Abstract
Background and aims
India has a significant burden of hepatitis C virus (HCV) infection and has committed to achieving national elimination by 2030. This will require a substantial scale‐up in testing and treatment. The “HEAD‐Start Project Delhi” aimed to enhance HCV diagnosis and treatment pathways among the general population.
Methods
A prospective study was conducted at 5 district hospitals (Arm 1: one‐stop shop), 15 polyclinics (Arm 2: referral for viral load (VL) testing and treatment) and 62 screening camps (Arm 3: referral for treatment). HCV prevalence, retention in the HCV care cascade, and turn‐around time were measured.
Results
Between January and September 2019, 37 425 participants were screened for HCV. The median (IQR) age of participants was 35 (26‐48) years, with 50.4% male and 49.6% female. A significantly higher proportion of participants in Arm 1 (93.7%) and Arm 3 (90.3%) received a VL test compared with Arm 2 (52.5%, P < .001). Of those confirmed positive, treatment was initiated at significantly higher rates for participants in both Arms 1 (85.6%) and 2 (73.7%) compared to Arm 3 (41.8%, P < .001). Arm 1 was found to be a cost‐saving strategy compared to Arm 2, Arm 3, and no action.
Conclusions
Delivery of all services at a single site (district hospitals) resulted in a higher yield of HCV seropositive cases and retention compared with sites where participants were referred elsewhere for VL testing and/or treatment. The highest level of retention in the care cascade was also associated with the shortest turn‐around times.
Keywords: decentralization, hepatitis C, India, primary health care, simplification
Key points.
Delivery of all hepatitis C services at a single site resulted in a higher yield and better retention compared with sites where participants were referred elsewhere for viral load testing and/or treatment. This study demonstrates that simplified, decentralized HCV care pathway can be effective in low resource settings.
1. INTRODUCTION
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease globally, with an estimated 58 million individuals chronically infected and approximately 700 000 HCV‐related deaths each year. 1 , 2 There is a disproportionately high burden of HCV in low‐ and middle‐income countries (LMICs). In recognition of this major global public health burden, in 2016 the World Health Organization (WHO) launched a Global Health Sector Strategy on Hepatitis 2016‐2021, with the goal of eliminating viral hepatitis as a public health threat by 2030. However, as of 2017, only 20% of individuals with HCV infection had been tested and approximately 25% of diagnosed individuals had been treated. 3
A recent systematic review reported the estimated prevalence of HCV infection in India to be between 1% and 1.9%, 4 with significant variations in prevalence by geographical region. In total, 12‐18 million people are estimated to be infected with HCV, 5 accounting for a significant proportion of the global HCV burden. The prevalence of HCV infection is higher in certain population subgroups, including patients undergoing hemodialysis, patients undergoing repeated blood transfusions (e.g. for thalassemia major), people who inject drugs (PWID), people living with HIV (PLHIV), and healthcare workers (HCWs). Delhi, the capital of India, has a population of about 18 million people and an estimated HCV prevalence of 1%, i.e. 1.8 million HCV‐infected residents. HCV prevalence among blood donors, pregnant women and PLHIV was recently reported to be 0.59%, 0.71% and 3.51%, respectively. 6
India has committed to achieving national elimination of HCV infection by 2030, underscored by the recent launch of a National Action Plan in 2018. Under this program, free screening, diagnosis and treatment for HCV would be made available at all healthcare levels. Direct‐acting antivirals (DAAs) were made freely available in 2017; however, diagnostics remain a key bottleneck for the uptake of services. Three states in India started Hepatitis C Elimination Through Access to Diagnostics (HEAD‐Start) projects with the aim of generating evidence to fill the diagnostics gap and provide an operational service delivery model for integrated diagnostics and treatment. Such evidence will be critical for informing policy at both state and central government levels.
The objective of the HEAD‐Start Project in Delhi was to evaluate the feasibility and effectiveness of different HCV testing and treatment approaches among the general population presenting for routine care at district hospitals and polyclinics, as well as several screening camps. Polyclinic is a primary health centre that provides a basic essential health services package including free‐of‐cost consultation, diagnostics and medicines. A screening camp offers a free‐of‐cost screening facility. The project implemented rapid HCV screening and treatment at 5 district hospitals (Arm 1), 15 Polyclinics (Arm 2), and 62 screening camps (Arm 3). In Arm 1, all HCV care for HCV‐seropositive adults was provided at a district hospital, including viral load (VL) testing, pre‐treatment assessments, treatment, and sustained virologic response (SVR) testing. In Arm 2, screening took place at polyclinics; seropositive adults were referred for venous blood collection for VL testing and treatment at one of five linked district hospitals. In Arm 3, participants were screened at camps and seropositive adults had a venous blood sample collected for VL testing; viremic participants were referred to one of the district hospitals for treatment. Effectiveness was evaluated and compared among the arms by assessing the retention of participants across the HCV care cascade, the turn‐around time for each step in the care cascade, and cost‐effectiveness. The outcomes of this study have informed implementation and scale‐up plans for the Government of Delhi HCV program.
2. METHODOLOGY
2.1. Study design and setting
This study was an observational, prospective cohort study design conducted between December 2018 and December 2019 to evaluate the effectiveness of a partially decentralized HCV diagnosis and care model in Delhi. HCV screening was carried out at District hospitals (5) and primary healthcare (PHC) facilities/polyclinics (15), selected based on site epidemiology, staff capacity and training for HCV testing, geography, and proximity to the five selected hospitals' catchment area (<100 km). Several screening camps were also held (62) (Figure 1 ). Patients presenting at district hospitals were attending outpatient departments for blood investigations, consultation for acute and chronic conditions with a specialist. Outpatients attending polyclinics were receiving onsite ancillary services, such as laboratory services for blood investigations, pharmacy services and medical care for acute and chronic conditions as well as routine care such as health screening. The screening camps were attended voluntarily by the general population. At hospitals (Arm 1), participants were offered HCV screening, blood collection for confirmatory testing and treatment at one site. At polyclinics (Arm 2), participants were offered screening; seropositive adults were then referred to linked hospitals for confirmatory blood collection and treatment. At screening camps (Arm 3), participants were offered screening and blood collection for confirmatory testing; HCV RNA‐positive patients were then referred to linked hospitals for treatment.
FIGURE 1.
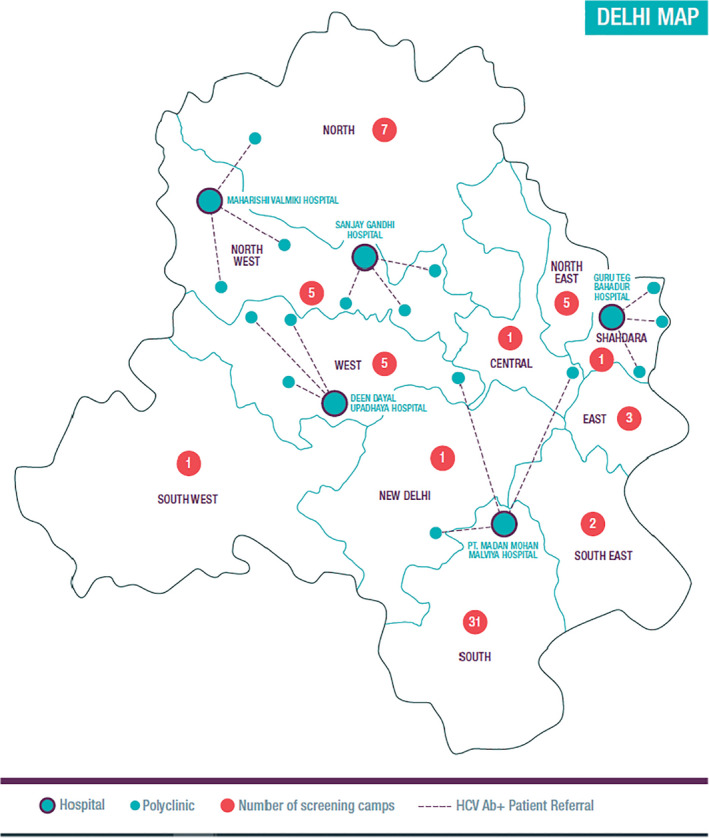
Geographical distribution of sites
2.2. Study participants
A total of 37 425 adults were enrolled in the study. At the district hospitals and polyclinics, participants were consecutively enrolled based on routine clinical indications for an HCV test, as per national guidelines. The guidelines state that serological testing will be made available for all patients presenting at a testing site, but with a focus on high‐risk groups. 7 At screening camps, all individuals presenting for an HCV screening test were offered enrolment into the study. Pregnant women, children, and adults already diagnosed with HCV or already initiated on treatment for the management of HCV infection were excluded from the study.
2.3. Study procedures
2.3.1. Screening and confirmation
All eligible participants were provided with pre‐test counselling and offered anti‐HCV screening using First Response and Meril HCV finger‐stick capillary blood rapid diagnostic test (RDT) kits (Premier Medical Corporation Ltd & Meril Life Sciences Ltd). If the RDT was positive, participants were provided with post‐test counselling and referred to one of the five selected hospitals for confirmatory testing. At the hospital, a 10‐mL venous blood sample was drawn into EDTA‐containing tubes, plasma was prepared within 72 hours, and it was sent to a reference laboratory in Delhi (Institute of Liver and Biliary Science, ILBS) for HCV RNA testing using an Abbott RealTime HCV quantitative VL assay (Abbott).
2.3.2. Pre‐treatment clinical evaluations
For participants with a positive HCV antibody test, a reflex sample was tested for pre‐treatment evaluations at the respective hospitals, including complete blood count (CBC), biochemistry liver function test (LFT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin direct/indirect, alkaline phosphatase, and serum creatinine, from which an APRI (AST to platelet ratio index) score was generated. Samples were tested for HCV RNA at the ILBS laboratory and the results were returned to the respective sites for a patient's subsequent visit. Participants also received ultrasound to assess cirrhosis. APRI scores were used to determine the presence of cirrhosis (>2). Cirrhotic participants were further assessed for compensation using ultrasound, FibroScan and clinical evaluations; decompensated participants were stabilized prior to commencement of treatment. Participants with decompensated cirrhosis, HIV or other complications were referred to ILBS for evaluation prior to starting treatment, which was either supervised by ILBS physicians or referred back to the HCV Unit to complete treatment.
2.3.3. Treatment regimen and evaluation of cure
Eligible participants without cirrhosis received sofosbuvir (400 mg/day) and daclatasvir (60 mg/day) for 12 weeks; those with cirrhosis received sofosbuvir (400 mg/day) and daclatasvir (60 mg/day) for 24 weeks. At 12 weeks after completion of treatment, participants were requested to return to the district hospital to have a 5‐mL venous blood sample collected for SVR‐12 VL testing at the ILBS laboratory using the Abbott RealTime HCV quantitative assay for assessment of cure. Participants with detectable SVR‐12 (>10 IU/mL) were retreated as per the National Viral Hepatitis Control Program (NVHCP) algorithm (Figure 2).
FIGURE 2.
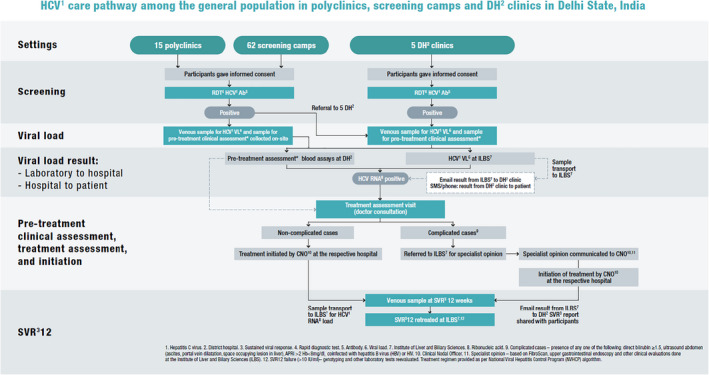
Clinical procedures and patient flow
2.4. Cost assessment
Estimates of the costs associated with testing were obtained from the study sites. A direct materials‐based approach was used to estimate the average cost of materials for an antibody and an RNA test per person tested. Unit costs included the costs of diagnostic tests after delivery and other consumables used (Table S1). The estimates of costs associated with treatment and auxiliary tests, e.g. LFTs, were provided by the Delhi Department of Health Services.
To assess the relative cost‐effectiveness of testing and care pathways of the three arms, we utilized Hep C Testing Calculator (www.hepccalculator.org), which utilizes a state‐transition model, MATCH (Markov‐based Analyses of Treatments for Chronic Hepatitis C) to simulate HCV disease progression. Natural history outcomes from this model have been validated previously. 8 , 9 We adapted the Hep C Testing Calculator to simulate the three arms of this study to evaluate their cost‐effectiveness. The model was developed following the principles on economic analyses with respect to viral hepatitis recommended by WHO 10
2.5. Data collection and analysis
Data were collected from primary source documents (screening registers, patient medical records, laboratory registers and laboratory reports) using paper forms and manually transcribed into an electronic database. Data were analysed using R 3.6.1 to obtain descriptive statistics and perform multiple regression analyses.
2.6. Ethical considerations
Permission to conduct this study was obtained from the ethics committee at ILBS (approval number: IEC/2018/63/MA04).
3. RESULTS
3.1. Study population characteristics
A total of 37 425 adults (58%, 26% and 16% in Arms 1, 2 and 3, respectively) were consecutively enrolled between 23 January and September 30, 2019. Participants’ median (IQR) age was 35 (26‐48) years, with similar proportions of males (50.4%) and females (49.6%). Most participants (58%) identified as having no/other HCV risk factors, 6.0% had undergone previous surgery, 2.2% had a history of blood transfusions, 0.4% were PLHIV, 1.4% had previous unsafe medical injections, 0.3% were PWID, 0.2% had a history of unsafe sex and 0.06% had thalassemia/history of dialysis. The overall RDT positivity rate was 2.0% (3.0%, 0.4% and 1.0% in Arms 1, 2 and 3, respectively, P < .001 all arms). RDT‐positive participants were younger than RDT‐negative participants with a median age (IQR) 33 (25‐45) vs 36 (26‐48) years, respectively, P < .05). No significant differences for RDT positivity were found between men and women. Risk factors associated with RDT positivity included a history of surgery (odds ratio (OR): 4.5 95% CI: 3.67‐5.59, thalassemia/dialysis (OR: 52.2 95% CI: 22.74‐119.78), HIV positivity (OR: 19.5 95% CI: 13.43‐28.17) and PWID (OR: 18.4 95% CI: 11.62‐29.15). Arm 1 had a significantly higher proportion of PLHIV compared with Arms 2 and 3 (P < .001) and a significantly higher proportion of participants with a history of unsafe medical injection compared with the proportion in Arms 2 and 3 (P < .001). All other risk factors and demographics were similar across the three arms (Table 1).
TABLE 1.
Participant characteristics
| Factor | Demographic variables | Simple logistic regression | Multiple logistic regression | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # Total | % Total | # Positive | % Positive | # Negative | % Negative | OR | CI (95%) | P‐value | P‐value adjusted | OR | CI (95%) | P‐value | P‐value adjusted | |
| General | 37 425 | 100 | 771 | 2.06 | 36 654 | 97.94 | ||||||||
| Median age | 35 | 33 | 36 | |||||||||||
| Age IQR (25%‐75%) | 26‐48 | 25‐45 | 26‐48 | |||||||||||
| Age range (Min‐max) | 18‐99 | 18‐80 | 18‐99 | |||||||||||
| Age | ||||||||||||||
| ≤30 | 14 073 | 37.6 | 358 | 2.54 | 13 715 | 97.46 | Reference | Reference | ||||||
| 31‐40 | 9219 | 24.63 | 187 | 2.03 | 9032 | 97.97 | 0.79 | 0.66‐0.95 | 0.01 | 0.01 | 0.75 | 0.62‐0.91 | 3.07E‐03 | 3.83E‐03 |
| 41‐50 | 6470 | 17.29 | 103 | 1.59 | 6367 | 98.41 | 0.62 | 0.5‐0.77 | 2.22E‐05 | 1.02E‐04 | 0.59 | 0.47‐0.75 | 1.07E‐05 | 2.67E‐05 |
| 51‐60 | 4464 | 11.93 | 81 | 1.81 | 4383 | 98.19 | 0.71 | 0.55‐0.9 | 5.45E‐03 | 9.08E‐03 | 0.66 | 0.51‐0.85 | 1.50E‐03 | 2.50E‐03 |
| >60 | 3199 | 8.55 | 42 | 1.31 | 3157 | 98.69 | 0.51 | 0.37‐0.7 | 4.09E‐05 | 1.02E‐04 | 0.44 | 0.32‐0.62 | 3.17E‐06 | 1.59E‐05 |
| Sex | ||||||||||||||
| Female | 18 544 | 49.55 | 327 | 1.76 | 18 217 | 98.24 | 0.75 | 0.65‐0.86 | 8.24E‐05 | 2.47E‐04 | 0.85 | 0.73‐1 | 0.04 | 0.13 |
| Male | 18 865 | 50.41 | 442 | 2.34 | 18 423 | 97.66 | Reference | Reference | ||||||
| Transgender | 16 | 0.04 | 2 | 12.5 | 14 | 87.5 | 5.95 | 1.35‐26.28 | 0.02 | 0.03 | 0.62 | 0.04‐9.84 | 0.74 | 1 |
| Number of risk factors | ||||||||||||||
| 0 | 12 799 | 34.2 | 98 | 0.77 | 12 701 | 99.23 | Reference | Reference | ||||||
| 1 | 23 710 | 63.35 | 502 | 2.12 | 23 208 | 97.88 | 2.8 | 2.26‐3.48 | 1.57E‐20 | 2.09E‐20 | 2.76 | 2.22‐3.43 | 6.52E‐20 | 8.69E‐20 |
| 2 | 839 | 2.24 | 144 | 17.16 | 695 | 82.84 | 26.85 | 20.54‐35.1 | 3.66E‐128 | 1.46E‐127 | 31.87 | 24.17‐42.03 | 5.65E‐133 | 2.26E‐132 |
| ≥3 | 77 | 0.21 | 27 | 35.06 | 50 | 64.94 | 69.99 | 42.09‐116.38 | 2.95E‐60 | 5.90E‐60 | 86.34 | 50.15‐148.64 | 3.29E‐58 | 6.58E‐58 |
| Risk Factor | ||||||||||||||
| Blood transfusion | 829 | 2.22 | 76 | 9.17 | 753 | 90.83 | 5.2 | 4.07‐6.68 | 4.64E‐39 | 9.28E‐39 | 4.75 | 3.56‐6.35 | 3.95E‐26 | 5.26E‐26 |
| Unsafe injection use | 535 | 1.43 | 98 | 18.32 | 437 | 81.68 | 12.1 | 9.57‐15.22 | 2.54E‐98 | 2.03E‐97 | 11.23 | 8.52‐14.81 | 5.70E‐66 | 4.56E‐65 |
| PWID | 117 | 0.31 | 29 | 24.79 | 88 | 75.21 | 16.2 | 10.61‐24.86 | 1.15E‐37 | 1.53E‐37 | 18.4 | 11.62‐29.15 | 2.24E‐35 | 3.58E‐35 |
| Unprotected sexual practice | 80 | 0.21 | 38 | 47.5 | 42 | 52.5 | 45.2 | 28.96‐70.51 | 2.88E‐63 | 1.15E‐62 | 36.33 | 22.18‐59.51 | 3.43E‐46 | 9.14E‐46 |
| PLHIV | 165 | 0.44 | 43 | 26.06 | 122 | 73.94 | 17.7 | 12.4‐25.23 | 1.41E‐56 | 3.77E‐56 | 19.45 | 13.43‐28.17 | 1.50E‐55 | 6.00E‐55 |
| Surgery | 2257 | 6.03 | 137 | 6.07 | 2120 | 93.93 | 3.5 | 2.91‐4.26 | 1.29E‐38 | 2.06E‐38 | 4.53 | 3.67‐5.59 | 3.61E‐45 | 7.22E‐45 |
| Dialysis/thalassemic | 23 | 0.06 | 12 | 52.17 | 11 | 47.83 | 52.7 | 23.17‐119.74 | 3.08E‐21 | 3.52E‐21 | 52.19 | 22.74‐119.78 | 1.06E‐20 | 1.21E‐20 |
| Other/No risk | 21 617 | 57.76 | 442 | 2.04 | 21 175 | 97.96 | 1 | 0.85‐1.13 | 0.81 | 0.81 | 0.95 | 0.82‐1.1 | 0.49 | 0.49 |
Bold indicates statistically significant values P < .05.
Abbreviations: PLHIV, people living with HIV; PWID, people who inject drugs.
3.2. HCV care cascade retention
Overall, 37 425 participants were screened for HCV using RDTs; 2.1% were RDT positive and of these 91.3% received a VL test (85.9% viremia). In total, 81.5% of participants were started on treatment; 90.5% completed treatment, 77.8% had an SVR‐12 test and 96% achieved cure (Table 2). Arm 1 had significantly higher levels of HCV seropositivity across the cascade of care compared with levels in Arms 2 and 3. RDT positivity rates in Arm 1 were 3.1%, compared with 0.4% and 1.1% in Arms 2 and 3, respectively (Figure 3).
TABLE 2.
HCV care cascade retention by site type and model of care
| Site description | Total screened | RDT+ | RNA test performed | HCV+ | Started Tx | Completed Tx | SVR tested | SVR achieved | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | |
| Total | ||||||||||||||||
| Total | 37 425 | 100 | 771 | 2.06 | 704 | 91.31 | 605 | 85.94 | 493 | 81.49 | 446 | 90.47 | 347 | 77.80 | 333 | 95.97 |
| Adjusted P‐value | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| Per arm | ||||||||||||||||
| 1 | 21 792 | 100 | 669 | 3.07 | 627 | 93.72 | 535 | 85.33 | 458 | 85.61 | 415 | 90.61 | 323 | 77.83 | 309 | 95.67 |
| 2 | 9822 | 100 | 40 | 0.41 | 21 | 52.50 | 19 | 90.48 | 14 | 73.68 | 12 | 85.71 | 9 | 75.00 | 9 | 100.00 |
| 3 | 5811 | 100 | 62 | 1.07 | 56 | 90.32 | 51 | 91.07 | 21 | 41.18 | 19 | 90.48 | 15 | 78.95 | 15 | 100.00 |
| P‐value (Pearson Chi squared test) | 1.34E‐57 | 0.10 | 0.94 | 0.02 | 0.99 | 1.00 | 0.99 | |||||||||
| Adjusted P‐value | 9.39E‐57 | 0.72 | 1.00 | 0.14 | 1.00 | 1.00 | 1.00 | |||||||||
Abbreviations: RDT, rapid diagnostic test; SVR, sustained virologic response; Tx, treatment.
FIGURE 3.
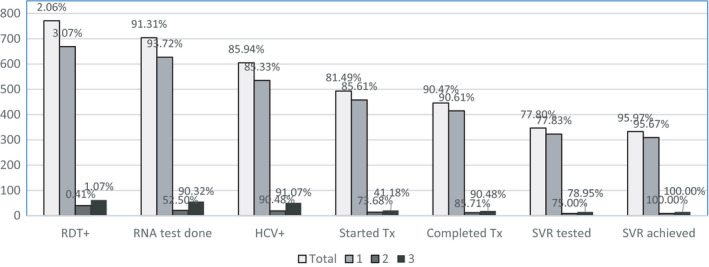
Retention of patients in the HCV care cascade by treatment site. 1 = Arm 1 (district hospitals). 2 = Arm 2 (polyclinics). 3 = Arm 3 (screening camps). RDT, rapid diagnostic test; SVR, sustained virologic response; Tx, treatment
A significantly higher proportion of participants from Arm 1 (93.7%) received a VL test compared with Arm 2 (52.5%, P < .001); however, similar levels were observed in Arm 3 compared with Arm 1 (90.3%). HCV viremia was similar across all arms (Arms 1, 2 and 3:85.3%, 90.5% and 91.1%, respectively). Similar levels of compensated cirrhosis were observed in all three arms (Arms 1, 2 and 3:4.5%, 7.1% and 14.2%, respectively, P < .001) Of those confirmed positive, treatment was initiated at similar rates for Arms 1 and 2 (85.6% and 73.7%, respectively), with significantly lower rates for Arm 3 (41.8%, P < .001, Arm 3 vs Arms 1 and 2). Treatment completion rates were similar across all three arms (Arms 1, 2 and 3:90.6%, 85.7% and 90.5%, respectively). Overall, 3.6% of participants discontinued treatment, 4.6% were lost to follow‐up, and 1% discontinued treatment due to serious adverse events, including death.
Of participants eligible for SVR‐12 testing at the study's completion, similar testing rates were reported for Arms 1 and 3 (77.8% and 78.9%, respectively), with lower rates for Arm 2 (75%, adjusted P = 1). Cure, defined as undetectable HCV VL, was achieved at similar rates in Arms 2 and 3 (both 100%) compared with 95.6% in Arm 1. Treatment failure, defined as detectable HCV RNA at SVR‐12, occurred in 4.0% of participants. Of these fourteen participants, seven were put on retreatment.
3.3. Turn‐around time between HCV care cascade steps
Overall, the time (median (IQR) days) between HCV serological testing and treatment initiation was shortest in Arm 1 (14, 8‐26.75) and Arm 2 (17.5, 12.5‐40.75) (adjusted P = .5) and longest in Arm 3 (31, 19‐85) (adjusted P <.001) (Table 3). The turn‐around time between HCV serological testing and sample collection for VL confirmation was 0 (0‐2) days in Arm 1, 2.5 (0‐9.5) days in Arm 2, and 0 (0‐0) days in Arm 3 (adjusted P < .02 for all pairwise comparison). Sample collection to VL testing was similar in all arms. The time between VL testing to return of results to patient was shortest 0 (0‐1) days for Arm 1, followed by Arm 2 1 (1‐2) days (adjusted P = .01), and 11 (2‐13) days for Arm 3 (adjusted P < .001 for comparisons with both Arm 1 and Arm 2). The time between VL results returned to participants and initiation of treatment was comparable between all three arms Arm 1 (8, 4‐21) days, Arm 2 (8, 3.25‐34) days and Arm 3 (16, 6‐79) days (adjusted P > .08 for all pairwise comparisons).
TABLE 3.
Turn‐around time between steps in the HCV care cascade
| Arm | RDT sample collection ‐ RNA sample collection | RNA sample collection ‐ RNA test performed | RNA test performed ‐ RNA results returned to patient | RNA results returned to patient ‐ treatment initiation | Total: RDT sample collection ‐ treatment initiation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | # | Median | IQR | # | Median | IQR | # | Median | IQR | # | Median | IQR | # | ||
| 1 | 0 | 0‐2 | 634 | 2 | 1‐3 | 627 | 0 | 0‐1 | 627 | 8 | 4‐21 | 458 | 14 | 8‐26.75 | 458 | |
| 2 | 2.5 | 0‐9.5 | 28 | 2 | 1‐3 | 21 | 1 | 1‐2 | 21 | 8 | 3.25‐34 | 14 | 17.5 | 12.5‐40.75 | 14 | |
| 3 | 0 | 0‐0 | 61 | 2 | 1‐2 | 56 | 11 | 2‐13 | 56 | 16 | 6‐79 | 21 | 31 | 19‐85 | 21 | |
3.4. Cost‐effectiveness analysis
Using an HCV antibody prevalence of 1% and viremic rate among antibody‐positive participants of 75% in the Hep C Testing Calculator, Arm 1 was found to be cost‐saving strategy—it resulted in more QALYs and lower costs than all other simulated strategies (Arm 2, Arm 3, and no action). The overall costs to the health system per 10 000 participants over a 30‐year time horizon and quality‐adjusted life‐years, respectively, were Arm 1: US $88 847, 168 447; Arm 2: US $159 222, 168 386; and Arm 3: US $162 695, 168 329. The total testing cost per treated patient was estimated at US$193.40 for Arm 1, $334.56 for Arm 2, and $376.54 for Arm 3. Compared with 'no action', Arm 1 would decrease 30‐year cumulative incidence of decompensated cirrhosis by 110 per 100 000 individuals, hepatocellular carcinoma by 60 per 100 000 individuals, and liver‐related deaths by 110. The corresponding reduction in disease burden under both Arm 2 and Arm 3 would be 50, 30 and 50, respectively.
4. DISCUSSION
This project demonstrated the effectiveness and feasibility of a simplified model of HCV care among the general population in Delhi, with attainment of good outcomes across the cascade of care. Of 37 425 participants screened for HCV using RDTs, 2.1% were RDT‐positive; of these, 91.3% received a VL test (85.9% viremic). There were 81.5% of participants initiated on treatment, with 90.5% completing their treatment; 77.8% had an SVR‐12 test and 96% achieved cure.
The first key finding from this study is the observation that when all services (testing and treatment) were provided at a single site (Arm 1 in hospitals, a “one‐stop shop”), there was a significantly higher retention rate of participants in the HCV care cascade compared with retention when participants were referred between sites (Arms 2 and 3). In polyclinics, seropositive participants were required to travel to hospitals for VL testing blood collection and subsequent treatment. This led to a loss of participants throughout the care cascade, particularly at this step (47.5% vs 6.3% loss in Arm 1) but also for follow‐up visits for treatment initiation and SVR testing (26.3% and 25%, respectively). Interestingly, in Arm 3, where seropositive participants had blood collection for VL testing at the site of screening this loss at this point in the cascade was not observed and retention was equivalent to that observed in Arm 1 (90.3% and 93.7%, respectively). However, in Arm 3, a significant number of participants were lost to follow‐up (treatment visit; 58.8% loss).
There may be several reasons for this attrition in Arms 2 and 3. When participants were referred to hospitals, there are several practical barriers, including the distance participants had to travel from polyclinics and home to the hospital and prohibitive transportation costs. The total time for participants to complete the HCV care cascade was significantly shorter in Arm 1, where services were all available at one site, compared with the time taken when participants were referred up from peripheral sites (Arms 2 and 3), indicating barriers to participants accessing services. Several other studies have reported similar observations of the impact of patient referral on retention in the HCV cascade. A recent systematic review by Oru et al demonstrated that fully decentralized (n = 29 studies) compared with partially decentralized (patient referral) (n = 11 studies) HCV care models resulted in higher testing uptake and linkage to care, but similar SVR rates. 11 In addition, Iwamato et al demonstrated that drawing blood for HCV confirmatory testing at the same site as screening reduces the time between screening and confirmation. 12
The second key finding was that while HCV seroprevalence was low overall, at 2.1%, it was highest at hospitals (3.1%) compared with polyclinics (0.4%) and screening camps (1.1%). The enrolled population included low proportions of key populations that typically have high HCV seroprevalence, such as PWID, PLHIV and dialysis patients. The small numbers of these sub‐populations that were among the enrolled population revealed the expected high yield of HCV seropositive cases (thalassaemic/dialysis patients, 52.2%; PWID, 24.8%; PLHIV, 26.1%); additional sub‐groups that resulted in high yields of positive cases included those who had unsafe sex (47.5%) or reported unsafe needle use (18.3%).
These findings will help guide the future implementation of screening strategies in Delhi, in terms of which sub‐populations to target during scale‐up efforts to ensure higher yields that result in lower costs for screening among the general population. Notably, screening of the general population in hospitals compared with screening in polyclinics resulted in a significantly higher yield of positive cases, underscoring the value and viability of this approach and potential cost saving of a hospital‐based screening program. Even when controlling for prevalence in the cost‐effectiveness analysis, the hospital arm was the most cost‐effective approach to providing HCV care, and if it were scaled up is projected to reach a larger percentage of viremic individuals.
The most significant challenges associated with this study were the delays and disruption associated with the COVID‐19 pandemic. Four of the five study hospitals were allocated to COVID‐19‐related activities; therefore, to ensure the safety of study staff and participants, study activities were relocated to ILBS and the one non‐COVID‐19 hospital. Public transport in Delhi was significantly impacted by COVID‐19 restrictions, leading to participants being unable to visit hospitals for follow‐up HCV care visits. Support was offered to participants for free private transportation to attend for follow‐up. However, delays and attrition for SVR‐12 testing was observed, which was likely due to the reluctance of participants to travel to health facilities during lockdowns.
This study has informed scale‐up plans for the Delhi Government HCV program in several ways. First, all HCV care (screening, VL sample collection and treatment of uncomplicated cases) will continue to be provided at the five district hospitals, with treatment of complicated cases at ILBS via referral. Treatment will be provided to all individuals free of charge at both district hospitals and ILBS. Second, HCV screening services will also be made available at public dispensaries and polyclinics; any positive individuals will be referred to a district hospital.
During the scale‐up of this one‐stop shop model at district hospitals, there are some areas that will require further optimisation, including improved case finding among high‐risk populations to increase the yield of positive cases. The screening of individuals at polyclinics requires careful consideration with regard to poor cost‐effectiveness, due to the extremely low yield of positive cases (0.4%) and poor linkage to care for seropositive cases, associated with the referral of participants for VL blood collection and subsequent treatment. The former could be improved by targeting both high‐risk and general population for screening and the latter could be improved by providing venous blood collection and treatment at polyclinics.
In conclusion, this study demonstrated strong retention of participants from the general population in the HCV cascade of care using a “one‐stop shop” model of HCV care at district hospitals. Our findings have led the Delhi Government HCV Program to plan the implementation of this model of care in district hospitals. However, further consideration must be given to improve yields and retention of participants from polyclinics prior to implementation, by providing decentralized HCV care at these sites.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
EG, SS, SKS and SShilton conceptualized the project. JM, EG, PK and SShilton developed the protocol. EG, DS, MM, PK, NT, BER and RJR were responsible for data curation. SS, SM, SKS and PE provided strategic guidance and oversight. JM, SS, SKS, EG, MM, SM, BER and SShilton provided supervision and support for study activities. MG and AT completed the formal statistical analysis. MA and JC completed the cost effectiveness analysis. JM wrote the first draft of the manuscript. JM, EG, DS, SS, MM, PK, NT, BER, RJR, MA, JC, SM, PE and SS reviewed and edited the manuscript. All authors have reviewed and approved the manuscript.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENTS
We would like to thank Dr Kavita Agarwal, Project Manager at ILBS and all staff involved in the study at the 5 District Hospitals in Delhi who were critical partners. These include the Medical Superintendents: Dr A.k Mehta, Dr P.S Nayyer, Dr Rajeev Sagar, Dr Sunil Kumar and Dr Ramesh Chugh; The Nodal Persons for Diagnostics: Dr Mala Vinayak, Dr Shalini Kakar, Dr Trishla, Dr Vivek Arora and Dr Kuldeep Kumar; and The Nodal Persons for Treatment: Dr Akansha, Dr Deepak Kumar Upadhyay, Dr N P Singh, Dr Rajiv Singhal, and Dr Suphala Bodo. We would also like to thank DNP+ for their support with community linkage, as well as all the study participants. We are grateful for the technical guidance and support from WHO India Country Office and WHO South East Asia Regional Office. AT acknowledges support from the Interdisciplinary Scientific and Educational School of Moscow University “Molecular Technologies of the Living Systems and Synthetic Biology”.
Markby J, Gupta E, Soni D, et al. Feasibility, effectiveness and cost of a decentralized HCV care model among the general population in Delhi, India. Liver Int. 2022;42:532–540. doi: 10.1111/liv.15112
Handling Editor: Alessio Aghemo
Funding information
This work is part of HEAD‐Start (Hepatitis C Elimination through Access to Diagnosis) funded by Unitaid.
DATA AVAILABILITY STATEMENT
There are no data requiring permission that have been reproduced from other sources. The data that support the findings of this study are available on reasonable request from the corresponding author.
REFERENCES
- 1. World Health Organization . Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. World Health Organization; 2021. [Google Scholar]
- 2. World Health Organization Guidelines on Hepatitis B and C testing. Available at http://www.who.int/hepatitis/publications/guidelines‐hepatitis‐c‐b‐testing/en/ Accessed 24 February
- 3. World Health Organization . Progress report on HIV, viral hepatitis and sexually transmitted infections. World Health Organization; 2019. [Google Scholar]
- 4. Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(Suppl 2):61‐80. [DOI] [PubMed] [Google Scholar]
- 5. Dhiman RK. Future of therapy for hepatitis C in India: a matter of accessibility and affordability? J Clin Exp Hepatol. 2014;4(2):85‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goel A, Seguy N, Aggarwal R. Burden of hepatitis C virus infection in India: a systematic review and meta‐analysis. J Gastroenterol Hepatol. 2019;34(2):321‐329. [DOI] [PubMed] [Google Scholar]
- 7. Government of India, National Viral Hepatitis Control Program . National Guidelines for the iagnosis and Management fo Viral Hepatitis. Government of India; 2018. [Google Scholar]
- 8. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost‐effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chhatwal J, Chen Q, Bethea ED, et al. Hep C Calculator: an online tool for cost‐effectiveness analysis of DAAs. Lancet Gastroenterol Hepatol. 2018;3(12):819. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . Viral Hepatitis Strategic Information and Modelling Reference Group. Meeting report. WHO headquarters; 2016. [Google Scholar]
- 11. Oru E, Trickey A, Shirali R, et al. Decentralisation, integration, and task‐shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta‐analysis. Lancet Global Health. 2021;9(4):e431‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwamoto M, Calzia A, Dublineau A, et al. Field evaluation of GeneXpert(®) (Cepheid) HCV performance for RNA quantification in a genotype 1 and 6 predominant patient population in Cambodia. J Viral Hepat. 2019;26(1):38‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
There are no data requiring permission that have been reproduced from other sources. The data that support the findings of this study are available on reasonable request from the corresponding author.


