Abstract
Members of the genus Geobacter are the dominant metal-reducing microorganisms in a variety of anaerobic subsurface environments and have been shown to be involved in the bioremediation of both organic and metal contaminants. To facilitate the study of the physiology of these organisms, a genetic system was developed for Geobacter sulfurreducens. The antibiotic sensitivity of this organism was characterized, and optimal conditions for plating it at high efficiency were established. A protocol for the introduction of foreign DNA into G. sulfurreducens by electroporation was also developed. Two classes of broad-host-range vectors, IncQ and pBBR1, were found to be capable of replication in G. sulfurreducens. In particular, the IncQ plasmid pCD342 was found to be a suitable expression vector for this organism. When the information and novel methods described above were utilized, the nifD gene of G. sulfurreducens was disrupted by the single-step gene replacement method. Insertional mutagenesis of this key gene in the nitrogen fixation pathway impaired the ability of G. sulfurreducens to grow in medium lacking a source of fixed nitrogen. Expression of the nifD gene in trans complemented this phenotype. This paper constitutes the first report of genetic manipulation of a member of the Geobacter genus.
Dissimilatory metal-reducing microorganisms play an important role in the natural cycling of organic matter and minerals in aquatic sediments, submerged soils, and subsurface environments and can be important agents for the bioremediation of both organic and metal contamination (13–15). Molecular analyses (16S ribosomal DNA) have revealed that dissimilatory metal-reducing microorganisms in the genus Geobacter are prominent members of the microbial community in a diversity of environments in which dissimilatory metal reduction is either naturally occurring or artificially stimulated (22, 28). Geobacter species are obligate anaerobes belonging to the delta subdivision of the Proteobacteria. These organisms have the ability to completely oxidize organic compounds to carbon dioxide with either humic substances or Fe(III) as the sole electron acceptor (15). Other metals which can serve as electron acceptors for Geobacter species include Mn(IV), U(IV), Co(III), and Tc(VII). Several Geobacter species can also reduce nitrate and fumarate. The organic compounds oxidized by Geobacter species invariably include acetate and other short-chain fatty acids. In addition, some Geobacter species are capable of completely oxidizing monoaromatic compounds such as benzoate, phenol, p-cresol, and toluene to carbon dioxide with Fe(III) as the electron acceptor.
Little is known about the biochemical pathways that couple the oxidation of organic compounds to the reduction of metals in Geobacter species or about the regulation of these processes. Because many electron carriers will nonspecifically reduce metals and humic substances in vitro, it has been difficult to use biochemical studies to determine which of the numerous redox active proteins present in Geobacter species are actually involved in the reduction of metals and humic substances in vivo. It may therefore be easier to deduce the physiological roles of redox active proteins and enzyme complexes via a genetic approach—gene disruption followed by phenotypic analysis. Until now, the lack of a genetic system for Geobacter species has prevented the application of this type of approach to the study of the physiology of these organisms.
Here we report the development of a genetic system for Geobacter sulfurreducens. G. sulfurreducens, which was isolated from hydrocarbon-contaminated soil (7), has all of the important metabolic features of Geobacter species, including the ability to oxidize monoaromatic compounds (15). Furthermore, G. sulfurreducens also has the capacity to grow with fumarate serving as the sole electron acceptor, a property which is essential for the generation of mutants that are defective in the transfer of electrons to metals and humic substances.
Preliminary studies have suggested that G. sulfurreducens might have genes for nitrogen fixation (4). The ability to fix nitrogen may be required for Geobacter to compete successfully in petroleum-contaminated subsurface environments which are carbon rich but contain little fixed nitrogen (4). Methods for genetically manipulating G. sulfurreducens were developed as part of a study assessing the capacity of this organism to fix nitrogen. In this study, the targeted disruption of a G. sulfurreducens homolog of the nifD gene, a gene required for nitrogen fixation by other microorganisms (9), was found to eliminate the ability of G. sulfurreducens to grow in a medium devoid of fixed nitrogen. The ability of G. sulfurreducens to grow in this medium was restored when a functional copy of the gene was reintroduced in trans. These results indicate that G. sulfurreducens fixes nitrogen in a manner similar to that of other nitrogen-fixing microorganisms. The genetic techniques described herein should be applicable to the study of other aspects of G. sulfurreducens physiology and should make it possible to take full advantage of the information present in the forthcoming sequence of the genome of this environmentally significant organism.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The plasmids and bacterial strains, G. sulfurreducens (ATCC 51573) and Escherichia coli, that were used in this study are listed and described in Table 1.
TABLE 1.
Strains and plasmids
| Species or plasmid | Strain or replicon(s); host range | Genotype or markers; characteristics and uses | Source or reference(s) |
|---|---|---|---|
| E. coli | DH5α | supE44 ΔlacU169 (φ 80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 5, 11 |
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thiΔ(lac-proAB) F′[traD36 proAB+ lacIq lacZΔM15] | 31 | |
| G. sulfurreducens | DL1 | Wild type, derived from single colony isolated on an NBAFYE plate | 7 |
| DL1/pCD354 | DL1 containing pCD354 | This work | |
| DL2A | nifD1::kan | This work | |
| DL2B | nifD1::kan | This work | |
| DL2C | nifD1::kan | This work | |
| DL2D | nifD1::kan | This work | |
| DL2D/pCDSnifD | DL2 containing pCDSnifD | This work | |
| Plasmids | |||
| pBBR1MCS-2 | ND,a broad | Kanr; unstable in G. sulfurreducens; source of kanamycin resistance cassette for pBRnif::kan | 12 |
| pBR322 | pMB1; E. coli | Ampr Tetr; suicide vector in G. sulfurreducens | 6 |
| pBRnif | pMB1; E. coli | Ampr; G. sulfurreducens nifH/nifD PCR fragment cloned into pBR322(ΔEcoRI-BamHI); intermediate in the construction of pBRnif::kan | This work |
| pBRnif::kan | pMB1; E. coli | Ampr Kanr; kanamycin resistance cassette cloned into EcoRV site of pBRnif; suicide vector for construction of nifD1::kan mutants in G. sulfurreducens | This work |
| pCD342 | IncQ; broad | Kanr; expression vector; suitable for use in G. sulfurreducens | This work |
| pCD354 | IncQ; broad | Kanr; gfpmut2 expression vector; suitable for use in G. sulfurreducens | This work |
| pCDnifD | IncQ; broad | Kanr; G. sulfurreducens nifD coding sequence cloned into pCD342 (ΔHindIII-EcoRI); intermediate in the construction of pCDSnifD | This work |
| pCDSnifD | IncQ; broad | Strr; streptomycin resistance cassette cloned into pCDnifD (ΔBstBI-BglII); nifD expression vector for G. sulfurreducens nifD1::kan mutants | This work |
| pBMK7 | pMB1, pBG1; E. coli, Desulfovibrio spp. | Kanr; E. coli and Desulfovibrio sp. shuttle vector; suicide vector in G. sulfurreducens | 23 |
ND, the incompatibility group of pBBR1MCS-2 has not been defined (2).
DNA manipulations and plasmid construction.
Total G. sulfurreducens genomic DNA was prepared using the Genome DNA Kit (Bio 101, Inc., Carlsbad, Calif.). Primers for amplification of DNA fragments from the G. sulfurreducens chromosome were designed using sequence data obtained from The Institute for Genomic Research website at http://www.tigr.org. PCR amplification was performed using AmpliTaq DNA polymerase (Roche Molecular Systems, Branchburg, N.J.) according to the manufacturer's instructions. Plasmid DNA was purified from E. coli and G. sulfurreducens strains using the Qiagen midi- or miniplasmid purification kits (Qiagen Inc., Valencia, Calif.). DNA samples for electrotransformation were subjected to a second ethanol precipitation and 70% ethanol wash followed by resuspension in 0.5× TE buffer (0.5 mM Tris HCl [pH 8.0], 0.5 mM EDTA) in order to reduce their salt concentration and prevent arcing.
The suicide vector, pBRnif::kan, was constructed for the targeted disruption of the G. sulfurreducens nifD gene. A 1.1-kb fragment consisting of the first ∼0.9 kb of the nifD coding region and ∼0.2 kb of upstream sequence was amplified from the G. sulfurreducens chromosome using the primers prDL1 (CCCCGCTGGAGATGGAAGAGC) and prDL2 (GGCGGATCCAGCCAGGGGATGCC). In primer sequences, restriction sites are italicized and annealing nucleotides are indicated in boldface. This fragment was digested with EcoRI (cleaves 18 bp downstream of prDL1) and BamHI (cleaves prDL2) and was inserted into the corresponding sites of pBR322 (6), resulting in the intermediate construct pBRnif. A kanamycin resistance cassette was amplified from pBBR1MCS-2 (12) with the primers prDL3 (CCCGGTAACCGGATGAATGTCAGC) and prDL4 (CCCGATATCGCGGTGGAATCG). The final suicide vector, pBRnif::kan (Fig. 2B), was constructed by inserting this cassette into a unique EcoRV site within pBRnif (at position 373 of the nifD coding sequence).
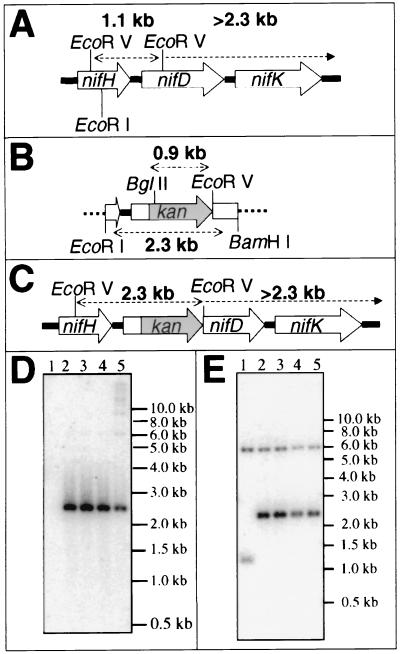
FIG. 2.
Confirmation of nifD gene disruption by Southern blot analysis. (A) Restriction map of the G. sulfurreducens nifHDK operon. (B) Restriction map of pBRnif::kan. The vector pBR322 is indicated by thick dotted lines. (C) Restriction map of G. sulfurreducens nifHDK operon containing the nifD1::kan insertion mutation. (D) Southern blot of genomic DNA prepared from wild-type (lane 1) and nifD1::kan strains (lanes 2 to 5) of G. sulfurreducens. Genomic DNA was digested with the restriction enzyme EcoRV and probed with a BglII/EcoRV restriction fragment of the kanamycin resistance cassette of pBRnif::kan. Expected radiolabeled bands are as follows: lane 1, none; lanes 2 to 5, 2.3 kb. (E) Southern blot of genomic DNA prepared from wild-type (lane 1) and nifD1::kan (lanes 2 to 5) strains of G. sulfurreducens. Genomic DNA was digested with the restriction enzyme EcoRV, blotted, and then probed with a BamHI/EcoRI restriction fragment of pBRnif::kan. Expected radiolabeled bands are as follows: lane 1, 1.1 and >2.3 kb; lanes 2 to 5, 2.3 and >2.3 kb. All restriction maps were based on sequence data obtained from http://www.tigr.org.
The nifD expression vector pCDSnifD was constructed in order to complement the nifD1::kan phenotype. The nifD coding sequence was amplified from G. sulfurreducens genomic DNA with the primers prDL5 (CCCGGTACCTGACAGGAGAATAC) and prDL6 (CCCAAGCTTAAAAGCGGACTCCG) and was inserted into pCR2.1-TOPO (Invitrogen, Carlsbad, Calif.). It was subsequently excised from this plasmid with EcoRI and HindIII and was inserted into the corresponding sites of the IncQ expression vector pCD342 (10). Because the resulting vector pCDnifD conferred resistance to kanamycin, it was not suitable for carrying out complementation studies in our nifD1::kan mutants. Therefore, the kanamycin resistance cassette of pCDnifD was excised with BglII and BstBI and replaced with a streptomycin resistance cassette that had been amplified from pJRD215 (8) with the primers prDL7 (CCCAGATCTTTCTCATGTTTGACAGC) and prDL8 (CCCTTCGAATGAGATTGATGTGTTCC).
Culturing conditions and growth media.
The two E. coli strains listed in Table 1 were propagated according to established methods (26).
G. sulfurreducens strains were cultured at 30°C under strict anaerobic conditions as previously described (19). Plating and incubations on solid media were performed inside an anaerobic chamber (Coy Laboratory Products Inc., Grass Lake, Mich.) containing a 7% H2–10%CO2–83% N2 atmosphere heated to 30°C. G. sulfurreducens strains were propagated in two types of media (see compositions below): NBAF supplemented with 0.1% yeast extract and 1 mM cysteine (NBAFYE) and FWAFC. NBAFYE was used for general propagation and plating and for the preparation of electrocompetent cells, whereas FWAFC was used for the analysis of nitrogen fixation phenotypes. Antibiotics were added to cultures or plates as needed. During extended incubations of liquid cultures, fresh antibiotics were added every 48 h.
All media were dispensed into anaerobic pressure tubes or bottles with butyl rubber stoppers and, unless otherwise indicated, bubbled with an 80% N2–20% CO2 gas mixture to remove dissolved oxygen and obtain a final pH of ∼6.7.
NBAF, a modified form of the medium described by Lovley et al. (16), contains 15 mM acetate as the electron donor and 40 mM fumarate as the electron acceptor. Its composition per liter of deionized water is 0.42 g of KH2PO4, 0.22 g of K2HPO4, 0.2 g of NH4Cl, 0.38 g of KCl, 0.36 g of NaCl, 0.04 g of CaCl2 · 2H2O, 0.1 g of MgSO4 · 7H2O, 1.8 g of NaHCO3, 0.5 g of Na2CO3, 2.04 g of NaC2H3O2 · 3H2O, 6.4 g of Na2C4H4O4, 0.5 ml of 0.1% resazurin, 1.0 ml of 100 mM Na2SeO4, 10.0 ml of a vitamin solution (17), and 10.0 ml of NB trace mineral solution. The composition of the NB trace mineral solution per liter of deionized water is 2.14 g of nitriloacetic acid, 0.1 g of MnCl2 · 4H2O, 0.3 g of FeSO4 · 7H2O, 0.17 g of CoCl2 · 6H2O, 0.2 g of ZnSO4 · 7H2O, 0.3 g of CuCl2 · 2H2O, 0.005 g of AlK(SO4)2 · 12H2O, 0.005 g of H3BO3, 0.09 g of Na2MoO4, 0.11 g of NiSO4 · 6H2O, and 0.2 g of Na2WO4 · 2H2O.
Liquid NBAFYE medium was prepared by adding appropriate volumes of sterile anoxic stock solutions of yeast extract and cysteine to NBAF medium to achieve final concentrations of 0.1% (wt/vol) and 1 mM, respectively. If NBAFYE medium was to be used for manipulations within the anaerobic chamber, the final cysteine concentration was increased to 5 mM. Solid NBAFYE was prepared by amending NBAF medium lacking CaCl2 and MgSO4 with 15 g of purified agar/liter. Following sterilization, the molten medium was cooled to 50°C and appropriate volumes of anaerobic stock solutions of CaCl2, MgSO4, yeast extract, and cysteine were added to yield final concentrations of 0.004%, 0.01%, 0.1%, and 5 mM, respectively. If necessary, antibiotics (concentrations listed below) were also added at this time.
FWAFC is a modification of FWA-Fe(III) citrate medium (19), a freshwater minimal medium containing 20 mM acetate as the electron donor and 55.9 mM Fe(III) citrate as the electron acceptor. Its composition per liter of deionized water is 13.7 g of FeC6O7, 2.5 g of NaHCO3, 0.25 g of NH4Cl, 0.6 g of NaH3PO4 · H2O, 0.1 g of KCl, 1.0 ml of 100 mM Na2SeO4, 10.0 ml of a vitamin solution (17), and 10.0 ml of a trace mineral solution (17).
Ammonium- and nitrogen-free FWAFC medium was prepared by sparging and overlaying ammonium-free FWAFC medium with an 80% Ar–20% CO2 gas mixture.
Antibiotics (200 μg of kanamycin, 400 μg of streptomycin, or 10 μg of chloramphenicol per ml) were added to liquid and solid medium as needed. All antibiotic stock solutions were sterile and anoxic (sparged and overlaid with an N2 atmosphere).
Determination of plating efficiency.
The cell density (cells/milliliter) of prestationary-phase liquid cultures was determined by staining cells with acridine orange and utilizing epifluorescence microscopy as previously described (19). The density of CFU in these cultures was determined by plating serial dilutions.
Preparation of electrocompetent cells.
Electrocompetent E. coli was prepared according to Sambrook et al. (26). Electrocompetent G. sulfurreducens was prepared from cultures maintained in NBAFYE medium as described below.
All manipulations were carried out on ice in an anaerobic chamber, and any buffers used were ice-cold and anoxic. Four hundred milliliters of mid-log-phase cultures (optical density at 600 nm = 0.2 to 0.35; 9 × 107 to 1.8 × 108 cells/ml) was harvested by centrifugation at 4°C for 8 min at 4,300 × g. The cells were washed twice with 400 ml of electroporation buffer (1 mM HEPES [pH 7.0], 1 mM MgCl2, and 175 mM sucrose) and resuspended in the same buffer at a final concentration of ∼1011 cells/ml. Because G. sulfurreducens was found to be particularly susceptible to shearing, pipetting of cell suspensions was minimized and, when necessary, performed with large-bore pipette tips. An appropriate volume of a 60% dimethyl sulfoxide (DMSO)–40% electroporation buffer solution was added to the final cell suspension to achieve a final DMSO concentration of 10%. The resulting electrocompetent cells were either electroporated immediately or stored at −70°C for future use.
Electrotransformation procedures.
All electrotransformations were performed in 0.15-cm-gap microelectroporation chambers using a Cell-Porator equipped with a Voltage Booster (Life Technologies Inc., Gaithersburg, Md.). Electrocompetent E. coli cells were transformed according to the manufacturer's instructions.
Electrocompetent G. sulfurreducens cells (25 μl), either freshly prepared or thawed on ice, were pulsed at 14.7 kV/cm for ∼6 ms (resistance = 4 kΩ; capacitance = 25 μF). Immediately following electroporation, cells were washed into the bottom of the microelectroporation chamber with 1 ml of room temperature phosphate-buffered NBAF (NBAF medium containing 50 mM potassium phosphate instead of bicarbonate). The cells were then transferred to a prewarmed anaerobic pressure tube containing 9 ml of NBAFYE. The electroporated cells were allowed to recover for 5 h at 30°C, prior to plating onto the appropriate solid growth medium.
Assessment of plasmid stability under nonselective conditions.
G. sulfurreducens was electrotransformed with either pBBR1MCS-2 or pJRD215 (Table 1) and plated onto selective medium (NBAFYE containing 200 μg of kanamycin/ml). The resulting kanamycin-resistant colonies were transferred to liquid selective medium until the cultures became turbid (3 to 6 days). Selective pressure was maintained throughout this incubation by adding fresh kanamycin to the cultures every 48 h. Twenty-four hours prior to the beginning of the experiment, cultures were diluted 10-fold into fresh selective medium. At time zero, the cultures were serially diluted into nonselective medium (NBAFYE) and were then plated onto both selective and nonselective media. The remainder of the various serial dilutions was incubated at 30°C. Every 48 h, a log-phase culture was selected from among the various dilutions for analysis and the process was repeated. The total number of CFU in the various cultures was determined on nonselective medium, and the percentage of kanamycin-resistant CFU was ascertained by comparison. The number of generations between time points was determined from the total CFU/milliliter using the following formula: log (final CFU per milliliter/initial CFU per milliliter)/log2.
Southern blotting.
Following digestion of genomic DNA with the restriction enzyme EcoRV, Southern blot analysis was performed according to Sambrook et al. (26). Probes were labeled with [α-32P]dCTP using the Multiprime DNA labeling system (Amersham Pharmacia Biotech, Piscataway, N.J.). [α-32P]dCTP was obtained from New England Nuclear (Boston, Mass.).
RESULTS AND DISCUSSION
Growth on solid medium and characterization of antibiotic sensitivity.
In order to be able to rapidly isolate clonal populations of G. sulfurreducens, conditions for high-efficiency plating onto solid growth medium were established. Simply solidifying the two liquid media for G. sulfurreducens, NBAF and FWAFC (see Materials and Methods), was not sufficient to achieve the rapid growth of G. sulfurreducens on plates. After considerable trial and error, it was found that supplementation of the solid form of NBAF medium with 0.1% yeast extract and a high concentration (5 mM) of the reductant cysteine yielded a solid medium, NBAFYE, on which both the growth rate and the plating efficiency of G. sulfurreducens were reproducibly high. Colonies were visible on solid NBAFYE medium after only 5 days of incubation at 30°C. In addition, when pre-stationary-phase liquid cultures with a density of (3.25 ± 0.54) × 108 cells/ml were plated onto this medium, (2.85 ± 0.48) × 108 CFU/ml were recovered (mean ± standard deviation; n = 3), demonstrating a high plating efficiency.
The growth of G. sulfurreducens on solid medium could be inhibited by a variety of commonly used antibiotics. Growth of 108 cells on solid medium could be inhibited by choramphenicol (10 μg/ml), nalidixic acid (10 μg/ml), tetracycline (10 μg/ml), kanamycin (200 μg/ml), spectinomycin (50 μg/ml), streptomycin (400 μg/ml), and ampicillin (400 μg/ml). The sensitivity of G. sulfurreducens to these antibiotics in liquid culture was similar (data not shown). These results indicated that it should be possible to select for the acquisition of multiple antibiotic resistance markers by G. sulfurreducens, a requirement for many genetic manipulations.
Development of an electrotransformation procedure.
A protocol for the electrotransformation of G. sulfurreducens was developed by modifying existing protocols (21) to account for the unique properties of G. sulfurreducens. The preparation of electrocompetent cells typically involves extensive washing of cells derived from mid-log-phase cultures in a suitable low-ionic-strength electroporation buffer, followed by resuspension of the cells at high density (109 to 1011 cells/ml) in this buffer (21). In order to attain sufficient G. sulfurreducens biomass at mid-log phase, it was necessary to supplement the NBAF medium routinely used to culture the organism with 0.1% (wt/vol) yeast extract. When grown on this rich medium at 30°C, G. sulfurreducens had a generation time of ∼4.5 h and reached a cell density of ∼108 cells/ml at mid-log phase (data not shown). The composition of the electroporation buffer was found to be critical for maintaining the viability of G. sulfurreducens throughout the washing and electrotransformation procedures. The optimal composition of the electroporation buffer was found to be 1 mM HEPES (pH 7.0), 1 mM MgCl2, and 175 mM sucrose. The addition of MgCl2 to the electroporation buffer was absolutely required if G. sulfurreducens was to remain viable during washing and storage. In the absence of MgCl2, 90% of the cells lysed prior to electroporation (data not shown). MgCl2 is thought to prevent bacterial lysis by stabilizing the outer membrane (27). The concentration of sucrose was another crucial variable in the composition of the electroporation buffer. Electrotransformed G. sulfurreducens could not tolerate large changes in osmolarity upon dilution back into nonselective growth medium. To avoid such changes in osmotic pressure, 175 mM sucrose was added to the electroporation buffer such that its osmolarity was equivalent to that of NBAFYE medium. The addition of 10% DMSO to the final cell suspension permitted storage of electrocompetent G. sulfurreducens at −70°C with little or no loss in transformation efficiency. Glycerol was not a suitable cryopreservant due to the sensitivity of G. sulfurreducens to rapid changes in osmolarity.
Electroporation parameters were optimized by transforming G. sulfurreducens with the broad-host-range IncQ plasmid pJRD215 (Table 1). Electrotransformation of G. sulfurreducens with pJRD215 resulted in the growth of kanamycin-resistant colonies. The presence of pJRD215 in these colonies was confirmed by PCR (data not shown) and by digesting plasmid DNA purified from these colonies with restriction enzymes (Fig. 1A, lane 1). Electrotransformation was optimal when G. sulfurreducens was pulsed at 14.7 kV/cm with a time constant of approximately 6 ms. Under these conditions, (1.98 ± 0.32) × 105 transformants/μg of pJRD215 DNA were obtained. Electroporation lethality was roughly 59% ± 5.23%, and transformants constituted 1 out of (1.19 ± 0.43) × 104 CFU. (Data are means ± standard errors; n = 11). These data were within the range reported for other bacteria in which gene replacement has been successful (3, 23, 24, 29).
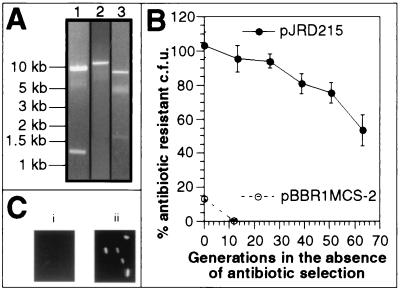
FIG. 1.
Stability of IncQ and pBBR1 vectors in G. sulfurreducens. (A) Purification of IncQ plasmids from G. sulfurreducens transformants. Plasmid DNA was purified from G. sulfurreducens transformed with pJRD215 (lane 1), pJRC2 (lane 2), and pCD342 (lane 3) by alkaline lysis (26). Following purification, plasmid DNA was digested with the restriction enzymes BglII and HindIII. The sizes of the expected restriction fragments for the three plasmids are as follows: 9.1 and 1.2 kb for pJRD215; 10.4 kb for pJRC2 (23); and 8.0 and 1.7 kb for pCD342 (10). (B) Stability of pJRD215 and pBBR1MCS-2 in G. sulfurreducens under nonselective conditions. Assessment of the stability of these plasmids in the absence of antibiotic selection is described in detail in Materials and Methods. Data are means ± standard errors of four independent experiments. (C) Expression of green fluorescent protein from an IncQ expression vector in G. sulfurreducens. Green fluorescent protein expression was visualized by Nomarski microscopy using a fluorescein isothiocyanate filter. Panel i, DL1, wild-type G. sulfurreducens. Panel ii, DL1/pCD354, G. sulfurreducens transformed with pCD354 (10).
As shown in Table 2, transformation efficiency increased to (2.96 ± 1.08) × 107 transformants/μg (mean ± standard error; n = 3) when pJRD215 DNA was purified from G. sulfurreducens. This 100-fold increase in transformation efficiency suggests that G. sulfurreducens may possess a robust restriction system.
TABLE 2.
Transformation of G. sulfurreducens with a variety of plasmids
| Plasmid | Replicon(s) | Host specificity | Antibiotic resistance | No. of transformantsa per μg of DNA | Stability (half-life in absence of antibiotic selection) |
|---|---|---|---|---|---|
| pJRD215 | IncQ | Broad | Kanr Strr | (1.98 ± 0.32) × 105 (11) | ∼60 generations |
| (2.96 ± 1.08) × 107 (3)c | |||||
| pJRC2 | IncQ | Broad | Chlr Strr | (4.06 ± 2.31) × 103 (3) | Not determined |
| pCD342 | IncQ | Broad | Kanr | (1.24 ± 0.37) × 104 (3) | Not determined |
| pBBR1MCS-2 | NDb | Broad | Kanr | (2.79 ± 0.41) × 104 (3) | <10 generations |
| pBMK7 | pMB1, pBG1 | E. coli, Desulfovibrio spp. | Kanr | 0 (5) | Not determined |
Data are means ± standard errors, and the number of experiments performed is indicated in parentheses. The number of transformants per microgram of DNA was determined by counting kanamycin-resistant colonies except in the case of pJRC2, in which chloramphenicol-resistant colonies were counted. Unless otherwise indicated, G. sulfurreducens was transformed with plasmid DNA purified from E. coli strain JM109 or DH5α. The stability of pJRD215 and pBBR1MCS-2 was determined as described in Materials and Methods.
ND, the incompatibility group of pBBR1MCS-2 has not been defined (2).
The number of transformants obtained when pJRD215 was purified from G. sulfurreducens.
Identification of potential “suicide” and expression vectors.
A variety of plasmids were tested for the ability to replicate in and confer antibiotic resistance upon G. sulfurreducens (Table 2). In addition to pJRD215, two other IncQ plasmids, pJRC2 and pCD342 (Table 1), were evaluated. The plasmid pJRC2 is identical to pJRD215 except that it confers resistance to chloramphenicol. pCD342 is an expression vector containing a polylinker which is preceded by the hybrid tac-lac promoter (tac and lacUV5 promoters in tandem) and followed by the rrnB transcriptional terminator (1, 20). Electroporation of G. sulfurreducens with all three IncQ plasmids resulted in the growth of numerous antibiotic-resistant transformants (Table 2), indicating that all of these plasmids were capable of replication in G. sulfurreducens. In addition, all three of the IncQ plasmids could be purified from cultures of G. sulfurreducens in sufficient quantity to be identified by digestion with restriction enzymes (Fig. 1A), suggesting that IncQ plasmids are stable in this organism. In fact, pJRD215 has a half-life of ∼60 generations in G. sulfurreducens in the absence of antibiotic selection (Table 2; Fig. 1B). Similarly, we were able to purify pCD342 from G. sulfurreducens (Fig. 1A, lane 3) after culturing a transformant for approximately 30 generations in the absence of antibiotic selection.
To determine whether pCD342 could serve as an expression vector for G. sulfurreducens, cells were transformed with pCD354 (10), a green fluorescent protein expression vector derived from pCD342. The green fluorescent protein was detected in the resulting transformants (Fig. 1C), indicating that pCD342 is indeed a suitable expression vector for G. sulfurreducens.
Due to their large size (≥ 10 kb) and low copy number, IncQ vectors are difficult to manipulate (8, 10). The pBBR1MCS series of broad-host-range expression vectors (12), in contrast, are fairly small (∼5.3 kb) and have a higher copy number. In addition, they contain a variety of antibiotic resistance cassettes and possess an extensive multicloning site located within the lacZ alpha peptide (12). Kanamycin-resistant transformants were isolated following electroporation of G. sulfurreducens with pBBR1MCS-2 (Table 2), indicating that this class of plasmids is capable of replication in G. sulfurreducens. However, pBBR1MCS-2 was found to be unstable in G. sulfurreducens (Fig. 1B). Despite the rigorous maintenance of selective pressure during propagation of pBBR1MCS-2 in liquid culture, only 15% of the CFU in these cultures were able to grow on solid medium containing kanamycin. Furthermore, in the absence of continued selection, kanamycin resistance was lost within 12 generations. Thus, despite the fact that pBBR1MCS vectors can replicate in G. sulfurreducens, their instability renders them unsuitable for use as expression vectors.
In addition to the two types of broad-host-range plasmids mentioned above, one plasmid with limited host specificity, pBMK7 (23), was evaluated for the ability to replicate in G. sulfurreducens. The plasmid pBMK7 is a shuttle vector that contains two replicons: the pBM1 replicon, which functions in E. coli, and the pBG1 replicon, which functions in Desulfovibrio species. Members of the genus Desulfovibrio, like those of the Geobacter genus, are obligate anaerobes belonging to the delta subdivision of the Proteobacteria. However, electrotransformation of G. sulfurreducens with pBMK7 repeatedly failed to yield kanamycin-resistant transformants (Table 2). Thus, even though Geobacter and Desulfovibrio species are closely related, the pBG1 replicon did not appear to function in G. sulfurreducens. Furthermore, these results indicated that plasmids containing the pBG1 or pBM1 replicons acted as suicide vectors in G. sulfurreducens and could, therefore, be used to introduce mutations into its chromosome.
Mutagenesis of the G. sulfurreducens chromosome by gene replacement.
The next step in the development of a genetic system for G. sulfurreducens was to determine whether the function of a specific gene could be eliminated by placing a mutation in the G. sulfurreducens chromosome. A gene suspected of being involved in nitrogen fixation by G. sulfurreducens was selected as the first target, because, as noted above, it is important to conclusively demonstrate that G. sulfurreducens has the capacity to fix nitrogen. In other microorganisms, nitrogen reduction is catalyzed by the nitrogenase enzyme complex, which is encoded by the highly conserved nifH, nifD, and nifK genes (9, 30). Because the nitrogenase complex functions specifically in the nitrogen fixation pathway, inactivation of the genes for any of its components yields a predictable and easily characterized phenotype, an inability to grow in medium lacking fixed nitrogen (9). By using the nifD genes of other organisms to probe the partial sequence of the G. sulfurreducens genome, a potential G. sulfurreducens nifHDK operon was identified. Studies were initiated to determine whether disruption of the putative nifD gene of G. sulfurreducens by the single-step gene replacement method (25) would affect the ability of G. sulfurreducens to grow in a medium devoid of fixed nitrogen.
In order to disrupt the putative G. sulfurreducens nifD gene, we constructed the suicide vector pBRnif::kan (Table 1; Fig. 2B), which contained a fragment of the nifD coding sequence disrupted by a kanamycin resistance cassette. To ensure that incorporation of the disrupted nifD coding sequence into the chromosome occurred via a double recombination event, the suicide vector was linearized with NruI and ScaI prior to electroporation. The recovery period following electroporation was extended to ∼18 h to allow ample time for recombination and expression of kanamycin resistance. This procedure resulted in the growth of 2.77 × 105 kanamycin-resistant colonies per μg of linear DNA. Eight of these kanamycin-resistant colonies were screened for the presence of the disrupted nifD (nifD1::kan) coding sequence by PCR, and all eight were found to contain the nifD1::kan insertion mutation (data not shown). These results indicated that recombination between linear DNA fragments and the G. sulfurreducens chromosome was a highly efficient process.
As shown in Fig. 2, Southern blot analysis confirmed both the presence and placement of the kanamycin resistance cassette within the 5′ end of the nifD gene in four of the eight nifD1::kan mutants described above, strains DL2A to -D. All four of these genetically identical mutants were unable to completely reduce the available Fe(III) in FWAFC medium lacking a source of fixed nitrogen (data not shown). This result suggested that disruption of the nifD gene had resulted in the expected phenotype, an inability to grow in the absence of exogenous fixed nitrogen. One of the four mutants, DL2D, was selected for a more detailed characterization of its phenotype (Fig. 3).
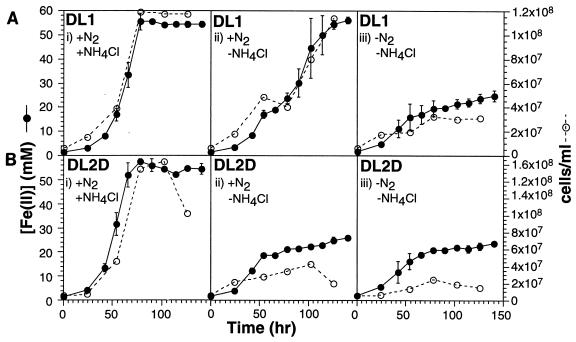
FIG. 3.
Nitrogen fixation by wild-type (DL1) and nifD1::kan (DL2D) G. sulfurreducens. FWAFC cultures of strains DL1 and DL2D were washed three times with FWAFC medium lacking both N2 and NH4Cl (N2 replaced by Ar) in order to obtain cell suspensions containing as little N2 and NH4Cl as possible. At time zero, the washed cells were inoculated into three types of FWAFC media: (i) FWAFC, (ii) ammonium-free FWAFC, and (iii) FWAFC lacking both N2 and NH4Cl at a final concentration of ∼6 × 106 cells/ml. Fe(II) concentration (in millimolars) and cell density (in cells/milliliter) were determined on 0.1-ml samples as previously described (18, 19). Fe(II) concentrations are means ± standard deviations of measurements obtained from three independent cultures, whereas cell densities were determined from one representative culture.
When strain DL2D and the wild-type strain DL1 were inoculated into FWAFC medium, they grew and reduced Fe(III) at similar rates (Fig. 3). When ammonium, the source of fixed nitrogen in the medium, was omitted, the wild-type strain exhibited a longer lag but eventually grew and reduced Fe(III) to levels comparable to those observed in ammonium-containing medium. The extended lag period most probably reflected the time required to induce the expression of nitrogen fixation genes. In contrast to the wild-type strain, the mutant strain DL2D failed to grow significantly in ammonium-free FWAFC medium and reduced only a small amount of the available Fe(III). In fact, the amount of growth and Fe(III) reduction by the mutant strain in ammonium-free medium was comparable to that in medium lacking both N2 and ammonium.
The small amount of cell growth observed in medium devoid of both N2 and ammonium (Fig. 3) can be attributed to the fact that residual ammonium may have been present in the washed starter cultures and that any cell lysis that occurred during washing would have resulted in the release of additional fixed nitrogen. The sustained low rate of Fe(III) reduction seen in this medium at the end of the experiment is probably due to the fact that G. sulfurreducens is still capable of reducing Fe(III) even when it is not actively growing.
Complementation of the nifD1::kan phenotype by in trans expression of the nifD gene.
In order to demonstrate that the phenotype of mutant DL2D was in fact due to disruption of the nifD gene and not to an unexpected secondary mutation or to an unanticipated effect on downstream gene expression, the nifD expression vector pCDSnifD was introduced into DL2D by electroporation. To determine if expression of the nifD gene in trans complemented the nifD1::kan phenotype, the ability of DL2D/pCDSnifD to grow in the absence of fixed nitrogen was evaluated (Fig. 4). The extent of cell growth and iron reduction by both DL2D/pCDSnifD and the wild-type strain (DL1) was similar under all three conditions tested (Fig. 4): (i) in the presence of both gaseous and fixed nitrogen, (ii) in the presence of gaseous nitrogen alone, and (iii) in the absence of both gaseous and fixed nitrogen. Thus, expression of the nifD gene in trans restored the ability of the nifD1::kan mutant DL2D to grow in the absence of exogenous fixed nitrogen. In fact, DL2D/pCDSnifD can be routinely cultured in ammonium-free FWAFC medium (data not shown).
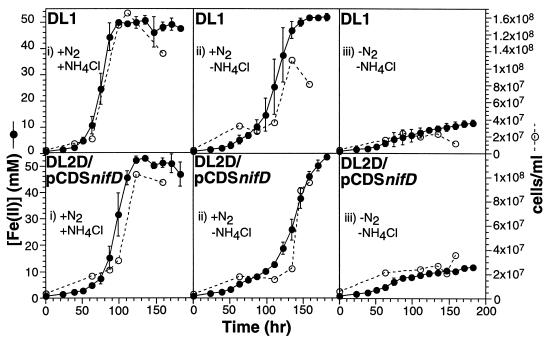
FIG. 4.
Complementation of nifD1::kan phenotype by expression of nifD gene in trans. In order to select for the maintenance of pCDSnifD, strain DL2D/pCDSnifD was maintained in FWAFC medium containing 400 mg of streptomycin/ml until the beginning of the experiments. To obtain cell suspensions containing as little N2, NH4Cl, and streptomycin as possible, FWAFC cultures of strains DL1 (wild type) and DL2D/pCDSnifD were washed three times with FWAFC medium without both N2 and NH4Cl (N2 replaced by Ar). At time zero, washed cells were inoculated into three types of FWAFC media: (i) FWAFC, (ii) ammonium-free FWAFC, and (iii) FWAFC lacking both N2 and NH4Cl at a final concentration of ∼3.6 × 106 cells/ml. Fe(II) concentration and cell density were determined on 0.1-ml samples as previously described (18, 19). Fe(II) concentrations are the means ± standard deviations of measurements obtained from three independent cultures, whereas cell densities were determined from one representative culture.
Although antibiotics, which constitute potential sources of fixed nitrogen, could not be used to select for the maintenance of pCDSnifD and the nifD1::kan mutation over the course of this experiment, PCR screening confirmed the presence of both the nifD1::kan mutation and pCDSnifD in strain DL2D/pCDSnifD at the end of the experiment (data not shown).
The results described above confirm that G. sulfurreducens has the ability to fix nitrogen. In addition, these results demonstrate that it is possible to complement mutations in G. sulfurreducens, a genetic technique required for properly evaluating the function of genes in this organism.
Concluding remarks.
In summary, this study has demonstrated that G. sulfurreducens is a genetically tractable microorganism. It is now possible to introduce foreign DNA into G. sulfurreducens by electroporation, to place mutations at specific locations within its chromosome, and to express proteins from extrachromosomal elements. These novel genetic techniques coupled with the availability of the sequence of the G. sulfurreducens genome will enable the rapid study of the physiological function of multiple G. sulfurreducens genes. The finding that the physiology of G. sulfurreducens can be studied by genetic techniques is significant, because microorganisms closely related to G. sulfurreducens are prominent members of a variety of Fe(III)-reducing microbial communities (22, 28). Thus, studies on the physiology of G. sulfurreducens, using the techniques outlined here, are likely to provide insights into microbial processes occurring in the Fe(III) reduction zone of subsurface environments. Studies similar to those described here but focusing on mechanisms for the reduction of Fe(III) and contaminant metals by G. sulfurreducens are currently in progress.
ACKNOWLEDGMENTS
This work was supported by the U.S. Department of Energy (grants DE-FG02–98ER20306 and DE-FG02–97ER-62475-A005).
All G. sulfurreducens sequence data used in this report were obtained from The Institute for Genomic Research website at http://www.tigr.org. We thank Judy Wall, Christoph Dehio, and Michael Kovach for sending us plasmids. We also acknowledge Betsy Harris for her guidance and assistance.
REFERENCES
- 1.Amann E, Brosius J, Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983;25:167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- 2.Antoine R, Locht C. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from Gram-positive organisms. Mol Microbiol. 1992;6:1785–1799. doi: 10.1111/j.1365-2958.1992.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 3.Battisti J M, Minnick M F. Development of a system for genetic manipulation of Bartonella bacilliformis. Appl Environ Microbiol. 1999;65:3441–3448. doi: 10.1128/aem.65.8.3441-3448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazylinski D A, Dean A J, Schüler D, Phillips E J, Lovley D R. N2-dependent growth and nitrogenase activity in the metal-metabolizing bacteria. Geobacter and Magnetospirillum species. Environ Microbiol. 2000;2:266–273. doi: 10.1046/j.1462-2920.2000.00096.x. [DOI] [PubMed] [Google Scholar]
- 5.Bethesda Research Laboratories. BRL pUC host: E. coli DH5αTM competent cells. Bethesda Res Lab Focus. 1986;8:9. [Google Scholar]
- 6.Bolivar F. Characterization of new cloning vehicles. Gene. 1978;4:121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- 7.Caccavo F J, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 9.Dean D R, Jacobson M R. Biochemical genetics of nitrogenase. In: Stacey G, Burris R H, Evans H J, editors. Biological nitrogen fixation. New York, N.Y: Chapman & Hall; 1992. pp. 763–864. [Google Scholar]
- 10.Dehio M, Knorre A, Lanz C, Dehio C. Construction of versatile high-level expression vectors for Bartonella henselae and the use of green fluorescent protein as a new expression marker. Gene. 1998;215:223–229. doi: 10.1016/s0378-1119(98)00319-9. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 12.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop R M I, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 13.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovley D R. Fe(III) and Mn(IV) reduction. In: Lovley D R, editor. Environmental microbe-metal interactions. Washington, D.C.: ASM Press; 2000. pp. 3–30. [Google Scholar]
- 15.Lovley D R. Fe(III)- and Mn(IV)-reducing prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The prokaryotes, in press. New York, N.Y: Springer-Verlag, Inc.; 2000. [Google Scholar]
- 16.Lovley D R, Fraga J L, Coates J D, Blunt-Harris E L. Humics as an electron donor for anaerobic respiration. Environ Microbiol. 1999;1:89–98. doi: 10.1046/j.1462-2920.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 17.Lovley D R, Greening R C, Ferry J G. Rapidly growing rumen methanogenic organism that synthesizes coenzyme M and has a high affinity for formate. Appl Environ Microbiol. 1984;48:81–87. doi: 10.1128/aem.48.1.81-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales V M, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 21.Nickoloff J A, editor. Methods in molecular biology series. 47. Electroporation protocols for microorganisms. Totowa, N.J: Humana Press; 1995. [Google Scholar]
- 22.Rooney-Varga J N, Anderson R T, Fraga J L, Ringelberg D, Lovley D R. Microbial communities associated with anaerobic benzene mineralization in a petroleum-contaminated aquifer. Appl Environ Microbiol. 1999;65:3056–3063. doi: 10.1128/aem.65.7.3056-3063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousset M, Casalot L, Rapp-Giles B J, Dermoun Z, De Philip P, Bélaich J P, Wall J D. New shuttle vectors for the introduction of cloned DNA in Desulfovibrio. Plasmid. 1998;39:114–122. doi: 10.1006/plas.1997.1321. [DOI] [PubMed] [Google Scholar]
- 24.Rousset M, Dermoun Z, Chippaux M, Bélaich J P. Marker exchange mutagenesis of the hydN genes in Desulfovibrio fructosovorans. Mol Microbiol. 1991;5:1735–1740. doi: 10.1111/j.1365-2958.1991.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 25.Russell C B, Thaler D S, Dahlquist F W. Chromosomal transformation of Escherichia coli recD strains with linearized plasmids. J Bacteriol. 1989;171:2609–2613. doi: 10.1128/jb.171.5.2609-2613.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Smith J C. Genetic transformation of Bacteroides spp. using electroporation. In: Nickoloff J A, editor. Methods in molecular biology series. 47. Electroporation protocols for microorganisms. Totowa, N.J: Humana Press; 1995. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- 28.Snoeyenbos-West O L, Nevin K P, Anderson R T, Lovley D R. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb Ecol. 2000;39:153–167. doi: 10.1007/s002480000018. [DOI] [PubMed] [Google Scholar]
- 29.Toyama H, Anthony C, Lidstrom M E. Construction of insertion and deletion mxa mutants of Methylobacterium extorquens AM1 by electroporation. FEMS Microbiol Lett. 1998;166:1–7. doi: 10.1111/j.1574-6968.1998.tb13175.x. [DOI] [PubMed] [Google Scholar]
- 30.Ueda T, Suga Y, Yahiro N, Matsuguchi T. Genetic diversity of N2-fixing bacteria associated with rice roots by molecular evolutionary analysis of a nifD library. Can J Microbiol. 1995;41:235–240. doi: 10.1139/m95-032. [DOI] [PubMed] [Google Scholar]
- 31.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]