Abstract
Although no gold standard exists to assess a patient's anticholinergic burden, a review identified 19 anticholinergic burden scales (ABSs). No study has yet evaluated whether a high anticholinergic burden measured with all 19 ABSs is associated with in‐hospital mortality and length of stay (LOS). We conducted a cohort study at a Swiss tertiary teaching hospital using patients' electronic health record data from 2015–2018. Included were patients aged ≥65 years, hospitalised ≥48 h without stays and >24 h in intensive care. Patients' cumulative anticholinergic burden score was classified using a binary (<3: low, ≥3: high) and categorical approach (0: no, 0.5–3: low, ≥3: high). In‐hospital mortality and LOS were analysed using multivariable logistic and linear regression, respectively. We included 27,092 patients (mean age 78.0 ± 7.5 years, median LOS 6 days). Of them, 913 died. Depending on the evaluated ABS, 1370 to 17,035 patients were exposed to anticholinergics. Patients with a high burden measured by all 19 ABSs were associated with a 1.32‐ to 3.03‐fold increase in in‐hospital mortality compared with those with no/low burden. We obtained similar results for LOS. To conclude, discontinuing drugs with anticholinergic properties (score ≥3) at admission might be a targeted intervention to decrease in‐hospital mortality and LOS.
Keywords: anticholinergic burden, in‐hospital mortality, length of stay, older patients
1. INTRODUCTION
With an ageing population, medical practice has changed over the past decades, as a drastic increase in polypharmacy has accompanied multimorbidity. The number of people taking at least five drugs has increased from 12% to 49% within the recent 20 years. 1 Hence, it is not surprising that the prevalence of drug use with potent anticholinergic (ACH) activity has nearly doubled in the last two decades. 2 Drugs with ACH properties block acetylcholine by binding to its receptor in the peripheral and central nervous system. Though some medications are used intentionally for their ACH action, others have ACH activity unrelated to their mechanisms of action. Older patients are in particular susceptible to ACH‐related adverse drug events (ADEs) due to physiological changes in pharmacokinetics and pharmacodynamics as well as ACH hypersensitivity. 3
At present, no gold standard exists to assess the ACH burden of a patient. Rudd et al. stated that expert‐based lists are the sole clinically useful tool to quantify the ACH burden. 4 These lists, called anticholinergic burden scales (ABSs), generally assign a number from one (low) to three (high) points to each substance. The cumulative ACH burden for a patient is calculated by identifying all prescribed ACH drugs, followed by adding up the scores of the substances (cumulative ACH burden). The resulting score helps identify patients at high risk of ACH‐related ADEs and could provide an opportunity to perform interventions, such as discontinuation of a drug.
In our previous systematic review, 5 we identified 19 ABSs with varying qualities. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Some of the ABSs were developed by a systematic review and built on previously published scales, 8 , 13 , 17 , 18 , 20 whereas other ABSs were derived from a serum radioreceptor anticholinergic activity (SAA) assay 12 , 19 or are entirely based on computational receptor binding affinities. 24 Most of the ABSs also include the opinion of an expert committee, whose composition varies greatly. ABSs that were not based on simple lists but rather on equations, such as the Drug Burden Index (DBI), 25 , 26 were excluded in this review.
Finally, some ABSs lack validation in clinical settings and others show contradicting results regarding the association with the investigated clinical outcomes, such as mortality. To our knowledge, no study has yet investigated all 19 ABSs in the same clinical setting. Hence, in this study, we aim to compare all published ABSs and evaluate their association with in‐hospital mortality and length of stay (LOS).
2. METHOD
2.1. Study design and setting
We conducted a cohort study of patients hospitalised between January 2015 and December 2018 at a Swiss tertiary teaching hospital, which includes 360 beds. Our dataset is derived from patients' electronic health records (EHRs) (i.e., data routinely collected during the hospitalisation). This study was undertaken per the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement. 27
2.2. Ethics approval
For this study, a protocol was written and approved by the national ethics review committee (EKNZ Project ID: 2018‐01000).
2.3. Participants
We included patients 65 years and older, who were hospitalised for at least 48 h. Patients were excluded if they were outpatients or stayed in an intensive care unit (ICU) for more than 24 h because no EHRs are used in the ICU.
2.4. Data collection
Demographic characteristics, laboratory values, medication intake and some variables from the nurse assessment tool ePA‐AC 28 within the first 24 h upon admission were extracted for each patient from the hospital's clinical information system and cross‐linked to the proper International Classification of Disease 10 (ICD‐10) diagnosis codes. Comorbidities were grouped according to the Charlson comorbidity index based on the ICD‐10 codes received at discharge using the R package {comorbidity}. 29 We grouped the following diseases: cancer and metastatic cancer (cancer), mild and moderate to severe liver disease (liver disease) and diabetes with and without chronic complications (diabetes). The coded comorbidity was carried forward in case of repeated hospitalisations of the same patient because we considered these mostly to be chronic conditions. Additionally, we grouped for all‐cause delirium at admission or during hospitalisation using the following ICD‐10 codes: F05.0, F05.1, F05.8, F05.9, F10.4, F11.4, F12.4, F13.4, F14.4, F15.4, F16.4, F17.4, F18.4 and F19.4.
The ePA‐AC tool 28 is performed by nurses at entry and then every other day, systematically assessing information on cognition, care, mobility and nutritional status of patients. We used the self‐care index (SPI), the Braden score and the nutrition deficiency score. An SPI score of 32 or more points means that patients can take care of themselves; a Braden score below 12 points is associated with a high risk of development of a decubitus and last a nutrition deficiency score with three or more points representing malnutrition. The variable polymedication stands for the amount of different drugs taken by a patient and was considered numerical.
In contrast to variables with high proportions of missing data, only those with more than 20% available data were considered. For categorical and binary variables, we generated the potential category ‘missing’. Table 1 summarises the patient characteristics of the dataset by using the R package {tableone}. 30
TABLE 1.
Patient characteristics of the entire cohort
| Patient characteristics | Overall (n: 27,092) |
|---|---|
| Died, n (%) | 913 (3.4) |
| Length of stay (LOS), median days [IQR] | 6.00 [4.00, 10.00] |
| Age, mean years (±SD) | 78.08 (7.69) |
| Age, n (%) | |
| 65–75 years | 10,932 (40.4) |
| 76–85 years | 11,012 (40.6) |
| 86–95 years | 4958 (18.3) |
| >95 years | 190 (0.7) |
| Female sex, n (%) | 14,014 (51.7) |
| Department, n (%) | |
| Medical department | 15,119 (55.8) |
| Surgical department | 11,973 (44.2) |
| Hearing device, n (%) | |
| None | 15,674 (57.9) |
| Hearing device | 2976 (11.0) |
| Missing | 8442 (31.2) |
| Visual aid, n (%) | |
| None | 4956 (18.3) |
| Glasses or contacts | 13,630 (50.3) |
| Missing | 8506 (31.4) |
| Acute myocardial infarction, n (%) | 1442 (5.3) |
| Congestive heart failure, n (%) | 4751 (17.5) |
| Peripheral vascular disease, n (%) | 3691 (13.6) |
| Cerebrovascular disease, n (%) | 3462 (12.8) |
| Dementia, n (%) | 2595 (9.6) |
| COPD, n (%) | 3041 (11.2) |
| Rheumatoid disease, n (%) | 798 (2.9) |
| Peptic ulcer disease, n (%) | 542 (2.0) |
| Liver disease, n (%) | 541 (2.0) |
| Diabetes, n (%) | 5692 (21.0) |
| Hemiplegia, Paraplegia, n (%) | 1232 (4.5) |
| Renal dysfunction, n (%) | 6176 (22.8) |
| Cancer, n (%) | 4625 (17.1) |
| Delirium, n (%) | 1695 (6.3) |
| Self‐care index, median [IQR] | 39.00 [34.00, 40.00] |
| Braden, median [IQR] | 22.00 [20.00, 23.00] |
| Nutrition deficiency score, median [IQR] | 1.00 [1.00, 2.00] |
| Catheterisation, n (%) | 7873 (29.1) |
| Surgery during stay, n (%) | 8676 (32.0) |
| Polymedication, mean [±SD] | 7.58 (3.84) |
| GFR [ml/min], median [IQR] | 64.00 [45.00, 80.00] |
| Creatinine [μmol/l], median [IQR] | 87.00 [70.00, 115.50] |
| Sodium [mmol/l], median [IQR] | 138.00 [135.00, 140.00] |
| Potassium [mmol/l], median [IQR] | 4.05 [3.80, 4.35] |
| ALAT [U/l], median [IQR] | 20.00 [14.00, 35.00] |
| ASAT [U/l], median [IQR] | 25.00 [20.00, 35.00] |
| CRP [mg/l], median [IQR] | 12.00 [2.70, 56.00] |
| CRP [mg/l], n (%) | |
| <5 | 7884 (29.1) |
| 5–10 | 2680 (9.9) |
| 10–50 | 6070 (22.4) |
| >50 | 6116 (22.6) |
| Missing | 4342 (16.0) |
| Temperature [°C], median [IQR] | 36.55 [36.25, 36.90] |
| Systolic blood pressure [mmHg], mean (±SD) | 134.14 (19.77) |
| Diastolic blood pressure [mmHg], mean (±SD) | 71.89 (11.67) |
| BMI, mean (±SD) | 26.10 (3.84) |
Abbreviations: ALAT, alanine transaminase; ASAT, aspartate transaminase; BMI, body mass index; CRP, C‐reactive protein; GFR, glomerular filtration rate; IQR, interquartile range; COPD, chronic obstructive pulmonary disease.
2.5. Main outcome and measures
The exact date of death was identified from the hospital discharge note and coded as a binary variable. The LOS was calculated from the admission to the discharge date. Due to a skewed distribution of the LOS, we log‐transformed the data, which were back‐transformed to ease interpretation. 31
2.6. Exposure
We considered all drugs in single active ingredient and combination products administered within the first 24 h of hospitalisation. The extracted raw data did not always contain machine‐readable information on the active ingredients of the ordered drugs, as a consequence of for instance nonstandardised free‐text entries, some of them with misspellings, hampering comprehensive drug identification. Based on the Anatomical Therapeutic Chemical Classification System (World Health Organisation, Geneva, Switzerland), we mapped the medication orders to their active ingredients in a semi‐automated process as described by Siebenhüner et al. 32
For every ABS, the cumulative ACH burden score was calculated using all 19 ABSs, which were identified and described in our systematic review. 5 A list of all the drugs can be found in supporting information Table S1. Drugs not scored were assumed to have no ACH activity and received zero points. All but four ABSs used a 4‐point grading system (0: no to 3: high). 13 , 14 , 19 , 24 For the scale by Minzenberg et al., 19 we set cut‐offs at 10 points for the Pharmacological Index (PI) and 47 points for the Clinical Index (CI) by comparing the substances with the other ABSs. The PI and CI were analysed separately. In the scale developed by Duran et al. (DS), 13 high potency drugs received a score of three points, low potency drugs received a score of two points, drugs listed in Table 4 in Duran's publication received a score of one point and drugs in Annex Sublist 1 in this publication received a score of half a point. The Anticholinergic Activity Scale (AAS) 14 was transformed as follows: four into three, three into two, two and one into one and zero remained zero. Finally, the Anticholinergic Toxicity Scale (ATS) 24 was not transformed because the scoring ranges between half a point and five points, which is very close to the scoring of other ABSs. We classified the patients into two or three groups of exposure using each scale, as follows: for the binary approach: no/low risk<3 or high risk≥3 and for the categorical approach: no risk = 0, low risk 0.5 to <3, or high risk ≥3.
2.7. Statistical analysis
Statistical analyses were performed using R version 3.6.2 and the integrated development environment R Studio. 33 The R functions and corresponding packages are denoted as function {package}. 34 To compare the characteristics of patients based on their ACH drug exposure, we used a Chi square test for categorical and dichotomous variables, for continuous variables with normal distribution a one‐way test (three groups) or t‐test (two groups) and in case of nonnormal distributions or unequal variance a Kruskal–Wallis test (three groups) or Mann–Whitney U test (two groups). The p‐values calculated in this report assume a significance level of .05. We report the mean ± the standard deviation or the median and interquartile range for continuous, and numbers with percentages for categorical variables.
To test for multicollinearity between the covariables, we calculated the variance of inflation factor (VIF) for each variable with vif {car}. 35 If variables have more than two degrees of freedom (Df), the generalised variance of inflation factor (GVIF) was calculated instead. Variables showing a >10 were excluded from the multivariable analysis.
A logistic regression model was used with a logit‐link function for the outcome of in‐hospital mortality, and a linear regression was conducted for the log‐transformed outcome of the LOS. For the LOS analysis, we excluded patients who died. We performed univariable and multivariable analyses adjusting for covariables for both outcomes that were selected based on prior work. 5 Since we fitted 19 models by simply exchanging the ABS predictor, we did not need to adjust for multiple testing.
Bearing in mind that our inclusion criterion of patients aged 65 years and older could be considered a rather young cut‐off to study death unlike Kidd et al., 36 who included much older patients exactly for this reason, we added a subgroup analysis for in‐hospital mortality according to stratified age groups.
3. RESULTS
A total of 130,105 patients 65 years and older were hospitalised for longer than 48 h from 2015 to 2018. After applying the inclusion and exclusion criteria, we identified 27,092 patients (Figure 1) with a mean age of 781 ± 7.7 years, of which 14,014 (51.7%) were women, and the median LOS was 6 days. Out of these, 913 died during hospitalisation (3.4%). Overall, exposure to drugs with ACH properties depended on the ABS used to quantify the cumulative ACH burden: between 1370 patients (5.1%, measured with the ATS) and 17,035 patients (62.9%, measured with the German Anticholinergic Burden Scale [GABS]) were exposed to drugs with ACH properties. Further characteristics of the patients are depicted in Table 1. Patient characteristics divided by exposure for both approaches (binary and categorical) with respect to the used ABS can be found in supporting information Tables S2 and S3, respectively.
FIGURE 1.
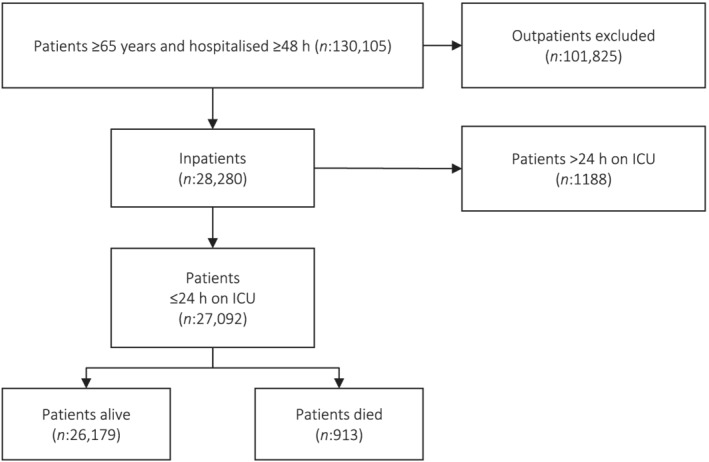
Flowchart of included and excluded patients
The following variables contained missing values: systolic blood pressure (5.2%), diastolic blood pressure (5.2% missing), body temperature (13.8%), SPI (46.3%), Braden score (45.9%), nutrition deficiency score (58.3%), visual aid (31.4%), hearing device (31.2%), body mass index (BMI) (36.1%), glomerular filtration rate (GFR) (12.3%), creatinine (12.2%), sodium (12.4%), potassium (12.4%), alanine transaminase (ALAT) (73.1%), aspartate transaminase (ASAT) (28.2%), C‐reactive protein (CRP) (16.0%) and polymedication (10.0%). We did not detect any collinearity between the variables.
3.1. Primary outcome mortality
Results of the binary and univariable analysis indicate that a high ACH burden score of three or more points was significantly associated with in‐hospital mortality after applying all of the 19 ABSs. After adjusting for covariables, the found associations in all ABSs remained significant (Table 2). Summer's Class of Drug List (SCDL) showed the largest effect size followed by the ATS and the Anticholinergic Drug Scale (ADS).
TABLE 2.
Multivariable regression using the binary approach for both outcomes: in‐hospital mortality and length of stay (LOS)
| Scale | Mortality | LOS | Low <3 (n died (%)) | High ≥3 (n died (%)) | ||
|---|---|---|---|---|---|---|
| Multivariable regression | ||||||
| OR | 95% CI | Exp(B) | 95% CI | |||
| ABC 6 | 1.39 | 1.11–1.72 | 1.08 | 1.04–1.11 | 25,348 (778 (3.1%)) | 1744 (125 (7.2%)) |
| AEC 7 | 1.49 | 1.17–1.87 | 1.00 | 0.96–1.03 | 25,512 (814 (3.2%)) | 1580 (99 (6.3%)) |
| ACB 9 | 1.55 | 1.32–1.80 | 1.03 | 1.01–1.05 | 22,013 (630 (2.9%)) | 5079 (283 (5.6%)) |
| AIS 8 | 1.58 | 1.37–1.82 | 1.07 | 1.06–1.09 | 19,343 (507 (2.6%)) | 7749 (406 (5.2%)) |
| CABS 10 | 1.35 | 1.09–1.65 | 1.03 | 1.00–1.06 | 24,820 (770 (3.1%)) | 2272 (143 (6.3%)) |
| Chew 12 | 1.37 | 1.12–1.66 | 1.12 | 1.09–1.15 | 24,534 (768 (3.1%)) | 2558 (145 (5.7%)) |
| AAS 14 | 1.34 | 1.11–1.62 | 1.01 | 0.98–1.04 | 24,381 (748 (3.1%)) | 2711 (165 (6.1%)) |
| ARS 21 | 1.92 | 1.50–2.42 | 1.01 | 0.97–1.05 | 25,821 (816 (3.2%)) | 1271 (97 (7.6%)) |
| ACL 22 | 1.53 | 1.23–1.88 | 1.12 | 1.09–1.15 | 24,979 (794 (3.2%)) | 2113 (119 (5.6%)) |
| CrAS 15 | 1.85 | 1.57–2.18 | 1.12 | 1.10–1.14 | 23,319 (675 (2.9%)) | 3773 (238 (6.3%)) |
| ADS 11 | 2.13 | 1.80–2.51 | 1.16 | 1.13–1.19 | 23,919 (691 (2.9%)) | 3173 (222 (7.0%)) |
| SCDL 23 | 3.03 | 2.56–3.57 | 1.12 | 1.09–1.14 | 23,790 (677 (2.8%)) | 3302 (236 (7.1%)) |
| PI 19 | 1.32 | 1.05–1.64 | 1.00 | 0.97–1.03 | 24,955 (791 (3.2%)) | 2137 (122 (5.7%)) |
| CI 19 | 1.35 | 1.07–1.69 | 0.99 | 0.96–1.02 | 25,086 (795 (3.2%)) | 2006 (118 (5.9%)) |
| GABS 18 | 1.51 | 1.31–1.74 | 1.08 | 1.06–1.10 | 18,586 (487 (2.6%)) | 8506 (426 (5.0%)) |
| DS 13 | 1.86 | 1.61–2.14 | 1.11 | 1.09–1.13 | 19,302 (477 (2.5%)) | 7790 (436 (5.6%)) |
| BAADS 20 | 1.69 | 1.46–1.95 | 1.07 | 1.05–1.09 | 18,949 (473 (2.5%)) | 8143 (440 (5.4%)) |
| KABS 17 | 1.65 | 1.42–1.91 | 1.08 | 1.06–1.10 | 21,416 (597 (2.8%)) | 5676 (316 (5.6%)) |
| ATS 24 | 2.22 | 1.72–2.84 | 1.00 | 0.96–1.04 | 26,137 (821 (3.1%)) | 955 (92 (9.6%)) |
| DRS 16 | 1.66 | 1.43–1.93 | 1.11 | 1.09–1.13 | 21,398 (598 (2.8%)) | 5694 (315 (5.5%)) |
Note: Far‐left column: individual ABS; two columns on the far right: absolute number of patients and percentage of prevalence of in‐hospital deaths for each group. Multivariable analysis is adjusted for age, sex, dementia, delirium, congestive heart failure, hemiplegia/paraplegia, chronic obstructive pulmonary disease, rheumatic diseases, diabetes, liver disease, cancer, renal disease, cerebrovascular disease, acute myocardial infarction, peripheral vascular disease, peptic ulcer and categorical C‐reactive protein. For the univariable analysis and analysis adjusted only for age and sex; see supporting information Tables S4.1 and S4.2. For LOS, the back‐transformed estimate coefficient to the power of e is depicted as Exp(B) and can be interpreted as ABC Exp(B): 1.04, 4% longer hospitalisation stay.
Abbreviations: AAS, Anticholinergic Activity Scale; ABC, Anticholinergic Burden Classification; ACB, Anticholinergic Cognitive Burden Scale; ACL, Anticholinergic Loading Scale; ADS, Anticholinergic Drug Scale; AEC, Anticholinergic Effect on Cognition; AIS, Anticholinergic Impregnation Scale; ARS, Anticholinergic Risk Scale; ATS, Anticholinergic Toxicity Scale; BAADS, Brazilian Anticholinergic Activity Drug Scale; CABS, Cancelli's Anticholinergic Burden Scale; CI, Clinical Index; CrAS, Clinician‐rated Anticholinergic Scale; DRS, Delirogenic Risk Scale; DS, Duran Scale; GABS, German Anticholinergic Burden Scale; KABS, Korean Anticholinergic Burden Scale; PI, Minzenberg's Pharmacological Index; SCDL, Summer's Class of Drug List.
In the categorical analysis, all ABSs, except Cancelli's Anticholinergic Burden Scale (CABS) and Minzenberg's PI, exhibited a gradual increase in the odds of a higher ACH burden score. Again, all 19 ABSs from the binary analysis remained significantly associated when exposed to a high ACH burden score (three or more points) but in most cases were not significantly associated when comparing no burden (zero points) to low burden (half a point to less than three points). Only four scales, the Anticholinergic Risk Scale (ARS), the CABS, the ADS and the Anticholinergic Cognitive Burden Scale (ACB) demonstrated a significant association with increased in‐hospital mortality when comparing no to low and no to high ACH burden while being adjusted for covariables (Table 3). The univariable and multivariable analyses adjusted to age and sex for both approaches are in supporting information Tables S4.1–S4.4.
TABLE 3.
Multivariable regression using the categorical approach for both outcomes: In‐hospital mortality and length of stay (LOS)
| Scale | Mortality | LOS | Exposed (n died (%)) | ||
|---|---|---|---|---|---|
| Multivariable | |||||
| OR | 95% CI | Exp(B) | 95% CI | ||
| ABC 6 | |||||
| No burden | Reference | 25,096 (781 (3.1%)) | |||
| Low burden | 0.60 | 0.25–1.21 | 0.97 | 0.88–1.06 | 252 (7 (2.8%)) |
| High burden | 1.38 | 1.10–1.71 | 1.03 | 0.98–1.09 | 1744 (125 (7.2%)) |
| AEC 7 | |||||
| No burden | Reference | 20,728 (611 (2.9%)) | |||
| Low burden | 1.13 | 0.95–1.34 | 1.00 | 0.97–1.03 | 4784 (203 (4.2%)) |
| High burden | 1.54 | 1.20–1.94 | 0.95 | 0.90–1.00 | 1580 (99 (6.3%)) |
| ACB 9 | |||||
| No burden | Reference | 15,993 (411 (2.6%)) | |||
| Low burden | 1.24 | 1.04–1.48 | 0.99 | 0.96–1.01 | 6020 (219 (3.6%)) |
| High burden | 1.66 | 1.40–1.96 | 0.97 | 0.94–0.99 | 5079 (283 (5.6%)) |
| AIS 8 | |||||
| No burden | Reference | 11,532 (295 (2.6%)) | |||
| Low burden | 1.03 | 0.85–1.24 | 0.99 | 0.97–1.01 | 7811 (211 (2.7%)) |
| High burden | 1.60 | 1.36–1.88 | 1.01 | 0.99–1.03 | 7749 (406 (5.2%)) |
| CABS 10 | |||||
| No burden | Reference | 23,444 (679 (2.9%)) | |||
| Low burden | 1.69 | 1.32–2.14 | 1.03 | 0.98–1.07 | 1376 (91 (6.6%)) |
| High burden | 1.45 | 1.17–1.78 | 0.97 | 0.92–1.02 | 2272 (143 (6.3%)) |
| Chew 12 | |||||
| No burden | Reference | 18,548 (535 (2.9%)) | |||
| Low burden | 1.06 | 0.90–1.25 | 1.02 | 1.00–1.05 | 5986 (233 (3.9%)) |
| High burden | 1.40 | 1.14–1.71 | 1.08 | 1.02–1.14 | 2558 (145 (5.7%)) |
| AAS 14 | |||||
| No burden | Reference | 21,777 (620 (2.8%)) | |||
| Low burden | 1.19 | 0.96–1.46 | 0.98 | 0.95–1.02 | 2604 (128 (4.9%)) |
| High burden | 1.39 | 1.14–1.68 | 0.98 | 0.94–1.02 | 2711 (165 (6.1%)) |
| ARS 21 | |||||
| No burden | Reference | 22,015 (639 (2.9%)) | |||
| Low burden | 1.27 | 1.06–1.52 | 0.97 | 0.95–1.00 | 3806 (177 (4.7%)) |
| High burden | 2.03 | 1.59–2.58 | 1.04 | 0.99–1.10 | 1271 (97 (7.6%)) |
| ACL 22 | |||||
| No burden | Reference | 19,473 (603 (3.1%)) | |||
| Low burden | 1.02 | 0.86–1.21 | 1.02 | 1.00–1.05 | 5506 (191 (3.5%)) |
| High burden | 1.54 | 1.23–1.90 | 1.10 | 1.04–1.17 | 2113 (119 (5.6%)) |
| CrAS 15 | |||||
| No burden | Reference | 16,621 (470 (2.8%)) | |||
| Low burden | 1.08 | 0.90–1.28 | 1.02 | 1.00–1.04 | 6698 (205 (3.1%)) |
| High burden | 1.90 | 1.59–2.25 | 1.05 | 1.01–1.09 | 3773 (238 (6.3%)) |
| ADS 11 | |||||
| No burden | Reference | 16,306 (426 (2.6%)) | |||
| Low burden | 1.26 | 1.07–1.48 | 1.04 | 1.02–1.06 | 7613 (265 (3.5%)) |
| High burden | 2.32 | 1.94–2.77 | 1.08 | 1.04–1.13 | 3173 (222 (7.0%)) |
| SCDL 23 | |||||
| No burden | Reference | 17,518 (527 (3.0%)) | |||
| Low burden | 0.72 | 0.59 – 0.87 | 0.93 | 0.89–0.97 | 6272 (150 (2.4%)) |
| High burden | 2.78 | 2.34–3.30 | 1.11 | 1.07–1.15 | 3302 (236 (7.1%)) |
| PI 19 | |||||
| No burden | Reference | 24,893 (789 (3.2%)) | |||
| Low burden | 2.46 | 0.40–8.15 | 1.06 | 0.85–1.31 | 62 (2 (3.2%)) |
| High burden | 1.32 | 1.05–1.64 | 0.97 | 0.93–1.00 | 2137 (122 (5.7%)) |
| CI 19 | |||||
| No burden | Reference | 24,913 (788 (3.2%)) | |||
| Low burden | 1.06 | 0.44–2.18 | 1.05 | 0.93–1.18 | 173 (7 (4.0%)) |
| High burden | 1.35 | 1.08–1.69 | 0.96 | 0.92–0.99 | 2006 (118 (5.9%)) |
| GABS 18 | |||||
| No burden | Reference | 10,057 (265 (2.6%)) | |||
| Low burden | 0.98 | 0.81–1.18 | 0.98 | 0.96–0.99 | 8529 (122 (2.6%)) |
| High burden | 1.49 | 1.27–1.77 | 1.00 | 0.98–1.03 | 8506 (426 (5.0%)) |
| DS 13 | |||||
| No burden | Reference | 11,404 (281 (2.5%)) | |||
| Low burden | 0.92 | 0.76–1.11 | 0.99 | 0.98–1.01 | 7898 (196 (2.5%)) |
| High burden | 1.79 | 1.52–2.11 | 1.03 | 1.00–1.05 | 7790 (436 (5.6%)) |
| BAADS 20 | |||||
| No burden | Reference | 11,257 (276 (2.5%)) | |||
| Low burden | 0.97 | 0.80–1.17 | 1.00 | 0.98–1.02 | 7692 (197 (2.6%)) |
| High burden | 1.67 | 1.42–1.96 | 1.00 | 0.98–1.03 | 8143 (440 (5.4%)) |
| KABS 17 | |||||
| No burden | Reference | 14,287 (366 (2.6%)) | |||
| Low burden | 1.16 | 0.97–1.38 | 1.01 | 0.99–1.03 | 7129 (231 (3.2%)) |
| High burden | 1.74 | 1.48–2.05 | 1.01 | 0.98–1.03 | 5676 (316 (5.6%)) |
| ATS 24 | |||||
| No burden | Reference | 25,722 (797 (3.1%)) | |||
| Low burden | 1.50 | 0.94–2.29 | 1.14 | 1.05–1.24 | 415 (24 (5.8%)) |
| High burden | 2.24 | 1.74–2.87 | 1.00 | 0.94–1.06 | 955 (92 (9.6%)) |
| DRS 16 | |||||
| No burden | Reference | 13,523 (346 (2.6%)) | |||
| Low burden | 1.14 | 0.96–1.35 | 1.01 | 1.00–1.03 | 7875 (252 (3.2%)) |
| High burden | 1.75 | 1.48–2.07 | 1.04 | 1.01–1.07 | 5694 (315 (5.5%)) |
Note: Far‐left column: individual ABS; two columns on the far right: absolute number of patients and percentage of prevalence of in‐hospital deaths for each group. Multivariable analysis is adjusted for age, sex, dementia, delirium, congestive heart failure, hemiplegia/paraplegia, chronic obstructive pulmonary disease, rheumatic diseases, diabetes, liver disease, cancer, renal disease, cerebrovascular disease, acute myocardial infarction, peripheral vascular disease, peptic ulcer and categorical C‐reactive protein. For the univariable analysis and analysis adjusted only for age and sex; see supporting information Tables S4.3 and S4.4. For LOS, the back‐transformed estimate coefficient to the power of e is depicted as Exp(B) and can be interpreted as ABC Exp(B): 1.04, 4% longer hospitalisation stay.
Abbreviations: AAS, Anticholinergic Activity Scale; ABC, Anticholinergic Burden Classification; ACB, Anticholinergic Cognitive Burden Scale; ACL, Anticholinergic Loading Scale; ADS, Anticholinergic Drug Scale; AEC, Anticholinergic Effect on Cognition; AIS, Anticholinergic Impregnation Scale; ARS, Anticholinergic Risk Scale; ATS, Anticholinergic Toxicity Scale; BAADS, Brazilian Anticholinergic Activity Drug Scale; CABS, Cancelli's Anticholinergic Burden Scale; CI, Clinical Index; CrAS, Clinician‐rated Anticholinergic Scale; DRS, Delirogenic Risk Scale; DS, Duran Scale; GABS, German Anticholinergic Burden Scale; KABS, Korean Anticholinergic Burden Scale; PI, Minzenberg's Pharmacological index; SCDL, Summer's Class of Drug List.
The subgroup analysis stratified according to age groups revealed that there might be an effect modification leading to overestimation of the results (supporting information Table S4.5). Although in all age groups we found an association with increased in‐hospital mortality, significance was lost with increasing age except when using newer ABSs and higher odds ratios were observed for patients in age group one (65–75 years) compared with the others.
3.2. Secondary outcome LOS
In the binary approach, univariable analysis showed that a high ACH burden score of three or more points measured with 19 ABSs was significantly associated with a 9% to 33% prolonged LOS. After adjusting for covariables, 13 ABSs remained significantly associated and resulted in a 3% to 16% longer stay (Table 2). The strongest associations were observed for the ADS, followed by the SCDL, Anticholinergic Load Scale (ACL), Clinician‐rated Anticholinergic Scale (CrAS) and Chew. In the categorical multivariable analysis, a gradual increase was observed in 11 ABSs. The univariable and multivariable analyses adjusted to age and sex are in supporting information Tables S4.1–S4.4.
4. DISCUSSION
The ACH properties of drugs are a risk for ADEs in older patients. Therefore, numerous ABSs have been developed to quantify the cumulative ACH burden in a patient by scoring each substance from zero points (no ACH properties) to three points (high ACH properties) and thus guide clinicians in their evaluation of drug‐related risk of adverse events.
4.1. Primary outcome mortality
We demonstrate that a cumulative ACH burden score of three or more points in a patient measured by all 19 ABSs was significantly associated with in‐hospital mortality. A reason for the small differences in the effect size between the scales could be the varying sample sizes (supporting information Tables S2 and S3) due to the different number of drugs scored to have ACH properties. If an ABS scored only a few drugs with mostly two or three points, sample size in terms of exposure becomes more imbalanced and the association is stronger as seen with the SCDL and ATS especially in the categorical approach. Furthermore, while some ABSs were restrictive in scoring drugs with one point (i.e. the SCDL), others assigned this many times. This implies that when using such a scale, it is important to take the cumulative ACH burden to reach a high score (three points or more points). In all ABSs only one substance (amitriptyline) was scored unanimously with a score of three points. Other reasons could be the different countries and time points the ABSs were developed. The oldest scale (SCDL) was developed in 1978, and the newest (Brazilian Anticholinergic Activity Scale [BAADS]) was in 2019. Starting in 2013, six ABSs (DS, Delirogenic Risk Scale [DRS], Anticholinergic Impregnation Scale [AIS], GABS, Korean Anticholinergic Burden Scale [KABS] and BAADS) were published based on a systematic review on prior published scales compared with the ABSs from the early 2000s, which were primarily single studies using different methods for the scale development ranging from expert opinions and literature reviews to SAA. However, these newer ABSs did not improve the original ABSs much with respect to their association with in‐hospital mortality.
In our previous review, we systematically assessed the quality of the ABSs. 5 Regarding the scales with the best quality (ACB, DS, GABS and Anticholinergic Effect on Cognition [AEC]), all of them exhibited a significant association in the present study, whereas the ABSs with the lowest quality (SCDL) had the strongest association. This points to the conclusion that quality does not seem to play an important role.
The only scale standing out is the ATS, 24 which showed a strong result and for which this study is the first validation. The ATS uses a computational scoring approach considering the chemical structure of a substance, the off‐target interactions and different muscarinic receptor subtypes and is completely objective. This approach seems good enough to identify all the substances scored with three points or more by other ABSs without taking into account clinical judgements or any pharmacodynamic characteristics. In the future, the ATS could potentially be used to study a specific ACH side effect affecting only one receptor subtype, because it differentiates between the muscarinic receptor subtypes. More studies using the ATS are warranted to confirm our findings.
Twenty‐one previous cohort and case–control studies investigated the association of six ABSs (ACB, ADS DS, ARS, Chew and AEC) with mortality in the older population. 5 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 However, these studies are not all comparable with ours. Out of them, five investigated postdischarge mortality, 39 , 44 , 46 , 50 , 51 six reports focused on a special population with dementia, 37 , 48 , 56 stroke, 42 depression, 38 and palliative care patients 55 and another six studies used data of outpatients or nursing homes. 40 , 41 , 43 , 47 , 49 , 54 Another two studies 45 , 53 are similar in terms of using data from patients older than 65 years. However, Mangoni et al., 45 finding no association with the ARS, additionally studied the impact of heat waves while Lu et al. 53 uses a national health insurance database (no association with ARS), which is not clearly distinguishing between inpatients and outpatients. The remaining two studies are comparable with ours. 36 , 52 Both evaluated the association of the ACH burden at admission and in‐hospital mortality in older patients, but only Lowry et al. used the inclusion criteria of 65 years and older. 52 The researchers found that a unit increase in the ARS is significantly associated with in‐hospital mortality. Kidd et al. 36 included patients older than 90 years and showed that the ACH burden measured with the ACB is not associated with in‐hospital mortality. Our study reproduced the findings in terms of the significance of the ARS. Furthermore, our subgroup analysis confirmed a loss of significance with increasing age for in‐hospital mortality. However, if we were to include only the ‘frail’ age groups three and four (>86 years), we would lose power and, furthermore, predicting mortality, especially drug‐induced, would become difficult because this patient population is inherently closer to the end of life.
Results of the categorical analyses exhibit no significant association with higher in‐hospital mortality when comparing no to low ACH burden, with the exception of the ACB, CABS, ARS and ADS. Whereas a score of three or more points compared with zero points was associated with increased in‐hospital mortality, which is in line with our observations of the binary approach. Therefore, a cumulative ACH burden score of three or more points seems to be a valid cut‐off that can be used in clinical practice to determine whether it is necessary to change the current medication of a patient admitted to the emergency room using any of the ABSs.
4.2. Secondary outcome LOS
A high cumulative ACH burden derived from 13 ABSs demonstrated a significant association with an increased LOS from 3% to 16%. The other ABSs exhibited no effect.
In the pre‐existing literature, contradictory results with regard to LOS have been reported. Thus far, only Salahudeen et al. 57 comparing eight ABSs (Anticholinergic Burden Classification [ABC], ADS, CrAS, ARS, Chew, ACB, ACL, AAS) found a positive significant association with LOS in all scales, which is confirmed by our findings for six out of the eight ABSs (ABC, ADS, CrAS, Chew, ACB and ACL). Nevertheless, in comparison with our study, they used pharmacy claims data. Several other studies, which differ from our study in terms of patient selection, analysed only one ABS and found no significant results. 45 , 46 , 50 , 58 , 59 Kidd et al. 36 and Lowry et al. 52 also investigated LOS and were not able to demonstrate a significant association when using the ABC and ARS. Our study confirms the findings for the ARS. Additionally, we observed an increased LOS for the AIS, SCDL, GABS, DS, BAADS, KABS and DRS when adjusted for covariables, which has not been previously shown. We hypothesise that the weaker association with LOS observed in this study compared with in‐hospital mortality might have been confounded by other unidentified factors, such as delay in placement in a rehabilitation centre or in nursing home that might have led to longer hospital stays unrelated to patients' health status.
4.3. Strengths
To our knowledge, this study is the first to investigate all 19 published ABSs, and the study population sample size was substantially larger than in previous studies. Adjusting for many comorbidities reduced the possible confounding. In addition to the medication list by entry, the free‐text entries were mapped to their ingredients because the medication lists are often incomplete, and combinations were included as well.
4.4. Limitations
This study has some limitations. One of the main limitations is its retrospective and single‐centre study design. Furthermore, we did not consider the dosage of medication and route of administration or conduct a follow‐up after discharge for the outcome mortality. However, most of the ABSs have also been developed without regard to the dosage applied in practice. In our dataset, we could have calculated the dosage taken by the patient, but constructing a weighting factor to incorporate this dosage in the cumulative ACH burden would be difficult, because doses of certain medications have large ranges (i.e. quetiapine) and each substance has its own half‐life. In addition, some medications are given daily or have been taken before admission while others are given on demand. Unfortunately, this differentiation of medication was not possible in our dataset but could potentially be improved when linking it to pharmacy claims or insurance data. Lastly, possible drug–drug interaction and patients' genetic predisposition (slow/fast metaboliser) might hamper the bioavailability of medications with ACH properties. Finally, no ABSs were specifically developed for Switzerland, potentially leading to incomplete or incorrect scoring due to the different drugs available nationally. Another limitation of this study is that it only considered medication within 24 h of admission, which could change during the course of the hospital stay. However, knowing already at admission that the odds for in‐hospital mortality are higher might give enough time to install preventive measures and substitute medications with a high ACH burden score.
5. CONCLUSION
The ABC, ACB, AIS, Chew, ACL, CrAS, ADS, SCDL, GABS, DS, BAADS, KABS and DRS showed a significant association with both outcomes. This is not surprising because the SCDL was the groundwork for the CrAS, which was further developed to the ADS, which was then included in several newer scales based on systematic reviews, such as GABS, DS, BAADS, KABS and DRS. As newer ABSs incorporate older versions and show more consistent results in both outcomes and subgroup analysis, we recommend to use one of these scales. Yet, any other ABS could be used, as the main trigger of both outcomes is the cumulative ACH burden score of three or more points, considered a high burden. Discontinuation or substituting drugs with strong ACH properties during admission might be a targeted intervention to reduce in‐hospital mortality and LOS. Nevertheless, with 19 ABSs to measure the ACH burden, it might be time to work on an internationally agreed standard.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest. Presentation of work: GSASA Congress 2020 (25.1‐26.11.2020), poster and oral presentation.
FUNDING INFORMATION
AL as a PhD student was supported by the 2017 National Research Grant from Swiss Association of Public Health Administration and Hospital Pharmacists (GSASA). The funders had no role in study design, data collection, analysis, decision to publish or preparing the manuscript.
Supporting information
Table S1: All drugs that have been scored >0 in at least one anticholinergic burden scale (ABS). Drugs not listed or drugs not scored in one ABS are considered a score of 0. Combinations are scored as the sum of the single ingredients.
Table S4.1: Univariable and multivariable regression using the binary approach for the outcome in‐hospital mortality.
Table S4.2: Univariable and multivariable regression using the binary approach for the outcome LOS.
Table S4.3: Univariable and multivariable regression using the categorical approach for the outcome in‐hospital mortality.
Table S4.4: Univariable and multivariable regression using the categorical approach for the outcome LOS.
Table S4.5: Multivariable regression stratified according to age groups using the binary approach for the outcome in‐hospital mortality.
Table S2: Table 1's binary approach
Table S3: Table 1's categorical approach
ACKNOWLEDGEMENTS
We would like to thank Melanie Berger for extracting the ICD‐10 codes and explaining the rules of coding. Additionally, we also thank Leo Steinberger for extracting the data from the hospital clinical information system. Open Access Funding provided by Universite de Geneve.
Lisibach A, Gallucci G, Beeler PE, Csajka C, Lutters M. High anticholinergic burden at admission associated with in‐hospital mortality in older patients: A comparison of 19 different anticholinergic burden scales. Basic Clin Pharmacol Toxicol. 2022;130(2):288-300. doi: 10.1111/bcpt.13692
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gao L, Maidment I, Matthews FE, Robinson L, Brayne C, On behalf of the Medical Research Council Cognitive Function and Ageing Study . Medication usage change in older people (65+) in England over 20 years: Findings from CFAS I and CFAS II. Age Ageing 2018;47(2):220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grossi CM, Richardson K, Savva GM, et al. Increasing prevalence of anticholinergic medication use in older people in England over 20 years: Cognitive function and ageing study I and II. BMC Geriatr. 2020;20(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kersten H, Wyller TB. Anticholinergic drug burden in older people's brain—How well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151‐159. [DOI] [PubMed] [Google Scholar]
- 4. Rudd KM, Raehl CL, Bond CA, Abbruscato TJ, Stenhouse AC. Methods for assessing drug‐related anticholinergic activity. Pharmacotherapy. 2005;25(11):1592‐1601. [DOI] [PubMed] [Google Scholar]
- 5. Lisibach A, Benelli V, Ceppi MG, Waldner‐Knogler K, Csajka C, Lutters M. Quality of anticholinergic burden scales and their impact on clinical outcomes: A systematic review. Eur J Clin Pharmacol. 2020;77(2):147‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non‐degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: Longitudinal cohort study. BMJ. 2006;332(7539):455‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bishara D, Harwood D, Sauer J, Taylor DM. Anticholinergic effect on cognition (AEC) of drugs commonly used in older people. Int J Geriatr Psychiatry. 2017;32(6):650‐656. [DOI] [PubMed] [Google Scholar]
- 8. Briet J, Javelot H, Heitzmann E, et al. The anticholinergic impregnation scale: Towards the elaboration of a scale adapted to prescriptions in French psychiatric settings. Therapie. 2017;72(4):427‐437. [DOI] [PubMed] [Google Scholar]
- 9. Campbell NL, Maidment I, Fox C, Khan B, Boustani M. The 2012 update to the anticholinergic cognitive burden scale. J Am Geriatr Soc. 2013;61:S142‐S143. [Google Scholar]
- 10. Cancelli I, Valentinis L, Merlino G, Valente M, Gigli GL. Drugs with anticholinergic properties as a risk factor for psychosis in patients affected by Alzheimer's disease. Clin Pharmacol Ther. 2008;84(1):63‐68. [DOI] [PubMed] [Google Scholar]
- 11. Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug‐related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481‐1486. [DOI] [PubMed] [Google Scholar]
- 12. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333‐1341. [DOI] [PubMed] [Google Scholar]
- 13. Duran CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69(7):1485‐1496. [DOI] [PubMed] [Google Scholar]
- 14. Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson's disease: A cohort study. J Neurol Neurosurg Psychiatry. 2010;81(2):160‐165. [DOI] [PubMed] [Google Scholar]
- 15. Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56(12):2203‐2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hefner G, Shams M, K. W‐S. Rating the delirogenic potential of drugs for prediction of side effects in elderly psychiatric inpatients. J J Pharma Pharmacovigilance. 2015;1(003). [Google Scholar]
- 17. Jun K, Hwang S, Ah YM, Suh Y, Lee JY. Development of an Anticholinergic Burden Scale specific for Korean older adults. Geriatr Gerontol Int. 2019;628‐634. [DOI] [PubMed] [Google Scholar]
- 18. Kiesel EK, Hopf YM, Drey M. An anticholinergic burden score for German prescribers: Score development. BMC Geriatr. 2018;18(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161(1):116‐124. [DOI] [PubMed] [Google Scholar]
- 20. Nery RT, Reis AMM. Development of a Brazilian anticholinergic activity drug scale. Einstein (Sao Paulo, Brazil). 2019;17(2):eAO4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rudolph J, Salow M, Angelini MC. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508‐513. [DOI] [PubMed] [Google Scholar]
- 22. Sittironnarit G, Ames D, Bush AI, et al. Effects of anticholinergic drugs on cognitive function in older Australians: Results from the AIBL study. Dement Geriatr Cogn Disord. 2011;31(3):173‐178. [DOI] [PubMed] [Google Scholar]
- 23. Summers KW. A clinical method of estimating risk of druc induced delirium. Life Sci. 1978;22(17):1511‐1516. [DOI] [PubMed] [Google Scholar]
- 24. Xu D, Anderson HD, Tao A, et al. Assessing and predicting drug‐induced anticholinergic risks: An integrated computational approach. Ther Adv Drug Saf. 2017;8(11):361‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781‐787. [DOI] [PubMed] [Google Scholar]
- 26. Hilmer SN, Mager DE, Simonsick EM, et al. Drug burden index score and functional decline in older people. Am J Med. 2009;122(12):1142‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. The Lancet. 2007;370(9596):1453‐1457. [DOI] [PubMed] [Google Scholar]
- 28. Hunstein D. Handbuch ergebnisorientiertes PflegeAssessment AcuteCare© (ePA AC©). Wiesbaden: ePA‐Competence‐Center; 2009. [Google Scholar]
- 29. Gasparini A. Comorbidity: An R package for computing comorbidity scores. J Open Source Softw. 2018;3(23):648. [Google Scholar]
- 30. Tableone: Create ‘Table 1’ to describe baseline characteristics with or without proensity score weights [computer program]. 2020.
- 31. Beeler PE, Cheetham M, Held U, Battegay E. Depression is independently associated with increased length of stay and readmissions in multimorbid inpatients. Eur J Intern Med. 2020;73:59‐66. [DOI] [PubMed] [Google Scholar]
- 32. Siebenhüner K, Blaser J, Nowak A, et al. Comorbidities associated with worse outcomes among inpatients admitted for acute gastrointestinal bleeding. Dig Dis Sci. 2021;1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RStudio: Integrated Development for R [computer program]. Boston, MA: RStudio; 2019. [Google Scholar]
- 34. R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 35. Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178‐183. [Google Scholar]
- 36. Kidd AC, Musonda P, Soiza RL, et al. The relationship between total anticholinergic burden (ACB) and early in‐patient hospital mortality and length of stay in the oldest old aged 90 years and over admitted with an acute illness. Arch Gerontol Geriatr. 2014;59(1):155‐161. [DOI] [PubMed] [Google Scholar]
- 37. Ah YM, Suh Y, Jun K, Hwang S, Lee JY. Effect of anticholinergic burden on treatment modification, delirium and mortality in newly diagnosed dementia patients starting a cholinesterase inhibitor: A population‐based study. Basic Clin Pharmacol Toxicol. 2019;124(6):741‐748. [DOI] [PubMed] [Google Scholar]
- 38. Chatterjee S, Bali V, Carnahan RM, Chen H, Johnson ML, Aparasu RR. Risk of mortality associated with anticholinergic use in elderly nursing home residents with depression. Drugs Aging. 2017;34(9):691‐700. [DOI] [PubMed] [Google Scholar]
- 39. Corsonello A, Cozza A, D'Alia S, et al. The excess mortality risk associated with anticholinergic burden among older patients discharged from acute care hospital with depressive symptoms. Eur J Intern Med. 2019;61:69‐74. [DOI] [PubMed] [Google Scholar]
- 40. Cross AJ, George J, Woodward MC, et al. Potentially inappropriate medication, anticholinergic burden, and mortality in people attending memory clinics. J Alzheimer's Dis: JAD. 2017;60(2):349‐358. [DOI] [PubMed] [Google Scholar]
- 41. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: The medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477‐1483. [DOI] [PubMed] [Google Scholar]
- 42. Gamble DT, Clark AB, Luben RN, Wareham NJ, Khaw KT, Myint PK. Baseline anticholinergic burden from medications predicts incident fatal and non‐fatal stroke in the EPIC‐Norfolk general population. Int J Epidemiol. 2018;47(2):625‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumpula E‐K, Bell JS, Soini H, Pitkala KH. Anticholinergic drug use and mortality among residents of long‐term care facilities: A prospective cohort study. J Clin Pharmacol. 2011;51(2):256‐263. [DOI] [PubMed] [Google Scholar]
- 44. Lattanzio F, Corica F, Schepisi R, et al. Anticholinergic burden and 1‐year mortality among older patients discharged from acute care hospital. Geriatr Gerontol Int. 2018;18(5):705‐713. [DOI] [PubMed] [Google Scholar]
- 45. Mangoni AA, Kim S, Hakendorf P, Mayner L, Woodman RJ. Heat waves, drugs with anticholinergic effects, and outcomes in older hospitalized adults. J Am Geriatr Soc. 2016;64(5):1091‐1096. [DOI] [PubMed] [Google Scholar]
- 46. Mangoni AA, van Munster BC, Woodman RJ, de Rooij SE. Measures of anticholinergic drug exposure, serum anticholinergic activity, and all‐cause postdischarge mortality in older hospitalized patients with hip fractures. Am J Geriatric Psych Off J Am Assoc Geriatric Psychiatry. 2013;21(8):785‐793. [DOI] [PubMed] [Google Scholar]
- 47. Myint PK, Fox C, Kwok CS, Luben RN, Wareham NJ, Khaw K‐T. Total anticholinergic burden and risk of mortality and cardiovascular disease over 10 years in 21,636 middle‐aged and older men and women of EPIC‐Norfolk prospective population study. Age Ageing. 2015;44(2):219‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan ECK, Eriksdotter M, Garcia‐Ptacek S, Fastbom J, Johnell K. Anticholinergic burden and risk of stroke and death in people with different types of dementia. J Alzheimer's Dis: JAD. 2018;65(2):589‐596. [DOI] [PubMed] [Google Scholar]
- 49. Vetrano DL, La Carpia D, Grande G, et al. Anticholinergic medication burden and 5‐year risk of hospitalization and death in nursing home elderly residents with coronary artery disease. J Am Med Dir Assoc. 2016;17(11):1056‐1059. [DOI] [PubMed] [Google Scholar]
- 50. Egberts A, van der Craats ST, van Wijk MD, Alkilabe S, van den Bemt PMLA, Mattace‐Raso FUS. Anticholinergic drug exposure is associated with delirium and postdischarge institutionalization in acutely ill hospitalized older patients. Pharmacol Res Perspect. 2017;5(3):e00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gutierrez‐Valencia M, Martinez‐Velilla N, Liborio Vetrano D, et al. Anticholinergic burden and health outcomes among older adults discharged from hospital: Results from the CRIME study. Eur J Clin Pharmacol. 2017;73(11):1467‐1474. [DOI] [PubMed] [Google Scholar]
- 52. Lowry E, Woodman RJ, Soiza RL, Mangoni AA. Associations between the anticholinergic risk scale score and physical function: Potential implications for adverse outcomes in older hospitalized patients. J Am Med Dir Assoc. 2011;12(8):565‐572. [DOI] [PubMed] [Google Scholar]
- 53. Lu WH, Wen YW, Chen LK, Hsiao FY. Effect of polypharmacy, potentially inappropriate medications and anticholinergic burden on clinical outcomes: A retrospective cohort study. CMAJ. 2015;187(4):E130‐E137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sarbacker GB, Espino DV, Wood RC, Oakes SL, Anand D, Markides KA. Total anticholinergic burden and survival within a cohort of elderly Mexican Americans. Geriatr Gerontol Int. 2017;17(10):1515‐1521. [DOI] [PubMed] [Google Scholar]
- 55. Sevilla‐Sanchez D, Molist‐Brunet N, Gonzalez‐Bueno J, Sola‐Bonada N, Espaulella‐Panicot J, Codina‐Jane C. Prevalence, risk factors and adverse outcomes of anticholinergic burden in patients with advanced chronic conditions at hospital admission. Geriatr Gerontol Int. 2018;18(8):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 56. Bishara D, Perera G, Harwood D, et al. The anticholinergic effect on cognition (AEC) scale—Associations with mortality, hospitalisation and cognitive decline following dementia diagnosis. Int J Geriatr Psychiatry. 2020;35(9):1069‐1077. [DOI] [PubMed] [Google Scholar]
- 57. Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc. 2015;63(1):85‐90. [DOI] [PubMed] [Google Scholar]
- 58. Gupte KP, Wu W. Impact of anticholinergic load of medications on the length of stay of cancer patients in hospice care. Int J Pharm Pract. 2015;23(3):192‐198. [DOI] [PubMed] [Google Scholar]
- 59. Koshoedo S, Soiza RL, Purkayastha R, Mangoni AA. Anticholinergic drugs and functional outcomes in older patients undergoing orthopaedic rehabilitation. Am J Geriatr Pharmacother. 2012;10(4):251‐257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: All drugs that have been scored >0 in at least one anticholinergic burden scale (ABS). Drugs not listed or drugs not scored in one ABS are considered a score of 0. Combinations are scored as the sum of the single ingredients.
Table S4.1: Univariable and multivariable regression using the binary approach for the outcome in‐hospital mortality.
Table S4.2: Univariable and multivariable regression using the binary approach for the outcome LOS.
Table S4.3: Univariable and multivariable regression using the categorical approach for the outcome in‐hospital mortality.
Table S4.4: Univariable and multivariable regression using the categorical approach for the outcome LOS.
Table S4.5: Multivariable regression stratified according to age groups using the binary approach for the outcome in‐hospital mortality.
Table S2: Table 1's binary approach
Table S3: Table 1's categorical approach
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


