Abstract
To determine the distribution and causes of extinction threat across functional groups of terrestrial vertebrates, we assembled an ecological trait data set for 18,016 species of terrestrial vertebrates and utilized phylogenetic comparative methods to test which categories of habitat association, mode of locomotion, and feeding mode best predicted extinction risk. We also examined the individual categories of the International Union for Conservation of Nature Red List extinction drivers (e.g., agriculture and logging) threatening each species and determined the greatest threats for each of the four terrestrial vertebrate groups. We then quantified the sum of extinction drivers threatening each species to provide a multistressor perspective on threat. Cave dwelling amphibians (p < 0.01), arboreal quadrupedal mammals (all of which are primates) (p < 0.01), aerial and scavenging birds (p < 0.01), and pedal (i.e., walking) squamates (p < 0.01) were all disproportionately threatened with extinction in comparison with the other assessed ecological traits. Across all threatened vertebrate species in the study, the most common risk factors were agriculture, threatening 4491 species, followed by logging, threatening 3187 species, and then invasive species and disease, threatening 2053 species. Species at higher risk of extinction were simultaneously at risk from a greater number of threat types. If left unabated, the disproportionate loss of species with certain functional traits and increasing anthropogenic pressures are likely to disrupt ecosystem functions globally. A shift in focus from species‐ to trait‐centric conservation practices will allow for protection of at‐risk functional diversity from regional to global scales.
Keywords: extinction drivers, feeding mode, habitat, locomotion, risk level, factores de extinción, hábitat, locomoción, modo de alimentación, nivel de riesgo
Short abstract
Article impact statement: An ecological trait database shows how habitat association, mode of locomotion, and feeding affect extinction risk in different lineages.
Abstract
Una Señal Ecológica Mundial del Riesgo de Extinción de los Vertebrados Terrestres
Resumen
Construimos un conjunto de datos de atributos ecológicos de 18,016 especies de vertebrados terrestres y utilizamos métodos de comparación filogenética para analizar cuáles categorías de asociación de hábitat, modo de locomoción y modo de alimentación predicen de mejor manera el riesgo de extinción. Lo anterior lo hicimos para determinar la distribución y las causas de las amenazas de extinción a lo largo de los grupos funcionales de vertebrados terrestres. También examinamos las categorías individuales de los factores de extinción (p. ej.: agricultura, tala de árboles) de la Lista Roja de la Unión Internacional para la Conservación de la Naturaleza que amenazan a cada especie y determinamos las principales amenazas para cada uno de los cuatro grupos de vertebrados terrestres. Después cuantificamos la suma de los factores de extinción que amenazan a cada especie para proporcionar una perspectiva de estresores múltiples sobre la amenaza. Los anfibios cavernícolas (p < 0.01), mamíferos arbóreos cuadrúpedos (todos son primates) (p < 0.01), aves aéreas y carroñeras (p < 0.01) y los escamados caminantes (p < 0.01) tuvieron una amenaza de extinción desproporcionada en comparación con los otros atributos ecológicos analizados. En todas las especies de vertebrados que estudiamos, los factores de riesgo más comunes fueron la agricultura, que amenaza a 4,491 especies, y la deforestación, que amenaza a 3,187 especies; le siguen las especies invasoras y las enfermedades, que juntas amenazan a 2,053 especies. Las especies con el mayor riesgo de extinción también se encontraban simultáneamente en riesgo por un mayor número de tipos de amenazas. Si esto se mantiene constante, la pérdida desproporcionada de especies con ciertos atributos funcionales y la creciente presión antropogénica probablemente alteren las funciones ecosistémicas a nivel mundial. Un cambio en el enfoque de las prácticas de conservación, de estar centradas en la especie a estar centradas en los atributos, permitirá la protección de la diversidad funcional en riesgo desde la escala regional hasta la global.
INTRODUCTION
Terrestrial vertebrates are highly threatened by the sixth mass extinction crisis and have estimated extinction rates considerably higher than background rates known from the fossil record (Alroy, 2015; Ceballos et al., 2017). Beyond the intrinsic loss associated with these widespread reductions in terrestrial vertebrate biodiversity, species extinctions will affect ecosystem structure and function, such as the loss of pollination services and seed dispersal (Biesmeijer et al., 2006), reduction of pest control (Karp et al., 2013), changes in nutrient cycling and movement (Young et al., 2010), and reduction of water quality and stream respiration (Whiles et al., 2013). These cascading ecosystem‐level effects are also projected to have serious economic, social, and health consequences for humans (Cardinale et al., 2012; Ratto et al., 2018).
Although the rate and projected magnitude of the current loss of terrestrial vertebrates is increasingly well quantified (Barnosky et al., 2011; Ceballos et al., 2020), the distribution of extinction threat across ecological functional groups is only just coming into focus (e.g., Chichorro et al., 2019; Atwood et al., 2020; Carmona et al., 2021). Quantifying loss in terms of ecological function is as critical as quantifying taxonomic magnitude for determining the long‐term effect of extinction on the biosphere (Bush et al., 2020). Assessing the effect of extinctions on ecosystem services similarly requires a focus on trait‐specific consequences, particularly as extinction risk relates to broad categories of habitat use, locomotion, and feeding specialization, for which species‐level data are increasingly robust (e.g., Dirzo et al., 2014).
We used a broad suite of ecological traits to test which traits and combinations of anthropogenic risk factors are shaping the modern extinction crisis. This integrative analytical approach provides a useful perspective for conservation practitioners to assess extinction risk at the functional trait level and, in turn, design management strategies to mitigate the worst consequences of functional loss. Terrestrial vertebrates are extensively studied with respect to extinction threat (Chichorro et al., 2019). Yet, relatively few researchers have conducted analyses within a robust phylogenetic framework, despite Purvis et al. (2000) having shown that nonphylogenetic tests can lead to erroneous results. Those who did use a phylogenetic framework conducted studies of a relatively limited taxonomic scope (e.g., González‐Suarez et al., 2013; González del Pleigo et al., 2019). Previous research indicates that both large and small body size, herbivory, slow life history, low fecundity, and certain habitat preferences are associated with elevated extinction threat in vertebrates (Ripple et al., 2017; Atwood et al., 2020; Carmona et al., 2021). However, the link between anthropogenic risk factors and species’ functional ecological traits is less well explored, and newly available, species‐level, molecular phylogenies for the majority of terrestrial vertebrates provide an improved opportunity to conduct trait‐based risk analyses. We aimed to address three hypotheses: extinction risk and the ecological traits of terrestrial vertebrates are associated; different groups of terrestrial vertebrates are affected by different extinction threats; and when a species simultaneously faces a large number of different threats, it is more likely to be at high risk of extinction.
METHODS
Summary of approach
To assess how the ecological selectivity of extinction risk varies among terrestrial vertebrates (amphibians, reptiles, mammals, and birds), we used the published literature to assign three core ecological trait axes (habitat association, mode of locomotion, and feeding mode) for each of 18,016 species of terrestrial vertebrates and then matched them to their International Union for the Conservation of Nature (IUCN) Red List category of extinction risk (IUCN, 2020). Across all four vertebrate groups, species in each ecological category had variable, and often high, threat categories (Figure 1). We then applied large‐scale, species‐level molecular phylogenies for amphibians (Jetz & Pyron, 2018), squamates (Tonini et al., 2016), mammals (Upham et al., 2019), and birds (Jetz et al., 2012) in logistic regression analyses. The binary red‐list extinction risk status (threatened or nonthreatened) was the response variable and the ecological traits were categorical predictor variables. Therefore, the associations between the likelihood of extinction and the ecological traits were tested while accounting for shared evolutionary history within each clade.
FIGURE 1.
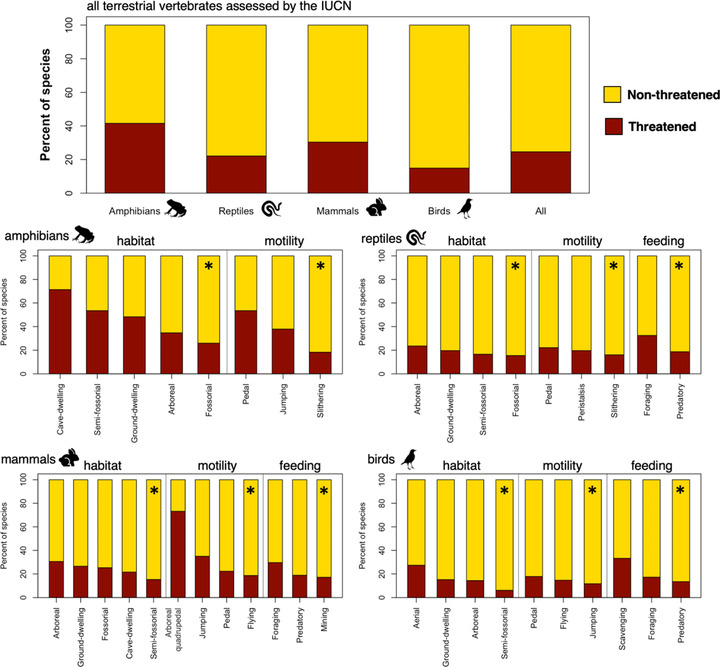
Percentage of threatened (red) and nonthreatened (yellow) species for all terrestrial vertebrate species in the study together and separately by terrestrial vertebrate group and ecological niche (order is descending) (*, ecological trait in each ecological niche with the lowest percentage of threatened species set as the reference level in the phyloglm analysis)
Taxon sampling
A nearly comprehensive list of 34,194 species names of terrestrial vertebrates (10,499 reptiles, 7628 amphibians, 10,548 birds, and 5519 mammals) was manually compiled and curated from the following sources: AmphibiaWeb (AmphibiaWeb, 2020); Reptile Database (Uetz et al., 2020); Mammal Species of the World checklist (Wilson & Reeder, 2005); and Clements Checklist from the Cornell Lab of Ornithology (Clements et al., 2016). The IUCN Red List is based on the BirdLife International Checklist (BirdLife International, 2020). Therefore, when we encountered a species with multiple synonyms, we used the IUCN Red List as the final determining name.
Ecological traits
Our ecological framework was modeled after the ecospace framework developed by Bambach et al. (2007) to classify marine animals and adapted for terrestrial vertebrates (Table 1). We assigned habitat association, mode of locomotion, and feeding mode to each terrestrial vertebrate species. Motility refers to the mode of locomotion (e.g., arboreal quadrupedal, slithering, etc.), rather than the level of motility in the framework outlined in Bambach et al. (2007) (e.g., nonmotile attached, fully motile fast, etc.) (Table 1). Because the functional morphology of foragers, browsers, and grazers is generally similar at a coarse scale of resolution, species with these feeding modes were grouped into a single category (Table 1).
TABLE 1.
Ecological categories in habitat association, locomotion, and feeding mode axes applied in a study of associations between extinction risk and ecological traits in terrestrial vertebrate species a
| Habitat Association | ||
|---|---|---|
| Ecological trait | Description | Example |
| 1) aerial | living within the atmosphere a significant proportion of time | seabirds |
| 2) arboreal | living on above ground vegetation a majority of time | primates |
| 3) ground dwelling | living on ground surface majority of time | giraffes |
| 4) semifossorial | living part above and part below ground level | gophers |
| 5) fossorial | living almost exclusively below ground | moles |
| 6) cave dwelling | living almost exclusively in caves | bats |
| Locomotion | ||
| 1) pedal | regularly moving from slow to fast pace; lifting and setting down feet in turn | ostriches |
| 2) arboreal quadrupedal | progression on small supports (tree branches) using all four limbs | gibbons |
| 3) jumping or hopping | pushing off a surface into the air and landing | frogs |
| 4) slithering | limbless movement by twisting or sliding | rattlesnakes |
| 5) peristalsis | movement caused by constriction and relaxation of a hollow muscular structure | worm lizards |
| 6) flying | primary movement by flying | hummingbirds |
| Feeding mode | ||
| 1) predatory | searches for and captures mobile prey | some snakes |
| 2) scavenging | searches for and eats prey that is no longer alive | condor |
| 3) foraging, browsing, or grazing | searches for and capture immobile aboveground prey | sparrow, deer, and rabbits |
| 4) mining | searches for and captures immobile subterranean prey | gophers |
In the data sets available from https://doi.org/10.5061/dryad.4b8gthtcq, metadata explain each of the ecological trait codes.
Ecological assignments were based on the single category in which the species spends the majority of its adult life stage. We excluded all species that spend the majority of their adult life in aquatic environments (e.g., some ducks and many amphibians). Seabirds were included and considered aerial for their habitat association. Species that exist primarily in human‐made structures (e.g., house mice [Mus musculus]) were considered ground dwelling for their habitat association. All primates were categorized as using arboreal quadrupedal locomotion, including those that also use brachiation (Rose, 1973; Schmidt, 2010). Our ecological categories followed established groupings in animal functional ecology (e.g., Nordell & Valone, 2013). Due to lack of data in the literature, some species could not be coded in this scheme and were excluded from the analysis. In total, we consulted 321 published references (peer‐reviewed articles, entries in animal life encyclopedias, and field guides) for this novel data compilation, including 171 references for amphibians, 110 for reptiles, 21 for mammals, and 19 for birds (complete reference list available from https://doi.org/10.5061/dryad.4b8gthtcq).
Extinction threat assessment
Presently, 86% of described terrestrial vertebrate species (31,771 species) have been assessed by IUCN (IUCN, 2020). However, these risk assessments have a taxonomically biased distribution. All described bird species have been assessed, whereas 91% of mammals, 84% of amphibians, and 70% of reptiles have been assessed (IUCN, 2020). We included all species in the risk analyses that had an IUCN threat category, were coded for all three ecological niche categories, and were sampled in the phylogenetic trees (see below), which amounted to a total of 18,016 species of terrestrial vertebrates (57% of all documented species with an IUCN Red List category): 3531 species of amphibians (51%), 4113 reptiles (53%), 2718 mammals (46%), and 7582 birds (68%). The remaining 43% of terrestrial vertebrate species were not included because species were listed as data deficient by the IUCN, ecological data were unavailable, available data were ambiguous, or species were unsampled in the phylogenetic trees. Tables for each of the four vertebrate groups with species present in the global list, present on the IUCN Red List (excluding data‐deficient species), present in the phylogenies, with full ecological assignments, and present across all data types are available from https://doi.org/10.5061/dryad.4b8gthtcq. Categories containing fewer than 10 species were removed from the analysis to avoid statistical artifacts due to small sample size.
The IUCN Red List (version 2020–2) categories for each terrestrial vertebrate species were used as the response variable to represent extinction risk. Species were assigned to one of eight categories: least concern (LC), near threatened (NT), vulnerable (VU), endangered (EN), critically endangered (CR), extinct in the wild (EW), and extinct (EX). The data deficient (DD) category denotes species where sufficient data have yet to be gathered to make a confident assessment (IUCN, 2012). Red‐list categories are an ordinal index, also commonly dichotomized into threatened and nonthreatened categories (e.g., Ripple et al., 2017; Atwood et al., 2020). Species listed as VU, EN, CR, EW, or EX were assigned to the threatened category and species listed as LC or NT were assigned to the nonthreatened category (IUCN, 2012).
Phylogenetic regression model selection analyses
We built a phylogenetic generalized linear model with habitat association, locomotion, and feeding mode as predictor variables and binary extinction risk (threatened or nonthreatened) as a response variable. All analyses were conducted in R version 4.0.3 (R Core Team, 2019), and we used the phyloglm function from the phylolm package (Ho & Ané, 2014). This approach allowed us to fit phylogenetic generalized linear models to the ecological data while accounting for the expected covariance due to shared evolutionary history. The VertLife phylogenetic trees (Jetz et al., 2012; Tonini et al., 2016; Jetz & Pyron, 2018; Upham et al., 2019) were built with a backbone‐and‐patch approach resulting in credible sets of 10,000 trees, which allowed us to propagate phylogenetic uncertainty (in tree topologies and node ages) into the extinction risk analyses. We used DNA‐only trees because DNA‐unsampled species, which are taxonomically imputed, differ in position within the taxonomic constraints. We used the phytools R package to match the node tips of the phylogenetic trees to the species in the ecological data set and to drop the remaining unmatched node tips (Revell, 2012). From a set of 100 randomly drawn trees from each credible set of 10,000 trees, we conducted phyloglm model selection analyses for each terrestrial vertebrate group. First, we conducted ecological model selection for all possible variable combinations across the 100 trees, then the change in corrected Akaike information criterion (ΔAICc) model weights (Burnham & Anderson, 2004) were determined for all models across the 100 trees (resulting in 100 ΔAICc values per ecological model). In the phylogenetic logistic regression analyses, we used AICc model weights across the 100 phylogenetic trees for model selection. The model with the highest median ΔAICc weight (across the 100 values) was considered the best‐fit model (AICc weight ranges in Figure 2). In all cases, the competing ecological models were not statistically supported (Figure 2). The package matrixStats (Bengtsson, 2020) was used to determine the ΔAICc, model weight, and R 2‐values. The ecological coefficients from the best‐fit model were plotted (Figure 3) using the vioplots package (Adler & Kelly, 2020).
FIGURE 2.
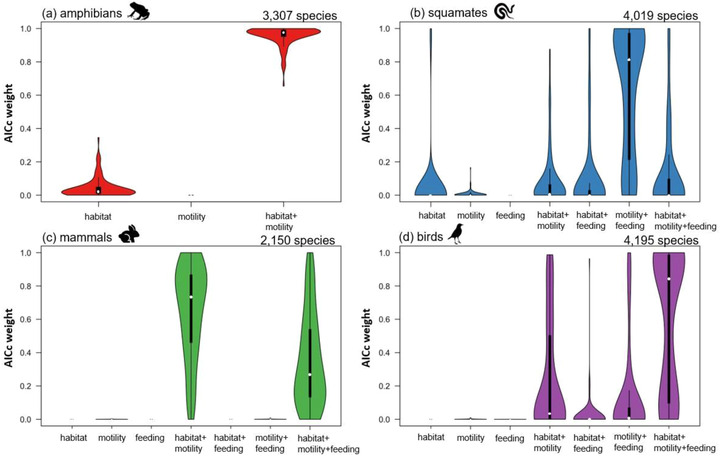
Model support from phyloglm analyses of corrected Akaike information criterion (AICc) values across the 100 phylogenetic trees for (a) amphibians, (b) squamates, (c) mammals, and (d) birds (white dot, median; thick black line, interquartile range; thin black line, 1.5 × interquartile range)
FIGURE 3.
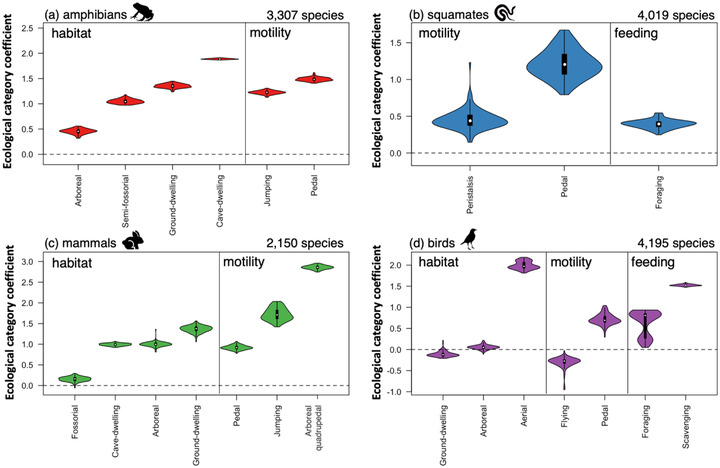
Ecological category coefficients from 100 trees for top phyloglm model for amphibians, squamates, mammals, and birds. Ecological categories with coefficient values >0 for all 100 trees are significantly associated with an elevated risk of extinction
In phyloglm analyses, reference categories were set to the ecological category with the lowest percentage of threatened species (Figure 1) because the percentage of species in the reference categories that were VU or worse (i.e., threatened) was generally quite high, as described below. This provides a baseline for each niche axis with which to compare other taxa with different ecological traits to the reference. For amphibians, the fossorial habitat association and the slithering mode of locomotion were set as the reference categories; 26% of fossorial and 18% of slithering amphibian species in these categories were listed as threatened (Figure 1). For squamates, the fossorial habitat association (16% threatened), the slithering mode of locomotion (16% threatened), and the predatory feeding mode (19% threatened) were set as the reference categories (Figure 1). For mammals, the semifossorial habitat association (15% threatened), the flying mode of locomotion (19% threatened), and the mining feeding mode (17% threatened) were set as the reference categories (Figure 1). Finally, for birds, the semifossorial (living partly above and partly below ground) habitat association (6% threatened), the jumping mode of locomotion (12% threatened), and the predatory feeding mode (14% threatened) were set as the reference categories (Figure 1). In addition to extinction risk varying across ecological traits, the percentage of data‐deficient species also varied: 19% in amphibians, 15% in reptiles, 14% in mammals, and 0.5% in birds (Appendix S1). The coefficients from the best‐fit phylogenetic logistic regression model for the individual ecological trait categories (across the 100 trees) indicated which ecological categories were at significantly elevated threat levels, relative to the reference.
Risk factors of extinction
We used two methods to examine patterns relating to the risk factors of extinction. First, we examined the individual categories of extinction drivers threatening each species and determined the greatest threat for each of the four terrestrial vertebrate groups. Second, we quantified the sum of risk factors threatening each species to provide a multistressor perspective on threat.
Primary risk factors were from IUCN (2020). Risk factors were recorded for all IUCN‐assessed species and consisted of the following 16 categories (in rank order from the most common threat to the least): agriculture and aquaculture, logging, invasive and native species and diseases, residential and industrial development, hunting and pet trade, fire, energy, and mining, pollution, climate change, other threats, transportation, direct human disturbance, dams and natural‐system modifications, geologic events, and fishing (detailed descriptions are at https://www.iucnredlist.org/resources/threat‐classification‐scheme).
In the second approach, we determined the total number of risk factors because threatened species often have more than one primary risk factor. We used a Mann–Whitney–Wilcoxon rank sum test with continuity correction to test the null hypothesis that the distributions of the total number of threat types are not significantly different between species categorized as NT or VU and species categorized as EN or CR. In a separate analysis, species categorized as EW or EX were added to the EN or CR group and compared again with the NT or VU group. All analyses were also conducted separately for each of the four taxonomic groups. Finally, a Kruskal–Wallis test with post hoc pairwise comparison Dunn test was used to determine whether there was a significant difference in the sum of threats between each of the IUCN Red List threat categories (NT, VU, EN, and CR).
RESULTS
Phylogenetic structuring of ecological traits and extinction risk
The best‐fit model for amphibians (median AICc weight = 0.98) and mammals (median AICc weight = 0.73) included habitat association and mode of locomotion. Feeding mode was excluded in amphibians because all assessed amphibians were predators (Figures 2a & c). The best‐fit model for squamates (median AICc weight = 0.82) included mode of locomotion and feeding mode (Figure 2b). The best‐fit model for birds (median AICc weight = 0.84) included all three ecological niche axes (Figure 2d).
Ecological categories with all 100 coefficient values >0 (i.e., the reference threat category) were interpreted to be associated with a significantly (p < 0.01) elevated risk of extinction (Figure 3). Amphibians with the arboreal, semifossorial, ground dwelling, and cave dwelling habitat associations (reference: fossorial) and the jumping and pedal modes of locomotion (reference: slithering) were at elevated risk relative to the reference categories (Figure 3a). Squamates with the peristalsis and pedal modes of locomotion (reference: slithering) and the foraging, browsing, or grazing feeding mode (reference: predatory) were at elevated risk (Figure 3b). Mammals with fossorial, cave dwelling, arboreal, and ground dwelling habitat associations (reference: semifossorial) and pedal, jumping, and arboreal quadrupedal modes of locomotion (reference: flying) were at elevated risk (Figure 3c). Finally, birds with aerial habitat association (reference: semifossorial), pedal mode of locomotion (reference: jumping), and foraging, browsing, or grazing and scavenging feeding modes (reference: predatory) were at elevated risk (Figure 3d).
Primary risk factors of extinction
Across all assessed terrestrial vertebrate species, agriculture was the most common cause of extinction threat, followed by logging, invasive species and disease, and development (Figure 4a). When each of the four terrestrial vertebrate groups was assessed independently, agriculture was again the most prevalent extinction driver (Figures 4a–e). By contrast, the relative importance of other extinction drivers differed among groups. In amphibians and reptiles, after agriculture, logging was followed by development, then invasive and native species and disease (Figures 4b & c). In mammals, agriculture was followed by hunting and trade, logging, then invasive species and disease (Figure 4d). In birds, agriculture was followed by logging, invasive species and disease, and hunting (Figure 4e).
FIGURE 4.
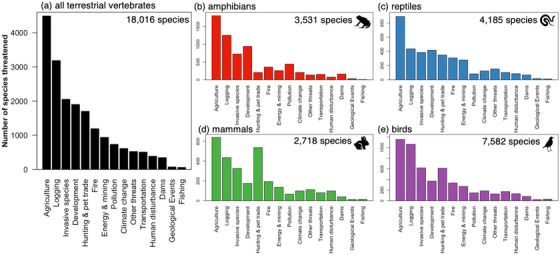
The primary extinction drivers and the sum of extinction drivers for (a) all 18,016 terrestrial vertebrate species together and separately by terrestrial vertebrate group: (b) amphibians, (c) reptiles, (d) mammals, and (e) birds. The total number of species threatened by each extinction driver for those classified as near threatened or worse by the International Union for the Conservation of Nature is shown in decreasing order for all terrestrial vertebrates. Threat order is the same (from the all‐species analysis) in all graphs to facilitate direct comparisons between groups (y‐axes values vary in range based on the number of species)
Ecological groups at an elevated risk of extinction (Figure 3) had a different set of threats compared with all terrestrial vertebrates assessed together. For example, agriculture threatened the greatest number of amphibian species that were ground dwelling, jumping or hopping, or predatory, or all three categories (Appendix S2); of reptile species that were ground dwelling or predatory or both that had pedal locomotion (Appendix S3); of mammal species that were arboreal, pedal, or foraging, browsing, or grazing, or all three categories (Appendix S4); and of bird species that were arboreal, flying, or predatory, or all three categories (Appendix S5). In contrast, other high‐risk groups, such as cave dwelling amphibians and mammals and scavenging birds, appeared to experience relatively little threat from agriculture (Appendices S2, S4, & S5).
Additive multistressor perspective on threat
There were a greater number of extinction drivers for species that were either EN or CR than for species that were NT or VU (W = 3.00 × 106, p < 0.001) (Figure 5a). This finding held when EX or EW species were included with the EN or CR species in comparison with the NT or VU species (W = 3.30 × 106, p < 0.001). When each vertebrate group was assessed individually, the number of extinction drivers threatening amphibian (W = 2.52 × 105, p < 0.001) (Figure 5b), reptile (W = 1.32 × 105, p < 0.05) (Figure 5c), and bird (W = 2.85 × 105, p < 0.05) (Figure 5e) species that were either EN or CR was greater than the number of drivers threatening species that were NT or VU. In mammals, however, there was no significant difference in the number of extinction drivers among these groupings (W = 8.52 × 104, p = 0.32) (Figure 5d). In addition, the total number of extinction drivers that species typically faced was significantly different for each individual red‐list threat category (i.e., NT, VU, EN, and CR) for all terrestrial vertebrates (χ2 = 79.8, df = 3, p < 0.0001), and the total number of extinction drivers that species typically faced also increased in an expected step‐wise pattern from low to high threat categories (Appendix S6. Amphibians (χ2 = 40.2, df = 3, p < 0.0001), mammals (χ2 = 8.2, df = 3, p = 0.04), and birds (χ2 = 24.1, df = 3, p < 0.0001) showed similar patterns. However, reptiles (χ2 = 7.1, df = 3, p = 0.07) displayed only marginally significant differences in the number of extinction drivers among red‐list categories (see Appendix S6 for all pairwise comparisons).
FIGURE 5.
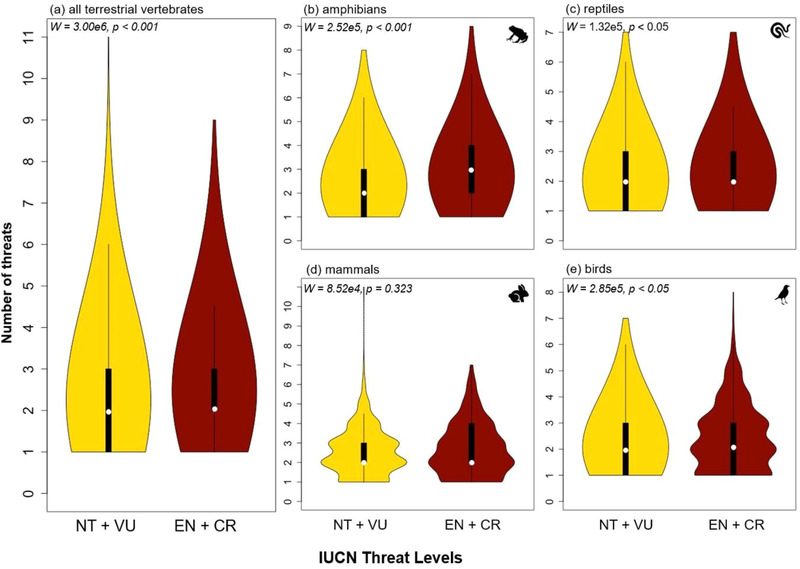
Number of nonthreatened (NT) and vulnerable (VU) and endangered (EN) and critically endangered (CR) terrestrial vertebrate species threatened by various extinction drivers (e.g., agriculture, logging, invasive species native species and disease) (white dot, median; thick black line, interquartile range; thin black line, 1.5 × interquartile range). The number of extinction drivers for each species is the total number of primary threat types as listed by the International Union for the Conservation of Nature (IUCN) for taxa that are near threatened or worse. The sum of extinction drivers for near threatened and vulnerable species and for the sum of extinction drivers for endangered and critically endangered species were grouped together
DISCUSSION
The consequences of the current biodiversity crisis are typically discussed in terms of population loss, species extinction, or loss of taxonomic diversity at higher levels (e.g., Dirzo & Raven, 2003; Grelle, 2005; Ceballos et al., 2015). In contrast, due to the differences in ecological and taxonomic diversity (e.g., Cooke et al., 2019), our results highlight another, equally important aspect of the biodiversity crisis: the ecological functions that are at risk of being lost. Our study also emphasizes the novel terrestrial ecospace concept, the varied importance of ecological traits as extinction risk predictors, and the threat types (and total number of threat types) associated with these traits in each of the four terrestrial vertebrate groups.
In our data, the ecological modes that best predicted extinction risk varied among the major taxonomic groupings. For example, in squamates, mode of locomotion and feeding mode best predicted extinction risk. In birds, however, habitat association, in addition to mode of locomotion, and feeding mode were the best predictors. In amphibians and mammals, habitat association and mode of locomotion were the best predictors. Because, thus far, there has been relatively less effort devoted to examining how these types of ecological traits shape extinction risk in extant lineages, there were fewer opportunities for direct comparison between our findings and previous research on extinction risk. However, Atwood et al. (2020) examined a subset of mammals, birds, and reptiles and found that diet (in particular herbivory) was an important determinant of extinction risk across these taxa. In contrast, we found that feeding mode was not a top predictor of mammal extinction risk. This difference may be due to differences between their categorization of feeding as diet (i.e., what is eaten) versus our categorization of a feeding mode (i.e., how feeding is performed). For example, in our analysis, the mining feeding mode (i.e., searches for and captures immobile subterranean prey) was a mix of herbivorous and carnivorous feeding behavior. Overall, our finding that extinction risk and ecological trait relationships varied among taxonomic groups parallels findings made based on different trait types and analytical methods, which highlights the diversity of pathways through which different taxa can become endangered (Davidson et al., 2009; Young et al., 2016).
The assembly of this new ecological‐trait database provides insight into how habitat association, mode of locomotion, and feeding mode can affect extinction risk in different lineages. Results from this effort can usefully be compared with previous work examining relationships between extinction risk and other classic species traits (e.g., geographic range size and body size [Young et al., 2016]). The association between small geographic range and elevated extinction risk (e.g., Purvis et al., 2000; Manne & Pimm, 2001) may have some connection mechanistically (at least in certain groups) to some of the relationships we documented. For example, perhaps the elevated risk observed in cave dwelling or semifossorial amphibians drives the relationship for certain species between small geographic range size and extinction risk. However, because geographic range size is one of the categories used to determine a species’ IUCN Red‐list category (IUCN, 2020), some degree of circularity is inherent in any analysis in which geographic range size is used as a predictor when it is also an input to the response variable. In addition to range size, body size has received a great deal of attention as a predictor of extinction risk in extant and extinct taxa (e.g., Payne et al., 2016; Smith et al., 2018; Payne & Heim, 2020). Body size extinction selectivity in the current marine biodiversity crisis, with larger species at greater risk, appears unlike any prior mass extinction for marine vertebrates and molluscs (Payne et al., 2016). Similarly, on land, hominins appear to have imposed extreme size selectivity on the largest species of mammals over the past ∼40,000 years, unlike the 66‐million‐year remainder of the Cenozoic (Smith et al., 2018). The recent and ongoing selectivity against large‐bodied marine vertebrates and molluscs appears coupled to motility level, but not habitat or feeding mode (Payne et al., 2016). Additionally, the extreme size selectivity observed in the late Quaternary against large mammals is consistent across trophic guilds (Smith et al., 2019). In aggregate, these results highlight the need for further study to identify both the possible interaction effects and additional unmeasured variables contributing to endangerment. Much of our effort herein was intended to broaden this evolving dialogue on predictors of extinction risk to include a new type of trait space for terrestrial animals that is closely aligned with the increasingly well‐studied ecospace concept for living and extinct marine animals (Bambach et. al, 2007; Bush et al., 2007; Knope et al., 2015; Knope et al., 2020).
Several ecological traits were disproportionately associated with elevated (or reduced) risk of extinction (Figure 3). The loss of ecological functions associated with those traits has the potential to widely disrupt ecosystem processes and services, potentially creating feedback loops that may exacerbate the original drivers of extinction (Dirzo et al., 2014). For example, because arboreal quadrupedal primates are at high risk of extinction (Figures 1 & 3c) and are often responsible for the gut transmission of large‐seeded fruits, their loss can negatively affect forest regeneration (Trolliet et al., 2016), thereby impairing the capacity for regeneration after deforestation. Lemurs, orangutans, and chimpanzees are examples of primates that are at an elevated risk of extinction and are also gut seed dispersers (Figures 1 & 3c). Scavenging bird species, such as vultures and condors, are also at elevated risk of extinction (Figures 1 & 3d), and the extinction of scavengers results in the loss of animal debris removal and altered disease dynamics, which can lead to trophic cascades and changes in nutrient cycling (Buechley & Şekercioğlu, 2016).
We also identified the rank order of threat types that are contributing to endangerment across all species included in the study (Figure 4a), across the four taxonomic groups (Figures 4b–e), and by ecological trait categories (Appendices S2–S5). Agriculture is now a dominant human influence on our planet, having expanded to cover approximately 40% of the world's ice‐free land surface (Foley et al., 2005) and was the single most common threat to terrestrial vertebrate species in our study (Figures 4a–e). Agriculture has also previously been identified as destabilizing genetic, taxonomic, and functional diversity (Campbell et al., 2017; Zabel et al., 2019). We found that agriculture is strongly and adversely affecting all four terrestrial vertebrate groups and identified the specific ecological groups that are more at risk from, and more resilient to, agriculture and other threats (Appendices S2–S5).
Understanding the connection between the high‐risk ecological categories and the primary extinction drivers can help managers target specific extinction drivers for species that are most vulnerable. For example, arboreal quadrupedal mammals are forest dependent and at an elevated risk of extinction (Figure 3c) due to agriculture and logging (Appendix S4), which leads to a loss of high‐quality forest habitat (Estrada et al., 2017). Bushmeat hunting and wildlife trade are also associated with at‐risk arboreal quadrupedal primates (Appendix S4) and directly reduce primate populations (Estrada et al., 2017). Ground dwelling amphibians were associated with an elevated risk (Figure 3a) from invasive species and disease (Appendix S2). For example, the chytrid fungus, which is classified under invasive species and disease, threatens many ground dwelling amphibian species (Kolby et al., 2015). Finally, scavenging birds are also at an elevated risk of extinction (Figure 3d) and hunting is their primary extinction driver (Appendix S5). Scavenging birds may be at higher risk of extinction if they consume carrion that contains lead bullets (Ogada et al., 2016) or other poisons, such as nonsteroidal anti‐inflammatory drugs (Naidoo et al., 2010). Their feeding mode is likely directly contributing to their endangerment due to human activities (Ogada et al., 2016). Our ecological‐threat driver analysis also highlights the ecological groups that are less threatened. For example, flying birds are less threatened compared with birds with jumping and pedal modes of locomotion, which may be related to their ability to move away from extinction drivers (Appendix S5). The inverse relationship we found between risk and mobility is consistent with results of recent work with marine animals in which fully motile species have lower extinction probabilities than nonmotile species across the past 500 million years (Knope et al., 2020).
Multiple primary extinction drivers may affect species in different ways, and these effects may interact with each other (Brook et al., 2008). For example, logging and agriculture can have immediate effects on both organisms and ecosystems, such as decreasing numbers, population restructuring, and the loss of genetic diversity (Estrada et al., 2017), whereas the biological response to climate change may lag with changes to temperature, precipitation, and other variables (Kuussaari et al., 2009). Climate change has been a dominant extinction driver in at least three of the five previous mass extinctions (e.g., Harnik et al., 2012), but it ranked low as an extinction driver in our analyses. However, its relative role in the current biodiversity crisis is expected to increase in the near future (e.g., Dirzo et al., 2014). In addition, interactions among extinction drivers may amplify their detrimental effects on species. For example, climate change can act synergistically with other extinction drivers to accelerate existing threats (Brook et al., 2008). Amphibians are susceptible to dangerous synergies among chytrid fungus, climate change, and land‐use change (Hof et al., 2011) because temperature and precipitation variability due to climate change in combination with land‐use practices can dictate the presence and range of chytrid transmission (Rohr et al., 2011). However, the additive threats of chytrid, climate change, and land‐use change affect the three major groups of amphibians (frogs, salamanders, and caecilians) differently (Hof et al., 2011). Therefore, although climate change currently ranks low in terms of the total number of species directly threatened by it (Figure 4), the detrimental effects of climate change are likely poised to intensify due to increasing climate change and interactions with other ongoing threats.
In addition to investigating the possible synergistic relationships between extinction drivers, we determined the total number of extinction drivers threatening each species. In agreement with previous work (Ducatez & Shine, 2017), we found that, within a given taxonomic group, species in higher threat categories were (on average) affected by a greater number of extinction drivers (Figure 5). This is a death‐by‐a‐thousand‐cuts scenario, in which a species may tolerate one or two extinction drivers, but as it is subjected to a greater number of threats, its vulnerability to extinction increases. This scenario is unlikely to be an artifact of greater attention to identifying the types of threats that yield high levels of endangerment. Although the IUCN initially focused attention on identifying extinction drivers for well‐known species (e.g., charismatic megafauna) that were already known to be threatened, as the number of species on the IUCN Red‐list has become more extensive over the past five decades, both threatened and nonthreatened species (including species of terrestrial vertebrates in the present study) have received similar attention when determining the number of associated threats (e.g., IUCN/SSC, 2004; BirdLife International, 2004; Baillie et al., 2004; Stuart et al., 2004; Rodrigues et al., 2006). Therefore, for future management, it may be important to account for the total number of extinction drivers that threaten a species.
It is important to recognize that the ecological categories associated with elevated risk are not necessarily direct causes of species extinction threat levels. Rather, in most cases, they more likely covary with other unidentified traits that may be more directly responsible. For example, arboreal quadrupedal primates are not necessarily at higher risk because of their mode of locomotion; rather, they are most likely at higher risk because they depend on forests that are often highly fragmented by agriculture and deforestation (Estrada et al., 2017). Due to the coarse ecological categorization of species, unmeasured variables explained much of the variance in extinction risk for terrestrial vertebrates in our analyses. In the best‐fit models, a median of 7–12% of the variance in extinction risk was explained across the four terrestrial vertebrate groups. Although the variance explained by these models was generally low, because we tested relatively few ecological predictor variables, it was unlikely that a high proportion of the variance in this complex real‐world system would be explained.
Data‐deficient species, as categorized by the IUCN Red‐list, were excluded from the analysis, but may provide vital additions to future extinction‐risk models. When a species lacks sufficient data on population size, geographic distribution, or threats, it is categorized as data deficient. This process creates uncertainty because the extinction threat is unknown (Nori & Loyola, 2015). Some species that are difficult to detect or identify, such as arboreal, cave dwelling, or fossorial species, may be overrepresented in the DD category and may be at a high risk of extinction (Butchart & Bird, 2010). Indeed, we may have underestimated extinction risk in these and other categories with high percentages of data‐deficient species. Future studies that prioritize data‐deficient species can elucidate uncertainties in determining the true extinction‐risk status.
Overall, the extinction‐risk patterns we identified may have particular applied value to conservation. With conservation resources limited and levels of endangerment high, these findings can help guide prioritization schemes for groups of vulnerable species. For example, some species groups, such as cave dwelling amphibians, arboreal quadrupedal mammals, and scavenging birds, appear to be experiencing disproportionate risk and warrant immediate attention and protection (Figure 1). Prioritization and conservation triage programs must also remain mindful that it is often complex interactions between ecological traits that shape risk. For example, habitat and motility combined to shape risk in amphibians (Figure 2), the most at‐risk of the terrestrial vertebrate groups we examined (Figure 1). Recognition of these patterns of vulnerability may be especially important in making informed a priori assessments of risk in data‐deficient species or understudied ecosystems. Recognition of this landscape of risk in these ecological traits can also help strategically direct applied research that can help identify the mechanisms that are actually driving this risk. Finally, recognizing which ecological trait groups are threatened helps restoration ecologists better assess how their actions may restore lost function via a suite of available methods (e.g., Donlan et al., 2006; Hansen & Galetti, 2009; McCauley et al., 2017) and more proactively manage and mitigate the cascading effects (Estes et al., 2011; Young et al., 2016) that may arise from these losses of function. Certainly, a final takeaway for managers is the cautionary observation that climate change appears to only be beginning to shape extinction risk and affect ecological trait vulnerability. Unfortunately, this may mean that many lessons used to predict and manage threats in recent times may be less instructive as climate impacts continue to accelerate (Ripple et al., 2020).
With sharp declines in global terrestrial vertebrate biodiversity, this phylogenetically informed, broad‐scale approach demonstrates that threat categories are not uniformly distributed across ecological categories. Far beyond individual at‐risk species, we find that whole suites of functional ecologies are at elevated risk of extinction globally. The preferential loss of groups of species associated with particular ecological traits and increasing human disruption of habitats have the potential to have global consequences for ecosystem structure and function. We identified the threat types most strongly associated with endangerment of ecological traits, which is a critical first step toward ameliorating these global functional disruptions.
Supporting information
Appendix S1. Percent of species in nonthreatened (least concern and near threatened) and threatened (vulnerable, endangered, critically endangered, extinct in the wild, and extinct) and data deficient as defined by the IUCN.
Appendix S2. The (a) number and (b) proportion of amphibian species threatened by each extinction driver separated by ecological category.
Appendix S3. The (a) number and (b) proportion of reptile species threatened by each extinction driver separated by ecological category.
Appendix S4. The (a) number and (b) proportion of mammal species threatened by each extinction driver separated by ecological category.
Appendix S5. The (a) number and (b) proportion of bird species threatened by each extinction driver separated by ecological category.
Appendix S6. Values represent Dunn's post‐hoc Z‐values from all possible pairwise comparisons for the number of risk factors between each risk‐level group.
ACKNOWLEDGMENTS
We thank E. Berg, M. Nakamoto, A. Romero, N. Rodriguez, M. Takakusagi, H. Tharp, L. Uehara, and K. Wichimai for assistance with data collection. We thank J. Koch and R. Ostertag for assistance with manuscript edits and D. Koizumi and T. Chong for administrative support. M.J.M. and M.L.K. conceived the study. M.J.M. collected the data. M.J.M., N.A.H., D.J.M., J.L.P., N.S.U., S.C.W., and M.L.K. all contributed equally to the data analyses and writing. This material is based on work supported by the U.S. National Science Foundation under grant 1345247 to M.L.K. Opinions, findings, and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Munstermann, Maya J , Heim, Noel A , McCauley, Douglas J , Payne, Jonathan L , Upham, Nathan S , Wang, Steve C , & Knope, Matthew L (2022). A global ecological signal of extinction risk in terrestrial vertebrates. Conservation Biology, 36, e13852. 10.1111/cobi.13852
Article impact statement: An ecological trait database shows how habitat association and modes of locomotion and feeding affect extinction risk in different lineages.
LITERATURE CITED
- Adler, D. , & Kelly, S. T . (2020). vioplot: Violin plot. R package version 0.3.5. Available from https://github.com/TomKellyGenetics/vioplot.
- Alroy, J . (2015). Current extinction rates of reptiles and amphibians. Proceedings of the National Academy of Sciences, 112, 13003–13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AmphibiaWeb . (2020). Data from The AmphibiaWeb. Available from https://amphibiaweb.org.
- Atwood, T. B. , Valentine, S. A. , Hammill, E. , McCauley, D. J. , Madin, E. M. , Beard, K. H. , & Pearse, W. D . (2020). Herbivores at the highest risk of extinction among mammals, birds, and reptiles. Science Advances, 6, eabb8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie, J. E. , Hilton‐Taylor, C. , & Stuart, S. N . (2004). IUCN Red List of threatened species. A global species assessment. Gland: IUCN. [Google Scholar]
- Bambach, R. K. , Bush, A. M. , & Erwin, D. H . (2007). Autecology and the filling of ecospace: Key metazoan radiations. Palaeontology, 50, 1–22. [Google Scholar]
- Barnosky, Anthony , Matzke, Nicholas , Tomiya, Susumu , Wogan, Guinevere , Swartz, Brian , Quental, Tiago , Marshall, Charles , McGuire, Jenny , Lindsey, Emily , Maguire, Kaitlin , Mersey, Ben & Ferrer, Elizabeth . (2011). Has the Earth's sixth mass extinction already arrived? Nature, 471, 51–57. [DOI] [PubMed] [Google Scholar]
- Bengtsson, H . (2020). matrixStats: Functions that apply to rows and columns of matrices (and to vectors). R package version 0.56.0. Available from https://CRAN.R‐project.org/package=matrixStats.
- Biesmeijer, J. C. , Roberts, S. P. M. , Reemer, M. , Ohlemüller, R. , Edwards, M. , Peeters, T. , Schaffers, A. P. , Potts, S. G. , Kleukers, R. , Thomas, C. D. , Settele, J. , & Kunin, W. E. (2006). Parallel declines in pollinators and insect‐pollinated plants in Britain and the Netherlands. Science, 313, 351–354. [DOI] [PubMed] [Google Scholar]
- BirdLife International . (2004). Threatened birds of the world 2004: Species factsheets for globally threatened birds. Cambridge: BirdLife International. [Google Scholar]
- Brook, B. W. , Sodhi, N. S. , & Bradshaw, C. J . (2008). Synergies among extinction drivers under global change. Trends in Ecology & Evolution, 23, 453–460. [DOI] [PubMed] [Google Scholar]
- Buechley, E. R. , & Şekercioğlu, Ç. H . (2016). The avian scavenger crisis: Looming extinctions, trophic cascades, and loss of critical ecosystem functions. Biological Conservation, 198, 220–228. [Google Scholar]
- Burnham, K. P. , & Anderson, D. R . (2004). Multi‐model inference: Understanding AIC and BIC in model selection. Sociological Methods & Research, 33, 261–304. [Google Scholar]
- Bush, A. M. , Bambach, R. K. , & Daley, G. M . (2007). Changes in theoretical ecospace utilization in marine fossil assemblages between the mid‐Paleozoic and late Cenozoic. Paleobiology, 33, 76–97. [Google Scholar]
- Bush, A. M. , Wang, S. C. , Payne, J. L. , & Heim, N. A . (2020). A framework for the integrated analysis of the magnitude, selectivity, and biotic effects of extinction and origination. Paleobiology, 46, 1–22. [Google Scholar]
- Butchart, S. H. , & Bird, J. P . (2010). Data deficient birds on the IUCN Red List: What don't we know and why does it matter? Biological Conservation, 143, 239–247. [Google Scholar]
- Campbell, B. M. , Beare, D. J. , Bennett, E. M. , Hall‐Spencer, J. M. , Ingram, J. S. , Jaramillo, F. , Ortiz, R. , Ramankutty, N. , Sayer, J. A. , & Shindell, D . (2017). Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecology and Society, 22, 1–11. [Google Scholar]
- Cardinale, Bradley J. , Duffy, J. Emmett , Gonzalez, Andrew , Hooper, David U. , Perrings, Charles , Venail, Patrick , Narwani, Anita , Mace, Georgina M. , Tilman, David , Wardle, David A. , Kinzig, Ann P. , Daily, Gretchen C. , Loreau, Michel , Grace, James B. , Larigauderie, Anne , Srivastava, Diane S. , & Naeem, Shahid . (2012). Biodiversity loss and its impact on humanity. Nature, 486, 59–67. [DOI] [PubMed] [Google Scholar]
- Carmona, C. P. , et al. (2021). Erosion of global functional diversity across the tree of life. Science Advances, 7, 2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos, G. , Ehrlich, P. R. , Barnosky, A. D. , García, A. , Pringle, R. M. , & Palmer, T. M . (2015). Accelerated modern human–induced species losses: Entering the sixth mass extinction. Science Advances, 1, e1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos, G. , Ehrlich, P. R. , & Dirzo, R . (2017). Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proceedings of the National Academy of Sciences, 114, E6089–E6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos, G. , Ehrlich, P. R. , & Raven, P. H . (2020). Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proceedings of the National Academy of Sciences, 117, 13596–13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichorro, F. , Juslén, A. , & Cardoso, P . (2019). A review of the relation between species traits and extinction risk. Biological Conservation, 237, 220–229. [Google Scholar]
- Clements, J. F. , Schulenberg, T. S. , Iliff, M. J. , Billerman, S. M. , Fredericks, T. A. , Gerbracht, J. A. , Lepage, D. , Sullivan, B. L. , & Wood, C. L. et al. (2016). Data from The eBird Clements checklist of birds of the world: Version 2016. Available from https://www.birds.cornell.edu/clementschecklist/download/.
- Cooke, R. S. , Eigenbrod, F. , & Bates, A. E . (2019). Projected losses of global mammal and bird ecological strategies. Nature Communications, 10, 2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, A. D. , Hamilton, M. J. , Boyer, A. G. , Brown, J. H. , & Ceballos, G . (2009). Multiple ecological pathways to extinction in mammals. Proceedings of the National Academy of Sciences, 106, 10702–10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirzo, R. , & Raven, P. H . (2003). Global state of biodiversity and loss. Annual Review of Environment and Resources, 28, 137–167. [Google Scholar]
- Dirzo, R. , Young, H. S. , Galetti, M. , Ceballos, G. , Isaac, N. J. , & Collen, B . (2014). Defaunation in the Anthropocene. Science, 345, 401–406. [DOI] [PubMed] [Google Scholar]
- Donlan, C. J. , Berger, J. , Bock, C. E. , Bock, J. H. , Burney, D. A. , Estes, J. A. , Foreman, D. , Martin, P. S. , Roemer, G. W. , Smith, F. A. , Soulé, M. E. , & Greene, H. W. (2006). Pleistocene rewilding: An optimistic agenda for twenty‐first century conservation. American Naturalist, 168, 660–681. [DOI] [PubMed] [Google Scholar]
- Ducatez, S. , & Shine, R . (2017). Drivers of extinction risk in terrestrial vertebrates. Conservation Letters, 10, 186–194. [Google Scholar]
- Estes, J. A. , Terborgh, J. , Brashares, J. S. , Power, M. E. , Berger, J. , Bond, W. J. , Carpenter, S. R. , Essington, T. E. , Holt, R. D. , Jackson, J. B. C. , Marquis, R. J. , Oksanen, L. , Oksanen, T. , Paine, R. T. , Pikitch, E. K. , Ripple, W. J. , Sandin, S. A. , Scheffer, M. , Schoener, T. W. , Shurin, J. B. , Sinclair, A. R. E. , Soulé, M. E. , Virtanen, R. , & Wardle, D. A. . (2011). Trophic downgrading of planet Earth. Science, 333, 301–306. [DOI] [PubMed] [Google Scholar]
- Estrada, A. , Garber, P. A. , Rylands, A. B. , Roos, C. , Fernandez‐Duque, E. , Fiore, A. D. , Anne‐Isola Nekaris, K. , Nijman, V. , Heymann, E. W. , Lambert, J. E. , Rovero, F. , Barelli, C. , Setchell, J. M. , Gillespie, T. R. , Mittermeier, R. A. , Arregoitia, L. V. , de Guinea, M. , Gouveia, S. , Dobrovolski, R. , Shanee, S. , Shanee, N. , Boyle, S. A. , Fuentes, A. , MacKinnon, K. C. , Amato, K. R. , Meyer, A. L. S. , Wich, S. , Sussman, R. W. , Pan, R. , Kone, I. , & Li, B. (2017). Impending extinction crisis of the world's primates: Why primates matter. Science Advances, 3, e1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley, J. A. , DeFries, R. , Asner, G. P. , Barford, C. , Bonan, G. , Carpenter, S. R. , Chapin, F. S. , Coe, M. T. , Daily, G. C. , Gibbs, H. K. , Helkowski, J. H. , Holloway, T. , Howard, E. A. , Kucharik, C. J. , Monfreda, C. , Patz, J. A. , Prentice, I. A , Ramankutty, N. , & Snyder, P. K. (2005). Global consequences of land use. Science, 309, 570–574. [DOI] [PubMed] [Google Scholar]
- Grelle, C. E. V . (2005). Predicting extinction of mammals in the Brazilian Amazon. Oryx, 39, 347–350. [Google Scholar]
- González‐del‐Pliego, P. , Freckleton, R. P. , Edwards, D. P. , Koo, M. S. , Scheffers, B. R. , Pyron, R. A. , & Jetz, W . (2019). Phylogenetic and trait‐based prediction of extinction risk for data‐deficient amphibians. Current Biology, 29, 1557–1563. [DOI] [PubMed] [Google Scholar]
- González‐Suárez, M. , Gómez, A. , & Revilla, E . (2013). Which intrinsic traits predict vulnerability to extinction depends on the actual threatening processes. Ecosphere, 4, 1–6. [Google Scholar]
- BirdLife International. (2020). Handbook of the birds of the world and BirdLife International digital checklist of the birds of the world. Version 5. Available from http://datazone.birdlife.org/userfiles/file/Species/Taxonomy/HBW-BirdLife_Checklist_v5_Dec20.zip. (accessed December 2020).
- Hansen, D. M. , & Galetti, M . (2009). The forgotten megafauna. Science, 324, 42–43. [DOI] [PubMed] [Google Scholar]
- Harnik, P. G. , Lotze, H. K. , Anderson, S. C. , Finkel, Z. V. , Finnegan, S. , Lindberg, D. R. , Liow, L. H. , Lockwood, R. , McClain, C. R. , McGuire, J. L. , O'Dea, A. , Pandolfi, J. M. , Simpson, C. , & Tittensor, D. P. (2012). Extinctions in ancient and modern seas. Trends in Ecology & Evolution, 27, 608–617. [DOI] [PubMed] [Google Scholar]
- Ho, L. S. T. , & Ané, C . (2014). A linear‐time algorithm for Gaussian and non‐Gaussian trait evolution models. Systematic Biology, 63, 397–408. [DOI] [PubMed] [Google Scholar]
- Hof, C. , Araújo, M. B. , Jetz, W. , & Rahbek, C . (2011). Additive threats from pathogens, climate and land‐use change for global amphibian diversity. Nature, 480, 516–519. [DOI] [PubMed] [Google Scholar]
- IUCN . (2012). IUCN Red List categories and criteria: Version 3.1. 2nd edition. Gland: IUCN. [Google Scholar]
- IUCN . (2020). The IUCN Red List of Threatened Species. Version 2020–2. Available from http://www.iucnredlist.org.
- IUCN/SSC . (2004). Global Amphibian Assessment, IUCN‐SSC, Conservation International, and NatureServe. Available from http://www.globalamphibians.org/.
- Jetz, W. , Thomas, G. H. , Joy, J. B. , Hartmann, K. , & Mooers, A. O . (2012). The global diversity of birds in space and time. Nature, 491, 444–448. [DOI] [PubMed] [Google Scholar]
- Jetz, W. , & Pyron, R. A . (2018). The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nature Ecology & Evolution, 2, 850–858. [DOI] [PubMed] [Google Scholar]
- Karp, D. S. , Mendenhall, C. D. , Sandí, R. F. , Chaumont, N. , Ehrlich, P. R. , Hadly, E. A. , & Daily, G. C . (2013). Forest bolsters bird abundance, pest control and coffee yield. Ecology Letters, 16, 1339–1347. [DOI] [PubMed] [Google Scholar]
- Knope, M. L. , Heim, N. A. , Frishkoff, L. O. , & Payne, J. L. (2015). Limited role of functional differentiation in early diversification of animals. Nature Communications, 6, 6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knope, M. L. , Bush, A. M. , Lo, F. , Heim, N. A. , & Payne, J. L . (2020). Ecologically diverse clades dominate the oceans via extinction resistance. Science, 367, 1035–1038. [DOI] [PubMed] [Google Scholar]
- Kolby, J. E. , Ramirez, S. D. , Berger, L. , Richards‐Hrdlicka, K. L. , Jocque, M. , & Skerratt, L. F . (2015). Terrestrial dispersal and potential environmental transmission of the amphibian chytrid fungus (Batrachochytrium dendrobatidis). PLoS One, 10, e0125386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuussaari, M. , Bommarco, R. , Heikkinen, R. K. , Helm, A. , Krauss, J. , Lindborg, R. , Öckinger, E. , Pärtel, M. , Pino, J. , Rodà, F. , Stefanescu, C. , Teder, T. , Zobel, M. , & Steffan‐Dewenter, I. (2009). Extinction debt: A challenge for biodiversity conservation. Trends in Ecology & Evolution, 24, 564–571. [DOI] [PubMed] [Google Scholar]
- Manne, L. L. , & Pimm, S. L . (2001). Beyond eight forms of rarity: Which species are threatened and which will be next? Animal Conservation, 4, 221‐229. [Google Scholar]
- McCauley, D. J. , Hardesty‐Moore, M. , Halpern, B. S. , & Young, H. S . (2017). A mammoth undertaking: Harnessing insight from functional ecology to shape de‐extinction priority setting. Functional Ecology, 31, 1003–1011. [Google Scholar]
- Naidoo, V. , Wolter, K. , Cromarty, D. , Diekmann, M. , Duncan, N. , Meharg, A. A. , Taggart, M. A. , Venter, L. , & Cuthbert, R . (2010). Toxicity of non‐steroidal anti‐inflammatory drugs to Gyps vultures: A new threat from ketoprofen. Biology Letters, 6, 339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordell, S. E. , & Valone, T. J . (2013). Animal behavior: Concepts, methods, and applications. Oxford: Oxford University Press. [Google Scholar]
- Nori, J. , & Loyola, R . (2015). On the worrying fate of data deficient amphibians. PLoS One, 10, e0125055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogada, D. , Shaw, P. , Beyers, R. L. , Buij, R. , Murn, C. , Thiollay, J. M. , Beale, C. M. , Holdo, R. M. , Pomeroy, D. , Baker, N. , Krüger, S. C. , Botha, A. , Virani, M. Z. , Monadjem, A. , & Sinclair, A. R. E. (2016). Another continental vulture crisis: Africa's vultures collapsing toward extinction. Conservation Letters, 9, 89–97. [Google Scholar]
- Payne, J. L. , Bush, A. M. , Heim, N. A. , Knope, M. L. , & McCauley, D. J . (2016). Ecological selectivity of the emerging mass extinction in the oceans. Science, 353, 1284–1286. [DOI] [PubMed] [Google Scholar]
- Payne, J. L. , & Heim, N. A . (2020). Body size, sampling completeness, and extinction risk in the marine fossil record. Paleobiology, 46, 23–40. [Google Scholar]
- Purvis, A. , Gittleman, J. L. , Cowlishaw, G. , & Mace, G. M . (2000). Predicting extinction risk in declining species. Proceedings of the Royal Society of London. Series B: Biological Sciences, 267, 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Ratto, F. , Simmons, B. I. , Spake, R. , Zamora‐Gutierrez, V. , MacDonald, M. A. , Merriman, J. C. , Tremlett, C. J. , Poppy, G. M. , Peh, K. S. , & Dicks, L. V . (2018). Global importance of vertebrate pollinators for plant reproductive success: A meta‐analysis. Frontiers in Ecology and the Environment, 16, 82–90. [Google Scholar]
- Revell, L. J . (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecology and Evolution, 3, 217–223.23467194 [Google Scholar]
- Ripple, W. J. , Wolf, C. , Newsome, T. M. , Hoffmann, M. , Wirsing, A. J. , & McCauley, D. J . (2017). Extinction risk is most acute for the world's largest and smallest vertebrates. Proceedings of the National Academy of Sciences, 114, 10678–10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripple, W. , Wolf, C. , Newsome, T. , Barnard, P. , Moomaw, W. , & Grandcolas, P . (2020). World scientists' warning of a climate emergency. Bioscience, 70, 8–12. [Google Scholar]
- Rodrigues, A. S. , Pilgrim, J. D. , Lamoreux, J. F. , Hoffmann, M. , & Brooks, T. M . (2006). The value of the IUCN Red List for conservation. Trends in Ecology & Evolution, 21, 71–76. [DOI] [PubMed] [Google Scholar]
- Rohr, J. R. , Halstead, N. T. , & Raffel, T. R . (2011). Modelling the future distribution of the amphibian chytrid fungus: The influence of climate and human‐associated factors. Journal of Applied Ecology, 48, 174–176. [Google Scholar]
- Rose, M. D . (1973). Quadrupedalism in primates. Primates; Journal of Primatology, 14, 337–357. [Google Scholar]
- Schmidt, M . (2010). Locomotion and postural behaviour. Advances in Science and Research, 5, 23–39. [Google Scholar]
- Smith, F. A. , Smith, R. E. , Lyons, S. K. , & Payne, J. L . (2018). Body size downgrading of mammals over the late Quaternary. Science, 360, 310–313. [DOI] [PubMed] [Google Scholar]
- Smith, F. A. , Smith, R. E. , Lyons, S. K. , Payne, J. L. , & Villaseñor, A . (2019). The accelerating influence of humans on mammalian macroecological patterns over the late Quaternary. Quaternary Science Reviews, 211, 1–6. [Google Scholar]
- Stuart, S. N. , Chanson, J. S. , Cox, N. A. , Young, B. E. , Rodrigues, A. S. , Fischman, D. L. , & Waller, R. W . (2004). Status and trends of amphibian declines and extinctions worldwide. Science, 306, 1783–1786. [DOI] [PubMed] [Google Scholar]
- Tonini, J. F. , Beard, K. H. , Ferreira, R. B. , Jetz, W. , & Pyron, R. A . (2016). Fully‐sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biological Conservation, 204, 23–31. [Google Scholar]
- Trolliet, F. , Serckx, A. , Forget, P. M. , Beudels‐Jamar, R. C. , Huynen, M. C. , & Hambuckers, A . (2016). Ecosystem services provided by a large endangered primate in a forest‐savanna mosaic landscape. Biological Conservation, 203, 55–66. [Google Scholar]
- Uetz, P. , Freed, P. , & Hošek, J . (2020). Data from The Reptile Database. Available from http://www.reptile‐database.org.
- Upham, N. S. , Esselstyn, J. A. , & Jetz, W . (2019). Inferring the mammal tree: Species‐level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biology, 17, e3000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiles, M. R. , Hall Jr., R. O. , Dodds, W. K. , Verburg, P , Huryn, A. D. , Pringle, C. M. , Lips, K. R. , Kilham, S. S. , Colón‐Gaud, C. , Rugenski, A. T. , Peterson, S. , & Connelly, S. (2013). Disease‐driven amphibian declines alter ecosystem processes in a tropical stream. Ecosystems, 16, 146–157. [Google Scholar]
- Wilson, D. E. , & Reeder, D. M . (2005). Mammal species of the world: A taxonomic and geographic reference. 3rd edition. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- Young, H. S. , McCauley, D. J. , Dunbar, R. B. , & Dirzo, R . (2010). Plants cause ecosystem nutrient depletion via the interruption of bird‐derived spatial subsidies. Proceedings of the National Academy of Sciences, 107, 2072–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, H. S. , McCauley, D. J. , Galetti, M. , & Dirzo, R . (2016). Patterns, causes, and consequences of Anthropocene defaunation. Annual Review of Ecology, Evolution, and Systematics, 47, 333–358. [Google Scholar]
- Zabel, F. , Delzeit, R. , Schneider, J. M. , Seppelt, R. , Mauser, W. , & Václavík, T . (2019). Global impacts of future cropland expansion and intensification on agricultural markets and biodiversity. Nature Communications, 10, 2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Percent of species in nonthreatened (least concern and near threatened) and threatened (vulnerable, endangered, critically endangered, extinct in the wild, and extinct) and data deficient as defined by the IUCN.
Appendix S2. The (a) number and (b) proportion of amphibian species threatened by each extinction driver separated by ecological category.
Appendix S3. The (a) number and (b) proportion of reptile species threatened by each extinction driver separated by ecological category.
Appendix S4. The (a) number and (b) proportion of mammal species threatened by each extinction driver separated by ecological category.
Appendix S5. The (a) number and (b) proportion of bird species threatened by each extinction driver separated by ecological category.
Appendix S6. Values represent Dunn's post‐hoc Z‐values from all possible pairwise comparisons for the number of risk factors between each risk‐level group.


