SUMMARY
Root architecture can be targeted in breeding programs to develop crops with better capture of water and nutrients. In rich nations, such crops would reduce production costs and environmental pollution and, in developing nations, they would improve food security and economic development. Crops with deeper roots would have better climate resilience while also sequestering atmospheric CO2. Deeper rooting, which improves water and N capture, is facilitated by steeper root growth angles, fewer axial roots, reduced lateral branching, and anatomical phenotypes that reduce the metabolic cost of root tissue. Mechanical impedance, hypoxia, and Al toxicity are constraints to subsoil exploration. To improve topsoil foraging for P, K, and other shallow resources, shallower root growth angles, more axial roots, and greater lateral branching are beneficial, as are metabolically cheap roots. In high‐input systems, parsimonious root phenotypes that focus on water capture may be advantageous. The growing prevalence of Conservation Agriculture is shifting the mechanical impedance characteristics of cultivated soils in ways that may favor plastic root phenotypes capable of exploiting low resistance pathways to the subsoil. Root ideotypes for many low‐input systems would not be optimized for any one function, but would be resilient against an array of biotic and abiotic challenges. Root hairs, reduced metabolic cost, and developmental regulation of plasticity may be useful in all environments. The fitness landscape of integrated root phenotypes is large and complex, and hence will benefit from in silico tools. Understanding and harnessing root architecture for crop improvement is a transdisciplinary opportunity to address global challenges.
Keywords: root, architecture, water, nitrogen, phosphorus, carbon
Significance Statement
Root architecture can be harnessed to develop crops with improved climate resilience and reduced input requirements, thereby improving global food security, agricultural sustainability, and climate change mitigation. Understanding and harnessing root architecture for crop improvement is a transdisciplinary opportunity to address global challenges.
ROOT ARCHITECTURE IS CRITICALLY IMPORTANT FOR THE GRAND CHALLENGE OF THE 21ST CENTURY: HOW TO SUSTAIN 10 BILLION PEOPLE IN A DEGRADED ENVIRONMENT
Humanity is at a crossroads. Human demands upon natural resources are growing in scope and intensity as those resources are being critically degraded. We need to develop new and more sustainable ways to live on this planet. Although this is true of nearly all aspects of human culture, it is especially relevant to our symbiosis with plants. The large majority of human interaction with terrestrial ecosystems consists of managing plants for food, wood, fiber, and increasingly fuel. Suboptimal water and nutrient availability are primary, pervasive limits to plant productivity. Roots are the interface of plants with soils, and root architecture, meaning the physical configuration of root systems in time and space, has overarching importance in determining how plants acquire soil resources. Root architecture therefore has a central role in the productivity and sustainability of all plant ecosystems, and thereby with our ability to exist.
Although root architecture is important for all terrestrial plant ecosystems, it is especially relevant in crop production because crops are more intensively managed and genetically improved, and are more essential for human welfare, than less intensively managed systems such as forests, pastures, and rangelands. Global agriculture urgently needs crops with better tolerance of drought and infertile soils. In the low‐input cropping systems characteristic of developing nations, drought and nutrient deficiency limit crop yields, and therefore food security and economic development (FAO, 2015; Nkonya et al., 2016; World Bank, 2017). In high‐input cropping systems, irrigation and intensive fertilization are costly, cause massive environmental pollution and resource depletion, and are unsustainable (Foley et al., 2011; Woods et al., 2010). Ongoing soil degradation is decreasing soil fertility and the ability of soils to respond to inputs (Narasimha et al., 2020). Drought, already the primary limit to global crop production, is worsening as a result of global climate change, by increasing atmospheric temperatures and thereby evapotranspiration, as well as by altering precipitation patterns (Mbow et al., 2019; Pachauri et al., 2015; Tebaldi and Lobell, 2008). The accelerating effects of global climate change are likely to exacerbate soil degradation and limit crop yields, especially in developing nations (Lynch et al., 2021a,b; St.Clair and Lynch, 2010). All of this is occurring against a backdrop of unprecedented expansion of the human population, the large majority of which is occurring in food‐insecure regions with drought and soil fertility challenges, as well as increasing food demand per capita. Sustaining 10 billion people in a degraded global environment is the paramount challenge of the 21st century. An inextricable component of that effort will be the development of crops with greater stress tolerance, and reduced reliance on irrigation and fertilizer.
In this review, I consider opportunities to breed crops with greater tolerance to drought and low soil fertility, as well as greater ability to sequester C from the atmosphere, by improving root architecture. This is a very broad topic that will not be comprehensively reviewed. Rather, I attempt to provide an overview of key issues, concepts, opportunities, and knowledge gaps. Model organisms, genetic manipulation, and highly controlled yet artificial growth conditions are invaluable tools to advance our understanding of basic root biology and its genetic and molecular regulation (Malamy, 2005; Wachsman et al., 2015). However, in the interests of brevity, to complement published reviews, as well as to maintain a focus on issues with immediate relevance to crop improvement, I focus on studies that demonstrate the functional utility of natural genotypic variation for specific root architectural phenotypes in annual crops of global importance, grown in the field or in realistic controlled environments (Rao et al., 2016). I also focus on root phenotypes rather than their genetic control and molecular breeding strategies, which have been the subject of other recent reviews (de Dorlodot et al., 2007; Mai et al., 2014; Varshney et al., 2021; Wasson et al., 2012).
THERE ARE TWO BROAD CLASSES OF SOIL RESOURCES: MOBILE (DEEP) AND IMMOBILE (SHALLOW)
Water readily moves through agricultural soils and therefore tends to be more available at depth. Exceptions are irrigated dryland systems in which water is regularly provided over a dry subsoil, but such systems are rare. Another important exception is the case of intermittent drought stress. Nutrients that are soluble in water and are not readily bound by the soil consequently leach with water and therefore are generally more available at depth. The most important of these is nitrate. In low‐input systems, nitrogen can be a shallow resource through gradual mineralization of organic matter in the topsoil. The two most important limiting resources for most agroecosystems, water and N, are therefore typically deep resources. The third most important constraint to global crop production is P, which, unlike nitrate, is immobilized by many soil constituents and therefore moves very slowly in soil by diffusion (Barber, 1995). Because of the continual deposition of P and other nutrients at the soil surface in decaying plant matter, and their slow downward movement via leaching, P accumulates in the topsoil (Lynch and Brown, 2001). Its bioavailability is also greater in the topsoil than the subsoil because of greater biological activity, soil organic matter, and oxygen, as well as factors that reduce P bioavailability in the subsoil such as acidity. Potassium, NH4, Ca, and Mg have mobilities intermediate between nitrate and phosphate because they are retained by soil cation exchange capacity. Sulfate, similar to nitrate, leaches readily. Therefore, roots must balance exploration of the topsoil, where P, K, and many other essential resources are localized, with exploration of the subsoil, where water and nitrate are localized (“Balancing deep and shallow exploitation” section).
ROOT ARCHITECTURAL PHENOTYPES TO IMPROVE THE CAPTURE OF DEEP SOIL RESOURCES
Steep, cheap, and deep
The ‘steep, cheap, and deep’ root ideotype for improved N and water capture consists of architectural, anatomical, and physiological traits that promote rapid exploration of deep soil domains (Lynch, 2013) (Figure 1). The speed of subsoil exploration is especially relevant for nitrate capture because, in many crop systems, there is a pulse of nitrate leaching early in the season, whether from fertilization or interseasonal mineralization of soil organic matter, during seedling establishment and early vegetative growth when plants have not yet fully developed their root systems. In this context, rapid subsoil exploration will capture nitrate that may otherwise leach out of the root zone entirely. By contrast, deep soil water is generally more important later in the growing season. ‘Steep’ refers to root growth angle, as well as other architectural phenotypes that promote deep rooting, whereas ‘cheap’ refers to traits that reduce the metabolic cost of soil exploration.
Figure 1.
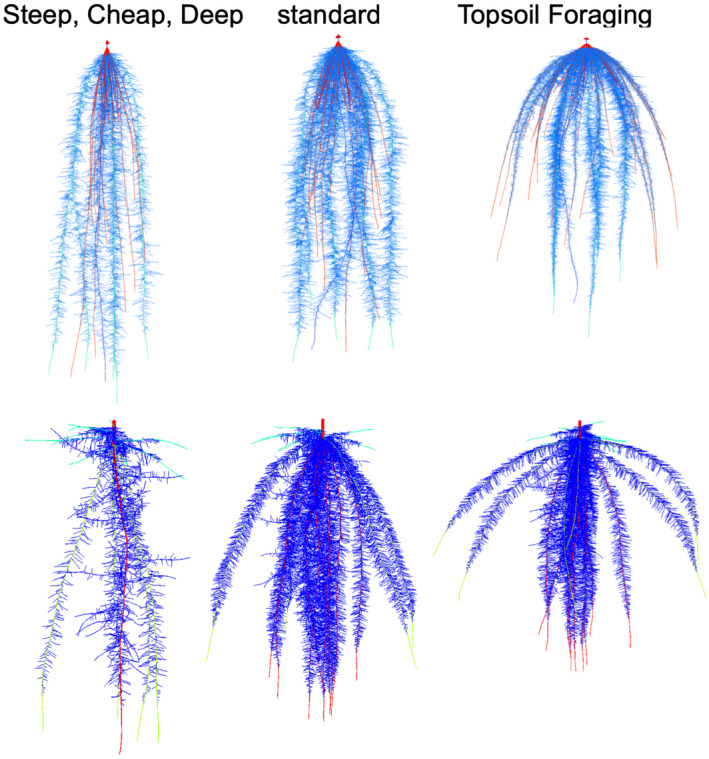
Steep, Cheap, and Deep and Topsoil Foraging ideotypes in maize (top) and common bean (bottom) at 42 days after germination as simulated by opensimroot. The center image represents standard phenotypes in maize and common bean germplasm. In maize (representing a non‐tillering monocot root architecture), the Steep, Cheap, and Deep phenotype was generated by reducing the number of axial roots, decreasing lateral root branching density, and increasing the steepness of crown root growth angles, whereas the Topsoil Foraging phenotype was generated by doing the opposite. In common bean (representing an annual dicot root architecture), the Steep, Cheap, and Deep phenotype was generated by reducing the number of hypocotyl‐borne roots, reducing the number of basal root whorls, decreasing lateral root branching density, and increasing the steepness of basal root growth angles, whereas the Topsoil Foraging phenotype was generated by doing the opposite. It has been proposed that the Steep, Cheap, and Deep phenotype is useful for the capture of subsoil resources including water and leached nitrate, whereas the Topsoil Foraging phenotype is useful for the capture of topsoil resources including recently mineralized NO3, NH4, P, K, Ca, and Mg and, in some cases, micronutrient metals. Model parameters are based on empirical observations. Images courtesy of Ernst Schafer. Reproduced from Lynch (2019).
Root growth angle
It is intuitively obvious from simple geometry that steeper root growth angles will result in more rapid development of deep roots, and therefore better utilization of deep soil resources, most importantly water and N (Figure 1). An elegant example of this is the gene DRO1, which regulates the growth angle of rice nodal roots (Uga et al., 2013). The presence of the DRO1 allele in rice isolines makes nodal roots steeper, deeper, and therefore better able to tolerate drought stress. In wheat, steeper nodal root growth angles are associated with better extraction of deep soil water and hence better yield under drought (Manschadi et al., 2010). Root angle regulates topsoil and subsoil foraging in common bean, with deeper root growth angles leading to better subsoil exploration, water capture, and drought tolerance (Ho et al., 2005). Steep root growth angles are also likely to be important for N capture because, in most agricultural soils, N is rapidly converted to nitrate, which readily leaches to deeper soil strata (Barber, 1995). Root depth, N capture, and plant growth and yield under N stress are substantially better in maize lines with steeper root growth angles (Dathe et al., 2016; Trachsel et al., 2013). Mutating the maize gene zmCIPK15, which regulates nodal root growth angle, leads to steeper growth angles at two nodal positions, which in turn leads to better N capture from deep soil and better plant growth in low N conditions in the field (Schneider et al., 2021a,b) (Figure 2). Taken together, these results (amongst others not mentioned here) support the hypothesis that steeper growth angles of axial roots improve subsoil exploration and thereby acquisition of the deep soil resources water and N.
Figure 2.
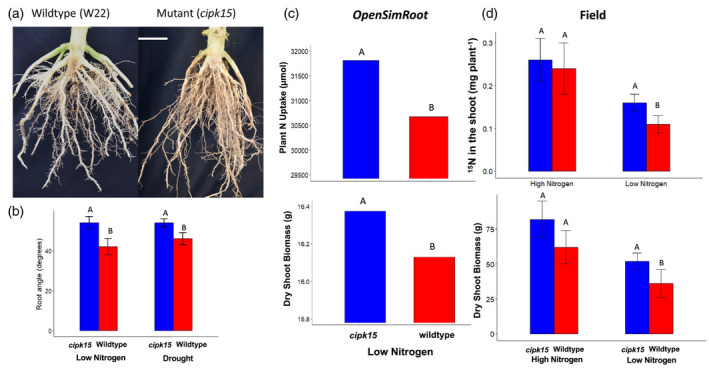
(a) Field‐excavated root crowns of wild‐type and zmCIPK mutant maize genotypes. (b) Growth angles (degrees from horizontal) of second node crown roots of wildtype and zmCIPK mutant maize genotypes under water deficit and low N stress in the greenhouse. (c) N uptake and biomass of wild‐type and zmCIPK mutant maize genotypes from low N soil at 40 days after germination as simulated in opensimroot. (d) N uptake from deep soil and biomass of wildtype and zmCIPK mutant maize genotypes 70 days after planting in the field in low N soil. Bars within a panel with different letters are significantly different at P ≤ 0.05. Adapted from Schneider et al. (2021a).
Number of axial roots
The second element of the steep, cheap and deep ideotype is ‘cheap’, denoting traits that reduce the metabolic cost of soil exploration. Root growth and maintenance consume a significant portion of internal plant resources (e.g. photosynthates, N, P, energy), especially under edaphic stress, which, in the case of the three primary soil resources (i.e. water, N, and P), increases resource allocation to roots relative to shoots. For example, root maintenance respiration accounted for up to 72% of the growth reduction caused by suboptimal K availability in maize plants in silico, and up to 38% of the growth reduction caused by suboptimal N or P availability (Postma and Lynch, 2011b). In this context, root phenotypes that reduce the metabolic cost of soil exploration should be beneficial for plant growth, by permitting greater soil exploration and hence greater capture of soil resources, as well as by liberating internal resources for other plant processes (Lynch, 2014; Lynch and Ho, 2005). An obvious architectural phene (‘phene’ is a fundamental unit of the phenotype, as opposed to phene aggregates, sensu York et al., 2013) that regulates root cost is simply the number of root axes produced. It was therefore proposed that root phenotypes with fewer axial roots would have greater resources to support the growth of the existing axial roots, resulting in deeper rooting and better capture of subsoil resources (Lynch, 2013). This should be especially true for water and nitrate, both being mobile resources that can be acquired by relatively lower root densities per volume of soil because they are mobile in soil and are brought to the root via transpiration‐driven mass flow. Indeed, maize genotypes with fewer crown roots have substantially better water capture, growth, and yield under drought (Gao and Lynch, 2016) (Figure 3c), and substantially better N capture, growth, and yield under N limitation (Saengwilai et al., 2014a) (Figure 3e).
Figure 3.

Effects of natural genotypic variation in maize for number of crown roots (a) and lateral root branching density (b) on plant performance in the field. Phenotypic variation in the field (a,b). Phenotypes with fewer crown roots had better yield under drought stress (c) and low N stress (e), but worse yield under low P stress (g). Phenotypes with fewer, longer lateral roots had better yield under drought stress (d) and low N stress (f) (note that this graph shows yield as a function root depth expressed as D 95, which is inversely related to lateral root branching density; Zhan and Lynch, 2015), but worse yield under low P stress. In (g), two phenotypic groups are presented: ‘MS’ for ‘many/short’ lateral roots, and ‘FL’ for ‘few/long’ lateral roots). Data from Gao and Lynch (2016) (c), Zhan et al. (2015) (d), Saengwilai et al. (2014b) (e), Zhan and Lynch (2015) (f), Sun et al. (2018) (g), Jia et al., 2018 (h). WS, water stress; WW, well‐watered; LN, low nitrogen; HN, high nitrogen. In (h), means with different letters are different at P ≤ 0.05.
Lateral root branching density
As with axial roots, a reduced number of lateral roots may be beneficial for root depth and therefore capture of deep soil resources (Lynch, 2013). Simulation modeling in simroot, a functional–structural model of root growth and soil resource capture (Lynch et al., 1997), indicated that a ‘few/long’ lateral root phenotype of reduced branching density but greater lateral root length would improve N capture under suboptimal N availability, by permitting deeper rooting hence better capture of leaching N resources (Postma et al., 2014). These simulation results were supported by analysis of maize genotypes with contrasting lateral root branching density under greenhouse and field conditions, which showed that genotypes with a ‘few/long’ phenotype had deeper rooting, and hence better water capture, growth, and yield under drought stress (Zhan et al., 2015) (Figure 3d), as well as better N capture, growth, and yield under N stress (Zhan and Lynch, 2015) (Figure 3f). It has been proposed that water capture and drought tolerance in wheat would be improved by root phenotypes that combine reduced lateral root branching in the topsoil with greater lateral branching in the subsoil (Wasson et al., 2012). This phenotype may be suited to dryland wheat production environments but, globally, many crops are grown in soils with suboptimal P availability in addition to drought, and reduced lateral branching in the topsoil may incur significant tradeoffs by reducing P acquisition (“Topsoil foraging” section). A phenotype with greater lateral branching density in the topsoil and less lateral branching density in the subsoil may therefore be more broadly adapted (Postma et al., 2014).
Anatomical phenotypes that reduce the cost of soil exploration
Although this review is focused on root architecture rather than root anatomy, the two are linked because root anatomical phenotypes have important effects on the metabolic costs of root construction and maintenance (Lynch et al., 2021a). Root metabolic costs directly affect root architecture by altering the number and length of roots that can be produced and sustained, and indirectly affect root architecture by influencing the capture of soil resources, and thereby plant growth and development. This has most clearly been demonstrated in maize, in which several anatomical phenotypes affect rooting depth and the capture of subsoil resources by reducing root metabolic costs (Lynch, 2014; Lynch et al., 2021a). Maize genotypes with greater root cortical aerenchyma, which converts living cortical parenchyma to air space by programmed cell death (Jackson and Armstrong, 1999), have roots with reduced nutrient content and reduced respiration, which is associated with deeper rooting, greater water capture, and greater growth and yield under drought stress in the field (Chimungu et al., 2015a,b; Zhu et al., 2010a) (Figure 4a,d), as well as deeper rooting, greater N capture, and hence greater growth and yield under N stress in the field (Saengwilai et al., 2014b). Maize genotypes also vary in the number of parenchyma cells in the root cortex through variation in the number of cortical cell files. Genotypes with fewer cortical cell files have reduced respiration and deeper rooting, greater water capture, and greater growth and yield under drought stress in the field (Chimungu et al., 2014b; Lynch, 2014) (Figure 4b,e). In silico analysis suggest similar benefits for N capture under N stress (Yang et al., 2020). The size of root cortical cells varies in maize, which may affect root cost by altering the proportion of the symplasm occupied by the vacuole, because the cytoplasm and organelles other than the vacuole have greater content of N and P, and greater respiration, than the vacuole (Lynch, 2013). Indeed, maize genotypes with larger root cortical cells had reduced respiration, deeper rooting, better water capture, and greater growth and yield under drought stress in the field (Chimungu et al., 2014a) (Figure 4c,f). Root cortical senescence in Triticeae cereals also reduces root metabolic costs (Schneider et al., 2017a) and improves nutrient capture in silico (Schneider et al., 2017b). The similar (and substantial) effects of these four anatomical phenotypes on rooting depth and the capture of subsoil resources, despite being under distinct genetic and developmental control, indicate that root anatomy has important effects on root architecture that could be harnessed in crop breeding (Lynch et al., 2021a).
Figure 4.
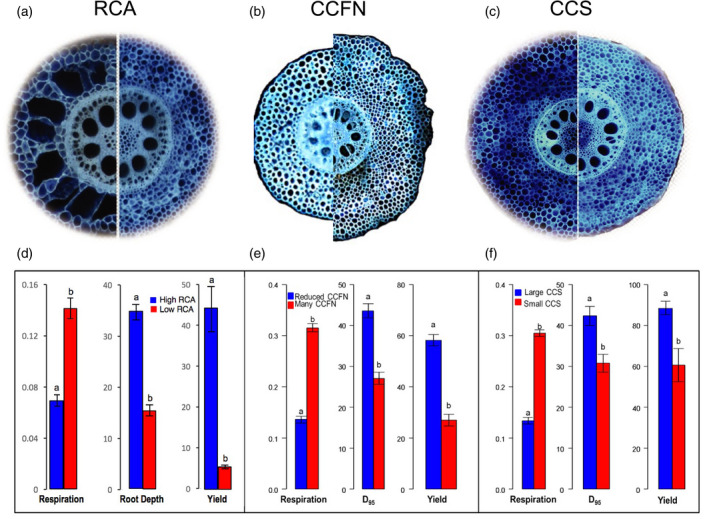
Phenotypic variation in maize for root cortical aerenchyma (RCA) (a), cortical cell file number (CCFN) (b) and cortical cell size (CCS) (c). (d) Under water stress, genotypes with greater RCA have less respiration (nmol CO2 s–1 cm–1), deeper rooting (d, cm roots at 40–50 cm soil depth; e, f, D 95, which is the depth in cm attained by the 95th percentile of roots), and greater yield (g per plant), as did genotypes with reduced CCFN (e) and greater CCS (f). Data shown are the mean ± SE (n = 3 or 4). Means with different letters are significantly different (P ≤ 0.05). Redrawn from Lynch (2018).
Challenges in the subsoil
As noted above, several root architectural and anatomical phenotypes promote deeper rooting. However, as roots descend into deeper soil domains, they confront several obstacles that must be overcome for continued root growth and resource capture (Lynch and Wojciechowski, 2015; Thorup‐Kristensen et al., 2020). Globally, the most important of these are mechanical impedance, hypoxia, and Al toxicity.
Impedance
Deeper soils are harder than surface soils because of the increasing pressure or ‘overburden’ caused by the weight of soil above, as well as reduced organic matter content and less biopore development by soil organisms. Several root phenotypes improve penetration of hard soils (Bengough et al., 2011). In wheat, steep growth angles improve penetration of hard soils (Whalley et al., 2013). Anatomical phenes are also important for penetration of hard soil. In maize grown in strong soils, deeper rooting was associated with greater cortical cell file number, greater cell size in the mid cortex, and greater root cortical aerenchyma formation (Vanhees et al., 2020). Cortical cell thickness, cortical cell count, cortical cell wall area, outer cortical cell size, and stele diameter are associated with increased root penetration and tensile strength (Chimungu et al., 2015a,b). Anatomical phenotypes that reduce root metabolic costs may also improve penetration of hard soil. In wheat, larger cortical cell size is associated with reduced respiration and greater penetration of hard soil (Colombi et al., 2019). Multiseriate cortical sclerenchyma (MCS) is characterized by several layers of small, lignified cells in the outer cortex of cereals (Schneider et al., 2021a,b). In maize and wheat, MCS increases root tensile strength and also increases rooting depth in strong soils (Schneider et al., 2021a,b). In maize, genotypes with MCS have greater rooting depth and greater shoot biomass in compacted soils (Schneider et al., 2021a,b).
It has been proposed that the plasticity of root growth in response to mechanical impedance may be adaptive by reallocating root foraging to softer, presumably wetter, soil domains (Lynch et al., 2021b). In support of this proposal, genotypes that thicken in response to impedance are less able to cross a hard soil layer than those for which the anatomical phenotype is unchanged (Vanhees et al., 2020, 2021). This response is mediated by ethylene (Vanhees et al., 2021), which recently has been shown to be a ‘stop signal’ for root growth into hard soil (Pandey et al., 2021). In the topsoil, this sort of plasticity may be advantageous by preventing root foraging into soil domains that are hard because of surface drying, whereas, in both the topsoil and the subsoil, growth plasticity in response to impedance may be beneficial by redirecting root growth to biopores and low resistance pathways at the interface of soil structural units (Lynch et al., 2021b).
Human management has changed soils in ways that make them generally more challenging for root growth from the perspective of mechanical impedance (Lynch et al., 2021b) (Figure 5). Tillage increases soil erosion through mechanical disturbance and by reducing vegetative cover, decreases soil organic matter, causes compaction by animal or vehicle traffic, and reduces biopore networks by direct disruption, as well as through decreased macrofaunal activity (Figure 5b). In rich nations, the increasing adoption of Conservation Agriculture, with reduced tillage and greater vegetation cover, is returning to some of the characteristics of native soils, with reduced erosion, greater organic matter, and better soil structure and biopore development (Figure 5c). However, in the low input agriculture characteristic of developing nations, many agroecosystems are characterized by ongoing soil degradation, loss of topsoil, loss of organic matter, and subsoil acidity (Lynch et al., 2021b) (Figure 5d). These trends are being exacerbated by global climate change, which will accelerate soil degradation, as well as drought‐induced soil hardening (Lynch and St.Clair, 2004; St.Clair and Lynch, 2010). Future crops should have adaptations to these contrasting soil environments.
Figure 5.

Conceptual scheme of four soil scenarios, their impedance profiles, and hypothetical root phenotypes adapted to them, as described in the text. (a) Native soil: mechanical impedance to root growth in native soils is mediated by high organic matter content, low‐resistance pathways formed by biopores, soil aggregates, and soil structure, and drought‐induced hardening of the topsoil (pink triangle), with N and water available in the topsoil, but greater water availability at depth. Nitrogen availability is limited and is greater in the epipedon from organic matter mineralization. We propose that root phenotypes adapted to this environment have plastic roots that can respond to local low resistance pathways, and will benefit from dimorphic root phenotypes that promote both topsoil and subsoil foraging. (b) Soils under conventional tillage, which, in comparison with native soil, have a thinner epipedon with less organic matter, hence less water holding capacity and greater susceptibility to soil hardening as a result of soil drying, fewer low resistance pathways from soil structure and biopores, and a plowpan from vehicle traffic. Nitrogen availability is greater at depth as a result of nitrate leaching from fertilizer. In these environments, nonplastic root phenotypes that can penetrate through hard surface layers to reach deep soil domains with greater water and N availability could be advantageous. Root phenotypes that promote topsoil foraging could be less useful for mature plants. (c) In high‐input agroecologies, traditional tillage in mechanized agriculture is evolving towards reduced tillage in Conservation Agriculture, which will return to some of the features of native soil, including greater topsoil organic matter, greater frequency of biopores, greater aggregate development and improved soil structure but harder bulk soil, and greater N availability in deep strata because of nitrate leaching from fertilizer. More plastic root phenotypes that avoid hard, dry soil domains to exploit biopores, soil fissures, and deeper, wetter, and therefore softer soils, could be advantageous. Penetrating axial roots, parsimonious root phenotypes, and phenotypes that support subsoil exploration could be useful in exploiting N and water in deep soil strata. (d) Soils under low‐input agriculture, with similar characteristics as mechanized agriculture but with greater loss of the epipedon and organic matter, hence greater susceptibility to soil hardening as a result of soil drying, no plowpan, low N availability limited to the epipedon because of limited fertilizer use, and the additional barrier of acid subsoil (yellow triangle). In these environments, non‐plastic root phenotypes that can penetrate through hard surface layers to reach deep soil domains with greater water availability will be advantageous, along with Al tolerance and dimorphic root phenotypes that also permit capture of shallow N from mineralization. Reproduced from Lynch et al. (2021b).
Hypoxia
Oxygen availability declines with soil depth, which can limit root activity. The root phenotype that has received the most research attention in this context is root cortical aerenchyma, which improves oxygenation of root tissue and the surrounding rhizosphere by forming continuous air‐filled lacunae (Jackson and Armstrong, 1999; Lynch and Wojciechowski, 2015). However, architectural phenotypes may also be important. Root plasticity, which channels root growth in hard subsoils into low resistance pathways such as biopores and interfaces between soil structural units, may be beneficial because these pathways are also pathways for gas exchange into deep soil.
Aluminum toxicity
In many weathered soils, acidity increases with depth, which, when below pH 5.2, leads to greater solubility of trivalent Al ions, which are phytotoxic. Aluminum toxicity directly impedes root growth by damaging the root apical meristem, and also by reducing the uptake of Ca and Mg, which have low bioavailability in weathered subsoils (Lynch and Wojciechowski, 2015). This is especially problematic for root growth because Ca is needed by the growing apical meristem and little is provided by phloem transport into the growing region. An important Al tolerance mechanism in several crop species is the production and release of carboxylate ions into the rhizosphere, which detoxify Al ions, as well as solubilizing P from metal complexes (Kochian et al., 2015; Ryan et al., 2011).
ROOT ARCHITECTURAL PHENOTYPES TO IMPROVE THE CAPTURE OF P AND OTHER SHALLOW SOIL RESOURCES
Topsoil foraging
Water, nitrate, and sulfate are highly mobile in agricultural soils and therefore are generally more available in deep soil horizons (Barber, 1995). The remaining plant nutrients have limited mobility because of reactions with soil constituents, and are continually deposited on the soil surface in plant residues, hence having greater availability in the topsoil. This is especially true for P (Lynch and Brown, 2001). The movement of phosphate in soil is limited by diffusion and is very slow, and in addition the bioavailability of P is improved by microbial activity, which is greater in the topsoil. Phosphorus bioavailability is therefore highly stratified in most soils. Low P bioavailability is a primary constraint to life on earth, is an important yield limitation in many developing nations, and has received more research attention than any other nutrient except N (Vance et al., 2003). Therefore, I focus here on architectural adaptations to P acquisition, although many of the same considerations may apply to other topsoil nutrients with agricultural relevance, especially K, which have received less research attention (Lynch, 2019).
Topsoil foraging is improved by architectural phenotypes that are the opposite of those discussed in sections “Root growth angle,” “Number of axial roots,” and “Lateral root branching density” (Figure 1). Whereas steep root growth angles result in deeper rooting and better capture of subsoil resources (“Root growth angle” section), shallow root growth angles improve topsoil foraging and thereby P capture under P stress (Bonser et al., 1996; Liao et al., 2001; Lynch and Brown, 2001; Rangarajan et al., 2018; Rubio et al., 2003; Zhu et al., 2005). Whereas maize genotypes with fewer axial roots have deeper rooting (“Number of axial roots” section), those with more crown roots (i.e. belowground nodal roots) have greater topsoil foraging, P capture, growth, and yield in low P soil (Sun et al., 2018) (Figure 3g). Common bean genotypes with more basal roots (the main axial root class in this species) have greater topsoil foraging, P capture, growth, and yield under P stress than phenotypes with fewer basal roots (Miguel et al., 2013; Rangarajan et al., 2018; Walk et al., 2006). In common bean, the production of hypocotyl‐borne roots improves topsoil foraging and P capture because these roots typically have very shallow growth angles and a lower metabolic cost than other axial roots (Miller et al., 2003; Rangarajan et al., 2018; Walk et al., 2006). Greater production of axial roots in monocots increases topsoil foraging because axial roots emerge in the topsoil, and also by creating more competing sinks for plant resources, thereby slowing the elongation of individual root axes into deeper soil domains. Whereas reduced lateral root branching density increases rooting depth (“Lateral root branching density” section), increased lateral root branching increases topsoil foraging both directly (when laterals are formed in the topsoil) and, indirectly, by reducing root depth, as has been demonstrated in silico (Postma et al., 2014) and amongst contrasting maize genotypes (Jia et al., 2018; Zhu and Lynch, 2004) (Figure 3h). The sharp contrast between architectural phenotypes that improve subsoil foraging and those that improve topsoil foraging creates obvious fitness tradeoffs for root strategies, which is globally most important in the case of P and water, two primary resource limitations with contrasting soil distributions (“Balancing deep and shallow exploitation” section).
Unlike the case of architectural phenotypes, it appears that anatomical phenotypes that reduce the metabolic cost of soil exploration benefit both topsoil and subsoil foraging. Continued soil exploration through root formation and elongation is especially important for immobile resources because the root (or its mycorrhizal symbiont) must be in close proximity to P to acquire it via diffusion (Barber, 1995). Indeed, common bean and maize genotypes with greater formation of root cortical aerenchyma have greater topsoil foraging, P capture, growth, and yield in low P soil, notwithstanding the reduction in mycorrhizal habitat by root cortical aerenchyma formation (Galindo‐Castañeda et al., 2018; Postma and Lynch, 2011a,b). In dicots, P stress inhibits the secondary growth of roots, and bean genotypes with greater inhibition of secondary growth under P stress have reduced root costs, greater P capture, and improved growth in low P soil (Strock et al., 2017; Strock and Lynch, 2017). Similar benefits for P capture were reported for reduced cortical cell file number and greater cortical cell size in maize roots in silico (Yang et al., 2020). Traits that reduce the metabolic cost of soil exploration merit attention as potential breeding targets (Lynch, 2014).
BALANCING DEEP AND SHALLOW EXPLOITATION
In some environments the majority of soil resources are concentrated in the topsoil; for example, those in which nutrients and water are provided to high value crops via drip irrigation, or humid low input agroecologies in which water is abundant and N is primarily available from mineralization of organic matter in the epipedon. However, in most global agroecosystems, roots must forage for resources in both shallow and deep soil domains. N leaching is common in fertilized agroecosystems, as well as systems in which mineral N accumulates in the topsoil N in a cold, dry or fallow season, then rapidly leaches in the growing season before crop roots are established. Water is often scarce in shallow soil domains because of greater root activity in the topsoil as well as direct soil evaporation. However, P, K, and most other nutrients are more available in the topsoil. This generates tradeoffs for root foraging strategies for topsoil and subsoil resources. For example, in common bean, steep basal root growth angles improve water capture under water stress, whereas shallow angles improve P capture (Ho et al., 2004, 2005). Tradeoffs between architectural phenotypes for deep and shallow soil exploration are present in a range of pulse crops, and differ between species with hypogeal (i.e. the cotyledons remain belowground) and epigeal (i.e. the cotyledons are lifted aboveground) germination (Burridge et al., 2020). Whereas extreme root architectural phenotypes are advantageous in extreme environments, intermediate root phenotypes that are capable of both topsoil and subsoil foraging without excessive root production are advantageous across a range of soil environments. For example, an in silico analysis of the effect of nodal root growth angle on N capture in maize found that, although extremely shallow root systems perform well in environments with minimal N leaching, dimorphic phenotypes with normal or shallow seminal and very steep nodal roots performed well in all scenarios, and consistently outperformed the steep phenotypes (Dathe et al., 2016). An in silico analysis of the effect of basal root phenotypes in common bean under conditions of both P and N stress found that optimal phenotypes were able to balance topsoil and subsoil foraging at minimal metabolic cost (Rangarajan et al., 2018). It has been proposed that root ideotypes for high‐input environments, where water remains an important resource limitation but P and other topsoil resources are abundant, could emphasize parsimonious root phenotypes that focus on subsoil exploration (Lynch, 2018; Wasson et al., 2012). Strategies to co‐optimize topsoil and subsoil foraging differ between monocot and dicot crops because, in monocot species, the topsoil is explored by continual production of adventitious roots from shoot nodes as they descend into the subsoil, in contrast to dicot species, in which most roots are formed as laterals from existing roots, with the exception of adventitious or hypocotyl‐borne roots, which do improve topsoil exploration and P capture in common bean (Miller et al., 2003). In maize, successive crown roots possess progressively steeper growth angles (York and Lynch, 2015), which improves topsoil foraging during early vegetative growth, coinciding with the topsoil availability of water, N, and P, with progressively deeper soil exploration over time, coinciding with the increasing importance of nitrate and water in deeper soil strata as the season progresses (Lynch, 2019). Crop breeding for most environments should target integrated root phenotypes that co‐optimize topsoil and subsoil foraging at minimal metabolic cost (i.e. without production of so many root axes that yield is adversely affected). These concepts are implicit in several published root ideotypes (Burridge et al., 2020; Lynch, 2018, 2019; Schmidt and Gaudin, 2017; Uga, 2021; Wasson et al., 2012).
ROOT ARCHITECTURE TO IMPROVE BIOSEQUESTRATION OF ATMOSPHERIC CO2
The ability of plants to convert atmospheric CO2 into soil organic matter is an attractive and feasible means to mitigate global climate change, while concurrently improving soil quality (Kell, 2011, 2012; Thorup‐Kristensen et al., 2020). A substantial portion of C fixed by plants in photosynthesis is allocated to roots, which in turn deposit a substantial portion into the rhizosphere (Farrar et al., 2003; Jones et al., 2004; Lambers et al., 2002; Lynch, 2014; Lynch and Ho, 2005). Root‐derived C decays more than twice as slowly in the soil compared to shoot‐derived C (Rasse et al., 2005) and the decay of plant residues in soil decreases with soil depth, along with oxygen availability and microbial activity. Architectural phenotypes that increase rooting depth would therefore directly increase biosequestration (Kell, 2011, 2012; Lynch and Wojciechowski, 2015). The magnitude of the benefits of increased rooting depth of cultivated plants on global C budgets could be substantial. In addition to the direct effects of rooting depth on C sequestration, greater rooting depth would improve the capture of water and N in many agroecosystems (“Root architectural phenotypes to improve the capture of deep soil resources” section), thereby improving plant growth, which would increase total C capture, as well as increase root and rhizosphere C allocation through allometry. An additional benefit of greater rooting depth for global C budgets would be reduced consumption of fossil fuels to power irrigation, as well as reduced consumption of fossil fuels, and also reduced production of NOx species, by reducing the need for N fertilizer. Lignin decays more slowly than hemicellulose and cellulose (Berg and McClaugherty, 2003; Kögel‐Knabner, 2002). In this context, the recent discovery of MCS is interesting (Schneider et al., 2021b). MCS varies among cereal genotypes, and lines with greater MCS have more lignin, stronger roots, better penetration of hard soil, and deeper rooting (Schneider et al., 2021b). Phenes such as MCS may be useful in breeding crops for greater C sequestration, by slowing the decay of root residues through greater rooting depth as well as via a more durable root composition.
ROOT ARCHITECTURAL PHENOTYPES FOR HIGH‐INPUT AGROECOSYSTEMS
High‐input agroecosystems employ management, crop genetics, fertilizers, and pesticides to mitigate many of the factors that restrict root function in native plants. However, water and N remain important limiting resources in most high‐input agroecosystems. The majority of global agriculture is not irrigated, and decreasing availability of fresh water, as well as salinization, limit future expansion of irrigated area (Hanjra and Qureshi, 2010). Global climate change is exacerbating crop water stress by increasing water demand and disrupting water availability (Mbow et al., 2019). Nitrogen in the form of N fertilizer is costly from economic, environmental, and energy perspectives (von Blottnitz et al., 2006; Robertson and Vitousek, 2009; Vitousek, 2009).
Parsimonious root phenotypes
It has been proposed that parsimonious root phenotypes would be advantageous for high‐input agroecosystems (Lynch, 2018). Crop ancestors confronted an array of biotic and abiotic stresses, belowground competition from other plant species, and spatiotemporal variability in the availability of soil resources. These selection pressures favored phenotypes with abundant roots, developmental plasticity in response to local availability of water and nutrients, and maintenance of unspecialized root tissues. High‐input agroecosystems have removed many of these constraints to root function, although water deficit remains a primary risk to crop production, and this risk is likely to grow in the future. Therefore, parsimonious root phenotypes that prioritize optimal water capture at the expense of ancestral adaptations may be useful breeding targets for high‐input agroecosystems. Such phenotypes are also likely to be beneficial for N capture (Lynch, 2018, 2019). Parsimonious architectural phenotypes would have fewer axial and lateral roots, reduced plasticity to local availability of water and nutrients, and greater loss of roots that do not contribute to water capture. Several elements of this hypothesis are supported by empirical evidence. As noted in “Root architectural phenotypes to improve the capture of deep soil resources” section, reduced production of axial roots and reduced lateral root branching density are both associated with deeper rooting, and hence greater capture of N and water, and greater growth and yield of maize under water or N stress. In this context, it is interesting that the past century of maize breeding has generated phenotypes with reduced production of axial roots (York et al., 2015). Although root anatomy is not the focus of this review, parsimonious anatomical phenotypes also increase rooting depth, capture of water and N, and growth and yield of maize under water and N stress (Lynch et al., 2021a).
Root architectural adaptations to Conservation Agriculture
Conservation Agriculture is ascendent in high‐input crop production, key elements of which are minimal soil disturbance and continual soil protection with vegetation (Page et al., 2020). This is changing the physical characteristics of agricultural soils because of increased soil organic matter content, improved soil aggregate structure, and greater generation and maintenance of biopore networks from the activity of roots and soil fauna (Lynch et al., 2021b; Or et al., 2021) (Figure 5c). Mechanical impedance is a primary constraint to root growth, especially in deep soil domains (Atwell, 1993; Gao et al., 2016; Lynch and Wojciechowski, 2015). Soils under Conservation Agriculture have more abundant low‐resistance pathways for root growth into the subsoil than conventionally tilled soils, created by the interfaces among soil structural units and persistent biopores (Lynch et al., 2021b). Drought‐induced hardening of the topsoil, which is projected to intensify in future climates, will increase the importance of subsoil exploration. These factors are generating soil environments that are similar to native soils in terms of mechanical impedance. Root adaptations of wild plants, especially growth plasticity in response to local soil hardness, may be beneficial for future crops, by permitting roots to avoid dry, hard soil domains in favor of softer soil with greater water availability, as well as low resistance pathways to the subsoil such as fissures and biopores (Lynch et al., 2021b).
ROOT ARCHITECTURAL PHENOTYPES FOR LOW‐INPUT AGROECOSYSTEMS
Food security in low‐input agroecosystems is an important global challenge
Low‐input agroecosystems include rangelands and forests in rich nations that receive few inputs as part of rational economic management, as well as smallholder crop production systems in developing countries, in which inputs would be rational but are not feasible because of poverty and limited access. Globally, low yields in smallholder systems are primary limits to food security and economic development. These systems are characterized by challenging edaphic environments, including water deficit, suboptimal nutrient availability of both topsoil (primarily P, but also, K, Ca, and Mg) and subsoil (water, N) resources, subsoil acidity, and pervasive soil degradation (Lynch, 2019). Root function in these systems is limited by greater biotic stress leading to root loss, and greater competition with weeds and polyculture taxa. Resource degradation, growing human populations, and climate change are exacerbating a situation which is already alarming, with 768 million reported as currently malnourished (FAO, 2021). Crops with greater stress tolerance and greater ability to convert scarce soil resources to yield are urgently needed in the global south (Lynch, 2019).
Root architectural ideotypes for low‐input agroecosystems
Because of the diverse array of constraints confronting crops in low‐input systems, root architectures that co‐optimize various functions should be useful. Dimorphic root phenotypes capable of both topsoil and subsoil exploration are needed (Burridge et al., 2020), along with traits that reduce the overall metabolic cost of maintaining many root axes, such as reduced secondary growth in dicots or root cortical aerenchyma in monocots (Lynch et al., 2021a). For example, an analysis of 577 common bean genotypes across 51 field environments showed that intermediate root architectural phenotypes had the best aggregate yield under drought and low fertility stress (Strock et al., 2019). Root phenotypic plasticity may be advantageous because, in these environments, resources may be patchy in time and space (Schneider and Lynch, 2020). Many of these environments have suffered serious soil erosion and the consequent loss of topsoil and organic matter. Global climate change is expected to accelerate soil degradation by increasing the severity of rainfall events and increasing plant water deficit, thereby reducing nutrient and C cycling, which will further exacerbate soil degradation. Root phenotypes with reduced response to local soil hardness, capable of penetrating through hard surface layers to reach deep soil domains with greater water availability, will be useful in this context (Lynch et al., 2021b) (Figure 5d). Architectural phenotypes resilient to root loss from diseases and insects will also be useful. Research conducted on this topic is scarce, although it is logical to assume that phenotypes with more root axes, and with root architectures with a smaller number of segments having many subtending segments, such that the loss of one segment leads to loss of many subtending segments (Lynch, 2005), would be beneficial. Root phenotypes for many low‐input systems would not be optimized for any one function such as water capture, but would be resilient against an array of biotic and abiotic challenges.
A successful case study: common bean breeding in Mozambique
An example of a successful root‐focused breeding program for low‐input agroecologies is that of common bean in Mozambique (Burridge et al., 2019). Common bean is an important food security crop in Mozambique, a low‐income economy with mainly smallholder farmers. Low P availability and drought are primary constraints to bean production in Mozambique, as they are in much of subSaharan Africa (Wortmann, 1998). A breeding program was established targeting root phenotypes improving P and water acquisition, focusing on phenotypes such as basal root whorl number, basal root growth angle, and long, dense root hairs (Lynch, 2019). New lines with better root phenotypes had a 2.5‐fold greater yield than the best existing lines in regional environments. The new lines also afforded several agroecological benefits, including better utilization of rock phosphate, reduced soil erosion, greater biological nitrogen fixation, and acceptable competition with maize in polyculture. Social science research identified farmer and consumer preferences, as well as constraints to adoption and dissemination of new bean lines. This example illustrates how targeting root phenotypes in a crop breeding program can have impact for smallholder farmers, thereby benefitting food security, household and village income, and soil health.
USEFUL ROOT PHENOTYPES REGARDLESS OF INPUT LEVEL
Root phenotypes likely to have utility in both high‐input and low‐input systems include:
Long, dense root hairs. Root hairs are important for the acquisition of immobile soil nutrients, especially P and K, which are critical limitations in low‐input agroecologies (Lynch, 2019). They also improve penetration of hard soil (Bengough et al., 2016; Haling et al., 2013), which should be beneficial in both high‐input and low‐input systems (Lynch et al., 2021b). Substantial genetic variation for root hair length and density has been observed in multiple crop species, which is easily screened (Lynch, 2019).
Cheap roots. Several root anatomical phenotypes reduce the metabolic cost of soil exploration, consequently improving water and nutrient capture, as well as penetration of hard soil (“Steep, cheap, and deep” section) (Colombi et al., 2019; Lynch, 2019). The benefits of these phenotypes for water capture and soil penetration should be broadly useful in many soils; their utility for nutrient capture should improve yield in the nutrient deficient soils of low‐input systems and decrease input requirements in high‐input systems.
Developmental regulation of root plasticity in dicot crops. In annual dicotyledonous crops, the root system is dominated by one or more orders of lateral roots emerging from the primary root in addition to dominant basal roots or early lateral roots in some species (Burridge et al., 2020). It has been proposed that axial roots should be relatively insensitive to local soil conditions because they regulate soil exploration of the root system at the organismic scale, whereas lateral roots should be plastic to exploit local water availability and low impedance pathways (Lynch et al., 2021b).
Developmental regulation of root plasticity in cereal crops. In cereal crops, the primary root is followed by seminal roots, then a series of nodal roots that emerge near or above the soil surface. The primary root must provide water for the growing seedling, so must grow vertically. It has been proposed that lateral roots emerging from the primary root, as well as seminal root axes and their laterals, may benefit from plastic responses to local soil conditions to exploit patchiness in P and N availability from, for example, mineralization of organic matter (Lynch et al., 2021b). As they are produced and descend downwards into the soil, nodal roots pass through the topsoil. Nodal root axes should be insensitive to local topsoil conditions including hardness because they must penetrate the topsoil to benefit the plant. Lateral roots arising from nodal roots could be plastic to local soil conditions to optimally exploit soil resources (Lynch et al., 2021b).
CROSS‐CUTTING ISSUES
The value of the microeconomic paradigm
The microeconomic paradigm, which considers plant strategies for resource capture within the context of costs and benefits, tradeoffs, and risks (Bloom et al., 1985; Lynch and Ho, 2005), has been a useful way to analyze the adaptive utility of root architectural phenotypes. Roots and their symbionts are heterotrophic organs that require a significant investment of plant resources. The ‘payoff’ of that investment in terms of capture of soil resources is affected by the distribution and availability of those resources in time and space, which contrasts among resources and is subject to tradeoffs. The availability and distribution of soil resources in many agroecosystems have a strong stochastic component, in part because of the stochastic nature of water availability (Lynch, 2018), which means that root strategies involve an element of risk. These factors all lend themselves to microeconomic analysis. The utility of the microeconomic paradigm is demonstrated by the successful deployment of topsoil foraging and steep, cheap, and deep ideotypes (Burridge et al., 2019), as well as by research revealing the importance of anatomical phenotypes that reduce the metabolic cost of soil exploration for soil resource capture (Lynch, 2014; Lynch et al., 2021a).
Integrated phenotypes
The utility of root architectural phene states for plant fitness is a function of their direct utility and their interactions with each other to form integrated phenotypes, and in turn how these integrated phenotypes interact with the environment. A clear example of phene synergism is in common bean, in which more basal root whorls and long, dense root hairs both improve P capture, but, in combination, their benefit is twice their additive effects (Miguel et al., 2015). As an example of phene interactions that can be either positive or negative, high conductance xylem phenotypes are beneficial for drought adaptation in the deep‐rooted tepary bean, but detrimental for drought adaptation in the more shallow‐rooted common bean (Strock et al., 2021). Multiple integrated phenotypes consisting of distinct phene states may improve tolerance to edaphic stress. For example, an analysis of 400 diverse maize lines in the field revealed distinct integrated root phenotypes related to drought adaptation, consisting of unique combinations of phene states that enable greater soil exploration, restrict uptake of water to conserve soil moisture, and improve penetrability of hard, dry soils (Klein et al., 2020).
The importance of interactions of root phenes with each other to create integrated phenotypes, as well as the importance of interactions of these integrated phenotypes with an array of environmental conditions, results in a vast and complex fitness landscape. It is not feasible to assess all possible integrated phenotypes empirically, nor is it feasible to analyze just one phene state against the array of background phenotypes with which it may potentially interact. In silico tools are needed, capable of evaluating many virtual root phenotypes in many virtual environments, including those that do not currently exist in nature. For example, an in silico analysis of root architectural phenotypes in common bean identified multiple integrated phenotypes that co‐optimize capture of N and P (Rangarajan et al., 2018). The integration of multiscale mechanistic models that represent root interactions with soils, together with crop and environmental models, will be important tools in deploying root phenotypes for future crops (Benes et al., 2020; Hammer et al., 2006; Postma et al., 2017; Rötter et al., 2015).
Phenotyping root architecture
An important obstacle to the deployment of root architectural phenotypes as selection criteria in crop breeding is phenotyping. Roots are complex, dynamic organs growing in and interacting with the soil, which is an opaque, diverse medium. In some cases, root architectural phenotypes observed in seedlings under controlled conditions are well correlated with field phenotypes. For example, basal root growth angle in common bean is associated with topsoil foraging and hence P capture from low P soils, and this phenotype is readily observed in seedlings growing in transparent growth pouches, comprising measurements that are well correlated with field phenotypes (Liao et al., 2004). Root hair phenotypes in maize and common bean are easily observed in seedlings, and are correlated with root hair phenotypes of mature plants in the field (Zhu et al., 2010b; Vieira et al., 2007). In common bean, seedling root phenotypes predicted yield across 51 field environments under drought and low fertility stress (Strock et al., 2019). However, seedlings do not express the phenotypes of root classes that appear later in development, such as later emerging nodal roots in cereals, and many root phenotypes may express plasticity in response to soil conditions (Rich and Watt, 2013; Correa et al., 2019. Therefore, field‐based assays of root architecture are useful. Several such assays have been developed. For example, the shovelomics method consists simply of excavating root crowns in the field with a shovel, rinsing the crown, and either phenotyping them directly or with the aid of image analysis tools developed for this purpose (Trachsel et al., 2011; Colombi et al., 2015; Burridge et al., 2016; Bucksch et al., 2014). As an example of the utility of this approach, shovelomics analysis of a maize diversity panel grown for 4 years in the field successfully identified zmCIPK15 as a regulator of nodal root growth angle and N capture in maize (Schneider et al., 2021a,b).
Ecosystem impacts
Root architectural phenotypes can substantially improve soil resource capture and C sequestration by crop plants. These qualities afford multiple benefits for agroecosystems and the global environment. In high‐input agroecosystems, crops with reduced demand for fertilizers and water would reduce production costs and the risk of yield loss from drought, while also reducing adverse environmental impacts from input use. The production and use of N fertilizer is a significant source of greenhouse gas production and water pollution (Von Blottnitz et al., 2006; Robertson and Vitousek, 2009; Vitousek, 2009). Phosphorus runoff is a significant cause of water pollution, and high‐grade P ore deposits are a non‐renewable resource that may be substantially depleted this century (Lynch, 2011; Richardson et al., 2011; Simpson et al., 2011; Vance et al., 2003). Water resources for irrigation are limited and are suffering degradation, whereas water deficit is expected to increase in rainfed agriculture because of global climate change (IPCC, 2014). The conversion of excess atmospheric CO2 to soil organic matter will mitigate climate change at the same time as improving soil quality. In all these cases, crops with greater resource capture will directly benefit farms and farmers while also affording substantial benefits for the global environment.
Low‐input agroecosystems include agroforestry, pasture, and biofuel production systems in which intensive inputs are not economically justified, as well as smallholder agriculture in developing nations, in which inputs are not available or affordable. In the first case, crops with greater soil resource capture would improve production, as well as improve the marginal benefit of input use. Biofuel production should focus on marginal environments to avoid displacement of food production. These environments generally have poor soil fertility and would benefit from greater nutrient capture by biofuel crops. Better N capture is especially relevant for biofuel production systems because N inputs are energy intensive (Jeswani et al., 2020). In smallholder agriculture, crops with greater soil resource capture would be transformative (Lynch, 2007). Yields in such systems are generally low as a result of water and nutrient limitation, so crops with better soil resource capture would have better yields, thereby directly improving food security and household income. Crops with greater nutrient acquisition could potentially ‘mine the soil’ through accelerated depletion of soil fertility. However, crop offtake (much of which is eventually returned to the soil anyway in many smallholder systems) may be offset by positive effects on nutrient cycling, maintenance of soil fertility through greater biomass production, greater P bioavailability, greater biological N fixation in legume crops, and reduced erosion, which is a dominant source of nutrient loss in low‐input systems. For example, new common bean lines with shallower roots and greater P capture had much better yield than conventional cultivars while substantially reducing P lost to runoff in smallholder systems (Henry et al., 2010). Additional food and income create opportunities for improved health, education, and input intensification, so that eventually these farmers may escape the poverty trap of low inputs/low yield. Resource‐efficient crops could reduce deforestation by reducing the need to colonize new land once the brief fertility pulse from logging and burning is exhausted. The manifold benefits of new bean lines with superior root phenotypes for bean production and soil fertility in smallholder production systems of Mozambique (“A successful case study: common bean breeding in Mozambique” section) are an example of these effects. In both high‐input and low‐input agroecologies, crops with better drought tolerance will be increasingly important in the foreseeable future because of global climate change.
PERSPECTIVES
Root architectural phenotypes are promising selection targets for the development of more resilient, productive, and climate‐smart crops. Important knowledge gaps remain, but existing information is sufficient to warrant deployment of root architectural phenotypes in crop breeding programs, as evident in the success of breeding stress resistant common bean (Burridge et al., 2019). Understanding the fitness landscape of specific root phenotypes in specific agroecologies is a non‐trivial challenge that does not receive attention, in either basic or applied research, commensurate with its importance and complexity. Root biology, and the interactions of roots with soils, including the soil microbiome, spans multiple spatiotemporal scales and disciplinary silos, and is exceptionally complex. Model organisms grown in artificial media, regardless of their merits for genetic analysis, have limited utility for understanding the biology of crop roots interacting with whole soils. Crop breeders will not select for a phenotype if its utility is unknown, regardless of whether we understand the genetic control of that phenotype or not. Several research trends are positive developments, including the revolution in our ability to analyze root and soil microbiomes, remote sensors that make it possible to monitor plant responses to soil conditions in real time in the field, genetic tools for the analysis of quantitative traits in the field, and increasingly powerful in silico tools to model the interplay of roots and soil across a range of scales and environments. The scope and urgency of the global challenge represented by the confluence of a growing human population, degradation of soil and water resources, and global climate change will make this research domain increasingly relevant and important. Understanding the interplay of roots and soil is a topic ripe with challenge and opportunity for young scientists interested in transdisciplinary research on fundamental biological problems that are critically important for human welfare.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
Acknowledgements
I dedicate this article to Kathleen Brown on the occasion of her retirement. Her insight, wisdom, kindness, and dedication to the truth have been an inspiration to me, as well as to generations of students and colleagues.
REFERENCES
- Atwell, B.J. (1993) Response of roots to mechanical impedance. Environmental and Experimental Botany, 33, 27–40. [Google Scholar]
- Barber, S. (1995) Soil Nutrient Bioavailability: A Mechanistic Approach, 2nd edition. Wiley. [Google Scholar]
- Benes, B. , Guan, K. , Lang, M. , Long, S.P. , Lynch, J.P. , Marshall‐Colón, A. et al. (2020) Multiscale computational models can guide experimentation and targeted measurements for crop improvement. The Plant Journal, 103, 21–31. [DOI] [PubMed] [Google Scholar]
- Bengough, A.G. , Loades, K. & McKenzie, B.M. (2016) Root hairs aid soil penetration by anchoring the root surface to pore walls. Journal of Experimental Botany, 67, 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengough, A.G. , McKenzie, B.M. , Hallett, P.D. & Valentine, T.A. (2011) Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany, 62, 59–68. [DOI] [PubMed] [Google Scholar]
- Berg, B. & McClaugherty, C. (2003) Plant Litter. Berlin, Heidelberg: Springer, Berlin Heidelberg. [Google Scholar]
- Bloom, A. , Chapin, F.I. & Mooney, H. (1985) Resource limitation in plants ‐ an economic analogy. Annual Review of Ecology and Systematics, 16, 363–392. [Google Scholar]
- Bonser, A.M. , Lynch, J.P. & Snapp, S. (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytologist, 132, 281–288. [DOI] [PubMed] [Google Scholar]
- Bucksch, A. , Burridge, J. , York, L.M. , Das, A. , Nord, E. , Weitz, J.S. et al. (2014) Image‐based high‐throughput field phenotyping of crop roots. Plant Physiology, 166, 470–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge, J.D. , Findeis, J.L. , Jochua, C.N. , Miguel, M.A. , Mubichi‐kut, F.M. , Quinhentos, M.L. et al. (2019) A case study on the efficacy of root phenotypic selection for edaphic stress tolerance in low‐input agriculture: common bean breeding in Mozambique. Field Crops Research, 244, 107612. [Google Scholar]
- Burridge, J. , Jochua, C.N. , Bucksch, A. & Lynch, J.P. (2016) Legume shovelomics : high — throughput phenotyping of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata subsp, unguiculata) root architecture in the field. Field Crops Research, 192, 21–32. [Google Scholar]
- Burridge, J.D. , Rangarajan, H. & Lynch, J.P. (2020) Comparative phenomics of annual grain legume root architecture. Crop Science, 60, 2574–2593. [Google Scholar]
- Chimungu, J.G. , Brown, K.M. & Lynch, J.P. (2014a) Large root cortical cell size improves drought tolerance in maize. Plant Physiology, 166(4), 2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu, J.G. , Brown, K.M. & Lynch, J.P. (2014b) Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiology, 166(4), 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu, J.G. , Loades, K.W. & Lynch, J.P. (2015) Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea mays). Journal of Experimental Botany, 66, 3151–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu, J.G. , Maliro, M.F.A. , Nalivata, P.C. , Kanyama‐Phiri, G. , Brown, K.M. & Lynch, J.P. (2015) Utility of root cortical aerenchyma under water limited conditions in tropical maize (Zea mays L.). Field Crops Research, 171, 86–98. [Google Scholar]
- Colombi, T. , Herrmann, A.M. , Vallenback, P. & Keller, T. (2019) Cortical cell diameter is key to energy costs of root growth in wheat. Plant Physiology, 180, 2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombi, T. , Kirchgessner, N. , Le Marié, C.A. , York, L.M. , Lynch, J.P. & Hund, A. (2015) Next generation shovelomics: set up a tent and REST. Plant and Soil, 388, 1–20. [Google Scholar]
- Correa, J. , Postma, J.A. , Watt, M. & Wojciechowski, T. (2019) Soil compaction and the architectural plasticity of root systems. Journal of Experimental Botany, 70, 6019–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dathe, A. , Postma, J.A. , Postma‐Blaauw, M.B. & Lynch, J.P. (2016) Impact of axial root growth angles on nitrogen acquisition in maize depends on environmental conditions. Annals of Botany, 118, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Dorlodot, S. , Forster, B. , Pagès, L. , Price, A. , Tuberosa, R. & Draye, X. (2007) Root system architecture: opportunities and constraints for genetic improvement of crops. Trends in Plant Science, 12, 474–481. [DOI] [PubMed] [Google Scholar]
- FAO (2015) The State of Food Insecurity in the World 2015. Taking Stock of Uneven Progress. Meeting the 2015 International Hunger Targets.
- FAO . (2021) http://www.FAO..org/hunger/en/
- Farrar, J. , Hawes, M. , Jones, D. & Lindow, S. (2003) How roots control the flux of carbon to the rhizosphere. Ecology, 84, 827–837. [Google Scholar]
- Foley, J.A. , Ramankutty, N. , Brauman, K.A. , Cassidy, E.S. , Gerber, J.S. , Johnston, M. et al. (2011) Solutions for a cultivated planet. Nature, 478, 337–342. [DOI] [PubMed] [Google Scholar]
- Galindo‐Castañeda, T. , Brown, K.M. & Lynch, J.P. (2018) Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant Cell and Environment, 41, 1579–1592. [DOI] [PubMed] [Google Scholar]
- Gao, W. , Hodgkinson, L. , Jin, K. , Watts, C.W. , Ashton, R.W. , Shen, J. et al. (2016) Deep roots and soil structure. Plant, Cell & Environment, 39, 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. & Lynch, J.P. (2016) Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). Journal of Experimental Botany, 67, 4545–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haling, R.E. , Brown, L.K. , Bengough, A.G. , Young, I.M. , Hallett, P.D. , White, P.J. et al. (2013) Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. Journal of Experimental Botany, 64, 3711–3721. [DOI] [PubMed] [Google Scholar]
- Hammer, G. , Cooper, M. , Tardieu, F. , Welch, S. , Walsh, B. , van Eeuwijk, F. et al. (2006) Models for navigating biological complexity in breeding improved crop plants. Trends in Plant Science, 11, 587–593. [DOI] [PubMed] [Google Scholar]
- Hanjra, M.A. & Qureshi, M.E. (2010) Global water crisis and future food security in an era of climate change. Food Policy, 35, 365–377. [Google Scholar]
- Henry, A. , Chaves, N.F. , Kleinman, P.J.A. & Lynch, J.P. (2010) Will nutrient‐efficient genotypes mine the soil? Effects of genetic differences in root architecture in common bean (Phaseolus vulgaris L.) on soil phosphorus depletion in a low‐input agro‐ecosystem in Central America. Field Crops Research, 115, 67–78. [Google Scholar]
- Ho, M.D. , McCannon, B.C. & Lynch, J.P. (2004) Theoretical modeling of tradeoffs limiting root architecture plasticity. Journal of Theoretical Biology, 226, 331–340. [DOI] [PubMed] [Google Scholar]
- Ho, M.D. , Rosas, J.C. , Brown, K.M. & Lynch, J.P. (2005) Root architectural tradeoffs for water and phosphorus acquisition. Functional Plant Biology, 32, 737–748. [DOI] [PubMed] [Google Scholar]
- IPCC . (2014) Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. In: Field, C.B. , Barros, V.R. , Dokken, D.J. , Mach, K.J. , Mastrandrea, M.D. , Bilir, T.E. et al. (Eds.) Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge, UK and New York, NY: Cambridge University Press, 1132 pp. [Google Scholar]
- Jackson, M.B. & Armstrong, W. (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology, 1, 274–287. [Google Scholar]
- Jeswani, H.K. , Chilvers, A. & Azapagic, A. (2020) Environmental sustainability of biofuels: a review. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 476, 20200351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, X. , Liu, P. & Lynch, J.P. (2018) Greater lateral root branching density in maize (Zea mays L.) improves phosphorus acquisition from low phosphorus soil. Journal of Experimental Botany, 69, 4961–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D.L. , Hodge, A. & Kuzyakov, Y. (2004) Plant and mycorrizal regulation of rhizodeposition. New Phytologist, 163, 459–480. [DOI] [PubMed] [Google Scholar]
- Kell, D.B. (2011) Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany, 108, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell, D.B. (2012) Large‐scale sequestration of atmospheric carbon via plant roots in natural and agricultural ecosystems: why and how. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, S.P. , Schneider, H.M. , Perkins, A.C. , Brown, K.M. & Lynch, J.P. (2020) Multiple integrated root phenotypes are associated with improved drought tolerance. Plant Physiology, 183, 1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian, L.V. , Piñeros, M.A. , Liu, J. & Magalhaes, J.V. (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annual Review of Plant Biology, 66, 571–598. [DOI] [PubMed] [Google Scholar]
- Kögel‐Knabner, I. (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biology and Biochemistry, 34, 139–162. [Google Scholar]
- Lambers, H. , Atkin, O.K. & Millenaar, F.F. (2002) Respiratory patterns in roots in relation to their functioning. In: Waisel, Y. , Eshel, A. & Kafkaki, K. (Eds.) Plant Roots: The Hidden Half. New York, New York: Marcel Dekker Inc, pp. 521–552. [Google Scholar]
- Liao, H. , Rubio, G. , Yan, X.L. , Cao, A.Q. , Brown, K.M. & Lynch, J.P. (2001) Effect of phosphorus availability on basal root shallowness in common bean. Plant & Soil, 232, 69–79. [PubMed] [Google Scholar]
- Liao, H. , Yan, X. , Rubio, G. , Pedraza, F. , Beebe, S. & Lynch, J.P. (2004) Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Functional Plant Biology, 31, 1–12. [DOI] [PubMed] [Google Scholar]
- Lynch, J.P. (2005) Root architecture and nutrient acquisition. In Nutrient acquisition by plants. Berlin, Heidelberg: Springer, pp. 147–183. [Google Scholar]
- Lynch, J.P. (2007) Roots of the second green revolution. Australian Journal of Botany, 55, 493–512. [Google Scholar]
- Lynch, J.P. (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology, 156, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, J.P. (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany, 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, J.P. (2014) Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant, Cell & Environment, 38, 1775–1784. [DOI] [PubMed] [Google Scholar]
- Lynch, J.P. (2018) Rightsizing root phenotypes for drought resistance. Journal of Experimental Botany, 69, 3279–3292. [DOI] [PubMed] [Google Scholar]
- Lynch, J.P. (2019) Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytologist, 223(2), 548–564. [DOI] [PubMed] [Google Scholar]
- Lynch, J.P. & Brown, K.M. (2001) Topsoil foraging ‐ an architectural adaptation of plants to low phosphorus availability. Plant & Soil, 237, 225–237. [Google Scholar]
- Lynch, J.P. , Ho, M.D. & phosphorus, L. (2005) Rhizoeconomics: Carbon costs of phosphorus acquisition. Plant & Soil, 269, 45–56. [Google Scholar]
- Lynch, J.P. , Mooney, S.J. , Strock, C.F. & Schneider, H.M. (2021b) Future roots for future soils. Plant, Cell & Environment, in press. 10.1111/pce.14213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, J.P. , Nielsen, K.L. , Davis, R.D. & Jablokow, A.G. (1997) SimRoot: Modelling and visualization of root systems. Plant & Soil, 188, 139–151. [Google Scholar]
- Lynch, J.P. & St.Clair, S (2004) Mineral stress: the missing link in understanding how global climate change will affect plants in real world soils. Field Crops Research, 90, 101–115. [Google Scholar]
- Lynch, J.P. , Strock, C.F. , Schneider, H.M. , Sidhu, J.S. , Ajmera, I. , Galindo‐Castañeda, T. et al. (2021a) Root anatomy and soil resource capture. Plant & Soil, 466, 21–63. 10.1007/s11104-021-05010-y [DOI] [Google Scholar]
- Lynch, J.P. & Wojciechowski, T. (2015) Opportunities and challenges in the subsoil: pathways to deeper rooted crops. Journal of Experimental Botany, 66, 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai, C.D. , Phung, N.T. , To, H.T. , Gonin, M. , Hoang, G.T. , Nguyen, K.L. et al. (2014) Genes controlling root development in rice. Rice, 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy, J.E. (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment, 28, 67–77. [DOI] [PubMed] [Google Scholar]
- Manschadi, A.M. , Christopher, J.T. , Hammer, G.L. & Devoil, P. (2010) Experimental and modelling studies of drought‐adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosystems, 144, 458–462. [Google Scholar]
- Mbow, C. , Rosenzweig, C. , Barioni, L.G. , Benton, T.G. , Herrero, M. , Krishnapillai, M. & Xu, Y. (2019) (Eds): Shukla, P.R. , Skea, J. , Calvo Buendia, E. , Masson‐Delmotte, V. , Pörtner, H.‐O. , Roberts, D.C. , Zhai, P. , Slade, R. , Connors, S. , van Diemen, R. , Ferrat, M. , Haughey, E. , Luz, S. , Neogi, S. , Pathak, M. , Petzold, J. , Portugal Pereira, J. , Vyas, P. , Huntley, E. , Kissick, K. , Belkacemi, M. & Malley, J. Food Security. Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. Geneva: IPCC. [Google Scholar]
- Miguel, M.A. , Postma, J.A. & Lynch, J.P. (2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiology, 167, 1430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel, M.A. , Widrig, A. , Vieira, R.F. , Brown, K.M. & Lynch, J.P. (2013) Basal root whorl number: a modulator of phosphorus acquisition in common bean (Phaseolus vulgaris). Annals of Botany, 112, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, C.R. , Ochoa, I. , Nielsen, K.L. , Beck, D. & Lynch, J.P. (2003) Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Functional Plant Biology, 30, 973–985. [DOI] [PubMed] [Google Scholar]
- Narasimha, M. , Prasad, V. & Pietrzykowski, M. (Eds) (2020) Climate Change and Soil Interactions. Elsevier.
- Nkonya, E. , Mirabaev, A. & von Braun, J. (2016) Economics of Land Degradation and Improvement – A Global Assessment for Sustainable Development. New York, New York, USA: Springer International. [Google Scholar]
- Or, D. , Keller, T. & Schlesinger, W.H. (2021) Natural and managed soil structure: On the fragile scaffolding for soil functioning. Soil and Tillage Research, 208, 104912. [Google Scholar]
- Pachauri, R.K. , Mayer, L. , and Intergovernmental Panel on Climate Change (Eds) (2015) Climate change 2014: synthesis report. Geneva, Switzerland: Intergovernmental Panel on Climate Change. [Google Scholar]
- Page, K.L. , Dang, Y.P. & Dalal, R.C. (2020) The ability of Conservation Agriculture to conserve soil organic carbon and the subsequent impact on soil physical, chemical, and biological properties and yield. Frontiers in Sustainable Food Systems. 10.3389/fsufs.2020.00031 [DOI] [Google Scholar]
- Pandey, B.K. , Huang, G. , Bhosale, R. , Hartman, S. , Sturrock, C.J. , Jose, L. et al. (2021) Plant roots sense soil compaction through restricted ethylene diffusion. Science, 371, 276–280. [DOI] [PubMed] [Google Scholar]
- Postma, J.A. , Dathe, A. & Lynch, J.P. (2014) The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiology, 166, 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma, J.A. , Kuppe, C. , Owen, M.R. , Mellor, N. , Griffiths, M. , Bennett, M.J. et al. (2017) OpenSimRoot: widening the scope and application of root architectural models. New Phytologist, 215, 1274–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma, J.A. & Lynch, J.P. (2011a) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Annals of Botany, 107, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma, J.A. & Lynch, J.P. (2011b) Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiology, 156, 1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan, H. , Postma, J.A. & Lynch, J.P. (2018) Co‐optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Annals of Botany, 122, 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, I.M. , Miles, J.W. , Beebe, S.E. & Horst, W.J. (2016) Root adaptations to soils with low fertility and aluminium toxicity. Annals of Botany, 118, 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasse, D.P. , Rumpel, C. & Dignac, M.‐F. (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant & Soil, 269, 341–356. [Google Scholar]
- Rich, S.M. & Watt, M. (2013) Soil conditions and cereal root system architecture: review and considerations for linking Darwin and Weaver. Journal of Experimental Botany, 64, 1193–1208. [DOI] [PubMed] [Google Scholar]
- Richardson, A.E. , Lynch, J.P. , Ryan, P.R. , Delhaize, E. , Smith, F.A. , Smith, S.E. et al. (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant & Soil, 349, 121–156. [Google Scholar]
- Robertson, G.P. & Vitousek, P.M. (2009) Nitrogen in agriculture: balancing the cost of an essential resource. Annual Review of Environment and Resources, 34, 97–125. [Google Scholar]
- Rötter, R.P. , Tao, F. , Höhn, J.G. & Palosuo, T. (2015) Use of crop simulation modelling to aid ideotype design of future cereal cultivars. Journal of Experimental Botany, 66, 3463–3476. [DOI] [PubMed] [Google Scholar]
- Rubio, G. , Liao, H. , Yan, X. & Lynch, J.P. (2003) Topsoil foraging and its role in plant competitiveness for phosphorus in common bean. Crop Science, 43, 598–607. [Google Scholar]
- Ryan, P.R. , Tyerman, S.D. , Sasaki, T. , Furuichi, T. , Yamamoto, Y. , Zhang, W.H. et al. (2011) The identification of aluminium‐resistance genes provides opportunities for enhancing crop production on acid soils. Journal of Experimental Botany, 62, 9–20. [DOI] [PubMed] [Google Scholar]
- Saengwilai, P. , Nord, E.A. , Chimungu, J. , Brown, K.M. & Lynch, J.P. (2014b) Root cortical aerenchyma enhances nitrogen acquisition from low nitrogen soils in maize. Plant Physiology, 166, 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai, P. , Tian, X. & Lynch, J. (2014a) Low crown root number enhances nitrogen acquisition from low nitrogen soils in maize (Zea mays L.). Plant Physiology, 166, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, J.E. & Gaudin, A.C.M. (2017) Toward an integrated root ideotype for irrigated systems. Trends in Plant Science, 22, 433–443. [DOI] [PubMed] [Google Scholar]
- Schneider, H.M. , Lor, V.S.N. , Hanlon, M.T. et al. (2021a) Root angle in maize influences nitrogen capture and is regulated by calcineurin B‐like protein (cbl) ‐interacting serine/threonine‐protein kinase 15 ( zmcipk15 ). Plant, Cell & Environment. 10.1111/pce.14135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, H.M. & Lynch, J.P. (2020) Should root plasticity be a crop breeding target? Frontiers in Plant Science, 11, 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, H.M. , Postma, J.A. , Wojciechowski, T. , Kuppe, C. & Lynch, J.P. (2017b) Root cortical senescence improves growth under suboptimal availability of N, P, and K. Plant Physiology, 174, 2333–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, H.M. , Strock, C.F. , Hanlon, M.T. et al. (2021b) Multiseriate cortical sclerenchyma enhance root penetration in compacted soils. Proceedings of the National Academy of Sciences of the United States of America, 118, e2012087118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, H.M. , Wojciechowski, T. , Postma, J.A. , Brown, K.M. , Lücke, A. , Zeisler, V. et al. (2017a) Root cortical senescence decreases root respiration, nutrient content, and radial water and nutrient transport in barley. Plant, Cell & Environment, 40, 1392–1408. [DOI] [PubMed] [Google Scholar]
- Simpson, R.J. , Oberson, A. , Culvenor, R.A. , Ryan, M.H. , Veneklaas, E.J. , Lambers, H. et al. (2011) Strategies and agronomic interventions to improve the phosphorus‐use efficiency of farming systems. Plant & Soil, 349, 89–120. [Google Scholar]
- St.Clair, S.B. & Lynch, J.P. (2010) The opening of Pandora’s Box: climate change impacts on soil fertility and crop nutrition in developing countries. Plant & Soil, 335, 101–115. [Google Scholar]
- Strock, C.F. , Burridge, J. , Massas, A.S.F. , Beaver, J. , Beebe, S. & Camilo, S.A. et al. (2019) Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. Field Crops Research, 237, 53–64. [Google Scholar]
- Strock, C.F. , Burridge, J.D. , Niemiec, M.D. , Brown, K.M. & Lynch, J.P. (2021) Root metaxylem and architecture phenotypes integrate to regulate water use under drought stress. Plant, Cell & Environment, 44, 49–67. [DOI] [PubMed] [Google Scholar]
- Strock, C.F. & Lynch, J.P. (2017) Root secondary growth: an unexplored component of soil resource acquisition. Annals of Botany, 126, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strock, C.F. , Morrow de la Riva, L. & Lynch, J.P. (2017) Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiology, 176, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, B. , Gao, Y. & Lynch, J. (2018) (2018) Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiology, 177, 90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebaldi, C. & Lobell, D.B. (2008) Towards probabilistic projections of climate change impacts on global crop yields. Geophysical Research Letters, 35, 2–7. [Google Scholar]
- Thorup‐Kristensen, K. , Halberg, N. , Nicolaisen, M. , Olesen, J.E. , Crews, T.E. , Hinsinger, P. et al. (2020) Digging deeper for agricultural resources, the value of deep rooting. Trends in Plant Science, 25, 406–417. [DOI] [PubMed] [Google Scholar]
- Trachsel, S. , Kaeppler, S. , Brown, K.M. & Lynch, J.P. (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant & Soil, 341, 75–87. [Google Scholar]
- Trachsel, S. , Kaeppler, S.M. , Brown, K.M. & Lynch, J.P. (2013) Maize root growth angles become steeper under low N conditions. Field Crops Research, 140, 18–31. [Google Scholar]
- Uga, Y. (2021) Challenges to design‐oriented breeding of root system architecture adapted to climate change. Breeding Science, 71, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga, Y. , Sugimoto, K. , Ogawa, S. , Rane, J. , Ishitani, M. , Hara, N. et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics, 45, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Vance, C.P. , Uhde‐Stone, C. & Allan, D.L. (2003) Phosphorus aquisition and use: critical adaptations by plants for securing a nonrewable resource. New Phytologist, 157, 423–447. [DOI] [PubMed] [Google Scholar]
- Vanhees, D.J. , Loades, K.W. , Bengough, A.G. , Mooney, S.J. & Lynch, J.P. (2020) Root anatomical traits contribute to deeper rooting of maize under compacted field conditions. Journal of Experimental Botany, 71, 4243–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhees, D.J. , Schneider, H.M. , Loades, K.W. , Bengough, A.G. , Bennett, M.J. , Pandey, B.K. et al. (2021) Genotypic variation in soil penetration by maize roots is negatively related to ethylene‐induced thickening. Plant, Cell and Environment. 10.1111/pce.14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney, R.K. , Barmukh, R. , Roorkiwal, M. , Qi, Y. , Kholova, J. , Tuberosa, R. , Reynolds, M.P. , Tardieu, F. & Siddique, K.H.M. (2021) Breeding custom‐designed crops for improved drought adaptation. Advanced Genetics, 2(3), 10.1002/ggn2.202100017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, R.F. , Jochua, C.N. & Lynch, J.P. (2007) Method for evaluation of root hairs of common bean genotypes. Pesq. agropec. bras . Brasília, 42, 1365–1368. [Google Scholar]
- Vitousek, P.M. , Naylor, R. , Crews, T. , David, M.B. , Drinkwater, L.E. , Holland, E. et al. (2009) Nutrient imbalances in agricultural development. Science, 324, 1519–1520. [DOI] [PubMed] [Google Scholar]
- Von Blottnitz, H. , Rabl, A. , Boiadjiev, D. , Taylor, T. & Arnold, S. (2006) Damage costs of nitrogen fertilizer in Europe and their internalization. Journal of Environmental Planning and Management, 49, 413–433. [Google Scholar]
- Wachsman, G. , Sparks, E.E. & Benfey, P.N. (2015) Genes and networks regulating root anatomy and architecture. New Phytologist, 208(1), 26–38. [DOI] [PubMed] [Google Scholar]
- Walk, T.C. , Jaramillo, R. & Lynch, J.P. (2006) Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant & Soil, 279, 347–366. [Google Scholar]
- Wasson, A.P. , Richards, R.A. , Chatrath, R. , Misra, S.C. , Prasad, S.V.S. , Rebetzke, G.J. et al. (2012) Traits and selection strategies to improve root systems and water uptake in water‐limited wheat crops. Journal of Experimental Botany, 63, 3485–3498. [DOI] [PubMed] [Google Scholar]
- Whalley, W.R. , Dodd, I.C. , Watts, C.W. , Webster, C.P. , Phillips, A.L. , Andralojc, J. et al. (2013) Genotypic variation in the ability of wheat roots to penetrate wax layers. Plant and Soil, 364, 171–179. [Google Scholar]
- Woods, J. , Williams, A. , Hughes, J.K. , Black, M. & Murphy, R. (2010) Energy and the food system. Philosophical Transactions of the Royal Society B, 365, 2991–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank (2017) World Development Indicators 2017. Washington, DC. [Google Scholar]
- Wortmann, C.S. (1998) Atlas of common bean (Phaseolus vulgaris L.). CIAT: Production in Africa. [Google Scholar]
- Yang, X. , Niemiec, M. & Lynch, J.P. (2020) Enlarged cortical cells and reduced cortical cell file number improve growth under suboptimal nitrogen, phosphorus and potassium availability. bioRxiv, 2020.07.06.189514. [Google Scholar]
- York, L.M. , Galindo‐Castañeda, T. , Schussler, J.R. & Lynch, J.P. (2015) Evolution of US maize (Zea mays L.) root architectural and anatomical phenes over the past 100 years corresponds to increased tolerance of nitrogen stress. Journal of Experimental Botany, 66, 2347–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York, L.M. & Lynch, J.P. (2015) Intensive field phenotyping of maize (Zea mays L.) root crowns identifies phenes and phene integration associated with plant growth and nitrogen acquisition. Journal of Experimental Botany, 66, 5493–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York, L.M. , Nord, E.A. & Lynch, J.P. (2013) Integration of root phenes for soil resource acquisition Integration of root phenes for soil resource acquisition. Frontiers Plant Science, 4, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, A. & Lynch, J.P. (2015) Reduced frequency of lateral root branching improves N capture from low‐N soils in maize. Journal of Experimental Botany, 66, 2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, A. , Schneider, H. & Lynch, J.P. (2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiology, 168, 1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J. , Brown, K.M. & Lynch, J.P. (2010a) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell and Environment, 33, 740–749. [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Kaeppler, S.M. & Lynch, J.P. (2005) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays L.). Functional Plant Biology, 32, 749–762. [DOI] [PubMed] [Google Scholar]
- Zhu, J.M. & Lynch, J.P. (2004) The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays) seedlings. Functional Plant Biology, 31, 949–958. [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Zhang, C. & Lynch, J.P. (2010b) The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Functional Plant Biology, 37, 313–322. [Google Scholar]


