Abstract
Azathioprine (AZA) is the preferred immunosuppressant for treating pemphigus vulgaris (PV), with discontinuation mainly attributed to hematological adverse events (AE). Reportedly, nucleoside diphosphate‐linked moiety X‐type motif 15 (NUDT15) polymorphisms have been strongly associated with thiopurine‐induced leukopenia. To investigate hematological AE of low‐dose AZA based on NUDT15 genotypes among patients with PV, a prospective cohort study was conducted in patients with PV, followed‐up for the first 8 weeks after AZA administration. All patients were divided into wild homozygous and heterozygous NUDT15 groups. Both groups initiated AZA at low dose (50 mg/day) and continued with different dose‐escalating approaches. Bone marrow suppression was considered the principal outcome. Overall, 62 patients with PV were enrolled (48 in the wild homozygous NUDT15 group vs. 14 in the heterozygous NUDT15 group). Except for median maintenance doses of AZA, no statistically significant differences were observed between the two groups in terms of age, sex, white blood cells, neutrophil count, platelet count, hemoglobin level, median final doses of corticosteroids (mg prednisone equivalent), pemphigus disease area index, and anti‐desmoglein 1/3 autoantibodies. In both groups, patients presented similar hematological AE and treatment responses after administration of different low‐dose AZA treatment strategies. Low‐dose AZA based on NUDT15 genotypes can reduce the risk of early hematological AE among patients with PV.
Keywords: adverse events, azathioprine, leukopenia, NUDT15, pemphigus vulgaris
1. INTRODUCTION
Pemphigus vulgaris (PV) is an acquired autoimmune disease, characterized by erythema, loose blisters, and erosions found to occur on the mucosa and skin. 1 , 2 In the early 1950s, approximately 75% of patients with PV reportedly died within a year. 3 Following the introduction of corticosteroid treatment, the mortality rate decreased to 15–45%. In the late 1960s, researchers observed that the combined use of corticosteroids and adjuvant immunosuppressants further decreased mortality attributed to PV to 5–10%. 3 , 4 , 5 The latest PV management guidelines recommend azathioprine (AZA) as the preferred immunosuppressant, with strength of recommendation B. 6 , 7 , 8 , 9 AZA exerts pharmacological effects by interfering with purine biosynthesis and modifying the DNA structure via the incorporation of thiopurine analogs into nucleic acids. 10 Following absorption, AZA first converts to 6‐mercaptopurine via a non‐enzymatic reaction. Next, AZA is metabolized to 6‐thioguanine nucleotides (6‐TGN) through three major enzymatic pathways, mediated by xanthine oxidase (XO), thiopurine S‐methyltransferase (TPMT), or hypoxanthine phosphoribosyltransferase (HGPRT), respectively. 11 , 12 During this process, the generation of an excessive serum concentration of 6‐TGN could result in a series of severe adverse events (AE), including hematological, hepatic, and pancreatic toxicities. 13 Several studies have revealed that TPMT variants are strongly associated with AZA‐induced AE. 14 , 15 , 16 To date, multiple TPMT variants have been reported, including TPMT*1, TPMT*2, TPMT*3A, TPMT*3B, TPMT*3C, and TPMT*4; 17 , 18 , 19 , 20 , 21 TPMT*3C is the most frequently observed variant among East Asians. 20 However, TPMT variants have been detected in only 3% of East Asians, 17 , 19 , 20 , 21 which fails to explain the high prevalence (>30%) of AZA‐induced bone marrow suppression observed among East Asian patients. 17 , 22 In 2014, Yang et al. 21 first reported that nucleoside diphosphate‐linked moiety X‐type motif 15 (NUDT15) polymorphisms are strongly associated with thiopurine‐induced leukopenia in Korean patients with inflammatory bowel disease (IBD). NUDT15 is a 164 amino acid protein that can prevent 8‐oxo‐guanine from being incorporated into DNA, thereby eliminating thiopurine‐associated AE. 23 Since then, an increasing number of studies have revealed that NUDT15 is a more common risk factor associated with AZA‐induced hematological AE among Asian patients than TPMT. 21 , 24 , 25 , 26 However, most of these studies were restricted to patients with gastrointestinal tract diseases or hematonosis, and few performed in patients with PV. 27 Herein, we describe a prospective cohort study in patients with PV at our hospital, evaluating the safety of different low‐dose AZA treatment strategies. Specifically, we aimed to identify whether NUDT15 (rs116855232) or TPMT*3C (rs1142345) polymorphisms are more common among Chinese individuals. As the first 8 weeks are reportedly key periods for the occurrence of early bone marrow suppression after AZA administration, 24 , 25 we investigated hematological AE during these first 8 weeks. Simultaneously, we briefly evaluated other AE, as well as the efficacy of treatment strategies employed.
2. METHODS
2.1. Study sites
This prospective cohort study was conducted at the Department of Dermatology, West China Hospital (WCH), Sichuan University, China, from 1 July 2016 to 31 August 2018. This mono‐centric study was performed at a 4950‐bed tertiary teaching hospital, one of the largest single‐site hospitals in the world. Study participants were from all over mainland China, with diverse ethnic and demographic backgrounds. The study protocol was approved by the Ethics Committee of WCH, Sichuan University (2017, no. 96) and registered in the Chinese Clinical Trial Registry (ChiCTR‐OIC‐1701175).
2.2. Study participants and enrollment criteria
All participants were diagnosed with PV at WCH during the study period and were recruited consecutively following signed informed consent, considering strict inclusion and exclusion criteria. The inclusion criteria were as follows: (i) aged 18–75 years; and (ii) confirmed PV. Diagnosis required clinical presentation, PV‐consistent histopathology, and either positive direct immunofluorescence (DIF) or serological detection of immunoglobulin (Ig)G autoantibodies against desmoglein (Dsg)3 and/or Dsg1 by enzyme‐linked immunosorbent assay (ELISA). For PV, the clinical presentation includes flaccid blisters and erosions involving the skin and/or oral mucosa, and histopathology involving epidermal acantholysis; in terms of DIF microscopy, either IgG and/or complement component 3 deposits can be observed at the keratinocyte cell membrane.
The following patients were excluded from the study: (i) those presenting contraindications for AZA therapy, including the presence of homozygous variants of TPMT*3C or NUDT15; (ii) those diagnosed with hematological diseases, especially leukopenia; (iii) women who were pregnant or had plans to get pregnant; (iv) those with clear indications of infections, malignant tumors, or chronic diseases, including severe hepatopathy, chronic renal failure, cardiovascular/pulmonary diseases, and neurological/psychiatric disorders; (v) those who were reluctant to provide informed consent or were unable to complete the entire study.
2.3. Determination of TPMT*3C and NUDT15 genotypes
For each patient, genotypes of TPMT*3C and NUDT15 were determined before AZA treatment. DNA, extracted from peripheral blood samples, was employed for TPMT*3C and NUDT15 genotyping using a TaqMan® double fluorescence probe hybridization assay, performed at the Department of Laboratory Medicine at our hospital. TaqMan PCR reactions proceeded in 30 µL TaqMan Universe PCR Master Mix (SINO‐ERA JIYIN TECH CO.LTD) containing approximately 30 ng genomic DNA, 0.1 µM TaqMan probes. The sequences of the TaqMan probes for TPMT*3C (rs1142345) were FAM‐GTAAGTAGA(T)ATAACTT‐BHQ1 and HEX‐GTAAGTAGA(C)ATAACTT‐BHQ1, and the TaqMan probes for NUDT15 (rs116855232) were FAM‐TTCTGGGGACTG(T)GTTGTTTAAAA‐BHQ1 and HEX‐TTCTGGGGACTG(C)GTTGTTTAAAA‐BHQ1. The TPMT*3C variants (8/171 or 4.7% with heterozygote alone) were lower than NUDT15 variants (45/171 or 26.3%) in the enrolled patients (Table S1; see Supplementary Information). Our study focused on patients with NUDT15 variants to evaluate the hematological safety of different AZA treatment strategies described below.
2.4. Treatment protocols
According to the strict inclusion and exclusion criteria described above, 48 patients with wild homozygous NUDT15 (wild‐type group) and 14 patients with heterozygous NUDT15 (heterozygous group) were included in the present study (62 patients were all with wild homozygous TPMT*3C) (Figure 1). In the first 4 weeks, AZA was initiated at a low dose (50 mg/day) in both groups during the first week. 9 , 28 , 29 The wild‐type group underwent a dose‐escalation approach (increased dose by 25 mg weekly to the target dose at 100 mg/day) mainly used and approved by gastroenterologists for the treatment of IBD, with proven safe and efficacious results. 28 , 29 , 30 , 31 , 32 A less radical and more conservative regimen was performed in the heterozygous group, including a constant AZA dose of 50 mg/day between week 2 and 4. Weekly routine blood tests were performed during this period. In the following 4 weeks, AZA was increased by 25 mg biweekly until the target dose (100 mg/day) was attained, and white blood cell (WBC) levels were monitored every 2 weeks.
FIGURE 1.

AZA treatment strategies between two groups of PV patients with wild homozygous or heterozygous NUDT15. aObvious drop defined as two successive drops of WBC over 3 × 109/L. bAcceptable drop defined as two successive drops of WBC within 3 × 109/L. Abbreviations: AZA, azathioprine; NUDT15, nucleoside diphosphate‐linked moiety X‐type motif 15; PV, pemphigus vulgaris; WBC, white blood cell
2.5. Treatment outcome measures
In the present study, bone marrow suppression (especially leukopenia) was considered the principal AE. According to the World Health Organization (WHO) criteria (Table S2) and studies related to AZA‐induced hematological AE (Table S3), bone marrow suppression was defined as follows: blood WBC count less than 3.5 × 109/L, neutrophil (NEU) count less than 1.5 × 109/L, platelet (PLT) count less than 100 × 109/L, or hemoglobin (Hb) less than 100 g/L, with leukopenia defined as a WBC count less than 3.5 × 109/L.
Secondary AE included hepatotoxicity (defined as alanine aminotransferase and/or aspartate aminotransferase ≥2 times the normal upper limit) and pancreatitis and were assessed by biochemical tests performed at week 0, 4, and 8. Other secondary AE included influenza‐like symptoms (dizziness, fever, chills, and general malaise), gastrointestinal AE (nausea, vomiting, reduced appetite, diarrhea, and stomachache), hair loss, skin rash, myalgia, and arthralgia, collected by clinicians during the follow‐up period.
Furthermore, evaluation of the pemphigus disease area index (PDAI) score and anti‐Dsg1/Dsg3 IgG autoantibodies by ELISA was performed at week 0, 4, and 8 to determine treatment response.
2.6. Statistical analysis
To describe baseline characteristics of the two groups, the mean ± standard deviation (SD) were employed for normally distributed variables and percentages for categorical variables. Additionally, we used an independent‐sample t‐test to compare baseline quantitative variables between the two groups and the χ2‐test for qualitative variables.
To assess bone marrow suppression and treatment response changes during the 8 weeks, line charts were employed to visually express changes in WBC, NEU, PLT, Hb, anti‐Dsg1/Dsg3 autoantibodies, and PDAI. Next, the outcome of these indicators was longitudinally measured at different time points, comparing whether these indicators were also affected by other confounding factors between the two groups; therefore, multilevel linear regression analysis (MLRA) was adopted (formula in Supplementary Information). 33 In the model, bone marrow suppression and treatment response indicators were set as dependent variables and a categorical variable of the group was set as an independent variable; other variables such as age, sex, corticosteroid dose, and time were also adjusted for possible confounding effects.
Finally, we compared the incidence of bone marrow suppression/leukopenia between the two groups using the χ2‐test or Fisher’s exact test. p < 0.05 was considered significant. All statistical analyses were performed using the R software (version 3.5.3; Foundation for Statistical Computing).
3. RESULTS
3.1. Patient characteristics
Overall, 62 eligible patients with PV were included in the present study. Patients were divided into two groups, including 48 patients in the wild homozygous NUDT15 group and 14 patients in the heterozygous NUDT15 group. The median maintenance doses of the patients with heterozygous NUDT15 (50 mg/day) were significantly lower than patients with wild homozygous NUDT15 (100 mg/day) (p < 0.001) from 1st to 8th week. Except for median maintenance doses of AZA, no other characteristics like demographic characteristics (age and sex), baseline laboratory measurements (blood cell counts), median final doses of corticosteroids (mg prednisone equivalent), or disease activity (PDAI) significantly differed between the two groups (Table 1).
TABLE 1.
Comparison of characteristics between two groups
| Indicators | Patients with heterozygous NUDT15 (n = 14) | Patients with wild homozygous NUDT15 (n = 48) | p‐value |
|---|---|---|---|
| Age, years | 43.36 ± 10.31 | 47.92 ± 12.32 | 0.212 |
| Sex | |||
| Male | 7 (50.00%) | 25 (52.08%) | 0.891 |
| Female | 7 (50.00%) | 23 (47.92%) | |
| Baseline laboratory findings | |||
| WBC, ×109/L | 11.22 ± 3.44 | 11.75 ± 3.84 | 0.654 |
| NEU, ×109/L | 8.30 ± 3.60 | 8.60 ± 3.77 | 0.788 |
| PLT, ×109/L | 228.57 ± 63.17 | 253.68 ± 81.72 | 0.295 |
| Hb, g/L | 148.57 ± 24.30 | 144.43 ± 17.73 | 0.485 |
| Anti‐Dsg1, µ/mL | 114.84 ± 96.18 | 96.24 ± 71.62 | 0.455 |
| Anti‐Dsg3, µ/mL | 128.67 ± 95.86 | 115.15 ± 64.92 | 0.554 |
| PDAI | 14.21 ± 15.70 | 10.38 ± 12.16 | 0.347 |
| Median maintenance doses of AZA, mg/day | 50 | 100 | <0.001 |
| Median final doses of corticosteroids, mg/day | 27.5 | 26.25 | 0.793 |
Abbreviations: anti‐Dsg1, anti‐desmoglein 1; anti‐Dsg3, anti‐desmoglein 3; AZA, azathioprine; Hb, hemoglobin; NEU, neutrophil; NUDT15, nucleoside diphosphate‐linked moiety X‐type motif 15; PDAI, pemphigus disease area index; PLT, platelet; WBC, white blood cell.
3.2. Changes in adverse events between two groups
Figure 2 presents changes in indicators of bone marrow suppression during the treatment period. In general, the indicators fluctuated more dramatically among patients with heterozygous NUDT15. All indicators decreased more substantially during weeks 2 and 3 in patients with heterozygous NUDT15. However, indicator lines between the two groups seemed to coincide after week 6. In total, eight of the 62 patients exhibited secondary AE (five patients exhibited hepatotoxicity, three patients exhibited influenza‐like symptoms) (Table S4). No patient exhibited pancreatitis, gastrointestinal side‐effects, hair loss, skin rash, myalgia, or arthralgia.
FIGURE 2.
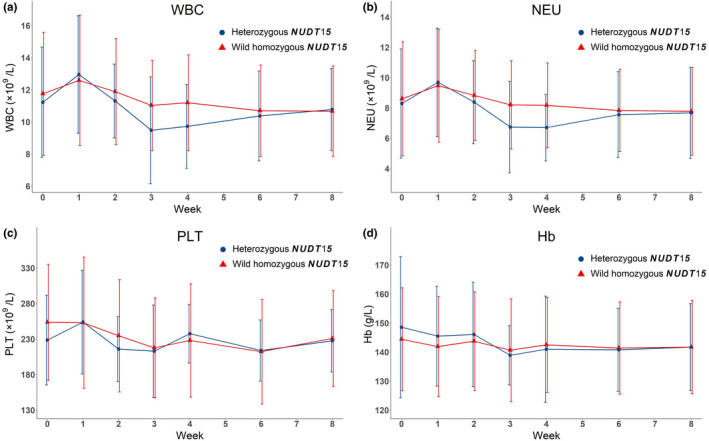
Changes in bone marrow suppression indicators (WBC, NEU, PLT, and Hb) between two groups within 8 weeks. Abbreviations: Hb, hemoglobin; NEU, neutrophil; NUDT15, nucleoside diphosphate‐linked moiety X‐type motif 15; PLT, platelet; WBC, white blood cell
3.3. Changes in treatment responses between two groups
Figure S1 shows changes in treatment response indicators. All indicators were decreased at each follow‐up visit. Based on grading criteria for disease severity by PDAI as established in Japan (0–8 for mild, 9–24 for moderate, and ≥25 for severe disease), 34 the condition was reduced from moderate to mild in both groups.
3.4. Comparison of changes in bone marrow suppression and treatment response indicators between two groups
The MLRA results on comparing bone marrow suppression and treatment response indicators between the two groups are shown in Table 2. After adjusting for possible confounding variables, we observed no significant difference in bone marrow suppression and treatment response indicators between the two groups. For instance, the WBC count of patients with heterozygous NUDT15 was 0.672 units less than that of patients with wild‐type NUDT15; however, the difference was not statistically significant. Similarly, NEU, PLT, Hb, anti‐Dsg1/Dsg3 autoantibodies, and PDAI were slightly lower or higher in patients with heterozygous NUDT15 than in another group, but differences were not statistically significant. These results indicated that heterozygous NUDT15 patients and wild homozygous NUDT15 patients had similar hematological AE after the administration of different treatments.
TABLE 2.
Comparison of changes in bone marrow suppression and treatment response indicators between two groups within 8 weeks
| WBC (×109/L) | NEU (×109/L) | PLT (×109/L) | Hb (g/L) | Anti‐Dsg1 (µ/mL) | Anti‐Dsg3 (µ/mL) | PDAI | |
|---|---|---|---|---|---|---|---|
| Fixed effects | |||||||
| Intercepts |
11.455 (1.682)*** |
6.654 (1.544)*** |
302.457 (43.732)*** |
147.417 (8.866)*** |
87.450 (44.497) |
87.422 (44.761) |
9.468 (6.073) |
| Age, years |
−0.052 (0.028) |
−0.020 (0.025) |
−1.817 (0.734)* |
−0.246 (0.149) |
−1.309 (0.714) |
−1.036 (0.734) |
−0.117 (0.101) |
| Sex | |||||||
| Male |
−0.714 (0.664) |
−0.319 (0.607) |
−17.808 (17.516) |
14.491 (3.557)*** |
−16.975 (17.341) |
−7.616 (17.527) |
−3.106 (2.284) |
| Female (ref.) | |||||||
| Corticosteroids, mg/day |
0.099 (0.021)*** |
0.098 (0.020)*** |
1.130 (0.485)* |
0.016 (0.097) |
2.284 (0.517)*** |
2.387 (0.499)*** |
0.208 (0.075) ** |
| Time, weeks |
−0.105 (0.047)* |
−0.078 (0.047) |
−2.163 (0.896)* |
−0.335 (0.177) |
−5.140 (0.875)*** |
−4.727 (0.798) |
−0.970 (0.163)*** |
| Group | |||||||
| Heterozygous NUDT15 |
−0.672 (0.688) |
−0.508 (0.628) |
−15.083 (18.264) |
0.578 (3.711) |
3.407 (17.907) |
5.252 (18.101) |
3.372 (2.239) |
| Wild homozygous NUDT15 (ref) | |||||||
| Random effects | |||||||
| Level‐2 residual variance a |
4.159 (0.888)*** |
3.287 (0.741)*** |
3220.241 (625.040)*** |
133.669 (25.087)*** |
2716.067 (572.359)*** |
3067.142 (612.570)*** |
37.737 (9.755)*** |
| Level‐1 residual variance |
5.047 (0.384)*** |
5.359 (0.409)*** |
1681.088 (127.089)*** |
64.666 (4.916)*** |
824.818 (128.774)*** |
685.385 (104.718)*** |
35.032 (4.978)*** |
| −2 log‐likelihood b | 1928.776 | 1933.542 | 4349.719 | 3024.885 | 1473.360 | 1524.704 | 1067.561 |
Numbers in parenthesis indicate the standard error. *p < 0.05; **p < 0.01; ***p < 0.001.
Abbreviations: anti‐Dsg1, anti‐desmoglein 1; anti‐Dsg3, anti‐desmoglein 3; Hb, hemoglobin; NEU, neutrophil; NUDT15, nucleoside diphosphate‐linked moiety X‐type motif 15; PDAI, pemphigus disease area index; PLT, platelet; WBC, white blood cell.
Level‐2 indicates individual patients; Level‐1 indicates measured weeks; if Level‐2 residual variances were statistically significant, multilevel linear regression analysis (MLRA) models were necessary and needed.
−2 log‐likelihood indicates the model fit.
3.5. Comparison of incidence of bone marrow suppression and leukopenia between two groups
We compared the number of patients who developed bone marrow suppression/leukopenia within the two groups (Table 3). Only one patient with heterozygous NUDT15 developed bone marrow suppression (owing to the reduced WBC, NEU, PLT), while four patients with wild‐type NUDT15 developed bone marrow suppression (mainly because of reduced PLT and Hb) (Table S5); however, this difference was not statistically significant. Among the 62 patients, only one patient in the heterozygous NUDT15 group developed leukopenia, failing to present statistical significance.
TABLE 3.
Comparison of incidence of bone marrow suppression/leukopenia between two groups within 8 weeks
| Patients with heterozygous NUDT15 | Patients with wild homozygous NUDT15 | p‐value | |
|---|---|---|---|
| Develop bone marrow suppression | |||
| Yes | 1 | 4 | 0.990 a |
| No | 13 | 44 | |
| Develop leukopenia | |||
| Yes | 1 | 0 | 0.226 b |
| No | 13 | 48 | |
Abbreviations: NUDT15, nucleoside diphosphate‐linked moiety X‐type motif 15.
Using χ2‐test with continuity correction (1 < expected count < 5).
Using Fisher’s exact test (the expected count <1).
4. DISCUSSION
Azathioprine has been recommended as the first corticosteroid‐sparing agent for patients with PV for over 20 years, 8 , 9 , 35 , 36 thus allowing historically high complete remission rates (28–45%) and low mortality rates (1.4–7%) with corticosteroid‐only treatment. 3 , 37 , 38 , 39 , 40 However, AE such as leukopenia may lead to life‐threatening infections that result in treatment discontinuation. For nearly 40 years, TPMT has been considered the most relevant gene in AZA‐induced leukopenia. 38 However, one study has reported that NUDT15 variants are more common among East Asians. 21 In our study sample, NUDT15 variants were found to predominate among Chinese patients when compared with TPMT*3C (26.32% vs. 4.68%), which was consistent with previous reports. 41 , 42
Although the association between NUDT15 and AZA‐induced hematological AE has been reported in gastrointestinal tract diseases and hematonosis, limited data is available in terms of dermatosis. Recently, our group presented a Chinese PV patient with heterozygous NUDT15 who developed leukopenia after AZA administration. 43 More recently, Shih et al. 44 have reported two other PV patients with homozygous variants of NUDT15 who developed severe bone marrow suppression. However, these were only limited to case reports. To our knowledge, this is the first cohort study presenting the hematological safety profiles of NUDT15 polymorphisms in Chinese patients with PV. Furthermore, although a general recommended AZA application method exists (2–3 mg/kg/day), 45 a specific and safe strategy to reach the therapeutic dose needs to be established. A survey regarding how gastroenterologists prescribe AZA reported that only 28% of physicians choose to start AZA at 2.5 mg/kg/day in clinical practice. 29 In East Asia, some gastroenterologists prefer to use a low starting dose (50 mg/day), and then gradually increase the dose. 28 , 32 In China, a step‐up strategy was performed by initiating AZA at 25 mg/day for 2 weeks, then increasing 25 mg biweekly. 32 Based on these studies, we considered that a gradual dose increment strategy is beneficial to reduce AZA‐induced hematological AE. Moreover, patients with PV typically require higher doses and prolonged corticosteroid treatment. The WBC‐decreasing effect of AZA would be compensated by the WBC‐increasing effect of the corticosteroids, 46 leading to normal WBC counts. This highlights the importance of monitoring the changing trends in WBC levels during AZA treatment. Therefore, we propose a treatment strategy that involves initiating AZA with a low dose, followed by dose adjustment and accompanied by monitoring the changing trend of WBC counts with therapeutic doses. Our study is the first to address how dermatologists can prescribe AZA specifically in clinical practice among patients with PV.
Following AZA treatment, we observed changes in WBC, NEU, PLT, and Hb levels between the two groups, with the mean values of the four indicators fluctuating during the first 6 weeks in the two groups; in week 3, these indicators revealed the lowest values, with the trend appearing to stabilize after week 6. This phenomenon was consistent with a previous report, in which the first 8 weeks were key periods for early leukopenia after AZA treatment, especially weeks 3 and 4.
Compared with the wild homozygous NUDT15 group, WBC, NEU, and PLT in patients with heterozygous NUDT15 were 0.672 × 109/L, 0.508 × 109/L, and 15.083 × 109/L lower, respectively; in contrast, Hb was 0.578 g/L higher. However, MLRA revealed that these indicators did not differ between the two groups. Moreover, the two patient groups demonstrated treatment responses following AZA therapy. These results indicated that the two treatment strategies had similar safety and efficacy.
Furthermore, we enumerated the number of patients who developed bone marrow suppression/leukopenia during the study period. One patient with heterozygous NUDT15 and four patients with wild homozygous NUDT15 developed bone marrow suppression, predominantly attributed to the reduced PLT and Hb levels. The lowest PLT and Hb values were 74 × 109/L and 96 g/L, respectively; and the results basically did not show symptoms and positive signs in practice. However, it is worth noting that the patient with heterozygous NUDT15 developed leukopenia (WBC 0.99 × 109/L) in week 3. AZA was immediately discontinued and the WBC count significantly improved after 1 week, implicating the possibility of other risk genes or influencing factors related to hematological AE of AZA. What is more, the incidence of leukopenia was quite lower in our study (1.6%) compared to other studies. 22 , 27 , 47 We considered the possible reasons were as follows: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for thiopurine dosing based on TPMT and NUDT15 genotypes recommended that for non‐malignant patients with homozygous mutation of TPMT/NUDT15 who have high risk of AZA‐related bone marrow suppression, AZA is avoided to be applied. For patients with heterozygous TPMT/NUDT15, AZA is used with reduced doses. CPIC also mentioned that clinicians should adjust doses of AZA based on monitoring of clinical myelosuppression and disease‐specific guidelines. 45 According to the guideline, all patients in our study firstly had TPMT*3C and NUDT15 tests and be filtered by our inclusion and exclusion criteria before taking AZA. Any patients with homozygous mutation of TPMT*3C/NUDT15 or heterozygous TPMT*3C were excluded. As for those with wild homozygous and heterozygous NUDT15, we prescribed different low‐dose‐escalating treatment strategies. Secondly, all patients taking AZA were regularly monitored with routine blood tests, and we would adjust the dose timely according to the test results. Based on the above, we have tried to reduce the incidence of leukopenia from three aspects: prevention, monitor, and adjustment. Besides, we could not deny the limitations of sample size in our single center study. Further analysis may require a larger sample size of patients in multi‐center in the future.
The present study included 62 patients, and it is crucial to expand the sample size in future investigations. Moreover, we only observed early hematological safety in the first 8 weeks, and a longer follow‐up period should be supplemented in the future. This study only involved NUDT15; other genes related to the metabolism of AZA need to be considered.
In conclusion, NUDT15 variants predominated over TPMT in Chinese patients and probably among all East Asians. Prescribing different low‐dose‐escalating treatment strategies for AZA according to NUDT15 polymorphisms, along with regular monitoring of routine blood tests, can reduce the risk of early hematological AE in patients with PV.
CONFLICT OF INTEREST
None declared.
Supporting information
Supplementary Material
Fig S1
ACKNOWLEDGMENTS
This work was supported by the Chengdu Science and Technology Program Projects (no. 2018‐CY02‐00058‐GX) and the 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (no. ZYJC18003). Author contributions were as follows: Wei Li and Yanhong Zhou contributed to the conception of the study and made constructive discussions in the manuscript; Xingli Zhou and Yiyi Wang mainly helped perform the study and wrote the manuscript; Liangliang Cheng, Ke Ju, and Tianjiao Lan performed the data analysis and Liangliang Cheng also contributed significantly to writing the manuscript; Hui Gou, Tongying Zhan, and Gaojie Li helped perform evaluation of pemphigus disease area index and record the occurrence of adverse events during the follow‐up period; and Yuanxia Gu, Yeting Sun, Yan Xu, and Yukun Sun contributed collecting laboratory results and record data.
Zhou X, Cheng L, Wang Y, Gou H, Ju K, Lan T, et al. Effect of NUDT15 polymorphisms on early hematological safety of low‐dose azathioprine in Chinese patients with pemphigus vulgaris: A prospective cohort study. J Dermatol. 2022;49:402–410. 10.1111/1346-8138.16265
Xingli Zhou, Liangliang Cheng, and Yiyi Wang contributed equally to this work. Yanhong Zhou and Wei Li contributed equally to this work.
REFERENCES
- 1. Cholera M, Chainani‐Wu N. Management of pemphigus vulgaris. Adv Ther. 2016;33:910–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang SY, Zhou XY, Zhou XL, Zhang Y, Deng Y, Liao F, et al. Subtype‐specific inherited predisposition to pemphigus in the Chinese population. Br J Dermatol. 2019;180:828–35. [DOI] [PubMed] [Google Scholar]
- 3. Bystryn JC, Steinman NM. The adjuvant therapy of pemphigus. An update. Arch Dermatol. 1996;132:203–12. [PubMed] [Google Scholar]
- 4. Martin LK, Werth VP, Villaneuva EV, Murrell DF. A systematic review of randomized controlled trials for pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol. 2011;64:903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fardet L, Fève B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74:1731–45. [DOI] [PubMed] [Google Scholar]
- 6. Hertl M, Jedlickova H, Karpati S, Marinovic B, Uzun S, Yayli S, et al. Pemphigus. S2 Guideline for diagnosis and treatment–guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2015;29:405–14. [DOI] [PubMed] [Google Scholar]
- 7. Committee for Guidelines for the Management of Pemphigus D , Amagai M, Tanikawa A, Shimizu T, Hashimoto T, Ikeda S, et al. Japanese guidelines for the management of pemphigus. J Dermatol. 2014;41:471–86. [DOI] [PubMed] [Google Scholar]
- 8. Harman KE, Brown D, Exton LS, Groves RW, Hampton PJ, Mohd Mustapa MF, et al. British Association of Dermatologists’ guidelines for the management of pemphigus vulgaris 2017. Br J Dermatol. 2017;177:1170–201. [DOI] [PubMed] [Google Scholar]
- 9. Murrell DF, Pena S, Joly P, Marinovic B, Hashimoto T, Diaz LA, et al. Diagnosis and management of pemphigus: recommendations of an international panel of experts. J Am Acad Dermatol. 2020;82:575–85.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol. 2006;55:369–89. [DOI] [PubMed] [Google Scholar]
- 11. Zabala‐Fernández W, Barreiro‐de Acosta M, Echarri A, Carpio D, Lorenzo A, Castro J, et al. A pharmacogenetics study of TPMT and ITPA genes detects a relationship with side effects and clinical response in patients with inflammatory bowel disease receiving Azathioprine. J Gastrointestin Liver Dis. 2011;20:247–53. [PubMed] [Google Scholar]
- 12. Gisbert JP, Gomollón F, Cara C, Luna M, González‐Lama Y, Pajares JM, et al. Thiopurine methyltransferase activity in inflammatory bowel disease. A study on 7046 Spanish patients. Med Clin. 2005;125:281–5. [DOI] [PubMed] [Google Scholar]
- 13. Luber RP, Honap S, Cunningham G, Irving PM. Can we predict the toxicity and response to thiopurines in inflammatory bowel diseases? Front Med. 2019;6:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Broekman M, Coenen MJH, Wanten GJ, van Marrewijk CJ, Klungel OH, Verbeek ALM, et al. Risk factors for thiopurine‐induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment Pharmacol Ther. 2017;46:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steponaitiene R, Kupcinskas J, Survilaite S, Varkalaite G, Jonaitis L, Kiudelis G, et al. TPMT and ITPA genetic variants in Lithuanian inflammatory bowel disease patients: prevalence and azathioprine‐related side effects. Adv Med Sci. 2016;61:135–40. [DOI] [PubMed] [Google Scholar]
- 16. Murphy LA, Atherton D. A retrospective evaluation of azathioprine in severe childhood atopic eczema, using thiopurine methyltransferase levels to exclude patients at high risk of myelosuppression. Br J Dermatol. 2002;147:308–15. [DOI] [PubMed] [Google Scholar]
- 17. Kim JH, Cheon JH, Hong SS, Eun CS, Byeon JS, Hong SY, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine‐induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol. 2010;44:e242–8. [DOI] [PubMed] [Google Scholar]
- 18. Collie‐Duguid ES, Pritchard SC, Powrie RH, Sludden J, Collier DA, Li T, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999;9:37–42. [DOI] [PubMed] [Google Scholar]
- 19. Oliveira E, Quental S, Alves S, Amorim A, Prata MJ. Do the distribution patterns of polymorphisms at the thiopurine S‐methyltransferase locus in sub‐Saharan populations need revision? Hints from Cabinda and Mozambique. Eur J Clin Pharmacol. 2007;63:703–6. [DOI] [PubMed] [Google Scholar]
- 20. Cao Q, Zhu Q, Shang Y, Gao M, Si J. Thiopurine methyltransferase gene polymorphisms in Chinese patients with inflammatory bowel disease. Digestion. 2009;79:58–63. [DOI] [PubMed] [Google Scholar]
- 21. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine‐induced leukopenia. Nat Genet. 2014;46:1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang HH, He Y, Wang HX, Liao CL, Peng Y, Tao LJ, et al. Comparison of TPMT and NUDT15 polymorphisms in Chinese patients with inflammatory bowel disease. World J Gastroenterol. 2018;24:941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maki H, Sekiguchi M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992;355:273–5. [DOI] [PubMed] [Google Scholar]
- 24. Shah SA, Paradkar M, Desai D, Ashavaid TF. Nucleoside diphosphate‐linked moiety X‐type motif 15 C415T variant as a predictor for thiopurine‐induced toxicity in Indian patients. J Gastroenterol Hepatol. 2017;32:620–4. [DOI] [PubMed] [Google Scholar]
- 25. Kakuta Y, Naito T, Onodera M, Kuroha M, Kimura T, Shiga H, et al. NUDT15 R139C causes thiopurine‐induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16:280–5. [DOI] [PubMed] [Google Scholar]
- 26. Chiengthong K, Ittiwut C, Muensri S, Sophonphan J, Sosothikul D, Seksan P, et al. NUDT15 c.415C>T increases risk of 6‐mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica. 2016;101:e24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kishibe M, Nozaki H, Fujii M, Iinuma S, Ohtsubo S, Igawa S, et al. Severe thiopurine‐induced leukocytopenia and hair loss in Japanese patients with defective NUDT15 variant: retrospective case‐control study. J Dermatol. 2018;45:1160–5. [DOI] [PubMed] [Google Scholar]
- 28. Hibi T, Naganuma M, Kitahora T, Kinjyo F, Shimoyama T. Low‐dose azathioprine is effective and safe for maintenance of remission in patients with ulcerative colitis. J Gastroenterol. 2003;38:740–6. [DOI] [PubMed] [Google Scholar]
- 29. Yip JS, Woodward M, Abreu MT, Sparrow MP. How are azathioprine and 6‐mercaptopurine dosed by gastroenterologists? Results of a survey of clinical practice. Inflamm Bowel Dis. 2008;14:514–8. [DOI] [PubMed] [Google Scholar]
- 30. Lee KM, Kim YS, Seo GS, Kim TO, Yang SK, Diseases IBDSGotKAftSoI . Use of thiopurines in inflammatory bowel disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015;13:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological A . American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–9. [DOI] [PubMed] [Google Scholar]
- 32. Yu LF, Zhong J, Cheng SD, Tang YH, Miao F. Low‐dose azathioprine effectively improves mucosal healing in Chinese patients with small bowel Crohn’s disease. J Dig Dis. 2014;15:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldstein H, Browne W, Rasbash J. Multilevel modelling of medical data. Stat Med. 2002;21:3291–315. [DOI] [PubMed] [Google Scholar]
- 34. Shimizu T, Takebayashi T, Sato Y, Niizeki H, Aoyama Y, Kitajima Y, et al. Grading criteria for disease severity by pemphigus disease area index. J Dermatol. 2014;41:969–73. [DOI] [PubMed] [Google Scholar]
- 35. Krakowski A, Covo J, Rozanski Z. Pemphigus vulgaris. Arch Dermatol. 1969;100:117. [PubMed] [Google Scholar]
- 36. Ikeda S, Imamura S, Hashimoto I, Morioka S, Sakuma M, Ogawa H. History of the establishment and revision of diagnostic criteria, severity index and therapeutic guidelines for pemphigus in Japan. Arch Dermatol Res. 2003;295:S12–6. [DOI] [PubMed] [Google Scholar]
- 37. Burton JL, Greaves MW, Marks J, Dawber RP. Azathioprine in pemphigus vulgaris. Br Med J. 1970;3:84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–62. [PMC free article] [PubMed] [Google Scholar]
- 39. Aberer W, Wolff‐Schreiner EC, Stingl G, Wolff K. Azathioprine in the treatment of pemphigus vulgaris. A long‐term follow‐up. J Am Acad Dermatol. 1987;16:527–33. [DOI] [PubMed] [Google Scholar]
- 40. Carson PJ, Hameed A, Ahmed AR. Influence of treatment on the clinical course of pemphigus vulgaris. J Am Acad Dermatol. 1996;34:645–52. [DOI] [PubMed] [Google Scholar]
- 41. Liang DC, Yang CP, Liu HC, Jaing TH, Chen SH, Hung IJ, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. 2016;16:536–9. [DOI] [PubMed] [Google Scholar]
- 42. Wong FC, Leung AW, Kwok JS, Chan MH, Li CK, Yuen YP. NUDT15 variant and thiopurine‐induced leukopenia in Hong Kong. Hong Kong Med J. 2016;22:185–7. [DOI] [PubMed] [Google Scholar]
- 43. Yan W, Zhou YH, Wang L, Xiao J, Li W. NUDT15 polymorphism and severe azathioprine‐induced myelosuppression in a Chinese man with pemphigus vulgaris. Br J Dermatol. 2018;178:e40–1. [DOI] [PubMed] [Google Scholar]
- 44. Shih YC, Zou YR, Wang B, Zheng J, Pan M. Azathioprine‐induced myelosuppression in two pemphigus vulgaris patients with homozygous polymorphism of NUDT15 . J Dermatol. 2019;46:e59–61. [DOI] [PubMed] [Google Scholar]
- 45. Relling MV, Schwab M, Whirl‐Carrillo M, Suarez‐Kurtz G, Pui CH, Stein CM, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019;105:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–15. [DOI] [PubMed] [Google Scholar]
- 47. Maley A, Swerlick RA. Azathioprine treatment of intractable pruritus: a retrospective review. J Am Acad Dermatol. 2015;73:439–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Fig S1


