Abstract
Emerging infectious diseases have resulted in severe population declines across diverse taxa. In some instances, despite attributes associated with high extinction risk, disease emergence and host declines are followed by host stabilisation for unknown reasons. While host, pathogen, and the environment are recognised as important factors that interact to determine host–pathogen coexistence, they are often considered independently. Here, we use a translocation experiment to disentangle the role of host traits and environmental conditions in driving the persistence of remnant bat populations a decade after they declined 70–99% due to white‐nose syndrome and subsequently stabilised. While survival was significantly higher than during the initial epidemic within all sites, protection from severe disease only existed within a narrow environmental space, suggesting host traits conducive to surviving disease are highly environmentally dependent. Ultimately, population persistence following pathogen invasion is the product of host–pathogen interactions that vary across a patchwork of environments.
Keywords: emerging infectious disease, geographic mosaics, host resistance, host tolerance, host–pathogen coexistence, temperature‐mediated effects, white‐nose syndrome
Following the invasion of novel and virulent pathogens, some host populations coexist with pathogens while others decline to extinction. Here, we use a translocation experiment to identify interacting mechanisms by which bat populations persist with white‐nose syndrome. We find that unique host traits have evolved since pathogen invasion and contribute to persistence, but that the degree of protection afforded is environmentally dependent.

INTRODUCTION
Emerging infectious diseases of wildlife have resulted in severe mortality events and regional to complete extinctions of host populations (Antolin et al., 2002; LaDeau et al., 2007; Langwig et al., 2012; Mccallum et al., 2009; Scheele et al., 2019; Van Riper III et al., 1986). In some instances, the presence of pathogen reservoirs, frequency‐dependent transmission, and small pre‐epidemic host population sizes suggest that host species will be driven to extinction (de Castro & Bolker, 2005). Additionally, high initial host population declines leave remnant populations more vulnerable to stochastic and Allee effects that increase the likelihood of host extinction (de Castro & Bolker, 2005; Friedman & Yakubu, 2012; Lande, 1998). However, following the initial epidemic and population declines, some host populations stabilise and persist for unknown reasons (Brannelly et al., 2021; Briggs et al., 2010; Langwig et al., 2012). For example, population persistence has been observed in several important disease systems including amphibians impacted by chytridiomycosis (Brannelly et al., 2021; Briggs et al., 2010; Scheele et al., 2015, 2017; Voyles et al., 2018), Tasmanian devils impacted by facial tumour disease (Epstein et al., 2016; Hohenlohe et al., 2019; Lazenby et al., 2018; Patton et al., 2020), birds impacted by avian malaria (Woodworth et al., 2005), and bats impacted by white‐nose syndrome (Langwig et al., 2012; Reichard et al., 2014). While initial evidence suggested that these hosts would be extirpated by infectious disease, some populations have stabilised despite infection prevalence remaining high while others continue to decline or have gone extinct (Brannelly et al., 2021; Briggs et al., 2010; Frick et al., 2015; Hoyt et al., 2020, 2021; Langwig et al., 2012, 2016; Lazenby et al., 2018; Samuel et al., 2015; Scheele et al., 2017, 2019).
Potential drivers of host–pathogen coexistence include the evolution of host resistance, tolerance, and/or general vigour (Best et al., 2008; Boots et al., 2009; Kutzer & Armitage, 2016; Råberg et al., 2007, 2009; Restif & Koella, 2004; Roy & Kirchner, 2000; Voyles et al., 2018; Wilber et al., 2021), environmental refugia from infection or severe disease (Heard et al., 2015; Mosher et al., 2018; Schelkle et al., 2012; Springer, 2009; Tobler et al., 2007; Zumbado‐Ulate et al., 2014), host demographic compensation (Arthur et al., 2004; Lachish et al., 2009; Mcdonald et al., 2016; Scheele et al., 2015; Spitzen‐Van Der Sluijs et al., 2017), density‐dependent transmission (Fenton et al., 2002; Hochachka & Dhondt, 2000; Lloyd‐Smith et al., 2005; McCallum et al., 2001), and attenuation of pathogen virulence (Anderson & May, 1982; Boots et al., 2004; Cressler et al., 2016; Kerr et al., 2006; Levin & Pimentel, 1981; Wild et al., 2009). However, studies investigating host coexistence with virulent pathogens frequently focus on a single aspect of the host–pathogen–environment interaction, despite evidence that such interactions may influence disease severity and scale up to population‐level effects on the host (Echaubard et al., 2014; Laine, 2007; Sadd, 2011). For example, studies of amphibian chytridiomycosis have illustrated shifts in host responses following pathogen invasion that may be indicative of host resistance (Voyles et al., 2018; Wilber et al., 2017), but that host–pathogen coexistence is additionally modified by an interaction with environmental conditions (Forrest & Schlaepfer, 2011; Scheele et al., 2015; Spitzen‐Van Der Sluijs et al., 2017; Wilber et al., 2017). Given the potential for interactions between the host and environment to drive host population persistence, which may change as pathogen invasion progresses, understanding these interactions is essential for identifying the conditions necessary for host–pathogen coexistence following the invasion of a virulent pathogen.
White‐nose syndrome (WNS) is an infectious disease of bats caused by the fungal pathogen Pseudogymnoascus destructans (Lorch et al., 2011; Minnis & Lindner, 2013; Warnecke et al., 2012). In North America, the disease was first detected in New York state in 2006 (Blehert et al., 2009) and has since resulted in large mortality events and regional extinctions of once common bat species (Frick et al., 2015; Langwig et al., 2012, 2016). An environmental pathogen reservoir establishes following the introduction of P. destructans to hibernacula (Hoyt et al., 2020; Langwig, Hoyt, et al., 2015) and most populations decline greater than 90%, often resulting in complete local extirpations (Frick et al., 2010; Langwig et al., 2012). Following the establishment of the pathogen within hibernacula, bats become infected with P. destructans upon entering previously contaminated hibernacula in the fall (Langwig, Frick, et al., 2015), and both indirect and direct transmission result in widespread infection early in the seasonal epidemic (Hoyt et al., 2018, 2020; Langwig, Frick, et al., 2015; Lorch et al., 2011). However, bats that survive hibernation emerge onto the landscape in spring and clear infection (Fuller et al., 2020; Langwig, Frick, et al., 2015; Meteyer et al., 2011).
The growth of P. destructans is sensitive to environmental temperature (Verant et al., 2012) and humidity (Marroquin et al., 2017), resulting in environmental trends in population declines such that populations and species roosting in warmer and wetter environments have more severe declines (Grieneisen et al., 2015; Hopkins et al., 2021; Langwig et al., 2012, 2016; Lilley et al., 2018). However, several years following pathogen introduction, some colonies of little brown bats (Myotis lucifugus) in the northeast United States stabilised at 5–30% of their pre‐epidemic population size following cumulative regional declines of 96% (Dobony et al., 2011; Frick et al., 2015; Hoyt et al., 2021; Langwig et al., 2012, 2017) despite infection prevalence remaining high (Langwig et al., 2017). The abiotic pathogen reservoir within hibernacula sustains a high prevalence of infection regardless of colony size, suggesting density‐dependent transmission is not a driver of population persistence (Hoyt et al., 2016, 2020; Langwig, Frick, et al., 2015). Compared to colonies undergoing epidemic conditions on the invasion front, bats in persisting colonies display slower on‐host pathogen growth rates, potentially a signature of host resistance (Langwig et al., 2017). However, persisting colonies also utilise colder hibernacula (Hopkins et al., 2021; Langwig et al., 2012), so lower pathogen growth rates may be a product of temperature operating independently of host characteristics. Several lines of evidence suggest that pathogen virulence attenuation is not a principal driver of host population persistence in this system. Few mutations have been identified in P. destructans since initial introduction to North America (Trivedi et al., 2017), and experimental inoculation of North American bats naïve to WNS shows similar virulence between North American and European isolates (Warnecke et al., 2012). Furthermore, P. destructans isolates in North America reproduce asexually (Drees et al., 2017; Palmer et al., 2014), and genetic analyses indicate shallow genetic diversity across the North American continent, with high connectivity and long‐distance dispersal of the pathogen (Drees et al., 2017; Leopardi et al., 2015; Ren et al., 2012). Due to the high connectivity of the pathogen population in North America, isolates from stabilising host populations and those undergoing severe declines are genetically indistinguishable (Drees et al., 2017; Leopardi et al., 2015; Ren et al., 2012), variation which should be detectable if pathogen virulence attenuation drives host–pathogen coexistence.
The relative role of host traits and environmental conditions in driving the persistence of little brown bat populations impacted by WNS is still unclear. Understanding the factors driving host persistence will provide empirical support for general theory on host coexistence with virulent pathogens and much needed information on this important and devastating wildlife disease. Ten years following the introduction of P. destructans and subsequent colony declines, we conducted a fully factorial translocation experiment to understand the mechanisms of population persistence. We leveraged the variable environmental conditions in hibernacula (Figure 1, Figure S1 and S2) and a previously conducted translocation experiment early in the epizootic to disentangle the relative roles of host traits and environmental conditions in driving disease severity and ultimately population persistence.
FIGURE 1.
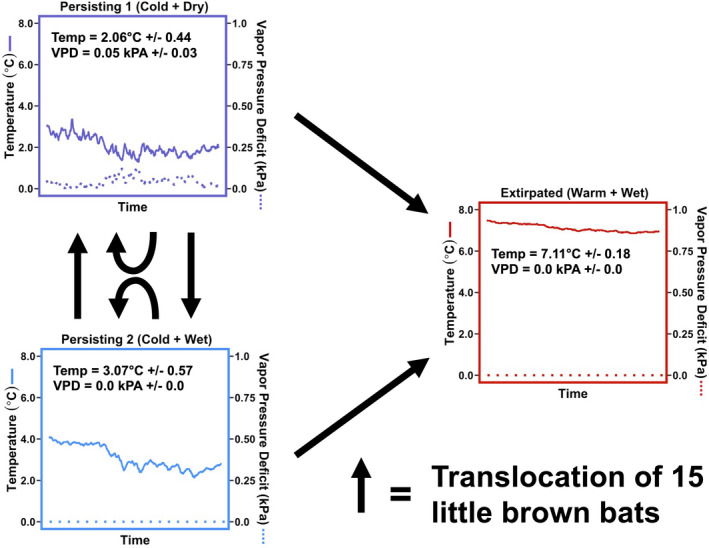
Schematic of the experimental design. Two sites in New York with known persisting colonies of little brown bats and one site that was previously extirpated of little brown bats were used in this study. The three sites are referred to as Persisting 1 (Cold + Dry), Persisting 2 (Cold + Wet), and Extirpated (Warm + Wet). Solid and dashed lines on plots correspond to temperature (Celsius) and vapour pressure deficit (kPa; higher values correspond to drier conditions), respectively, within each site over the course of this study. Mean ± SD of temperature and vapour pressure deficit within each site is shown on each plot. Sites varied in their environmental conditions, with the persisting sites being colder than the extirpated site and Persisting 1 (Cold + Dry) being significantly drier than Persisting 2 (Cold + Wet). Persisting 2 (Cold + Wet) was similar in its humidity conditions to Extirpated (Warm + Wet). In early hibernation, 45 bats from each of the persisting sites (n = 90) were collected and randomly assigned a translocation site and cage (3 bats per cage). The translocation was fully factorial, with 15 bats from each site being caged within the same site, in the opposite persisting site, or the extirpated site. Bats remained caged within these sites until late hibernation, when survivors were returned to their site of origin
MATERIALS AND METHODS
Translocation experiment
We used two hibernacula of persisting little brown bat (Myotis lucifugus) colonies in New York and one hibernaculum previously extirpated of bats by white‐nose syndrome (WNS) in Vermont in this study (Figure 1). Prior to hibernation, we installed ten, five‐sided reptile cages (dimensions: 12”W, 18”L, 20”H) in known or historical roosting locations within each site (Figure S8). We mounted each cage so that the open side was in contact with the hibernaculum surface, allowing bats to roost on the substrate. We mounted cages high on site ceilings and sealed them to prevent bat escape and eliminate the possibility of predation. Water was provided in poultry waterers in each cage.
In December 2018, in early hibernation, we returned to the two sites of persisting M. lucifugus colonies in New York. We collected 45 male M. lucifugus from each site and each received a unique forearm band. Individuals were randomly assigned to both a site and a cage within that site with three individual bats from the same origin site sharing each cage. These assignments were in a full factorial design, such that of the 45 bats collected from each site, 15 were assigned to be caged within the same site, 15 within the opposite persisting site, and 15 within the previously extirpated site. We placed each bat individually in a cloth bag and transported them to their assigned site in a cooler so that they would remain in torpor and limit energy expenditure. We replicated the transportation disturbance across all treatment groups. All individuals were caged with at least one infected individual and bats remained in their cages for the duration of hibernation. In mid‐hibernation, each site was visited once to record survival status through visual inspection (Persisting 1: 50 days; Persisting 2: 64 days; Extirpated: 71 days following initial translocation); no cages were opened or bats handled to minimise disturbance. In March 2019, in late hibernation, 110 days following the initial translocation, we returned to each of the three sites to collect data and terminate the experiment. We released all surviving individuals at their site of origin.
Disease severity metrics
Upon returning to each of the three sites, we recorded the survival status of each individual. To calculate the on‐host pathogen growth rate of Pseudogymnoascus destructans, we collected swab samples from each individual bat in both December 2018 and March 2019 using a standardised swabbing protocol described in (Langwig, Frick, et al., 2015). We used real‐time polymerase chain reaction (qPCR) to quantify fungal loads (Muller et al., 2013) (described in Supplemental Information).
In December 2018 and March 2019, we weighed each bat using an electronic scale. We subtracted the late hibernation weight of each individual from their early hibernation weight to calculate weight loss over the course of hibernation in grams. Additionally, in March 2019, we collected data on the severity of tissue invasion by transilluminating both wings of each individual using a 9‐watt 368nm fluorescent light. Infected tissue fluoresces orange under ultraviolet light, verified by histology (Turner et al., 2014). We used the proportion of the plagiopatagium displaying orange fluorescence as the response variable in models of this disease severity metric (Supplemental Information).
Statistical analysis
We conducted all analyses using package ‘lme4’ (Bates et al., 2015) in R v.3.6.0 (R Core Team 2019). We constructed separate models for each disease severity metric. To measure pathogen growth rate for each individual bat, we subtracted its early hibernation fungal load value from its late hibernation value to quantify the change in fungal loads. Bats that had no detectable fungus at the time of swabbing were assigned a value of 4.35e‐03 pg (equivalent to a C t value of 40) for that swab sample (Langwig et al., 2016), or a single P. destructans conidium. We then added a constant of 10 and log10‐transformed the growth rate values. We used a linear regression with pathogen growth rate as the response variable and site (hibernaculum to which bats were translocated), origin site (hibernaculum from which bats originated), and their interaction as fixed effects to explore how pathogen growth varied across sites. Inclusion of cage ID as a random effect prevented model convergence and was dropped from the model, and model results were verified in a Bayesian framework using package ‘BRMS’ with weakly‐informed priors (normal distribution with a mean of 0 and a standard deviation of 5; Bürkner, 2017). To understand the relationship between roosting temperature and change in fungal loads, we constructed a linear mixed model with the average roosting temperature (data collected by iButtons within each cage), origin site, their interaction and the average vapour pressure deficit within a site as fixed effects and cage ID as a random effect. We used a generalised linear model with a gamma error distribution and log link to analyse differences in early hibernation pathogen loads between bats that originated in the two persisting sites. A generalised linear model was used because a linear model violated assumptions of normally distributed and homoscedastic error.
To test for differences in the severity of tissue invasion across the sites, we used a logistic regression with orange pixels indicating infection as successes and non‐orange pixels as failures (generalised linear mixed model with binomial distribution and logit link), site and origin site as fixed effects and cage ID as a random effect. Additionally, to test for differences in tissue invasion between caged bats and free‐flying bats opportunistically sampled at the end of hibernation in each of the persisting sites, we used a logistic regression with the same response variable (generalised linear mixed model with binomial distribution and logit link) and site, caging status (caged vs. free‐flying), and their interaction as fixed effects. To explore the differences in weight loss across the three sites, we used a generalised linear mixed model (gamma distribution, log link) with weight loss as the response variable, site and origin site as fixed effects and cage ID as a random effect. A generalised linear mixed model was used because a linear model violated assumptions of normally distributed and homoscedastic error, and a gamma error distribution was used because weight loss is a continuous variable bounded by zero and infinity. To test for differences in late hibernation body mass between caged and free‐flying bats in each persisting site, we used a linear regression with body mass as the response variable and an interaction term of site and caging status as the explanatory variable. We used a generalised linear mixed model (binomial distribution, logit link, cage ID as a random effect) to explore the relationship between early hibernation body mass and survival. Finally, to investigate how survival varied across sites, we used a generalised linear mixed model (binomial distribution, logit link) with survival as the response variable, site and origin site as fixed effects, and cage ID as a random effect.
RESULTS
Summary
Bats were translocated between two hibernacula with persisting colonies as well as to one hibernaculum previously extirpated of bats by WNS (Figure 1). Bats were caged within each site, where they remained for the duration of winter, allowing us to quantify disease severity from the same individuals at both the beginning and end of hibernation. In all three sites, survival observed during the translocation experiment was higher than that observed within the same sites during the initial epidemic. This suggests little brown bats in persisting colonies may have unique host traits that promote surviving infection with P. destructans. However, disease severity and subsequently survival varied across the three hibernacula, suggesting environmental conditions potentially interact with circulating host traits to ultimately drive persistence.
Pathogen growth rate
At the time of collection in early hibernation, 88.89% (n = 40) of individuals from Persisting 1 (Cold + Dry) had detectable P. destructans from swab samples, as did 97.78% (n = 44) from Persisting 2 (Cold + Wet). All cages in each site had at least one infected bat at the beginning of the experiment. Additionally, at the time of collection, bats captured in Persisting 1 (Cold + Wet) had higher pathogen loads than bats that originated in Persisting 2 (Cold + Dry) (generalised linear model with gamma error distribution and log link: β = 0.282 ± 0.094 SE, p = 0.003; Figure S3).
Over the course of the experiment, the highest on‐host pathogen growth rates were recorded in the previously extirpated site (Warm + Wet), which was 4.5°C warmer than either of the two persisting sites, on average. Pathogen growth rates in Persisting 2 (Cold + Wet) were slightly higher than in Persisting 1 (Cold + Dry), likely attributable to the 1°C warmer and more humid conditions in the former. We also detected an interactive effect of origin site (the site from which bats were collected at the beginning of the experiment) and translocation site on the pathogen growth rate, such that within the previously extirpated site (Warm + Wet), bats originating in Persisting 2 (Cold + Wet) had lower pathogen growth rates than bats that originated in Persisting 1 (Cold + Dry) (Figure 2; Figure S4; Table S1). Pathogen growth rate increased with average roosting temperature, consistent with the sensitivity of P. destructans to ambient thermal conditions (Langwig et al., 2016; Verant et al., 2012). However, due to the effect of origin site, the estimated relationship between average roosting temperature and pathogen growth rate was positive for bats that originated in Persisting 1 (Cold + Dry) but did not increase for bats that originated in Persisting 2 (Cold + Wet) (Figure 2; Table S2). Additionally, we found that pathogen growth on bat skin was generally lower in drier conditions. Interestingly, we observed a decrease in pathogen loads on five bats in Persisting 1 (Cold + Dry), four in Persisting 2 (Cold + Wet), and one in Extirpated (Warm + Wet).
FIGURE 2.
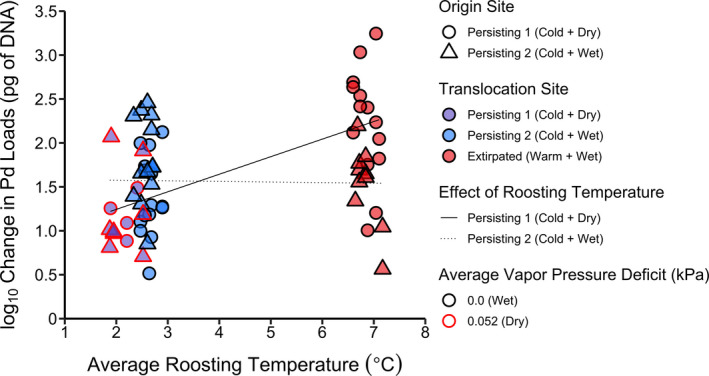
Relationship between average roosting temperature and pathogen growth rate on bats. The shape of data points denotes the persisting site from which individuals originated: Persisting 1 (Cold + Dry) = circle, Persisting 2 (Cold + Wet) = triangle. The colour of data points represents the translocation sites: Persisting 1 (Cold + Dry) = purple, Persisting 2 (Cold + Wet) = blue, Extirpated (Warm + Wet) = red. The solid and dotted lines are model estimates for the effect of roosting temperature for bats that originated in Persisting 1 (Cold + Dry) and Persisting 2 (Cold + Wet) sites, respectively. The outline of points represents average humidity conditions (vapour pressure deficit) within each site: 0.0 kPa (wet conditions) = black, 0.052 kPa (dry conditions) = red. As Extirpated (Warm + Wet) and Persisting 2 (Cold + Wet) were both at 100% relative humidity for the duration of the experiment, the humidity values are the same for these sites. A log10 change in Pd loads value of 1 is associated with no change in pathogen load from early to late hibernation, and data points above or below this value indicate an increase or decrease in pathogen load, respectively
Infection severity and host body condition
The degree of tissue invasion and weight loss varied across the three sites (Figure 3). Corresponding to the temperature‐dependent pathogen growth rate, the severity of infection at Extirpated (Warm + Wet) was significantly higher than that observed at the similarly wet site, Persisting 2 (Cold + Wet) (Figure 3a; Table S3). However, contrary to the temperature‐dependent pathogen growth rate observed, bats at Persisting 1 (Cold + Dry) displayed significantly higher infection severity than that observed at Persisting 2 (Cold + Wet) and did not significantly differ from that observed in Extirpated (Warm + Wet). Across all sites, there was no clear effect of origin site on the degree of tissue invasion. Patterns of tissue invasion were mirrored by the degree of weight loss observed across the sites, as bats in Persisting 2 (Cold + Wet) had lower weight loss compared to either of the other sites (Figure 3b; Table S4). The particularly dry conditions within Persisting 1 (Cold + Dry) may have resulted in the deviation from the temperature–pathogen growth relationship, exacerbating infection severity and weight loss despite a relatively low level of pathogen growth. Bats originating in Persisting 2 (Cold + Wet) lost slightly more weight than bats originating in Persisting 1 (Cold + Dry), but there was no support that weight loss was the product of an interaction between origin site and translocation site (interaction p‐value > 0.05).
FIGURE 3.
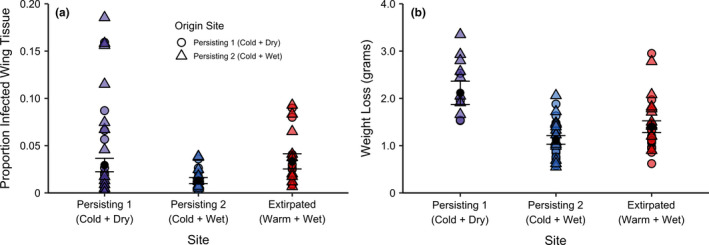
The (a) proportion of wing tissue displaying signs of infection with Pseudogymnoascus destructans and (b) amount of weight lost over hibernation in bats from each site. Black points and error bars represent model estimates ± standard error. Tissue invasion at Extirpated (Warm + Wet) was higher than that at the similarly humid Persisting 2 (Cold + Wet), suggesting that the higher fungal growth rate at the extirpated site resulted in greater tissue invasion and ultimately higher weight loss. The low humidity conditions at Persisting 1 (Cold + Dry) may have driven the observed increase in tissue invasion despite a lower pathogen growth rate. Low humidity conditions can exacerbate evaporative water loss from infected tissue, which may have increased arousal frequency and weight loss
Survival
The highest survival was observed in Persisting 2 (Cold + Wet) (29 survivors of 30, 96.67% surviving), mirroring the pattern of infection severity and host body condition (Figure 4a; Table S5). The lowest observed survival occurred in Persisting 1 (Cold + Dry) (12 survivors of 30, 40% surviving) while Extirpated (Warm + Wet) displayed an intermediate level of survival (24 survivors of 30, 80% surviving). We found no effect of early hibernation body mass on survival (Table S6). The higher degree of mortality at Extirpated (Warm + Wet) may have been related to the high pathogen growth rate within the site, while the high mortality at Persisting 1 (Cold + Dry) may be attributable to the particularly dry conditions, which appeared to exacerbate infection severity. Additionally, low humidity conditions increase evaporative water loss during infection with P. destructans (Mcguire et al., 2017), so the high degree of mortality within the cold and dry site may be attributable to the high infection severity driving higher rates of water loss (Cryan et al., 2010, 2013; Ehlman et al., 2013; Mcguire et al., 2017; Verant et al., 2014; Warnecke et al., 2013; Willis et al., 2011). However, in all sites, the level of survival observed was higher than that observed during the initial epidemic within the same sites, suggesting traits adaptive to surviving WNS may be promoting persistence in these populations (Figure 4a).
FIGURE 4.
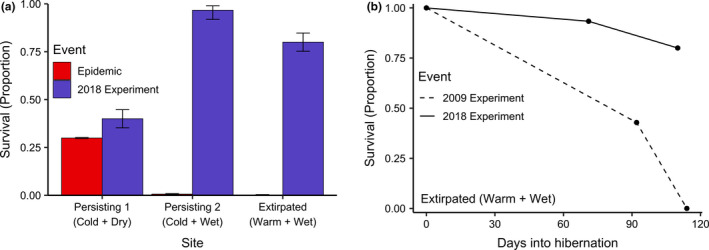
(a) The proportion of bats surviving the initial epidemic (red bars) and 2018 experiment (blue bars) in each site. Error bars represent standard errors. Observed survival corresponded to the degree of tissue invasion and weight loss at each site. Despite the lowest pathogen growth rate, bats in Persisting 1 (Cold + Dry) had a high degree of infection severity, ultimately resulting in the lowest observed survival. In all three sites, we observed higher survival in this experiment than during the initial epidemic within the same sites, suggesting that WNS may have selected for host traits suitable to surviving the disease. (b) Proportion of bats surviving translocation to the Extirpated (Warm + Wet) site in the 2018 experiment (solid line) and 2009 experiment (dashed line). Bats translocated to the extirpated site in 2009 were naïve to WNS, and consequently exhibited 100% mortality. Higher survival was observed in bats translocated to the site in 2018, suggesting that host traits beneficial to surviving WNS may be circulating in these populations
An additional line of evidence for adaptive traits promoting persistence in this system comes from comparison to a study conducted in 2009 in which bats naïve to WNS were translocated to the same extirpated site used in this study. In the historical experiment, all experimental bats died within 114 days (compared to 110 days, the length of this experiment; Figure 4b) (Hicks et al., 2021). Effectively, this represents two replicates of extirpation within this site, in which all naïve bats succumbed to disease during the initial epidemic and subsequent re‐introduction in 2009. However, in this translocation experiment, 24 of the 30 (80%) little brown bats we translocated from persisting colonies to the same extirpated site survived hibernation, suggesting traits adaptive to surviving infection may be circulating in these populations. Additionally, compared to the bats in the extirpated site during the historical translocation experiment, bats in our study had low disease severity scores as indicated by UV fluorescence (Figure 5; methods of comparison described in Supplemental Information). Among the two experiments, the faster speed of mortality in the historical experiment resulted in most of the histology scoring occurring in dead or moribund bats while in the contemporary experiment, wing invasion was predominately measured in live bats (N = 64 alive, 17 dead; additional information on experimental design in Supplemental Information).
FIGURE 5.
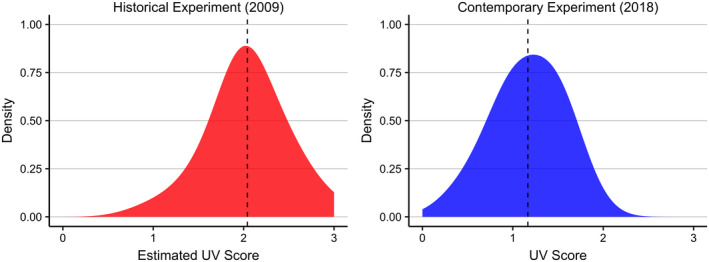
Density plots of infection severity as indicated by degree of orange fluorescence under ultraviolet light in bats from the historical and contemporary translocation experiments in Extirpated (Warm + Wet). A UV score of 0 = no orange fluorescence indicative of infection, 1 = 1–10% of wing area displays orange fluorescence, 2 = 10–50% of wing area displays fluorescence, and 3 = 50–100% of wing area displays fluorescence. Dashed lines correspond to average values. Bats translocated to the extirpated site in 2009 were naïve to WNS and displayed a higher degree of infection severity compared to the non‐naïve bats translocated to the same site in 2018
Comparisons with free‐flying bats
At the end of the experiment in late hibernation, we sampled free‐flying bats found roosting near the cages within each persisting site. At Persisting 1 (Cold + Dry), we found that free‐flying bats had significantly lower tissue invasion compared to the bats within cages (Figure 6; Table S7). Conversely, free‐flying bats at Persisting 2 (Cold + Wet) displayed a similar degree of infection severity to caged bats within the same site. Free‐flying bats had higher late hibernation body mass than caged bats in both persisting sites (Figure S5; Table S8), and this difference was more pronounced in Persisting 1 (Cold + Dry).
FIGURE 6.
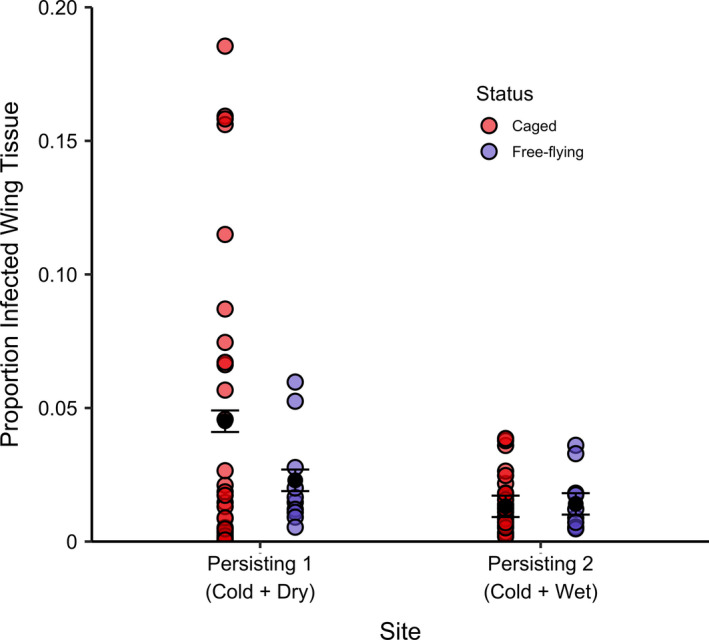
Proportion of infected wing tissue as indicated by orange fluorescence on caged and free‐flying bats in each of the two persisting sites (Meteyer et al., 2009; Turner et al., 2014). Point colours represent caging status: caged = red, free‐flying = blue. Black points and error bars represent model estimates ± standard error. Free‐flying bats were opportunistically captured at the end of hibernation at experiment termination. In Persisting 1 (Cold + Dry), where disease severity in caged bats was high, caged bats had significantly higher levels of tissue invasion compared to free‐flying bats within the same site. However, this difference was not detected at Persisting 2 (Cold + Wet). This suggests that little brown bats in the cold and dry persisting site do not roost in dry microclimates for the entirety of hibernation, but rather move roosting locations periodically, as has been observed prior to the WNS epidemic. Moving amongst a variety of microclimates in this site may allow this persisting colony to mitigate disease severity
DISCUSSION
Our data suggest that environmental conditions interact with host traits to jointly drive persistence of host populations. Importantly, the survival we observed during this experiment was significantly higher than survival during the initial epidemic within the same sites in all cases, as well as during a similar experiment in 2009 within the extirpated site. We found evidence for increased on‐host growth of P. destructans with increasing roosting temperature in bats that originated in Persisting 1 (Cold + Dry), but no relationship in bats that originated in Persisting 2 (Cold + Wet). Infection severity, host body condition, and survival also appeared to be influenced by site humidity, with higher disease severity and lower survival associated with over‐winter exposure to the driest conditions in Persisting 1 (Cold + Dry). However, within the dry conditions, bats sampled from outside of cages in late hibernation displayed significantly lower infection severity compared to their caged counterparts, whereas no such difference was detected in Persisting 2 (Cold + Wet). This suggests that bats within Persisting 1 (Cold + Dry) may be utilising a variety of microclimates and not remaining in these dry environments for the entire winter period, as the caged bats experienced. Furthermore, we observed declines in pathogen loads on 10 individuals, nine of which were in persisting sites. These data collectively suggest that persisting little brown bat colonies in the northeast United States may have evolved traits beneficial to surviving WNS (Auteri & Knowles, 2020; Gignoux‐Wolfsohn et al., 2021), but that these host traits interact with environmental conditions such that protection against severe disease and mortality depends to a strong degree on temperature and humidity.
We detected a positive relationship between average roosting temperature during hibernation and on‐host pathogen growth rate. This is corroborated by data from the initial WNS epidemic, where the most severely impacted colonies were those hibernating in relatively warm hibernacula (Langwig et al., 2012). Infection severity and over‐winter weight loss showed a similar trend when humidity was high, with lower values occurring at Persisting 2 (Cold + Wet) compared to Extirpated (Warm + Wet). However, despite the coldest ambient conditions, infection severity and host weight loss were high under the dry conditions within Persisting 1 (Cold + Dry) and were comparable to the extirpated site. Under unfavourable ambient conditions, fungal pathogens may forgo reproduction and instead commit resources to within‐host growth and the formation of spores that can survive stressful conditions. For example, Metarhizium anisopliae is a fungal pathogen of tick eggs that invades the egg tissue and undergoes growth (Ment et al., 2010; Valerio Garcia et al., 2005). Under humid, favourable conditions, the fungus will emerge from the egg to undergo asexual reproduction. However, under dry, unfavourable conditions, the fungus will instead remain within the egg host, continue to undergo growth and produce environmentally resistant spores (Ment et al., 2010). We suggest that, similarly, exposure to dry conditions of Persisting 1 (Cold + Dry) over winter were unfavourable to the survival of P. destructans in superficial infections, and that the pathogen augmented tissue invasion to satisfy moisture requirements, resulting in a high degree of infection severity. Additionally, evaporative water loss from hibernating bats is highest in dry conditions (Ben‐Hamo et al., 2013; Thomas & Cloutier, 1992) and is exacerbated by infection with P. destructans (Mcguire et al., 2017), resulting in dehydration and increased arousal frequency to re‐hydrate (Cryan et al., 2010, 2013; Ehlman et al., 2013; Mcguire et al., 2017; Verant et al., 2014; Warnecke et al., 2013; Willis et al., 2011). Increased frequency of arousal from torpor drives the premature depletion of fat reserves during hibernation, resulting in weight loss and starvation (Reeder et al., 2012; Warnecke et al., 2012). Therefore, the increased tissue invasion and evaporative water loss in Persisting 1 (Cold + Dry) may have operated synergistically to result in severe disease and ultimately the lowest observed survival.
Colonies of little brown bats in dry hibernacula may be persisting because of the availability of different microclimates. Microclimatic conditions are not uniform throughout an entire hibernation site, but vary with factors such as depth, air flow, and the height of the ceiling (Perry, 2013). Some evidence suggests that bats may move to different roosting locations periodically during winter (Ryan et al., 2019), possibly in response to shifting costs associated with hibernation (Boyles et al., 2020), which could expose them to a variety of microclimates (Boyles et al., 2017). For example, some data suggest that bats may transition from roosting in relatively warm sections of hibernacula in early hibernation to the relatively cold sections by late winter (Ryan et al., 2019). In our study, bats were unable to select varying microclimates over the course of hibernation. However, we observed hundreds of little brown bats roosting in the area surrounding the cages during late winter in Persisting 1 (Cold + Dry), whereas less than a dozen individuals appeared to use that specific location in early hibernation, suggesting that bats do not roost in the same location for the entirety of hibernation in this site. Given that disease severity is highly dependent on environmental conditions within hibernacula, this movement behaviour may have been pre‐adaptive to surviving WNS if bats utilise microclimates that mitigate disease severity for at least part of hibernation. For example, movement within hibernacula may reduce the growth of P. destructans in late hibernation if bats move to the relatively cold conditions that slow pathogen growth, potentially affording them enough time to emerge from hibernation in spring and clear infection. Within Persisting 1 (Cold + Dry), free‐flying bats sampled at the end of hibernation had significantly lower infection severity than caged bats, which is the expected pattern if movement within hibernacula is indeed beneficial to mitigating disease severity. Furthermore, free‐flying bats in both persisting sites had higher late hibernation body masses, and this was more pronounced in Persisting 1 (Cold + Dry). Behavioural responses that moderate the severity of disease have also been proposed for snake populations impacted by snake fungal disease (McKenzie et al., 2021), caused by the fungal pathogen Ophidiomyces ophiodiicola (Lorch et al., 2015). Snakes infected with O. ophiodiicola exhibit changes to their behaviour that include increased surface activity and more time spent in exposed environments compared to their disease‐free conspecifics (Lorch et al., 2015; McKenzie et al., 2021), potentially a sign of a behavioural fever response to infection (Burns et al., 1996). However, we make the important distinction here that because movement within hibernacula was observed in bats prior to the WNS epidemic, this behaviour may have been pre‐adaptive to surviving the disease rather than a direct response to the disease itself. Future research should investigate how the availability and utilisation of varying environmental conditions can influence the dynamics of WNS, and how this may scale up to a population‐level response.
During the initial epidemic, the cold conditions within hibernacula utilised by persisting colonies may have prevented total colony collapse, allowing standing genetic variation for favourable host traits to propagate (Bell, 2013; Grieneisen et al., 2015; Langwig et al., 2012, 2016; Lilley et al., 2018). Previous research has also found genetic evidence from persisting little brown bat colonies indicative of a selective sweep following the invasion of P. destructans (Auteri & Knowles, 2020; Gignoux‐Wolfsohn et al., 2021). However, our data suggest that populations that appear to have evolved adaptive host traits are only afforded protection within a narrow environmental space. These processes have the potential to result in local adaptation, in which the evolutionary response of populations to WNS and the resulting dominant phenotype is specific to the local environmental conditions of hibernacula (Lilley et al., 2020). We detected an effect of origin site on pathogen growth rate within Extirpated (Warm + Wet), potentially a signature of local adaptation to differing conditions in source hibernacula. However, for local adaptation to occur, the strength of selection must be high enough to combat the homogenising effects of gene flow (Felsenstein, 1976; García‐Ramos & Kirkpatrick, 1997; Hendry et al., 2001; Wright, 1969), and current genetic evidence suggests a panmictic genetic landscape for bat populations (Burns et al., 2014; Johnson et al., 2015; Talbot et al., 2016, 2017; Wilder et al., 2015), but see (Davy et al., 2015; Miller‐Butterworth et al., 2014).
Several host traits have been proposed as potential mechanisms of population persistence of little brown bats, including the evolution of host resistance or tolerance (Hoyt et al., 2016; Langwig et al., 2016, 2017; Zukal et al., 2016), changes in fat deposition (Cheng et al., 2019), changes in hibernation physiology (Auteri & Knowles, 2020; Gignoux‐Wolfsohn et al., 2021), and reduced arousal frequency from hibernation (Lilley et al., 2016). While the objective of this study was not to identify the specific host trait potentially driving persistence, it is the first to connect host traits to individual‐level survival rather than population‐level trends, filling an essential gap in our understanding of WNS. However, potential mechanisms other than beneficial host traits are not necessarily absent and should be further explored. For example, while current genetic and phenotypic evidence suggests pathogen virulence attenuation is not a primary driver of host population persistence in this system (Drees et al., 2017; Forsythe et al., 2018; Leopardi et al., 2015; Palmer et al., 2014; Ren et al., 2012; Trivedi et al., 2017; Warnecke et al., 2012), it is possible that the signal of this mechanism has not yet been detected with the data on pathogen diversity currently available for North American isolates. Additionally, given that the data presented suggest an important role of environmental conditions in driving disease severity and potentially host persistence, shifting environmental conditions within hibernacula since the emergence of WNS in North America could contribute to changing host population trajectories (Mammola et al., 2019; Perry, 2013). However, there is no clear trend in changing underground conditions since pathogen invasion in our dataset (Figure S6), suggesting shifting thermal conditions are not likely to explain persistence in this system. However, because hibernacula temperatures are sensitive to aboveground conditions (Perry, 2013), global climate change has the potential to alter the host–pathogen interaction in this system (Altizer et al., 2013; Mammola et al., 2019; Price et al., 2019). Finally, while current evidence does not suggest density‐dependent transmission occurs in this system (Hoyt et al., 2016, 2020; Langwig, Frick, et al., 2015), other density‐dependent effects might be present if population density impacts infection severity rather than prevalence. For example, if conspecific behaviour can exacerbate disease severity, then populations of lower density may be freed from detrimental conspecific effects. However, relatively small colonies of little brown bats exhibit population declines of the same magnitude of much larger colonies, a consistency which should not be observed if density‐dependent effects drive population persistence (Langwig et al., 2012). Additionally, recent evidence suggests that hibernating bats continue to exhibit extensive social interactions following the arrival of WNS, suggesting a change in social behaviour is not a principle contributor to population persistence (Hoyt et al., 2018). Finally, we found no support for a correlation between pathogen load and colony size, further suggesting density‐dependent effects on disease severity are not a primary a driver of persistence (Figure S7). Identifying the specific host traits contributing to the persistence of little brown bat colonies is essential to the successful management of the species and should be a primary focus of future research.
As P. destructans continues to spread throughout North America, bat population declines and regional extirpations will continue to occur. However, this study strongly suggests that prior to the invasion of P. destructans, host traits conducive to surviving WNS circulated in little brown bat populations, which now offer some colonies imperfect protection from the disease. These host traits do not operate independently to promote population persistence with WNS, but rather interact strongly with environmental conditions, specifically temperature and humidity, to ultimately drive host–pathogen coexistence. Therefore, we should not expect to see all little brown bat populations across North America stabilise or rebound from declines, but rather the persistence of colonies with the correct combination of host traits and environmental conditions.
Host population response to the invasion of a virulent pathogen will not be predictable by a single aspect of the host, environment, or pathogen. Rather, host–pathogen interactions and coexistence will be strongly mediated by environmental conditions, the result of which may be as variable as the environment itself (Parratt et al., 2016; Wilber et al., 2016). Underlying variation in host and pathogen populations will set the stage for subsequent coevolutionary processes and the likelihood of coexistence (Barrett & Schluter, 2008; Bell, 2013), but this interaction and the resulting host population response may be influenced by environmental conditions that vary over space and time (Parratt et al., 2016; Wolinska & King, 2009), as illustrated by this study. Therefore, to achieve predictability in how emerging infectious diseases will impact host populations, it is essential to disentangle host–environment–pathogen interactions across a geographic and temporal mosaic of host–pathogen coevolution.
AUTHORSHIP
ATG, JRH and KEL conceptualized the project and methodology. ATG, JRH, SAY, CJH, ABB and KEL performed the investigation. ATG and KEL curated the data and carried out formal analyses. ATG wrote the original draft of the manuscript. ATG, JRH, SAY, CJH, ABB and KEL reviewed & editied the manuscript. JRH and KEL a supervised the project.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank several individuals for their invaluable contributions to the field element of this study. From the New York State Department of Environmental Conservation (NYDEC), we thank Amanda Bailey, Samantha Hoff, and Casey Pendergast. From the Vermont Fish & Wildlife Department, we thank Kerry Monahan and Joel Flewelling. We thank Jeff Foster and Katy Parise from NAU for assistance in P. destructans sample processing. Funding was provided by the joint NSF‐NIH‐NIFA Ecology and Evolution of Infectious Disease award DEB‐1911853 and Virginia Tech.
Grimaudo, A.T. , Hoyt, J.R. , Yamada, S.A. , Herzog, C.J. , Bennett, A.B. & Langwig, K.E. (2022) Host traits and environment interact to determine persistence of bat populations impacted by white‐nose syndrome. Ecology Letters, 25, 483–497. Available from: 10.1111/ele.13942
DATA AVAILABILITY STATEMENT
The data and code used to produce the results and figures of this manuscript are publicly available on Dryad under DOI: https://doi.org/10.5061/dryad.j9kd51cdw
REFERENCES
- Altizer, S. , Ostfeld, R.S. , Johnson, P.T.J. , Kutz, S. & Harvell, C.D. (2013) Climate change and infectious diseases: from evidence to a predictive framework. Science, 341, 514–519. [DOI] [PubMed] [Google Scholar]
- Anderson, R.M. & May, R.M. (1982) Coevolution of hosts and parasites. Parasitology, 85, 411–426. [DOI] [PubMed] [Google Scholar]
- Antolin, M.F. , Gober, P. , Luce, B. , Biggins, D.E. & Van Pelt, W.E. (2002) The influence of sylvatic plague on North American wildlife at the landscape level, with special emphasis on black‐footed ferret and prairie dog conservation. U.S. Fish and Wildlife Publishing, 57, 104–127. [Google Scholar]
- Arthur, A. , Ramsey, D. & Efford, M. (2004) Impact of bovine tuberculosis on a population of brushtail possums (Trichosurus vulpecula Kerr) in the Orongorongo Valley, New Zealand. Wildlife Research, 31, 389–395. [Google Scholar]
- Auteri, G.G. & Knowles, L.L. (2020) Decimated little brown bats show potential for adaptive change. Scientific Reports, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, R.D.H. & Schluter, D. (2008) Adaptation from standing genetic variation. Trends in Ecology & Evolution, 23, 38–44. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B.M. & Walker, S.C. (2015) Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Bell, G. (2013) Evolutionary rescue and the limits of adaptation. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20120080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Hamo, M. , Muñoz‐Garcia, A. , Williams, J.B. , Korine, C. & Pinshow, B. (2013) Waking to drink: rates of evaporative water loss determine arousal frequency in hibernating bats. Journal of Experimental Biology, 216, 573–577. [DOI] [PubMed] [Google Scholar]
- Best, A. , White, A. & Boots, M. (2008) Maintenance of host variation in tolerance to pathogens and parasites. Proceedings of the National Academy of Sciences, 105, 20786–20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blehert, D.S. , Hicks, A.C. , Behr, M. , Meteyer, C.U. , Berlowski‐Zier, B.M. , Buckles, E.L. et al. (2009) Bat white‐nose syndrome: an emerging fungal pathogen? Science, 323, 227. [DOI] [PubMed] [Google Scholar]
- Boots, M. , Best, A. , Miller, M.R. & White, A. (2009) The role of ecological feedbacks in the evolution of host defence: what does theory tell us? Philosophical Transactions of the Royal Society of London. Series B, 364, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots, M. , Hudson, P.J. & Sasaki, A. (2004) Large shifts in pathogen virulence relate to host population structure. Science, 303, 842–844. [DOI] [PubMed] [Google Scholar]
- Boyles, J.G. , Boyles, E. , Dunlap, R.K. , Johnson, S.A. & Brack, V. Jr (2017) Long‐term microclimate measurements add further evidence that there is no “optimal” temperature for bat hibernation. Mammalian Biology, 86, 9–16. [Google Scholar]
- Boyles, J.G. , Johnson, J.S. , Blomberg, A. & Lilley, T.M. (2020) Optimal hibernation theory. Mammal Review, 50, 91–100. [Google Scholar]
- Brannelly, L.A. , McCallum, H.I. , Grogan, L.F. , Briggs, C.J. , Ribas, M.P. , Hollanders, M. et al. (2021) Mechanisms underlying host persistence following amphibian disease emergence determine appropriate management strategies. Ecology Letters, 24, 130–148. [DOI] [PubMed] [Google Scholar]
- Briggs, C.J. , Knapp, R.A. & Vredenburg, V.T. (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proceedings of the National Academy of Sciences, 107, 9695–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkner, P.C. (2017) brms: an R package for Bayesian multilevel models using Stan. Journal of Statistical Software, 80, 1–28. [Google Scholar]
- Burns, G. , Ramos, A. & Muchlinski, A. (1996) Fever response in North American snakes. Journal of Herpetology, 30, 133–139. [Google Scholar]
- Burns, L.E. , Frasier, T.R. & Broders, H.G. (2014) Genetic connectivity among swarming sites in the wide ranging and recently declining little brown bat (Myotis lucifugus). Ecology and Evolution, 4, 4130–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, T.L. , Gerson, A. , Moore, M.S. , Reichard, J.D. , DeSimone, J. , Willis, C.K.R. et al. (2019) Higher fat stores contribute to persistence of little brown bat populations with white‐nose syndrome. Journal of Animal Ecology, 88, 591–600. [DOI] [PubMed] [Google Scholar]
- Cressler, C.E. , Mcleod, D.V. , Rozins, C. , Van Den Hoogen, J. & Day, T. (2016) The adaptive evolution of virulence: a review of theoretical predictions and empirical tests. Parasitology, 143, 915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan, P.M. , Meteyer, C.U. , Blehert, D.S. , Lorch, J.M. , Reeder, D.M. , Turner, G.G. et al. (2013) Electrolyte depletion in white‐nose syndrome bats. Journal of Wildlife Diseases, 49, 398–402. [DOI] [PubMed] [Google Scholar]
- Cryan, P.M. , Meteyer, C.U. , Boyles, J.G. & Blehert, D.S. (2010) Wing pathology of white‐nose syndrome in bats suggests life‐threatening disruption of physiology. BMC Biology, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy, C.M. , Martinez‐Nunez, F. , Willis, C.K.R. & Good, S.V. (2015) Spatial genetic structure among bat hibernacula along the leading edge of a rapidly spreading pathogen. Conservation Genetics, 16, 1013–1024. [Google Scholar]
- de Castro, F. & Bolker, B. (2005) Mechanisms of disease‐induced extinction. Ecology Letters, 8, 117–126. [Google Scholar]
- Dobony, C.A. , Hicks, A.C. , Langwig, K.E. , von Linden, R.I. , Okoniewski, J.C. & Rainbolt, R.E. (2011) Little brown myotis persist despite exposure to white‐nose syndrome. Journal of Fish and Wildlife Management, 2, 190–195. [Google Scholar]
- Drees, K.P. , Lorch, J.M. , Puechmaille, S.J. , Parise, K.L. , Wibbelt, G. , Hoyt, J.R. et al. (2017) Phylogenetics of a fungal invasion: origins and widespread dispersal of white‐nose syndrome. MBio, 8, e01941–e2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echaubard, P. , Leduc, J. , Pauli, B. , Chinchar, V.G. , Robert, J. & Lesbarrères, D. (2014) Environmental dependency of amphibian‐ranavirus genotypic interactions: evolutionary perspectives on infectious diseases. Evolutionary Applications, 7, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlman, S.M. , Cox, J.J. & Crowley, P.H. (2013) Evaporative water loss, spatial distributions, and survival in white‐nose‐syndrome‐affected little brown myotis: a model. Journal of Mammalogy, 94, 572–583. [Google Scholar]
- Epstein, B. , Jones, M. , Hamede, R. , Hendricks, S. , McCallum, H. , Murchison, E.P. et al. (2016) Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nature Communications, 7, 12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J. (1976) The theoretical population genetics of variable selection and migration. Annual Review of Genetics, 10, 253–280. [DOI] [PubMed] [Google Scholar]
- Fenton, A. , Fairbairn, J.P. , Norman, R. & Hudson, P.J. (2002) Parasite transmission: reconciling theory and reality. Journal of Animal Ecology, 71, 893–905. [Google Scholar]
- Forrest, M.J. & Schlaepfer, M.A. (2011) Nothing a hot bath won’t cure: infection rates of amphibian chytrid fungus correlate negatively with water temperature under natural field settings. PLoS One, 6, e28444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe, A. , Giglio, V. , Asa, J. & Xu, J. (2018) Phenotypic divergence along geographic gradients reveals potential for rapid adaptation of the white‐nose syndrome pathogen, Pseudogymnoascus destructans, in North America. Applied and Environment Microbiology, 84, e00863–e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick, W.F. , Pollock, J.F. , Hicks, A.C. , Langwig, K.E. , Reynolds, D.S. , Turner, G.G. et al. (2010) An emerging disease causes regional population collapse of a common North America bat species. Science, 329, 679–682. [DOI] [PubMed] [Google Scholar]
- Frick, W.F. , Puechmaille, S.J. , Hoyt, J.R. , Nickel, B.A. , Langwig, K.E. , Foster, J.T. et al. (2015) Disease alters macroecological patterns of North American bats. Global Ecology and Biogeography, 24, 741–749. [Google Scholar]
- Friedman, A. & Yakubu, A.‐A. (2012) Host demographic Allee effect, fatal disease, and migration: persistence or extinction. SIAM Journal on Applied Mathematics, 72, 1644–1666. [Google Scholar]
- Fuller, N.W. , McGuire, L.P. , Pannkuk, E.L. , Blute, T. , Haase, C.G. , Mayberry, H.W. et al. (2020) Disease recovery in bats affected by white‐nose syndrome. Journal of Experimental Biology, 223, jeb211912. [DOI] [PubMed] [Google Scholar]
- García‐Ramos, G. & Kirkpatrick, M. (1997) Genetic models of adaptation and gene flow in peripheral populations. Evolution (N, Y), 51, 21–28. [DOI] [PubMed] [Google Scholar]
- Gignoux‐Wolfsohn, S.A. , Pinsky, M.L. , Kerwin, K. , Herzog, C. , Hall, M. , Bennett, A.B. et al. (2021) Genomic signatures of evolutionary rescue in bats surviving white‐nose syndrome. Molecular Ecology, 30, 5643–5657. [DOI] [PubMed] [Google Scholar]
- Grieneisen, L.E. , Brownlee‐Bouboulis, S.A. , Johnson, J.S. & Reeder, D.M. (2015) Sex and hibernaculum temperature predict survivorship in white‐nose syndrome affected little brown myotis (Myotis lucifugus). Royal Society Open Science, 2, 140470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard, G.W. , Thomas, C.D. , Hodgson, J.A. , Scroggie, M.P. , Ramsey, D.S.L. & Clemann, N. (2015) Refugia and connectivity sustain amphibian metapopulations afflicted by disease. Ecology Letters, 18, 853–863. [DOI] [PubMed] [Google Scholar]
- Hendry, A.P. , Day, T. & Taylor, E.B. (2001) Population mixing and the adaptive divergence of quantitative traits in discrete populations: a theoretical framework for empirical tests. Evolution (N. Y), 55, 459–466. [DOI] [PubMed] [Google Scholar]
- Hicks, A.C. , Darling, S. , Flewelling, J. , von Linden, R. , Meteyer, C.U. , Redell, D. et al. (2021). Environmental transmission of Pseudogymnoascus destructans to hibernating little brown bats. bioRxiv, July 2021, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka, W.M. & Dhondt, A.A. (2000) Density‐dependent decline of host abundance resulting from a new infectious disease. Proceedings of the National Academy of Sciences, 97, 5303–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe, P.A. , Mccallum, H.I. , Jones, M.E. , Lawrance, M.F. , Hamede, R.K. & Storfer, A. (2019) Conserving adaptive potential: lessons from Tasmanian devils and their transmissible cancer. Conservation Genetics, 20, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, S.R. , Hoyt, J.R. , White, J.P. , Kaarakka, H.M. , Redell, J.A. , DePue, J.E. et al. (2021) Continued preference for suboptimal habitat reduces bat survival with white‐nose syndrome. Nature Communications, 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, J.R. , Kilpatrick, A.M. & Langwig, K.E. (2021) Ecology and impacts of white‐nose syndrome on bats. Nature Reviews Microbiology, 19, 1–15. [DOI] [PubMed] [Google Scholar]
- Hoyt, J.R. , Langwig, K.E. , Sun, K. , Lu, G. , Parise, K.L. , Jiang, T. et al. (2016) Host persistence or extinction from emerging infectious disease: insights from white‐nose syndrome in endemic and invading regions. Proceedings of the Royal Society B, 283, 20152861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, J.R. , Langwig, K.E. , Sun, K. , Parise, K.L. , Li, A. , Wang, Y. et al. (2020) Environmental reservoir dynamics predict global infection patterns and population impacts for the fungal disease white‐nose syndrome. Proceedings of the National Academy of Sciences, 117, 7255–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, J.R. , Langwig, K.E. , White, J.P. , Kaarakka, H.M. , Redell, J.A. , Kurta, A. et al. (2018) Cryptic connections illuminate pathogen transmission within community networks. Nature, 563, 710–713. [DOI] [PubMed] [Google Scholar]
- Johnson, L.N.L. , Mcleod, B.A. , Burns, L.E. , Arseneault, K. , Frasier, T.R. & Broders, H.G. (2015) Population genetic structure within and among seasonal site types in the little brown bat (Myotis lucifugus) and the northern long‐eared bat (M. septentrionalis). PLoS One, 10, e0126309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, B. , Neuhauser, C. , Bohannan, B.J.M. & Dean, A.M. (2006) Local migration promotes competitive restraint in a host‐pathogen “tragedy of the commons”. Nature, 442, 75–78. [DOI] [PubMed] [Google Scholar]
- Kutzer, M.A.M. & Armitage, S.A.O. (2016) Maximising fitness in the face of parasites: a review of host tolerance. Zoology, 119, 281–289. [DOI] [PubMed] [Google Scholar]
- Lachish, S. , McCallum, H. & Jones, M. (2009) Demography, disease and the devil: life‐history changes in a disease‐affected population of Tasmanian devils (Sarcophilus harrisii). Journal of Animal Ecology, 78, 427–436. [DOI] [PubMed] [Google Scholar]
- LaDeau, S.L. , Kilpatrick, A.M. & Marra, P.P. (2007) West Nile virus emergence and large‐scale declines of North American bird populations. Nature, 447, 710–713. [DOI] [PubMed] [Google Scholar]
- Laine, A.L. (2007) Pathogen fitness components and genotypes differ in their sensitivity to nutrient and temperature variation in a wild plant‐pathogen association. Journal of Evolutionary Biology, 20, 2371–2378. [DOI] [PubMed] [Google Scholar]
- Lande, R. (1998) Demographic stochasticity and Allee effect on a scale with isotropic noise. Oikos, 83, 353–358. [Google Scholar]
- Langwig, K.E. , Frick, W.F. , Bried, J.T. , Hicks, A.C. , Kunz, T.H. & Marm Kilpatrick, A. (2012) Sociality, density‐dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white‐nose syndrome. Ecology Letters, 15, 1050–1057. [DOI] [PubMed] [Google Scholar]
- Langwig, K.E. , Frick, W.F. , Hoyt, J.R. , Parise, K.L. , Drees, K.P. , Kunz, T.H. et al. (2016) Drivers of variation in species impacts for a multi‐host fungal disease of bats. Philosophical Transactions of the Royal Society B: Biological Sciences, 371, 20150456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langwig, K.E. , Frick, W.F. , Reynolds, R. , Parise, K.L. , Drees, K.P. , Hoyt, J.R. et al. (2015) Host and pathogen ecology drive the seasonal dynamics of a fungal disease, white‐nose syndrome. Proceedings of the Royal Society B, 282, 20142335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langwig, K.E. , Hoyt, J.R. , Parise, K.L. , Frick, W.F. , Foster, J.T. & Kilpatrick, A.M. (2017) Resistance in persisting bat populations after white‐nose syndrome invasion. Philosophical Transactions of the Royal Society of London. Series B, 372, 20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langwig, K.E. , Hoyt, J.R. , Parise, K.L. , Kath, J. , Kirk, D. , Frick, W.F. et al. (2015) Invasion dynamics of white‐nose syndrome fungus, midwestern United States, 2012–2014. Emerging Infectious Diseases, 21, 1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenby, B.T. , Tobler, M.W. , Brown, W.E. , Hawkins, C.E. , Hocking, G.J. , Hume, F. et al. (2018) Density trends and demographic signals uncover the long‐term impact of transmissible cancer in Tasmanian devils. Journal of Applied Ecology, 55, 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi, S. , Blake, D. & Puechmaille, S.J. (2015) White‐Nose Syndrome fungus introduced from Europe to North America. Current Biology, 25, R217–R219. [DOI] [PubMed] [Google Scholar]
- Levin, S. & Pimentel, D. (1981) Selection of intermediate rates of increase in parasite‐host systems. American Naturalist, 117, 308–315. [Google Scholar]
- Lilley, T.M. , Anttila, J. & Ruokolainen, L. (2018) Landscape structure and ecology influence the spread of a bat fungal disease. Functional Ecology, 32, 2483–2496. [Google Scholar]
- Lilley, T.M. , Johnson, J.S. , Ruokolainen, L. , Rogers, E.J. , Wilson, C.A. , Schell, S.M. et al. (2016) White‐nose syndrome survivors do not exhibit frequent arousals associated with Pseudogymnoascus destructans infection. Frontiers in Zoology, 13, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley, T.M. , Wilson, I.W. , Field, K.A. , Reeder, D.M. , Vodzak, M.E. , Turner, G.G. et al. (2020) Genome‐wide changes in genetic diversity in a Population of Myotis lucifugus Affected by White‐Nose Syndrome. G3: Genes, Genomes, Genetics, 10, 2007–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd‐Smith, J.O. , Cross, P.C. , Briggs, C.J. , Daugherty, M. , Getz, W.M. , Latto, J. et al. (2005) Should we expect population thresholds for wildlife disease? Trends in Ecology & Evolution, 20, 511–519. [DOI] [PubMed] [Google Scholar]
- Lorch, J.M. , Lankton, J. , Werner, K. , Falendysz, E.A. , Mccurley, K. & Blehert, D.S. (2015) Experimental infection of snakes with Ophidiomyces ophiodiicola causes pathological changes that typify snake fungal disease. MBio, 6, e01534–e1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch, J.M. , Meteyer, C.U. , Behr, M.J. , Boyles, J.G. , Cryan, P.M. , Hicks, A.C. et al. (2011) Experimental infection of bats with Geomyces destructans causes white‐nose syndrome. Nature, 480, 376–379. [DOI] [PubMed] [Google Scholar]
- Mammola, S. , Piano, E. , Cardoso, P. , Vernon, P. , Domínguez‐Villar, D. , Culver, D.C. et al. (2019) Climate change going deep: the effects of global climatic alterations on cave ecosystems. Anthr. Rev, 6, 98–116. [Google Scholar]
- Marroquin, C.M. , Lavine, J.O. & Windstam, S.T. (2017) Effect of humidity on development of Pseudogymnoascus destructans, the causal agent of bat white‐nose syndrome. Northeastern Naturalist, 24, 54–64. [Google Scholar]
- McCallum, H. , Barlow, N. & Hone, J. (2001) How should pathogen transmission be modelled? Trends in Ecology & Evolution, 16, 295–300. [DOI] [PubMed] [Google Scholar]
- McCallum, H. , Jones, M. , Hawkins, C. , Hamede, R. , Lachish, S. , Sinn, D.L. et al. (2009) Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease‐induced extinction. Ecology, 90, 3379–3392. [DOI] [PubMed] [Google Scholar]
- Mcdonald, J.L. , Bailey, T. , Delahay, R.J. , Mcdonald, R.A. , Smith, G.C. & Hodgson, D.J. (2016) Demographic buffering and compensatory recruitment promotes the persistence of disease in a wildlife population. Ecology Letters, 19, 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcguire, L.P. , Mayberry, H.W. & Willis, C.K.R. (2017) White‐nose syndrome increases torpid metabolic rate and evaporative water loss in hibernating bats. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 313, R680–R686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie, J.M. , Price, S.J. , Connette, G.M. , Bonner, S.J. & Lorch, J.M. (2021) Effects of snake fungal disease on short‐term survival, behavior, and movement in free‐ranging snakes. Ecological Applications, 31, e02251. [DOI] [PubMed] [Google Scholar]
- Ment, D. , Gindin, G. , Glazer, I. , Perl, S. , Elad, D. & Samish, M. (2010) The effect of temperature and relative humidity on the formation of Metarhizium anisopliae chlamydospores in tick eggs. Fungal Biology, 114, 49–56. [DOI] [PubMed] [Google Scholar]
- Meteyer, C.U. , Buckles, E.L. , Blehert, D.S. , Hicks, A.C. , Green, D.E. , Shearn‐Bochsler, V. et al. (2009) Histopathologic criteria to confirm white‐nose syndrome in bats. Journal of Veterinary Diagnostic Investigation, 21, 411–414. [DOI] [PubMed] [Google Scholar]
- Meteyer, C.U. , Valent, M. , Kashmer, J. , Buckles, E.L. , Lorch, J.M. , Blehert, D.S. et al. (2011) Recovery of little brown bats (Myotis lucifugus) from natural infection With Geomyces destructans, white‐nose syndrome. Journal of Wildlife Diseases, 47, 618–626. [DOI] [PubMed] [Google Scholar]
- Miller‐Butterworth, C.M. , Vonhof, M.J. , Rosenstern, J. , Turner, G.G. & Russell, A.L. (2014) Genetic structure of little brown bats (Myotis lucifugus) corresponds with spread of white‐nose syndrome among hibernacula. Journal of Heredity, 105, 354–364. [DOI] [PubMed] [Google Scholar]
- Minnis, A.M. & Lindner, D.L. (2013) Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biology, 117, 638–649. [DOI] [PubMed] [Google Scholar]
- Mosher, B.A. , Bailey, L.L. , Muths, E. & Huyvaert, K.P. (2018) Host–pathogen metapopulation dynamics suggest high elevation refugia for boreal toads. Ecological Applications, 28, 926–937. [DOI] [PubMed] [Google Scholar]
- Muller, L.K. , Lorch, J.M. , Lindner, D.L. , O’Connor, M. , Gargas, A. & Blehert, D.S. (2013) Bat white‐nose syndrome: a real‐time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Geomyces destructans . Mycologia, 105, 253–259. [DOI] [PubMed] [Google Scholar]
- Palmer, J.M. , Kubatova, A. , Novakova, A. , Minnis, A.M. , Kolarik, M. & Lindner, D.L. (2014) Molecular characterization of a heterothallic mating system in Pseudogymnoascus destructans, the fungus causing white‐nose syndrome of bats. G3: Genes, Genomes, Genetics, 4, 1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parratt, S.R. , Numminen, E. & Laine, A.‐L. (2016) Infectious disease dynamics in heterogeneous landscapes. Annual Review of Ecology Evolution and Systematics, 47, 283–306. [Google Scholar]
- Patton, A.H. , Lawrance, M.F. , Margres, M.J. , Kozakiewicz, C.P. , Hamede, R. , Ruiz‐Aravena, M. et al. (2020) A transmissible cancer shifts from emergence to endemism in Tasmanian devils. Science, 370, eabb9772. [DOI] [PubMed] [Google Scholar]
- Perry, R.W. (2013) A review of factors affecting cave climates for hibernating bats in temperate North America. Environmental Reviews, 21, 28–39. [Google Scholar]
- Price, S.J. , Leung, W.T.M. , Owen, C.J. , Puschendorf, R. , Sergeant, C. , Cunningham, A.A. et al. (2019) Effects of historic and projected climate change on the range and impacts of an emerging wildlife disease. Global Change Biology, 25, 2648–2660. [DOI] [PubMed] [Google Scholar]
- Råberg, L. , Graham, A.L. & Read, A.F. (2009) Decomposing health: tolerance and resistance to parasites in animals. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg, L. , Sim, D. & Read, A.F. (2007) Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science, 318, 812–814. [DOI] [PubMed] [Google Scholar]
- Reeder, D.M. , Frank, C.L. , Turner, G.G. , Meteyer, C.U. , Kurta, A. , Britzke, E.R. et al. (2012) Frequent arousal from hibernation linked to severity of infection and mortality in bats with white‐nose syndrome. PLoS One, 7, e38920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard, J.D. , Fuller, N.W. , Bennett, A.B. , Darling, S.R. , Moore, M.S. , Langwig, K.E. et al. (2014) Interannual survival of Myotis lucifugus (Chiroptera: Vespertilionidae) near the epicenter of white‐nose syndrome. Northeastern Naturalist, 21, N56–N59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, P. , Haman, K.H. , Last, L.A. , Rajkumar, S.S. , Keel, M.K. & Chaturvedi, V. (2012) Clonal spread of Geomyces destructans among bats, Midwestern and Southern United States. Emerging Infectious Diseases, 18, 883–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restif, O. & Koella, J.C. (2004) Concurrent evolution of resistance and tolerance to pathogens. American Naturalist, 164, E90–E102. [DOI] [PubMed] [Google Scholar]
- Roy, B.A. & Kirchner, J.W. (2000) Evolutionary dynamics of pathogen resistance and tolerance. Evolution (N, Y), 54, 51–63. [DOI] [PubMed] [Google Scholar]
- Ryan, C.C. , Burns, L.E. & Broders, H.G. (2019) Changes in underground roosting patterns to optimize energy conservation in hibernating bats. Canadian Journal of Zoology, 97, 1064–1070. [Google Scholar]
- Sadd, B.M. (2011) Food‐environment mediates the outcome of specific interactions between a bumblebee and its trypanosome parasite. Evolution (N, Y), 65, 2995–3001. [DOI] [PubMed] [Google Scholar]
- Samuel, M.D. , Woodworth, B.L. , Atkinson, C.T. , Hart, P.J. & LaPointe, D.A. (2015) Avian malaria in Hawaiian forest birds: infection and population impacts across species and elevations. Ecosphere, 6, 1–21. [Google Scholar]
- Scheele, B.C. , Hunter, D.A. , Skerratt, L.F. , Brannelly, L.A. & Driscoll, D.A. (2015) Low impact of chytridiomycosis on frog recruitment enables persistence in refuges despite high adult mortality. Biological Conservation, 182, 36–43. [Google Scholar]
- Scheele, B.C. , Pasmans, F. , Skerratt, L.F. , Berger, L. , Martel, A.N. , Beukema, W. et al. (2019) Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science, 363, 1459–1463. [DOI] [PubMed] [Google Scholar]
- Scheele, B.C. , Skerratt, L.F. , Grogan, L.F. , Hunter, D.A. , Clemann, N. , McFadden, M. et al. (2017) After the epidemic: ongoing declines, stabilizations and recoveries in amphibians afflicted by chytridiomycosis. Biological Conservation, 206, 37–46. [Google Scholar]
- Schelkle, B. , Mohammed, R.S. , Coogan, M.P. , McMullan, M. , Gillingham, E.L. , van Oosterhout, C. et al. (2012) Parasites pitched against nature: Pitch Lake water protects guppies (Poecilia reticulata) from microbial and gyrodactylid infections. Parasitology, 139, 1772–1779. [DOI] [PubMed] [Google Scholar]
- Spitzen‐Van Der Sluijs, A. , Canessa, S. , Martel, A. & Pasmans, F. (2017) Fragile coexistence of a global chytrid pathogen with amphibian populations is mediated by environment and demography. Proceedings of the Royal Society B‐Biological Sciences, 284, 20171444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, Y.P. (2009) Do extreme environments provide a refuge from pathogens? A phylogenetic test using serpentine flax. American Journal of Botany, 96, 2010–2021. [DOI] [PubMed] [Google Scholar]
- Talbot, B. , Vonhof, M.J. , Broders, H.G. , Fenton, B. & Keyghobadi, N. (2016) Range‐wide genetic structure and demographic history in the bat ectoparasite Cimex adjunctus . BMC Evolutionary Biology, 16, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot, B. , Vonhof, M.J. , Broders, H.G. , Fenton, M.B. & Keyghobadi, N. (2017) Population structure in two geographically sympatric and congeneric ectoparasites (Cimex adjunctus and Cimex lectularius) in the North American great lakes region. Canadian Journal of Zoology, 95, 901–907. [Google Scholar]
- Thomas, D.W. & Cloutier, D. (1992) Evaporative water loss by hibernating little brown bats, Myotis lucifugus. Physiological Zoology, 65, 443–456. [Google Scholar]
- Tobler, M. , Schlupp, I. , García De León, F.J. , Glaubrecht, M. & Plath, M. (2007) Extreme habitats as refuge from parasite infections? Evidence from an extremophile fish. Acta Oecologica, 31, 270–275. [Google Scholar]
- Trivedi, J. , Lachapelle, J. , Vanderwolf, K.J. , Misra, V. , Willis, C.K.R. , Ratcliffe, J.M. et al. (2017) Fungus causing white‐nose syndrome in bats accumulates genetic variability in North America with no sign of recombination, mSphere, 2, e00271–e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, G.G. , Meteyer, C.U. , Barton, H. , Gumbs, J.F. , Reeder, D.M. , Overton, B. et al. (2014) Nonlethal screening of bat‐wing skin with the use of ultraviolet fluorescence to detect lesions indicative of white‐nose syndrome. Journal of Wildlife Diseases, 50, 566–573. [DOI] [PubMed] [Google Scholar]
- Valerio Garcia, M. , Carlos Monteiro, A. , Szabo, J.P.M. , Prette, N. & Henrique Bechara et al. (2005) Mechanism of infection and colonization of Rhipicephalus sanguineus eggs by Mertarhizium anisopliae as revealed by scanning electron microscopy and histopathology. Brazilian Journal of Microbiology, 36, 368–372. [Google Scholar]
- van Riper, C. , van Riper, S.G. , Goff, M.L. & Laird, M. (1986) The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecological Monographs, 56, 327–344. [Google Scholar]
- Verant, M.L. , Boyles, J.G. , Waldrep, W. Jr , Wibbelt, G. & Blehert, D.S. (2012) Temperature‐dependent growth of Geomyces destructans, the fungus that causes bat white‐nose syndrome. PLoS One, 7, e46280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verant, M.L. , Meteyer, C.U. , Speakman, J.R. , Cryan, P.M. , Lorch, J.M. & Blehert, D.S. (2014) White‐nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiology, 14, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyles, J. , Woodhams, D.C. , Saenz, V. , Byrne, A.Q. , Perez, R. , Rios‐Sotelo, G. et al. (2018) Shifts in disease dynamics in a tropical amphibian assemblage are not due to pathogen attenuation. Science, 359, 1517–1519. [DOI] [PubMed] [Google Scholar]
- Warnecke, L. , Turner, J.M. , Bollinger, T.K. , Lorch, J.M. , Misra, V. , Cryan, P.M. et al. (2012) Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white‐nose syndrome. Proceedings of the National Academy of Sciences, 109, 6999–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke, L. , Turner, J.M. , Bollinger, T.K. , Misra, V. , Cryan, P.M. , Blehert, D.S. et al. (2013) Pathophysiology of white‐nose syndrome in bats: a mechanistic model linking wing damage to mortality. Biology Letters, 9, 20130177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber, M.Q. , Carter, E.D. , Gray, M.J. & Briggs, C.J. (2021) Putative resistance and tolerance mechanisms have little impact on disease progression for an emerging salamander pathogen. Functional Ecology, 35, 847–859. [Google Scholar]
- Wilber, M.Q. , Knapp, R.A. , Toothman, M. & Briggs, C.J. (2017) Resistance, tolerance and environmental transmission dynamics determine host extinction risk in a load‐dependent amphibian disease. Ecology Letters, 20, 1169–1181. [DOI] [PubMed] [Google Scholar]
- Wilber, M.Q. , Langwig, K.E. , Kilpatrick, A.M. , Mccallum, H.I. & Briggs, C.J. (2016) Integral Projection Models for host‐parasite systems with an application to amphibian chytrid fungus. Methods in Ecology and Evolution, 7, 1182–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild, G. , Gardner, A. & West, S.A. (2009) Adaptation and the evolution of parasite virulence in a connected world. Nature, 459, 983–986. [DOI] [PubMed] [Google Scholar]
- Wilder, A.P. , Kunz, T.H. & Sorenson, M.D. (2015) Population genetic structure of a common host predicts the spread of white‐nose syndrome, an emerging infectious disease in bats. Molecular Ecology, 24, 5495–5506. [DOI] [PubMed] [Google Scholar]
- Willis, C.K.R. , Menzies, A.K. , Boyles, J.G. & Wojciechowski, M.S. (2011) Evaporative water loss is a plausible explanation for mortality of bats from white‐nose syndrome. Integrative and Comparative Biology, 51, 364–373. [DOI] [PubMed] [Google Scholar]
- Wolinska, J. & King, K.C. (2009) Environment can alter selection in host–parasite interactions. Trends in Parasitology, 25, 236–244. [DOI] [PubMed] [Google Scholar]
- Woodworth, B.L. , Atkinson, C.T. , LaPointe, D.A. , Hart, P.J. , Spiegel, C.S. , Tweed, E.J. et al. (2005) Host population persistence in the face of introduced vector‐borne diseases: Hawaii amakihi and avian malaria. Proceedings of the National Academy of Sciences, 102, 1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S. (1969). Evolution and the genetics of populations, volume 2: the theory of gene frequencies. University of Chicago Press, Chicago. [Google Scholar]
- Zukal, J. , Bandouchova, H. , Brichta, J. , Cmokova, A. , Jaron, K.S. , Kolarik, M. et al. (2016) White‐nose syndrome without borders: Pseudogymnoascus destructans infection tolerated in Europe and Palearctic Asia but not in North America. Scientific Reports, 6, 19829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbado‐Ulate, H. , Bolaños, F. , Gutiérrez‐Espeleta, G. & Puschendorf, R. (2014) Extremely low prevalence of Batrachochytrium dendrobatidis in frog populations from neotropical dry forest of Costa Rica supports the existence of a climatic refuge from disease. EcoHealth, 11, 593–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data and code used to produce the results and figures of this manuscript are publicly available on Dryad under DOI: https://doi.org/10.5061/dryad.j9kd51cdw


