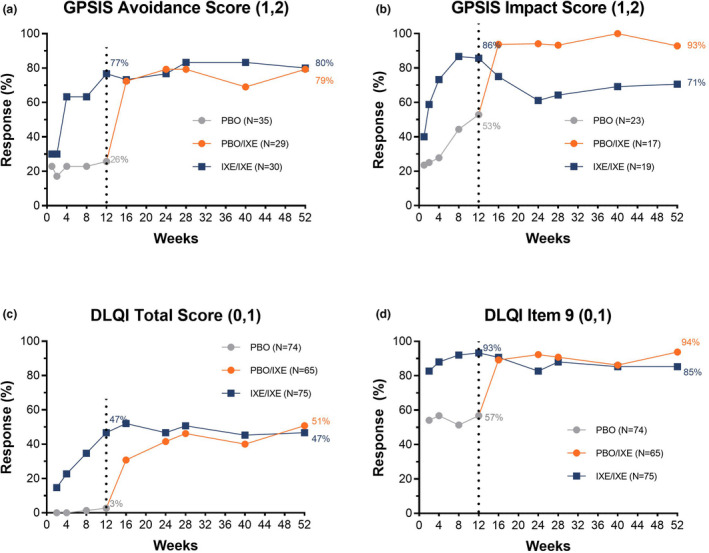
GPSIS‐Avoidance (1,2), and GPSIS‐Impact (1,2) outcomes, along with DLQI (0,1) and DLQI item 9 (0,1) responses rates, through Week 52 of IXORA‐Q (non‐responder imputation [based on patients with Mx + Px1 for GPSIS‐impact]) including blinded T2T and open‐label T2T periods. (a) Proportion of patients who reported that they never (1) or rarely (2) avoided sexual activity due to GenPs (GPSIS‐Avoidance [1,2]) [based on patients with GPSIS‐avoidance score ≥3 at baseline]; (b) Proportion of patients who reported a very low or not at all (1) or low (2) level of worsening of GenPs symptoms following sexual activity (GPSIS‐Impact [1,2]) [based on patients with GPSIS‐avoidance score ≥3 at baseline]; (c) Proportion of patients achieving a response of (0,1) in DLQI total score indicating no effect on HRQoL; (d) Proportion of patients achieving a response of (0,1) on DLQI item 9, indicating ‘no’ (0) or ‘a little’ (1) sexual difficulties due to skin. The dotted line separates the double‐blind treatment period (Weeks 0–12) and the open‐label treatment period (Weeks 12–52).
In the IXE/IXE population, 47% of patients achieved a DLQI (0,1) at Week 12 that persisted through Week 52 (Fig. 1c). DLQI item 9 (0,1) was achieved by 85% of IXE/IXE patients at Week 52 (Fig. 1d). 51% of patients in the PBO/IXE group achieved DLQI (0,1) at Week 52, and 94% of patients achieved DLQI item 9 (0,1) at Week 52 (Fig. 1c and 1d).
At Week 12, IXE treatment resulted in significantly greater mean change from baseline in six SF‐36 domains, 9 and the physical component summary (PCS; Table 1). Upon switching to IXEQ4W, the mean change from baseline for all SF‐36 domains and both component summary scores increased from Week 12 to Week 52 in the PBO/IXE group. In the IXE/IXE group, improvements observed at Week 12 generally persisted through Week 52 but were numerically lower.
Table 1.
Mean change from baseline in SF‐36 domains at Weeks 12 and 52
| Domain | Week 12 | Week 52 | |||
|---|---|---|---|---|---|
| PBO/IXE (N = 65) LSM (±SE) | IXE/IXE (N = 74) LSM (±SE) | P‐value | PBO/IXE (N = 65) Mean (±SE) | IXE/IXE (N = 74) Mean (±SE) | |
| Physical functioning | 1.7 (1.8) | 9.7 (1.8) | 0.002 | 10.6 (3.0) | 5.9 (2.1) |
| Role limitations due to physical health | 1.3 (2.0) | 13.5 (2.0) | <0.001 | 10.10 (3.7) | 9.8 (2.8) |
| Bodily pain | 4.1 (2.5) | 20.9 (2.5) | <0.001 | 19.6 (3.7) | 13.9 (3.3) |
| General health perceptions | 2.6 (1.6) | 5.5 (1.6) | 0.215 | 5.2 (2.5) | 3.7 (1.7) |
| Vitality | 2.9 (1.9) | 7.6 (1.9) | 0.081 | 9.4 (2.7) | 4.5 (2.2) |
| Social functioning | 2.9 (2.1) | 14.5 (2.1) | <0.001 | 16.2 (3.3) | 9.5 (3.3) |
| Role limitations due to emotional problems | 4.1 (1.6) | 9.4 (1.6) | 0.02 | 10.1 (3.0) | 4.4 (2.6) |
| Mental health | 3.7 (1.3) | 7.5 (1.3) | 0.041 | 6.5 (2.0) | 5.9 (1.8) |
| PCS | 0.7 (0.8) | 5.2 (0.8) | <0.001 | 4.5 (1.2) | 2.5 (1.1) |
| Mental health component summary (MCS) | 2.2 (0.7) | 4.0 (0.7) | 0.085 | 4.9 (1.3) | 3.6 (0.9) |
Data is change from baseline (mBOCF). The ANCOVA model includes treatment, baseline BSA category, and baseline value.
Abbreviations: BSA, body surface area; IXE, ixekizumab; LSM, least squares mean; mBOCF, modified baseline observation carried forward; MCS, mental health component summary; PBO, placebo; PCS, physical component summary; SE, standard error.
The rapid onset of efficacy and significant improvement of signs and symptoms of GenPs with IXE, were reported previously. 7 , 8 Our results suggest IXE‐treated patients achieved significant clinical improvements in GenPs symptoms, HRQoL, and the sexual impact of GenPs that persisted for up to 1 year, supporting the previous results of the IXORA‐Q study and IXE as an efficacious treatment option for moderate‐to‐severe GenPs.
Acknowledgements
Writing support was provided by Gina Moore, MS, and Anitha Alex, PhD, employees of Syneos Health, and Geraldine Fahy, PhD, employee of Eli Lilly. The patients in this letter have given written informed consent to publication of their case details.
Footnotes
Response for GPSIS‐impact analysis based on the Mx+Px; abbreviations: Mx=number of patients who report being sexually active on the GPSIS Item 1; Px=number of patients who do not report on GPSIS Item 1.
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, except for pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1. Ryan C, Sadlier M, De Vol E et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol 2015; 72: 978–983. 10.1016/j.jaad.2015.02.1127. [DOI] [PubMed] [Google Scholar]
- 2. Cather JC, Ryan C, Meeuwis K et al. Patients' perspectives on the impact of genital psoriasis: a qualitative study. Dermatol Ther (Heidelb) 2017; 7: 447–461. 10.1007/s13555-017-0204-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meeuwis KA, de Hullu JA, van de Nieuwenhof HP et al. Quality of life and sexual health in patients with genital psoriasis. Br J Dermatol 2011; 164: 1247–1255. 10.1111/j.1365-2133.2011.10249.x. [DOI] [PubMed] [Google Scholar]
- 4. Meeuwis KA, van de Kerkhof PC, Massuger LF, de Hullu JA, van Rossum MM. Patients' experience of psoriasis in the genital area. Dermatology 2012; 224: 271–276. 10.1159/000338858. [DOI] [PubMed] [Google Scholar]
- 5. Wylie G, Evans CD, Gupta G. Reduced libido and erectile dysfunction: rarely reported side‐effects of methotrexate. Clin Exp Dermatol 2009; 34: e234. 10.1111/j.1365-2230.2008.03082.x. [DOI] [PubMed] [Google Scholar]
- 6. Guenther L, Potts Bleakman A, Weisman J et al. Ixekizumab Results in Persistent Clinical Improvement in Moderate‐to‐Severe Genital Psoriasis During a 52 Week, Randomized, Placebo‐Controlled, Phase 3 Clinical Trial. Acta Derm Venereol. 2020; 100: adv00006. doi: 10.2340/00015555-3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yosipovitch G, Foley P, Ryan C et al. Ixekizumab improved patient‐reported genital psoriasis symptoms and impact of symptoms on sexual activity vs placebo in a randomized, double‐blind study. J Sex Med. 2018; 15: 1645–1652. doi: 10.1016/j.jsxm.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 8. Ryan C, Menter A, Guenther L et al. Efficacy and safety of ixekizumab in a randomized, double‐blinded, placebo‐controlled phase IIIb study of patients with moderate‐to‐severe genital psoriasis. Br J Dermatol 2018; 179: 844–852. doi: 10.1111/bjd.16736. [DOI] [PubMed] [Google Scholar]
- 9. Maruish MKM, Bjorner J, Gandek B, Turner‐Bowker DM, Ware JE. User's Manual for the SF‐36v2 Health Survey, 3rd edn. Quality Metric Incorporated, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, except for pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.


