Abstract
The incidence of cutaneous squamous cell carcinoma (cSCC) is rapidly increasing. A growing part of this patient group is formed by immunocompromised patients, for example organ‐transplant recipients (OTR). Although over 90% of the cSCC show a relatively harmless clinical behaviour, there is also a chance of developing advanced cSCC and metastases. Locally advanced cSCC are defined as cSCC that have locally advanced progression and are no longer amenable to surgery or radiation therapy. Better understanding of the clinical behaviour of cSCC is essential to discriminate between low‐ and high‐risk cSCC. Staging systems are important and have recently been improved. Genetic characterisation of SCC will likely become an important tool to help distinguish low and high‐risk cSCC with an increased potential to metastasise in the near future. Available treatments for high‐risk and advanced cSCC include surgery, radiotherapy, chemotherapy and targeted therapy with epidermal growth factor receptors inhibitors. Anti‐PD‐1 antibodies show promising results with response rates of up to 50% in both locally advanced and metastatic cSCC but, in its present form, is not suitable for OTR.
Introduction
Cutaneous squamous cell carcinomas (cSCC) are keratinocyte carcinomas, originating from the keratinocytes located in the epidermis or adnexal structures. They account for approximately 20% of all cutaneous malignancies. Although exact cumulative incidences are hard to estimate, a rising trend in cSCC is documented worldwide for decades. 1
Risk factors for cSCC are increasing age, male gender, exposure to ultraviolet radiation (UVR), infection with β‐human papillomaviruses (HPV), smoking, genetic factors (fair skin, genetic syndromes) and immunosuppression. In the context of organ transplantation, the immunosuppressive agents azathioprine and cyclosporin and the antifungal drug voriconazole are associated with an increased risk of cSCC. 2
Although more than 90% of the cSCC display a relatively harmless behaviour, there is also a group of patients who develop advanced cSCC. 3 Advanced cSCC include locally advanced and/or metastatic cSCC. Locally advanced cSCC are defined as cSCC that have locally advanced progression (tumours that are large or have penetrated deep into underlying tissues, muscles or nerves) and are no longer amenable to surgery or radiation therapy. Metastatic cSCC are tumours that have spread beyond the original location to adjacent skin, lymph nodes or other organs. 4
Better understanding of the clinical course of cSCC is essential to identify those cSCC that are prone to aggressive growth and/or metastatic behaviour. The immune system plays an important role in the development of cSCC. Organ transplant recipients (OTR) have a 60–100 times increased risk to develop cSCC compared to the age and sex matched immunocompetent population. 5 The number of immunocompromised patients worldwide is rising due to an increase in the number of organ transplantations but also the number of patients with inflammatory bowel disease and rheumatoid arthritis who are treated with immunosuppressive drugs for prolonged periods increases over time. 6 , 7
Staging systems for cSCC are important and have been recently improved. 8 Genetic characterisation of cSCC with an increased potential to metastasise will possibly become an important tool to help us diagnosing cSCC with a poor prognosis in the near future. 9
The relatively poor prognosis of locally advanced and metastatic cSCC emphasises the need for novel therapeutic strategies in this group. PD‐1 inhibitors show promising results but may not be useful for cSCC in OTR, because of the increased risk of transplant rejection.
This review will give an update on the epidemiology, risk factors, staging systems and current treatment options of advanced cSCC. Management of advanced cSCC in the immunocompromised population receives extra focus in this review.
Epidemiology and patient related risk factors associated with local recurrence and metastases
The risk of cSCC metastases varies between 0.1% and 9.9% in the immunocompetent population, with a 2.8% chance of dying because of this disease. 10 Most cSCC represent low‐risk cSCC. However, high‐risk cSCC may have a metastatic rate of up to 37%. 11 Approximately 90% of cSCC metastases appear within 2 years after the initial diagnosis. 12 More than two‐third of the patients suffering from cSCC metastases die because of locally invasive cSCC or nodal metastases, rather than distant organ metastases. 13 Thompson et al. 14 published an excellent study regarding tumour related risk factors for recurrence, metastases and disease‐specific death and a summary is displayed in Table 1.
Table 1.
Risk factors for recurrence or metastasis of cutaneous squamous cell carcinoma, adjusted from Thompson et al. 14
| Risk factors |
|---|
| Breslow thickness >2 mm |
| Invasion beyond subcutaneous fat |
| Perineural invasion |
| Diameter >20 mm |
| Poor differentiation |
| Immunosuppression |
| Location on the lip, ear or temple |
It is expected that the risk factors for metastases are similar in OTR and the immunocompetent population, yet immunosuppressed patients with cSCC could have worse outcomes. 15 In one study, the metastatic rate of cSCC is estimated at 13% in the presence of immunosuppression 16 ; however, a recent meta‐analysis showed a pooled metastasis risk estimate of 7.3%. 17 Another study related the high metastatic rate of cSCC in OTR to the higher amount of local recurrences in OTR compared to immunocompetent patients. 18
Better understanding of pathogenesis of high‐risk cSCC
The influence of the immune system on the development of cSCC and cSCC metastases is still underreported and merits more attention.
In immunocompetent patients, the immune system is able to recognise antigens related to viral infections, as well as tumour antigens. This is called immune surveillance. Immunocompromised patients, for example OTR have an impaired immune surveillance due to their life‐long immunosuppressive medication, which is needed to retain the transplanted organ, but thereby facilitating the survival and proliferation of atypical cells. The cSCC have a high mutational load with on average 50 mutations per megabase pair DNA. 19 This is even more than the average mutational load in malignant melanoma, 20 which should be sufficient to lead to frequent formation of neoantigens that can be recognised by T lymphocytes. 21 cSCC are, therefore, highly immunogenic tumours, which makes immunocompromised patients, especially vulnerable for developing cSCC. 22 An important defence line consists of elimination of altered cells by innate and adaptive arms of the immune system. 23 Antigens are secreted by tumour cells, will be expressed on the cell membrane and recognised by antigen presenting cells (APC). T lymphocytes and natural killer (NK) cells, among others, are then activated to help eliminate the tumour cells. The human leukocyte antigen (HLA) system has an important role in the recognition of antigens. HLA class I can be found on all cells in the human body. Its function is to present antigens to the CD8 positive T lymphocytes and to make connections with NK cells. HLA class II are expressed on APCs (dendritic cells, macrophages, B cells and CD4 positive T lymphocytes). HLA class III has involvements within the complement system and cytokine formation. When a T lymphocyte recognises the peptide presented by the HLA antigen in the tumour cell, co‐receptors act as activators and inhibitors of the immune response (Fig. 1). Programmed cell death 1 protein (PD‐1) and Cytotoxic T‐Lymphocyte Antigen 4 (CTLA4) are inhibitory receptors and known as immune checkpoint receptors. PD‐1 is expressed on the surface of T cells, B cells, natural killer cells, dendritic cells and monocytes.
Figure 1.
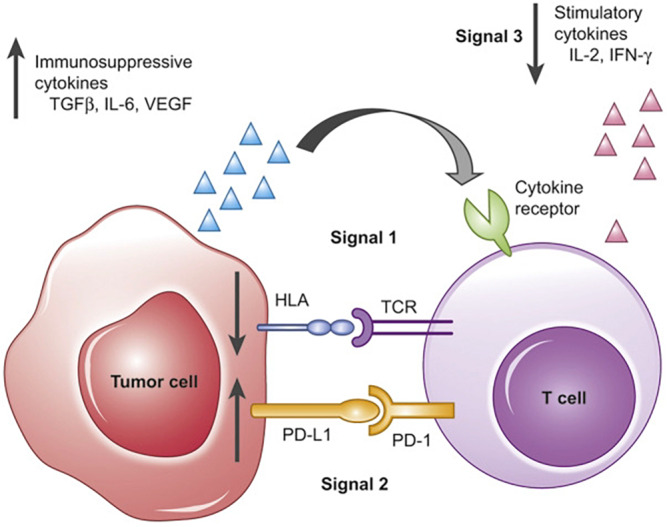
Immune surveillance. Reproduced from Moy et al. 46 with permission from Elsevier. T‐cell activation requires three simultaneous signals in order to carry out its anti‐tumour effects. Signal 1 comprises the T‐cell receptor – HLA interaction, with presentation of antigens from the tumour cell. Signal 2 is a summation of costimulatory and coinhibitory signals. These signals must occur in the presence of Signal 3, made up of immune‐activating cytokines, such as IL‐2 or IFN‐γ. Programmed cell death 1 protein (PD‐1) is an inhibitory receptor. Immune evasion can occur at any of these signals (black arrows), impairing the immune system from effectively eradicating malignant cells.
UVR plays a key role in cSCC carcinogenesis by inducing DNA mutations and escaping from immune surveillance. 24 DNA mutations caused by UVR in skin cancers include inactivation of tumour suppressor genes (p53, CDKN2A and PTCH) or activation of proto‐oncogenes (Ras). These genes are regulators of the cell cycle and when altered are able to induce tumorigenic effects. The accumulation of mutations ultimately involves various signalling pathways, which mediate epidermal growth factor receptor (EGFR) overexpression. These pathways include RAS‐RAF‐MEK‐MAPK, PLC‐gamma/PKC, and PI3K/AKT/mTOR and when altered, they can trigger increased proliferation, migration, survival, resistance to apoptosis and altered differentiation. 25
UVR has also important effects on immune function and causes alterations of the cutaneous cell mediated immunity. 23 A decrease of function of the Langerhans cells, cytotoxic and helper T lymphocytes as they are depleted and may have undergone changes in morphology, and an simultaneous increase of UV‐induced regulatory T cells lead to alterations in favour of both the development of skin tumours and a higher risk of metastasis. UV‐mediated immunosuppression can be both local and systemic by secretion of immunosuppressive cytokines. 23 For example azathioprine and voriconazole are both photosensitive agents and as such can induce tumorigenic effects.
Chronic inflammation can also trigger certain molecular and cellular networks that have a role in the initiation and progression of cSCC, as well as tumour angiogenesis and metastasis. 26
Infection with βHPV is thought to play a role in the initiation stage of cSCC carcinogenesis, although the opinions on this subject are controversial. 27 There is evidence that the processes of DNA repair and UVR‐induced apoptosis are less effective in βHPV infected cells, which leads to accumulation of DNA damage with actinic keratoses, Bowen's disease and cSCC as the final end result. βHPV most likely does not play a role in the maintenance of the malignant phenotype or in the development of advanced stages of cSCC. 28
Better identification of high‐risk cSCC
The significant morbidity and mortality of patients with advanced cSCC highlights the urgent need for early identification of high‐risk cSCC.
Multiple tumour classification systems have been developed in which various criteria are determined that carry a higher risk of locoregional or distant metastases. Commonly used classification systems are the American Joint Committee on Cancer (AJCC) tumour classification system, the Union for International Cancer Control (UICC) classification system and the Brigham and Women's Hospital (BWH) Tumour Classification System. Differences between the systems are displayed in table 2. In January 2018, the eighth edition of the AJCC (AJCC8) came into force. 8 Important changes compared to the seventh edition (AJCC7) were the following: SCC of the vermillion lip were categorised under cSCC instead of oral SCC. Risk factors for T1 to upstage to T2 were removed. Instead, risk factors as tumour invasion of >6 mm (instead of >4 mm) and/or invasion beyond the subcutaneous fat, and perineural invasion was defined as tumour cells in the nerve sheath of a nerve lying deeper than the dermis or measuring 0.1 mm or larger in calibre, were introduced to upstage a T1 or T2 tumour to T3. Well‐known risk factors such as differentiation grade, angioinvasion and a location on the ear or lip do not contribute to the tumour, nodes, metastasis classification anymore. Recurrent cSCC and immunosuppression are often mentioned as risk factors for metastases; however, they are not yet incorporated in these staging systems.
Table 2.
Changes between the American Joint Committee on Cancer (AJCC8), Union for International Cancer Control (UICC8) and Brigham and Women's Hospital (BWH) classification systems. 30 , 36
| AJCC8 | UICC8 | BWH | |||
|---|---|---|---|---|---|
| T1 | ≤2 cm in greatest diameter | T1 | ≤2 cm in greatest diameter | T1 | 0 High‐risk factors§ |
| T2 | >2–4 cm in greatest diameter | T2 | >2–4 cm in greatest diameter | T2a | 1 High‐risk factors |
| T2b | 2–3 High‐risk factors | ||||
| T3 | Tumour >4 cm in greatest diameter or minor bone invasion or perineural invasion or deep invasion† | T3 | Tumour >4 cm in greatest diameter or minor bone invasion or perineural invasion or deep invasion‡ | T3 | ≥4 High‐risk factors |
| T4a | Tumour with gross cortical bone and/or marrow invasion | T4a | Tumour with gross cortical bone and/or marrow invasion | ||
| T4b | Tumour with skull bone invasion and/or skull base foramen involvement | T4b | Tumour with skull bone invasion and/or skull base foramen involvement | ||
Deep invasion defined as invasion beyond the subcutaneous fat or >6 mm (as measured from the granular layer of adjacent normal epidermis to the base of the tumour), perineural invasion defined as tumour cells in the nerve sheath of a nerve lying deeper than the dermis or measuring 0.1 mm or larger in calibre or presenting with clinical or radiographic involvement of named nerves without skull base invasion or transgression.
Deep invasion defined as invasion beyond the subcutaneous fat or >6 mm (as measured from the granular layer of adjacent normal epidermis to the base of the tumour); perineural invasion for T3 classification is defined as clinical or radiographic involvement of named nerves without foramen or skull base invasion or transgression.
BWH high‐risk factors include tumour diameter ≥2 cm, poorly differentiated histology, perineural invasion of nerve(s) 0.1 mm in calibre or tumour invasion beyond subcutaneous fat (excluding bone invasion, which upgrades tumour to BWH stage T3).
The positive predictive value of the AJCC8 for a poor outcome remains only 17% 8 , 29 , 30 as the majority of cSCC designated ‘high‐risk’ do not develop advanced disease, and does not allow accurate prediction of which cSCC will progress to locoregional spread or disease‐specific death. 8 , 31 , 32 An alternative staging system from BWH performs better, but the positive predictive value for a poor outcome is still only 24%‐38%. 8 , 30
Staging systems for locally advanced cSCC have not been extensively studied. In staging systems for melanoma and Merkel cell carcinoma, it is known that in‐transit metastasis has prognostic value; however, this is not yet incorporated in cSCC staging. A recent study compared the outcome of patients with cSCC in‐transit metastases with T3N0 tumours, T4 tumours with bone invasion, lymph node metastases and distant metastases. cSCC patients with in‐transit metastases experienced outcomes similar to locally advanced non‐metastatic cSCC patients. 33
Besides these clinical and histological characteristics, better understanding of the genomic alterations and the mechanisms of immune evasion that drive locally advanced and metastatic cSCC is urgently needed to provide more accurate predictive algorithms. Recently, a study was published in which a gene expression profile was developed and validated for predicting high‐risk cSCC, showing a positive predictive value of 60% in the highest risk group. 9 Large‐scale studies investigating genetic risk factors for cSCC metastases in OTR have not yet been performed.
Currently, lymph node palpation, ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) are frequently used methods for detection of metastasis. Recent studies state that in patients with high‐risk cSCC, in the cases of absence of clinically palpable lymphadenopathy and negative imaging, it would be reasonable to consider sentinel lymph node biopsy; however, convincing evidence is still lacking. 34 , 35
Better treatment and prevention
Management of cSCC is important, especially in patients suffering from multiple cSCC. Surgery remains the golden standard for low‐risk cSCC. The European consensus group suggests 6–10 mm clinical safety margins for cSCC with high‐risk factors. 36
The great advantage of Mohs' over traditional surgical excision is that 100% of the surgical margins can be evaluated, resulting in lower recurrence rates (3% vs. 8% during a follow‐up period of 5 years, respectively). 37 However, it should be mentioned that no randomised controlled trials comparing Mohs' and standard surgical excision have been performed. One study found a 52% tissue‐sparing effect for Mohs' vs. standard surgical excision. 38 When it is not possible to perform a re‐excision in case of narrow margins, adjuvant radiotherapy can be considered. Curettage and electrodessication is a safe therapy for OTR suffering from multiple T1 cSCC (well differentiated tumours on low‐risk locations) with a cure rate of around 95%. 39
Locally advanced and metastatic cSCC require other treatments that need to be evaluated by a multidisciplinary team. Available treatment options include chemotherapy (such as cisplatin), targeted therapy with EGFR inhibitors (i.e. cetuximab) and anti‐PD‐1 antibodies (cemiplimab, pembrolizumab, nivolumab). Cemiplimab is the first systemic treatment approved by FDA and EMA for advanced and metastatic cSCC. 40 Anti‐PD‐1 antibodies show promising results with response rates of up to 50% in both locally advanced and metastatic cSCC, 41 with emerging evidence of durable responses. 42 The side effect profile of anti‐PD‐1 antibodies appears to be favourable compared to chemotherapy. PD‐1 inhibitors may not be useful for cSCC in OTR, because of the high chance of transplant rejection. 43
Education for prevention and early detection of cSCC is a corner stone for all OTR. The use of sun‐protective closing, hats and sunscreens should be promoted. Prescription of systemic retinoids, nicotinamide and field treatments for actinic keratoses such as 5‐fluorouracil should be discussed for high‐risk patients.
Animal studies with an HPV vaccine have shown a protective effect against the development of cSCC in HPV infected animals, but an effective vaccine to protect against actinic keratoses and cSCC in human beings is currently not available. 44
Reliable identification of the highest risk cSCC by gene expression profile could allow clinicians in the future to deploy more aggressive surgery and/or adjuvant radiotherapy for these tumours, thus reducing metastatic risk. 9 , 45
Conflict of interest
None.
Funding source
None.
References
- 1. Leiter U, Keim U, Eigentler T et al. Incidence, mortality, and trends of nonmelanoma skin cancer in Germany. J Invest Dermatol 2017; 137: 1860–1867. [DOI] [PubMed] [Google Scholar]
- 2. Kolaitis NA, Duffy E, Zhang A et al. Voriconazole increases the risk for cutaneous squamous cell carcinoma after lung transplantation. Transpl Int 2017; 30: 41–48. [DOI] [PubMed] [Google Scholar]
- 3. Maubec E. Update of the management of cutaneous squamous‐cell carcinoma. Acta Derm Venereol 2020; 100: adv00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soura E, Gagari E, Stratigos A. Advanced cutaneous squamous cell carcinoma: how is it defined and what new therapeutic approaches are available? Curr Opin Oncol 2019; 31: 461–468. [DOI] [PubMed] [Google Scholar]
- 5. Mudigonda T, Levender MM, O'Neill JL, West CE, Pearce DJ, Feldman SR. Incidence, risk factors, and preventative management of skin cancers in organ transplant recipients: a review of single‐ and multicenter retrospective studies from 2006 to 2010. Dermatol Surg 2013; 39: 345–364. [DOI] [PubMed] [Google Scholar]
- 6. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020; 5: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nair B, Taylor‐Gjevre R, Wu L, Jin S, Quail JM. Incidence and prevalence of rheumatoid arthritis in Saskatchewan, Canada: 2001–2014. BMC Rheumatol 2019; 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karia PS, Morgan FC, Califano JA, Schmults CD. Comparison of tumor classifications for cutaneous squamous cell carcinoma of the head and neck in the 7th vs 8th Edition of the AJCC Cancer Staging Manual. JAMA Dermatol 2018; 154: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wysong A, Newman JG, Covington KR et al. Validation of a 40‐gene expression profile test to predict metastatic risk in localized high‐risk cutaneous squamous cell carcinoma. J Am Acad Dermatol 2021; 84: 361–369. [DOI] [PubMed] [Google Scholar]
- 10. Venables ZC, Autier P, Nijsten T et al. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol 2019; 155: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high‐risk and metastatic disease. Am J Clin Dermatol 2016; 17: 491–508. [DOI] [PubMed] [Google Scholar]
- 12. Genders RE, Osinga JAJ, Tromp EE, O'Rourke P, Bouwes Bavinck JN, Plasmeijer EI. Metastasis risk of cutaneous squamous cell carcinoma in organ transplant recipients and immunocompetent patients. Acta Derm Venereol 2018; 98: 551–555. [DOI] [PubMed] [Google Scholar]
- 13. Eigentler TK, Leiter U, Hafner HM, Garbe C, Rocken M, Breuninger H. Survival of patients with cutaneous squamous cell carcinoma: results of a prospective cohort study. J Invest Dermatol 2017; 137: 2309–2315. [DOI] [PubMed] [Google Scholar]
- 14. Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease‐specific death: a systematic review and meta‐analysis. JAMA Dermatol 2016; 152: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harwood CA, Toland AE, Proby CM et al. The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol 2017; 177: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 16. Rowe DE, Carroll RJ, Day CL, Jr . Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992; 26: 976–990. [DOI] [PubMed] [Google Scholar]
- 17. Genders RE, Weijns ME, Dekkers OM, Plasmeijer EI. Metastasis of cutaneous squamous cell carcinoma in organ transplant recipients and the immunocompetent population: is there a difference? A systematic review and meta‐analysis. J Eur Acad Dermatol Venereol 2019; 33: 828–841. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez JL, Reddy ND, Cunningham K, Silverman R, Madan E, Nguyen BM. Multiple cutaneous squamous cell carcinoma in immunosuppressed vs immunocompetent patients. JAMA Dermatol 2019; 155: 625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. South AP, Purdie KJ, Watt SA et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol 2014; 134: 2630–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexandrov LB, Nik‐Zainal S, Wedge DC et al. Signatures of mutational processes in human cancer. Nature 2013; 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 22. Bottomley MJ, Thomson J, Harwood C, Leigh I. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci 2019; 20: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rangwala S, Tsai KY. Roles of the immune system in skin cancer. Br J Dermatol 2011; 165: 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mancebo SE, Wang SQ. Skin cancer: role of ultraviolet radiation in carcinogenesis. Rev Environ Health 2014; 29: 265–273. [DOI] [PubMed] [Google Scholar]
- 25. Corchado‐Cobos R, García‐Sancha N, González‐Sarmiento R, Pérez‐Losada J, Cañueto J. Cutaneous squamous cell carcinoma: from biology to therapy. Int J Mol Sci 2020: 21: 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neagu M, Constantin C, Caruntu C, Dumitru C, Surcel M, Zurac S. Inflammation: a key process in skin tumorigenesis. Oncol Lett 2019; 17: 4068–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouwes Bavinck JN, Feltkamp MCW, Green AC et al. Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: a multicenter, prospective cohort study. Am J Transplant 2018; 18: 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tampa M, Mitran CI, Mitran MI et al. The role of beta HPV types and HPV‐Associated inflammatory processes in cutaneous squamous cell carcinoma. J Immunol Res 2020; 2020: 5701639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 2018; 78: 237–247. [DOI] [PubMed] [Google Scholar]
- 30. Ruiz ES, Karia PS, Besaw R, Schmults CD. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs the Brigham and Women's Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol 2019; 155: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feinstein S, Higgins S, Ahadiat O, Wysong A. A retrospective cohort study of cutaneous squamous cell carcinoma with lymph node metastasis: Risk factors and clinical course. Dermatol Surg 2019; 45: 772–781. [DOI] [PubMed] [Google Scholar]
- 32. Wisgerhof HC, Edelbroek JR, de Fijter JW et al. Subsequent squamous‐ and basal‐cell carcinomas in kidney‐transplant recipients after the first skin cancer: cumulative incidence and risk factors. Transplantation 2010; 89: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 33. Smile TD, Xiong DX, Varra V et al. Disease progression in cutaneous squamous cell carcinoma patients with satellitosis and in‐transit metastasis. Anticancer Res 2021; 41: 289–295. [DOI] [PubMed] [Google Scholar]
- 34. Fox M, Brown M, Golda N et al. Nodal staging of high‐risk cutaneous squamous cell carcinoma. J Am Acad Dermatol 2019; 81: 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kofler L, Kofler K, Schulz C, Breuninger H, Häfner HM. Sentinel lymph node biopsy for high‐thickness cutaneous squamous cell carcinoma. Arch Dermatol Res 2021; 313: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stratigos AJ, Garbe C, Dessinioti C et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 2. Treatment. Eur J Cancer 2020; 128: 83–102. [DOI] [PubMed] [Google Scholar]
- 37. van Lee CB, Roorda BM, Wakkee M et al. Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after Mohs micrographic surgery vs. standard excision: a retrospective cohort study. Br J Dermatol 2019; 181: 338–343. [DOI] [PubMed] [Google Scholar]
- 38. Correa J, Pastor M, Céspedes E, Magliano J, Bazzano C. Tissue‐sparing outcome of mohs micrographic surgery in squamous cell carcinomas. Actas Dermosifiliogr (Engl Ed) 2020; 111: 847–851. [DOI] [PubMed] [Google Scholar]
- 39. de Graaf YG, Basdew VR, van Zwan‐Kralt N, Willemze R, Bavinck JN. The occurrence of residual or recurrent squamous cell carcinomas in organ transplant recipients after curettage and electrodesiccation. Br J Dermatol 2006; 154: 493–497. [DOI] [PubMed] [Google Scholar]
- 40. Ahmed SR, Petersen E, Patel R, Migden MR. Cemiplimab‐rwlc as first and only treatment for advanced cutaneous squamous cell carcinoma. Expert Rev Clin Pharmacol 2019; 12: 947–951. [DOI] [PubMed] [Google Scholar]
- 41. Benzaquen M. Update on the anti‐programmed cell death‐1 receptor antibodies in advanced cutaneous squamous‐cell carcinoma. Dermatol Ther 2020; 33: e13325. [DOI] [PubMed] [Google Scholar]
- 42. Rischin D, Migden MR, Lim AM et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed‐dosing, long‐term outcome of weight‐based dosing. J Immunother Cancer 2020; 8: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aguirre LE, Guzman ME, Lopes G, Hurley J. Immune checkpoint inhibitors and the risk of allograft rejection: a comprehensive analysis on an emerging issue. Oncologist 2019; 24: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gupta R, Rady PL, Doan HQ, Tyring SK. Development of a β‐HPV vaccine: updates on an emerging frontier of skin cancer prevention. J Clin Virol 2020; 126: 104348. [DOI] [PubMed] [Google Scholar]
- 45. Rebeca T, Giselle P, Litchman GH, Rigel DS. Impact of gene expression profile testing on the management of squamous cell carcinoma by dermatologists. J Drugs Dermatol 2019; 18: 980–984. [PubMed] [Google Scholar]
- 46. Moy JD, Moskovitz JM, Ferris RL. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur J Cancer 2017; 76: 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]


