Abstract
Background
Most case series of patients with ischemic stroke (IS) and COVID‐19 are limited to selected centers or lack 3‐month outcomes. The aim of this study was to describe the frequency, clinical and radiological features, and 3‐month outcomes of patients with IS and COVID‐19 in a nationwide stroke registry.
Methods
From the Swiss Stroke Registry (SSR), we included all consecutive IS patients ≥18 years admitted to Swiss Stroke Centers or Stroke Units during the first wave of COVID‐19 (25 February to 8 June 2020). We compared baseline features, etiology, and 3‐month outcome of SARS‐CoV‐2 polymerase chain reaction‐positive (PCR+) IS patients to SARS‐CoV‐2 PCR− and/or asymptomatic non‐tested IS patients.
Results
Of the 2341 IS patients registered in the SSR during the study period, 36 (1.5%) had confirmed COVID‐19 infection, of which 33 were within 1 month before or after stroke onset. In multivariate analysis, COVID+ patients had more lesions in multiple vascular territories (OR 2.35, 95% CI 1.08–5.14, p = 0.032) and fewer cryptogenic strokes (OR 0.37, 95% CI 0.14–0.99, p = 0.049). COVID‐19 was judged the likely principal cause of stroke in 8 patients (24%), a contributing/triggering factor in 12 (36%), and likely not contributing to stroke in 13 patients (40%).
There was a strong trend towards worse functional outcome in COVID+ patients after propensity score (PS) adjustment for age, stroke severity, and revascularization treatments (PS‐adjusted common OR for shift towards higher modified Rankin Scale (mRS) = 1.85, 95% CI 0.96–3.58, p = 0.07).
Conclusions
In this nationwide analysis of consecutive ischemic strokes, concomitant COVID‐19 was relatively rare. COVID+ patients more often had multi‐territory stroke and less often cryptogenic stroke, and their 3‐month functional outcome tended to be worse.
Keywords: COVID‐19, ischemic stroke, SARS‐CoV‐2
In a nationwide Swiss analysis of consecutive ischemic strokes, concomitant COVID‐19 was relatively rare. COVID+ patients more often had multi‐territory stroke and less often cryptogenic stroke, and their 3‐month functional outcome tended to be worse.

INTRODUCTION
Since December 2019, coronavirus disease 2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has resulted in considerable morbidity and mortality worldwide, including Switzerland. SARS‐CoV‐2 causes severe disease needing hospitalization and respiratory support, especially in older patients with chronic comorbidities and vascular risk factors such as hypertension and diabetes. The patients at risk of severe forms of COVID‐19 [1] share the same demographics and risk factors as patients at higher risk of ischemic stroke. In addition, COVID‐19 may also be responsible for specific pathophysiological mechanisms leading to ischemic stroke. Indeed, severe cases of COVID‐19 are associated with a cytokine storm syndrome, characterized by an uncontrolled immune response involving the continuous activation and proliferation of macrophages and lymphocytes. This systemic inflammatory response exacerbates the inflammatory activity within atherosclerotic plaques making them prone to rupture [2]. Inflammation also causes endothelial dysfunction and activates the coagulation cascade [3]. In addition, previous studies have provided evidence of cardiac injury among patients hospitalized with COVID‐19 [4].
Since the beginning of the COVID‐19 pandemic, several studies have described features of acute ischemic stroke patients with concomitant SARS‐CoV‐2 infection, but they generally lacked comparisons to stroke patients who are COVID‐19‐negative [5, 6]. Some studies, either based on international or regional registries, performed a comparison with COVID‐negative stroke patients but were limited to selected centers or often lacked the 3‐month outcome [7, 8, 9, 10, 11.
The aim of this study was to describe the clinical and radiological features and 3‐month outcomes of patients with ischemic stroke (IS) and concomitant or recent (<4 weeks since symptom onset) COVID‐19 in a nationwide Swiss cohort.
METHODS
Study population
We analyzed data from the national Swiss Stroke Registry (SSR), a web‐based registry implemented in the clinical data management system secuTrial, whose data processing is aided by the software package secuTrial. Since 2014, all consecutive patients hospitalized in stroke units and comprehensive stroke centers (all certified according to national Swiss Stroke Unit and Stroke Center criteria [https://www.sfcns.ch/Stroke.html], and those of the European Stroke Organization) [12] must be enrolled in the SSR, which was designed for quality control and research in acute stroke management. For the present analysis we included all consecutive IS patients aged ≥18 years who were admitted to SSR hospitals within 7 days from stroke onset (or last proof of good health). The diagnosis of IS in SSR patients corresponds to the historical World Health Organization (WHO) definition, namely a new syndrome of rapidly developing clinical symptoms and/or signs of focal disturbance of cerebral function lasting longer than 24 h with no apparent cause other than vascular origin, regardless of whether infarction was evident on cerebral neuroimaging. Therefore, patients with COVID‐19 and encephalopathy with non‐focal findings and/or non‐acute onset were not included in the SSR registry and in this analysis, independently of imaging finding. For this study we included patients admitted between 25 February and 8 June 2020. This timeframe corresponds to the first wave of COVID‐19 in Switzerland, which we defined as being from the day the first case of COVID‐19 was detected in the country to the moment when the number of new daily cases fell to under 30 new cases per day (https://corona‐data.ch).
This project was planned, designed, and approved by the SSR steering committee, which is composed of multidisciplinary researchers (neurologists, neuroradiologists, and neurosurgeons), in cooperation with the clinical trial unit of Basel (data management) and the Swiss Stroke Society.
Before analysis, data were anonymized following the principles of the Swiss Human Research Ordinance. Given that only anonymized data were used, local ethics commission approval and patient consent was not needed according to the Swiss Human Research Act. We informed patients in writing about the potential scientific use of their routinely collected data and their right of refusal.
Baseline characteristics
Local investigators at participating SSR centers collected standardized prespecified variables using electronic case report forms as described previously [13]. The secured, web‐based SSR databank is hosted at the clinical trial unit in Basel. We used the following variables in this analysis: patient demographic characteristics (age, sex, and body mass index), medical history (e.g., history of prior ischemic stroke, intracerebral hemorrhage, hypertension, dyslipidemia, diabetes, atrial fibrillation, and smoking), prior anti‐thrombotic therapy on admission, clinical information (National Institutes of Health Stroke Scale [NIHSS] score, blood glucose, and blood pressure on admission), the diagnostic tests performed and their main results (e.g., imaging modality, presence of large vessel occlusion). Information on recanalization treatments included the use of intravenous thrombolysis and/or mechanical thrombectomy (MT) as well as details of dosage, workflow metrics, and mode of application. We also collected additional information from each stroke center and stroke unit, including the date and result of the polymerase chain reaction (PCR) test for SARS‐CoV‐2 from nasopharyngeal swabs and SARS‐CoV‐2 serology, the presence of symptoms attributable to COVID‐19 (fever, respiratory symptoms), and the need of intubation and additional laboratory results at stroke onset (including blood cell counts, C‐reactive protein [CRP], activated partial thromboplastin time [aPTT], prothrombin time‐international normalized ratio [PT‐INR], fibrinogen, and D‐dimers).
Since the SSR database did not include the details on COVID‐19, a dedicated form was added to the online secuTrial environment to allow each center to enter additional data required for this study.
Likelihood of association between stroke and COVID‐19
Each center was also asked to evaluate the estimated probability of an association between COVID‐19 and the ischemic stroke, according to the following criteria:
COVID‐19 as probable principal cause (i.e., the patient would not have had the cerebrovascular event without COVID‐19): no other potential cause of stroke applying the TOAST criteria (i.e., large artery atherosclerosis with stenosis >50% in the ischemic territory, small artery occlusion, high‐risk cardioembolic source, other determined causes including arterial dissection) AND few (≤2) established risk factors AND symptomatic COVID‐19 at the time of stroke onset.
COVID‐19 as contributing/triggering factor: other potential causes of stroke AND/OR multiple risk factors (>2) AND symptomatic COVID‐19 at the time of event.
COVID‐19 as unlikely contributor: none of the above.
Follow‐up
All patients enrolled in the SSR received standardized follow‐up assessments by local investigators for in‐hospital outcomes (recurrent IS, symptomatic intracerebral hemorrhage according to European Cooperative Acute Stroke Study (ECASS) II criteria, (13) death) and 3‐month functional outcome using the modified Rankin Scale (mRS). Stroke neurologists or trained research staff performed follow‐up assessments during clinic visits, or via structured telephone interviews.
Study groups and outcomes
The group of interest (COVID+) consisted of patients with IS and COVID‐19 established by a positive PCR test on nasopharyngeal swab or by a typical clinical presentation and positive serology. The control group, (COVID−) comprised all the remaining IS patients admitted during the study period, including those with a negative PCR on nasopharyngeal swab and those asymptomatic for COVID‐19 not tested by nasopharyngeal swab.
The outcomes of this study were:
In patients with IS and COVID‐19 (COVID+ group): the time from first symptoms of COVID‐19 and stroke onset, the type and severity of COVID‐19 symptoms (asymptomatic carrier; respiratory/fever only, intubation other or longer than for MT treatment).
In patients with IS and COVID‐19 (COVID+ group) when compared with controls (non‐COVID+ group): demographics (age, sex), clinical and stroke data (pre‐stroke disability, admission NIHSS, pre‐treatment, vascular risk factors, vascular territory[ies], stroke mechanism, presence of large vessel occlusion, frequency of acute revascularization treatments), symptomatic intracerebral hemorrhage (sICH) according to the ECASS definition [14], and 7‐day and 3‐month mortality and disability.
In the COVID+ group and a matched population of non‐COVID+ group: acute laboratory exams at stroke onset including blood cell counts, CRP, aPTT, PT‐INR, and D‐dimers.
For the last two comparisons, we excluded IS patients with COVID‐19 infection of more than 1 month before or after stroke onset as a potential relationship with COVID‐19 was difficult to ascertain, even though late stroke after COVID‐19 has been reported [15].
Statistical analysis
We conducted the statistical analysis according to the following pre‐defined statistical analysis plan.
We summarized continuous data as median values and interquartile range (IQR) and categorical data as absolute numbers and percentage. We compared baseline variables and short‐term outcomes between the COVID+ and the non‐COVID+ groups using Pearson's chi‐squared test for categorical variables, and Mann–Whitney U‐tests for continuous variables, as appropriate. Demographic and clinical variables associated with COVID‐19 in the univariate analyses (p value < 0.10), were entered as independent variables into a multivariable logistic model with COVID‐19 infection as dependent variable, adopting a backward elimination approach using a removal criterion of p value > 0.10. For a more parsimonious model, we merged non‐significant categories of multinomial variables to the reference level.
We assessed 3‐month outcome by means of an ordered logit regression model, including COVID‐19 infection as independent variable with the response variable being the shift towards unfavorable outcome (i.e., higher mRS) at 3 months. A likelihood ratio test was used to estimate the assumption of proportional odds: no evidence of non‐proportional odds emerged across all the categories of mRS (p > 0.05). This analysis was first conducted on the whole cohort (unadjusted analysis) and then using a control group of non‐COVID+ patients matched for age, baseline NIHSS, pre‐stroke disability, and acute recanalization treatment using a 1:10 propensity score weighting method.
We compared the laboratory values of COVID+ patients to those of a control group of non‐COVID+ patients matched for age, gender, stroke etiology, and prior anticoagulant treatment at admission using a 1:2 propensity score weighting method.
We performed statistical analysis with R statistical software (version 3.3.2). P values < 0.05 were considered significant. As this was an exploratory analysis, correction for multiple analyses was not necessary.
This observational study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
RESULTS
SARS‐CoV‐2 testing and results
Over the study period, we included 2341 ischemic stroke patients from 20 stroke units and stroke centers in Switzerland. PCR testing for SARS‐CoV‐2 on nasopharyngeal swabs was performed according to local hospital policy and on the decision of the treating physician in 777 patients (33%).
Thirty‐six patients (corresponding to a rate of 1.5% of the overall population, and 4.6% of the population of patients tested by nasopharyngeal swab) had confirmed COVID‐19 (35 established by PCR on nasopharyngeal swabs and one by a typical clinical presentation and positive serology), of whom 30 presented fever or respiratory symptoms, while the remaining six had asymptomatic SARS‐CoV‐2 infection. Among symptomatic patients, 10 required transfer to an intensive care unit and eight had orotracheal intubation.
Temporal relationship between SARS‐CoV‐2 infection and stroke onset
Among the 30 symptomatic patients, COVID‐19 symptoms preceded stroke onset in 13 (six were still symptomatic at the time of stroke and seven had symptom resolution from 31 days to 1 day before stroke onset). Six patients had COVID‐19 symptoms that started on the same day as the stroke, and the other 11 patients presented COVID‐19 symptoms from 1 to 32 days after stroke onset. The detailed temporal course of COVID‐19 infection and its diagnosis in relation to stroke onset is detailed in Figure 1.
FIGURE 1.
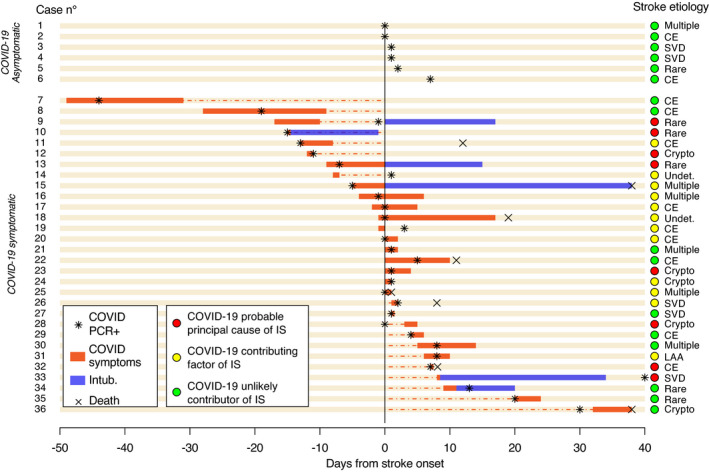
Each line represents a case. Patients are grouped in asymptomatic (cases 1 to 6) and symptomatic (cases 7 to 36). The x‐axis represents days from stroke onset. Red bars, number of days with symptoms of COVID‐19 (fever or respiratory symptoms). Blue bars, days of orotracheal intubation. Dotted red line, days between stroke onset and end/start of COVID‐19 symptoms (in patients not symptomatic at stroke onset). Black star, polymerase chain reaction (PCR) for SARS‐CoV‐2 by nasopharyngeal swab. Black cross, death of patient. Colored circles represent the likelihood of association: red, COVID‐19 probable principal cause of ischemic stroke (IS); yellow, COVID‐19 contributing factor of IS; green, COVID‐19 unlikely contributor of IS. Abbreviations: CE, cardioembolic; crypto, cryptogenic; LAA, large artery atherosclerosis; SVD, small vessel disease; undet., undetermined
Baseline characteristics of COVID+ and non‐COVID+ IS patients
Three patients had COVID‐19 more than 1 month before or after the stroke and we excluded them from the analyses. The univariate comparison of the 33 COVID+ patients to non‐COVID+ patients (COVID PCR− and/or asymptomatic non‐tested IS patients) is detailed in Table 1. The patients in the COVID+ group were slightly older (78 vs. 75 years, p = 0.299), and had a similar burden of vascular risk factors and similar medical therapy at stroke onset (excepting that COVID+ patients were more frequently under vitamin K antagonists and less under direct oral anticoagulants, p = 0.238 and p = 0.007, respectively). Stroke severity, frequency of large vessel occlusion, and acute revascularization treatment were comparable between the two groups. COVID+ patients more frequently had ischemic lesions in multiple vascular territories (27.3% vs. 13.2%, p = 0.036). Compared to non‐COVID+ controls, COVID+ patients had stroke etiology less frequently attributed to large artery atherosclerosis (3% vs. 14.7%) or cryptogenic origin (15.2% vs. 31.7%), and more frequently to rare/other mechanisms (18.2% vs. 8.9%) or to more than one etiology (18.2% vs. 5.7%; unadjusted p value for overall comparison of stroke etiology distribution = 0.003). Of the six COVID+ patients with stroke from rare/other etiologies, the stroke mechanism was a prothrombotic coagulation disorder in two patients, vasculitis in one, arterial dissection in one, and related to cardiac interventions in two. We found similar differences on univariate comparison when we used as the control group only patients that tested negative (Table S1a). In multivariate logistic regression analysis on the whole cohort, lower frequency of cryptogenic stroke and involvement of multiple arterial territories were independently associated with COVID‐19 infection (Table 2).
TABLE 1.
Baseline demographics and clinical characteristics of the whole cohort, and the non‐COVID+ and COVID+ ischemic stroke patients
| Demographics and clinical characteristics | Total (N = 2338) | Non‐COVID+ (n = 2305) | COVID+ (n = 33) | P value |
|---|---|---|---|---|
| Age (years) | 75 (65–83) | 75 (65–83) | 78 (67–88) | 0.299 |
| Female sex | 965 (41.3%) | 956 (41.5%) | 9 (27.3%) | 0.142 |
| pre‐stroke mRS | 0 (0–1) | 0 (0–1) | 1 (0–2.1) | 0.007 |
| Vascular risk factors | ||||
| Arterial hypertension | 1715 (73.4%) | 1694 (73.6%) | 21 (63.6%) | 0.279 |
| Diabetes | 528 (22.6%) | 519 (22.5%) | 9 (27.3%) | 0.662 |
| Dyslipidemia | 1500 (64.2%) | 1480 (64.2%) | 20 (60.6%) | 0.803 |
| Smoking | 472 (20.2%) | 469 (20.4%) | 3 (9.1%) | 0.166 |
| Atrial fibrillation | 547 (23.4%) | 537 (23.3%) | 10 (30.3%) | 0.462 |
| Coronary artery disease | 393 (16.8%) | 387 (16.8%) | 6 (18.2%) | 1.000 |
| Prosthetic heart valve | 80 (3.4%) | 77 (3.4%) | 3 (9.1%) | 0.188 |
| Low cardiac EF | 46 (3.2%) | 44 (3.1%) | 2 (8.3%) | 0.387 |
| Peripheral artery disease | 137 (5.9%) | 133 (5.8%) | 4 (12.1%) | 0.245 |
| Previous stroke | 451 (19.3%) | 444 (19.3%) | 7 (21.2%) | 0.955 |
| Previous TIA | 124 (5.3%) | 123 (5.3%) | 1 (3%) | 0.843 |
| Previous ICH | 46 (2%) | 46 (2%) | 0 (0%) | 0.849 |
| BMI | 25.2 (22.9–28.2) | 25.2 (22.9–28.2) | 23.6 (20.1–26.2) | 0.029 |
| Therapy at stroke onset | ||||
| Aspirin | 705 (30.2%) | 693 (30.1%) | 12 (37.5%) | 0.477 |
| Clopidogrel | 158 (6.8%) | 156 (6.8%) | 2 (6.2%) | 1.000 |
| Anticoagulant | 357 (15.3%) | 351 (15.2%) | 6 (18.2%) | 0.822 |
| DOAC | 255 (10.9%) | 254 (11%) | 1 (3%) | 0.238 |
| VKA | 103 (4.4%) | 98 (4.3%) | 5 (15.6%) | 0.007 |
| Lipid‐lowering drugs | 727 (31.1%) | 716 (31.1%) | 11 (34.4%) | 0.838 |
| Onset‐to‐door delay (h) | 7.3 (2.2–21.8) | 7.4 (2.2–22) | 5.2 (1.7–15.7) | 0.411 |
| Baseline NIHSS | 3 (1–7) | 3 (1–7) | 5 (1.9–9.4) | 0.119 |
| Baseline SBP (mmHg) | 157 (140–178) | 157 (140–178) | 146 (131.5–164.2) | 0.009 |
| Baseline DBP (mmHg) | 84 (74–95) | 84 (74–95) | 75 (64.7–83) | 0.004 |
| MRI as baseline imaging modality | 517 (25.7%) | 509 (25.6%) | 8 (28.6%) | 0.893 |
| Baseline vascular imaging results | ||||
| No abnormality | 870 (47.5%) | 860 (47.6%) | 10 (43.5%) | 0.750 |
| Stenosis 50%–99% in suspected ischemic territory | 293 (16%) | 288 (15.9%) | 5 (21.7%) | |
| Occlusion in suspected ischemic territory | 667 (36.5%) | 659 (36.5%) | 8 (34.8%) | |
| Arterial territory | ||||
| Unilateral carotid | 1407 (60.2%) | 1387 (60.2%) | 20 (60.6%) | 0.000 |
| Vertebrobasilar | 609 (26%) | 605 (26.2%) | 4 (12.1%) | |
| Bilateral carotid | 173 (7.4%) | 164 (7.1%) | 9 (27.3%) | |
| Carotid and vertebrobasilar | 141 (6%) | 141 (6.1%) | 0 (0%) | |
| Cryptogenic | 8 (0.3%) | 8 (0.3%) | 0 (0%) | |
| Multiple arterial territories | 314 (13.4%) | 305 (13.2%) | 9 (27.3%) | 0.036 |
| Stroke etiology | ||||
| Cardiac embolism | 593 (25.4%) | 583 (25.3%) | 10 (30.3%) | 0.003 |
| Large artery atherosclerosis (≥50% stenosis) | 339 (14.5%) | 338 (14.7%) | 1 (3%) | |
| Small vessel disease | 321 (13.7%) | 316 (13.7%) | 5 (15.2%) | |
| Other determined etiology | 212 (9.1%) | 206 (8.9%) | 6 (18.2%) | |
| Unknown etiology | 736 (31.5%) | 731 (31.7%) | 5 (15.2%) | |
| More than one possible etiology | 137 (5.9%) | 131 (5.7%) | 6 (18.2%) | |
| Acute recanalization treatments | ||||
| IV thrombolysis | 499 (21.4%) | 493 (21.4%) | 6 (18.2%) | 0.812 |
| MT | 372 (15.9%) | 369 (16%) | 3 (9.1%) | 0.401 |
Univariate analysis with p values from Pearson's chi‐squared test for continuous variables and Mann−Whitney U‐test for numeric variables.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; DOAC, direct oral anticoagulants; EF, ejection fraction; ICH, intracranial hemorrhage; IV, intravenous: mRS, modified Rankin Scale; MT, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; sICH, symptomatic intracranial hemorrhage; TIA, transient ischemic attack; VKA, vitamin K antagonists.
TABLE 2.
Multivariate analysis comparing COVID+ versus non‐COVID+ stroke patients in the Swiss Stroke Registry : parameters independently associated with COVID‐19 infection
| Variable | OR (95% CI) | P value |
|---|---|---|
| Multiple arterial territories | 2.35 (1.08–5.14) | 0.032 |
| Stroke etiology | ||
| Large artery atherosclerosis a | 0.16 (0.02–1.21) | 0.075 |
| Cryptogenic a | 0.37 (0.14–0.99) | 0.049 |
| More than one possible etiology a | 2.50 (0.99–6.32) | 0.054 |
Values in bold type denote statistical significance.
Versus other categories merged: cardioembolic, rare etiologies, small vessel disease.
Laboratory parameters of COVID+ and non‐COVID+ IS patients
The comparison of acute laboratory values of the COVID+ with the non‐COVID+ group matched for age, gender, stroke severity, prior anticoagulation on admission, and stroke etiology showed a significantly prolonged aPTT (32.5 s vs. 28 s, p = 0.005), elevated CRP (31.1 vs. 3 mg/L, p < 0.001), and lower lactate dehydrogenase (316 vs. 421 U/L, p < 0.000). The other laboratory parameters were not significantly different (Table S2), although D‐dimers were twice as high in COVID+ patients as in controls.
Likelihood of association between stroke and COVID‐19
According to our predefined criteria, we judged that COVID‐19 was likely to be the principal cause of stroke in eight patients (24%), that it was a contributing/triggering factor of stroke in 12 (36%), and that it was likely not contributing to stroke in 13 (40%). Among the eight patients in which COVID‐19 was judged the likely principal cause of IS, three had stroke from a rare etiology (one had central nervous system vasculitis, one prothrombotic state with positive anti‐phospholipid antibodies, and one an extensive carotid artery thrombosis on a small plaque), one from a cardioembolic mechanism (left ventricular thrombosis without any structural heart disease), one had a microangiopathic‐looking stroke without risk factors or chronic signs of small vessels disease, and in the remaining three the stroke mechanism was undetermined.
Outcome of COVID+ and non‐COVID+ IS patients
Cerebral hemorrhagic complication and stroke recurrence did not differ between the two groups. COVID+ patients were less likely to be discharged home and had a non‐significant increase in in‐hospital mortality. Details are displayed in Table 3 (and in Table S1b for the analysis using patients that tested negative as the control group).
TABLE 3.
Short‐term and 3‐month outcomes of the whole cohort, and non‐COVID+ and COVID+ ischemic stroke patients
| Outcomes | Total (N = 2338) | Non‐COVID+ (n = 2305) | COVID+ (n = 33) | P value |
|---|---|---|---|---|
| Short‐term outcomes | ||||
| NIHSS at 24 h | 2 (0–6) | 2 (0–6) | 4 (1.9–9.4) | 0.025 |
| sICH | 34 (1.5%) | 33 (1.4%) | 1 (3%) | 0.978 |
| In‐hospital stroke recurrence | 46 (2%) | 45 (2%) | 1 (3%) | 1.000 |
| In‐hospital mortality | 133 (5.7%) | 129 (5.6%) | 4 (12.1%) | 0.221 |
| Discharge destination | ||||
| Home | 940 (42.8%) | 937 (43.2%) | 3 (10.7%) | 0.005 |
| Other acute care hospital | 304 (13.8%) | 297 (13.7%) | 7 (25%) | |
| Rehabilitation hospital | 843 (38.4%) | 828 (38.2%) | 15 (53.6%) | |
| Nursing home, palliative care center, or other medical facility | 108 (4.9%) | 105 (4.8%) | 3 (10.7%) | |
| 3‐month outcomes a | ||||
| mRS, median (IQR) | 2 (0–3) | 2 (0–3) | 3 (2–5.3) | 0.003 b |
| Stroke recurrence | 52 (2.8%) | 51 (2.7%) | 1 (4%) | 1.000 |
| ICH | 8 (0.4%) | 8 (0.4%) | 0 (0%) | 1.000 |
| Death | 271 (13.2%) | 264 (13.1%) | 7 (24.1%) | 0.088 c |
Abbreviations: ICH, intracranial hemorrhage; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; sICH, symptomatic intracranial hemorrhage.
Calculated for patients with available 3‐month outcomes, COVID+: n = 29, non‐COVID+: n = 2019.
P value of unadjusted ordinal regression analysis for higher mRS shift (cOR 2.57, 95% CI 1.37–4.81).
P value of unadjusted logistic regression analysis for mortality (OR 1.46, 95% CI 0.61–3.50).
Follow‐up at 3 months was available in 29/33 (88%) COVID+ patients and in 2019/2305 (88%) controls. We observed a significant mRS shift towards a worse outcome in COVID+ patients (cOR 2.57, 95% CI 1.37–4.81, p < 0.01). A non‐significant trend was present in the propensity score matched analysis (ps‐adj cOR 1.85, 95% CI 0.96–3.58, p = 0.07), with matching for age, baseline NIHSS, acute pre‐stroke disability, and revascularization treatment (Figure 2). The clinical characteristics of the propensity matched control group are shown in Table S3. The mortality rate in the whole cohort analysis was almost twice as high in the COVID+ group compared to the non‐COVID+ group, but not statistically significant (24.1% vs. 13.1%, OR 2.12, 95% CI 0.89–5.00, p = 0.088). This trend was less pronounced after propensity score adjustment (ps‐adjOR 1.46, 95% CI 0.59–3.59, p = 0.41).
FIGURE 2.
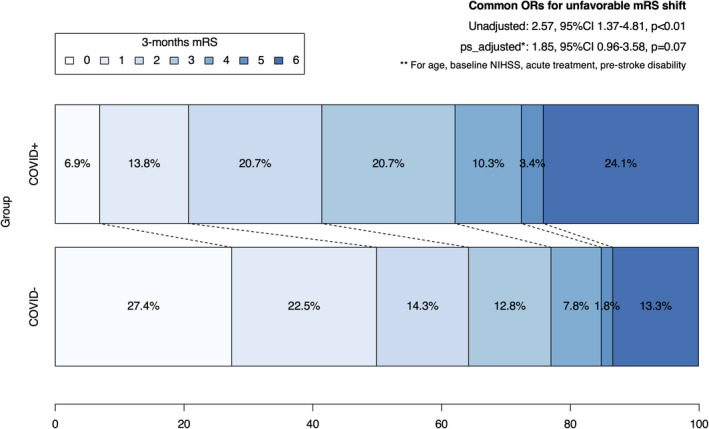
Three‐month modified Rankin Scale (mRS) in COVID+ and non‐COVID+ stroke patients. Abbreviations: CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio
DISCUSSION
In this nationwide Swiss study of >2000 IS patients admitted to stroke units and centers during the first wave of COVID‐19 we showed that concomitant symptomatic or asymptomatic SARS‐CoV‐2 infection occurred in 1.5% of patients (4.6% of the population of those tested by nasopharyngeal swab). The temporal association between COVID‐19 and stroke onset was heterogeneous, as stroke onset could precede, follow, or be concomitant with the symptoms of COVID‐19. On multivariate analysis, COVID+ patients had a significantly higher frequency of multi‐territorial ischemic lesions and rare causes of stroke as well as a lower frequency of IS attributed to large artery atherosclerosis or cryptogenic stroke. We considered SARS‐CoV‐2 infection as the principal cause of stroke in one‐third of patients and a triggering factor in half of them. In the unadjusted analysis, functional outcome at 3 months was significantly worse in COVID+ patients; after propensity score matching for age, stroke severity, previous disability, and acute treatment there was a strong trend towards a worse outcome (p = 0.07).
The frequency of COVID‐19 in IS probably depends on the COVID‐19 incidence in the general population, which peaked at 154 cases per 1,000,000 per day in Switzerland in the first wave (https://corona‐data.ch), an average value for industrialized countries. It is possible that we underestimated the incidence of patients with COVID‐19 infection and IS as the SSR focuses on patients with stroke as the main clinical problem, potentially missing patients with minor strokes and severe COVID‐19. Still, the relatively low incidence of 1.5% of COVID‐19 in IS patients during this period (4.6% of the population of those tested by nasopharyngeal swab) suggests that the infection has a relatively low impact on stroke admissions.
The temporal relationship between COVID‐19 and stroke has been poorly characterized until now, as previous studies did not report time between COVID‐19 symptoms and stroke onset in a systematic manner; median times ranged from 9 to 21 days with high variability within each study and across different studies [15, 16, 17. The observations in our cohort seem to confirm this heterogeneity as we observed similar proportions of patients with stroke occurring shortly before, simultaneously, or after the onset or resolution of COVID‐19 symptoms. This finding suggests that an IS may complicate COVID‐19 infection at any stage of the disease, from the pre‐symptomatic to the late phase.
In COVID+ IS patients we observed a lower proportion of cryptogenic stroke (15%) compared to previous studies in which this ranged from 22% to 35% [5, 6, 7, 18. A possible explanation for this finding may be the high rate of patients with complete workup in our cohort (94% in COVID+ patients), compared to previous studies where stroke workup was complete in 72%–87% of cases [7, 19. Another explanation could be the increased rate of identified rare stroke mechanisms including coagulation disorder and vasculitis, which could have been caused by COVID‐19.
Independently of stroke etiology, multi‐territorial infarcts were significantly associated with concomitant SARS‐CoV‐2 infection. This finding is in line with previous results [20, 21, 22, and probably reflects the coagulation disorder and consecutive thromboembolism or the diffuse vasculopathy that may be associated with COVID‐19 [23, 24.
Earlier studies already suggested that the association between COVID‐19 and IS may be causative in some patients and incidental in others [18, 25, and our results are in agreement with this hypothesis. Indeed, local investigators considered IS a direct consequence of COVID‐19 in one‐third of patients, in almost half of them COVID‐19 was estimated to be a trigger factor of IS, while in one‐third the association between COVID‐19 and IS was supposed to be incidental. In the first instance most patients had stroke mechanisms classically reported in association with COVID‐19, such as hypercoagulable state, inflammation, or vasculitis [23, 26, 27. Still, despite the likely association of COVID‐19 and IS, many patients have a high load of cerebrovascular risk factors and other, usual stroke mechanisms such as cardioembolism or large artery atherosclerosis; therefore, COVID‐19 may act as a trigger in these at‐risk patients.
Previous studies have already shown that in IS patients COVID‐19 is associated with a worse outcome at discharge and higher in‐hospital and 3‐month mortality [1, 11, 20, even though key quality performance measures of acute stroke care were stable during lockdowns, at least in well‐established European stroke centers including Switzerland [28]. Our study assessed the medium‐term functional impact on COVID‐19‐IS patients and found a nearly significant worse 3‐month outcome after propensity score matching for prognostic baseline features and acute revascularization treatment (Figure 2). Of note, COVID+ IS patients had more premorbid disability in the univariate analysis, as reported in a previous study [29]. Presumably, patients with pre‐existing disability have a higher risk of complicated COVID‐19 requiring hospitalization, and among the possible complications a higher risk of IS. The absolute rate of mortality at 3 months was twice as high in the COVID+ group, but this difference was not significant after propensity score adjustment. Our results suggest that COVID‐19 itself contributes to the worse outcome of the patients through the wide range of complications affecting multiple organs and systems [30]. This effect may also be present in non‐severe infections; indeed in the COVID+ group fewer than one‐third required intensive care unit care and/or orotracheal intubation and one‐fifth of patients had asymptomatic infection.
Our results have potentially relevant clinical implications. We provide a comprehensive clinical description of IS patients with concomitant SARS‐CoV‐2 infection, which may be useful for identifying and appropriately treating these patients. We showed that IS may occur at any phase of the disease and independently of disease severity, underlining that a high clinical suspicion of IS should be maintained in cases of new neurological symptoms appearing in COVID‐19 patients. In contrast, we should suspect a concomitant SARS‐CoV‐2 infection during pandemic periods in patients with multi‐territorial stroke.
The worse 3‐month outcome in IS patients with COVID‐19 highlights even more the importance of offering acute revascularization therapies and specialized stroke care to these patients in order to minimize the impact of the combined condition. Further studies are warranted to explore the effectiveness and the potential complication of such treatments in COVID‐19 patients.
Our study has limitations, including the relatively low number of IS patients with concomitant COVID‐19 limiting the statistical power for detecting clinical and prognostic differences to non‐COVID+ patients. The study included exclusively patients from the first wave of the COVID‐19 pandemic, when systematic testing for SARS‐CoV‐2 in hospitalized patients was not yet in place and did not achieve complete coverage. It is therefore possible that some asymptomatic or mild COVID‐19 patients were wrongly included in the control group in our sample. We partially overcame this limitation by performing a sensitivity analysis with COVID‐19‐negative patients confirmed by PCR test. Also, some blood test parameters that we often saw as abnormal in COVID‐19 patients were not available or missing in most patients. Finally, our study lacks information on the patent foramen ovale status, which can be a potentially relevant stroke mechanism especially in COVID‐19 patients given that they are mainly at risk of venous thrombosis.
The strength of our study is that we performed a nationwide analysis using data from a national registry based on a prospectively constructed database with predefined variables, including consecutive patients from all stroke units and stroke centers in Switzerland, providing insights into the hospitalization and 3‐month follow‐up periods.
In conclusion, this nationwide analysis showed that COVID‐19 was relatively rare in consecutive patients with acute ischemic stroke, although multi‐territorial involvement was a distinctive feature of COVID‐19 IS patients. COVID‐19 had a relevant impact on 3‐month outcome, with positive patients seeming to fare worse across the entire modified Rankin scale spectrum. The onset of IS could occur at any phase of SARS‐CoV‐2 infection, which may act as the main cause of stroke, as the trigger when added to multiple vascular comorbidities, or as an incidental event.
CONFLICT OF INTERESTS
DS: congress travel support from Bristol‐Myers Squibb, research grants from the Swiss Heart Foundation and from the University of Lausanne*. PM: research grants from the Swiss National Science Foundation and the Swiss Heart Foundation; consulting and speaker fees from Medtronic. GMDM: research grants from the Swiss National Science Foundation and Swiss Heart Foundation; consultant/travel honoraria from Bayer, BMS/Pfizer, Medtronic. All fees are paid to the University Hospital Basel. STE*: research grants from the Swiss National Science Foundation and the Swiss Heart Foundation; travel or speaker honoraria from Bayer, Boehringer‐Ingelheim, Daiichi‐Sankyo; advisory boards for Bayer, Boehringer‐Ingelheim, BMS/Pfizer, MindMaze; educational grant from Pfizer; research support from Daiichi‐Sankyo. UF*: research grants from the Swiss National Science Foundation and the Swiss Heart Foundation; consulting and speaker fees from Medtronic, Stryker, CSL Behring. LHB: research grants from the Swiss National Science Foundation, the University of Basel, the Swiss Heart Foundation, and the “Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung” (Basel, Switzerland); an unrestricted research grant from AstraZeneca; consultancy or advisory board fees or speaker's honoraria from Amgen, Bayer, Bristol‐Myers Squibb, Claret Medical, and InnovHeart; travel grants from AstraZeneca and Bayer. AAT: consultancy or advisory board fees or speaker's honoraria from AstraZeneca, Schwabe Pharma, and Mitsubishi Tanabe. FM: research grant from the Swiss Heart Foundation. NP (outside the submitted work): research grants from the Swiss National Science Foundation and Swiss Heart Foundation; speaker honoraria from Vifor; advisory boards for AstraZeneca. *All fees are paid to their institutions (CHUV, Inselspital, University Hospital Basel, Felix Plater Spital).
AUTHOR CONTRIBUTIONS
Davide Strambo: Conceptualization (equal); Data curation (equal); Formal analysis (lead); Funding acquisition (equal); Writing – original draft (equal). Gian Marco De Marchis: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Writing – review & editing (equal). Leo H. Bonati: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Writing – review & editing (equal). Marcel Arnold: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Writing – review & editing (equal). Emmanuel Carrera: Data curation (equal); Writing – review & editing (equal). Santi Galletta: Data curation (equal); Writing – review & editing (equal). Krassen Nedeltchev: Data curation (equal); Writing – review & editing (equal). Timo Kahles: Data curation (equal); Writing – review & editing (equal). Carlo W. Cereda: Data curation (equal); Writing – review & editing (equal). Giovanni Bianco: Data curation (equal); Writing – review & editing (equal). Georg Kägi: Data curation (equal); Writing – review & editing (equal). Andreas R. Luft: Data curation (equal); Writing – review & editing (equal). Manuel Bolognese: Data curation (lead); Writing – review & editing (lead). Lehel‐Barna Lakatos: Data curation (equal); Writing – review & editing (equal). Stephan Salmen: Data curation (equal); Writing – review & editing (equal). Pamela Correia: Data curation (equal); Writing – review & editing (equal). Rolf Sturzenegger: Data curation (equal); Writing – review & editing (equal). Albert Sylvan: Data curation (equal); Writing – review & editing (equal). Friedrich Medlin: Data curation (equal); Writing – review & editing (equal). Christian Berger: Data curation (equal); Writing – review & editing (equal). Florian Lindheimer: Data curation (equal); Writing – review & editing (equal). Markus Baumgärtner: Data curation (equal); Writing – review & editing (equal). Ludwig Schelosky: Data curation (equal); Writing – review & editing (equal). Christophe Bonvin: Data curation (equal); Writing – review & editing (equal). Marie‐Luise Mono: Data curation (equal); Writing – review & editing (equal). Biljana Rodic: Data curation (equal); Writing – review & editing (equal). Andrea von Reding: Data curation (equal); Writing – review & editing (equal). Guido Schwegler: Data curation (equal); Writing – review & editing (equal). Federico Massini: Data curation (equal); Writing – review & editing (equal). Alexander A. Tarnutzer: Data curation (equal); Writing – review & editing (equal). Shadi Taheri: Data curation (equal); Writing – review & editing (equal). Nils Peters: Data curation (equal); Writing – review & editing (equal). Morin Beyeler: Data curation (equal); Writing – review & editing (equal). Valerian Altersberger: Data curation (equal); Writing – review & editing (equal). Stefan T. Engelter: Data curation (equal); Writing – review & editing (equal). Urs Fischer: Conceptualization (equal); Funding acquisition (equal); Writing – review & editing (equal). Patrik Michel: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Writing – original draft (equal).
Supporting information
ACKNOWLEDGMENTS
The authors are grateful to M. Price Hirt for English language correction and editing. Open access funding provided by Universite de Lausanne.
Strambo D, De Marchis GM, Bonati LH, et al. Ischemic stroke in COVID‐19 patients: Mechanisms, treatment, and outcomes in a consecutive Swiss Stroke Registry analysis. Eur J Neurol.2022;29:732–743. doi: 10.1111/ene.15199
Funding information
The Swiss Heart Foundation
DATA AVAILABILITY STATEMENT
Anonymized participant data are available upon reasonable request including a data analysis, authorship, and publication plan.
REFERENCES
- 1. Marti‐Fabregas J, Guisado‐Alonso D, Delgado‐Mederos R, et al. Impact of COVID‐19 infection on the outcome of patients with ischemic stroke. Stroke. 2021;52(12):3908‐3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madjid M, Vela D, Khalili‐Tabrizi H, Casscells SW, Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34:11‐18. [PMC free article] [PubMed] [Google Scholar]
- 3. Corrales‐Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496‐505. [DOI] [PubMed] [Google Scholar]
- 4. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan. JAMA Cardiol. 2020;5(7):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramos‐Araque ME, Siegler JE, Ribo M, et al. Stroke etiologies in patients with COVID‐19: the SVIN COVID‐19 multinational registry. BMC Neurol. 2021;21:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shahjouei S, Tsivgoulis G, Farahmand G, et al. SARS‐CoV‐2 and stroke characteristics: a report from the multinational COVID‐19 Stroke Study Group. Stroke. 2021;52:e117‐e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuentes B, Alonso de Lecinana M, Garcia‐Madrona S, et al. Stroke acute management and outcomes during the COVID‐19 outbreak: a cohort study from the Madrid stroke network. Stroke. 2021;52:552‐562. [DOI] [PubMed] [Google Scholar]
- 8. Ntaios G, Michel P, Georgiopoulos G, et al. Characteristics and outcomes in patients with COVID‐19 and acute ischemic stroke: the global COVID‐19 Stroke Registry. Stroke. 2020;51:e254‐e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katz JM, Libman RB, Wang JJ, et al. Cerebrovascular complications of COVID‐19. Stroke. 2020;51:e227‐e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yaghi S, Ishida K, Torres J, et al. SARS‐CoV‐2 and stroke in a New York healthcare system. Stroke. 2020;51:2002‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calmettes J, Peres R, Goncalves B, et al. Clinical outcome of acute ischemic strokes in patients with COVID‐19. Cerebrovasc Dis. 2021;1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waje‐Andreassen U, Nabavi DG, Engelter ST, et al. European Stroke Organisation certification of stroke units and stroke centres. Eur Stroke J. 2018;3:220‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonati LB, Bonvin R, Cereda CH, et al. Das Schweizerische Hirnschlagregister (Swiss Stroke Registry) ‐ ein Werkzeug für die Qualitätssicherung und Forschung. Swiss Med Forum. 2016;16:168‐169. [Google Scholar]
- 14. Hacke W, Kaste M, Fieschi C, et al. Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245‐1251. [DOI] [PubMed] [Google Scholar]
- 15. Tu TM, Seet CYH, Koh JS, et al. Acute ischemic stroke during the convalescent phase of asymptomatic COVID‐2019 infection in men. JAMA Netw Open. 2021;4:e217498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valencia‐Enciso N, Ortiz‐Pereira M, Zafra‐Sierra MP, Espinel‐Gomez L, Bayona H. Time of stroke onset in coronavirus disease 2019 patients around the globe: a systematic review and analysis. J Stroke Cerebrovasc Dis. 2020;29:105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID‐19 patients. Stroke. 2020;51:e219‐e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shahjouei S, Anyaehie M, Koza E, et al. SARS‐CoV‐2 is a culprit for some, but not all acute ischemic strokes: a report from the multinational COVID‐19 Stroke Study Group. J Clin Med. 2021;10:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naccarato M, Scali I, Olivo S, et al. Has COVID‐19 played an unexpected "stroke" on the chain of survival? J Neurol Sci. 2020;414:116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID‐19: a systematic review and meta‐analysis. Int J Stroke. 2021;16:137‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Escalard S, Maier B, Redjem H, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID‐19: experience from Paris. Stroke. 2020;51:2540‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Anna L, Kwan J, Brown Z, et al. Characteristics and clinical course of Covid‐19 patients admitted with acute stroke. J Neurol. 2020;267:3161‐3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sashindranath M, Nandurkar HH. Endothelial dysfunction in the brain: setting the stage for stroke and other cerebrovascular complications of COVID‐19. Stroke. 2021;52(5):1895‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vogrig A, Gigli GL, Bna C, Morassi M. Stroke in patients with COVID‐19: clinical and neuroimaging characteristics. Neurosci Lett. 2021;743:135564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. John S, Kesav P, Mifsud VA, et al. Characteristics of large‐vessel occlusion associated with COVID‐19 and ischemic stroke. AJNR Am J Neuroradiol. 2020;41:2263‐2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanafi R, Roger PA, Perin B, et al. COVID‐19 neurologic complication with CNS vasculitis‐like pattern. AJNR Am J Neuroradiol. 2020;41:1384‐1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Altersberger VL, Stolze LJ, Heldner MR, et al. Maintenance of acute stroke care service during the COVID‐19 pandemic lockdown. Stroke. 2021;52:1693‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perry RJ, Smith CJ, Roffe C, et al. Characteristics and outcomes of COVID‐19 associated stroke: a UK multicentre case‐control study. J Neurol Neurosurg Psychiatry. 2021;92:242‐248. [DOI] [PubMed] [Google Scholar]
- 30. Kaushik P, Kaushik M, Parveen S, Tabassum H, Parvez S. Cross‐talk between key players in patients with COVID‐19 and ischemic stroke: a review on neurobiological insight of the pandemic. Mol Neurobiol. 2020;57:4921‐4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized participant data are available upon reasonable request including a data analysis, authorship, and publication plan.


