Abstract
Introduction
There is still an undiscovered territory about the sequelae and lung ultrasound (LUS) findings after SARS-CoV2 acute infection. This study aims to investigate the post-COVID period from a clinical, psychosocial, and radiological point of view, analyze LUS on COVID-19 follow-up and detect whether these outcomes are related to the patient situation.
Methods
We conducted an observational study on patients diagnosed with SARS-CoV2 pneumonia and admitted to the University Hospital of La Candelaria (Tenerife, Spain) from 1st March to 31st August 2020. We performed a descriptive analysis on post-COVID manifestations, LUS score, health-related quality of life measured through the Euroqol 5D-5L questionnaire, and lung function parameters on follow-up, and we compared these variables to the outcomes during the hospital admission.
Results
77 patients were included; the mean age was 57 years and the follow-up mean time from hospital discharge was 16 weeks. 87% of the cases had symptoms on follow-up, the most common was dyspnea (65%); these manifestations were more frequent in females (p = 0,015). 76,5% of the cases had lung aeration alteration in LUS on follow-up; lower PaO2/FiO2 and greater CRP and IL-6 levels on admission were related to LUS score ≥1.
Conclusions
Almost 90% of the patients had persistent symptoms after 16 weeks of hospital discharge due to COVID-19, the most common manifestation presented was dyspnea. Altered lung aeration pattern in LUS was observed on more than 70% of the patients on follow-up.
Keywords: COVID-19, SARS-CoV2, LUS, Lung ultrasound, Post-COVID
Introduction
In December 2019, Wuhan (China) became the epicenter of an infectious outbreak of a new type of coronavirus termed SARS-CoV2 [1]. The infection had a particularly high spread rate, which caused a rapid worldwide expansion, reaching Europe at the end of January 2020. Today, more than one year after the first case of coronavirus disease 2019 (COVID-19), there are more than 219 million cases worldwide and more than 4 million confirmed deaths due to this infection [2].
Regarding COVID-19 data, primary pulmonary manifestations of SARS-CoV-2 acute infection include hypoxemia, dyspnea, and cough; extrapulmonary symptoms may vary, in an acute situation. Fever, asthenia, headache, and myalgia are the most reported symptoms [3]. Long-term outcomes for pulmonary abnormalities include functional impairment as well as residual radiographic findings of ground-glass opacities and fibrotic changes suggesting persistent interstitial disease [4,5]. Systemic complications have also been reported, including arrhythmias, acute coronary syndrome, myocarditis, acute cerebrovascular disease, and neurologic and neuromuscular manifestations [6,7].
Many patients are suffering from different symptoms even after several weeks from hospital discharge. Long covid, or post-covid syndrome, is defined by the National Institute for Health and Care Excellence (NICE) as “signs and symptoms that develop during or after an infection consistent with COVID-19 which continue for more than 12 weeks and are not explained by an alternative diagnosis”[8].
Focusing on lung affection due to COVID-19, there is a wide range of data that describes the most common patterns seen on chest computerized tomography (CT) scan [9], in order of frequency: ground-glass opacity, interlobular septal thickening, air bronchogram, and crazy-paving pattern. Although chest-CT scan is the gold standard for lung imaging, lung ultrasound (LUS) has shown that is able to detect interstitial lung disease, subpleural consolidations, and acute respiratory distress syndrome from any etiologic cause [10], [11], [12]; hence LUS performance on the follow-up clinic could help physicians decide which patients may benefit from further radiological studies such a chest-CT scan.
Therefore, the current study aims to: (1) analyze lung ultrasound findings at follow-up and the relation of these results with the patient clinical situation at admission and follow-up; (2) study whether inflammatory biomarkers and COVID-19 severity on admission can foresee a worse clinical outcome after several months of cured COVID-19; (3) investigate the post-COVID manifestations that appeared after 16 weeks of hospital discharge not only from a clinical and radiological point of view but also from a psychosocial outlook.
Methods
Study design and participants
We conducted a prospective, observational, and descriptive study including patients diagnosed with SARS-CoV2 pneumonia according to World Health Organization guidelines [2], admitted at the Respiratory Disease Department of the University Hospital Nuestra Señora de La Candelaria (Tenerife, Spain) from 1st March to 31st August 2020.
All patients were included based on the following inclusion criteria: (1) patient age ≥ 18 years, (2) confirmed diagnosis of COVID-19 performed by SARS-CoV-2 real-time reverse polymerase chain reaction (RT-PCR) from nasopharyngeal swabs samples, and (3) hospital admission to the University Hospital Nuestra Señora de La Candelaria. Patients with these criteria were excluded: (1) SARS-CoV2 RT-PCR negative result, (2) interstitial lung disease diagnosed before COVID-19 admission, (3) active neoplasia, and (4) neoplasia history treated with chemotherapy and/or radiotherapy.
The ethics committee of the University Hospital of La Candelaria approved this study, and all participants signed informed consent to participate in this study.
Data collection
The demographics, clinical features, laboratory findings, and outcomes of the participants were collected from the electronic medical record system in our hospital.
The following variables were collected at hospital admission: (1) blood test parameters, including a total number of leukocytes, lymphocytes, and platelets, d-dimers, lactate dehydrogenase (LDH), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), C-reactive protein (CRP), procalcitonin and interleukin 6 (IL-6); (2) PaO2/FiO2 ratio (ratio of partial pressure of arterial oxygen in mmHg to the fraction of inspired oxygen), on room air; (3) COVID-19 severity at admission, divided into three categories according to oxygen requirements: mild cases, which did not require oxygen therapy, moderate for those cases managed with non-invasive oxygen therapies (nasal cannula, reservoir mask, and high flow nasal cannula), and severe for those who required orotracheal intubation; (4) demographic data such as age, sex, and smoking habit (active smokers if they had smoked at least one cigarette in the last 6 months, former smokers if they had smoked in the past but were remaining abstinent for at least 6 months, or non-smokers if they had never smoked); (5) presence of comorbidities such as arterial hypertension, dyslipidemia, type 2 diabetes mellitus, obesity (defined by BMI ≥ 30 kg/m2), ischemic heart disease, immunosuppression, chronic obstructive pulmonary disease (COPD), and asthma.
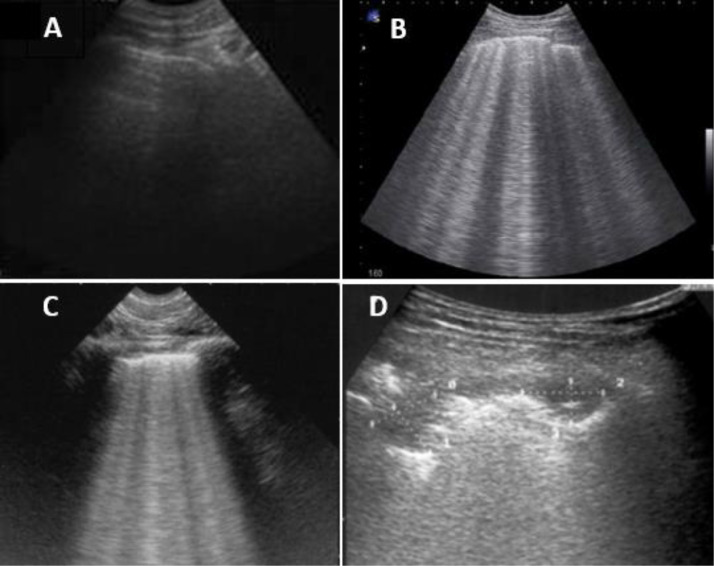
Outpatient follow-up was performed in the next 4 months after hospital discharge due to COVID-19. The following variables were collected: (1) pulmonary function test (PFT), performed within one week before the follow-up clinic: forced vital capacity (FVC), forced expiratory volume in one second (FEV1) and diffusing capacity for carbon monoxide (DLCO) according to national guidelines [13]; (2) lung ultrasound (LUS) performance was determined by LUSS12 protocol [14]. LUS is performed on 12 lung quadrants. LUS score (LUSS) 0 to 3 was given; pattern A score 0: fine pleural line and visible A-lines (Fig. 1 A), pattern B score 1: minimum of three non-confluents B lines with a distance between two of them up to 7 mm and fragmented pleural-line (Fig. 1B), pattern B score 2: confluent B-lines (Fig. 1C) and pattern C score 3: consolidations (Fig. 1D). Scores ranged from 0 to 36 [15,16]. A score was given for each lung quadrant when the patterns were seen in the intercostal space of two consecutive ribs in at least 2 consecutive intercostal spaces. LUSS ≥ 1 was considered as a sign of altered lung aeration pattern, as proposed by Volpicelli et al. [16]. A TE5 (Mindray, San Jose, CA, USA) ultrasound portable unit was used, the lung exploration was performed with a convex 2.5–7.5 MHz transducer (Mindray C5–1 s, San Jose, CA, USA). The study was conducted in the post-COVID clinic by a single operator experienced in LUS; (3) symptoms, classified as respiratory (dyspnea, cough, and expectoration) and non-respiratory symptoms (asthenia, hair loss, arthralgia, ageusia, persistent headache, muscular weakness, myalgia, anosmia, memory loss, cramps and others referred by the patients). The patients’ current clinical features were distinguished from those pre-COVID admission symptoms or due to underlying diseases before COVID-19; (4) European Quality of Life 5 Dimensions (EQ-5D-5L) questionnaire [17] was performed to analyze the impact of the COVID-19 on patients’ quality of life after hospital discharge. All participants were contacted by telephone within a week after the follow-up clinic. The results were presented as per EQ-5D-5L guidelines [17] through an EQ visual analogue scale (EQ-VAS) system, where the endpoints 0 to 100 are labeled ‘The best health you can imagine’ and ‘The worst health you can imagine’, and the analysis of the following dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The results were compared with the Canary Islands’ general population [18].
Fig. 1.
LUS lung patterns. A: fine pleural line and visible A-lines; B: minimum of three non-confluents B lines with a distance between two of them up to 7 mm and fragmented pleural-line; C: confluent B-lines; D: consolidations.
Statistical analysis
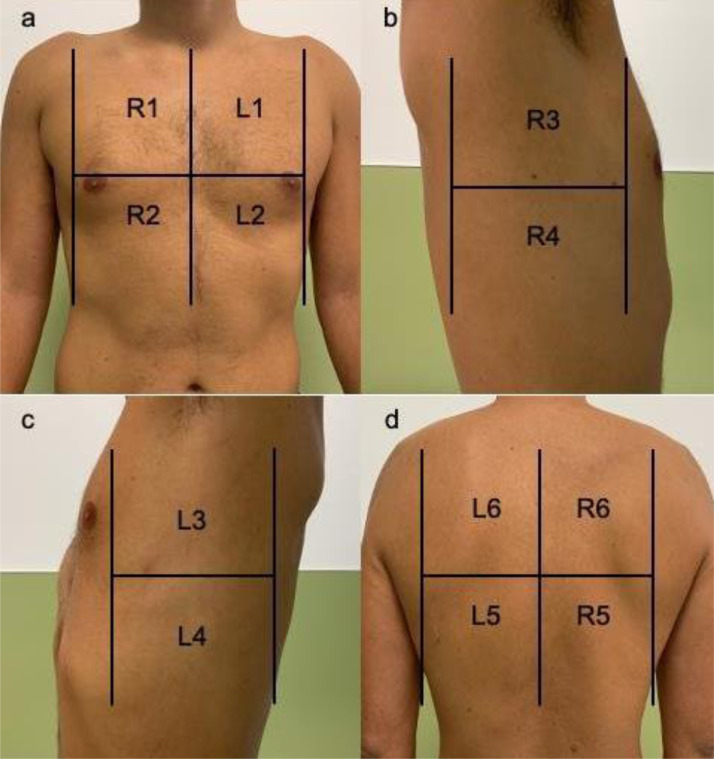
Continuous variables were expressed as means ± standard deviation (SD) and categories as counts and percentages. For the bivariate analysis, we used Student´s t-test for continuous variables to compare the means of independent samples. For the association between categorical variables, we used Chi-square and Fisher´s exact test. Statistical significance was set at 5% and marginally significant accepted for p < 0.1. Analyses were conducted with SPSS software (version 21; SPSS, Chicago, IL, USA) (Fig. 2 ).
Fig. 2.
Areas of lung ultrasound examination. a: upper anterior and lower anterior quadrant of the right (R1–R2) and left (L1–L2) hemithorax; b, c: upper lateral and lower lateral quadrants of the right (R3–R4) and left (L3–L4) hemithorax; d: lower and upper quadrants of the posterior thorax of the right (R5–R6) and left (L5–L6) hemithorax. Image from Wangüemert Pérez et al. [19].
Results
Study sample
A total of 93 patients met the inclusion criteria, 16 of them were excluded due to different reasons: previously diagnosed interstitial lung disease (4 patients), active neoplasia (3 patients), history of radiotherapy treatment (2 patients). 7 patients did not attend the first follow-up consultation. Finally, 77 cases were included in this study.
Demographics and baseline characteristics
The patients’ baseline characteristics are reflected in Table 1 . The median age of the participants is 57 years, ranging from 26 years to 77 years; 37 cases (48%) are female. Most of the participants were non-smokers (79%). Regarding underlying conditions, 48% of the patients have arterial hypertension, 30% dyslipidemia, and 17% type 2 diabetes mellitus. Obesity is observed in 14% of the patients. In this study, most cases have moderate COVID-19 severity at admission (49 patients, 64%). The mean time from hospital discharge to follow-up is 16.1 weeks (SD ± 6.1 weeks).
Table 1.
Baseline characteristics of COVID-19 follow-up patients. Data are given as mean ± SD (mean ± standard deviation) and number of patients (percentage). Abbreviations: COPD, chronic obstructive pulmonary disease.
| BASELINE CHARACTERISTICS OF COVID-19 PATIENTS (n = 77) | |
| Age, years, mean (± SD) | 57,45 (± 13,14) |
| Sex | |
| – Male, n (%) | 40 (52) |
| – Female, n (%) | 37 (48) |
| Smoking | |
| – Non smoker, n (%) | 61 (79) |
| – Active or former smoker, n (%) | 16 (21) |
| Underlying diseases | |
| – No conditions, n (%) | 21 (27) |
| – Hypertension, n (%) | 37 (48) |
| – Dyslipidemia, n (%) | 23 (30) |
| – Diabetes, n (%) | 13 (17) |
| – Obesity, n (%) | 11 (14) |
| – Ischemic cardiomyopathy, n (%) | 5 (7) |
| – Immunosupression, n (%) | 4 (5) |
| – COPD, n (%) | 2 (3) |
| – Asthma, n (%) | 2 (3) |
| Severity of COVID-19 | |
| – Mild, n (%) | 17 (22) |
| – Moderate, n (%) | 49 (64) |
| – Severe, n (%) | 11 (14) |
| Time from hospital discharge to follow-up, weeks (± SD) | 16,09 (± 6,06) |
Clinical, functional, and analytical characteristics
Regarding clinical manifestations, 87% of the cases presented with symptoms at follow-up. These symptoms were classified as respiratory and non-respiratory and are summarized in Table 2 . The most reported was dyspnea (65%), followed by asthenia (42%), hair loss (31%), and arthralgias (26%). 6 patients had major complications: pulmonary thromboembolism (3), deep venous thrombosis (1), subdural hematoma (1), and cerebrovascular accident (1). COVID-19 severity at admission in the present study was not related to the presence of symptoms during the follow-up.
Table 2.
Clinical outcomes in COVID-19 follow-up patients. Data are given as mean ± SD (mean ± standard deviation) and number of patients (percentage). Abbreviations: mMRC, modified Medical Research Council dypnoea scale; EQ-5D-5L, EuroQol - 5 Dimension - 5 Level questionnaire; EQ VAS, EuroQol questionnaire Visual Analogue Scale; * Canary Islands’ general population according to Canary Health Survey 2015 [20].
| POST-COVID CLINICAL MANIFESTATIONS AT FOLLOW-UP | ||||||
| Total (n = 77) | ||||||
| Symptoms, n (%) | 67 (87,0) | |||||
| respiratory variables | ||||||
| – Dyspnea, n (%) | 51 (64,9) | |||||
| mMRC 0 | 26 (33,8) | |||||
| mMRC 1 | 33 (42,9) | |||||
| mMRC 2 | 17 (22,1) | |||||
| mMRC 3 | 1 (1,3) | |||||
| mMRC 4 | 0 (0) | |||||
| – Cough, n (%) | 15 (19,4) | |||||
| – Expectoration, n (%) | 1 (1,3) | |||||
| Non-respiratory variables | ||||||
| – Asthenia, n (%) | 32 (41,5) | |||||
| – Hair loss, n (%) | 24 (31,1) | |||||
| – Arthralgia, n (%) | 20 (25,9) | |||||
| – Ageusia, n (%) | 12 (15,5) | |||||
| – Persistent headache, n (%) | 11 (14,2) | |||||
| – Muscular weakness, n (%) | 11 (14,2) | |||||
| – Myalgia, n (%) – Anxiety, n (%) | 9 (11,6) 8 (10,38) |
|||||
| – Anosmia, n (%) | 7 (9,0) | |||||
| – Memory loss, n (%) | 7 (9,0) | |||||
| – Cramp, n (%) – Menstrual cycle alterations, n (%) – Dysesthesia, n (%) – Dizziness, n (%) – Limbs’ swelling, n (%) | 6 (7,8) 6 (7,8) 4 (5,19) 4 (5,19) 3 (3,89) |
|||||
| POST-COVID CLINICAL MANIFESTATIONS RELATED TO COVID-19 SEVERITY | ||||||
| Mild (n = 17) | Moderate (n = 49) | Severe (n = 11) | P value | |||
| Symptoms,n(%) | 14 (82) | 43 (88) | 10 (91) | 0,779 | ||
| Respiratory symptoms,n(%) | 11 (65) | 33 (67) | 9 (82) | 0,592 | ||
| Non-respiratory symptoms,n(%) | 13 (76) | 37 (75) | 10 (91) | 0,531 | ||
| POST-COVID CLINICAL MANIFESTATIONS RELATED TO GENDER | ||||||
| Male (n = 40) | Female (n = 37) | P value | ||||
| Symptoms, mean ± SD | 2,50 ± 2,07 | 3,75 ± 2,36 | 0,015 | |||
| Respiratory symptoms, mean ± SD | 0,77 ± 0,61 | 0,94 ± 0,74 | 0,275 | |||
| Non-respiratory symptoms, mean ± SD | 1,72 ± 1,70 | 2,81 ± 1,86 | 0,009 | |||
| HEALTH-RELATED QUALITY OF LIFE (5Q-5D-5L QUESTIONNAIRE) RELATED TO GENDER AND AGE GROUP IN 43 COVID-19 FOLLOW-UP PATIENTS COMPARED TO GENERAL POPULATION | ||||||
| COVID-19 follow-up patients (n = 43) | General population* (n = 1.739.088) | P-value | ||||
| Male EQ-VAS, mean ± SD | ||||||
| – Age group < 44 | 63,75 ± 17,02 | 80,03 ± 16,17 | 0,013 | |||
| – Age group 45 – 64 | 82,13 ± 12,55 | 74,34 ± 17,70 | 0,061 | |||
| – Age group > 64 | 67,08 ± 19,12 | 68,51 ± 19,40 | 0,768 | |||
| Female EQ-VAS, mean ± SD | ||||||
| – Age group < 44 | 81,67 ± 7,64 | 77,67 ± 18,33 | 0,593 | |||
| – Age group 45 – 64 | 64,00 ± 20,74 | 70,02 ± 21,38 | 0,260 | |||
| – Age group > 64 | 70,09 ± 18,82 | 61,45 ± 22,01 | 0,128 | |||
As shown in Table 2, we detected female patients significantly had a greater number of clinical manifestations compared to male patients (p = 0,015) and specifically, a higher number of non-respiratory symptoms (p = 0,009).
Regarding lung function parameters at follow-up, the mean levels for our study population were: DLCO 92.8% of predicted (SD ± 17.7%), FEV1 99.4% of predicted (SD ± 16%) and FVC 95.1% of predicted (SD ± 17.9).
Analytical parameters levels at admission such as ALT, AST, LDH, CRP, procalcitonin, ferritin, IL-6 and CK, were not involved in the symptoms’ prevalence in the convalescence period after discharge, but a significant correlation between genders was found (Table 2). ALT, AST and LDH levels on admission were remarkably higher in male group: ALT 69,25 (SD ± 47,41) versus 44,27 (SD ± 32,14), p = 0,009; AST 62,22 (SD± 43,53) versus 43,62 (SD ± 26,35), p = 0,025; LDH 404,72 (SD ± 122,87) versus 317,10 (SD ± 98,51), p = 0,027.
Health-related quality of life (EQ-5D-5L questionnaire)
43 patients completed the EQ-5D-5L questionnaire. Cases were classified by gender and age group and compared with the Canary Islands’ general population [18] as presented in Table 2.
Male patients under the age of 44, had a lower EQ-VAS health-related quality of life after suffering COVID-19 compared to the same age general male population (63.8 ± 17.1 vs 80 ± 16.2, respectively, p = 0,013). The rest of the age groups and females in this study population did not present poorer EQ-VAS health-related quality of life after COVID-19 discharge compared to the general population (Table 2).
In terms of the 5-dimension analysis, the most common moderate impaired aspect for the male population (n = 24) was pain/discomfort (33.3%), followed by usual activities (20.8%), anxiety/depression and mobility (16.7%), and self-care (8.3%). For the female population (n = 19), the most affected element was usual activities (21.1%), and then mobility, self-care and anxiety/depression (15.8%), and lastly, pain/discomfort (10.5%).
Correlation between LUS findings at follow-up and clinical and analytical outcomes
A total of 59 patients (76.5%) had an altered lung aeration pattern in LUS at follow-up (LUSS ≥ 1). To better represent the symptoms’ outcomes according LUSS, we classified the LUSS alterations in three different groups as presented in Table 3 (LUSS 0, LUSS 1-15, and LUSS ≥ 15) following Wangüemert Pérez et al., studies [19,20]. We observed normal LUS (LUSS 0) findings in 50% of the asymptomatic patients and 19% of symptomatic cases, although this correlation was not significant (p = 0,106). Within the group admitted with severe grade of COVID-19, no patient was found to have normal LUS findings on follow-up. Most moderate and severe cases had a LUSS score between 1 and 15 (84% and 64%, respectively). These findings were statistically significant (p = 0.002). We detected a greater LUSS in the male group compared to the female (p = 0,016 and p = 0,001, respectively).
Table 3.
Lung ultrasound outcomes at COVID-19 follow-up related to symptoms, gender and quality of life measured by EQ-5D-5L questionnaire at follow-up and analytical parameters at admission. Data are given as mean ± SD (mean ± standard deviation) and number of patients (percentage). Abbreviations: LUS, Lung Ultrasound Score; ALT, alanine transaminase; AST, aspartate transaminase; LDH, lactate dehydrogenase; CRP, C reactive protein;; IL-6, interleukin-6; CK, creatine kinase; EQ-5D-5L, EuroQol - 5 Dimension - 5 Level questionnaire; EQ-VAS, EuroQol questionnaire Visual Analogue Scale.
| LUNG ULTRASOUND FINDINGS AT FOLLOW UP RELATED TO POST-COVID SYMPTOMS AT FOLLOW-UP | |||||
|---|---|---|---|---|---|
| LUSS 0 | LUSS 1 - 15 | LUSS > 15 | P value | ||
| Total (n = 77),n(%) | 18 (23,3) | 56 (72,7) | 3 (3,8) | – | |
| Asymptomatic (n = 10) | 5 (50) | 5 (50) | 0 (0) | 0,106 | |
| Symptomatic (n = 67) | 13 (19,4) | 51 (76,1) | 3 (4,4) | ||
| – Respiratory symptoms (n = 53),n(%) | 11 (20,7) | 39 (73,5) | 3 (5,6) | 0,254 | |
| – Non-respiratory symptoms (n = 60),n(%) | 11 (18,3) | 46 (76,6) | 3 (5) | 0,096 | |
| LUNG ULTRASOUND FINDINGS AT FOLLOW-UP ACCORDING TO ADMISSION SEVERITY | |||||
| LUSS 0 | LUSS 1 - 15 | LUSS > 15 | P value | ||
| Mild (n = 17),n(%) | 9 (53) | 8 (47) | 0 (0) | ||
| Moderate (n = 49),n(%) | 7 (14) | 41 (84) | 1 (2) | 0.002 | |
| Severe (n = 11),n(%) | 2 (18) | 7 (64) | 2 (18) | ||
| LUNG ULTRASOUND FINDINGS AT FOLLOW-UP ACCORDING TO GENDER | |||||
| Male group (n = 40) | Female group (n = 37) | P value | |||
| LUSS, mean ±SD | 5,40 ± 5,22 | 1,84 ± 2,43 |
0,001 0,016 |
||
| LUSS 0 | 9 | 18 | |||
| LUSS ≥ 1 | 31 | 19 | |||
| LUNG ULTRASOUND FINDINGS AT FOLLOW UP RELATED TO BIOMARKERS AT ADMISSION | |||||
| LUSS 0 | LUSS ≥ 1 | P value | |||
| ALT, U/L, mean ±SD | 59,92 ± 50,26 | 55,80 ± 38,02 | 0,687 | ||
| AST, U/L, mean ±SD | 21,81 ± 5,65 | 24,13 ± 14,94 | 0,625 | ||
| LDH, mg/dl, mean ±SD | 327,59 ± 158,42 | 381,54 ± 181,53 | 0,198 | ||
| CRP, mg/dl, mean ±SD | 5,80 ± 5,75 | 10,81 ± 8,78 | 0,004 | ||
| Procalcitonin, mg/ml, mean ±SD | 0,11 ± 0,12 | 32 ± 0,36 | 0,291 | ||
| Ferritin, ng/ml, mean ±SD | 1403,90 ± 3897,74 | 1080,30 ± 631,52 | 0,626 | ||
| IL-6, pg/ml, mean ±SD | 17,27 ± 24,53 | 49,92 ± 48,03 | 0,020 | ||
| Creatin Kinase, mean ±SD | 126,68 ± 129,37 | 416,89 ± 1020,60 | 0,080 | ||
| LUNG ULTRASOUND FINDINGS AT FOLLOW UP RELATED TO QUALITY-OF-LIFE (5Q-5D-5L QUESTIONNAIRE) AT FOLLOW-UP | |||||
| LUSS 0 | LUSS ≥ 1 | P value | |||
| EQ - VAS, mean ±SD (n = 43) | 76,35 ± 17,56 | 67,50 ± 17,45 | 0,112 | ||
| – In male patients, mean ±SD (n = 24) | 83,38 ± 14,38 | 65,63 ± 16,82 | 0,018 | ||
| – In female patients, mean ±SD (n = 19) | 70,11 ± 18,51 | 70,50 ± 18,92 | 0,964 | ||
Regarding analytical parameters, we detected those with greater levels of CRP and IL-6 at admission were related to LUSS ≥ 1 on follow-up: CRP levels 10.8 (SD ± 8.8) vs normal LUS at follow up: 5.8 (SD ± 5.7); IL-6 levels: 49.9 (SD ± 48) vs normal LUS at follow up 17.3 (SD ± 24.5), p = 0,004 and 0 = 0,02, respectively. Correlations between LUS findings and other biomarkers were not significant.
Regarding the health-related quality of life, we observed that male patients with higher LUSS presented with lower quality of life: EQ-VAS 65.6 (SD ± 16.9) vs 83.4 (SD ± 14.4), with significant correlation (p = 0,018). No significant association was found between LUS alterations and health-related quality of life in other groups.
Discussion
The present study describes follow-up data of patients after 16 weeks of hospital discharge due to COVID-19.
Regarding baseline characteristics, nearly 70% of the patients had an underlying condition, such as hypertension in 48%, dyslipidemia in 30%, and type 2 diabetes in 17% of the cases. Our study group has similar findings if compared to larger groups of patients in other countries such as United Kingdom or the USA, where the comorbidities where present in more than the 70% of the included patients [21,22].
We recorded post-COVID manifestations in more than 85% of the patients. Dyspnea was the most common manifestation, followed by asthenia, hair loss, and arthralgias. In our research, both respiratory and non-respiratory manifestations were more frequent in the female group. This gender fact and a broad spectrum of symptoms have been reported also in other studies [23], [24], [25]. In terms of health-related quality of life, we conducted an EQ-5D-5L questionnaire in 43 patients. Peculiarly, we found that the male group under 44 years had a poorer quality of life measured as EQ-VAS compared to the same age and region general population; this stands far from the fact that the female group is more frequently affected by post-COVID-19 symptoms and a lower quality of life would be expected due to this situation. Some studies have investigated the health-related quality of life through a 5Q-5D-5L questionnaire [26,27] but none of them have detected a difference between genders.
Post-COVID symptoms in our study were not influenced by COVID-19 severity or blood test biomarkers on admission. Data regarding these facts is variable; Townsend and colleagues [23] did not found any correlation between COVID-19 acute severity and medium-term outcomes such as respiratory sequelae, quality of life, or 6 min-walk-test result either. Several studies hold up on the opposite [19,26]. Kamal et al. [26], observed both presences of comorbidities and severity of acute SARS-CoV2 infection were involved in a greater number of post-COVID-19 manifestations. The link between acute infection severity and acute effects may be more clear [27], but the medium and long-term consequences remain uncertain; this could be due to other influential factors that are not reported in the same way in different studies and might be detected if standardized grades of COVID-19 severity including multiple variables were created. The fact that seems to be more uniformly agreed is the difference that lies between genders; in our research, we observed a significant association between analytical parameters such as ALT, AST, LDH and ferritin levels on admission, which were remarkably higher in the male group. In this direction, there are several studies [28,29] that have found similar outcomes, and have associated higher biomarker levels with males. This aspect could be explained by biological differences between sex hormones interactions and immune system response [23].
We detected that more than 70% of the patients had an altered lung aeration in LUS (LUSS ≥ 1) after 16 weeks of SARS-CoV2 acute infection; this situation reassures residual lung abnormalities during the post-COVID period that are seen in CT scan performed during the convalescence period in some studies [30].
The ratio of the partial pressure of oxygen in arterial blood (PaO2) to the inspired oxygen fraction (FiO2) has been used to quantify the degree of abnormalities in pulmonary gas exchange. In our study, we detected those lower levels of PaO2/FiO2 ratio were related to LUS alterations on follow-up, though this correlation was marginally significant. In the same direction, biomarkers related to oxygen status, and lung affection in acute SARS-CoV2 infection, have been studied before [19]. We found a significant correlation between greater CRP and IL-6 levels on admission with a higher prevalence of having a LUSS ≥ 1 at follow-up.
Though we did not observe an association between SARS-CoV2 pneumonia and biomarker levels at admission with post-COVID symptoms in our study, these situations might be clinically relevant in terms of lung parenchymal alterations detected by LUS. Patients with severe lung tissue impairment and higher biomarker levels during acute SARS-CoV2 infection are expected to have residual parenchymal alterations in the post-COVID period, but these outcomes are not always necessarily linked with symptomatic manifestations. As we mentioned earlier, the connection between the acute clinical situation and post-COVID outcomes remains unclear. In our research, there is a high prevalence of post-COVID symptoms, especially dyspnea, regardless of LUS or lung function abnormalities. This situation in our study group could be explained by an extra-parenchymal etiology of the shortness of breath due to muscular weakness and fatigue.
The health-related quality of life differences between the male and the female group detected in the current study and mentioned earlier, were also evident regarding LUS results. We found a poorer quality of life in male cases with altered lung aeration pattern in LUS and a significantly greater LUSS in males cases compared to females. This could be explained by a higher biomarkers level on admission and biological sex-based differences in lung physiology and immune response that causes worse outcomes for COVID-19 in males [23]; but justify why males have poor quality-of-life when females have more post-COVID symptoms (respiratory and non-respiratory) might be a challenge, and perhaps differences lying on psychosocial and cultural aspects, social support, adversity perception and resilience between women and men, may be the reason behind this fact [31].
To the best of our knowledge, the current study is one of the first to approach LUS performance in COVID-19 patients’ follow-up. LUS is a non-harmful, non-invasive, widely available, and portable procedure, that provides extensive information about the patient respiratory evolution. LUS could be helpful to decide and screen which patients may need further investigations. However, more extensive studies are necessary to better define LUS outcomes in post-COVID-19 patients and the technique reproducibility.
Conclusions
Our study concludes: (1) male gender, greater severity on admission and higher biomarkers levels such as CRP and IL-6 may increase the chances of having a greater LUSS in the next months after hospital discharge due to COVID-19; (2) almost 90% of the patients that had an acute infection due to SARS-CoV2, have symptoms 16 weeks after hospital discharge; these symptoms are not related to COVID-19 severity on admission but are though associated with a female gender predominance; (3) health-related quality of life is poorer after suffering COVID-19 at the male early adulthood stage.
Study limitations
This study has some limitations worth noting: (1) it is a single-center study, a larger sample size is required to determine a strong association; (2) all the LUS were performed by a single operator with extensive experience in this field and therefore, inter-observer variability data is not available; (3) LUS findings on follow-up are not compared to this same data during the hospital stay or prior to discharge, hence the lung radiological evolution that patients may have from the acute phase of the infection to four months after discharge, has not been collected in this study.
Declaration of Competing Interest
None.
Appendix
References
- 1.Zhu N., Zhang D., Wang W., Xingwang L., Yang B., Jingdong S., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation; 2020. Novel coronavirus (2019-nCoV): situation report - 19.https://apps.who.int/iris/handle/10665/330988 [revised on 20th May 2021] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.You J., Zhang L., Ni-Jia-Ti M.Y., Zhang J., Fuyin H., Chen L., et al. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J Infect. 2020;81(2):e150–e152. doi: 10.1016/j.jinf.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu M., Liu Y., Xu D., Zhang R., Lan L., Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21(6):746–755. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T., Fan Y., Chen M., Xiaoyan W., Zhang L., Tao H., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [published correction appears in JAMA Cardiol. 2020 Jul 1;5(7):848] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Guohui F., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; 2020. COVID-19 rapid guideline: managing the long-term effects of COVID-19.https://www.nice.org.uk/guidance/ng188 [PubMed] [Google Scholar]
- 9.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demi M., Prediletto R., Soldati G., Demi L. Physical mechanisms providing clinical information from ultrasound lung images: hypotheses and early confirmations. IEEE Trans Ultrason Ferroelectr Freq Control. 2020;67(3):612–623. doi: 10.1109/TUFFC.2019.2949597. [DOI] [PubMed] [Google Scholar]
- 11.Soldati G., Demi M., Inchingolo R., Smargiassi A., Demi L. On the physical basis of pulmonary sonographic interstitial syndrome. J Ultrasound Med. 2016;35(10):2075–2086. doi: 10.7863/ultra.15.08023. [DOI] [PubMed] [Google Scholar]
- 12.Giovannetti G., De Michele L., De Ceglie M., et al. Lung ultrasonography for long-term follow-up of COVID-19 survivors compared to chest CT scan. Respir Med. 2021;181 doi: 10.1016/j.rmed.2021.106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibila O., Molina-Molina M., Valenzuela C., Ríos-Cortes A., Arbillaga-Etxarri A., Torralba-García Y., et al. Documento de consenso de la Sociedad Española de Neumología y Cirugía Torácica (SEPAR) para el seguimiento clínico post-COVID-19 [Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Consensus for post-COVID-19 Clinical Follow-up] Open Respir Arch. 2020;2(4):278–283. doi: 10.1016/j.opresp.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpicelli G., Elbarbary M., Blaivas M., Lichtenstein D., Mathis G., Kirkpatrick A., et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 15.Soldati G., Smargiassi A., Inchingolo R., Buonsenso D., Perrone T., Briganti D.F., et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med. 2020;39(7):1413–1419. doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpicelli G., Caramello V., Cardinale L., Mussa A., Bar F., Frascisco M.F. Detection of sonographic B-lines in patients with normal lung or radiographic alveolar consolidation. Med Sci Monit. 2008;14(3):CR122–CR128. [PubMed] [Google Scholar]
- 17.Herdman M., Gudex C., Lloyd A., Janssen M.F., Kind P., Parkin D., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Encuesta de Salud de Canarias. Instituto Canario de Estadística; Canarias: 2015. Población de 16 y más años según autovaloración del estado de salud por intervalos de la escala visual analógica (EQ-5D), sexos y grupos de edad.http://www.gobiernodecanarias.org/istac/temas_estadisticos/sociedad/salud/estadodesalud/C00035A.html [revised on 20th May 2021] [Google Scholar]
- 19.Wangüemert Pérez A.L., Figueira Gonçalves J.M., Hernández Pérez J.M., Ramallo Fariña Y., Del Castillo Rodriguez J.C. Prognostic value of lung ultrasound and its link with inflammatory biomarkers in patients with SARS-CoV-2 infection. Respir Med Res. 2021;79 doi: 10.1016/j.resmer.2020.100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manivel V., Lesnewski A., Shamim S., Carbonatto G., Govindan T. CLUE: COVID-19 lung ultrasound in emergency department. Emerg Med Australas. 2020;32(4):694–696. doi: 10.1111/1742-6723.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. Published 2020 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [published correction appears in JAMA. 2020 May 26;323(20):2098] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend L., Dowds J., O'Brien K., Sheill G., Dyer A.H., O'Kelly B., et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18(6):997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong A.W., Shah A.S., Johnston J.C., Carlsten C., Ryerson C.J. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J. 2020;56(5) doi: 10.1183/13993003.03276-2020. Published 2020 Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daher A., Balfanz P., Cornelissen C., Müller A., Bergs I., Marx N., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174 doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamal M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021;75(3):e13746. doi: 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poggiali E., Zaino D., Immovilli P., Rovero L., Losi G., Dacrema A., et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clin Chim Acta. 2020;509:135–138. doi: 10.1016/j.cca.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfe J., Safdar B., Madsen T.E., Sethuraman K.N., Becker B., Greenberg M.R., et al. Sex- or gender-specific differences in the clinical presentation, outcome, and treatment of SARS-CoV-2. Clin Ther. 2021;43(3):557–571. doi: 10.1016/j.clinthera.2021.01.015. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raimondi F., Novelli L., Ghirardi A., Russo F.M., Pellegrini D., Biza R., et al. Covid-19 and gender: lower rate but same mortality of severe disease in women-an observational study. BMC Pulm Med. 2021;21(1):96. doi: 10.1186/s12890-021-01455. Published 2021 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang T., Liu Z., Wu C.C., Jin C., Zhao H., Wang Y., et al. Evolution of CT findings in patients with mild COVID-19 pneumonia. Eur Radiol. 2020;30(9):4865–4873. doi: 10.1007/s00330-020-06823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galambos N.L., Barker E.T., Krahn H.J. Depression, self-esteem, and anger in emerging adulthood: seven-year trajectories. Dev Psychol. 2006;42(2):350–365. doi: 10.1037/0012-1649.42.2.350. [DOI] [PubMed] [Google Scholar]