Abstract
Assessing the persistence of chemicals in the environment is a key element in existing regulatory frameworks to protect human health and ecosystems. Persistence in the environment depends on many fate processes, including abiotic and biotic transformations and physical partitioning, which depend on substances' physicochemical properties and environmental conditions. A main challenge in persistence assessment is that existing frameworks rely on simplistic and reductionist evaluation schemes that may lead substances to be falsely assessed as persistent or the other way around—to be falsely assessed as nonpersistent. Those evaluation schemes typically assess persistence against degradation half‐lives determined in single‐compartment simulation tests or against degradation levels measured in stringent screening tests. Most of the available test methods, however, do not apply to all types of substances, especially substances that are poorly soluble, complex in composition, highly sorptive, or volatile. In addition, the currently applied half‐life criteria are derived mainly from a few legacy persistent organic pollutants, which do not represent the large diversity of substances entering the environment. Persistence assessment would undoubtedly benefit from the development of more flexible and holistic evaluation schemes including new concepts and methods. A weight‐of‐evidence (WoE) approach incorporating multiple influencing factors is needed to account for chemical fate and transformation in the whole environment so as to assess overall persistence. The present paper's aim is to begin to develop an integrated assessment framework that combines multimedia approaches to organize and interpret data using a clear WoE approach to allow for a more consistent, transparent, and thorough assessment of persistence. Integr Environ Assess Manag 2022;18:868–887. © 2021 ExxonMobil Biomedical Sciences, Inc. Integrated Environmental Assessment and Management published by Wiley Periodicals LLC on behalf of Society of Environmental Toxicology & Chemistry (SETAC).
Keywords: Degradation, Integrated framework for persistence assessment, Multimedia fate and transport model, Overall persistence, Weight‐of‐evidence
Key Points
Weight of Evidence (WoE) is needed to utilize different standard and non‐standard data types.
A WoE framework is presented to organize the comparisons and make use of all available data.
Overall persistence (P ov) supports holistic assessments that account for emission patterns and physicochemical properties of a substance.
Combined WoE and P ov frameworks will support improved persistence assessments.
INTRODUCTION
The occurrence and risks of chemical substances in the environment are based on several factors including mode of entry (e.g., emission pattern; ECETOC, 2011; Pennington, 2001), fate processes (e.g., interactions between compartments, advection, degradation), bioavailability, and interactions with biota. One of the most important aspects of chemical safety assessments of anthropogenic chemical substances (henceforth referred to as "substances") is the evaluation of the ability of a substance to be transformed in different environmental compartments (e.g., air, water, soil, sediment; Webster et al., 1998). The rates of transformation and emissions will directly influence the concentrations of a substance in these compartments and therefore exposure of biota: The lower the rates of transformation, the higher environmental concentrations will be and the longer a substance will persist. It is assumed that continuous release of a persistent substance may result in its large‐scale distribution and/or accumulation to levels that may have known or currently unknown adverse effects in biota (including humans; Abelkop et al., 2015). An important issue for highly persistent substances is that if ecotoxicological concerns are demonstrated after release into the environment, it would require many years to remove that substance from the environment after a restriction or ban on its use (Matthies et al., 2016).
Criteria for the identification of persistent anthropogenic chemical substances have been incorporated into chemical legislations in different parts of the world. The existing legislations, which define different protection objectives and different specific criteria for various environments, have been established as a result of scientific and policy discussions. Currently, persistence criteria are in most cases defined as half‐lives in single compartments, and these are derived from compartment‐based laboratory biodegradation studies, generally performed under aerobic conditions or are based on stringent screening tests (ECHA, 2017a). Such studies should be performed under controlled conditions to make it possible to obtain relevant and reproducible information for comparison with appropriate criteria. This narrow focus excludes several important environmental processes such as resuspension, dynamic partitioning, and photodegradation processes (Solomon et al., 2013) and adaptation of microbial communities (Poursat et al., 2019). The significance of these processes for persistence assessment is discussed in the companion paper (Davenport et al., 2021). Further, existing criteria were developed based on structurally specific classes of legacy pollutants and do not reflect the behavior of a wide range of other chemical classes (Matthies et al., 2016). It is questionable whether these criteria can be readily applied to other substances without considering their possible different intrinsic properties (Whale et al., 2021). For example, ionic substances, volatile and poorly soluble substances, substances of unknown or variable composition, complex reaction products, or biological materials (UVCBs), and polymers have special properties that can require adapted methods (Davenport et al., 2021; ECETOC, 2020) and dedicated frameworks for persistence evaluations.
The ability of a substance to be transformed depends on a combination of its intrinsic properties together with the physical and biogeochemical properties of the environmental system (biomass, temperature, organic matter, etc.; Boethling et al., 2009), which may be heterogeneous. Test design should minimize artifactual impacts of environmental factors (Davenport et al., 2021), for example, limited active biomass (Ott et al., 2019, 2020) or limited bioavailability (Ortega‐Calvo et al., 2015; Schäffer et al., 2018) to understand the intrinsic degradation potential for a given substance. Overall, the current general approach to regulatory persistence (P) assessments, with notable exceptions (ECCC, 2016), follows a simplistic approach in which many important influencing factors are not satisfactorily considered, and anthropogenic chemical substances of interest can therefore be falsely assessed to be intrinsically persistent. Further, the data can be conflicting, both within and between compartments, which complicates consistent persistence assessments. In the present paper, the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) Task Force “Moving persistence (P) assessments into the 21st Century” proposes a generalized conceptual framework to move P assessments into the 21st century exploring the application of weight‐of‐evidence (WoE), and overall persistence (P ov) to the evaluation steps. A companion paper addresses scientific opportunities related to improving the accuracy and reliability of laboratory methods to improve persistence assessment (Davenport et al., 2021).
A multimedia framework is needed to account for environmental emissions, partitioning between compartments and to integrate the combined effects of compartment‐specific behavior on environmental persistence (Gouin et al., 2000; Hollander et al., 2008; Mackay, Hughes, et al., 2014; Scheringer et al., 2009). All relevant properties of a substance and the nature of different environments should be included in the assessment, and the fate processes taking place must be considered in a WoE approach. A WoE framework is also needed to evaluate multiple lines of evidence (LoE) within and between compartments (Bilcke, 2002; Hardy et al., 2017; WHO, 2009), using different sources of information, such as laboratory screening and simulation studies, quantitative structure–activity relationship (QSAR) models, monitoring studies, and other information to be assembled in an expert judgment. A special focus should be given to the partitioning properties of a substance and the inclusion of all possible degradation processes in addition to aerobic biodegradation.
Currently, there is no such framework that defines which data and how such data should be used and weighted to determine whether a substance is persistent. Therefore, the objective of this paper is to propose an integrated WoE framework that includes a multimedia approach that can account for different emission scenarios but can also leverage available laboratory (standard or nonstandard) and field‐monitored degradation studies for a persistence assessment. This approach supplements the precautionary principle where in the absence of any data on degradation in the environment a substance is assumed to be persistent. Where high‐quality degradation and partitioning data for all relevant compartments are available, these can be used with multimedia modeling to evaluate the persistency of the substance in the environment as a whole, considering the effects of bioavailability and environmental factors. This approach can also be used to identify compartments where the substance is persistent and where mitigating and regulatory measures may be required. If all of the required data are not available, estimated data can be applied, considering the uncertainty associated with such estimates in order to be consistent with the precautionary principle. The objective of such a framework, along with the recommended methodological improvements in the companion paper (Davenport et al., 2021), is to ensure a more consistent, transparent, and thorough persistence assessment that is sufficiently flexible to leverage multiple types of persistence data (e.g., experimental, modeling, field).
The present paper is structured so that current definitions of persistence are described first to establish a common understanding of the concepts. The unit‐world concept is introduced as the general framework of the physical world that will be used to organize the WoE approach that is needed to advance the science of persistence assessment. The P ov metric is then discussed as an integrated approach to environmental persistence assessment in the multimedia unit‐world conceptual model. P ov is a metric representing residence time based on multimedia fate and transport models and is the operational outcome of the unit‐world concept that is required to organize persistence data. The WoE approach to persistence assessment, based on the unit‐world concept, in an Integrated Framework for Persistence Assessment, is then outlined. Finally, the paper provides some working examples of P ov calculations for phenanthrene and clear recommendations for future work to develop guidance and fit‐for‐purpose criteria to support the advancement of the science of persistence assessments.
The focus of this work is narrowly placed on characterizing persistence as a property of a molecule in the multimedia environment. The metrics, concepts, and approaches described in the present paper have obvious application to formal regulatory assessments of persistence, bioaccumulation, and toxicity (PBT) of chemicals as well as formal regulatory risk assessment work. However, these are considered beyond the scope of this work, because both PBT and risk assessment science are very detailed subjects in their own right.
CURRENT UNDERSTANDING OF PERSISTENCE
Definitions
Degradability
Degradability can be defined as the ability of a chemical to be structurally modified or broken down into smaller molecules in a particular environment as a result of abiotic processes (e.g., hydrolysis by water, direct or indirect photolysis by light), biotic processes (e.g., degradation by microorganisms—usually bacteria or fungi), or a combination of both. Biodegradability can be more specifically defined as the ability of a chemical to degrade as a result of interactions with biological elements (Goswami & O'Haire, 2016). Abiotic degradation processes typically result in the single transformation of one chemical into another one (=primary degradation), which can be an important precursor step to facilitate more complete degradation processes carried out by microorganisms that can achieve complete degradation (=ultimate degradation) with the complete conversion of organic carbon into ubiquitous inorganic chemicals (e.g., CO2, CH4, water, mineral salts) and/or incorporation into microbial biomass (Duber‐Smith et al., 2012).
Degradability describes how completely (=degradation extent, i.e., primary or ultimate, mineralization or conversion to biomass, etc.) and how quickly (=degradation rate) a chemical will degrade in a particular environment. Although the degradation rate can be used to assess the propensity of a chemical to degrade in the environment, there is no scientifically objective threshold that can be used to define a chemical as degradable, because this concept is subject to personal values and perceptions. For some chemicals, degradation will only occur under very specific conditions and/or over extremely long periods on a human scale (Saxe, 2011).
Persistence
Environmental persistence of chemicals (parents or relevant metabolites) is a subjective concept (Lipnick, Hermens, et al., 2000; Lipnick, Jansson, et al., 2000). For example, many definitions have been proposed in varying contexts (i.e., scientific research, regulatory assessment, and societal concern; ECETOC, 2003; Nordberg et al., 2009). From a general perspective, environmental persistence can be tentatively defined as the propensity for a chemical to remain in the environment before being transformed by chemical and/or biological processes in a particular environmental compartment (e.g., air, water, soil, sediment). No clear scientific evidence exists about how long a chemical must persist in the environment for it to be considered of concern. That is because environmental persistence as such has no scientific meaning—any chemical could be considered persistent based on the above definition—and only makes sense when used relative to a given framework. In environmental regulations, arbitrary assessment criteria are thus used, such as half‐life thresholds reflecting levels of presumed or known risks considered unacceptable to human society.
Underlying properties and factors
Regulations generally consider persistence as a chemical‐intrinsic property whereas, in real‐world as well as under experimental conditions, it is actually determined by a combination of substance‐specific properties (so‐called intrinsic persistence) and environmental factors (so‐called environment‐dependent persistence; Thouand et al., 2011).
Intrinsic persistence
A substance may remain in the environment over long periods because its chemical structure is recalcitrant to any hydrolytic and/or photolytic degradation or to any enzymatic attack from microorganisms. The latter situation may occur if the substance is not able to interact with microbial cells (e.g., because of unsuitable size or shape or absence of suitable membrane transporters) or is not able to enter microbial metabolic pathways (e.g., is not a substrate for available intracellular or extracellular enzymes or not converted into precursor metabolites; Fewson, 1988). There may be cases where the enzymatic potential of a single species may not be sufficient and where the complete biodegradation of a chemical could only be achieved through the complex interplay of microbial communities (Andrady, 2007).
Environment‐dependent persistence
Environmental conditions encompass a wide range of environmental factors (e.g., pH, temperature, light intensity), which can exert appreciable influence on microbial diversity and richness, and eventually determine the likelihood for competent degraders to be present in a particular environment (Garbeva et al., 2004; McArthur, 2006). Environmental conditions may also affect the bioavailability and bioaccessibility of substances to microorganisms, which may further affect their degradation rates.
Bioavailability is a very complex concept that varies between scientific disciplines (e.g., toxicity vs. biodegradation) and even within disciplines. Bioavailability may represent (i) the ability of a substance to interact with a living system, (ii) the fraction of a substance accessible to a living system for absorption, (iii) the rate at which a substance is absorbed into a living system, or (iv) a measure of the potential to cause a toxic effect on a living system (Ortega‐Calvo et al., 2015; Semple et al., 2004). For persistence assessments, bioavailability generally refers to degradation limited by, for example, sorption or entrapment in the environmental matrix, volatilization processes, and/or partial solubilization.
Bioaccessibility simply represents the potential for a substance to become bioavailable from a given place or at a given time (Katayama et al., 2010; Semple et al., 2004). It depends on the forces at play in a particular environment (e.g., adsorption forces, ionic interactions, covalent bonds, entrapment; Schäffer et al., 2018), and it encompasses both the fraction that is available now for biodegradation and the fraction that could be accessible to microorganisms in future (Semple et al., 2004).
Current persistence assessment criteria
Criteria for environmental persistence assessment have been proposed since the late 1970s by several organizations and regulatory bodies (e.g., Convention for the Protection of the Marine Environment of the North‐East Atlantic [OSPAR], United Nations Environment Programme [UNEP], European Chemicals Agency [ECHA]) and have been mostly derived from a well‐known set of chemicals—the “dirty dozen” legacy of persistent organic pollutants (POPs)—demonstrating high persistence in soil and sediment (Matthies et al., 2016). In the European chemical legislative framework, persistence is considered as the timescale above which an irreversible threat to human health or wildlife could arise if a substance would display properties of concern like severe toxicity, potential for widespread distribution, or potential for transfer and magnification in the food chains (ECHA, 2017a; Scheringer et al., 2006). In this framework, persistence can be assessed against either screening criteria (“pass” in ready or inherent biodegradability screening tests for nonpersistence) or half‐life based definitive criteria (compartment‐specific, half‐life thresholds often requiring simulation studies). In this respect, clear definitions of metrics and concepts used to describe persistence are important to achieve consistent and effective outcomes, and to limit confusion. For example, common terms such as degradation half‐time, disappearance half‐time (DT50), including lag phase, and half‐life (t 1/2), without lag phase, are used in regulatory and scientific literature. The lag phase is considered a reflection of microbial (presence of competent degraders) and chemical processes (bioavailability) and an important consideration in the WoE processes.
Most half‐life thresholds were defined in the early 2000s by individual regulatory agencies using reference substances and best judgment practices (Matthies et al., 2016). Thus, the current persistence cutoffs are highly variable between jurisdictions and may not reflect the actual risks of current chemical products, because they represent a compromise between policy and science. Thus, the following list represents a series of issues with current‐use persistency assessment criteria:
Regulatory cutoffs are based on compartmental half‐lives of a few legacy POPs, which do not represent the large structural and physicochemical diversity of substances.
Regulatory cutoffs were derived from more than 40‐year‐old degradation studies whose testing protocols were different from the actual Organisation for Economic Co‐operation and Development (OECD) tests used for persistence assessment, which were designed less than 20 years ago (Matthies et al., 2016).
Screening and higher tier test methods are not broadly applicable to all chemical types (Davenport et al., 2021; Shrestha et al., 2019).
Half‐lives depend demonstrably on environmental conditions (e.g., temperature, substrate concentration, and microbial community; Boethling et al., 2009).
The methods used to measure half‐lives are often not standard, defined, or transparent (Hughes et al., 2020; Webster et al., 1998), which can reduce the certainty associated with persistence classification. Currently, guidance is lacking to assess the suitability of nonstandard experimental methods for use in persistence assessments.
Half‐life criteria are typically based on biodegradation in a single environmental compartment. Yet, lack of degradation in one compartment does not necessarily mean that the substance will persist in the overall environment when the compartment where the chemical is considered persistent is a minor receptor of this chemical or is in equilibrium with the rest of the environment (Webster et al., 1998). Current assessment strategies could thus result in overestimation of real‐world persistence.
Overall, these issues limit the applicability and environmental relevance of persistence criteria. There is therefore an urgent need for a novel approach.
P OV AS AN INTEGRATED METRIC FOR ENVIRONMENTAL PERSISTENCE
P ov, as described below and in literature, is a useful concept to organize and interpret persistence data under differing emission scenarios (Scheringer et al., 2009). This approach is intended to be used in a tiered approach to perform fit‐for‐purpose risk assessments (Bonnell et al., 2018; Gouin et al., 2000, 2012). For example, initial simulations can be performed with available data, either derived from QSAR or available experimental data in the first tiers (ECETOC, 2003; OECD, 2018). The first‐tier P ov results can be compared with screening thresholds (e.g., vs. POPs) to evaluate the need for further work. Uncertainty in these P ov assessments should be subsequently evaluated for the likely risks posed by the emission scenario or the proximity to the persistence thresholds. If the analysis indicates potential concern, then P ov simulations can be used to characterize fate processes or compartments of concern that can prompt higher tier assessments to address the data gaps. Then the updated metrics can be analyzed again in the P ov framework to assess the likelihood of persistence in the overall environment. A brief example of this approach is given in the Supporting Information, where Tier 0 half‐life values for phenanthrene were used to provide initial results, which were subsequently used to develop an updated analysis by highlighting key data gaps.
Pov based on a unit‐world model
Medium‐ or compartment‐specific persistence criteria often fail to account for how partitioning and environmental distribution upon emission to the environment affect the persistence of a substance in the whole environment. Shortfalls associated with using compartment‐specific half‐lives as evaluation criteria for persistence in PBT assessments have long been recognized (Section Current persistence assessment criteria; ECETOC, 2003; Fenner et al., 2005; Klasmeier et al., 2006; Mackay & Webster, 2006; Scheringer et al., 2009; Van De Meent et al., 2000; Wegmann et al., 2009), and various alternative approaches have been presented for persistence assessment. One such approach, introduced to overcome the aforementioned shortfalls, focuses on evaluating persistence using unit‐world models (UWM; Mackay & Paterson, 1981). Considering the whole environment, UWM are simplified conceptual models of a given emission scenario that includes general environmental compartments (water, air, soil; see Section Proposed multimedia informed WoE persistence assessment framework). As such, UWM can be adapted and applied to many specific emission scenarios. The UWM concept provides a conceptual framework for organizing data and recognizing the potential impact of environmental fate processes on chemical persistence.
The UWM concept can be adapted to numerical models that are used to evaluate the persistence of a substance of interest by considering both compartment‐specific half‐lives and physicochemical characteristics (Van De Meent et al., 2000). Specifically, within the UWM concept, P ov has recently been proposed to be a suitable replacement metric for the compartment‐specific half‐lives in P assessment (ECCC, 2016).
First introduced by Mackay (1979), P ov is defined as the residence time of a contaminant within a defined environment and is based on compartment‐specific half‐lives, compartment‐specific dimensions, and mass transfer processes. In contrast to single‐compartment, half‐life criteria, this metric treats the environment as a single, unified set of connected media, integrating single‐media half‐lives based on degradation processes and phase partitioning, and allowing for the persistence of a substance in the whole environment to be estimated (Junker et al., 2019; Webster et al., 1998; Wegmann et al., 2009). P ov can be calculated using various multimedia fate and transport models (MFTMs), which are applied mathematical models that relate multiphase partitioning and environmental fate properties to residence time to predict P ov or overall half‐life (t 1/2,ov) of a substance of interest. Briefly, MFTMs describe substance behavior in evaluative, unit‐world environments (i.e., environments divided into conceptual compartments describing single environmental media such as air, water, sediment, soil) and then assume mass conservation across the entire system, while accounting for thermodynamics, intermedia transfer (e.g., sediment–water exchanges, volatilization, and dry and wet deposition), input processes (emissions), and degradation characteristics (abiotic and biotic) of the substance of interest (ECETOC, 2003).
P ov can be estimated in relation to residence time (τ; the “turnover time” of a substance at steady state in a flow‐through system including degradation and advection), elimination time (the elimination time from the overall environment following emission cessation), fraction remaining (the mass‐fraction of remaining substance following cessation of emissions), and/or temporal remote state (the long‐term removal rate after substance emissions cease; Scheringer et al., 2009; Stroebe et al., 2004; Van De Meent et al., 2000). For example, the τ of a chemical can be calculated for each compartment within an environmental system of interest using MFTMs that consider environmental processes alongside reaction losses and partitioning. Kinetic MFTMs can then be used to evaluate overall residence time (τov) for a defined mode of entry. This P ov value can then be applied as a persistence criterion that holistically accounts for the residency (i.e., turnover time) of a chemical within the specific environment of interest (Mackay, Hughes, et al., 2014). Although there are limited applications of P ov in regulatory frameworks (Bonnell et al., 2018; ECCC, 2016; Scheringer et al., 2009), this parameter is useful because it considers all possible fate and transport of substances in the environment. In addition, P ov can be used as an indicator of contamination reversibility following the cessation of substance release. The P ov should always be evaluated with care because this value will depend on the emission scenario of the substance, which should be based on typical use patterns.
P ov has several advantages over current‐use, single‐compartment persistence assessment schemes: (i) P ov provides a certain flexibility with respect to metrics; by providing different endpoints through which persistence can be assessed (e.g., residence time, elimination time, fraction remaining, and temporal residence state), P ov can offer a comprehensive understanding of persistence under various emission scenarios; (ii) by integrating all single‐media, half‐life data into a single metric of persistence, P ov offers the advantage of simplifying environmental risk and regulatory assessments; and (iii) by adequately accounting for chemical fate and transport (e.g., chemical fate and transport within the defined environment of interest), emission patterns, and other significant environmental factors, P ov remains comprehensive and holistic, providing a more realistic assessment of the persistence of a chemical released into the environment than single‐compartment, half‐life values (Scheringer et al., 2009; Webster et al., 1998). Therefore, P ov represents a persistence assessment alternative that is not only appropriate but also advantageous for use in chemical regulation and environmental risk assessment.
Use of multimedia fate and transport models in P ov assessment
Currently, there are a range of different MFTMs available for use in P ov calculation, which tend to differ in terms of complexity, geometry, and environmental parameterization (Table S1; Fenner et al., 2005; Wegmann et al., 2009). For example, MFTMs can range from relatively simple (e.g., Level I; accounting for distribution of a fixed quantity of a conserved substance in a closed environment at equilibrium) to highly complex (e.g., Level IV; accounting for variable emission of a substance [nonsteady‐state] in an environment under nonequilibrium conditions; Mackay, 2001). In addition, MFTMs tend to vary in terms of substance emission profile, with some models allowing for a higher level of control of emission scenarios than others (Table S1). Finally, MFTMs can vary significantly in terms of geographical scale and environmental conditions, ranging from regional to continental in scale and accounting for differing specificity in evaluative environments (e.g., CalTOX [multimedia total exposure model for hazardous‐waste sites] is parameterized to reflect Californian conditions, whereas ELPOS [Environmental Long‐Range Transport and Persistence of Organic Substances] is designed to reflect European environments; Beyer & Matthies, 2002; McKone & Enoch, 2002; Table S1).
In general, different MFTMs provide similar P ov results for various chemical substances (Fenner et al., 2005). Thus, it is likely that most MFTMs can be equivalently applied to evaluate P ov. However, differential model features translate into diverse requirements for substance data inputs. For example, simpler (steady state), regional environmental fate models (e.g., Level II equilibrium models; Mackay, Di Guardo, Paterson, & Cowan, 1996; Mackay, Di Guardo, Paterson, Kicsi, et al., 1996; Mackay, Di Guardo, Paterson, Kicsi, Cowan, et al., 1996) require relatively limited numbers of substance‐specific parameters (e.g., molecular weight [MW], vapor pressure [P 0], water solubility [S w], temperature [T], n‐octanol–water partitioning coefficient [K ow)], and degradation rate constants in soil, water, sediment, and air [k soil, k water, k sediment, k air]), whereas more complex (dynamic), global environmental fate models (e.g., global distribution model for persistent organic chemicals [Globo‐POP]; Wania & Mackay, 1993, 1995; Wania et al., 1999) require larger numbers of substance‐specific parameters (e.g., MW, partition coefficients: K ow, K aw [air–water partition coefficient], K oa [octanol–air partition coefficient]), degradation rate constants (k soil, k water, k sediment, k OH [degradation rate constant with hydroxyl radicals]), enthalpies of phase transfer octanol–air, air–water, octanol–water (ΔH oa, ΔH aw, ΔH ow), and activation energies (E a) for all degradation reactions; Table S1). Indeed, across all MFTMs there is a direct relationship between the level of detail and required number of input parameters. Thus, when choosing a model for use in P ov evaluation, it should be fit‐for‐purpose.
It is important to note that even the highly complex MFTMs do not account for all emission scenarios, and additional analysis may be needed to adequately understand the environmental fate and persistence of substances with unique emission scenarios. For example, a substance, which is disposed of down the drain post‐use and moves through a sewer system where several different physicochemical and biodegradation processes can occur, may require additional consideration when evaluating P. Following disposal, the substance will enter a wastewater treatment plant (WWTP) where further degradation is possible. Depending on degradation and physicochemical properties, the substance of interest or its metabolites could remain in the aqueous phase and be discharged from the WWTP as effluent, entering the aquatic compartment where further degradation and/or partitioning can occur. Alternatively, substances or metabolites that are sorptive and exist on sludge solids could enter aerobic or anaerobic digesters and be further degraded. In the case that these digested sludge solids would be land applied, any remaining substances or metabolites would then enter the soil compartment and be subjected to further degradation and/or partitioning processes. Obviously, these various emission scenarios must be accounted for to accurately assess the persistence of these down‐the‐drain chemicals. Currently, various models are available and used routinely to assess the fate and persistence of substances during wastewater treatment (i.e., SimpleTreat [Struijs, 2014] is incorporated into the European Union System for the Evaluation of Substances [EUSES]) that can be leveraged to better understand this complex emission scenario.
Use of MFTMs for P assessment requires consideration of substance‐specific input parameters (ECETOC, 2003), which are often more influential to P ov results than the specific choice of system parameters (Webster et al., 2004). Uncertainties in the model inputs (experimental or QSAR) can be evaluated in sensitivity analyses to characterize the relative importance of the assumptions. Further, using a tiered approach, the uncertainties can be compared against estimated risks or relative to benchmark chemicals. Development of specific guidance on how to provide input parameters to MFTMs is beyond the scope of the present work, although this has been addressed in other high‐throughput applications (Bonnell et al., 2018; Gouin et al., 2012; OECD, 2018). Owing to the flexible nature of the P ov framework, there is no available single P ov‐based criteria because the specific combination of compartment‐based criteria, emission scenarios, and physicochemical properties can result in a range of acceptable P ov (see Supporting Information for an example).
Overall, MFTMs represent powerful tools that should be used to evaluate P in chemical regulation and environmental risk assessment. Not only can they be employed to calculate P ov, providing an assessment of the P of a substance in the overall environment, but MFTMs can also be applied to characterize the influence of different environmental factors, use, and/or release patterns, and the uncertainty in available input data to support more realistic and relevant P assessments than single‐compartment P models.
WoE FRAMEWORK FOR PERSISTENCE ASSESSMENT
Over the past several decades, the concept of chemical persistence assessment using multimedia models has been broached many times, but with limited uptake in regulatory settings (Boethling, 2016). However, a unit‐world concept remains a useful mechanism to evaluate various types of data that inform fate and degradation processes (Figure 1). Therefore, an integrated persistence‐assessment framework is needed based on a unit‐world concept to organize and define data relevance in the WoE approach recommended by OECD (OECD, 2019).
Figure 1.
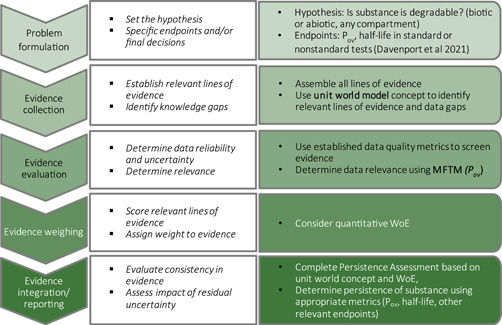
Schematic of a weight‐of‐evidence approach adapted for persistence assessment (adapted from OECD (2019))
Principles of WoE
WoE refers to a decision‐making approach in which one collects all available LoEs, triages them by reliability and relevance, and integrates this information into an overall picture to inform risk‐based decision making. In the past, this process was viewed as a subjective approach in the face of conflicting or incomplete information but is meant to allow flexibility in contrast to bright‐line criteria (Weed, 2005).
The concept of gathering existing information, assessing its reliability in a transparent and systematic manner, and interpreting results in a WoE framework has been discussed extensively over the past decade (Rhomberg et al., 2013; SCENIHR, 2012) including the development of templates to organize data for regulatory activities (link). Lutter et al. (2015) were one of the first groups to propose an approach on how to evaluate WoE frameworks. Their recommendations came from a wider discussion that took place in 2012 among a group of scientific and regulatory experts who were involved with agriculture and pest management. Their proposal included formulating a hypothesis related to specific endpoints under evaluation, providing more transparent discussions of WoE and a more rigorous and systematic determinant of WoE for the evaluation of substances (i.e., consistency). They proposed that this approach should not only include all available LoEs but also an evaluation of data quality and study reliability.
More recently, ECETOC proposed a WoE‐based framework applied to the PBT/vPvB (persistent, bioaccumulative, toxic/very persistent, very bioaccumulative) assessment (ECETOC, 2014). Their approach followed the general WoE framework outlined by the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR, 2012). Again, building on previous frameworks, the approaches outlined by SCENIHR not only recommended identifying, collecting, and evaluating all sources of information related to the specific question (hypothesis) at hand but also introduced the consideration of scientific quality and relevance of the studies as a critical component of the assessment. This group also recommended the use of a scoring system for the overall WoE assessment (i.e., strong, moderate, weak, uncertain).
In 2013, a group of experts surveyed more than 50 existing WoE frameworks to identify best practices (Rhomberg et al., 2013). They concluded that a WoE approach consists of several critical steps, including evaluating strength and quality of existing data and information, and a systematic process on how to integrate these data to best inform risk. Since then, many different WoE frameworks have been proposed with different phases in this stepwise process but, essentially, most of them adhere to the following logic: (i) problem formulation including hypothesis definition, (ii) data selection, (iii) evaluation of data and study quality evaluation (criteria for review of individual studies), and (iv) data integration.
In a more recent publication, the OECD outlined the key elements necessary for implementing a WoE approach to the evaluation of chemical substances (OECD, 2019), which is used as the basis for the proposed persistence assessment framework in this paper (Figure 1). The OECD (2019) document was being put forward as “good WoE practices” and still accounts for professional judgment as a critical component in constructing the appropriate framework for differing specific endpoints. The OECD provides a stepwise approach to conducting WoE for chemical evaluation including five key elements (Figure 1): (1) problem formulation (hypothesis development), (2) evidence collection, (3) evidence evaluation, (4) evidence weighing, (5) evidence integration and reporting (OECD, 2019). This framework illustrates that developing criteria for study quality (e.g., reliability) and relevance is especially important when multiple values for the same metric are available for a given substance. The study designs and data interpretation should be examined closely to determine the reliability and relevance of the findings as well as sources of variability. WoE is a tool used to evaluate data, but specific applications need specific considerations to appropriately organize and compare different data types. For example, the specific WoE approach taken for persistence assessments will differ from the specific approach needed for toxicity assessments.
Examples of previous WoE approaches to persistence assessment
Before the publication of the OECD WoE guiding principles (OECD, 2019), many of the key elements outlined by the OECD were already applied in chemical safety assessments (Brandt et al., 2016; Bridges & Solomon, 2016; Giesy et al., 2014; Hughes et al., 2020; Wassenaar & Verbruggen, 2021). Giesy et al. (2014) used a WoE approach to assess the organophosphorus pesticide chlorpyrifos. There was a significant amount of data available for this compound, with multiple laboratory test values for half‐lives spanning 2 orders of magnitude in various media, partially exceeding persistence thresholds. They used the geometric mean of the half‐lives to reconcile the data variation, with the results that the persistence criteria were not exceeded in any media. In addition, they looked at field data, which revealed relatively short half‐lives not exceeding persistence criteria. Additionally, chlorpyrifos was known to hydrolyze, albeit with significant variation in half‐life at different pH values. The conclusion was that the criteria for persistence were not exceeded.
Brandt et al. (2016) proposed a WoE approach to assess the persistence of a group of substituted phenolic benzotriazoles. They hypothesized that these substances would be very persistent in the environment as defined by their REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals; Regulation [EC] No 1907/2006) endpoints for persistence. There was insufficient experimental information available to reach a conclusion on the persistence of these substances. They used QSARs and biodegradation models for phenolic benzotriazoles to group them as a class and, because of their hydrophobic nature, identified sediments as the most relevant compartment for persistence assessment. Here, they relied on several LoEs, including environmental monitoring of phenolic benzotriazoles in different environmental compartments and biota (literature values), and one laboratory biodegradation and one field dissipation study. The final element of the WoE approach was a sediment core analysis to determine the in situ half‐life. Although the authors considered the relevance of the data based on physicochemical properties (and modeling degradation kinetics and pathways), the authors subsequently diverged from the OECD process for evidence evaluation. A key principle in evaluating evidence is to determine data reliability, uncertainty, and relevance. The authors opined that weighting the evidence could not be done objectively and argued for more of a “summary narrative” approach. Here is where the key elements of the OECD guidance may prove helpful in future, because it separates the weighting process from the process of setting more objective quality criteria to assess data reliability.
The OECD document points out that weighing is used to differentiate data sources, not to judge overall quality, which they consider as a separate (earlier) step. Weight can be qualitative or assigned a score and is based on reliability and relevance (e.g., similar to Klimisch scoring; Klimisch et al., 1997). Here, the OECD document states that weighing of data should be based on “clear and transparent” methodology. Suter et al. (2020) provided similar recommendations in their comparison of a WoE versus a systematic review for evaluating multiple LoEs to help inform decision making. They also pointed out that, regardless of the methodology selected, the important component is to follow scientific standards of transparency concerning the choice and implementation of the approach. In the end, Brandt et al. (2016) integrated the multiple LoEs into this WoE approach and concluded that the phenolic benzotriazoles under consideration are persistent in the environment. However, they also suggested that more guidance is needed to provide a more quantitative WoE approach while still maintaining some flexibility for its application.
In Wassenaar and Verbruggen (2021), a WoE approach, as indicated by Chapter R.11 of the ECHA Guidance on Information Requirements and Chemical Safety Assessment, was used to evaluate the persistence, bioaccumulation, and toxicity of alkylated 3‐ring PAHs (polyaromatic hydrocarbons). Eighteen studies with persistence data were screened for relevance to determine environmental degradation half‐life values. As described in the paper, none of the 18 studies qualified, and therefore there was no WoE for a half‐life determination. Instead, a WoE argument was made that the data as a whole indicated a trend of increasing half‐life with increasing alkylation for alkylated 3‐ring PAHs.
Hughes et al. (2020) also used the same R.11 guidance process to assess the persistence of phenanthrene. The studies were assessed and qualitatively weighted by relevance (similarity to standard test guidelines). In silico half‐life predictions, nonrelevant guideline studies, and monitoring studies were also incorporated into the WoE. The consistency or coherence of the data was assessed by assembling all half‐lives per compartment and understanding the outliers. Their conclusion was that phenanthrene is not a persistent molecule in any environmental compartment, and they reinforce this using a P ov calculation. The aforementioned environmental half‐life data were used as the basis of the P ov example given in the Supporting Information.
A more recent quantitative analysis for the cyclic volatile methyl siloxanes (cVMS; Bridges & Solomon, 2016) most closely follows the key elements outlined in the OECD WoE document (OECD, 2019). Their underlying hypothesis was that the persistence of these substances (octamethylcyclotetrasiloxane [D4], decamethylcyclopentasiloxane [D5], and dodecamethylcyclohexasiloxane [D6]) exceeded the threshold set by the Stockholm Convention. In developing their framework, they relied heavily on previously published WoE frameworks (SCENIHR, 2012). Their analysis consisted of LoE, which included general trends in environmental monitoring measurement, laboratory and field studies, and multimedia fate models where the unique physicochemical properties of the cVMS (low solubility and high vapor pressure) directly affected the overall environmental fate (partitioning) of these materials. Although the half‐life in air for the cVMS (abiotic processes dominating) exceeded the two‐dimensional criterion for persistence Matthies et al. (2016), reasoned that the tendency of these materials to remain in the atmosphere, where they are degraded, limits their ability for long‐range transport and the ability to affect organisms. The authors conclude that these substances are not persistent in a way that would be harmful. The authors did question the appropriateness of biodegradation test setups that prevent evaporative losses, when those are artificial constructs that would not exist in the natural environment.
A key aspect that differentiated the Bridges and Solomon (2016) evaluation, using WoE for persistence, from others was the development of an extensive, transparent scoring criteria for quality, reliability, and relevance. The authors emphasized the importance of assessing the LoE for relevance (to the stated hypotheses) and reliability and that reliability scoring be conducted in an objective, reproducible, and transparent manner. Also, the studies needed to have appropriate quality control or quality assurance, data processing, and clarity to be considered reliable. In the scoring of the field studies, they observed that reliable sampling and good analytical practice were important and identified more than 10 predefined criteria for quality and relevance to laboratory studies. Some of the more relevant quality criteria suggested by Bridges and Solomon (2016) and others (Forney et al., 2001; Hermsen et al., 2018; Koelmans et al., 2019; Kowalczyk et al., 2015; Martin et al., 2017; Moermond et al., 2016; Ott et al., 2020) are outlined in Table 2.
Table 2.
Physicochemical properties and their impact on chemical fate
| Physicochemical property | Impact on fate |
|---|---|
| Molecular weight, water and octanol solubility, steric hindrance, ionizability, adsorptive properties, chemical form (e.g., liquid, solid, particle, etc.) | Bioavailability |
| Henry's constant in water, vapor pressure, K oa | Air/water partitioning |
| Ionizability, adsorptive properties, water solubility | Soil or sediment/water partitioning |
| Hydrolyzable groups | Potential modulation of degradation by environmental pH |
| Photochemical reactivity | Potential modulation of degradation by sunlight |
In the end, the results from the Bridges and Solomon (2016) assessment were visualized in graphical plots of score for quality against the score for relevance to each study (Van Der Kraak et al., 2014). This visualization facilitated coherence or integration of all relevant, reliable evidence of P assessment, and the information provided a more holistic approach to the integration of physicochemical properties, multimedia fate modeling, testing, and field observation to assess the overall P ov of cVMSs in the environment.
In summary, the OECD WoE principles provide a practical tiered framework for testing and assessment of persistence. For example, multiple data for a given test method can be evaluated together. In cases where there is preexisting data, higher tier information (e.g., simulation data or calculations of P ov) should be more heavily weighted over lower tier information (e.g., QSAR or screening level tests) to characterize persistence. In silico tools such as QSARs and multimedia fate models, as well as read across, relevant physicochemical properties can be considered together using subject matter expertise (SME) and a WoE approach. When multiple values for the same metric are available for a given substance, the study designs and data interpretation should be examined closely to determine relevance of the findings as well as sources of variability so that the data can be appropriately considered a WoE evaluation. Types of information that would prove useful in assessing the overall reliability and quality of a study are included in Table 1.
Table 1.
Persistence information for laboratory and field studies: Quality control and assessment measures
| Laboratory | Fielda |
|---|---|
| Experimental design | External checks |
|
|
| Test substance characterization and purity | Laboratory analysis of duplicate samples |
| Robust analytical method | |
| Mode of substance application | Sampling method and analysis method: Outlined and reproducible |
| Analytics | Internal checks (performed by the project field volunteers, staff, and laboratory) |
|
|
| Source of biomass (soil, water, sediment sludge, etc.) | |
|
Regardless, the concepts of gathering existing information, assessing reliability of information in a transparent and systematic manner, and interpreting results in a WoE framework are generally applicable to persistence assessments.
PROPOSED MULTIMEDIA INFORMED WoE PERSISTENCE ASSESSMENT FRAMEWORK
Data for incorporation into a WoE‐informed unit‐world persistence assessment
The key principle driving integrated persistence assessment is understanding the degradability of a substance in the overall environment, based on substance emissions and known transformation and physicochemical properties. In this framework, a unit‐world approach is used to focus attention on relevant physicochemical properties driving the fate of the molecule (e.g., solubility, adsorption, volatility), and focus the evaluation on key degradation and fate processes that influence the persistence. Such an approach was applied by Hughes et al. (2020) to evaluate the persistence of phenanthrene, with a P ov‐like calculation being performed using a Level III model (see also the example given in Supporting Information).
In addition to the data from standardized tests, nontesting data can guide the interpretation of testing results as well as provide independent LoEs. These additional data are needed owing to the variability of biodegradation testing outcomes discussed in this and the companion paper (Davenport et al., 2021). The use of multiple LoEs to classify environmental persistence can reduce the overall uncertainty associated with P outcomes. Information from in silico predictions, laboratory and field data, and MFTM can be used in a WoE approach to assess persistence potential (Di Guardo et al., 2018; Giesy et al., 2014; Mackay, Giesy, et al., 2014; SCHEER, 2018). The desired metric of persistence is an indicator of overall (bio)degradability, but regulations are written in measurements of compartment‐specific biodegradation, that is, half‐life (Rücker & Kümmerer, 2012). The sections below discuss a few of these LoEs, with several examples of how they are used in the WoE flowchart in Figure 1 and described in the WoE section below (Brandt et al., 2016; Giesy et al., 2014).
(Bio)degradation testing data
Biodegradation testing is often considered the most straightforward manner to assess the persistence of a substance. Positive ready, enhanced, and inherent biodegradation tests may be considered sufficient to conclude on nonpersistence of a chemical, provided that additional criteria are also fulfilled (ECHA, 2017a). In case of a negative result, higher tier simulation tests may be needed to draw definitive conclusions for regulatory persistence assessment. However, biodegradation testing data may be fraught with experimental variability (see section on Current Understanding of Persistence and Davenport et al., 2021). Multiple tests may thus lead to diverging results that hamper drawing firm conclusions on biodegradability. Yet, testing data is the primary evidence used to calculate half‐lives and conclude on the persistence of a substance, which highlights the importance of additional LoEs to support persistence assessments. Depending on the molecules, abiotic factors can drive, or contribute substantially, to degradation.
Physicochemical properties
Physicochemical properties determine how chemicals partition in the environment and give insight into which degradation processes play a significant role in the fate of a chemical (Klöpffer et al., 1982; Figure 1). A basic understanding of physicochemical properties can give a preliminary estimate of the substance's fate and its potential persistence. As discussed in Section Overall Persistence (Pov) as an integrated metric for environmental persistence, these physicochemical characteristics are the basic data input for MFTMs. Separating the impact of sorption (e.g., reduced bioavailability or bioaccessibility) from degradation properties is required to evaluate the intrinsic persistence of a molecule accurately (Section Current understanding of persistence).
Environmental factors
Effective degradation processes can be abiotic reactions such as hydrolysis or photolysis, which tend to be compartment specific, or they can be biotic reactions, with microbial processes dominating as they display the greatest metabolic diversity and flexibility. However, unless the environmental conditions are appropriate, (bio)degradation may not take place. Some of these environmental conditions are further discussed below (also see Davenport et al., 2021).
Oxygen availability
The presence and relative abundance of appropriate electron acceptors is an important environmental variable controlling biodegradation. Aerobic degradation is typically faster than other pathways. Oxygen availability modulates both the abiotic processes of oxidation and biodegradation. Further, environmental compartments can vary in degradation capability such as comparing aerobic water with anaerobic sediment layers (Merrettig‐Bruns & Jelen, 2009). With a few exceptions (e.g., organochlorine substances [Tiehm & Schmidt, 2011]), anaerobic biodegradation tends to be slower than aerobic biodegradation and is considered less relevant to risk assessment (Ghattas et al., 2017). This suggests that persistence evaluations typically should not be based on anaerobic systems, although they may be part of the WoE. Further, if anaerobic data are used in an assessment, the evaluation criteria should be revised because the implicit exposure dynamic is different.
Microbial community
A microbial community needs to contain competent degraders for biodegradation to occur. The concentration and distribution of those competent degraders will affect the rate of degradation and the likelihood of passing a biodegradation test (Ott et al., 2020). Adaptation and acclimation are two terms that are often confused while discussing the ability of a microbial community to biodegrade a substance. Outside this field, the term “adaptation” normally refers to genetic changes that result in new phenotypes; however, this term is often used in persistence testing to mean any change in a microbial community caused by long‐term exposure to the test item (OECD, 2006). A shift in the relative abundance of species in a microbial community, either resulting from previous exposure to the test substance or from a long biodegradation testing time, would be considered an adaptation, even if there are no new genes and the gross genetic composition of the community remains the same. Acclimation is the short‐term process by which a microbial inoculum adjusts to the test conditions, such as testing outside ambient conditions (e.g., temperature soils tested at low temperature). In both adaptation and acclimation, microbial community structure will be altered. Under REACH, acclimation is permitted, whereas adaptation is not (ECHA, 2017b). However, it is reasonable to expect that microbes in many compartments have been exposed to industrial and naturally occurring chemicals. Furthermore, there are many examples of the genetic plasticity of microbes enabling xenobiotic biodegradation, so adaptation in the traditional sense is a valid mechanism that should be addressed when examining persistent substances (Itrich et al., 2015; Liang et al., 2012; Nagata et al., 2019; Nielsen et al., 2017; Poursat et al., 2019; Top & Springael, 2003). The most suitable approach to address this issue under environmentally relevant conditions should be the subject of future research.
Temperature
It is generally accepted that higher temperatures increase the rate of chemical reactions, which translates to microbes having greater metabolic activity at higher temperatures provided those temperatures do not exceed the organisms' optimum temperatures. However, this does not necessarily mean that biodegradation processes in temperate regions take longer than in tropical regions. Empirical evidence demonstrates that biodegradation rates can be similar for some chemical classes across different global regimes and temperatures (Brown et al., 2020; Lewis & Prince, 2018). The ECHA guidance recommends that all new simulation studies (OECD test guidelines [TG] 307, 308, and 309) be performed at 12°C, because this is considered the average temperature of European surface waters (ECHA, 2017a). A controversial decision is that studies at other temperatures should be adjusted using an Arrhenius equation with a generic Energy Activation (65.4 kJ/mol; Brown et al., 2020; ECHA, 2017a). This temperature correction guidance derives mainly from pesticide data and is based mostly on test systems with manipulated test temperatures (i.e., systems that are not tested at the temperature of the inoculum collection site), instead of systems that are tested at the temperature of the inoculum collection site. In the former case, it is questionable whether these calculated temperature adjustments reflect the biological phenomena accurately (Brown et al., 2020).
Organic matter
Organic matter plays multiple roles in the fate of a chemical. It can be a substrate on which chemicals sorb, particularly with hydrophobic chemicals, thus altering the bioavailability of the chemical (Huang et al., 2003). The amount of nonextractable residues (NER) is correlated with the presence of organic matter (Kästner et al., 2014). Organic matter can also catalyze other degradative reactions, such as oxidation, reduction, etc. (Kästner et al., 2014). At the same time, organic matter also provides a substrate for microbial growth, leading to biofilms, which are the most effective modes of biodegradation (Horemans et al., 2013).
Salinity, pH, and light availability
Each environment will have its own adapted microbial population that can survive in its habitat's pH and salinity. Light availability controls processes such as photodegradation or photooxidation. It can also affect the microbial population by encouraging the growth of photosynthesizers and increasing the organic content of a system (Pastore et al., 2018; Southwell et al., 2020).
Volumes and use patterns
If the objective of persistence assessment is to identify substances that could accumulate in the environment, then it makes sense to look at how these substances are used and initially released into the environment. For example, a readily biodegradable, high‐volume chemical with continuous release may achieve “pseudo‐persistence” or be “continuously present” in the environment (Mackay, Hughes, et al., 2014). Conversely, some substances identified as persistent may not accumulate in the environment if they are used at low volumes with infrequent emissions. Another example is that a highly volatile compound, subject to rapid photooxidation leading to benign products that does not degrade in sediments, would not be a concern if the only releases are to the air. To avoid misclassification, it would be useful to have more granularity in the persistence criteria to tie in the volume and use (exposure) to the fate of the substance (persistence). Unfortunately, it can be difficult to obtain accurate information on how substances are used and distributed in the environment, especially if the same substance is used across multiple sites, for multiple uses, and at varying release levels. From a risk perspective, low volume substances may result in lower risks; therefore, more uncertainty in the persistence assessment could be tolerated depending on the hazard of the substance.
Predictive models
Predictive models are used heavily to assess the properties and behavior of substances for regulatory decision‐making and to estimate fate and transport of contaminants, engineer molecules for greener chemistry, and achieve specific functionality. They are an accepted part of a WoE argument (Pizzo et al., 2013). Although quantitative structure–biodegradation relationships (QSBRs) and knowledge‐based approaches can be used independently, a WoE approach is best supported by combining the information. Predictive models offer generalizations that can provide insight into the behavior of a substance. For assessment of biodegradability, predictive models are built on two types of information: (1) QSBRs relating chemical structures with biodegradation test data and (2) the existence of biodegradation metabolic pathways for classes of chemicals.
QSBRs correlate the biodegradation test data of a substance to molecular descriptors, such as the physicochemical properties of that substance, or to the molecular fragments, or the particular atoms or bonds, comprising the substance. These correlations allow biodegradability predictions of untested molecules based on the presence of those studied molecular descriptors or fragments. Two common examples of QSBRs that use the fragment contribution method are the BIOWIN modules in the US EPA (Environmental Protection Agency) EpiSuite software (US EPA, 2020) and MultiCASE (Klopman et al., 1995). QSBRs have limited domains of applicability, because the relationship between molecular structure and biodegradability is only as good as the underpinning empirical data used in the training set. Additionally, biodegradation test data are variable and location‐specific (Davenport et al., 2021), particularly in terms of the calculation of a half‐life for a substance (Rücker & Kümmerer, 2012). Several reviews of QSBRs are available for further information (Ballabio et al., 2017; Mamy et al., 2015; Rücker & Kümmerer, 2012).
The other type of predictive model is based on the knowledge of existing metabolic or biodegradation pathways. The likelihood of a reaction, other than those that are cometabolic, is often related to whether the reaction is thermodynamically favorable (Finley et al., 2009). There are a few databases of microbial metabolic pathways, such as the Kyoto Encyclopedia of Genes and Genomes (KEGG), a database for central metabolism pathways, and the Environmental Contaminant Biotransformation Pathway Resource (enviPath), which is a reworking of the University of Minnesota Biocatalysis/Biodegradation Database and Pathway Prediction System (UMBBD/PPS; Wicker et al., 2016). Using these databases, several models have been developed to predict the likelihood of biodegradation based on existing pathways and even time frames for biodegradation, such as Biodegradability Evaluation and Simulation System, EnviPath, and so forth (Judson, 2019). Another frequently used model, CATABOL, combines QSBR with a simulated catabolic transformation (Jaworska et al., 2002). A further refinement of CATABOL is Catalogic, which coordinates transformation reactions with a logical metabolism pathway that includes primary and ultimate half‐lives (Dimitrov et al., 2011). Omic‐based assessment methods can be used to characterize rates and mechanisms to increase the robustness of biodegradability prediction. Genetic sequencing information and the assignment of function to proteins encoded by those genes can also be used to infer the functions of microbes in a system and to infer the presence of biodegradation pathways (Faust et al., 2011; Saidi et al., 2017).
Field data and monitoring studies
Field data, in which the substance of interest is released and tracked in a relatively controlled setting, and monitoring studies, in which existing substance releases are tracked, provide as realistic an evaluation of the fate of a chemical as possible; however, this realism comes with the extreme complexity of real‐world systems. Field data can be incorporated into a WoE persistence assessment with some limitations. Successful applications of field data need to be evaluated in terms of a mass balance approach, where the inputs are known and the physical and biochemical processes are reasonably uniform, so that comparisons with the measurements can be used to infer degradation rates. These evaluations are often developed with focused studies that develop data in the context of a multimedia modeling approach to characterize overall fate to highlight important processes (e.g., biodegradation, photolysis, etc.; Chapra, 2008) and may use radiolabeled substrata or isotope analysis to assist in assessing the mass balance (Melsbach et al., 2019). In the context of persistence in overall fate, other fate processes such as sequestration, or burial in sediments, should be considered because they limit bioavailability and reduce the circulation of substances in the broader environment. Environmental conditions in the field study need to be relatively constant, or at least predictable in some manner, in the assessment to minimize the effect of spatial and temporal heterogeneity. Monitoring datasets are often not suitable for these evaluations because these conditions are often not met.
Field data are improved by benchmarking the behavior of chemicals under evaluation relative to chemicals with a known persistence status (e.g., polychlorinated biphenyls [PCBs]; McLachlan et al., 2017). Benchmarking should evaluate chemicals based on similar environmental conditions and emission profiles. Benchmarking can also address the apparent variability inherent in persistence testing because performance of the substance under evaluation will be compared with the performance of the benchmark substance (Zou et al., 2015). The challenge with field data is that variation in important physical and biochemical features (e.g., sorption limitations, redox conditions, resuspension, microbial community dynamics) can change spatially and temporally. This variation can result in apparent hot spots where substances are not degraded in one location (e.g., higher measurements), but are found in lower concentrations in other areas because the conditions are more favorable for degradation. For these reasons, typical monitoring data may not be suitable for regulatory persistence assessments (ECHA, 2017a).
There are however a few examples of how field and monitoring data can be used to support persistence assessments. Depending on the specific study questions, fieldwork can include watershed scale studies, characterization of chemical fate through WWTPs, or measurement of substance profiles in far‐field, remote locations. The first example is based on the use of fate and transport models to track the release and fate of pesticides in the Scheldt estuary (Steen et al., 2002). The loads of the pesticide in a river were determined through measurements of flows and concentrations in upstream environments. The apparent biodegradation rate or half‐life for pesticides was determined by comparing the model predicted concentrations with the concentrations of the pesticides measured in the downstream and estuary compartments. A similar approach was also used to estimate field biodegradation rates of nonylphenol ethoxylates in the Scheldt and Rhine estuaries (Jonkers et al., 2005). Far‐field areas can be used as well. For example, open ocean monitoring datasets use depth‐resolved sampling to evaluate the degradation of substances once they enter the water column through settling from the atmosphere (González‐Gaya et al., 2019). This was done by measuring the fluxes at the surface and tracking the concentrations over depth and as a function of particle concentration and biomass abundance. There is also the use of monitoring data from remote regions to indicate possible persistence of a chemical (Hung et al., 2016), although in a WoE context, presence alone is often not sufficient evidence of persistence.
Biodegradation kinetics in the environment
Biodegradation kinetics are directly affected by substance concentration, and these changes in kinetics should be kept in mind when making persistence determinations (Howard & Banerjee, 1984; Kolvenbach et al., 2014). Biodegradation kinetics are often assumed to follow first‐order rate processes (Schwarzenbach et al., 2016), in which the reaction rate is controlled primarily by the concentration of one of the reactants (with pseudo‐first‐order kinetics occurring when the other reactant is either present in excess or is being kept constant). First‐order kinetics usually apply only at low concentration with some reports that true first‐order kinetics were not obtained unless test concentrations were as low as 1 µg/L (Subba‐Rao et al., 1982). However, multiple studies have demonstrated that degradation kinetics change as substance concentrations change, and these rates are not always first order. Results of biodegradation studies conducted at high substrate concentrations may not accurately reflect degradation at low (environmentally realistic) concentrations (Hales et al., 1997; Jones & Alexander, 1986; Scow et al., 1986). Others have demonstrated that organic compounds are degraded at low concentrations, but not at unrealistic higher concentrations where toxicity may occur (Hammershøj et al., 2019).
Summary of the WoE approach
The key driving principle behind this Integrated Framework for Persistence Assessment is the need to understand the degradability of a molecule in different relevant environmental compartments based on the substance's emissions and known physicochemical properties. A conceptual model, or problem formulation, is required to provide a basis for evaluating data between compartments or comparing different data types (e.g., photolysis vs. biodegradation). This will provide a basis for a critical review of apparent outlier data. For example, is the outlier caused by a real process or by an artifact of the experimental design? And if notable differences are observed, then higher tier evaluations should be performed to reconcile the differences. In a conservative assessment, the default assumption for any substance is that it is not degradable, and testing results and predictive models are generated to demonstrate the opposite. If using a WoE approach, a precautionary approach does not necessarily yield the correct outcome. Therefore, in a WoE approach based on reliable and relevant data, evidence demonstrating lack of, or slow, degradation should be given less weight than evidence of degradability, because a persistence assessment should consider the intrinsic properties of a chemical and not necessarily identify the scenarios where degradation will not occur. This is because it is always possible to find or design a system where a degradable substance would not degrade (e.g., environmental‐dependent persistent), which may not be realistic or relevant under typical use. Effective screening should therefore focus on minimizing the impact of system variables to focus, to the extent possible, on intrinsic degradation properties. To conclude that a substance is not degradable using a WoE approach, there should be consistent evidence from both testing data and bioavailability of the substance. For degradation to occur, three phenomena must coincide: amenable chemical structures, effective degradation processes, and a conducive environment. Any evidence of degradability speaks directly to the amenability of the chemical structure.
A multimedia approach should be used to account for the physicochemical properties driving the fate of the molecules (e.g., volatility) and focus the evaluation on key processes that influence persistence (Figure 2). Controversially, from a risk perspective, physical advection processes such as burial may also be considered on the WoE because these processes result in reduced exposure to human and environmental receptors.
Figure 2.
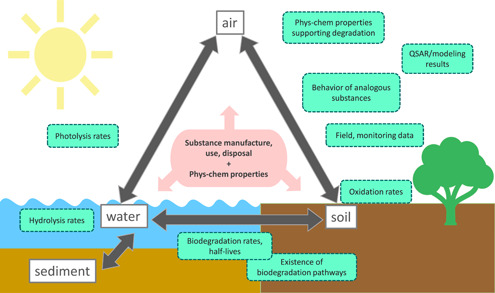
Schematic of a conceptual unit‐world model that helps organize the Integrated Framework for Persistence Assessment. Green squares indicate types of data that inform substance fate in the various compartments, which can receive the substance depending on its manufacture, use, disposal, and physicochemical properties
The OECD WoE framework can be used within the multimedia context described above because it is a representation of the actual processes that control the fate and exposure of chemicals in the environment (Section on Proposed Multimedia Persistence Framework; Figure 1). The problem formulation needs to start with a hypothesis to guide the downstream elements of the WoE scheme. In the present work on persistence assessments, this should focus on characterizing the potential for a molecule to degrade. This should be organized in a unit‐world approach to evaluate the potential combination of multiple degradation and fate processes that control the exposure of chemicals. This will help focus attention on data that are relevant and realistic, and avoid data that are confounded by unrepresentative experimental design features.
Collection of data can include all of the elements outlined in the present work, including field data, QSAR, P ov, and so forth. It may also include data from new experimental methods and concepts described in Davenport et al. (2021). The study quality should be evaluated to understand if the data were accurately reported and sufficiently documented, and based on realistic and relevant conditions that support the guiding hypothesis.
This is related to the next step in data evaluation where data need to be compared with the conceptual models and hypotheses that are outlined in the problem formulation step. This will allow reconciliation of potential outliers for data‐rich substances, and will increase confidence in the interpretation of available data for data‐poor substances. In particular for data‐poor substances, initial data evaluated in a P ov context will allow comparison with legacy POPs and other chemical types to guide the interpretation and evaluation.
Weighting of data should be informed by the multimedia approach to identify the relevant data. The multimedia approach provides a natural framework for integrating different LoEs because it is designed conceptually, and numerically, to predict the impact of different LoEs (fate processes in air vs. water vs. sediment, use pattern, benchmarks, etc.). The multimedia data and P ov can be used to determine where fate processes and degradation occur and the P ov of the substance in a multimedia unit world. This allows evaluators to focus on a holistic approach to environmental fate and comparative behavior instead of focusing solely on compartment‐wise testing.
CONCLUSION
Persistence assessment—as part of chemical safety assessment—is a key element in existing regulatory frameworks to protect human health and ecosystems from harmful substances of concern. Persistence, however, is a very complex concept to address: It is determined by a combination of chemical‐specific properties (intrinsic persistence) and environmental factors (environment‐dependent persistence), and involves multiple processes in multiple compartments (e.g., air, water, soil, sediment, biota). Depending on the substance, a thorough assessment of persistence would thus require different types of data produced across different compartments and according to different methods, leading to a wide variety of results. For ease of interpretation, the current regulatory frameworks focus mostly on single‐compartment degradation half‐lives to assess persistence and prioritize substances for regulatory actions. This approach, however, is reductionistic and, for a comprehensive evaluation of persistence, a flexible and integrated assessment framework needs to be developed upon existing ones. This framework should integrate multimedia fate and transport models to identify major distribution patterns and transformation processes of substances once released into the environment, and help establish data gaps and testing needs following an intelligent testing strategy. The P ov metric represents residence time based on such models, and examples demonstrate how the P ov approach would work for a data‐rich substance using a simple multimedia model. Further development for a wider range of substances, including data‐poor examples, and more complex models used in environmental risk assessment are required, but are beyond the scope of this publication.
The proposed assessment framework should integrate a transparent WoE methodology to combine all available information in an acceptable way for regulators. This will facilitate bringing science back to the core of the debate regarding the introduction of modeling parameters or new cutoff criteria and broaden the flexibility of persistence assessment. Furthermore, the proposed framework supplements the precautionary principle by considering current scientific insights and all relevant data where these are available, which results in reduced uncertainty. In addition, the existing compartment‐based criteria should be critically reviewed for application in the context of the proposed inclusion of WoE and P ov into persistence assessments. This type of framework has been successfully employed in some regions (Bonnell et al., 2018), and there is potential for application in others.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Supporting information
The Supporting Information file contains 1) a summary table to multimedia models that can support the overall persistence analysis promoted in this article, and 2) a case study on phenanthrene to demonstrate how a tiered P ov‐based Persistence assessment can be performed.
ACKNOWLEDGMENT
This work was conducted as part of the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) task force “Moving Persistence (P) Assessments into the 21st Century” (2019–2021). The authors thank the ECETOC scientific committee and other task force members Russell Davenport, Pippa Curtis‐Jackson, Philipp Dalkmann, Jordan Davies, Kathrin Fenner, Laurence Hand, Kathleen McDonough, Andreas Schäffer, and Cyril Sweetlove for their valuable comments during the preparation of this manuscript. We are also grateful for suggestions on how to improve the manuscript from Heli Hollnagel, Bennard Van Ravenzwaay, Björn Hidding, David Saunders, Charlie Meyer, Philip Botham, Christopher Hughes, Erik Van Miert, Gordon Sanders, Joop Hermens, Timothy Gant, Andreas Häner, and Anna Böhnhardt. The authors' time to contribute to this review was supported by their organizations.
This article includes online‐only Supporting Information.
DATA AVAILABILITY STATEMENT
There are few data reported in this paper. It is a synthesis of modeling and weight‐of‐evidence approaches. Relevant data are provided in the text or Tables S1 and S2, and the original sources are fully cited.
REFERENCES
- Abelkop, A. D. K. , Graham, J. D. , & Royer, T. V. (2015). Persistent, bioaccumulative, and toxic (PBT) chemicals: Technical aspects, policies, and practices. CRC Press. 10.1201/9781351228893 [DOI] [Google Scholar]
- Andrady, A. E. (2007). In Mark J. E. (Ed.), Biodegradability of polymers. Physical properties of polymers handbook (pp. 951–964). Springer. 10.1007/978-0-387-69002-5_56 [DOI] [Google Scholar]
- Ballabio, D. , Biganzoli, F. , Todeschini, R. , & Consonni, V. (2017). Qualitative consensus of QSAR ready biodegradability predictions. Toxicological & Environmental Chemistry, 99, 1193–1216. 10.1080/02772248.2016.1260133 [DOI] [Google Scholar]
- Beyer, A. , & Matthies, M. (2002). Criteria for atmospheric longrange transport potential and persistence of pesticides and industrial chemicals. Schmidt. [Google Scholar]
- Bilcke, C. V. (2002). The Stockholm convention on persistent organic pollutants. Review of European Community and International Environmental Law, 11(3), 328–342. 10.2307/2668517 [DOI] [Google Scholar]
- Boethling, R. (2016). Present, bioaccumulative, and toxic: Sufficient for PBT? Integrated Environmental Assessment and Management, 12(1), 204–205. 10.1002/ieam.1725 [DOI] [PubMed] [Google Scholar]
- Boethling, R. , Fenner, K. , Howard, P. , Klečka, G. , Madsen, T. , Snape, J. R. , & Whelan, M. J. (2009). Environmental persistence of organic pollutants: Guidance for development and review of POP risk profiles. Integrated Environmental Assessment and Management, 5(4), 539–556. 10.1897/IEAM_2008-090.1 [DOI] [PubMed] [Google Scholar]
- Bonnell, M. A. , Zidek, A. , Griffiths, A. , & Gutzman, D. (2018). Fate and exposure modeling in regulatory chemical evaluation: New directions from retrospection. Environmental Science: Processes & Impacts, 20(1), 20–31. 10.1039/c7em00510e [DOI] [PubMed] [Google Scholar]
- Brandt, M. , Becker, E. , Jöhncke, U. , Sättler, D. , & Schulte, C. (2016). A weight‐of‐evidence approach to assess chemicals: Case study on the assessment of persistence of 4,6‐substituted phenolic benzotriazoles in the environment. Environmental Sciences Europe, 28(1), 1–14. 10.1186/s12302-016-0072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges, J. , & Solomon, K. R. (2016). Quantitative weight‐of‐evidence analysis of the persistence, bioaccumulation, toxicity, and potential for long‐range transport of the cyclic volatile methyl siloxanes. Journal of Toxicology and Environmental Health—Part B Critical Reviews, 19(8), 345–379. 10.1080/10937404.2016.1200505 [DOI] [PubMed] [Google Scholar]
- Brown, D. M. , Camenzuli, L. , Redman, A. D. , Hughes, C. , Wang, N. , Vaiopoulou, E. , Saunders, D. , Villalobos, A. , & Linington, S. (2020). Is the Arrhenius‐correction of biodegradation rates, as recommended through REACH guidance, fit for environmentally relevant conditions? An example from petroleum biodegradation in environmental systems. Science of the Total Environment, 735, 139293. 10.1016/j.scitotenv.2020.139293 [DOI] [PubMed] [Google Scholar]
- Chapra, S. C. (2008). Surface water‐quality modeling. Waveland Press. [Google Scholar]
- Davenport, R. , Curtis‐Jackson, P. , Dalkmann, P. , Davies, J. , Fenner, K. , Hand, L. , McDonough, K. , Ortega‐Calvo, J. J. , Ott, A. , Parsons, J. , Schäffer, A , Sweetlove, C , Trapp, S , Wang, N , & Redman, A. (2021). Scientific concepts and methods for moving persistence assessments into the 21st century. [DOI] [PMC free article] [PubMed]
- Di Guardo, A. , Gouin, T. , MacLeod, M. , & Scheringer, M. (2018). Environmental fate and exposure models: Advances and challenges in 21st century chemical risk assessment. Environmental Science: Processes & Impacts, 20(1), 58–71. 10.1039/C7EM00568G [DOI] [PubMed] [Google Scholar]
- Dimitrov, S. , Pavlov, T. , Dimitrova, N. , Georgieva, D. , Nedelcheva, D. , Kesova, A. , Vasilev, R. , & Mekenyan, O. (2011). Simulation of chemical metabolism for fate and hazard assessment. II CATALOGIC simulation of abiotic and microbial degradation. SAR & QSAR in Environmental Research, 22(7–8), 719–755. 10.1080/1062936X.2011.623322 [DOI] [PubMed] [Google Scholar]
- Duber‐Smith, D. , Chang, Y. , Olson, A. , Rosholt, A. , Api, A. M. , Vey, M. , Ugurlayan, A. , Srinivasan, V. , Antignac, E. , Troyano, E. , Mcmillan, D. , Sarlo, K. , Li, L. , Wimalasena, R. , Flanagan, J. , Garrison, M. , Dayan, N. , Kilfoyle, B. E. , Terebetski, J. L. , … Saxe, J. K. (2012). Kirk‐Othmer encyclopedia of chemical technology—Natural cosmetics. Wiley.
- ECCC . (2016). Science approach document: Ecological risk classification of organic substances. https://www.ec.gc.ca/ese-ees/default.asp?lang=En%26n=a96e2e98-1
- ECETOC . (2003). Persistence of chemicals in the environment (Technical Report No. 90). ECETOC, Brussels, October. ISSN‐0773‐8072‐90. https://www.ecetoc.org/wp-content/uploads/2014/08/ECETOC-TR-090.pdf
- ECETOC . (2011). Refined approaches for risk assessment of PBT/vPvB chemicals (Technical Report No.112). ECETOC, Brussels, October. ISSN‐0773‐8072‐112. https://www.ecetoc.org/publication/tr-112-refined-approaches-for-risk-assessment-of-pbtvpvb-chemicals/
- ECETOC . (2014). Information to be considered in a weight‐of‐evidence‐based PBT/vPvB assessment of chemicals (Annex XIII of REACH) (Special Report No. 18). ECETOC, Brussels, July. https://www.ecetoc.org/publication/special-report-18-information-to-be-considered-in-a-weight-of-evidence-based-pbtvpvb-assessment-of-chemicals-annex-xiii-of-reach/
- ECETOC . (2020). The applicability of analytical tools, test methods and models for polymer risk assessment (Technical Report No. 133‐2). ECETOC, Brussels, May. ISSN‐2079‐1526‐133‐2 (online). https://www.ecetoc.org/publication/tr-133-2-the-applicability-of-analytical-tools-test-methods-and-models-for-polymer-risk-assessment/
- ECHA . (2017a). Guidance on information requirements and chemical safety assessment, chapter R.11: PBT/vPvB assessment (version 3.0). European Chemical Agency. https://echa.europa.eu/documents/10162/13632/information_requirements_r11_en.pdf
- ECHA . (2017b). Guidance on information requirements and chemical safety assessment, chapter R. 7b: endpoint specific guidance (version 4.0). ECHA. https://echa.europa.eu/documents/10162/13632/information_requirements_r7b_en.pdf
- Faust, K. , Croes, D. , & van Helden, J. (2011). Prediction of metabolic pathways from genome‐scale metabolic networks. Biosystems, 105(2), 109–121. 10.1016/j.biosystems.2011.05.004 [DOI] [PubMed] [Google Scholar]
- Fenner, K. , Scheringer, M. , Macleod, M. , Matthies, M. , McKone, T. , Stroebe, M. , Beyer, A. , Bonnell, M. , Le Gall, A. C. , Klasmeier, J. , Mackay, D. , Van De Meent, D. , Pennington, D. , Scharenberg, B. , Suzuki, N. , & Wania, F. (2005). Comparing estimates of persistence and long‐range transport potential among multimedia models. Environmental Science & Technology, 39(7), 1932–1942. 10.1021/es048917b [DOI] [PubMed] [Google Scholar]
- Fewson, C. A. (1988). Biodegradation of xenobiotic and other persistent compounds: The causes of recalcitrance. Trends in Biotechnology, 6(7), 148–153. 10.1016/0167-7799(88)90084-4 [DOI] [Google Scholar]
- Finley, S. D. , Broadbelt, L. J. , & Hatzimanikatis, V. (2009). Thermodynamic analysis of biodegradation pathways. Biotechnology and Bioengineering, 103(3), 532–541. 10.1002/bit.22285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney, L. J. , Liu, W. T. , Guckert, J. B. , Kumagai, Y. , Namkung, E. , Nishihara, T. , & Larson, R. J. (2001). Structure of microbial communities in activated sludge: Potential implications for assessing the biodegradability of chemicals. Ecotoxicology and Environmental Safety, 49(1), 40–53. 10.1006/eesa.2001.2034 [DOI] [PubMed] [Google Scholar]
- Garbeva, P. , Veen, J. A. , & van Elsas, J. (2004). Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology, 42, 243–270. 10.1146/annurev.phyto.42.012604.135455 [DOI] [PubMed] [Google Scholar]
- Ghattas, A.‐K. , Fischer, F. , Wick, A. , & Ternes, T. A. (2017). Anaerobic biodegradation of (emerging) organic contaminants in the aquatic environment. Water Research, 116, 268–295. 10.1016/j.watres.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Giesy, J. P. , Solomon, K. R. , Mackay, D. , & Anderson, J. (2014). Evaluation of evidence that the organophosphorus insecticide chlorpyrifos is a potential persistent organic pollutant (POP) or persistent, bioaccumulative, and toxic (PBT). Environmental Sciences Europe, 26, 29. 10.1186/s12302-014-0029-y [DOI] [Google Scholar]
- González‐Gaya, B. , Martínez‐Varela, A. , Vila‐Costa, M. , Casal, P. , Cerro‐Gálvez, E. , Berrojalbiz, N. , Lundin, D. , Vidal, M. , Mompeán, C. , Bode, A. , Jiménez, B. , & Dachs, J. (2019). Biodegradation as an important sink of aromatic hydrocarbons in the oceans. Nature Geoscience, 12(2), 119–125. 10.1038/s41561-018-0285-3 [DOI] [Google Scholar]
- Goswami, P. , & O'Haire, T. (2016). Advances in technical nonwovens—Developments in the use of green (biodegradable), recycled and biopolymer materials in technical nonwovens (pp. 97–114). Woodhead Publishing. 10.1016/B978-0-08-100575-0.00003-6 [DOI] [Google Scholar]
- Gouin, T. , Mackay, D. , Webster, E. , & Wania, F. (2000). Screening chemicals for persistence in the environment. Environmental Science and Technology, 34(5), 881–884. 10.1021/es991011z [DOI] [Google Scholar]
- Gouin, T. , Van Egmond, R. , Price, O. R. , & Hodges, J. E. N. (2012). Prioritising chemicals used in personal care products in China for environmental risk assessment: Application of the RAIDAR model. Environmental Pollution, 165, 208–214. 10.1016/j.envpol.2011.12.030 [DOI] [PubMed] [Google Scholar]
- Hales, S. G. , Feijtel, T. , King, H. , Fox, K. , & Verstratete, W. (1997). Biodegradation kinetics: Generation and use of data for regulatory decisions making. In SETAC Europe Workshop, Port Sunlight, UK, Brussels, Belgium, September 4–6, pp. 87–100.
- Hammershøj, R. , Birch, H. , Redman, A. D. , & Mayer, P. (2019). Mixture effects on biodegradation kinetics of hydrocarbons in surface water: Increasing concentrations inhibited degradation whereas multiple substrates did not. Environmental Science and Technology, 53(6), 3087–3094. 10.1021/acs.est.9b00638 [DOI] [PubMed] [Google Scholar]
- Hardy, A. , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Benfenati, E. , Chaudhry, Q. M. , Craig, P. , Frampton, G. , … Younes, M. (2017). Guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal, 15, e04971. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsen, E. , Mintenig, S. M. , Besseling, E. , & Koelmans, A. A. (2018). Quality criteria for the analysis of microplastic in biota Samples: A critical review. Environmental Science and Technology, 52(18), 10230–10240. 10.1021/acs.est.8b01611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander, A. , Scheringer, M. , Shatalov, V. , Mantseva, E. , Sweetman, A. , Roemer, M. , Baart, A. , Suzuki, N. , Wegmann, F. , & Van De Meent, D. (2008). Estimating overall persistence and long‐range transport potential of persistent organic pollutants: A comparison of seven multimedia mass balance models and atmospheric transport models. Journal of Environmental Monitoring, 10, 1139–1147. 10.1039/b803760d [DOI] [PubMed] [Google Scholar]
- Horemans, B. , Breugelmans, P. , Hofkens, J. , Smolders, E. , & Springael, D. (2013). Environmental dissolved organic matter governs biofilm formation and subsequent linuron degradation activity of a linuron‐degrading bacterial consortium. Applied and Environmental Microbiology, 79(15), 4534–4542. 10.1128/AEM.03730-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, P. H. , & Banerjee, S. (1984). Interpreting results from biodegradability tests of chemicals in water and soil. Environmental Toxicology and Chemistry, 3(4), 551–562. 10.1002/etc.5620030405 [DOI] [Google Scholar]
- Huang, W. , Peng, P. , Yu, Z. , & Fu, J. (2003). Effects of organic matter heterogeneity on sorption and desorption of organic contaminants by soils and sediments. Journal of Applied Geochemistry, 18(7), 955–972. 10.1016/S0883-2927(02)00205-6 [DOI] [Google Scholar]
- Hughes, C. B. , Brown, D. M. , Camenzuli, L. , Redman, A. D. , Arey, J. S. , Vione, D. , Wang, N. , & Vaiopoulou, E. (2020). Can a chemical be both readily biodegradable AND very persistent (vP)? Weight‐of‐evidence determination demonstrates that phenanthrene is not persistent in the environment. Environmental Sciences Europe, 32(148), 1–19. 10.1186/s12302-020-00427-1 [DOI] [Google Scholar]
- Hung, H. , Katsoyiannis, A. A. , Brorström‐Lundén, E. , Olafsdottir, K. , Aas, W. , Breivik, K. , Bohlin‐Nizzetto, P. , Sigurdsson, A. , Hakola, H. , Bossi, R. , Skov, H. , Sverko, E. , Barresi, E. , Fellin, P. , & Wilson, S. (2016). Temporal trends of Persistent Organic Pollutants (POPs) in arctic air: 20 years of monitoring under the Arctic Monitoring and Assessment Programme (AMAP). Environmental Pollution, 217, 52–61. 10.1016/j.envpol.2016.01.079 [DOI] [PubMed] [Google Scholar]
- Itrich, N. R. , McDonough, K. M. , Van Ginkel, C. G. , Bisinger, E. C. , Lepage, J. N. , Schaefer, E. C. , Menzies, J. Z. , Casteel, K. D. , & Federle, T. W. (2015). Widespread microbial adaptation to l‐glutamate‐N,N‐diacetate (L‐GLDA) following its market introduction in a consumer cleaning product. Environmental Science and Technology, 49(22), 13314–13321. 10.1021/acs.est.5b03649 [DOI] [PubMed] [Google Scholar]
- Jaworska, J. , Dimitrov, S. , Nikolova, N. , & Mekenyan, O. (2002). Probabilistic assessment of biodegradability based on metabolic pathways: Catabol system. SAR and QSAR in Environmental Research, 13(2), 307–323. 10.1080/10629360290002794 [DOI] [PubMed] [Google Scholar]
- Jones, S. H. , & Alexander, M. (1986). Kinetics of mineralization of phenols in lake water. Applied and Environmental Microbiology, 51(5), 891–897. 10.1128/aem.51.5.891-897.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers, N. , Laane, R. W. P. M. , de Graaf, C. , & de Voogt, P. (2005). Fate modeling of nonylphenol ethoxylates and their metabolites in the Dutch Scheldt and Rhine estuaries: Validation with new field data. Estuarine Coastal Shelf Science, 62(1–2), 141–160. 10.1016/j.ecss.2004.08.014 [DOI] [Google Scholar]
- Judson, P. (2019). Chapter 17: Predicting biodegradation. In Hirst J. (Ed.), Knowledge‐based expert systems in chemistry: Artificial intelligence in decision making (RSC Theoretical and Computational Chemistry Series, Vol. 15, 2nd ed., pp. 221–232). Royal Society of Chemistry. [Google Scholar]
- Junker, T. , Coors, A. , & Schüürmann, G. (2019). Compartment‐specific screening tools for persistence: Potential role and application in the regulatory context. Integrated Environmental Assessment and Management, 15(3), 470–481. 10.1002/ieam.4125 [DOI] [PubMed] [Google Scholar]
- Kästner, M. , Nowak, K. M. , Miltner, A. , Trapp, S. , & Schäffer, A. (2014). Classification and modelling of nonextractable residue (NER) formation of xenobiotics in soil—A synthesis. Critical Reviews Environmental Science Technology, 44(19), 2107–2171. 10.1080/10643389.2013.828270 [DOI] [Google Scholar]
- Katayama, A. , Bhula, R. , Burns, G. R. , Carazo, E. , Felsot, A. , Hamilton, D. , Harris, C. , Kim, Y.‐H. , Kleter, G. , Koedel, W. , Linders, J. , Peijnenburg, J. G. M. W. , Sabljic, A. , Stephenson, R. G. , Racke, D. K. , Rubin, B. , Tanaka, K. , Unsworth, J. , Wauchope, R. D. (2010). Bioavailability of xenobiotics in the soil environment. In Whitacre D. M. (Ed.), Reviews of environmental contamination and toxicology (pp. 1–86). Springer New York. [DOI] [PubMed] [Google Scholar]
- Klasmeier, J. , Matthies, M. , Macleod, M. , Fenner, K. , Scheringer, M. , Stroebe, M. , Le Gall, A. C. , McKone, T. , Van De Meent, D. , & Wania, F. (2006). Application of multimedia models for screening assessment of long‐range transport potential and overall persistence. Environmental Science and Technology, 40(1), 53–60. 10.1021/es0512024 [DOI] [PubMed] [Google Scholar]
- Klimisch, H. J. , Andreae, M. , & Tillmann, U. (1997). A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regulatory Toxicology and Pharmacology, 25, 1–5. 10.1006/rtph.1996.1076 [DOI] [PubMed] [Google Scholar]
- Klöpffer, W. , Rippen, G. , & Frische, R. (1982). Physicochemical properties as useful tools for predicting the environmental fate of organic chemicals. Ecotoxicology and Environmental Safety, 6(3), 294–301. 10.1016/0147-6513(82)90019-7 [DOI] [PubMed] [Google Scholar]
- Klopman, G. , Zhang, Z. , Balthasar, D. M. , & Rosenkranz, H. S. (1995). Computer‐automated predictions of aerobic biodegradation of chemicals. Environmental Toxicology and Chemistry, 14(3), 395–403. 10.1002/etc.5620140307 [DOI] [Google Scholar]
- Koelmans, A. A. , Mohamed Nor, N. H. , Hermsen, E. , Kooi, M. , Mintenig, S. M. , & De France, J. (2019). Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Research, 155, 410–422. 10.1016/j.watres.2019.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolvenbach, B. A. , Helbling, D. E. , Kohler, H. P. E. , & Corvini, P. F.‐X. (2014). Emerging chemicals and the evolution of biodegradation capacities and pathways in bacteria. Current Opinion in Biotechnology, 27, 8–14. 10.1016/j.copbio.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Kowalczyk, A. , Martin, T. J. , Price, O. R. , Snape, J. R. , van Egmond, R. A. , Finnegan, C. J. , Schäfer, H. , Davenport, R. J. , & Bending, G. D. (2015). Refinement of biodegradation tests methodologies and the proposed utility of new microbial ecology techniques. Ecotoxicology and Environmental Safety, 111, 9–22. 10.1016/j.ecoenv.2014.09.021 [DOI] [PubMed] [Google Scholar]
- Lewis, A. , & Prince, R. C. (2018). Integrating dispersants in oil spill response in Arctic and other icy environments. Environmental Science and Technology, 52(11), 6098–6112. 10.1021/acs.est.7b06463 [DOI] [PubMed] [Google Scholar]
- Liang, B. , Jiang, J. , Zhang, J. , Zhao, Y. , & Li, S. (2012). Horizontal transfer of dehalogenase genes involved in the catalysis of chlorinated compounds: Evidence and ecological role. Critical Reviews in Microbiology, 38(2), 95–110. 10.3109/1040841X.2011.618114 [DOI] [PubMed] [Google Scholar]
- Lipnick, R. L. , Hermens, J. L. M. , & Jones, K. (2000). Persistent, bioaccumulative, and toxic chemicals: Volume I: Fate and exposure. American Chemical Society.
- Lipnick, R. L. , Jansson, B. , Mackay, D. , & Petreas, M. (2000). Persistent, bioaccumulative, and toxic chemicals: Volume II: Assessment and new chemicals (ACS Symposium Series, No. 773). American Chemical Society.
- Lutter, R. , Abbott, L. , Becker, R. , Borgert, C. , Bradley, A. , Charnley, G. , Dudley, S. , Felsot, A. , Golden, N. , Gray, G. , Juberg, D. , Mitchell, M. , Rachman, N. , Rhomberg, L. , Solomon, K. , Sundlof, S. , & Willett, K. (2015). Improving weight of evidence approaches to chemical evaluations. Risk Analysis, 35(2), 186–192. 10.1111/risa.12277 [DOI] [PubMed] [Google Scholar]
- Mackay, D. (1979). Finding fugacity feasible. Environmental Science and Technology, 13, 1218–1223. 10.1021/es60158a003 [DOI] [Google Scholar]
- Mackay, D. (2001). Multimedia environmental models: The fugacity approach. CRC Press. 10.1201/9781420032543 [DOI] [Google Scholar]
- Mackay, D. , Di Guardo, A. , Paterson, S. , & Cowan, C. E. (1996). Evaluating the environmental fate of a variety of types of chemicals using the EQC model. Environmental Toxicology and Chemistry, 15, 1627–1637. 10.1002/etc.5620150929 [DOI] [Google Scholar]
- Mackay, D. , Di Guardo, A. , Paterson, S. , Kicsi, G. , & Cowan, C. E. (1996). Assessing the fate of new and existing chemicals: A five‐stage process. Environmental Toxicology and Chemistry, 15, 1618–1626. 10.1002/etc.5620150928 [DOI] [Google Scholar]
- Mackay, D. , Di Guardo, A. , Paterson, S. , Kicsi, G. , Cowan, C. E. , & Kane, D. M. (1996). Assessment of chemical fate in the environment using evaluative, regional and local‐scale models: Illustrative application to chlorobenzene and linear alkylbenzene sulfonates. Environmental Toxicology and Chemistry, 15, 1638–1648. 10.1002/etc.5620150930 [DOI] [Google Scholar]
- Mackay, D. , Hughes, D. M. , Romano, M. L. , & Bonnell, M. (2014). The role of persistence in chemical evaluations. Integrated Environmental Assessment and Management, 10(4), 588–594. 10.1002/ieam.1545 [DOI] [PubMed] [Google Scholar]
- Mackay, D. , & Paterson, S. (1981). Calculating fugacity. Environmental Science & Technology, 15(9), 1006–1014. 10.1021/es00091a001 [DOI] [PubMed] [Google Scholar]
- Mackay, D. , & Webster, E. (2006). Environmental persistence of chemicals. Environmental Science and Pollution Research International, 13, 43–49. 10.1065/espr2006.01.008 [DOI] [PubMed] [Google Scholar]
- Mackay, D. , Giesy, J. P. , & Solomon, K. R. (2014). Fate in the environment and long‐range atmospheric transport of the organophosphorus insecticide, chlorpyrifos and its oxon. In Giesy J. P., & Solomon K. R. (Eds.), Ecological risk assessment for chlorpyrifos in terrestrial and aquatic systems in the United States (pp. 35–76). Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Mamy, L. , Patureau, D. , Barriuso, E. , Bedos, C. , Bessac, F. , Louchart, X. , Martin‐Laurent, F. , Miege, C. , & Benoit, P. (2015). Prediction of the fate of organic compounds in the environment from their molecular properties: A review. Critical Reviews Environmental Science Technology, 45(12), 1277–1377. 10.1080/10643389.2014.955627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, T. J. , Snape, J. R. , Bartram, A. , Robson, A. , Acharya, K. , & Davenport, R. J. (2017). Environmentally relevant inoculum concentrations improve the reliability of persistent assessments in biodegradation screening tests. Environmental Science and Technology, 51(5), 3065–3073. 10.1021/acs.est.6b05717 [DOI] [PubMed] [Google Scholar]
- Matthies, M. , Solomon, K. , Vighi, M. , Gilman, A. , & Tarazona, J. V. (2016). The origin and evolution of assessment criteria for persistent, bioaccumulative and toxic (PBT) chemicals and persistent organic pollutants (POPs). Environmental Science: Processes & Impacts, 18(9), 1114–1128. 10.1039/c6em00311g [DOI] [PubMed] [Google Scholar]
- McArthur, J. V. (2006). Microbial ecology: An evolutionary approach. Academic Press (Elsevier).
- McKone, T. E. , & Enoch, K. G. (2002). CalTOX (registered trademark), a multimedia total exposure model spreadsheet user's guide. Version 4.0 (Beta). https://www.osti.gov/servlets/purl/803756
- McLachlan, M. S. , Zou, H. , & Gouin, T. (2017). Using benchmarking to strengthen the assessment of persistence. Environmental Science and Technology, 51(1), 4–11. 10.1021/acs.est.6b03786 [DOI] [PubMed] [Google Scholar]
- Melsbach, A. , Ponsin, V. , Torrentó, C. , Lihl, C. , Hofstetter, T. B. , Hunkeler, D. , & Elsner, M. (2019). 13C‐ and 15N‐isotope analysis of desphenylchloridazon by liquid chromatography–isotope‐ratio mass spectrometry and derivatization gas chromatography–isotope‐ratio mass spectrometry. Analytical Chemistry, 91(5), 3412–3420. 10.1021/acs.analchem.8b04906 [DOI] [PubMed] [Google Scholar]
- Merrettig‐Bruns, U. , & Jelen, E. (2009). Anaerobic biodegradation of detergent surfactants. Materials (Basel), 2(1), 181–206. 10.3390/ma2010181 [DOI] [Google Scholar]
- Moermond, C. T. A. , Kase, R. , Korkaric, M. , Ågerstrand, M. , & Agerstrand, M. (2016). CRED: Criteria for reporting and evaluating ecotoxicity data. Environmental Toxicology and Chemistry, 35(5), 1297–1309. 10.1002/etc.3259 [DOI] [PubMed] [Google Scholar]
- Nagata, Y. , Kato, H. , Ohtsubo, Y. , & Tsuda, M. (2019). Lessons from the genomes of lindane‐degrading sphingomonads. Environmental Microbiology Reports, 11(5), 630–644. 10.1111/1758-2229.12762 [DOI] [PubMed] [Google Scholar]
- Nielsen, T. K. , Rasmussen, M. , Demanèche, S. , Cecillon, S. , Vogel, T. M. , & Hansen, L. H. (2017). Evolution of sphingomonad gene clusters related to pesticide catabolism revealed by genome sequence and mobilomics of Sphingobium herbicidovorans MH. Genome Biology and Evolution, 9(9), 2477–2490. 10.1093/gbe/evx185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg, M. , Templeton, D. , Andersen, O. , & Duffus, J. (2009). IUPAC glossary of terms used in ecotoxicology (IUPAC Recommendations 2012). Pure and Applied Chemistry, 81(5), 829–970. 10.1351/PAC-REC-08-07-09 [DOI] [Google Scholar]
- OECD . (2006). OECD guidelines for the testing of chemicals. https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-3-degradation-and-accumulation_2074577x
- OECD . (2018). Prioritisation of chemicals using the integrated approaches for testing and assessment (IATA)‐based ecological risk classification. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2018)27%26docLanguage=En
- OECD . (2019). Guiding principles and key elements for establishing a weight of evidence for chemical assessment No. 311. https://images.chemycal.com/Media/Files/ENV-JM-MONO(2019)31.pdf. (311):1‐37
- Ortega‐Calvo, J. J. , Harmsen, J. , Parsons, J. R. , Semple, K. T. , Aitken, M. D. , Ajao, C. , Eadsforth, C. , Galay‐Burgos, M. , Naidu, R. , Oliver, R. , Peijnenburg, W. J. , Römbke, J. , Streck, G. , & Versonnen, B. (2015). From bioavailability science to regulation of organic chemicals. Environmental Science and Technology, 49, 10255–10264. 10.1021/acs.est.5b02412 [DOI] [PubMed] [Google Scholar]
- Ott, A. , Martin, T. J. , Acharya, K. , Lyon, D. Y. , Robinson, N. , Rowles, B. , Snape, J. R. , Still, I. , Whale, G. F. , Albright, V. C. , Bäverbäck, P. , Best, N. , Commander, R. , Eickhoff, C. , Finn, S. , Hidding, B. , Maischak, H. , Sowders, K. A. , Taruki, M. , … Davenport, R. J. (2020). Multi‐laboratory validation of a new marine biodegradation screening test for chemical persistence assessment. Environmental Science and Technology, 54(7), 4210–4220. 10.1021/acs.est.9b07710 [DOI] [PubMed] [Google Scholar]
- Ott, A. , Martin, T. J. T. J. , Whale, G. F. G. F. , Snape, J. R. J. R. , Rowles, B. , Galay‐Burgos, M. , & Davenport, R. J. R. J. (2019). Improving the biodegradability in seawater test (OECD 306). Science of the Total Environment, 666, 399–404. 10.1016/j.scitotenv.2019.02.167 [DOI] [PubMed] [Google Scholar]
- Pastore, M. , Santaeufemia, S. , Bertucco, A. , & Sforza, E. (2018). Light intensity affects the mixotrophic carbon exploitation in chlorella protothecoides: Consequences on microalgae‐bacteria based wastewater treatment. Water Science and Technology, 78(8), 1762–1771. 10.2166/wst.2018.462 [DOI] [PubMed] [Google Scholar]
- Pennington, D. W. (2001). An evaluation of chemical persistence screening approaches. Chemosphere, 44(7), 1589–1601. 10.1016/S0045-6535(00)00530-0 [DOI] [PubMed] [Google Scholar]
- Pizzo, F. , Lombardo, A. , Manganaro, A. , & Benfenati, E. (2013). In silico models for predicting ready biodegradability under REACH: A comparative study. Science of the Total Environment, 463–464, 161–168. 10.1016/j.scitotenv.2013.05.060 [DOI] [PubMed] [Google Scholar]
- Poursat, B. A. J. J. , van Spanning, R. J. M. M. , de Voogt, P. , & Parsons, J. R. (2019). Implications of microbial adaptation for the assessment of environmental persistence of chemicals. Critical Reviews Environmental Science Technology, 49(23), 2220–2255. 10.1080/10643389.2019.1607687 [DOI] [Google Scholar]
- Rhomberg, L. R. , Goodman, J. E. , Bailey, L. A. , Prueitt, R. L. , Beck, N. B. , Bevan, C. , Honeycutt, M. , Kaminski, N. E. , Paoli, G. , Pottenger, L. H. , Scherer, R. W. , Wise, K. C. , & Becker, R. A. (2013). A survey of frameworks for best practices in weight‐of‐evidence analyses. Critical Reviews in Toxicology, 43(9), 753–784. 10.3109/10408444.2013.832727 [DOI] [PubMed] [Google Scholar]
- Rücker, C. , & Kümmerer, K. (2012). Modeling and predicting aquatic aerobic biodegradation—A review from a user's perspective. Green Chemistry, 14, 875–887. 10.1039/c2gc16267a [DOI] [Google Scholar]
- Saidi, R. , Boudellioua, I. , Martin, M. J. , & Solovyev, V. (2017). Rule mining techniques to predict prokaryotic metabolic pathways. Methods in Molecular Biology, 1613, 311–331. 10.1007/978-1-4939-7027-8_12 [DOI] [PubMed] [Google Scholar]
- Saxe, J. K. (2011). Biodegradability evaluation for cosmetic ingredients and finished products. In N. Dayan, & L. Kromidas (Eds.), Formulating, packaging and marketing of natural cosmetic products (pp. 387–410). Wiley. 10.1002/9781118056806.ch20 [DOI]
- SCENIHR . (2012). European Union, Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) memorandum on the use of the scientific literature for human health risk assessment purposes—Weighing of evidence and expression of uncertainty. 19 March. https://op.europa.eu/en/publication-detail/-/publication/555f24ce-367e-4417-88ef-7e4932d7bddf
- Schäffer, A. , Kästner, M. , & Trapp, S. (2018). A unified approach for including non‐extractable residues (NER) of chemicals and pesticides in the assessment of persistence. Environmental Sciences Europe, 30(51), 1–14. 10.1186/s12302-018-0181-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheringer, M. , Jones, K. C. , Matthies, M. , Simonich, S. , & van de Meent, D. (2009). Multimedia partitioning, overall persistence, and long‐range transport potential in the context of POPs and PBT chemical assessments. Integrated Environmental Assessment and Management, 5(4), 557–576. 10.1897/IEAM_2009-007.1 [DOI] [PubMed] [Google Scholar]
- Scheringer, M. , McLeod, M. , Matthies, M. , & Klasmeier, J. (2006). Persistence criteria in the REACH legislation: Critical evaluation and recommendations. https://www.umweltbundesamt.de/sites/default/files/medien/2674/dokumente/gutachten_gesamtpersistenz.pdf
- Schwarzenbach, R. P. , Gschwend, P. M. , & Imboden, D. M. (2016). Environmental organic chemistry.
- Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) . (2018). Memorandum on weight of evidence and uncertainties. Revision 2018. European Commission. https://ec.europa.eu/health/sites/default/files/scientific_committees/scheer/docs/scheer_o_014.pdf [Google Scholar]
- Scow, K. M. , Simkins, S. , & Alexander, M. (1986). Kinetics of mineralization of organic compounds at low concentrations in soil. Applied and Environmental Microbiology, 51(5), 1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple, K. , Doick, K. , Jones, K. , Burauel, P. , Craven, A. , & Harms, H. (2004). Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environmental Science and Technology, 38, 228A–231A. 10.1021/es040548w [DOI] [PubMed] [Google Scholar]
- Shrestha, P. , Meisterjahn, B. , Klein, M. , Mayer, P. , Birch, H. , Hughes, C. B. , & Hennecke, D. (2019). Biodegradation of Vvlatile chemicals in soil: Separating volatilization and degradation in an improved test setup (OECD 307). Environmental Science and Technology, 53(1), 20–28. 10.1021/acs.est.8b05079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, K. , Matthies, M. , & Vighi, M. (2013). Assessment of PBTs in the European Union: A critical assessment of the proposed evaluation scheme with reference to plant protection products. Environmental Sciences Europe, 25(1), 1–17. 10.1186/2190-4715-25-10 [DOI] [Google Scholar]
- Southwell, R. V. , Hilton, S. L. , Pearson, J. M. , Hand, L. H. , & Bending, G. D. (2020). Inclusion of seasonal variation in river system microbial communities and phototroph activity increases environmental relevance of laboratory chemical persistence tests. Science of the Total Environment, 733, 139070. 10.1016/j.scitotenv.2020.139070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen, R. J. C. A. , Evers, E. H. G. , van Hattum, B. , Cofino, W. P. , & Brinkman, U. A. T. (2002). Net fluxes of pesticides from the Scheldt Estuary into the North Sea: A model approach. Environmental Pollution, 116(1), 75–84. 10.1016/s0269-7491(01)00123-3 [DOI] [PubMed] [Google Scholar]
- Stroebe, M. , Scheringer, M. , & Hungerbühler, K. (2004). Measures of overall persistence and the temporal remote state. Environmental Science and Technology, 38, 5665–5673. 10.1021/es035443s [DOI] [PubMed] [Google Scholar]
- Struijs, J. (2014). SimpleTreat 4.0: A model to predict fate and emission of chemicals in wastewater treatment plants. https://www.rivm.nl/bibliotheek/rapporten/601353005.pdf
- Subba‐Rao, R. V. , Rubin, H. E. , & Alexander, M. (1982). Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Applied and Environmental Microbiology, 43(5), 1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter, G. , Nichols, J. , Lavoie, E. , & Cormier, S. (2020). Systematic review and weight of evidence are integral to ecological and human health assessments: They need an integrated framework. Integrated Environmental Assessment and Management, 16(5), 718–728. 10.1002/ieam.4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouand, G. G. , Durand, M. J. , Maul, A. , Gancet, C. , & Blok, H. (2011). New concepts in the evaluation of biodegradation/persistence of chemical substances using a microbial inoculum. Frontiers in Microbiology, 2, 164. 10.3389/fmicb.2011.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiehm, A. , & Schmidt, K. R. (2011). Sequential anaerobic/aerobic biodegradation of chloroethenes—Aspects of field application. Current Opinion in Biotechnology, 22(3), 415–421. 10.1016/j.copbio.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Top, E. M. , & Springael, D. (2003). The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Current Opinion in Biotechnology, 14(3), 262–269. 10.1016/S0958-1669(03)00066-1 [DOI] [PubMed] [Google Scholar]
- US EPA . (2020). Estimation Programs Interface Suite for Microsoft Windows. US EPA.
- Van De Meent, D. , McKone, T. , Parkerton, T. , Matthies, M. , Scheringer, M. , Wania, F. , Purdy, R. , & Bennett, D. H. (2000). Persistence and transport potential of chemicals in a multimedia environment. Evaluation of persistence and long‐range transport potential of organic chemicals in the environment (pp. 1–44). SETAC Press. https://escholarship.org/uc/item/4650x5s2 [Google Scholar]
- Van Der Kraak, G. J. , Hosmer, A. J. , Hanson, M. L. , Kloas, W. , & Solomon, K. R. (2014). Effects of atrazine in fish, amphibians, and reptiles: An analysis based on quantitative weight of evidence. Critical Reviews in Toxicology, 44(Suppl 5), 1–66. 10.3109/10408444.2014.967836 [DOI] [PubMed] [Google Scholar]
- Wania, F. , & Mackay, D. (1993). Modelling the global distribution of toxaphene: A discussion of feasibility and desirability. Chemosphere, 27, 2079–2094. 10.1016/0045-6535(93)90403-R [DOI] [Google Scholar]
- Wania, F. , & Mackay, D. (1995). A global distribution model for persistent organic chemicals. Science of the Total Environment, 160–161, 211–232. 10.1016/0048-9697(95)04358-8 [DOI] [Google Scholar]
- Wania, F. , Mackay, D. , Li, Y.‐F. , Bidleman, T. F. , & Strand, A. (1999). Global chemical fate of α‐hexachlorocyclohexane. 1. Evaluation of a global distribution model. Environmental Toxicology and Chemistry, 18, 1390–1399. 10.1002/etc.5620180707 [DOI] [Google Scholar]
- Wassenaar, P. N. H. , & Verbruggen, E. M. J. (2021). Persistence, bioaccumulation and toxicity‐assessment of petroleum UVCBs: A case study on alkylated three‐ring PAHs. Chemosphere, 276, 130113. 10.1016/j.chemosphere.2021.130113 [DOI] [PubMed] [Google Scholar]
- Webster, E. , Mackay, D. , Di Guardo, A. , Kane, D. , & Woodfine, D. (2004). Regional differences in chemical fate model outcome. Chemosphere, 55, 1361–1376. 10.1016/j.chemosphere.2003.10.061 [DOI] [PubMed] [Google Scholar]
- Webster, E. , Mackay, D. , & Wania, F. (1998). Evaluating environmental persistence. Environmental Toxicology and Chemistry, 17, 2148–2158. [DOI] [Google Scholar]
- Weed, D. L. (2005). Weight of evidence: A review of concept and methods. Risk Analysis, 25(6), 1545–1557. 10.1111/j.1539-6924.2005.00699.x [DOI] [PubMed] [Google Scholar]
- Wegmann, F. , Cavin, L. , MacLeod, M. , Scheringer, M. , & Hungerbühler, K. (2009). The OECD software tool for screening chemicals for persistence and long‐range transport potential. Environmental Modelling and Software, 24, 228–237. 10.1016/j.envsoft.2008.06.014 [DOI] [Google Scholar]
- Whale, G. , Parsons, J. , Ginkel, K. , Davenport, R. , Vaiopoulou, E. , Fenner, K. , & Schaeffer, A. (2021). Improving our understanding of the environmental persistence of chemicals. Integrated Environmental Assessment and Management, 17(6), 1123–1135. 10.1002/ieam.4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2009). Principles and methods for the risk assessment of chemicals in food. International Programme on Chemical Safety. Environmental Health Criteria, 240, 1–34. https://www.who.int/publications/i/item/9789241572408 [Google Scholar]
- Wicker, J. , Lorsbach, T. , Gütlein, M. , Schmid, E. , Latino, D. , Kramer, S. , & Fenner, K. (2016). enviPath—The environmental contaminant biotransformation pathway resource. Nucleic Acids Research, 44, D502–D508. 10.1093/nar/gkv1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, H. , Radke, M. , Kierkegaard, A. , MacLeod, M. , & McLachlan, M. S. (2015). Using chemical benchmarking to determine the persistence of chemicals in a Swedish lake. Environmental Science and Technology, 49(3), 1646–1653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supporting Information file contains 1) a summary table to multimedia models that can support the overall persistence analysis promoted in this article, and 2) a case study on phenanthrene to demonstrate how a tiered P ov‐based Persistence assessment can be performed.
Data Availability Statement
There are few data reported in this paper. It is a synthesis of modeling and weight‐of‐evidence approaches. Relevant data are provided in the text or Tables S1 and S2, and the original sources are fully cited.


