Abstract
A growing body of evidence indicates that early‐life exposure to selective serotonin reuptake inhibitor has long‐term consequences on the offspring's pain in addition to affective disorders like anxiety disorder and major depression. Serotonin, besides its role in regulating pain and emotions, promotes neuronal network formation. The prefrontal cortex and the amygdala are two key brain regions involved in the modulation of pain and its affective comorbidities. Thus, the aim of this review is to understand how early‐life selective serotonin reuptake inhibitor exposure alters the developing prefrontal cortex and amygdala and thereby underlies the long‐term changes in pain and its affective comorbidities in later life. While there is still limited data on the effects of early‐life selective serotonin reuptake inhibitor exposure on pain, there is a substantial body of evidence on its affective comorbidities. From this perspective paper, four conclusions emerged. First, early‐life selective serotonin reuptake inhibitor exposure results in long‐term nociceptive effects, which needs to be consistently studied to clarify. Second, it results in enhanced depressive‐like behaviour and diminished exploratory behaviour in adult rodents. Third, early‐life selective serotonin reuptake inhibitor exposure alters serotonergic levels, transcription factors expression, and brain‐derived neurotrophic factor levels, resulting in hyperconnectivity within the amygdala and the prefrontal cortex. Finally, it affects antinociceptive inputs of the prefrontal cortex and the amygdala in the spinal cord. We conclude that early‐life selective serotonin reuptake inhibitor exposure affects the maturation of prefrontal cortex and amygdala circuits and thereby enhances their antinociceptive inputs in the spinal cord.
Keywords: affective disorders, amygdala, early‐life, pain, prefrontal cortex, selective serotonin reuptake inhibitors
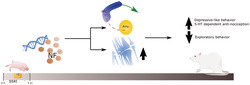
Exposure to selective serotonin reuptake inhibitors (SSRI) between gestational day (G) 0 and postnatal day (P) 21 affects epigenetic, serotonergic, and neurotrophic factors (NF). Consequently, the prefrontal cortex (PFC) and the amygdala (Amy) present intra‐hyperconnectivity, and the PFC feedforward disinhibition leads to increased output of the PFC. This results in heightened depressive‐like behaviour and serotonin (5‐HT)‐dependent nociception modulation of the spinal cord input as well as decreased exploratory behaviour.
Abbreviations
- 5‐HT
5‐hydroxytryptamine; serotonin
- BDNF
brain‐derived neurotrophic factor
- BLA
basolateral amygdala
- CpG
5′‐cytosine‐phosphate‐guanine‐3′
- EPM
elevated plus maze
- FLX
fluoxetine
- FST
forced‐swim test
- G
gestational day
- mPFC
medial prefrontal cortex
- NSF
novelty suppressed feeding
- OFT
open‐field test
- P
postnatal day
- PAG
periaqueductal grey
- PFC
prefrontal cortex
- PSD‐95
postsynaptic density protein 95
- PVA
Ca2+‐binding protein parvalbumin
- SERT
serotonin transporter
- SERT‐KO
serotonin transporter‐knock out
- SSRI
selective serotonin reuptake inhibitors
- Tph
transient receptor potential vanilloid type 1
- trkB
tropomyosin‐related kinase B receptor
- TRPV1
transient receptor potential vanilloid type 1
- vlPAG
ventrolateral periaqueductal grey
1. INTRODUCTION
Antidepressants represent up to 29% of all analgesic prescriptions used to treat chronic pain and were shown to have a direct analgesic effect in patients with chronic pain (Breivik et al., 2006; Fishbain et al., 2000; McDermott et al., 2006). The overlap of antidepressants' effectiveness for chronic pain and affective disorders like anxiety and major depression may arise from the strong co‐morbidity observed between chronic pain and these conditions. Indeed, emotional disturbances often arise as a consequence of chronic pain or can be a risk factor for its chronification (Means‐Christensen et al., 2008; Tsang et al., 2008; Woo, 2010). Central to both the modulation of (chronic) pain and affective disorders is the neurotransmitter 5‐hydroxytryptamine (5‐HT; serotonin) (Baldwin & Rudge, 1995; Huang et al., 2019; Millan, 2002).
In addition to its role in regulating pain and emotions, serotonin promotes neuronal network formation, including processes like neurite outgrowth, synaptogenesis, cellular differentiation, and cell migration (Faber & Haring, 1999; Fricker et al., 2005; Gaspar et al., 2003; Suri et al., 2015). As serotonin modulates network maturation and adult emotional behaviour, the serotonin transporter (SERT) is crucial to the regulation of extracellular serotonin concentrations in early‐life (Daubert & Condron, 2010; Suri et al., 2015). SERT is responsible for the reuptake of extracellular serotonin and is the main target of selective serotonin reuptake inhibitors (SSRI); 16.4% and 12% of pregnant women suffer from ante‐partum and/or post‐partum depression, respectively (Okagbue et al., 2019; Shorey et al., 2018). Accordingly, 2% of women are prescribed with SSRIs during pregnancy, which were shown to transfer across the placenta as well as into the breast milk, hence exposing the developing foetus and infant to the medication (Kristensen et al., 1999; Rampono et al., 2004; Ververs et al., 2006). Consequently, serotonin modulation during development, such as experienced by early‐life SSRI exposure, affects the maturation of the central nervous system and has long‐lasting consequences on the offspring's pain response as well as on its affective comorbidities (Butkevich & Mikhailenko, 2018; Glover & Clinton, 2016; Homberg et al., 2010, 2014; Lee, 2009; Suri et al., 2015; Toffoli et al., 2014). As the understanding of the role of early‐life SSRI exposure on pain in later life is limited, insights from their effects on affective comorbidities, like anxiety disorders and major depression, might allow for better comprehension of the potential long‐term effects of early‐life SSRI on the maturation of the CNS and the related behavioural consequences.
Emotional disturbances, such as anxiety disorder and major depression, may increase pain sensation and vice versa, thereby suggesting the involvement of common pathways and neuronal networks (Ong et al., 2019; Qi et al., 2014). Pain, as well as affective comorbidities are modulated by a wide range of brain regions, among which the prefrontal cortex (PFC) and the amygdala (Bernard et al., 1996; Ong et al., 2019; Simons et al., 2014). The amygdala receives sensory and nociceptive information via ascending pathways to integrate the affective component of pain but also modulates pain by projecting to descending pain centers such as the periaqueductal grey (PAG) (Bernard et al., 1996; Gauriau & Bernard, 2002; Veinante et al., 2013). Furthermore, the amygdala is required for the modulation of chronic pain by the PFC and presents a deepened connectivity with the PFC upon heightened anxiety and depression in late childhood (Jalbrzikowski et al., 2017; Ong et al., 2019). Likewise, patients suffering from chronic pain show increased functional connectivity between the amygdala and the PFC. Furthermore, amygdala projections inhibit the PFC, which in turn leads to a reduced PAG‐mediated serotonin‐dependent antinociception in neuropathic animals (Huang et al., 2019; Simons et al., 2014). The descending antinociceptive system is believed to be activated by SSRI, leading to the alleviation of pain (Marks et al., 2009).
The importance of connections between the PFC and the amygdala in the modulation of both pain and its affective comorbidities highlights the need to better understand the long‐term effects of early‐life SSRI exposure on those brain regions. Disruption of the developing network between the PFC and the amygdala by exposition to SSRI around birth is likely to have dramatic long‐term consequences and may result in both pain and emotional disturbances (Gaspar et al., 2003). The aim of the present perspective paper is to understand how early‐life exposure to SSRIs alters developing connections of the PFC and the amygdala, and thereby underlies long‐term changes in pain and its co‐morbidities in later life. To this end, we summarize the state of the field in early‐life SSRI exposure and pain, before exploring the long‐term effects on affective comorbidities. Most importantly, by describing the effects of SSRI exposure around birth on the maturation of the neuronal network and connections between the PFC and the amygdala, we link these underlying mechanisms to implications in pain and its affective comorbidities.
1.1. Search strategy
Systematic searches were conducted on Embase, Medline and PubMed, combining the following terms (and related synonyms): “serotonergic system,” “early‐life,” “prefrontal cortex” or “amygdala,” “mood disorder,” “neurodevelopment” and “nociception.” The search resulted in 1484 hits, of which titles and abstracts were screened for relevance (by MB and ARdK). A special attention was given to the studies examining behaviour and/or neurobiological change in the amygdala and/or the PFC following SSRI exposure anytime between gestational day 0 (G 0) and postnatal day 21 (P 21) or that made use of SERT knock‐out models. This resulted in a total of 53 articles included in this review. Administration of SSRI between G 0 and P 1 is classified as prenatal treatment, while administration of SSRI from P 1 to P 21 is defined as postnatal administration. Treatment during both the prenatal and postnatal periods is referred as perinatal.
2. EARLY‐LIFE SSRI EXPOSURE AND LONG‐TERM EFFECTS ON PAIN
Long‐term behavioural effects of prenatal, postnatal, and perinatal SSRI exposure have been reported in various animal models (Butkevich & Mikhailenko, 2018; Knaepen et al., 2013; Lee, 2009; Toffoli et al., 2014). It should be noted that animals' pain behaviour was based on reflex‐based responses (like thermal hypersensitivity of the hind paws), hence representing nociception rather than pain. While some studies describe no effects of perinatal SSRI exposure on adult thermal sensitivity (Lisboa et al., 2007), other studies reported antinociceptive thermal effects or the maintenance of thermal threshold in young rats perinatally exposed to SSRI (Table 1) (Butkevich & Mikhailenko, 2018; Lee, 2009; Toffoli et al., 2014; Vartazarmian et al., 2005). In contrast, decreased mechanical withdrawal thresholds were reported in adulthood after perinatal SSRI exposure, suggesting pronociceptive long‐term effects on mechanical sensitivity (Knaepen et al., 2013). In addition, animals postnatally exposed to SSRIs showed longer postoperative hypersensitivity in adulthood upon re‐injury while maternal prenatal stress alone was shown to decrease postoperative hypersensitivity (Knaepen et al., 2013). Thermal sensitivity to heat stimuli is mediated by the transient receptor potential vanilloid type 1 (TRPV1) which was recently shown to exert antidepressive effects and may therefore participate to the differential outcomes of early life SSRI exposure on mechanical versus thermal sensitivity (Manna & Umathe, 2012). All the studies observing nociceptive behaviour after early life SSRI exposure injected animals with Fluoxetine but with different dosages and mode of injection, without affecting nociception (Table 1). It is therefore of great interest to investigate the effect of other SSRIs on long‐term nociceptive behaviour.
TABLE 1.
Overview table of studies investigating nociception following early life SSRI exposure
| Reference | Type of study | Sex | SSRI (/day) | Time of exposure | Test | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G0–22 | P1–3 | P4–7 | P8–14 | P15–21 | |||||||
| Thermal threshold | |||||||||||
| Butkevich and Mikhailenko (2018). | Rat | F | FLX to dams | G9–G20 | Hot plate | Hypersensitivity: decreased thermal threshold in control. No change in FLX injected adults. | |||||
| 10 mg/kg | |||||||||||
| Toffoli et al. (2014). | Rat | M | FLX to dams | G0–P21 | Hot plate | Hyposensitivity: increased thermal threshold. | |||||
| 5 mg/kg | |||||||||||
| Lee (2009). | Rat | M/F | FLX | P0–P6 | Hot plate | Hyposensitivity: increased thermal threshold. | |||||
| 10 mg/kg | |||||||||||
| Knaepen et al. (2013) | Rat | M/F | FLX to dams | G21–P21 | Laser beam | No effects. | |||||
| 10 mg/kg | |||||||||||
| Lisboa et al. (2007) | Mouse | M/F | FLX to dams | G0–P21 | Hot plate | No effects. | |||||
| 20–40 mg/kg | |||||||||||
| Vartazarmian et al. (2005) | Guinea pigs | M/F | FLX to dams | G1–P1 | Hot plate | Hypersensitivity: decreased thermal threshold in control. No change in FLX injected adults. | |||||
| 10 mg/kg | |||||||||||
| Mechanical threshold | |||||||||||
| Knaepen et al. (2013) | Rat | M/F | FLX to dams | G21–P21 | vFh | Decreased mechanical withdrawal thresholds. Postoperative mechanical sensitivity. | |||||
| 10 mg/kg | |||||||||||
| Oberlander et al. (2002) | Clinical | M/F | FLX, PAR, BNZ, SET. | Prenatal or postnatal | Face reactivity | Decreased facial sensitivity/reactivity at 2 days old. | |||||
| Oberlander et al. (2005) | Clinical | M/F | FLX, PAR, BNZ, SET. | Prenatal or postnatal | Face reactivity | Decreased facial sensitivity/reactivity at 2 months old. | |||||
| Morag et al. (2004) | Clinical | M | PAR (20 mg/day) | Prenatal | Face reactivity | No facial sensitivity before 13th days of life. | |||||
Note: Most preclinical studies investigated thermal threshold and only one researched mechanical threshold. Three clinical studies were encounter. Studies are display according to the following criteria: (1) animal model used (rats, mouse, other); (2) Time of SSRI exposure (prenatal administration, perinatal administration, P1 to P3, P4 to P7, P8 to P14, and P15 to P21, SERT‐KO). FLX: fluoxetine; PAR: paroxetine; BNZ: benzamidine; SER: sertraline; vFh: von Frey hair filaments; M: male; F: female; P: postnatal day; G: gestational day.
Clinical reports on the long‐term effects of perinatal SSRI exposure on pain are limited. Two clinical studies indicate a reduced pain response following exposition, as infants presented diminished facial reactivity at 2 days and 2 months of age (Oberlander et al., 2002, 2005). To date, one case report has been published, indicating a lack of pain responses after SSRI exposure around birth in a newborn (Morag et al., 2004).
Taken together, clinical, and preclinical studies show that early‐life SSRI exposure results in long‐term effects on nociception, which might either be antinociceptive or pronociceptive (Knaepen et al., 2013; Morag et al., 2004; Oberlander et al., 2002, 2005). Owning to the fact that the number of studies is limited, the conclusions are to be taken with the utmost care as more evidence is needed to substantiate these findings.
3. EARLY‐LIFE SSRI EXPOSURE AND LONG‐TERM EFFECTS ON AFFECTIVE COMORBIDITIES
Affective disorders such as anxiety disorders and major depression are known to predispose individuals to pain (Means‐Christensen et al., 2008; Woo, 2010). To improve the understanding of potential long‐term effects of early‐life SSRI exposure on nociception, evidence related to the long‐term effects on affective comorbidities are discussed.
Rodents perinatally exposed to SSRIs often display alterations in anxiety‐related parameters, as assessed in a variety of tests including the open‐field test (OFT), the elevated plus maze (EPM), the light–dark box and the novelty suppressed feeding (NSF) test (Butkevich & Mikhailenko, 2018; Ko et al., 2014; Millard et al., 2019; Sarkar et al., 2014; Zohar et al., 2016). The majority of these studies reported no effects of perinatal SSRI exposure on anxiety in the OFT or the EPM, independent of timing of exposure (prenatal, perinatal, or postnatal) (Ansorge et al., 2008; Bairy et al., 2007; Boulle et al., 2016a,b; Coleman et al., 1999; Ehrlich et al., 2015; Glover et al., 2015; Grimm & Frieder, 1987; Lisboa et al., 2007; Meyer et al., 2018; Nagano et al., 2012; Popa et al., 2008; Rayen et al., 2011; Toffoli et al., 2014). On the other hand, a small number of studies observed enhanced anxiety following prenatal, postnatal, or perinatal SSRI exposure (Butkevich & Mikhailenko, 2018; Ko et al., 2014; Millard et al., 2019; Rebello et al., 2014; Sarkar et al., 2014; Zohar et al., 2016). Conversely, a decrease in anxiety behaviour following prenatal or postnatal SSRI exposure was reported (da Silva et al., 2014; Glover et al., 2015; Ko et al., 2014). The high variability in the type of SSRI used, dosage, method of SSRI administration, timing, and type of behavioural testing, as well as the parameters assessed in the anxiety‐related behavioural tests might explain the differences in results reported by the various studies (Table 2). Furthermore, Rebello et al. (2014) detected behavioural effects of postnatal exposure to fluoxetine (FLX) only when medication was administered between P 2 and P 11. Those results suggest that in mice, P 2–P 11 is a time sensitive period that may characterize this animal model (Rebello et al., 2014). In summary, early‐life SSRI exposure increases or decreases anxiety‐related behaviour, depending on numerous factors such species‐specific and time‐sensitive periods, SSRI dosage and age of the offspring studied.
TABLE 2.
Overview table of studies investigating behaviour following early life SSRI exposure
| Reference | Type of study | Sex | SSRI (/day) | Time of exposure | Outcome measures | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G0–22 | P1–3 | P4–7 | P8–14 | P15–21 | |||||||
| Ehrlich et al. (2015) | Rat | F | ESCI to dams | G0–P1 | OPT, SIT, novel object recognition; EPM. | No effects. | |||||
| 12.2 mg/kg | |||||||||||
| Bairy et al. (2007) | Rat | M/F | FLX to dams | G6–G20 | OFT, Morris water maze test, EPM. | Decreased anxiety at P 16 but increase at P 35. | |||||
| 8 mg/kg; 12 mg/kg | |||||||||||
| Butkevich and Mikhailenko (2018) | Rat | F | FLX to dams | G9–G20 | EPM, FST, Morris water maze. | Increased anxiety. No effect on depressive‐like behaviour. | |||||
| 10 mg/kg | |||||||||||
| Grimm and Frieder (1987). | Rat | M/F | Zimelidine to dams | G10–G20 | OFT. | Decreased anxiety at P 20. | |||||
| 5 mg/kg | P4–P8 | Increased anxiety at P 50 (males). | |||||||||
| Nagano et al. (2012) | Rat | M | DEX to dams; FLX to dams | DEX: G16–21 | OFT, light–dark choice test. | No effects. | |||||
| 50 ug/kg; 0.1 mg/mL | FLX: P2–21 | ||||||||||
| Millard et al. (2019). | Rat | M | FLX to dams | G0–P14 | OFT, EPM, FST. | Increased anxiety; increase depressive like‐behaviour. | |||||
| 10 mg/kg | |||||||||||
| Toffoli et al. (2014) | Rat | M | FLX to dams | G0–P21 | EPM, OFT. | No effects. | |||||
| 5 mg/kg | |||||||||||
| Francis‐Oliveira et al. (2013) | Rat | M/F | FLX to dams | G0–P21 | Sucrose preference test; NSF. | Decreased anxiety; decrease depressive‐like behaviour. | |||||
| 5 mg/kg | |||||||||||
| Glover et al. (2015) | Rat | M | PAR to dams | G0–P21 | FST, EPM, OFT. | Increased depressive‐like behaviour (when genetically predisposed) | |||||
| 10 mg/kg | |||||||||||
| Zohar et al. (2016) | Rat | M/F | CIT to dams | G7–P21 | EPM, FST. | Increased anxiety (male); increase depressive‐like behaviour. | |||||
| 10 mg/kg | |||||||||||
| Gemmel et al. (2017) | Rat | M/F | FLX to dams | G10–P21 | Play behaviour test, SIT. | FLX affects social behaviour in adolescence (males). | |||||
| 5 g/kg | |||||||||||
| Gemmel et al. (2019) | Rat | M/F | FLX to dams | G10–P21 | SIT. | Increased play behaviour (males). Decreased play behaviour (female). | |||||
| 5 g/kg | |||||||||||
| Ko et al. (2014) | Rat | M | FLX | P0–P4 | OFT, EPM, FST, SIT, acoustic startle response and prepulse inhibition. | Decreased anxiety; increased depressive‐like behaviour. Increase anxiety to novel environment | |||||
| 20 mg/kg | |||||||||||
| Lee (2009) | Rat | M/F | FLX | P0–P6 | Oft. | Reduced exploratory behaviour. | |||||
| 10 mg/kg | |||||||||||
| Rayen et al. (2011) | Rat | M/F | FLX | P1–P21 | OFT, FST. | No effects. | |||||
| 5 mg/kg | |||||||||||
| Boulle et al. (2016b) | Rat | F | FLX to dams | P1–P21 | OFT, EZM, FST. | Increased depressive‐like behaviour. No effects on anxiety. | |||||
| 5 g/kg | |||||||||||
| Boulle et al. (2016a) | Rat | M | FLX to dams | P1–P21 | OFT, EZM, FST. | Decreased anxiety. No effects on depressive‐like behaviour. | |||||
| 5 g/kg | |||||||||||
| da Silva et al. (2014) | Rat | F | FLX | P1–P21 | Behavioural satiety sequence; EPM. | Decreased anxiety. | |||||
| 10 mg/kg | |||||||||||
| Sarkar et al. (2014) | Rat | M | FLX | P2–P21 | OFT, EPM, FST. | Increased anxiety; increase depressive‐like behaviour. Mediated by 5‐HT2a and 5‐HT2c | |||||
| 10 mg/kg | |||||||||||
| Bijlsma et al. (2015) | Rat | M | SERT‐KO | SERT‐KO | Startle apparatus. | SERT‐KO present increased fear response in adulthood | |||||
| Coleman et al. (1999) | Mouse | M/F | PAR to dams | G0–G16.5 | EPM, 8‐arm maze, FST. | No effects. | |||||
| 20 mg/kg | |||||||||||
| Meyer et al. (2018) | Mouse | M/F | SET | G0–P14 | SIT; EPM. | No effects. | |||||
| G0‐P1: 5 mg/kg to dam; P1‐P14: 1.5 mg/kg | |||||||||||
| Lisboa et al. (2007) | Mouse | M/F | FLX to dams | G0–P21 | OFT, FST, EPM, intruder‐resident test. | Increased depressive‐like behaviour (female). No effects on anxiety. | |||||
| 20–40 mg/kg | |||||||||||
| Soiza‐Reilly et al. (2019) | Mouse | M/F | FLX | P2–P14 | FST, locomotor activity, NSF. | Increased depressive‐like behaviour. | |||||
| 10 mg/kg | |||||||||||
| Rebello et al. (2014) | Mouse | M/F | FLX | P2–P21; P2–P11; P12–P21 | OFT, NSF, SESC, FST, sucrose preference test; fear conditioning. | Increased anxiety. Increased depressive‐like behaviour. | |||||
| 10 mg/kg | |||||||||||
| Ansorge et al. (2008) | Mouse | M | FLX; desipramine; CIT and clomipramine | P4–P21 | OFT, EPM; NSF, NIH, shock escape. | Increased anxiety; decreased exploration. | |||||
| 10 mg/kg; 10 mg/kg; 10 mg/kg; 20 mg/kg | |||||||||||
| Karpova et al. (2009) | Mouse | M | FLX | P4–P21 | Light–dark test; OFT; FST. | Decreased exploratory behaviour; increased anxiety; decreased depressive‐like behaviour. | |||||
| 5 mL/kg | |||||||||||
| Ansorge et al. (2004) | Mouse | M/F | FLX | P4–P21 | OFT, EPM, NSF. SESC. | Increased anxiety. | |||||
| 10 mg/kg | |||||||||||
| Popa et al. (2008) | Mouse | F | ESCI | P5–P19 | Dark/light box, EPM, sucrose preference, TST, FST. | No effects on anxiety. Increased depressive‐like behaviour. | |||||
| SERT‐KO | 10 mg/kg | ||||||||||
| Wellman et al. (2007) | Mouse | M | SERT‐KO | SERT‐KO | FST. | Increased depressive‐like behaviour following stress. | |||||
| Rotem‐Kohavi et al. (2019) | Clinical | M/F | FLX, PAR, SET, CIT | Prenatal | Infant behaviour questionnaire at 6 months of age. | No effects. | |||||
Note: Most preclinical studies investigated anxiety and depressive‐like behaviour. One clinical study was encounter. Studies are display according to the following criteria: (1) animal model used (rats, mouse, other); (2) Time of SSRI exposure (prenatal administration, perinatal administration, P1 to P3, P4 to P7, P8 to P14, and P15 to P21, SERT‐KO). BNZ: benzodiazepine; CIT: citalopram; DEX: dexamethasone; EPM: elevated plus maze; EZM: elevated zero maze; ESCI: escitalopram; F: female; FLX: fluoxetine; FST: forced swim test; G: gestational day; M: male; NIH: novelty‐suppressed hypophagia; NSF: Novelty‐suppressed feeding; OFT: open‐field test; P: postnatal day; PAR: paroxetine; SERT‐KO: SERT‐knock out; SESC: shock escape paradigm; SET: sertraline; SIT: social interaction test; TST: tail suspension test; vFh: von Frey hair.
Interestingly, exposure to perinatal SSRI diminishes exploratory behaviour in adult animals (Ansorge et al., 2004, 2008; Grimm & Frieder, 1987; Karpova et al., 2009; Lee, 2009; Millard et al., 2019). While anxiety represents a fearful response to an (anticipated) aversive event, and may involve differences in exploratory behaviour, the latter also depends on the innate drive of rodents to apprehend their environment. Although most studies reported no effect of perinatal SSRI exposure on anxiety per se, they consistently report decreased exploratory behaviour (Ansorge et al., 2004, 2008; Glover et al., 2015; Karpova et al., 2009; Millard et al., 2019). For instance, animals postnatally exposed to SSRI developed an enlarged latency to feeding in the NSF test in late adulthood (Ansorge et al., 2004, 2008; Karpova et al., 2009; Rebello et al., 2014; Soiza‐Reilly et al., 2019). Furthermore, postnatal SSRI exposure decreases exploratory behaviour in all but SERT −/− knockout animals, suggesting that the effect of postnatal SSRI exposure on exploratory behaviour is 5‐HT dependent (Ansorge et al., 2004). This conclusion is supported by animals inclined to risk‐taking behaviour exhibiting serotonergic circuit disparities, which likely explain different outcomes in exploratory behaviour with breeds prone to anxious temperament (Glover et al., 2015; Kerman et al., 2011). The work by Glover et al. (2015) suggests that intensified extracellular 5‐HT concentrations reduce exploratory behaviour, which may also result from disrupted fear acquisition following alteration of the SERT system during development (Bijlsma et al., 2015; Glover et al., 2015; Rebello et al., 2014). Lower innate drive to exploration in rodents following early‐life SSRI exposure suggests anxiety and/or depressive‐like behaviour. However, the decrease in exploratory behaviour upon perinatal SSRI exposure was not supported by all studies; two studies reported no effects of prenatal SSRI exposure on adult exploratory behaviour in the OFT and the 8‐maze, results potentially explained by the use of female instead of male rodents and/or the use a different behavioural test (Coleman et al., 1999; Ehrlich et al., 2015). While a study found a negative effects of SERT‐knock out (SERT‐KO) on the time spent in the light compartment, other studies did not find an effect of postnatal and perinatal SSRI treatment on exploratory behaviour in the light/dark box and the NSF (Francis‐Oliveira et al., 2013; Nagano et al., 2012; Popa et al., 2008). Indeed, SERT‐KO animals seemed to display a more anxious temperament in the light/dark box and the EPM as compared with wild type animals postnatally treated with SSRIs (Popa et al., 2008). The contradictory findings regarding exploratory behaviour may result from the interdependency between exploratory behaviour, anxiety, and depressive‐like behaviour.
Numerous rodent studies have reported enhanced depression‐related behaviour in adulthood following perinatal SSRI exposure (Boulle et al., 2016b; Glover et al., 2015; Ko et al., 2014; Lisboa et al., 2007; Millard et al., 2019; Rebello et al., 2014; Sarkar et al., 2014; Soiza‐Reilly et al., 2019; Zohar et al., 2016). Notably, perinatal SSRI exposure increases depressive‐like symptoms in the forced swim test (FST) and the sucrose preference test (Boulle et al., 2016b; Glover et al., 2015; Millard et al., 2019; Popa et al., 2008; Rebello et al., 2014; Zohar et al., 2016). Furthermore, the development of depressive‐like behaviour following perinatal SSRI exposure is dependent on SERT availability, as suggested by the development of depressive‐like behaviour in bred animals naturally prone to anxious temperament but not in risk‐taking animals (Glover et al., 2015). Findings from early‐life SSRI exposure are supported by SERT‐KO models reporting increased depressive‐like behaviours in the FST (Wellman et al., 2007). No significant differences were observed in depressive‐like behaviour between SERT‐KO and wild‐type animals exposed to SSRIs perinatally (Popa et al., 2008).
In conclusion, exposure to SSRI in early life results in decreased exploratory behaviour and increased depressive‐like behaviour in rodents. Considering the intensified depressive‐like behaviour following early‐life SSRI exposure, reduced exploratory behaviour might be linked to depression rather than anxiety‐related mechanisms. Depressive‐like and anxiety‐related phenotypes are shown to predispose the individuals to pain, and increased depressive‐like behaviour may in turn affect pain behaviour in an early life SSRI exposure‐dependent manner (Woo, 2010). Furthermore, if neuronal networks involved in depression and anxiety‐related processing and that are shared with pain are affected by early life SSRI exposure, one may expect pain alterations following perinatal serotonergic modulation.
4. THE ROLE OF THE PFC AND THE AMYGDALA IN THE LONG‐TERM CONSEQUENCES OF EARLY‐LIFE SSRI EXPOSURE IN THE MODULATION OF PAIN
The PFC and the amygdala are closely linked to anxiety and depression, but also play a role in the descending serotonergic modulation of nociception (Huang et al., 2019; Jalbrzikowski et al., 2017). As early‐life SSRI impacts later life nociception and depressive‐like behaviour, it is of major interest to understand the effects of SSRI exposure around birth on the maturation of the PFC and the amygdala.
By the presence of SERT and 5‐HT receptors in the PFC and the amygdala, early life SSRI exposure affects the maturation of those regions. In the PFC, SERT is expressed in glutamatergic pyramidal neurons between P 0 and P 10 (Narboux‐Nême et al., 2008; Soiza‐Reilly et al., 2019). The high expression of monoamino‐oxidase (MAO) A and MAO B, the absence of tryptophan hydroxylase (Tph) or aromatic acid decarboxylase and the presence of SERT in a small subset of PFC neurons suggests that those SERT‐positive neurons remove serotonin from the extracellular space during postnatal life (Soiza‐Reilly et al., 2019). This subset of PFC neurons is therefore vulnerable to the modulation of the SERT system by perinatal SSRI exposure. For instance, postnatal exposure of the SSRI Citalopram downregulated the expression of SERT in the mPFC in later life, while perinatal exposure of SSRI Sertraline exposure upregulated cortical SERT expression in later life (Meyer et al., 2018; Simpson et al., 2011; Zhou et al., 2015). In neurons of the amygdala, SERT is not expressed throughout life but is present in nerve endings innervating the amygdala (Asan et al., 2013; Narboux‐Nême et al., 2008). Moreover, prenatal SSRI‐treatment upregulated the autoregulatory 5‐HT1a receptor in the amygdala, suggesting the development of fewer serotonergic neurons compared with control offspring (Ehrlich et al., 2015; Rumajogee et al., 2004).
4.1. Early‐life SSRI exposure affects cortical maturation and intraconnectivity of PFC and amygdala
Serotonin production and degradation in the PFC were shown to be vulnerable to perinatal SSRI exposure (Gemmel et al., 2018; Glazova et al., 2014; Meyer et al., 2018; Velasquez et al., 2019). Lower levels of serotonin were found in the G 17 embryo of mice treated with SSRIs at G 8, implying that early‐life SERT blockade does not, contrary to adulthood, lead to a long‐lasting raise in serotonin concentrations in the extracellular space (Velasquez et al., 2019). Prenatal SSRI exposure resulted in extracellular levels of serotonin as low as that encountered in SERT‐KO animals at G 17 (Velasquez et al., 2019). In contrast, the rate‐limiting enzyme for serotonin production (Tph2) is upregulated in 20‐week‐old rodent PFC after perinatal SSRI exposure, suggesting higher levels of extracellular serotonin (Meyer et al., 2018; Walther et al., 2003). In line with these results, heightened serotonin turnover and levels were reported in adolescent animals (Gemmel et al., 2018; Glazova et al., 2014). Thereupon early‐life SSRI exposure has a developmental timing effect, with a transitory decrease in serotonin neonates but an increase starting in adolescence. Changes in PFC serotonergic levels after perinatal SSRI exposure were not observed in all studies and are likely the result of a species‐specific and time‐sensitive period for observing consequences of SSRI exposure (Gemmel et al., 2016; Grimm & Frieder, 1987; Ko et al., 2014; Suri et al., 2015). The transitory decrease in extracellular serotonin during early life has structural and physiological consequences mediated by 5‐HT receptors: while some 5‐HT receptors present a time‐sensitive shift in cortical response from excitatory to inhibitory, other 5‐HT receptors such as 5‐HT 1a/1b have auto‐inhibitory effects on the growth of serotonergic neurons (Suri et al., 2015). In addition, the FLX‐mediated stimulation of 5‐HT2a downregulated this receptor, leading to long lasting anxiety‐related and depressive‐like behaviour (Sarkar et al., 2014). The role of 5‐HT receptor alterations following early life SSRI exposure and implications on pain comorbidities has been reviewed in more detail by Suri and colleagues.
4.2. Changes in serotonergic metabolism impact upon epigenetic mechanisms within the PFC and the amygdala
While to the best of our knowledge, no studies have investigated early‐life SSRI‐induced alteration in serotonin levels in the amygdala, one study reported downregulation of multiple genes in the amygdala between P 14 and P 21 in animals prone to anxious behaviour perinatally exposed to SSRI (Table 3) (Glover et al., 2015). Unfortunately, while the genes seemed to be associated with haemoglobin complex and intracellular organelles, the functional implications of this downregulation of genes in the amygdala are not clear (Glover et al., 2015). In the PFC, increased methylation patterns following perinatal SSRI administration were reported, which suggests epigenetic changes to be associated with early‐life SSRI exposure (Toffoli et al., 2014). Similarly, SERT‐KO animals showed reinforced 5′‐cytosine‐phosphate‐guanine‐3′ (CpG) methylation pattern at P 7 and P 21, indicating epigenetic changes associated with SERT‐KO (Calabrese et al., 2013). SERT‐KO animals presented down‐ and up‐regulation of genes involved in cytoskeleton interaction, neurite/axon outgrowth and synaptic development (Soiza‐Reilly et al., 2019).
TABLE 3.
Overview table of studies investigating alteration in the PFC and/or the amygdala following early life SSRI exposure
| Reference | Type of study | Sex | SSRI (/day) | Time of exposure | Outcome measures | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G0–22 | P1–3 | P4–7 | P8–14 | P15–21 | |||||||
| Prefrontal cortex | |||||||||||
| Grimm and Frieder (1987) | Rats | M/F | Zimelidine to dams | G10–G20 | 5‐HT labelling: measurement of spontaneous release. | No effects. | |||||
| 5 mg/kg | P4–P8 | ||||||||||
| Cabrera‐Vera et al. (1997) | Rats | M | FLX to dams | G13–G20 | HPLC: monoamine and biogenic amines. Radioligand binding assay for 5‐HT uptake sites. | Increased 5‐HT content in the frontal cortex at PD 26 but not PD 70. No effects on SERT binding. | |||||
| 10 mg/kg | |||||||||||
| Millard et al. (2019) | Rats | M | FLX to dams | G0–P14 | Western blot: NR1, NR2a, NR2b, PSD‐95, mGluR1, mGluR5, Homer1b/c and b‐actin. | Decreased cortical level of glutamatergic markers (NR1, NR2a, PSD‐95, GluR1) | |||||
| 10 mg/kg | |||||||||||
| Toffoli et al. (2014) | Rats | M | FLX to dams | G0–P21 | DNA samples and global methylation profile. | Increased methylation % in the cortex. | |||||
| 5 mg/kg | |||||||||||
| Zohar et al. (2016) | Rats | M/F | CIT to dams | G7–P21 | IHC: serotonin, tryptophan, hydroxylase, 5‐HT1a, and corticotrophin releasing factor 2. | Sex different 5‐HT1a distribution: affected by CIT in males but not females. | |||||
| 10 mg/kg | |||||||||||
| Gemmel et al. (2018) | Rats | M/F | FLX to dam | G10–P21 | HPLC‐Ed measurement of monoamine levels. IHC of synaptophysin, PSD‐95, GR. | Increased 5‐HT in female PFC, decreased turnover. No effect on males. | |||||
| 5 mg/kg | |||||||||||
| Ko et al. (2014) | Rats | M | FLX | P0–P4 | HPLC analysis of 5‐HIAA and 5‐HT. Western blotting for Tph levels. IHC for Trp. Golgi–Cox staining | No effects on 5‐HT metabolism. Exuberant dendritic branches. Increased dendritic complexity, greater dendritic length. Reduced dendritic densities. | |||||
| 20 mg/kg | |||||||||||
| Bock et al. (2013) | Rat | M | FLX | P1–P15 | rtPCR, ICH for S100B positive cells | Long term increased S100B cell density in the PFC and increased mRNA expression following FLX treatment. | |||||
| 5 mg/kg | |||||||||||
| Zhou et al. (2015) | Rat | M/F | CIT | P1–P10 | IHC: Tph, SERT. | Reduced SERT, DA, and GABA interneurons cell density in the mPFC following CIT exposure. | |||||
| 10 mg/kg | |||||||||||
| Glazova et al. (2014) | Rat | M/F | Fluvoxamine | P1–P14 | HPC: DA, NA, DOPAC, 5‐HT and 5‐HIAA. | Increased 5‐HT metabolism at P16. No effect on NA or DA. | |||||
| 10 mg/kg | |||||||||||
| Gemmel et al. (2016) | Rat | M/F | FLX to dam | P1–P21 | HLPC‐Ed measurement of: DA, DAPAC; HVA, 5‐HT, 5‐HIAA. IHC for double cortin and synaptophysin positive cells. | No effects. | |||||
| 5 mg/kg | |||||||||||
| Sarkar et al. (2014) | Rat | M | FLX | P2–P21 | RT‐qPCR: 5‐HT2a, 2c and 1a receptor | Stimulation of 5‐HT2a by FLX downregulate the receptor, leading to long lasting anxiety and depressive‐like behaviour. Blockade of 5‐HT2a inhibit the decreased mRNA levels. | |||||
| 10 mg/kg | |||||||||||
| Kozisek et al. (2008) | Rat | M/F | ESCI; desipramine | P9–P12 | ELISA: BDNF. rtPCR: BDNF and TrkB. | Increased in extracellular 5‐HT. Increased BDNF levels in the PFC at P13. | |||||
| 1–15 mg/kg | P17–P20 | ||||||||||
| Simpson et al. (2011) | Rat | M/F | CIT | P8–P21 | IHC and quantification: SERT and TPH. Colossal connectivity and ultrastructural analysis. | Decreased SERT density in mPFC (male) | |||||
| 5‐15 mg/kg | |||||||||||
| Maciag et al. (2006) | Rat | M | CIT (5 mg/kg) | P8–P21 | IHC: Tph, SERT and neuron specific nuclear protein. | Decreased SERT immunoreactivity in the mPFC. | |||||
| Clomipramine (15 mg/kg) | |||||||||||
| Witteveen et al. (2013) | Rat | M/F | SERT‐KO | SERT‐KO | 3D collagen co‐cultures of embryonic raphe nuclei to mPFC projections. IHC anti‐Satb2 and anti‐5‐HT and anti‐Tuji. | Maturation of the dorsal raphe to mPFC projection is SERT dependent. | |||||
| Brivio et al. (2019) | Rat | M | SERT‐KO | SERT‐KO | RT‐qPCR and Western blot: GluNI, PSD95, CDC42 and SEPT7. | Decreased NMDA level in the PFC and decrease synaptic density. Altered spine formation throughout life. | |||||
| Calabrese et al. (2013) | Rat | M | SERT‐KO | SERT‐KO | RT‐qPCR: BDNF (exon I, IV and VI), Npas4, Creb, Craf, Dnmt1, GABAay2, Gad67, Vgat. Western blot: BDNF. Methylated DNA Immunoprecipitation: BDNF exon IV. | Decreased of BDNF level (gene expression and mBDNF) in the PFC. Epigenetic modulation involved. | |||||
| Velasquez et al. (2019) | Mouse | M/F | CIT to dams | G8–G17 | HPLC: fetal brain CIT, 5‐HT and 5‐HIAA. IHC: serotonin, netrinG1, TBR1. | Decreased serotonergic tissue levels. | |||||
| 260 mg/L | |||||||||||
| Meyer et al. (2018) | Mouse | M/F | G0–P1: SET to dams | G0–P14 | RT‐qPCR for serotonin transporter and serotonin receptor expression. | Increased cortical levels of 5‐HT1a, 2a, 2c, 5‐HTT and Tph2. | |||||
| P1–P14: 1.5 mg/kg | |||||||||||
| Soiza‐Reilly et al. (2019) | Mouse | M/F | FLX | P2–P14 | IHC: 5‐HT, GFP, DsRed; Cux1; Foxp2; cfos. In situ hybridization: SERT. Array tomography: VGLUT1,2 or GAD. Western blot: VGLUT1 and GAD. Patch clamp electrophysiology. Cell sorting and RNA‐sequencing. | SERT expressed in glutamatergic pyramidal neurons, and FLX down or up regulate synaptic and circuit modelling. PFC‐SERT+ neurons involved in the top‐down modulation of emotional circuits | |||||
| SERT‐KO | 10 mg/kg | ||||||||||
| Rebello et al. (2014) | Mouse | M/F | FLX | P2–P21; P2–P11; P12–P21 | Golgi stain. Electrophysiology: whole cell and patch clamp. | Morphological changes in pyramidal neurons. Altered mPFC output. No change in Glutamatergic inputs. | |||||
| 10 mg/kg | |||||||||||
| Molteni et al. (2009) | Mouse | F | SERT‐KO | SERT‐KO | Plasma corticosterone levels. In situ hybridization: Arc, Zif.268 and B‐actin. | Lower arc in the PFC, and hyper responsiveness of arc following stress. Structural remodelling. | |||||
| Altamura et al. (2007) | Mouse | M/F | SERT‐KO | SERT‐KO | Nissls, Giesma staining. | Increase cellular density. Decrease cortical thickness. | |||||
| Rotem‐Kohavi et al. (2019) | Clinical | M/F | FLX, PAR,SET, CIT | Prenatal | Structural, microstructural and resting state functional and metabolic imaging (T1 MRI) at 40.9 weeks (postmenstrual age) | Higher connectivity in the frontal superior orbital left lobe. | |||||
| Amygdala | |||||||||||
| Ehrlich et al. (2015) | Rat | F | ESCI to dams | G0–P1 | RT‐PCR: Nkcc1, Kcc2, 5‐HT1a. | ESCI upregulate 5‐HT1a. | |||||
| 12.2 mg/kg | |||||||||||
| Francis‐Oliveira et al. (2013) | Rat | M/F | FLX to dams | G0–P21 | IHC (anti‐Fos) after stressor. | Affect amygdala circuits (down regulate activity following stressor in male, upregulate in female) | |||||
| 5 mg/kg | |||||||||||
| Glover et al. (2015) | Rat | M | PAR to dams | G0–P21 | Assessment of PARA levels in the brain and serum. RNA labelling and array hybridization, micro array analysis. | 3 genes are upregulated at P7 (bLR strain), and a greater number are downregulated from P14 through adulthood. | |||||
| 10 mg/kg | |||||||||||
| Ko et al. (2014) | Rat | M | FLX | P0–P4 | HPLC: 5‐HIAA and 5‐HT. Western blotting and IHC: Tph. Golgi–Cox staining. | No change in dendritic complexity, length, diameter and number. Decreased spine density in FLX treated animals. | |||||
| 20 mg/kg | |||||||||||
| Bijlsma et al. (2015) | Rat | M | SERT‐KO | SERT‐KO | In situ hybridization: CRF 1 receptor mRNA. | No effects. | |||||
| Narboux‐Nême et al. (2008) | Mouse | M/F | SERT‐KO | SERT‐KO | Histology: with Xgal. | No SERT expression. | |||||
| Nietzer et al. (2011) | Mouse | M | SERT‐KO | SERT‐KO | Golgi–Cox. Morphometry. | SERT KO show an increase spine and dendritic branching. | |||||
| Wellman et al. (2007) | Mouse | M | SERT‐KO | SERT‐KO | Histology and morphological analysis. | Hyperconnectivity in the amygdala. | |||||
| Lugo‐Candelas et al. (2018) | Clinical | M/F | Prenatal | 5‐HTT polymorphism. Maternal depression. T2 MRI scanner for structural and diffusion MRI | Increased: right amygdala grey matter volume, and connectivity to the right insula. | ||||||
Note: Most preclinical studies were performed on rats or mice. Two clinical studies were encounter. Studies are display according to the following criteria: (1) animal model used (rats, mouse, other); (2) Time of SSRI exposure (prenatal administration, perinatal administration, P1 to P3, P4 to P7, P8 to P14 and P15 to P21, SERT‐KO). Arc: activity‐regulated cytoskeleton‐associated protein; b‐actin: beta‐actin; BDNF: brain‐derived neurotrophic factor; BNZ: benzodiazepine; 5‐HIAA: 5‐hydroxyindoleacetic acid; 5‐HT1a: serotonin 1A receptor; CDC42: cell division control protein 42 homolog; CIT: citalopram; Cux1: cut like homeobox 1; DA: dopamine; DEX: dexamethasone; ELISA: enzyme‐linked immunosorbent assay; ESCI: escitalopram; F: female; FLX: fluoxetine; Foxp2: forkhead box protein P2; G: gestational day; GFP: green fluorescent protein; GR: glucocorticoids receptor; HLPC: high‐performance liquid chromatography; HLPC‐Ed: high‐performance liquid chromatography with electrochemical detection; ICH: immunohistochemistry; Kcc2: Ke‐Cl cotransporter 2; M: Male; mGluR1: glutamate receptor, metabotropic 1; mGluR5: glutamate receptor, metabotropic 5; Nkcc1: Na‐K‐Cl cotransporter; NR1: glutamate receptor 1; NR2a: glutamate receptor 2a; NR2b: glutamate receptor 2b; P: postnatal day; PCR: polymerase chain reaction; PSD‐95: postsynaptic density protein 95; rtPCR: real‐time polymerase chain reaction; rtqPCR: real‐time quantitative polymerase chain reaction; S100B: S100 calcium‐binding protein B; SEPT7: Septin‐7; SERT‐KO: serotonin transporter‐knock out; SET: sertraline; Tph: tryptophan hydroxylase; Trp: tryptophan synthase; Zif‐268: zinc finger binding protein clone 268.
Early‐life SSRI exposure has consequences at the gene transcription level with repercussions on neuronal maturation of the PFC and the amygdala (Calabrese et al., 2013; Glover et al., 2015; Soiza‐Reilly et al., 2019; Toffoli et al., 2014). For instance, postnatal SSRI exposure increases S100B‐positive astrocytes in the PFC (Bock et al., 2013; Gemmel et al., 2016, 2018). It is hypothesized that the transient heightened S100β following perinatal SSRI exposure results in enhanced serotonergic growth in the PFC, via stimulation of 5‐HT receptors on astrocytes, eventually resulting in S100β release (Bock et al., 2013; Gemmel et al., 2016, 2018; Glover & Clinton, 2016; Nishimura et al., 1995). Furthermore, synaptic growth is closely related to the SERT system, as suggested by the diminished presence of the synaptic growth marker postsynaptic density protein‐95 (PSD‐95) in SERT‐KO animal (Brivio et al., 2019). While Gemmel et al. (2018) reported no effect of perinatal SSRI exposure on PSD‐95 protein levels, Millard et al. (2019) showed a decline in PSD‐95 RNA levels in adult animals perinatally exposed to SSRI. It is therefore possible that, even though PSD‐95 proteins are not disturbed by the exposure to SSRI in early‐life, the changes in PSD‐95 mRNA alone following SSRI exposure around birth are sufficient to affect synaptic growth and development (Gemmel et al., 2018; Millard et al., 2019). Likewise, perturbations of the serotonergic system in early‐life alters the expression of fundamental markers of the glutamatergic synapses. For instance, SERT‐KO animals presented decreased mRNA and protein levels of Sept7 and CDC42, two transcriptional markers involved in axonal and dendritic development of the glutamatergic fibers (Brivio et al., 2019). In summary, decreased Sept7, CDC 42 and PSD‐95 levels after early‐life SSRI exposure indicate altered maturation of glutamatergic circuit in the PFC (Brivio et al., 2019). Other transcriptional factors and proteins (Creb, Arc, Zif‐268) involved in activity‐dependent remodelling were also shown to be downregulated in PFC of SERT‐KO animals (Calabrese et al., 2010; Molteni et al., 2009).
Maturation of the PFC and amygdala after early‐life SSRI exposure are also regulated by neurotrophic factors like BDNF (Liu et al., 2017). BDNF expression was increased following administration of SSRI in the second postnatal week of the rat while the BDNF tropomyosin‐related kinase B receptor (trkB) expression was not affected by perinatal SSRI exposure, suggesting no alteration of the intracellular BDNF cascade (Kozisek et al., 2008). Contrary to wild‐type mice exposed to SSRIs perinatally, studies provided evidence that SERT‐KO‐mice present diminished BDNF mRNA isoform expression, but not its protein levels (Calabrese et al., 2010, 2013). It is not completely clear how BDNF transcriptional pathways are affected by early‐life SSRI exposure, but the alteration in the BDNF system following perinatal changes in the SERT system may participate in the observed amygdala and the PFC intra‐hyperconnectivity (Calabrese et al., 2013; Pezawas et al., 2008). Research on the effects of early‐life SSRI exposure on transcriptional and neurotrophic factors is limited to the PFC, and future research should include changes and effects in the amygdala.
As a result of affected serotonin levels, transcriptional factors and BDNF levels within the PFC and amygdala, the maturation of neurons, dendrites and synapses in these brain areas are increased upon perinatal SSRI exposure (Altamura et al., 2007; Ko et al., 2014; Nietzer et al., 2011; Rotem‐Kohavi et al., 2019; Velasquez et al., 2019; Wellman et al., 2007; Witteveen et al., 2013). Preclinical studies show that perinatal exposure to SSRI increases dendritic complexity in the PFC (Altamura et al., 2007; Ko et al., 2014; Velasquez et al., 2019; Witteveen et al., 2013). For instance, diminished serotonergic availability induced by prenatal SSRI exposure altered cortical cell proliferation and/or survival, as demonstrated by a rise in cortical cells in the PFC without affecting cell proportion (Velasquez et al., 2019). Furthermore, SERT‐KO mice have shown to present increased fascicle numbers and length as well as heightened innervation and longer fibre in the PFC (Altamura et al., 2007; Witteveen et al., 2013). Clinical data also showed a reinforced connectivity in the frontal superior orbital cortex, a region of the PFC, in children exposed to SSRI around birth (Rotem‐Kohavi et al., 2019). Similarly, perinatal SSRI exposure led to strengthened dendritic complexity in the amygdala and eventually to hyperconnectivity (Nietzer et al., 2011; Wellman et al., 2007). Contradictory results were reported with decreased spine density, number of nodes and intersection in the PFC and the amygdala adult animal following postnatal SSRI treatment (Ko et al., 2014; Rebello et al., 2014). Nevertheless, the hyperconnectivity observed in rodent's basolateral amygdala (BLA) is in line with a clinical study reporting an enlargement in grey matter in the amygdala of newborns exposed to SSRI during pregnancy (Lugo‐Candelas et al., 2018). It should be noted that these clinical findings were contradicted by no change in amygdala connectivity in infants exposed to prenatal SSRI (Rotem‐Kohavi et al., 2019).
4.3. Early‐life SSRI exposure influences the PFC modulation of pain via GABAergic interneurons
Exposure to SSRIs in early‐life induces effects on PFC that are not solely limited to the serotonergic system but also affects the glutamatergic and GABAergic networks (Brivio et al., 2019; Calabrese et al., 2013; Millard et al., 2019; Zhou et al., 2015). Indeed, perinatal SSRI exposure has been shown to diminish both glutamatergic and GABAergic receptor expression in the PFC and similar finding were encountered in the SERT‐KO animals (Brivio et al., 2019; Calabrese et al., 2013; Millard et al., 2019; Zhou et al., 2015). More importantly, administration of SSRIs during the first postnatal week reduced the immunoreactivity of Ca2+‐binding protein parvalbumin (PVA) in the GABAergic interneuron in late adulthood, implying that early‐life SSRI exposure downregulates both glutamatergic and GABAergic networks in the PFC (Zhou et al., 2015). In this context, it is to be noted that PVA‐immunoreactive GABAergic interneurons are involved in the top‐down modulation of nociception by the PFC and the amygdala (Huang et al., 2019). In a pronociceptive manner, the BLA projects to both pyramidal neurons and PVA‐immunoreactive GABAergic interneurons, which also form mono‐synapses with pyramidal neurons (Huang et al., 2019). The ablation of amygdala inputs to the pyramidal neurons via the PVA‐immunoreactive GABAergic interneurons and mono‐synaptic projection weakens the feedforward inhibition of pyramidal neurons, in turn strengthening PFC projections to the ventrolateral PAG (vlPAG) (Huang et al., 2019). A similar situation may be expected upon decreased presence of PVA‐immunoreactive GABAergic interneurons following postnatal SSRI exposure, where glutamatergic input from the BLA contributes to the inhibition of pyramidal neurons. The infralimbic and the prelimbic region of the PFC respond with decreased and increased frequency to a general stimulus, respectively, in mice treated with postnatal SSRIs compared with mice treated with vehicle, which can also contribute to a disrupted balance in the mPFC output (Rebello et al., 2014). Altogether, the strengthened input to the vlPAG results in an enhanced noradrenergic and serotonergic antinociceptive descending modulation of the incoming nociceptive signals in the spinal cord (Huang et al., 2019).
Over all, although the amygdala is hyperconnected, its input to the PFC remains limited due to diminished presence of PVA‐GABAergic interneurons (Zhou et al., 2015). On the other hand, PFC hyperconnectivity strengthens inputs to the PAG, eventually providing antinociceptive serotonergic and adrenergic modulation in the spinal cord (Huang et al., 2019). Upon acute stress, hyperexpression of the transcriptional factors Arc and Zif‐268 suggests enhanced activation of PFC networks and thereby an increased antinociceptive modulation of the PFC (Molteni et al., 2009) (Figure 1).
FIGURE 1.
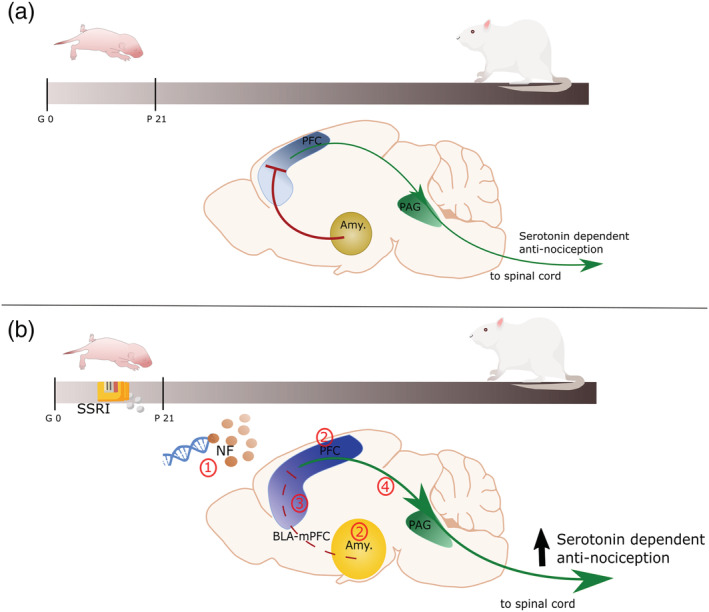
Schematic representation of the role of the PFC and amygdala roles on nociceptive processing in normal animals (a) and after early‐life SSRI exposure (b). (a) In normal condition, PVA interneurons connect the amygdala to the PFC, and with reciprocal connection form an important part of the nociceptive network in the adult rat. The PVA interneurons inhibit the pyramidal neurons of the PFC, which in turn, via the periaqueductal grey, results in a reduced serotonin‐dependent antinociceptive modulation of the spinal cord pain gate. (b) Upon early life SSRI exposure, adult animals present (1) epigenetic alteration of genes involved in cytoskeleton maturation and synaptic development, affected neurotrophic factors and serotonin levels in the PFC, (2) intra‐hyperconnectivity in the amygdala and the PFC, and (3) a diminished PVA interneurons reactivity leading to disinhibition of the PFC by the amygdala. Reduced PFC feedforward inhibition is the results of disrupted inhibitory and excitatory balance in the PVA interneurons, the monosynaptic connection of the BLA with the pyramidal neurons, and other inputs into the pyramidal neurons. (4) Consequently, PFC outputs to the spinal cord via PAG are strengthened, resulting in enhanced serotonin‐dependent antinociceptive input. Amy.: amygdala; BLA: basolateral amygdala; BLA‐PFC: network of neurons including (1) output of the BLA into PVA interneurons and pyramidal neurons, (2) input into pyramidal neurons from other cortical areas and (3) pyramidal neurons; NF: neurotrophic factors PAG: periaqueductal grey; PFC: prefrontal cortex; PVA: parvalbumin GABAergic; SSRI: selective serotonin reuptake inhibitors
In conclusion, early‐life SSRI exposure affects serotonin levels, transcription factors and BDNF levels throughout the development, resulting in hyperconnectivity of the PFC and the amygdala. This hyperconnectivity strengthens the disinhibition of the PFC following the reduced presence of PVA‐GABAergic interneurons and thereby enhances the antinociceptive modulation of the PFC on the spinal cord.
5. PERSPECTIVES AND CONCLUSIONS
While untreated maternal depression has been shown to increase the risk of fetal growth disorder, miscarriage, and complications during delivery, growing evidence suggests that exposure to SSRI around birth has long lasting consequences on pain as well as its affective comorbidities (Schaffir, 2018). As of today, no clinical study reported on the long‐term effects of perinatal SSRI exposure on pain past a few months of age. The serotonin system is highly conserved across species and therefore, the alterations observed in rodents' studies could give pertinent insights into the neurodevelopment of children following early‐life SSRI exposure (Bacqué‐Cazenave et al., 2020; Gingrich et al., 2017). The data gathered in this perspective paper provide important evidence to understand the effects of early‐life SSRI exposure on pain in later life and may act as a guide for future treatment of this vulnerable patient population.
This review has concentrated on the role of the PFC and amygdala in the long‐term effects of perinatal SSRI exposure. Results are limited by the inconsistent use of males and females, thereby restricting conclusions regarding sex effects and the variability in dosage and type of SSRI. Based on the evidence reviewed, four conclusions can be drawn: Early‐life SSRI exposure has long‐term effects on nociception, which needs to be more consistently studied. Second, heightened depressive‐like behaviour and decreased exploratory behaviour in rodent emerge following exposure to SSRI in early‐life. Third, intra‐hyperconnectivity of the amygdala and the PFC results from altered serotonin levels, transcription factors expression and BDNF levels following perinatal SSRI exposure. Finally, the alteration of GABAergic system resulting from exposure to SSRI in early life induces antinociceptive input from the PFC and the amygdala to the spinal cord in later life.
There is still much to understand on the consequences of early‐life SSRI exposure on pain processing. Considering the pivotal role of the amygdala and its connectivity with PFC in nociception, chronic pain, major depression and anxiety disorder, future research should clarify the role of the amygdala in SSRI mediated long‐term consequences. Likewise, most studies have investigated the PFC and amygdala as separate entities, while it is of equal importance to move beyond their separate effects and focus upon their connectivity related to the SSRI mediated effects. Regarding the intra‐hyperconnectivity of the amygdala and the PFC, it would be interesting and relevant to examine the nature of the hyperconnected fibers. Although the PFC and the amygdala play a pivotal role in the nociceptive consequence of early life SSRI, other players such as the thalamocortical circuits and the spinal cord, which both are known to be involved in the modulation of pain, may be affected by serotonergic transmission or early life SSRI exposure (de Kort et al., 2021; Lee, 2009).
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
AUTHOR CONTRIBUTIONS
MB, DvdH, and BJO designed and conceptualized the manuscript, contributed to the structure and content. MB drafted the manuscript and figures. MB and RdK determined the systematic search strategy and performed the search. All authors commented on previous versions of the manuscript, critically revised, and quality assessed the manuscript. All authors have read and approved the final version of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15544.
ACKNOWLEDGEMENT
M.B. is financially supported by the the Pain Knowledge Center from Maastricht.
Baudat, M. , de Kort, A. R. , van den Hove, D. L. A. , & Joosten, E. A. (2022). Early‐life exposure to selective serotonin reuptake inhibitors: Long‐term effects on pain and affective comorbidities. European Journal of Neuroscience, 55(1), 295–317. 10.1111/ejn.15544
Edited by: Michel Barrot
DATA AVAILABILITY STATEMENT
The data that support the findings of this review are available from the corresponding author upon reasonable request.
REFERENCES
- Altamura, C. , Dell'Acqua, M. L. , Moessner, R. , Murphy, D. L. , Lesch, K. P. , & Persico, A. M. (2007). Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: A quantitation study. Cerebral Cortex, 17(6), 1394–1401. 10.1093/cercor/bhl051 [DOI] [PubMed] [Google Scholar]
- Ansorge, M. S. , Morelli, E. , & Gingrich, J. A. (2008). Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(1), 199–207. 10.1523/JNEUROSCI.3973-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge, M. S. , Zhou, M. , Lira, A. , Hen, R. , & Gingrich, J. A. (2004). Early‐life blockade of the 5‐HT transporter alters emotional behavior in adult mice. Science, 306(5697), 879–881. 10.1126/science.1101678 [DOI] [PubMed] [Google Scholar]
- Asan, E. , Steinke, M. , & Lesch, K. P. (2013). Serotonergic innervation of the amygdala: Targets, receptors, and implications for stress and anxiety. Histochemistry and Cell Biology, 139(6), 785–813. 10.1007/s00418-013-1081-1 [DOI] [PubMed] [Google Scholar]
- Bacqué‐Cazenave, J. , Bharatiya, R. , Barrière, G. , Delbecque, J. P. , Bouguiyoud, N. , di Giovanni, G. , Cattaert, D. , & de Deurwaerdère, P. (2020). Serotonin in animal cognition and behavior. International Journal of Molecular Sciences, 21(5), 1649. 10.3390/ijms21051649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairy, K. L. , Madhyastha, S. , Ashok, K. P. , Bairy, I. , & Malini, S. (2007). Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology, 79(1), 1–11. 10.1159/000096645 [DOI] [PubMed] [Google Scholar]
- Baldwin, D. , & Rudge, S. (1995). The role of serotonin in depression and anxiety. International Clinical Psychopharmacology., 9, 41–46. 10.1097/00004850-199501004-00006 [DOI] [PubMed] [Google Scholar]
- Bernard, J. F. , Bester, H. , & Besson, J. M. (1996). Involvement of the spino‐parabrachio‐amygdaloid and‐hypothalamic pathways in the autonomic and affective emotional aspects of pain. Progress in Brain Research, 107, 243–255. 10.1016/S0079-6123(08)61868-3 [DOI] [PubMed] [Google Scholar]
- Bijlsma, E. Y. , Hendriksen, H. , Baas, J. M. , Millan, M. J. , & Groenink, L. (2015). Lifelong disturbance of serotonin transporter functioning results in fear learning deficits: Reversal by blockade of CRF1 receptors. European Neuropsychopharmacology, 25(10), 1733–1743. 10.1016/j.euroneuro.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Bock, N. , Koc, E. , Alter, H. , Roessner, V. , Becker, A. , Rothenberger, A. , & Manzke, T. (2013). Chronic fluoxetine treatment changes S100B expression during postnatal rat brain development. Journal of Child and Adolescent Psychopharmacology, 23(7), 481–489. 10.1089/cap.2011.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle, F. , Pawluski, J. L. , Homberg, J. R. , Machiels, B. , Kroeze, Y. , Kumar, N. , Steinbusch, H. W. , Kenis, G. , & van den Hove, D. (2016a). Prenatal stress and early‐life exposure to fluoxetine have enduring effects on anxiety and hippocampal BDNF gene expression in adult male offspring. Developmental Psychobiology, 58(4), 427–438. 10.1002/dev.21385 [DOI] [PubMed] [Google Scholar]
- Boulle, F. , Pawluski, J. L. , Homberg, J. R. , Machiels, B. , Kroeze, Y. , Kumar, N. , Steinbusch, H. W. M. , Kenis, G. , & van den Hove, D. L. A. (2016b). Developmental fluoxetine exposure increases behavioral despair and alters epigenetic regulation of the hippocampal BDNF gene in adult female offspring. Hormones and Behavior, 80, 47–57. 10.1016/j.yhbeh.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Breivik, H. , Collett, B. , Ventafridda, V. , Cohen, R. , & Gallacher, D. (2006). Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. European Journal of Pain, 10(4), 287–333. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Brivio, P. , Homberg, J. R. , Riva, M. A. , & Calabrese, F. (2019). Alterations of glutamatergic markers in the prefrontal cortex of serotonin transporter knockout rats: A developmental timeline. Cellular and Molecular Neurobiology, 39(5), 715–720. 10.1007/s10571-019-00673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkevich, I. P. , & Mikhailenko, V. A. (2018). Effect of fluoxetine in prenatal period on nociceptive system reactivity and psychoemotional behavior in young female rats. Bulletin of Experimental Biology and Medicine, 165(2), 209–212. 10.1007/s10517-018-4131-9 [DOI] [PubMed] [Google Scholar]
- Cabrera‐Vera, T. M. , Garcia, F. , Pinto, W. , & Battaglia, G. (1997). Effect of Prenatal Fluoxetine (Prozac) exposure on brain serotonin neurons in prepubescent and adult male rat offspring. The Journal of Pharmacology and Experimental Therapeutics, 280(1), 138–145. PUMID: 8996191. [PubMed] [Google Scholar]
- Calabrese, F. , Guidotti, G. , Middelman, A. , Racagni, G. , Homberg, J. , & Riva, M. A. (2013). Lack of serotonin transporter alters BDNF expression in the rat brain during early postnatal development. Molecular Neurobiology, 48(1), 244–256. 10.1007/s12035-013-8449-z [DOI] [PubMed] [Google Scholar]
- Calabrese, F. , Molteni, R. , Cattaneo, A. , Macchi, F. , Racagni, G. , Gennarelli, M. , Ellenbroek, B. A. , & Riva, M. A. (2010). Long‐term duloxetine treatment normalizes altered brain‐derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Molecular Pharmacology, 77(5), 846–853. 10.1124/mol.109.063081 [DOI] [PubMed] [Google Scholar]
- Coleman, F. H. , Christensen, H. D. , Gonzalez, C. L. , & Rayburn, W. F. (1999). Behavioral changes in developing mice after prenatal exposure to paroxetine (Paxil). American Journal of Obstetrics and Gynecology, 181(5 Pt 1), 1166–1171. 10.1016/s0002-9378(99)70102-x [DOI] [PubMed] [Google Scholar]
- da Silva, A. I. , Monteiro Galindo, L. C. , Nascimento, L. , Moura Freitas, C. , Manhaes‐de‐Castro, R. , Lagranha, C. J. , & Lopes de Souza, S. (2014). Fluoxetine treatment of rat neonates significantly reduces oxidative stress in the hippocampus and in behavioral indicators of anxiety later in postnatal life. Canadian Journal of Physiology and Pharmacology, 92(4), 330–337. 10.1139/cjpp-2013-0321 [DOI] [PubMed] [Google Scholar]
- Daubert, E. A. , & Condron, B. G. (2010). Serotonin: A regulator of neuronal morphology and circuitry. Trends in Neurosciences, 33(9), 424–434. 10.1016/j.tins.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kort, A. R. , Joosten, E. A. , Patijn, J. , Tibboel, D. , & van den Hoogen, N. J. (2021). The development of descending serotonergic modulation of the spinal nociceptive network: A life span perspective. Pediatric Research, 1–9. 10.1038/s41390-021-01638-9 [DOI] [PubMed] [Google Scholar]
- Ehrlich, D. E. , Neigh, G. N. , Bourke, C. H. , Nemeth, C. L. , Hazra, R. , Ryan, S. J. , Rowson, S. , Jairam, N. , Sholar, C. A. , Rainnie, D. G. , Stowe, Z. N. , & Owens, M. J. (2015). Prenatal stress, regardless of concurrent escitalopram treatment, alters behavior and amygdala gene expression of adolescent female rats. Neuropharmacology, 97, 251–258. 10.1016/j.neuropharm.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, K. M. , & Haring, J. H. (1999). Synaptogenesis in the postnatal rat fascia dentata is influenced by 5‐HT1a receptor activation. Brain Research. Developmental Brain Research, 114(2), 245–252. 10.1016/s0165-3806(99)00036-x [DOI] [PubMed] [Google Scholar]
- Fishbain, D. A. , Cutler, R. , Rosomoff, H. L. , & Rosomoff, R. S. (2000). Evidence‐based data from animal and human experimental studies on pain relief with antidepressants: A structured review. Pain Medicine, 1(4), 310–316. 10.1046/j.1526-4637.2000.00042.x [DOI] [PubMed] [Google Scholar]
- Francis‐Oliveira, J. , Ponte, B. , Barbosa, A. P. , Veríssimo, L. F. , Gomes, M. V. , Pelosi, G. G. , Britto, L. R. , & Moreira, E. G. (2013). Fluoxetine exposure during pregnancy and lactation: Effects on acute stress response and behavior in the novelty‐suppressed feeding are age and gender‐dependent in rats. Behavioural Brain Research, 252, 195–203. 10.1016/j.bbr.2013.05.064 [DOI] [PubMed] [Google Scholar]
- Fricker, A. D. , Rios, C. , Devi, L. A. , & Gomes, I. (2005). Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Molecular Brain Research, 138(2), 228–235. 10.1016/j.molbrainres.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Gaspar, P. , Cases, O. , & Maroteaux, L. (2003). The developmental role of serotonin: News from mouse molecular genetics. Nature Reviews Neuroscience, 4(12), 1002–1012. 10.1038/nrn1256 [DOI] [PubMed] [Google Scholar]
- Gauriau, C. , & Bernard, J. F. (2002). Pain pathways and parabrachial circuits in the rat. Experimental Physiology, 87(2), 251–258. 10.1113/eph8702357 [DOI] [PubMed] [Google Scholar]
- Gemmel, M. , De Lacalle S., Mort S. C., Hill L. A., Charlier T. D., & Pawluski J. L. (2019). Perinatal fluoxetine has enduring sexually differentiated effects on neurobehavioral outcomes related to social behaviors. Neuropharmacology, 144, 70–81. 10.1016/j.neuropharm.2018.10.009 [DOI] [PubMed] [Google Scholar]
- Gemmel, M. , Kokras, N. , Dalla, C. , & Pawluski, J. L. (2018). Perinatal fluoxetine prevents the effect of pre‐gestational maternal stress on 5‐HT in the PFC, but maternal stress has enduring effects on mPFC synaptic structure in offspring. Neuropharmacology, 128, 168–180. 10.1016/j.neuropharm.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Gemmel M., Hazlett M., Bögi E., De Lacalle S., Hill L. A., Kokras N., Hammond G. L., Dalla C., Charlier T. D., & Pawluski J. L. (2017). Perinatal fluoxetine effects on social play, the HPA system, and hippocampal plasticity in pre‐adolescent male and female rats: Interactions with pre‐gestational maternal stress. Psychoneuroendocrinology, 84, 159–171. 10.1016/j.psyneuen.2017.07.480 [DOI] [PubMed] [Google Scholar]
- Gemmel, M. , Rayen, I. , Lotus, T. , van Donkelaar, E. , Steinbusch, H. W. , de Lacalle, S. , Kokras, N. , Dalla, C. , & Pawluski, J. L. (2016). Developmental fluoxetine and prenatal stress effects on serotonin, dopamine, and synaptophysin density in the PFC and hippocampus of offspring at weaning. Developmental Psychobiology, 58(3), 315–327. 10.1002/dev.21372 [DOI] [PubMed] [Google Scholar]
- Gingrich, J. A. , Malm, H. , Ansorge, M. S. , Brown, A. , Sourander, A. , Suri, D. , Teixeira, C. M. , Caffrey Cagliostro, M. K. , Mahadevia, D. , & Weissman, M. M. (2017). New insights into how serotonin selective reuptake inhibitors shape the developing brain. Birth Defects Research, 109(12), 924–932. 10.1002/bdr2.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazova, N. Y. , Merchieva, S. , Volodina, M. , Sebentsova, E. , Manchenko, D. , Kudrun, V. , & Levitskaya, N. (2014). Effects of neonatal fluvoxamine administration on the physical development and activity of the serotoninergic system in white rats. Acta Naturae, 6(3 [22]), 98–105. 10.32607/20758251-2014-6-3-98-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, M. E. , & Clinton, S. M. (2016). Of rodents and humans: A comparative review of the neurobehavioral effects of early life SSRI exposure in preclinical and clinical research. International Journal of Developmental Neuroscience, 51, 50–72. 10.1016/j.ijdevneu.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, M. E. , Pugh, P. C. , Jackson, N. L. , Cohen, J. L. , Fant, A. D. , Akil, H. , & Clinton, S. M. (2015). Early‐life exposure to the SSRI paroxetine exacerbates depression‐like behavior in anxiety/depression‐prone rats. Neuroscience, 284, 775–797. 10.1016/j.neuroscience.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, V. E. , & Frieder, B. (1987). Prenatal and early postnatal exposure to zimelidine: Behavioral, neurochemical and histological findings in rats. International Journal of Neuroscience, 33(3–4), 225–235. 10.3109/00207458708987407 [DOI] [PubMed] [Google Scholar]
- Homberg, J. , Schubert, D. , & Gaspar, P. (2010). New perspectives on the neurodevelopmental effects of SSRIs. Trends in Pharmacological Sciences, 31(2), 60–65. 10.1016/j.tips.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Homberg, J. R. , Molteni, R. , Calabrese, F. , & Riva, M. A. (2014). The serotonin–BDNF duo: Developmental implications for the vulnerability to psychopathology. Neuroscience & Biobehavioral Reviews, 43, 35–47. 10.1016/j.neubiorev.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Huang, J. , Gadotti, V. M. , Chen, L. , Souza, I. A. , Huang, S. , Wang, D. , Ramakrishnan, C. , Deisseroth, K. , Zhang, Z. , & Zamponi, G. W. (2019). A neuronal circuit for activating descending modulation of neuropathic pain. Nature Neuroscience, 22(10), 1–10. 10.1038/s41593-019-0481-5 [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski, M. , Larsen, B. , Hallquist, M. N. , Foran, W. , Calabro, F. , & Luna, B. (2017). Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: Associations with anxiety and depression. Biological Psychiatry, 82(7), 511–521. 10.1016/j.biopsych.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, N. N. , Lindholm, J. , Pruunsild, P. , Timmusk, T. , & Castrén, E. (2009). Long‐lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. European Neuropsychopharmacology, 19(2), 97–108. 10.1016/j.euroneuro.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Kerman, I. A. , Clinton, S. M. , Bedrosian, T. A. , Abraham, A. D. , Rosenthal, D. T. , Akil, H. , & Watson, S. J. (2011). High novelty‐seeking predicts aggression and gene expression differences within defined serotonergic cell groups. Brain Research, 1419, 34–45. 10.1016/j.brainres.2011.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaepen, L. , Rayen, I. , Charlier, T. D. , Fillet, M. , Houbart, V. , van Kleef, M. , Steinbusch, H. W. , Patijn, J. , Tibboel, D. , Joosten, E. A. , & Pawluski, J. L. (2013). Developmental fluoxetine exposure normalizes the long‐term effects of maternal stress on post‐operative pain in Sprague‐Dawley rat offspring. PLoS ONE, 8(2), e57608. 10.1371/journal.pone.0057608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, M. C. , Lee, L. J. , Li, Y. , & Lee, L. J. (2014). Long‐term consequences of neonatal fluoxetine exposure in adult rats. Developmental Neurobiology, 74(10), 1038–1051. 10.1002/dneu.22185 [DOI] [PubMed] [Google Scholar]
- Kozisek, M. E. , Middlemas, D. , & Bylund, D. B. (2008). The differential regulation of BDNF and TrkB levels in juvenile rats after four days of escitalopram and desipramine treatment. Neuropharmacology, 54(2), 251–257. 10.1016/j.neuropharm.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Kristensen, J. , Ilett, K. , Hackett, L. , Yapp, P. , Paech, M. , & Begg, E. (1999). Distribution and excretion of fluoxetine and norfluoxetine in human milk. British Journal of Clinical Pharmacology, 48(4), 521–527. 10.1046/j.1365-2125.1999.00040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, L. J. (2009). Neonatal fluoxetine exposure affects the neuronal structure in the somatosensory cortex and somatosensory‐related behaviors in adolescent rats. Neurotoxicity Research, 15(3), 212–223. 10.1007/s12640-009-9022-4 [DOI] [PubMed] [Google Scholar]
- Lisboa, S. F. S. , Oliveira, P. E. , Costa, L. C. , Venancio, E. J. , & Moreira, E. G. (2007). Behavioral evaluation of male and female mice pups exposed to fluoxetine during pregnancy and lactation. Pharmacology, 80(1), 49–56. 10.1159/000103097 [DOI] [PubMed] [Google Scholar]
- Liu, B. , Liu, J. , Wang, M. , Zhang, Y. , & Li, L. (2017). From serotonin to neuroplasticity: Evolvement of theories for major depressive disorder. Frontiers in Cellular Neuroscience, 11, 305. 10.3389/fncel.2017.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo‐Candelas, C. , Cha, J. , Hong, S. , Bastidas, V. , Weissman, M. , Fifer, W. P. , Myers, M. , Talati, A. , Bansal, R. , Peterson, B. S. , Monk, C. , Gingrich, J. A. , & Posner, J. (2018). Associations between brain structure and connectivity in infants and exposure to selective serotonin reuptake inhibitors during pregnancy. JAMA Pediatrics, 172(6), 525–533. 10.1001/jamapediatrics.2017.5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D., Simpson K. L., Coppinger D., Lu Y., Wang Y., Lin R. C. S., & Paul I. A. (2006). Neonatal Antidepressant Exposure has Lasting Effects on Behavior and Serotonin Circuitry. Neuropsychopharmacology, 31(1), 47–57. 10.1038/sj.npp.1300823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna, S. S. , & Umathe, S. N. (2012). A possible participation of transient receptor potential vanilloid type 1 channels in the antidepressant effect of fluoxetine. European Journal of Pharmacology, 685(1–3), 81–90. 10.1016/j.ejphar.2012.04.023 [DOI] [PubMed] [Google Scholar]
- Marks, D. M. , Shah, M. J. , Patkar, A. A. , Masand, P. S. , Park, G. Y. , & Pae, C. U. (2009). Serotonin‐norepinephrine reuptake inhibitors for pain control: Premise and promise. Current Neuropharmacology, 7(4), 331–336. 10.2174/157015909790031201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, A. M. , Toelle, T. R. , Rowbotham, D. J. , Schaefer, C. P. , & Dukes, E. M. (2006). The burden of neuropathic pain: Results from a cross‐sectional survey. European Journal of Pain, 10(2), 127–135. 10.1016/j.ejpain.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Means‐Christensen, A. J. , Roy‐Byrne, P. P. , Sherbourne, C. D. , Craske, M. G. , & Stein, M. B. (2008). Relationships among pain, anxiety, and depression in primary care. Depression and Anxiety, 25(7), 593–600. 10.1002/da.20342 [DOI] [PubMed] [Google Scholar]
- Meyer, L. R. , Dexter, B. , Lo, C. , Kenkel, E. , Hirai, T. , Roghair, R. D. , & Haskell, S. E. (2018). Perinatal SSRI exposure permanently alters cerebral serotonin receptor mRNA in mice but does not impact adult behaviors. The Journal of Maternal‐Foetal & Neonatal Medicine, 31(11), 1393–1401. 10.1080/14767058.2017.1317342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan, M. J. (2002). Descending control of pain. Progress in Neurobiology, 66(6), 355–474. 10.1016/S0301-0082(02)00009-6 [DOI] [PubMed] [Google Scholar]
- Millard, S. J. , Lum, J. S. , Fernandez, F. , Weston‐Green, K. , & Newell, K. A. (2019). Perinatal exposure to fluoxetine increases anxiety‐ and depressive‐like behaviours and alters glutamatergic markers in the prefrontal cortex and hippocampus of male adolescent rats: A comparison between Sprague‐Dawley rats and the Wistar‐Kyoto rat model of depression. Journal of Psychopharmacology, 33(2), 230–243. 10.1177/0269881118822141 [DOI] [PubMed] [Google Scholar]
- Molteni, R. , Calabrese, F. , Maj, P. F. , Olivier, J. D. , Racagni, G. , Ellenbroek, B. A. , & Riva, M. A. (2009). Altered expression and modulation of activity‐regulated cytoskeletal associated protein (arc) in serotonin transporter knockout rats. European Neuropsychopharmacology, 19(12), 898–904. 10.1016/j.euroneuro.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Morag, I. , Batash, D. , Keidar, R. , Bulkowstein, M. , & Heyman, E. (2004). Paroxetine use throughout pregnancy: Does it pose any risk to the neonate? Journal of Toxicology ‐ Clinical Toxicology, 42(1), 97–100. 10.1081/clt-120028753 [DOI] [PubMed] [Google Scholar]
- Nagano, M. , Liu, M. , Inagaki, H. , Kawada, T. , & Suzuki, H. (2012). Early intervention with fluoxetine reverses abnormalities in the serotonergic system and behavior of rats exposed prenatally to dexamethasone. Neuropharmacology, 63(2), 292–300. 10.1016/j.neuropharm.2012.03.027 [DOI] [PubMed] [Google Scholar]
- Narboux‐Nême, N. , Pavone, L. M. , Avallone, L. , Zhuang, X. , & Gaspar, P. (2008). Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs). Neuropharmacology, 55(6), 994–1005. 10.1016/j.neuropharm.2008.08.020 [DOI] [PubMed] [Google Scholar]
- Nietzer, S. , Bonn, M. , Jansen, F. , Heiming, R. , Lewejohann, L. , Sachser, N. , Asan, E. S. , Lesch, K. P. , & Schmitt, A. (2011). Serotonin transporter knockout and repeated social defeat stress: Impact on neuronal morphology and plasticity in limbic brain areas. Behavioural Brain Research, 220(1), 42–54. 10.1016/j.bbr.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Nishimura, A. , Ueda, S. , Takeuchi, Y. , Sawada, T. , & Kawata, M. (1995). Age‐related decrease of serotonergic fibres and S‐100 beta immunoreactivity in the rat dentate gyrus. Neuroreport, 6(10), 1445–1448. 10.1097/00001756-199507100-00021 [DOI] [PubMed] [Google Scholar]
- Oberlander, T. F. , Grunau, R. E. , Fitzgerald, C. , Ellwood, A. L. , Misri, S. , Rurak, D. , & Riggs, K. W. (2002). Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatric Research, 51(4 I), 443–453. 10.1203/00006450-200204000-00008 [DOI] [PubMed] [Google Scholar]
- Oberlander, T. F. , Grunau, R. E. , Fitzgerald, C. , Papsdorf, M. , Rurak, D. , & Riggs, W. (2005). Pain reactivity in 2‐month‐old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics, 115(2), 411–425. 10.1542/peds.2004-0420 [DOI] [PubMed] [Google Scholar]
- Okagbue, H. I. , Adamu, P. I. , Bishop, S. A. , Oguntunde, P. E. , Opanuga, A. A. , & Akhmetshin, E. M. (2019). Systematic review of prevalence of antepartum depression during the trimesters of pregnancy. Open Access Macedonian Journal of Medical Sciences, 7(9), 1555–1560. 10.3889/oamjms.2019.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, W. Y. , Stohler, C. S. , & Herr, D. R. (2019). Role of the prefrontal cortex in pain processing. Molecular Neurobiology, 56(2), 1137–1166. 10.1007/s12035-018-1130-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas, L. , Meyer‐Lindenberg, A. , Goldman, A. L. , Verchinski, B. A. , Chen, G. , Kolachana, B. S. , Egan, M. F. , Mattay, V. S. , Hariri, A. R. , & Weinberger, D. R. (2008). Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Molecular Psychiatry, 13(7), 709–716. 10.1038/mp.2008.32 [DOI] [PubMed] [Google Scholar]
- Popa, D. , Léna, C. , Alexandre, C. , & Adrien, J. (2008). Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: Evidence from sleep, stress, and behavior. Journal of Neuroscience, 28(14), 3546–3554. 10.1523/JNEUROSCI.4006-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, J. , Chen, C. , Lu, Y. C. , Zhang, T. , Xu, H. , Cui, Y. Y. , Chen, Y. Z. , Wang, W. , Dong, Y. L. , & Li, Y. Q. (2014). Activation of extracellular signal‐regulated kinase1/2 in the medial prefrontal cortex contributes to stress‐induced hyperalgesia. Molecular Neurobiology, 50(3), 1013–1023. 10.1007/s12035-014-8707-8 [DOI] [PubMed] [Google Scholar]
- Rampono, J. , Proud, S. , Hackett, L. P. , Kristensen, J. H. , & Ilett, K. F. (2004). A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate. International Journal of Neuropsychopharmacology, 7(3), 329–334. 10.1017/S1461145704004286 [DOI] [PubMed] [Google Scholar]
- Rayen, I. , van den Hove, D. L. , Prickaerts, J. , Steinbusch, H. W. , & Pawluski, J. L. (2011). Fluoxetine during development reverses the effects of prenatal stress on depressive‐like behavior and hippocampal neurogenesis in adolescence. PLoS ONE, 6(9), e24003. 10.1371/journal.pone.0024003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello, T. J. , Yu, Q. , Goodfellow, N. M. , Caffrey Cagliostro, M. K. , Teissier, A. , Morelli, E. , Demireva, E. Y. , Chemiakine, A. , Rosoklija, G. B. , Dwork, A. J. , Lambe, E. K. , Gingrich, J. A. , & Ansorge, M. S. (2014). Postnatal day 2 to 11 constitutes a 5‐HT‐sensitive period impacting adult mPFC function. Journal of Neuroscience, 34(37), 12379–12393. 10.1523/JNEUROSCI.1020-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem‐Kohavi, N. , Williams, L. J. , Muller, A. M. , Abdi, H. , Virji‐Babul, N. , Bjornson, B. H. , Brain, U. , Werker, J. F. , Grunau, R. E. , Miller, S. P. , & Oberlander, T. F. (2019). Hub distribution of the brain functional networks of newborns prenatally exposed to maternal depression and SSRI antidepressants. Depression & Anxiety, 36(8), 753–765. 10.1002/da.22906 [DOI] [PubMed] [Google Scholar]
- Rumajogee, P. , Vergé, D. , Hanoun, N. , Brisorgueil, M. J. , Hen, R. , Lesch, K. P. , Hamon, M. , & Miquel, M. C. (2004). Adaption of the serotoninergic neuronal phenotype in the absence of 5‐HT autoreceptors or the 5‐HT transporter: Involvement of BDNF and cAMP. European Journal of Neuroscience, 19(4), 937–944. 10.1111/j.0953-816X.2004.03194.x [DOI] [PubMed] [Google Scholar]
- Sarkar, A. , Chachra, P. , & Vaidya, V. A. (2014). Postnatal fluoxetine‐evoked anxiety is prevented by concomitant 5‐HT2A/C receptor blockade and mimicked by postnatal 5‐HT2A/C receptor stimulation. Biological Psychiatry, 76(11), 858–868. 10.1016/j.biopsych.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Schaffir, J. (2018). Consequences of antepartum depression. Clinical Obstetrics and Gynecology, 61(3), 533–543. 10.1097/GRF.0000000000000374 [DOI] [PubMed] [Google Scholar]
- Shorey, S. , Chee, C. Y. I. , Ng, E. D. , Chan, Y. H. , San Tam, W. W. , & Chong, Y. S. (2018). Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta‐analysis. Journal of Psychiatric Research, 104, 235–248. 10.1016/j.jpsychires.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Simons, L. E. , Pielech, M. , Erpelding, N. , Linnman, C. , Moulton, E. , Sava, S. , Lebel, A. , Serrano, P. , Sethna, N. , Berde, C. , Becerra, L. , & Borsook, D. (2014). The responsive amygdala: Treatment‐induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain, 155(9), 1727–1742. 10.1016/j.pain.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, K. L. , Weaver, K. J. , de Villers‐Sidani, E. , Lu, J. Y. , Cai, Z. , Pang, Y. , Rodriguez‐Porcel, F. , Paul, I. A. , Merzenich, M. , & Lin, R. C. (2011). Perinatal antidepressant exposure alters cortical network function in rodents. Proceedings of the National Academy of Sciences of the United States of America, 108(45), 18465–18470. 10.1073/pnas.1109353108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza‐Reilly, M. , Meye, F. J. , Olusakin, J. , Telley, L. , Petit, E. , Chen, X. , Mameli, M. , Jabaudon, D. , Sze, J. Y. , & Gaspar, P. (2019). SSRIs target prefrontal to raphe circuits during development modulating synaptic connectivity and emotional behavior. Molecular Psychiatry, 24(5), 726–745. 10.1038/s41380-018-0260-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri, D. , Teixeira, C. M. , Cagliostro, M. K. C. , Mahadevia, D. , & Ansorge, M. S. (2015). Monoamine‐sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology, 40(1), 88–112. 10.1038/npp.2014.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffoli, L. , Rodrigues, G. Jr. , Oliveira, J. , Silva, A. , Moreira, E. , Pelosi, G. , & Gomes, M. (2014). Maternal exposure to fluoxetine during gestation and lactation affects the DNA methylation programming of rat's offspring: Modulation by folic acid supplementation. Behavioural Brain Research, 265, 142–147. 10.1016/j.bbr.2014.02.031 [DOI] [PubMed] [Google Scholar]
- Tsang, A. , von Korff, M. , Lee, S. , Alonso, J. , Karam, E. , Angermeyer, M. C. , Borges, G. L. , Bromet, E. J. , Demytteneare, K. , de Girolamo, G. , de Graaf, R. , Gureje, O. , Lepine, J. P. , Haro, J. M. , Levinson, D. , Oakley Browne, M. A. , Posada‐Villa, J. , Seedat, S. , & Watanabe, M. (2008). Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression‐anxiety disorders. The Journal of Pain, 9(10), 883–891. 10.1016/j.jpain.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Vartazarmian, R. , Malik, S. , Baker, G. B. , & Boksa, P. (2005). Long‐term effects of fluoxetine or vehicle administration during pregnancy on behavioral outcomes in guinea pig offspring. Psychopharmacology, 178(2–3), 328–338. 10.1007/s00213-004-2003-7 [DOI] [PubMed] [Google Scholar]
- Veinante, P. , Yalcin, I. , & Barrot, M. (2013). The amygdala between sensation and affect: A role in pain. Journal of Molecular Psychiatry, 1(1), 1–14. 10.1186/2049-9256-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez, J. C. , Zhao, Q. , Chan, Y. , Galindo, L. C. M. , Simasotchi, C. , Wu, D. , Hou, Z. , Herod, S. M. , Oberlander, T. F. , Gil, S. , Fournier, T. , Burd, I. , Andrews, A. M. , & Bonnin, A. (2019). In utero exposure to citalopram mitigates maternal stress effects on fetal brain development. ACS Chemical Neuroscience, 10(7), 3307–3317. 10.1021/acschemneuro.9b00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ververs, T. , Kaasenbrood, H. , Visser, G. , Schobben, F. , de Jong‐van den Berg, L. , & Egberts, T. (2006). Prevalence and patterns of antidepressant drug use during pregnancy. European Journal of Clinical Pharmacology, 62(10), 863–870. 10.1007/s00228-006-0177-0 [DOI] [PubMed] [Google Scholar]
- Walther, D. J. , Peter, J. U. , Bashammakh, S. , Hortnagl, H. , Voits, M. , Fink, H. , & Bader, M. (2003). Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science, 299(5603), 76–76. 10.1126/science.1078197 [DOI] [PubMed] [Google Scholar]
- Wellman, C. , Izquierdo, A. , Garrett, J. , Martin, K. , Carroll, J. , Millstein, R. , Lesch, K. P. , Murphy, D. L. , & Holmes, A. (2007). Impaired stress‐coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock‐out mice. Journal of Neuroscience, 27(3), 684–691. 10.1523/JNEUROSCI.4595-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteveen, J. S. , Middelman, A. , van Hulten, J. A. , Martens, G. J. , Homberg, J. R. , & Kolk, S. M. (2013). Lack of serotonin reuptake during brain development alters rostral raphe‐prefrontal network formation. Frontiers in Cellular Neuroscience, 7, 143. 10.3389/fncel.2013.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, A. K. (2010). Depression and anxiety in pain. Reviews in Pain, 4(1), 8–12. 10.1177/204946371000400103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. , Lu, J. Y.‐F. , Darling, R. D. , Simpson, K. L. , Zhu, X. , Wang, F. , Yu, L. , Sun, X. , Merzenich, M. M. , & Lin, R. C. (2015). Behavioral training reverses global cortical network dysfunction induced by perinatal antidepressant exposure. Proceedings of the National Academy of Sciences, 112(7), 2233–2238. 10.1073/pnas.1416582111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar, I. , Shoham, S. , & Weinstock, M. (2016). Perinatal citalopram does not prevent the effect of prenatal stress on anxiety, depressive‐like behaviour and serotonergic transmission in adult rat offspring. The European Journal of Neuroscience, 43(4), 590–600. 10.1111/ejn.13150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this review are available from the corresponding author upon reasonable request.


