Abstract
Background and Aims
Drug checking services provide people who use drugs with chemical analysis results of their drug samples while simultaneously monitoring the unregulated drug market. We sought to identify and synthesize literature on the following domains: (a) the influence of drug checking services on the behaviour of people who use drugs; (b) monitoring of drug markets by drug checking services; and (c) outcomes related to models of drug checking services.
Methods
Systematic review. A systematic literature search was conducted in MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, PsycINFO, Scopus, Web of Science and Dissertations and Theses Global. Eligible studies were peer‐reviewed articles and conference abstracts or grey literature, published in any language since 1990 and including original data on the domains. We assessed risk of bias for quantitative peer‐reviewed articles reporting on behaviour or models of drug checking services using National Institutes of Health tools.
Results
We screened 2463 titles and abstracts and 156 full texts, with 90 studies meeting inclusion criteria. Most (n = 65, 72.2%) were from Europe and used cross‐sectional designs (n = 79, 87.7%). Monitoring of drug markets by drug checking services (n = 63, 70%) was the most reported domain, followed by the influence of drug checking services on behaviour (n = 31, 34.4%), including intent to use, actual use and disposal of the drug, and outcomes related to models of drug checking services (n = 17, 18.9%). The most common outcome measures were detection of unexpected substances (n = 50, 55.6%), expected substances (n = 44, 48.9%), new psychoactive substances (n = 40, 44.4%) and drugs of concern (n = 32, 36.5%) by drug checking services.
Conclusions
Drug checking services appear to influence behavioural intentions and the behaviour of people who use drugs, particularly when results from drug checking services are unexpected or drugs of concern. Monitoring of drug markets by drug checking services is well established in Europe, and increasingly in North America. Concerns about drug contents and negative health consequences facilitate the use of drug checking services; lack of concern; trust in drug sellers; lack of accessibility of drug checking services; and legal and privacy concerns are barriers to use.
Keywords: Drug checking, drug safety testing, drug testing, harm reduction, pill testing, street drug analysis
INTRODUCTION
A public health intervention operating for more than 50 years, drug checking services (DCS) allow the public to submit drug samples from unregulated drug markets (i.e. illegal and legal drugs sold through criminal channels) for chemical analysis. DCS emerged across the United States in the late 1960s and early 1970s during the rise of a psychedelic counterculture that championed the use of psychoactive substances to expand consciousness [1, 2]. DCS were later expanded in European settings throughout the 1990s, beginning in the Netherlands, primarily in response to the popularity of dance events and associated use of 3,4‐methylenedioxymethamphetamine (MDMA) and other drugs [3, 4]. More recently, DCS have been implemented in Australasia, the Americas and the United Kingdom, often with an emphasis on preventing harms from new psychoactive substances (NPS), including synthetic opioids. A global review of DCS conducted in 2017 identified 31 services operating across 20 countries [5]. Notably, the contamination of unregulated drug markets with fentanyl and the resulting opioid overdose crisis has motivated the recent expansion of DCS in Canada [6] and the United States [7].
DCS provide people who use drugs (PWUD) with information on the chemical composition of their drug samples to facilitate more informed decision‐making [8]. While some analysis methods can be operated by PWUD, DCS typically offer tailored harm reduction advice with the provision of analysis results to PWUD [9]. By aggregating data on the composition of drug samples, DCS provide insight into trends in the unregulated drug supply and inform policymaking and harm reduction activities at the population level [10]. DCS can inform public health alerts [11] when drugs of concern are detected, thus offering potential benefits to the broader community of PWUD and service providers [12]. DCS differ globally in terms of their legality and degree of government support, as well as where and how samples are collected and analysed. Models include mobile services at events, fixed services where samples can be dropped off or mailed and the distribution of analysis methods for personal use, all of which employ a variety of technologies with differing benefits and drawbacks [8, 13, 14].
Given the growing availability of DCS and interest in their impacts, we conducted a systematic review to investigate what is known from the existing literature about the influence of DCS on PWUD. The aims of this study were to identify and synthesize evidence across three domains: (a) influence of DCS on behaviour of PWUD, (b) monitoring of drug markets by DCS and (c) outcomes related to models of DCS.
METHODS
The reporting of this systematic review was guided by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) [15]. The protocol was registered in PROSPERO (CRD42018105366) [16].
Search strategy and selection criteria
We developed, piloted and refined the search strategy in consultation with a library sciences expert. The search strategy was peer‐reviewed by a librarian outside the review team using the guideline for Peer Review of Electronic Search Strategies (PRESS) [17] and revised accordingly. We searched MEDLINE (OVID), Embase (OVID), Cochrane Central Register of Controlled Trials (OVID), Cochrane Database of Systematic Reviews (OVID), PsycINFO (OVID), Scopus, Web of Science (including Science Citation Index, Social Sciences Citation Index, Conference Proceedings Citation Index–Science, Conference Proceedings Citation Index–Social Science and Humanities, Emerging Sources Citation Index) and Dissertations and Theses Global (ProQuest) for peer‐reviewed journal articles and conference abstracts published in any language from 1 January 1990 (the lower bound for when DCS proliferated) to 26 July 2018, with a full search update on 16 October 2019. We used medical subject headings (MeSH) and keywords, adapted for each database, related to DCS (including service names from a global review of DCS [5]), controlled drugs and harm reduction services. To capture all domains, search terms specific to outcomes were not employed. The results were de‐duplicated in EndNote. The full search strategies for each database, exactly as run, appear in Supporting information, Appendix A. We also searched reference lists of included studies. Grey literature reporting on the influence of DCS on behaviour of PWUD was included and identified using Google, Google Scholar and websites for DCS [5], as well as through contact with content experts. Search terms for Google and Google Scholar were ‘pill‐testing’ OR ‘pill testing’ OR ‘drug‐checking’ OR ‘drug checking’.
Eligibility criteria applied in the screening process were defined by population, intervention and evaluation (PIE). Studies were included if the population of interest comprised people of any age who engage in non‐medical use of drugs and voluntarily access DCS. Studies that involved the implementation of DCS were included with no restrictions on analysis methods. Studies were excluded if the intervention involved analysing human biological specimens, analysis results were not offered to clients, clients were not accessing DCS but independently accessing analysis methods or DCS were not implemented (e.g. feasibility study). Studies were included if they evaluated the (a) influence of DCS on behaviour of PWUD (broadly defined to include behavioural intentions and enacted behaviour), (b) monitoring of drug markets by DCS and (c) outcomes related to models of DCS (including barriers and facilitators to use). Eligibility was restricted to studies reporting original quantitative or qualitative data. As such, commentaries, letters to the editor, editorials, reviews and unpublished conference abstracts were excluded. Titles, abstracts and full texts were translated as needed.
Screening, data extraction and quality assessment
Screening and data extraction were conducted in DistillerSR (Evidence Partners, Ottawa, ON, Canada) using standardized, pilot‐tested charting forms. The screening form contained questions based on the eligibility criteria and was used to assess the relevance of titles and abstracts, as well as full texts. Two independent reviewers (N.M. and J.T./I.R.) began by screening titles and abstracts. Any deemed relevant by at least one reviewer advanced to the next stage in which both reviewers (N.M. and J.T./I.R./K.S.) independently screened full texts in duplicate for inclusion. While the effects of DCS on the monitoring of drug markets were included as an outcome in our protocol, this was clarified and broadened during screening to capture outcomes related to the monitoring of drug markets by DCS. Data extraction was conducted in duplicate by both reviewers (N.M. and J.T./K.S.). The data extraction form included intervention details (model of DCS, population), study characteristics (year, location, design), sample characteristics (type, size, age, sex) and findings for domains.
Risk of bias assessment was performed in duplicate for peer‐reviewed articles reporting quantitative data on behaviour and/or models of DCS. Epidemiological assessment of risk of bias was deemed inappropriate for drug market monitoring studies and other designs (e.g. qualitative research) [18]. Tools from the National Institutes of Health were used to assess risk of bias for individual studies (see Supporting information, Appendix B). The quality assessment tools for observational cohort and cross‐sectional studies [19] or for before–after (pre–post) studies with no control group [20] were used, as appropriate to study design. Studies were assigned one point for each criterion they satisfied and could receive up to 14 or 12 points for cross‐sectional and before–after studies, respectively, with higher scores indicating less susceptibility to bias.
Conflicts were resolved by consensus between reviewers, with input from the last author as required. The last author triple‐checked data extraction and quality assessment for 10% of studies.
Data analysis
We used narrative synthesis without meta‐analysis [21] for data analysis. Outcome measures were not pre‐specified, but inductively coded in an iterative process throughout data extraction to ensure that all relevant outcomes were captured. Within each domain, study outcomes were compared with outcome measures identified in earlier studies. New outcome measures were added when findings concerned a distinct construct. Specific data from studies were coded within the relevant outcome measure and summarized, such as by converting data points into ranges or condensing participant responses into a short sentence. The addition of new outcome measures decreased as data extraction proceeded and outcomes reached saturation. Analysis following data extraction led to the re‐organization and addition of some outcome measures (e.g. separating intentions in response to actual or hypothetical analysis results from DCS, drugs of concern from other drugs detected). We derived and prioritized domains based on consultation with content experts and knowledge users through the Canadian Research Initiative in Substance Misuse (CRISM). We organized the narrative synthesis by outcome measures within each domain. For individual outcome measures, we summarized the range of findings and highlighted differences across settings and populations.
RESULTS
Study characteristics
As shown in the PRISMA flow diagram (Figure 1), of 2463 titles and abstracts and 156 full texts assessed for relevance, 90 studies met the inclusion criteria (Supporting information, Appendix C). Primary reasons for exclusion at the full text screening stage were that the study did not report original research (n = 30), did not evaluate DCS as defined for this review (n = 19) or did not report on pre‐specified domains (n = 17). Details of included studies are presented by domain in Supporting information, Tables S1–S3. Included studies were published between 1997‐2019; 46.7% (n = 42) were published in 2017‐19, 24.4% (n = 22) in 2014‐16, and 28.9% (n = 26) before 2014. Table 1 provides an overview of the most recent reported models of DCS, including analysis methods and populations.
FIGURE 1.
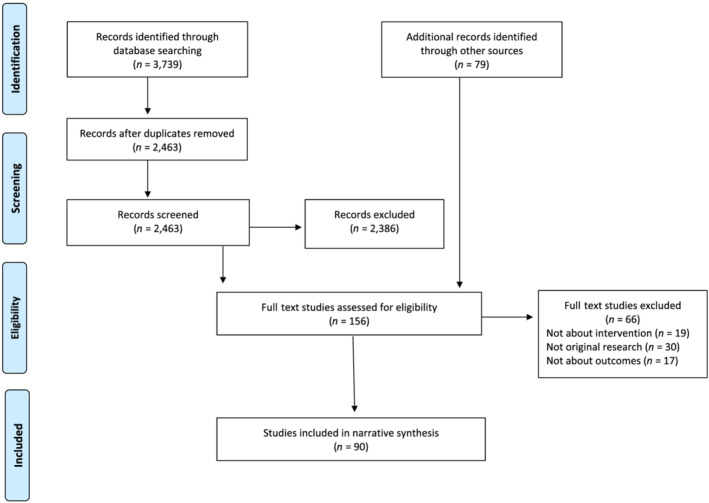
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram
TABLE 1.
Models described for DCS in included studies
| Country | Provider | Collection Model | Analysis Model | Analysis Methods | Population |
|---|---|---|---|---|---|
| Australia | ACT GTM Pill Testing Pilot | Mobile | On‐site | FTIR | Partygoers |
| Australia | Enlighten | Mobile | Off‐site; on‐site | GC–MS; reagents | Partygoers |
| Austria | Checkit! | Mobile | Off‐site; on‐site | GC–MS; HP‐LC; LC‐DAD; UV spectroscopy | Partygoers |
| Belgium | Modus Fiesta | Fixed; mobile | Off‐site; on‐site | GC–MS; reagents; TLC | Partygoers |
| Canada | ANKORS (AIDS Network Outreach and Support Society) | Mobile | On‐site | Fentanyl test strips; GC–MS; Raman spectroscopy; reagents | Partygoers |
| Canada | BCCSU (British Columbia Centre on Substance Use) | Fixed; mobile | On‐site | Fentanyl test strips; FTIR | Structurally vulnerable people who use/inject drugs; partygoers |
| France | SINTES (National Identification System for Drugs and Other Substances) | Mobile | Off‐site | GC–MS | PWUD |
| Germany | Hanover Drobs | Fixed; mobile | On‐site | Reagents | Partygoers |
| Italy | BAONPS (Be Aware On Night Pleasure Safety) | Mobile | Off‐site; on‐site | GC–MS; LC–MS; Raman spectroscopy | Partygoers |
| Netherlands | DIMS (Drug Information and Monitoring System) | Fixed | Off‐site; on‐site | GC–MS; GC‐NPD; LC‐DAD; NMR spectroscopy; reagents; TLC | PWUD recreationally |
| Portugal | Check!n | Mobile | On‐site | Reagents; TLC | Partygoers |
| Portugal | Kosmicare Association | Mobile | On‐site | Reagents; TLC | Partygoers |
| Slovenia | DrogArt | Fixed | Off‐site | Unspecified | ‘High‐risk’ PWUD; partygoers |
| Spain | Ailaket | Mobile | Unspecified | TLC | PWUD recreationally |
| Spain | Energy control | Fixed; mobile; postal | Off‐site | GC–MS; LC–MS; TLC; UV spectroscopy | PWUD recreationally |
| Switzerland | Streetwork | Fixed; mobile | On‐site | GC–MS; HP‐LC; LC‐DAD; UV spectroscopy | Partygoers |
| United Kingdom | The Loop | Mobile | On‐site | FTIR; mass loss analysis; reagents | Partygoers |
| United States | DanceSafe | Mobile | On‐site | Reagents | Partygoers |
| United States | EcstasyData | Postal | Off‐site | GC–MS; reagents | PWUD; drug sellers; club owners; parents |
| United States | Organizations in Rhode Island | Personal use | Off‐site | Fentanyl test strips | Young PWUD |
| United States | SAC (Syringe Access Collaborative) | Fixed; personal use | On‐site; off‐site | Fentanyl test strips | PWUD |
| United States | Syringe services programme in Boston | Personal use | Off‐site | Fentanyl test strips | Street‐based PWUD |
| United States | Syringe services programmes in New York City | Personal use | Off‐site | Fentanyl test strips | People physically dependent on opioids |
| United States | Urban Survivors Union | Personal use | Off‐site | Fentanyl test strips | People who inject drugs |
DCS = drug checking services; FTIR = Fourier transform infrared spectroscopy; GC–MS = gas chromatography–mass spectrometry;
GC‐NPD = gas chromatography nitrogen phosphorous detection; HP‐LC = high‐performance liquid chromatography;
LC–MS = liquid chromatography–mass spectrometry; LC‐DAD = liquid chromatography with diode array detection;
NMR = nuclear magnetic resonance; PWUD = people who use drugs;
TLC = thin layer chromatography; UV = ultraviolet visible.
Characteristics of included studies are described in Table 2, both overall and stratified by peer‐reviewed articles (n = 54, 60%), peer‐reviewed conference abstracts (n = 19, 21.1%) and grey literature (n = 17, 18.9%). Most studies (n = 65, 72.2%) were from Europe (Austria, Belgium, France, Germany, Italy, the Netherlands, Portugal, Slovenia, Spain, Switzerland, the United Kingdom). Other countries represented included Australia, Canada, Colombia, Mexico, New Zealand and the United States. Most studies used cross‐sectional (n = 49, 54.4%) or repeated cross‐sectional (n = 30, 33.3%) designs. Monitoring of drug markets by DCS (n = 63, 70%) was the most common domain, followed by influence of DCS on behaviour (n = 31, 34.4%) and outcomes related to models of DCS (n = 17, 18.9%).
TABLE 2.
Characteristics of included studies
| Characteristic | Total N (%) (n = 90) | Articles N (%) (n = 54) | Conference abstracts N (%) (n = 19) | Grey literature N (%) (n = 17) |
|---|---|---|---|---|
| Country | ||||
| Australia | 2 (2.2) | 1 (1.9) | 0 (0) | 1 (5.9) |
| Austria | 1 (1.1) | 0 (0) | 0 (0) | 1 (5.9) |
| Belgium | 2 (2.2) | 0 (0) | 0 (0) | 2 (11.8) |
| Canada | 9 (10) | 7 (13) | 0 (0) | 2 (11.8) |
| Colombia | 1 (1.1) | 0 (0) | 0 (0) | 1 (5.9) |
| France | 2 (2.2) | 2 (3.7) | 0 (0) | 0 (0) |
| Italy | 2 (2.2) | 1 (1.9) | 0 (0) | 1 (5.9) |
| Netherlands | 19 (21.1) | 16 (29.6) | 0 (0) | 3 (17.6) |
| New Zealand | 3 (3.3) | 0 (0) | 0 (0) | 3 (17.6) |
| Portugal | 3 (3.3) | 3 (5.5) | 0 (0) | 0 (0) |
| Slovenia | 1 (1.1) | 1 (1.9) | 0 (0) | 0 (0) |
| Spain | 30 (33.3) | 12 (22.2) | 18 (94.7) | 0 (0) |
| Switzerland | 1 (1.1) | 1 (1.9) | 0 (0) | 0 (0) |
| United Kingdom | 2 (2.2) | 1 (1.9) | 0 (0) | 1 (5.9) |
| United States | 9 (10) | 7 (13) | 1 (5.3) | 1 (5.9) |
| Multi‐country a | 3 (3.3) | 2 (3.7) | 0 (0) | 1 (5.9) |
| Domains b | ||||
| (a) Influence of DCS on behaviour of PWUD | 31 (34.4) | 14 (25.9) | 0 (0) | 17 (100) |
| (b) Monitoring of drug markets by DCS | 63 (70) | 44 (81.5) | 19 (100) | 0 (0) |
| (c) Outcomes related to models of DCS | 17 (18.9) | 17 (31.5) | 0 (0) | 0 (0) |
| Study design | ||||
| Cross‐sectional | 49 (54.4) | 24 (44.4) | 11 (57.9) | 14 (82.4) |
| Repeated cross‐sectional | 30 (33.3) | 21 (38.9) | 8 (42.1) | 1 (5.9) |
| Longitudinal | 1 (1.1) | 1 (1.9) | 0 (0) | 0 (0) |
| Time‐series | 2 (2.2) | 2 (3.7) | 0 (0) | 0 (0) |
| Qualitative | 7 (7.8) | 5 (9.3) | 0 (0) | 2 (11.8) |
| Case report | 1 (1.1) | 1 (1.9) | 0 (0) | 0 (0) |
One North American article including Canada, Mexico and the United States, two multi‐country European studies (one article including Austria, Belgium, Netherlands, Portugal, Spain and Switzerland, and one book, including Austria, Netherlands and Germany).
Combined total exceeds number of studies because some evaluated more than one domain.
DCS = drug checking services; PWUD = people who use drugs.
Outcome measures
Among 90 studies, we categorized 55 outcome measures (Figure 2). Outcome measures in each domain are presented in the following section. Findings related to the influence of DCS on behaviour, and common outcome measures related to drug market monitoring and models of DCS, are prioritized. Results of individual studies are in Supporting information, Tables S1–S3.
FIGURE 2.
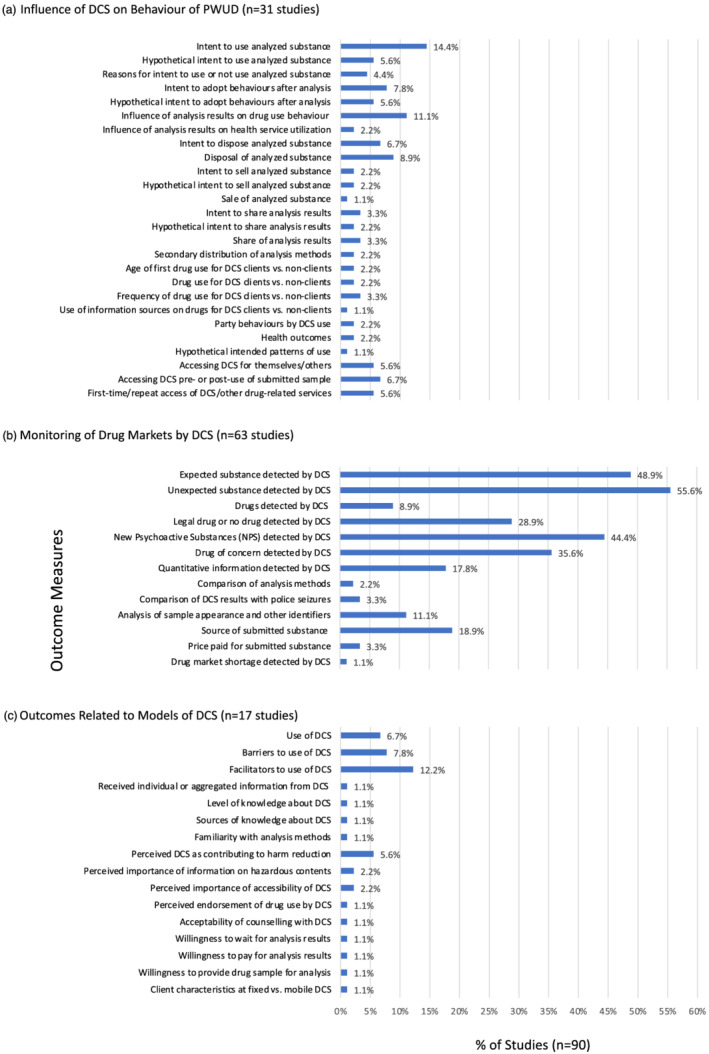
Outcome measures examined by included studies (n = 90). DCS = drug checking services; PWUD = people who use drugs
Influence of DCS on behaviour of PWUD
The most common outcome measures related to the influence of DCS on behaviour were intent to use the analysed substance (14.4% of studies, n = 13), the influence of analysis results on drug use behaviour (11.1%, n = 10) and disposal of the analysed substance (8.9%, n = 8). Enacted behaviours as observed or per self‐reported historical recall were measured in 16 studies (17.8%) [22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37]. Intended behaviours in response to actual or hypothetical analysis results from DCS were assessed in 22 studies (24.4%) [31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52].
Studies found that DCS influenced intended behaviour and, although less researched, enacted behaviour. Among studies of PWUD in party settings (referred to as ‘partygoers’ in studies), greater intention to not use the analysed substance was consistently reported if analysis results were unexpected [33, 35, 40, 42, 43, 45, 48, 52] or ‘questionable’/‘suspicious’ [49, 50, 51]. For example, a cross‐sectional study from Australia (n = 83) in 2018 found partygoers were more likely to change their intention to use when analysis results were unexpected [odds ratio (OR) = 2.63, 95% confidence interval (CI) = 0.85–8.16] [35], as did two cross‐sectional studies from Portugal (n = 310, n = 100) in 2016 and 2014 [40, 43]. Similarly, other intended behaviour changes—such as using less of a substance or seeking more information about it—were more common among partygoers when analysis results from DCS suggested that substances were ‘questionable’/‘suspicious’ [49, 51].
The proportion of participants reporting analysis results from DCS influenced their drug use varied by population and setting. Among partygoers, 16% of participants in the Netherlands in 1996 [29], 50% in Austria in 1997–99 [37] and 87% in New Zealand (n = 47) in 2018–19 [33] reported that analysis results impacted their drug use. A cross‐sectional study in 2017 from the United States among people who inject drugs (n = 125) found 43% changed their behaviour, and this was more likely when fentanyl was detected [adjusted OR (aOR) = 5.08, 95% CI = 2.12–12.17] [22]. Qualitative and longitudinal studies of young PWUD (n = 81) in the United States in 2017 supported this finding, and found that fentanyl detection was associated with positive changes in overdose risk behaviours (i.e. using less, using with others, doing a test shot) [31, 34]. Overall, and in alignment with findings on intended drug use behaviour in response to ‘questionable’/‘suspicious’ analysis results, self‐reported behaviour was more likely to change when analysis results detected fentanyl. Beyond individual analysis results, a repeated cross‐sectional study from Colombia (n = 1533) in 2013 and 2016 examined the influence of alerts from DCS and found that a majority of partygoers reported an impact on their behaviour [36].
Only one study linked intended behaviours to observed health outcomes for PWUD accessing DCS. A Canadian cross‐sectional study of DCS at a supervised injection site (n = 1411) in 2016–17 found that people who inject drugs were more likely to report the intention to use a smaller quantity than usual when fentanyl was detected by DCS (OR = 9.36, 95% CI = 4.25–20.65) [41]. In turn, those intending to use a smaller quantity were found to be less likely to overdose (OR = 0.41, 95% CI = 0.18–0.89) and be administered naloxone (OR = 0.38, 95% CI = 0.15–0.96).
Disposal of the analysed substance was observed [24, 26, 27, 32, 35] or self‐reported [22, 31, 34] as an outcome of DCS in eight studies. Like other behaviours, disposal was more frequent when analysis results from DCS were unexpected [24, 27, 32, 52]. Among partygoers in a cross‐sectional study (n = 2078) in 2015 and case report (n = 2786) in 2014 from Canada, observed disposal ranged from 4% [27] to 7% [26]. A longitudinal study from the United States (n = 81) in 2017 reported 10% disposal among young PWUD when fentanyl was detected [34], while a cross‐sectional study from the United States (n = 125) in 2017 found 0% disposal among people who inject drugs [22]. Qualitative research from Canada (n = 20) in 2017–18 found that intention to dispose varied by drug preference among structurally vulnerable (i.e. marginalized as a result of their positions in social hierarchies) PWUD, with those using opioids being less likely than those using stimulants to dispose if fentanyl was detected [38].
While three European studies and one Canadian study reported intent to sell the analysed substance among partygoers, only one longitudinal study from the United States (n = 81) in 2017 assessed this self‐reported behaviour and found 10% of young PWUD sold the substance after fentanyl was detected [34].
With one exception [49], among the five studies assessing intent to share analysis results from DCS with others the majority of partygoers reported an intention to do so. Two studies reporting on intent to share analysis results in a hypothetical situation found that information‐sharing with friends and drug sellers was more common when results were ‘questionable’/‘suspicious’ [49, 51]. Beyond sharing information, qualitative and longitudinal studies of young PWUD (n = 81) in the United States in 2017 found 58% reported distributing fentanyl test strips received from DCS to others, particularly those perceived to have a higher risk of using drugs containing fentanyl [31, 34].
While less directly relevant to the influence of DCS on behaviour, drug‐using patterns of partygoers who did and did not use DCS were compared in three European book‐length cross‐sectional studies undertaken in Belgium (n = 486) in 2006, the Netherlands (n = 285) in 2002 and a multi‐country study from Austria, the Netherlands and Germany (n = 702) in 2002 [49, 50, 51]. In all three studies, DCS clients reported higher frequencies of ecstasy use than non‐clients. Furthermore, the multi‐country study found the frequency of accessing DCS was negatively correlated with the frequency of ecstasy use and unsafe party behaviour [51]. Patterns of accessing DCS were examined in 14 studies [22, 24, 25, 28, 32, 33, 36, 39, 40, 41, 42, 47, 48, 51]. With one exception [24], among reporting studies the majority of partygoers accessed DCS for both themselves and other people [40, 47]. Among partygoers a cross‐sectional study from Portugal (n = 310) in 2016 reported that 77% accessed DCS pre‐use [40], whereas a Colombian repeated cross‐sectional study (n = 831) in 2016 found that 49% waited for their analysis results before using [36]. Studies reporting on this outcome measure for people who inject drugs were mixed, with two cross‐sectional studies from the United States (n = 125, n = 242) in 2017–18 finding that the majority accessed DCS pre‐use [22, 25] and one Canadian cross‐sectional study (n = 1411) in 2016–17 reporting primarily post‐use access [41]. In terms of receiving drug‐related services (e.g. harm reduction, drug treatment) prior to accessing DCS, 70% or more of partygoers had not previously accessed drug‐related services in four reporting studies [32, 33, 42, 51]. In contrast, only one cross‐sectional study from the United States among people who inject drugs (n = 125) in 2017 examined this pattern of accessing DCS, and found that a lesser majority of 54% were not existing clients of a syringe service programme [22].
Among studies reporting on behaviour, five referenced theories, models or frameworks, including theories of reasoned action or planned behaviour [40, 44], the information–motivation–behavioral skills model [31, 34] and risk environment frameworks [38, 40].
Drug market monitoring
The most common outcome measures overall related to drug market monitoring and included: detection of unexpected substances (55.6% of studies, n = 50 studies), expected substances (48.9%, n = 44), NPS (44.4%, n = 40), drugs of concern (36.5%, n = 32) and legal or no drugs (28.9%, n = 26) by DCS, and source of submitted substance (18.9%, n = 17).
Outcome measures coded concordance as detection of the expected substance (i.e. expected drug only, expected drug with other unexpected drug) or of an unexpected substance (i.e. unexpected drug only) by DCS. These concordance measures were reported in 61.1% of studies (n = 55). Seven studies assessed concordance among multiple drug classes, including psychedelics, stimulants and depressants [40, 53, 54, 55, 56, 57, 58]; 11 studies focused exclusively on MDMA [44, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68]. One study compared concordance between drugs purchased on‐ and off‐line [69] and another compared concordance between controlled drugs and NPS [70].
NPS refer to ‘substances of abuse’ that are not internationally controlled (e.g. synthetic cathinones, cannabinoids, opioids) [71]. Four studies examining NPS were technical papers characterizing the chemistry of novel compounds [72, 73, 74, 75]. Only a single study outside Europe reported on the detection of NPS [53]. European studies reported increasing detection of NPS by DCS over time [55, 70, 76, 77, 78, 79, 80, 81, 82, 83, 84], with NPS presenting as expected [55, 70, 78] and unexpected drugs [55, 79, 80, 82, 84]. A proliferation of different types of NPS, including cathinones and phenethylamines, was reported over time [55, 79, 81, 85].
Drugs of concern included fentanyl and analogues, as well as other drugs identified as causing health harms by included studies. Nine studies reported the detection of fentanyl or analogues by DCS in Canada [24, 41, 53, 54], Slovenia [86] and Spain [56, 76, 87] from 2016 to 2019 and in the Netherlands in 2011 [58]. Six of the studies reported that fentanyl or analogues were detected in samples expected to be other drugs [24, 41, 54, 58, 76, 87] and none reported fentanyl or analogues as expected. Other drugs of concern reported in five or more studies included atropine, DOx, levamisole and para‐methoxy(meth)amphetamine (P(M)MA); these were detected in Europe [43, 55, 56, 57, 58, 61, 63, 66, 67, 68, 88, 89], the United States [44, 65] and Canada [53]. Notably, some drugs of concern overlapped with NPS or legal drugs. Legal drugs (not including NPS) reported in five or more studies were caffeine, ephedrine, levamisole, lidocaine, phenacetin and procaine.
The source of substances submitted to DCS was an outcome in 17 studies, and was reported by individuals accessing DCS or based on where the sample was collected/mailed. Studies specified a location (e.g. region, country, city) [44, 56, 60, 65, 70, 72, 76, 81, 90, 91], on‐line (e.g. webshops, cryptomarkets) [69, 75, 81, 87, 91, 92, 93] or on‐site/off‐site music festival grounds where DCS were accessed [26, 32].
Outcomes related to models of DCS
Facilitators (12.2% of studies, n = 11) and barriers (7.8%, n = 7) to use of DCS were the most common outcome measures related to models of DCS. Beyond the most common, this domain also included outcome measures on use, knowledge, perceptions and preferences related to models of DCS.
Facilitators included motivations for use. Concerns about drug contents and negative health consequences from consumption were a primary facilitator to use of DCS. In the music festival setting, a cross‐sectional study from the United Kingdom (n = 230) in 2016 found the most common motivator for accessing DCS was concern about the sample, including having already experienced negative effects [32]. A time‐series study conducted in the Netherlands (n = 22 280) in 2004–10 found people who use ecstasy more commonly reported health concerns as their rationale for accessing DCS after a drug market shortage of MDMA‐like substances [94]. Drug market changes and resulting concerns about drug contents and health consequences are also highly relevant to other contexts as a facilitator for accessing DCS. Qualitative research in the United States in 2017 reported concern about fentanyl in the unregulated drug market and associated risk of overdose as a facilitator for use of DCS among young PWUD (n = 81) [31], as well as people who use opioids (n = 55) [23]. Related facilitators are distrust in drug markets [31, 86] and sellers [95]. Unique facilitators among structurally vulnerable PWUD included allowing the provision of analysis results post‐use and returning the sample after analysis [38]. Select other facilitators to use of DCS were central location [34, 38] as well as accuracy and comprehensiveness of results [38, 54].
Barriers to using DCS were often linked to described facilitators and included lack of concern over drug contents [38, 53], high trust in drug sellers [95], inaccessible location [38, 86] and limitations of results [23, 38]. Another barrier was legal risks due to drug criminalization [31], which was linked to anonymity concerns [86]. Legal risks and privacy concerns were also perceived barriers for drug sellers to access DCS [96].
Study quality
Assessment of study quality was conducted for 13 articles meeting a priori criteria (Supporting information, Tables S1 and S3). Among 11 cross‐sectional studies, scores ranged from 3 to 7 of 14 possible points, with a mean of 4.8 (standard deviation = 1.2). One time‐series study received 5 points and one longitudinal study received 4 points, out of 12 possible points. All studies were of relatively poor quality and limitations related to cross‐sectional designs and an absence of clear, valid, reliable and consistently implemented outcome measures.
DISCUSSION
This systematic review identified 90 studies evaluating the impacts of DCS from 1990 to 2019. While scholarship is growing, knowledge gaps persist. Studies evaluating DCS were overwhelmingly geographically concentrated in Europe (72.2%). Given that most European DCS target PWUD in party settings, the available evidence on DCS is largely focused upon this subpopulation. While these findings may not be generalizable [97]—particularly to different subpopulations of PWUD—recent expansion of DCS for overdose prevention in Canada and the United States has led to an emergent evidence base on the impact of DCS on structurally vulnerable PWUD.
Monitoring of drug markets by DCS was the predominant domain reported in the literature. Strong evidence exists demonstrating that DCS provide a unique form of drug market monitoring by providing information on the level of concordance between expected (i.e. anticipated by individuals accessing DCS) and detected contents in drug samples. Available evidence also demonstrates the capacity of DCS to detect NPS and drugs of concern. Despite perceptions that DCS could increase accountability among drug sellers and improve the quality of the unregulated drug supply [32, 98], this was not borne out in the evidence and remains a gap in the literature. Our primary domain—the influence of DCS on behaviour of PWUD—was measured in a third of studies, while behavioural intention in response to analysis results from DCS was assessed most often (24.4%). Adjustments in behaviour were found across reporting studies and were generally more common when results from DCS were unexpected or drugs of concern.
Given the proliferation of DCS since 1992 in non‐English‐speaking countries, the comprehensiveness of this systematic review is a key strength achieved through inclusion of studies since 1990 in all languages, grey literature and peer‐reviewed conference abstracts. However, this work has limitations typical of systematic reviews. Publication bias may limit the representativeness of the included literature, as studies not showing positive impacts of DCS could be under‐represented. Timing of the search is another limitation, and several peer‐reviewed articles have been published since [99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111], including one Canadian study among drug sellers [100]. Our eligibility criteria excluded studies where DCS were not implemented although they may have reported outcomes on our domains, such as facilitators and barriers to use of DCS [112]. We did not conduct a meta‐analysis due to significant heterogeneity in methods, populations and outcomes throughout included studies. Due to resource limitations, we only reviewed grey literature on the primary domain and did not appraise the quality of grey literature or qualitative studies. Studies for which quality was assessed were not of high quality, which may increase the level of uncertainty with respect to outcomes reported. Key methodological limitations among studies reporting on behaviour of PWUD include cross‐sectional designs and behavioural intention measures subject to the intention–behaviour gap [113]. Challenges facing the generation of evidence on DCS include limited resources for research and evaluation as well as barriers to conducting research on PWUD and DCS due to drug criminalization [114].
CONCLUSIONS
This systematic review found that monitoring of drug markets by DCS is well established in Europe and increasingly in North America; there is an emerging evidence base on the capacity of DCS to influence behavioural intention and, to a lesser extent, enacted behaviour, among PWUD. Further research on enacted behaviours, linking behaviours to health outcomes and among people who inject drugs or use opioids would benefit the knowledge base, as would more rigorous and higher‐quality study designs. As DCS gain popularity, ongoing scientific evaluations across settings are critical to understanding the impact and limitations of this intervention.
DECLARATION OF INTERESTS
D.W. is a founder of DoseCheck Technologies, a privately held company seeking to develop drug checking instruments.
AUTHOR CONTRIBUTIONS
Nazlee Maghsoudi: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; visualization. Justine Tanguay: Data curation; formal analysis; investigation; project administration. Kristy Scarfone: Data curation; formal analysis; investigation; project administration. Indhu Rammohan: Investigation. Carolyn Ziegler: Data curation; resources; software. Dan Werb: Conceptualization; funding acquisition; resources; supervision. Ayden I. Scheim: Conceptualization; methodology; supervision; validation.
Supporting information
Data S1. Supporting Information
Table S1. Findings for Primary Domain – Influence of DCS on Behaviour of PWUD
Table S2. Findings for Secondary Domain – Monitoring of Drug Markets by DCS
Table S3. Findings for Tertiary Domain – Outcomes Related to Models of DCS
Figure 2: Outcome Measures Examined by Included Studies (n=90)
Figure 2A. Influence of DCS on Behaviour of PWUD (n=31 studies)
Figure 2B. Monitoring of Drug Markets by DCS (n=63 studies)
Figure 2C. Outcomes Related to Models of DCS (n=17 studies)
ACKNOWLEDGEMENTS
Support was provided by CRISM, Canadian Institutes of Health Research (CIHR) Vanier Canada Graduate Scholarship (N.M.), CIHR New Investigator Award (D.W.), CIHR Fellowship (A.I.S.), Ontario Ministry of Research, Innovation and Science Early Researcher Award (D.W.) and St Michael's Hospital Foundation. We acknowledge the leadership and working group of CRISM Emerging Health Threat Implementation Science Program on Opioid Interventions and Services: Drug Checking. We acknowledge that the land where we worked is the traditional territory of many nations, including the Mississaugas of the Credit, Anishnabeg, Chippewa, Haudenosaunee and Wendat peoples, and home to diverse First Nations, Inuit and Métis peoples. A preprint of this paper appears at https://www.qeios.com/read/TXE86U.
Maghsoudi N, Tanguay J, Scarfone K, Rammohan I, Ziegler C, Werb D, et al. Drug checking services for people who use drugs: a systematic review. Addiction. 2022;117:532–544. 10.1111/add.15734
Funding information Canadian Institutes of Health Research; Canadian Research Initiative in Substance Misuse; Ontario Ministry of Research, Innovation and Science; St Michael's Hospital Foundation
REFERENCES
- 1. Smith D. Street drug analysis and community based drug programs. J Psychedelic Drugs. 1974;6:153–9. [Google Scholar]
- 2. Renfroe C. MDMA on the street: analysis anonymous. J Psychoact Drugs. 1986;18:363–9. [DOI] [PubMed] [Google Scholar]
- 3. Kriener H, Billeth R, Gollner C, Lachout S, Neubauer P, Schmid R. An Inventory of On‐site Pill‐testing Interventions in the EU. Vienna, Austria: European Monitoring Centre for Drugs and Drug Addiction; 2001. [Google Scholar]
- 4. Brunt T. Drug Checking as a Harm Reduction Tool for Recreational Drug Users: opportunities and Challenges. Vienna, Austria: European Monitoring Centre on Drugs and Drug Addiction; 2017. [Google Scholar]
- 5. Barratt M, Kowalski M, Maier L, Ritter A. Global Review of Drug Checking Services Operating in 2017. Sydney, Australia: National Drug and Alcohol Research Centre, UNSW Sydney; 2018. [Google Scholar]
- 6. Maghsoudi N, McDonald K, Stefan C, Beriault D, Mason K, Barnaby L, et al. Evaluating networked drug checking services in Toronto, Ontario: study protocol and rationale. Harm Reduct J. 2020;17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fentanyl Overdose Reduction Checking Analysis Study (FORECAST). Baltimore, MD: Johns Hopkins Bloomberg School of Public Health and Bloomberg American Health Initiative; 2018. [Google Scholar]
- 8. Kerr T, Tupper K. Drug Checking as a Harm Reduction Intervention. Vancouver, BC: British Columbia Centre on Substance Use; 2017. [Google Scholar]
- 9. Leece P. Evidence Brief: Drug checking Services as a Harm Reduction Intervention. Cambridge, ON: Public Health Ontario; 2017. [Google Scholar]
- 10. Schroers A. Drug checking: monitoring the contents of new synthetic drugs. J Drug Issues. 2002;32:635–46. [Google Scholar]
- 11.Nightlife Empowerment and Well‐being Implementation Project and Trans European Drug Information. Factsheet on Drug Checking in Europe. Nightlife Empowerment and Well‐being Implementation Project and Trans European Drug Information; 2011.
- 12. Nightlife Empowerment and Well‐being Implementation Project and Trans European Drug Information . Drug checking Service. Good Practice Standards. Barcelona, Spain: Nightlife Empowerment and Well‐being Implementation Project; 2012. [Google Scholar]
- 13.Nightlife Empowerment and Well‐being Implementation Project and Trans European Drug Information. Guidelines for Drug Checking Methodology. Barcelona, Spain: Nightlife Empowerment and Well‐being Implementation Project and Trans European Drug Information; 2012.
- 14. Harper L, Powell J, Pijl EM. An overview of forensic drug testing methods and their suitability for harm reduction point‐of‐care services. Harm Reduct J. 2017;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maghsoudi N, Werb D, Scheim A, Tanguay J, Ziegler C. The implementation of drug checking services for people who use drugs: a systematic review. PROSPERO: international prospective register of systematic reviews. 2018. CRD42018105366. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=105366 [DOI] [PMC free article] [PubMed]
- 17. McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- 18. Ma L‐L, Wang Y‐Y, Yang Z‐H, Huang D, Weng H, Zeng X‐T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross‐sectional Studies. Bethesda, MD: National Institutes of Health; 2014. [Google Scholar]
- 20. National Institutes of Health . Quality Assessment Tool for Before‐After (Pre‐Post) Studies With No Control Group. Bethesda, MD: National Institutes of Health; 2014. [Google Scholar]
- 21. Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme Version. Lancaster, UK: 2006;1:b92. 10.13140/2.1.1018.4643 [DOI] [Google Scholar]
- 22. Peiper N, Clarke S, Vincent L, Ciccarone D, Kral A, Zibbell J. Fentanyl test strips as an opioid overdose prevention strategy: findings from a syringe services program in the southeastern United States. Int J Drug Policy. 2019;63:122–8. [DOI] [PubMed] [Google Scholar]
- 23. McKnight C, Des JD. Being ‘hooked up’ during a sharp increase in the availability of illicitly manufactured fentanyl: adaptations of drug using practices among people who use drugs (PWUD) in New York City. Int J Drug Policy. 2018;60:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mema S, Sage C, Xu Y, Tupper K, Ziemianowicz D, McCrae K, et al. Drug checking at an electronic dance music festival during the public health overdose emergency in British Columbia. Can J Public Health. 2018;109:740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harm Reduction Coalition . Fentanyl Test Strip Pilot: San Francisco August 2017–January 2018. New York, NY: Harm Reduction Coalition ; 2018. [Google Scholar]
- 26. Munn M, Lund A, Golby R, Turris S. Observed benefits to on‐site medical services during an annual 5‐day electronic dance music event with harm reduction services. Prehosp Disaster Med. 2016;31:228–34. [DOI] [PubMed] [Google Scholar]
- 27. Sage C. Harm Reduction and Drug Checking; A wrap‐around service for festivals. Case Study: Shambhala Music Festival /ANKORS Drug Checking Harm Reduction Service data 2015. ANKORS (AIDS Network Outreach & Support Society); 2016.
- 28. Huberty C, Favresse D. Assessment of risk reduction actions in the context of testing activities carried out by the non‐profit organization Modus Vivendi: Research report. 2010.
- 29. van de Wijngaart G, Braam R, de Bruin D, Fris M, Maalsté N, Verbraeck H. Ecstasy use at large‐scale dance events in the Netherlands. J Drug Issues. 1999;29:679–701. [Google Scholar]
- 30. van de Wijngaart G, Braam R, de Bruin D, Fris M, Maalsté N, Verbraeck H. Ecstasy and the Dutch Rave Scene: A Socio‐Epidemiological Study on the Nature and Extent of, and the Risks Involved in Using Ecstasy and Other Party Drugs at Dance Events. Utrecht, the Netherlands: Addiction Research Centre; 1998. [Google Scholar]
- 31. Goldman J, Waye K, Periera K, Krieger M, Yedinak J, Marshall B. Perspectives on rapid fentanyl test strips as a harm reduction practice among young adults who use drugs: a qualitative study. Harm Reduct J. 2019;16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Measham F. Drug safety testing, disposals and dealing in an English field: Exploring the operational and behavioural outcomes of the UK's first on‐site ‘drug checking’ service. Int J Drug Policy. 2019;67:102–7. [DOI] [PubMed] [Google Scholar]
- 33. Knox A. 2018/2019 Results . KnowYourStuffNZ; 2019. Available at: https://knowyourstuff.nz/2018-19-results/. Accessed 16 Oct 2019. [Google Scholar]
- 34. Krieger M, Goedel W, Buxton J, Lysyshyn M, Bernstein E, Sherman S, et al. Use of rapid fentanyl test strips among young adults who use drugs. Int J Drug Policy. 2018;61:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Makkai T, Macleod M, Vumbaca G, Hill P, Caldicott D, Noffs M, et al. Report on Canberra GTM Harm Reduction Service. Leura, NSW: Harm Reduction Australia; 2018. [Google Scholar]
- 36. Morris V, Gordillo C. Results Report Satisfaction Survey and Change of Behaviour Échele Cabeza 2016. Échele Cabeza; 2016. [Google Scholar]
- 37. Kriener H, Schmid R. Check your pills. Check your life. ChEck iT! High quality on‐site testing of illicit substances. Information, counselling and safer use measures at raves in Austria. 2005. DrugText [internet] 2005. Available at: https://web.archive.org/web/20081021045950/http:/www.drugtext.org/library/articles/kriener.htm. Accessed 19 July 2019. [Google Scholar]
- 38. Bardwell G, Boyd J, Tupper K, Kerr T. ‘We don't got that kind of time, man. We're trying to get high!’: exploring potential use of drug checking technologies among structurally vulnerable people who use drugs. Int J Drug Policy. 2019;71:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sherman S, Morales K, Park J, McKenzie M, Marshall B, Green T. Acceptability of implementing community‐based drug checking services for people who use drugs in three United States cities: Baltimore, Boston and Providence. Int J Drug Policy. 2019;68:46–53. [DOI] [PubMed] [Google Scholar]
- 40. Valente H, Martins D, Carvalho H, Pires C, Carvalho M, Pinto M, et al. Evaluation of a drug checking service at a large scale electronic music festival in Portugal. Int J Drug Policy. 2019;73:88–95. [DOI] [PubMed] [Google Scholar]
- 41. Karamouzian M, Dohoo C, Forsting S, McNeil R, Kerr T, Lysyshyn M. Evaluation of a fentanyl drug checking service for clients of a supervised injection facility, Vancouver, Canada. Harm Reduct J. 2018;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. 2017/2018 Results . KnowYourStuffNZ; 2018. Available at: https://knowyourstuff.nz/our-results/2017-2018-results/. Accessed 23 July 2019. [Google Scholar]
- 43. Martins D, Barratt M, Pires C, Carvalho H, Ventura M, Fornís I, et al. The detection and prevention of unintentional consumption of DOx and 25x‐NBOMe at Portugal's boom festival. Hum Psychopharmacol Clin Exp. 2017;32:E2608. [DOI] [PubMed] [Google Scholar]
- 44. Saleemi S, Pennybaker S, Wooldridge M, Johnson M. Who is ‘Molly’? MDMA adulterants by product name and the impact of harm‐reduction services at raves. J Psychopharmacol. 2017;31:1056–60. [DOI] [PubMed] [Google Scholar]
- 45. 2016/2017 Results . KnowYourStuffNZ; 2017. Available at: https://knowyourstuff.nz/our-results/our-results_2016_17/. Accessed 23 July 2019.
- 46. Royal Society for Public Health . Drug Safety Testing at Festivals and Night Clubs. London, UK: Royal Society for Public Health; 2017. [Google Scholar]
- 47. Michelow W, Dowden C. ‘Start Small, Take it Easy’: Results from the ANKORS Harm Reduction Survey at the 2013 Shambhala Music Festival. Nelson, BC, ANKORS (AIDS Network Outreach and Support Society); 2015. [Google Scholar]
- 48.BAONPS (Be Aware On Night Pleasure Safety). Italian Results. 2017. Accessed 30 July 2019.
- 49. Houioux G, Favresse D, De Smet P, Piette D. Risk reduction with analysis of new synthetic drugs: Evaluation. 2006.
- 50. Korf D, Benschop A, Brunt T. Testing pills in the Netherlands: An investigation into strengthening the monitor for recreational drugs. Amsterdam, the Netherlands: Rozenberg Publishers; 2003. [Google Scholar]
- 51. Benschop A, Rabes M, Korf D. Pill Testing, Ecstasy and Prevention: A Scientific Evaluation in Three European Cities. Amsterdam, the Netherlands: Rozenberg Publishers; 2002. [Google Scholar]
- 52. Koeter M. Get your ecstasy tested!: Evaluation of the test service of the Brijder Foundation. Amsterdam, the Netherlands: Amsterdam Institute for Addiction Research; 1997. [Google Scholar]
- 53. McCrae K, Tobias S, Tupper K, Arredondo J, Henry B, Mema S, et al. Drug checking services at music festivals and events in a Canadian setting. Drug Alcohol Depend. 2019;205:107589. [DOI] [PubMed] [Google Scholar]
- 54. Tupper K, McCrae K, Garber I, Lysyshyn M, Wood E. Initial results of a drug checking pilot program to detect fentanyl adulteration in a Canadian setting. Drug Alcohol Depend. 2018;190:242–5. [DOI] [PubMed] [Google Scholar]
- 55. Brunt T, Nagy C, Bücheli A, Martins D, Ugarte M, Beduwe C, et al. Drug testing in Europe: monitoring results of the trans European drug information (TEDI) project. Drug Test Anal. 2017;9:188–98. [DOI] [PubMed] [Google Scholar]
- 56. Caudevilla F, Ventura M, Fornís I, Barratt M, Vidal C, Quintana P, et al. Results of an international drug testing service for cryptomarket users. Int J Drug Policy. 2016;35:38–41. [DOI] [PubMed] [Google Scholar]
- 57. Martins D, Valente H, Pires C. CHECK!NG: the last frontier for harm reduction in party settings. Saúde Sociedade. 2015;24:646–60. [Google Scholar]
- 58. Brunt T, Niesink R. The drug information and monitoring system (DIMS) in the Netherlands: implementation, results, and international comparison. Drug Test Anal. 2011;3:621–34. [DOI] [PubMed] [Google Scholar]
- 59. Vidal C, Ventura M, Fornís I, Gil C, Calzada N, Fitó A, et al. Crystals and tablets in the Spanish ecstasy market 2000–2014: Are they the same or different in terms of purity and adulteration? Forensic Sci Int. 2016;263:164–8. [DOI] [PubMed] [Google Scholar]
- 60. Sibbald K, Rushton W, King J, Charlton N. Adulterants in tablets sold as ecstasy in the US from 2009–2013. Clin Toxicol. 2014;52:704–5. [Google Scholar]
- 61. Brunt T, Koeter M, Niesink R, van den Brink W. Linking the pharmacological content of ecstasy tablets to the subjective experiences of drug users. Psychopharmacology. 2012;220:751–62. [DOI] [PubMed] [Google Scholar]
- 62. Brunt T, Poortman A, Niesink R, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol. 2011;25:1543–7. [DOI] [PubMed] [Google Scholar]
- 63. Vogels N, Brunt T, Rigter S, van Dijk P, Vervaeke H, Niesink R. Content of ecstasy in the Netherlands: 1993–2008. Addiction. 2009;104:2057–66. [DOI] [PubMed] [Google Scholar]
- 64. Giraudon I, Bello P‐Y. Monitoring ecstasy content in France: Results from the National Surveillance System 1999–2004. Subst Use Misuse. 2007;42:1567–78. [DOI] [PubMed] [Google Scholar]
- 65. Tanner‐Smith E. Pharmacological content of tablets sold as ‘ecstasy’: results from an online testing service. Drug Alcohol Depend. 2006;83:247–54. [DOI] [PubMed] [Google Scholar]
- 66. Delile J‐M, Gachie J‐P. Ecstasy and risk reduction: The place of "testing" and substance analyses. Alcool Addictol. 2002;24:311–8. [Google Scholar]
- 67. Spruit I. Monitoring synthetic drug markets, trends, and public health. Subst Use Misuse. 2001;36:23–47. [DOI] [PubMed] [Google Scholar]
- 68. Spruit I. Ecstasy use and policy responses in the Netherlands. J Drug Issues. 1999;29:653–77. [Google Scholar]
- 69. van der Gouwe D, Brunt T, van Laar M, van der Pol P. Purity, adulteration and price of drugs bought on‐line versus off‐line in the Netherlands. Addiction. 2017;112:640–8. [DOI] [PubMed] [Google Scholar]
- 70. Palma A, Ventura M, Galindo L, Fonseca F, Grifell M, Quintana P, et al. Something new about something old: a 10‐year follow‐up on classical and new psychoactive tryptamines and results of analysis. J Psychoact Drugs. 2017;49:297–305. [DOI] [PubMed] [Google Scholar]
- 71. United Nations Office on Drugs and Crime (UNODC) . The Challenge of New Psychoactive Substances. Vienna, Austria: UNODC; 2013. [Google Scholar]
- 72. Fabregat‐Safont D, Carbón X, Ventura M, Fornís I, Hernández F, Ibáñez M. Characterization of a recently detected halogenated aminorex derivative: para‐fluoro‐4‐methylaminorex (4′ F‐4‐MAR). Sci Rep. 2019;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fabregat‐Safont D, Carbón X, Gil C, Ventura M, Sancho J, Hernández F, et al. Reporting the novel synthetic cathinone 5‐PPDI through its analytical characterization by mass spectrometry and nuclear magnetic resonance. Forens Toxicol. 2018;36:447–57. [Google Scholar]
- 74. Fabregat‐Safont D, Carbón X, Ventura M, Fornís I, Guillamón E, Sancho J, et al. Updating the list of known opioids through identification and characterization of the new opioid derivative 3, 4‐dichloro‐N‐(2‐[diethylamino] cyclohexyl)‐N‐methylbenzamide (U‐49900). Sci Rep. 2017;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fabregat‐Safont D, Fornís I, Ventura M, Gil C, Calzada N, Sancho J, et al. Identification and characterization of a putative new psychoactive substance, 2‐(2‐[4‐chlorophenyl] acetamido)‐3‐methylbutanamide, in Spain. Drug Test Anal. 2017;9:1073–80. 10.1002/dta.2182 [DOI] [PubMed] [Google Scholar]
- 76. Roldán M, Grifell M, Gonzalez I, Fuentes J, Frings M, Ventura M, et al. Analysing heroin samples as harm reduction intervention—prevalence of adulteration with fentanyl derivatives. Eur Neuropsychopharmacol. 2019;29:S474–5. [Google Scholar]
- 77. Pérez S, de Dios M, Monteagudo E, Sanagustín D, Trabsa A, Grifell M, et al. New designer benzodiazepines use in Barcelona. Eur Psychiatry. 2017;41:S874. [Google Scholar]
- 78. Linsen F, Koning R, van Laar M, Niesink R, Koeter M, Brunt T. 4‐Fluoroamphetamine in the Netherlands: more than a one‐night stand. Addiction. 2015;110:1138–43. [DOI] [PubMed] [Google Scholar]
- 79. Hondebrink L, Nugteren‐van Lonkhuyzen J, Van Der Gouwe D, Brunt T. Monitoring new psychoactive substances (NPS) in the Netherlands: data from the drug market and the poisons information centre. Drug Alcohol Depend. 2015;147:109–15. [DOI] [PubMed] [Google Scholar]
- 80. Vidal C, Fornís I, Ventura M. New psychoactive substances as adulterants of controlled drugs. A worrying phenomenon? Drug Test Anal. 2014;6:819–24. [DOI] [PubMed] [Google Scholar]
- 81. Caudevilla F, Ventura M, Indave B, Fornís I. Presence and composition of cathinone derivatives in drug samples taken from a drug test service in Spain (2010–2012). Hum Psychopharmacol Clin Exp. 2013;28:341–4. [DOI] [PubMed] [Google Scholar]
- 82. Blanckaert P, van Amsterdam J, Brunt T, van den Berg J, Van Durme F, Maudens K, et al. 4‐methyl‐amphetamine: a health threat for recreational amphetamine users. J Psychopharmacol. 2013;27:817–22. [DOI] [PubMed] [Google Scholar]
- 83. Caudevilla F, Riba J, Ventura M, González D, Farré M, Barbanoj M, et al. 4‐Bromo‐2, 5‐dimethoxyphenethylamine (2C‐B): presence in the recreational drug market in Spain, pattern of use and subjective effects. J Psychopharmacol. 2012;26:1026–35. [DOI] [PubMed] [Google Scholar]
- 84. Bossong M, Brunt T, Van Dijk J, Rigter S, Hoek J, Goldschmidt H, et al. mCPP: an undesired addition to the ecstasy market. J Psychopharmacol. 2010;24:1395–401. [DOI] [PubMed] [Google Scholar]
- 85. Grifell M, Ventura M, Carbón X, Quintana P, Galindo L, Palma A, et al. Patterns of use and toxicity of new para‐halogenated substituted cathinones: 4‐CMC (clephedrone), 4‐CEC (4‐chloroethcatinone) and 4‐BMC (brephedrone). Hum Psychopharmacol Clin Exp. 2017;32:E2621. [DOI] [PubMed] [Google Scholar]
- 86. Sande M, Šabić S. The importance of drug checking outside the context of nightlife in Slovenia. Harm Reduct J 2018;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Quintana P, Ventura M, Grifell M, Palma A, Galindo L, Fornís I, et al. The hidden web and the fentanyl problem: detection of ocfentanil as an adulterant in heroin. Int J Drug Policy. 2017;40:78–83. [DOI] [PubMed] [Google Scholar]
- 88. Grifell M, Palma A, Ventura M, Fornís I, Dinamarca F, Torrens M, et al. A trip to the unknown: 2, 5‐dimethoxy‐4‐chloroamphetamine (DOC) sold as LSD: study on samples delivered by users asking for substance analysis. Eur Neuropsychopharmacol. 2015;25:S620. [Google Scholar]
- 89. Brunt T, Rigter S, Hoek J, Vogels N, van Dijk P, Niesink R. An analysis of cocaine powder in the Netherlands: content and health hazards due to adulterants. Addiction. 2009;104:798–805. [DOI] [PubMed] [Google Scholar]
- 90. Sanagustín D, de Dios M, Monteagudo E, Pérez S, Trabsa A, Galindo L, et al. U‐47700: the new emerging opioid drug. Eur Psychiatry. 2017;41:S395. [Google Scholar]
- 91. Galindo L, Grifell M, Quintana P, Palma A, Tirado J, Ventura M, et al. The synthetic cannabinoids: JWH, four years of analysis. Eur Psychiatry. 2016;33:S115–6. [Google Scholar]
- 92. Monteagudo E, de Dios M, Trabsa A, Grifell M, Galindo L, Quintana P, et al. Is methylone a new public health threat in Spain? Eur Psychiatry. 2017;41:S871. [Google Scholar]
- 93. Brunt T, Atkinson A, Nefau T, Martinez M, Lahaie E, Malzcewski A, et al. Online test purchased new psychoactive substances in 5 different European countries: a snapshot study of chemical composition and price. Int J Drug Policy. 2017;44:105–14. [DOI] [PubMed] [Google Scholar]
- 94. Brunt T, Niesink R, van den Brink W. Impact of a transient instability of the ecstasy market on health concerns and drug use patterns in the Netherlands. Int J Drug Policy. 2012;23:134–40. [DOI] [PubMed] [Google Scholar]
- 95. Bardwell G, Boyd J, Arredondo J, McNeil R, Kerr T. Trusting the source: the potential role of drug dealers in reducing drug‐related harms via drug checking. Drug Alcohol Depend. 2019;198:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Palamar J, Acosta P, Sutherland R, Shedlin M, Barratt M. Adulterants and altruism: A qualitative investigation of ‘drug checkers’ in North America. Int J Drug Policy. 2019;74:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Palamar J, Fitzgerald N, Keyes K, Cottler L. Drug checking at dance festivals: a review with recommendations to increase generalizability of findings. Exp Clin Psychopharmacol. 2021;29:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wallace B, van Roode T, Pagan F, Hore D, Pauly B. The potential impacts of community drug checking within the overdose crisis: qualitative study exploring the perspective of prospective service users. BMC Public Health. 2021;21:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Beaulieu T, Wood E, Tobias S, Lysyshyn M, Patel P, Matthews J, et al. Is expected substance type associated with timing of drug checking service utilization? A cross‐sectional study. Harm Reduct J. 2021;18:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Betsos A, Valleriani J, Boyd J, Bardwell G, Kerr T, McNeil R. ‘I couldn't live with killing one of my friends or anybody’: a rapid ethnographic study of drug sellers’ use of drug checking. Int J Drug Policy. 2021;87:102845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Borden S, Saatchi A, Vandergrift G, Palaty J, Lysyshyn M, Gill C. A new quantitative drug checking technology for harm reduction: pilot study in Vancouver, Canada using paper spray mass spectrometry. Drug Alcohol Rev. 2021. 1–9. 10.1111/dar.13370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Laing M, Ti L, Marmel A, Tobias S, Shapiro A, Laing R, et al. An outbreak of novel psychoactive substance benzodiazepines in the unregulated drug supply: preliminary results from a community drug checking program using point‐of‐care and confirmatory methods. Int J Drug Policy. 2021;93:103169. [DOI] [PubMed] [Google Scholar]
- 103. Measham F, Turnbull G. Intentions, actions and outcomes: a follow up survey on harm reduction practices after using an English festival drug checking service. Int J Drug Policy. 2021;95:103270. [DOI] [PubMed] [Google Scholar]
- 104. Patel P, Guzman S, Lysyshyn M, Buxton J, Kuo M, Tobias S, et al. Identifying cocaine adulteration in the unregulated drug supply in British Columbia, Canada. Can J Addict. 2021;12:39–44. [Google Scholar]
- 105. Ti L, Tobias S, Maghsoudi N, Milloy MJ, McDonald K, Shapiro A, et al. Detection of synthetic cannabinoid adulteration in the unregulated drug supply in three Canadian settings. Drug Alcohol Rev. 2021;40:580–5. [DOI] [PubMed] [Google Scholar]
- 106. Tobias S, Grant CJ, Laing R, Arredondo J, Lysyshyn M, Buxton J, et al. Time‐series analysis of fentanyl concentration in the unregulated opioid drug supply in a Canadian setting. Am J Epidemiol. 2021. 1–7. 10.1093/aje/kwab129 [DOI] [PubMed] [Google Scholar]
- 107. Tobias S, Shapiro AM, Grant CJ, Patel P, Lysyshyn M, Ti L. Drug checking identifies counterfeit alprazolam tablets. Drug Alcohol Depend. 2021;218:108300. [DOI] [PubMed] [Google Scholar]
- 108. McCrae K, Hayashi K, Bardwell G, Nosova E, Milloy MJ, Wood E, et al. The effect of injecting alone on the use of drug checking services among people who inject drugs. Int J Drug Policy. 2020;79:102756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. McCrae K, Wood E, Lysyshyn M, Tobias S, Wilson D, Arredondo J, et al. The utility of visual appearance in predicting the composition of street opioids. Subst Abuse. 2020. 10.1080/08897077.2020.1864569 [DOI] [PubMed] [Google Scholar]
- 110. Measham F. City checking: piloting the UK's first community‐based drug safety testing (drug checking) service in 2 city centres. Br J Clin Pharmacol. 2020;86:420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tobias S, Shapiro AM, Wu H, Ti L. Xylazine identified in the unregulated drug supply in British Columbia, Canada. Can J Addict. 2020;11:28–32. [Google Scholar]
- 112. Barratt M, Bruno R, Ezard N, Ritter A. Pill testing or drug checking in Australia: acceptability of service design features. Drug Alcohol Rev 2018;37:226–236. [DOI] [PubMed] [Google Scholar]
- 113. Sheeran P, Webb T. The intention–behavior gap. Soc Personal Psychol Compass. 2016;10:503–18. [Google Scholar]
- 114. Vidal C, Ventura M, Measham F, Brunt T, Bücheli A, Paulos C, et al. The utility of drug checking services as monitoring tools and more: a response to Pirona et al. Int J Drug Policy. 2017;45:46–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Table S1. Findings for Primary Domain – Influence of DCS on Behaviour of PWUD
Table S2. Findings for Secondary Domain – Monitoring of Drug Markets by DCS
Table S3. Findings for Tertiary Domain – Outcomes Related to Models of DCS
Figure 2: Outcome Measures Examined by Included Studies (n=90)
Figure 2A. Influence of DCS on Behaviour of PWUD (n=31 studies)
Figure 2B. Monitoring of Drug Markets by DCS (n=63 studies)
Figure 2C. Outcomes Related to Models of DCS (n=17 studies)


