Abstract
Dopamine type 2 receptor (D2R) agonists have anticonvulsant effect, whereas D2R antagonists increase seizure risk, but the mechanism of this action has not been delineated. We tested whether D2R agonists activate parvalbumin (PV)-containing inhibitory interneurons to suppress seizures. We treated frontal lobe onset seizures with a D2R agonist sumanirole, and it suppressed seizures. We used activity reporter TRAP2 mice and found that injection of D2R agonist led to extensive activation of PV interneurons in the cortex and striatum ipsilateral to the seizure focus. D2R agonists activate PV interneurons, which in turn inhibit principal neurons, potentially explaining their anticonvulsant effect.
Keywords: dopamine agonist, epilepsy, PV interneurons, seizures, TRAP2 mice
1 |. INTRODUCTION
Dopamine receptor modulation changes susceptibility to seizures. Dopamine type 2 receptor (D2R) agonists are anticonvulsants and suppress pilocarpine and kindled seizures,1,2 whereas D2R antagonists are proconvulsant.3 According to the US Food and Drug Administration package insert and series of carefully performed studies, patients taking high-affinity D2R antagonists such as haloperidol, clozapine, and olanzapine for schizophrenia or psychosis have a higher risk of seizures.4,5 D2R agonists are anticonvulsants in limbic seizures, but their effectiveness in frontal lobe seizures was not tested.1,2 We previously found that D2R agonist injected through a bilateral cannula directly into the striatum stopped frontal lobe seizures for 2.5 h following the injection.6 Here, we wanted to determine whether systemic injection would also be anticonvulsant. Also, the mechanism behind dopamine receptor modulation of seizures is not fully understood. Thus, we tested whether D2R agonists activate inhibitory parvalbumin (PV) interneurons to suppress seizures.
2 |. MATERIALS AND METHODS
2.1 |. Animals
The University of Virginia Animal Care and Use Committee approved the experimental protocol. TRAP2 (Transient Recombination of Activated Populations, second generation) mice were generated by crossing Fos2A−ICreERT2 (#030323) and Ai9 mice (#007909, Jackson Laboratories). We studied TRAP2 and C57Bl/6 mice of both sexes (6–12 weeks old), gave them ad libitum food and water, and maintained them on a 12 h light/12 h dark cycle. KAPA Biosystems kit was used for genotyping.
2.2 |. Seizure induction and drug injections
We placed 1.7-mg cobalt wire in the right premotor cortex (anteroposterior, +2.6 mm; mediolateral, −1.8 mm) of C57Bl/6 mice with four bilateral electroencephalographic (EEG) electrodes under isoflurane anesthesia and continuously monitored for seizures via video/EEG.7 All mice developed seizures before any injections were made. In the first cohort, one group of mice received three consecutive sumanirole injections (5 mg/kg ip) at 18, 20, and 22 h after cobalt placement. Another group of mice received three normosol control injections. A blinded investigator counted the number of seizures within 2 h before the injections (16–18 h after cobalt) to determine the baseline, within 6 h during the treatment with three injections (injections at 18, 20, and 22 h after cobalt) because sumanirole has a sustained effect for at least 2 h, and 2 h after the injections (24–26 h after cobalt) to determine return to the baseline (Figure 1A). In the second cohort, a different group of mice was injected once with sumanirole at 18 h (and another group at 20 h) after cobalt. The number of seizures in these groups was compared before (16–18 h (or 18–20 h) after cobalt) and during the injection (18–20 h (or 20–22 h) after cobalt; Figure 1 C,D).
FIGURE 1.
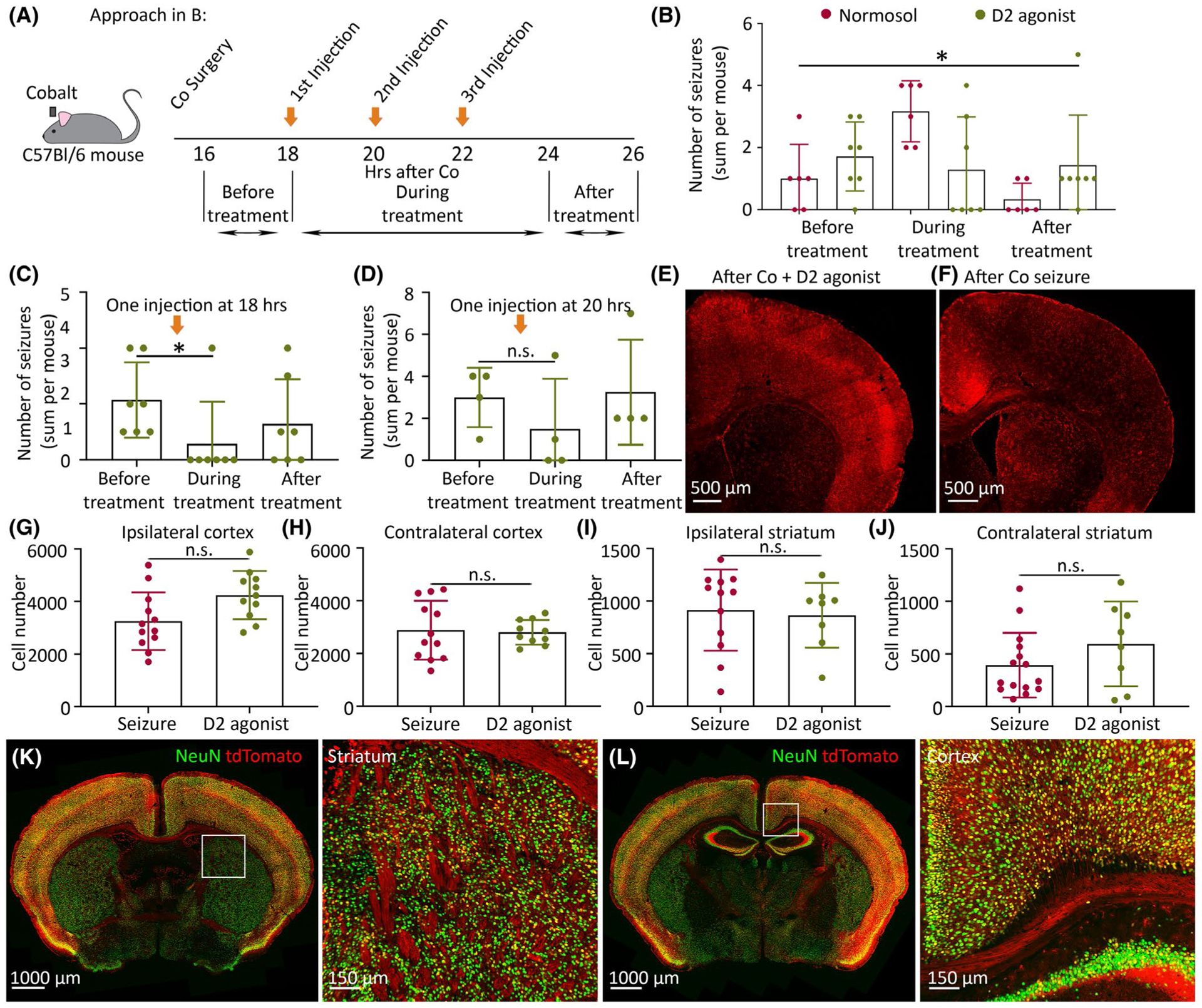
Systemic injection of dopamine type 2 receptor (D2R) agonist is anticonvulsant in frontal lobe seizures. (A) Experimental approach: two groups of cobalt-implanted mice were injected intraperitoneally with either sumanirole or normosol at 18, 20, 22 h after cobalt (three orange arrows), and seizure number was counted before, during, and after the treatment (three horizontal black arrows). (B) The effect of three systemic D2R agonist injections on the number of seizures as shown in A. One group of mice (n = 7) was injected with sumanirole (green) and another group of mice (n = 6) with normosol (red). The number of seizures (sum per mouse) was counted before (16–18 h after cobalt), during (18–24 h after cobalt, three injections at 18, 20, and 22 h), and after the treatment (24–26 h after cobalt). (C) The effect of a single sumanirole injection at 18 h after cobalt on the seizure number before the injection (16–18 h after cobalt), during the injection (18–20 h after cobalt), and after the injection (20–22 h after cobalt; Friedman test, n = 7 mice). (D) The effect of a single sumanirole injection at 20 h after cobalt on the seizure number before the injection (18–20 h after cobalt), during the injection (20–22 h after cobalt), and after the injection (22–24 h after cobalt; Friedman test, n = 4 mice). (E) tdTomato expression in cobalt-implanted TRAP2 mice after D2R agonist injection. (F) tdTomato expression after a seizure. (G–J) Cell number in the right (ipsilateral to the seizure focus) and left (contralateral to the seizure focus) cortex and striatum in D2R agonist-injected TRAP2 mice (green) versus TRAP2 mice after a seizure (red; n = 4–5 mice). Each point on the graph represents mean cell number at a specific distance from anterior (2.46 mm) to posterior (.14 mm) bregma for either D2R agonist or seizure mouse groups ± SEM. Unpaired t-tests or Kolmogorov–Smirnov tests were done to compare means of the two groups. (K, L) Activation of the cortex and striatum in D2R agonist-injected TRAP2 mice. Data are mean ± SEM. *p < .05, n.s. nonsignificant; PV, parvalbumin
To label activated cells after D2R agonist injection, cobalt-implanted TRAP2 mice were injected with sumanirole at 18 h after cobalt and with 4-hydroxytamoxifen (4-OHT, 50 mg/kg sc) within 90 min after sumanirole. Cobalt-implanted control TRAP2 mice were injected with 4-OHT within 90 min after a secondarily generalized tonic–clonic seizure. Animals were perfused 5 days after 4-OHT injection to allow tdTomato expression.
2.3 |. Immunohistochemistry, microscopy, and analysis
TRAP2 mice were perfused with 4% paraformaldehyde, sliced on a cryostat at 40 μm, immunolabeled with rabbit anti-NeuN (1:500, #24307, Cell Signaling) and rabbit anti-PV (1:500, #ab11427; Abcam), and imaged on a confocal microscope at 10×/.45 numerical aperture (NA) or 20×/.95 NA as described previously.6
We used ImageJ and Imaris Spots detection tools to count tdTomato-positive cells in D2R agonist-injected versus noninjected mice. We subtracted background and adjusted the threshold to count cells.
The fraction of activated PV cells was detected using the Imaris Spots tool. The number of cells expressing both tdTomato and PV was divided by the total number of PV-positive cells in the right (ipsilateral to the focus) and left cortex and the striatum.
2.4 |. Statistics
We analyzed data using Prism 9 for all statistical tests and presented them as mean ± SEM, with n indicating the number of animals. We used an unpaired t-test for normally distributed data and the Kolmogorov–Smirnov (KS) test if data were not distributed normally to compare means of two groups. We also used multiple comparison analysis of variance (ANOVA) with Bonferroni correction or Friedman test. Results were considered statistically significant for p < .05.
3 |. RESULTS
3.1 |. Systemic administration of D2 receptor agonist is anticonvulsant during frontal lobe seizures
We placed cobalt in the right premotor cortex to cause frontal lobe focal motor to bilateral tonic–clonic seizures.7 Mice always had only behavioral seizures accompanied by EEG ictal activity, and none of the seizures was only electrographic. To determine whether systemic D2R agonist injection was anticonvulsant, we administered full selective D2R agonist sumanirole (5 mg/kg ip) or normosol as a control. Because sumanirole has a sustained effect for at least 2 h,6 we had two injection cohorts. In the first cohort, we did three consecutive injections to determine the anticonvulsant effect. One group of mice received three consecutive sumanirole injections at 18, 20, and 22 h after cobalt insertion, and another group of mice received three normosol injections (Figure 1A). During the three-injection treatment, D2R agonist-injected mice had fewer seizures than the control mice (Figure 1B; before [sum of seizures per mouse from 16 to 18 h after cobalt]: D2R agonist 1.71 ± .42, normosol 1.00 ± .45; during [sum of seizures per mouse from 18 to 24 h after cobalt]: D2R agonist 1.29 ± .64, normosol 3.17 ± .40; after [sum of seizures per mouse from 24 to 26 h after cobalt]: D2R agonist 1.43 ± .61, normosol .33 ± .21; p = .0142, F value = 3.380, df = 5, multiple comparison ANOVA with Bonferroni correction; D2: n = 7 mice, normosol: n = 6 mice). We also assessed mean seizure number within the treatment (during first injection: D2R agonist .00 ± .00, normosol 1.33 ± .49; during second injection: D2R agonist .86 ± .46, normosol 1.00 ± .45; during third injection: D2R agonist .43 ± .30, normosol .83 ± .17). The first injection at the peak of seizures (at 18 h after cobalt) appeared to be more effective at preventing seizures than the following injections. In the second cohort, we compared seizure numbers before and after a single sumanirole injection at 18 h after cobalt. Animals had fewer seizures following than before the injection (Figure 1C; before: 2.14 ± .51; during: .57 ± .57; after: 1.29 ± .61, p = .0463, df = 2, false discovery rate adjusted p-value [Q value] = .05, Friedman test, n = 7 mice). We wanted to determine whether decreased effect after the second and third D2R agonist injections was due to tachyphylaxis or due to a rundown of the seizure burden. Therefore, we did a single sumanirole injection at 20 h after cobalt and found a similar number of seizures before and during the treatment (Figure 1D; before: 3.00 ± .71; during: 1.50 ± 1.19; after: 3.25 ± 1.25; p = .6528, df = 2, Q = .05, Friedman test, n = 4 mice). We could not detect the anticonvulsant effect due to the natural decline in seizure frequency in our model and not due to tachyphylaxis. Animals exhibited no change in EEG/behavior, and D2R agonist treatment did not modify seizure severity patterns, although hypoactivity was possible in some mice.
Next, we visualized activated neurons after a single D2R agonist injection using TRAP2 mice that express Cre under the c-Fos promoter regulation. We injected TRAP2 mice with sumanirole (5 mg/kg ip) at 18 h after cobalt insertion, followed by 4-OHT injection within 90 min after sumanirole to translocate Cre inside the nucleus and relieve repression from tdTomato to label activated cells (Figure 1E,K,L). For controls, we injected TRAP2 mice with 4-OHT 90 min after a seizure (Figure 1F). All mice were injected with 4-OHT after equivalently severe seizures. D2R agonist-injected and seizure control mice had a similar number of activated tdTomato-positive neurons in the right and left cortex and striatum (Figure 1G–J).
3.2 |. Increased activation of PV interneurons in the ipsilateral cortex and striatum after D2R agonist injection
PV interneurons express D2Rs.8 We compared PV interneuron activation in cobalt-implanted TRAP2 mice that received systemic D2R agonist injection (Figure 2A,C) versus those with only a seizure (Figure 2B,D). We found more tdTomato-positive PV interneurons in the right (ipsilateral) cortex and striatum of D2R agonist-injected mice than in those of mice with seizures (Figure 2E,G; ipsilateral cortex: D2R agonist 57.86 ± 10.41%, seizure 29.49 ± 3.32%, p = .0241; ipsilateral striatum: D2R agonist 80.69 ± 6.00%, seizure 44.07 ± 6.94%, p = .0062). In contrast, the contralateral cortex and striatum of D2R agonist-injected mice and mice with seizures had a similar fraction of activated PV cells (Figure 2F,H; contralateral cortex: D2R agonist 44.61 ± 15.48%, seizure 18.83 ± 4.04%; contralateral striatum: D2R agonist 60.25 ± 16.95%, seizure 22.61 ± 8.04%). We next plotted the percentage of activated PV interneurons across bregma from anterior to posterior cortex and striatum, where each black line is a simple linear regression for a separate mouse (Figure 2I–L). Only in the ipsilateral cortex did all D2R agonist-injected mice and mice with seizures have positive slopes, meaning that the closer to the seizure focus (in the anterior premotor cortex), the more PV cells were active (Figure 2I). In contrast, in the striatum, PV interneurons were on average uniformly active, with no anterior to posterior activation pattern.
FIGURE 2.
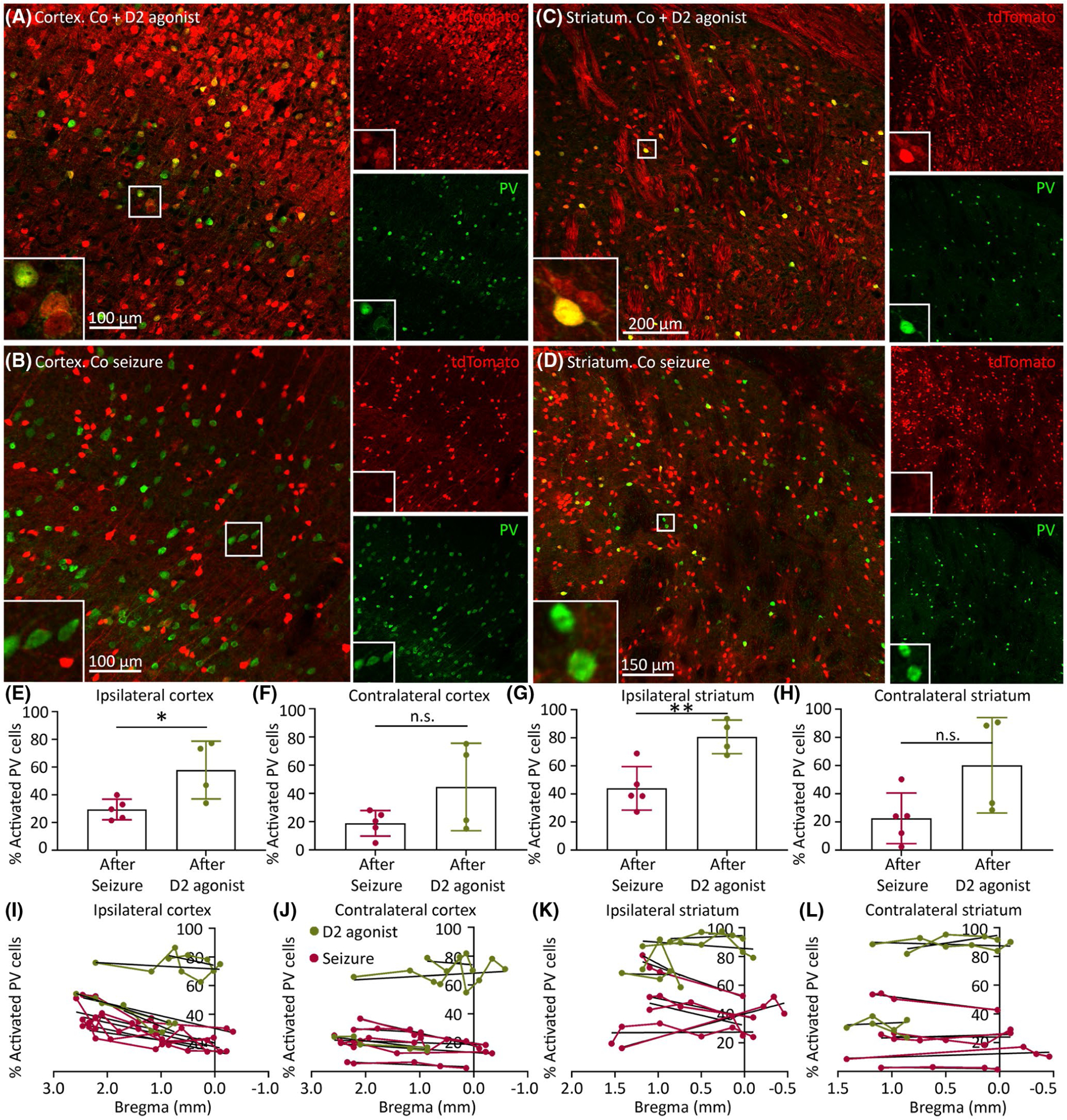
Dopamine type 2 receptor (D2R) agonist increases the number of activated parvalbumin (PV) interneurons in the right (ipsilateral) cortex and striatum. tdTomato expression (red) in PV interneurons (green) is shown. (A) Cortex after D2R agonist injection. (B) Cortex after seizures. (C) Striatum after D2R agonist injection. (D) Striatum after seizures. (E–H) Percentage of activated PV interneurons in cobalt-implanted TRAP2 mice after D2R agonist injection (green) or after seizure (red; tdTomato-positive PV interneurons divided by the total number of PV interneurons in the specified structure, n = 4–5 mice). (I–L) Percentage of activated PV cells from anterior to posterior cortex and striatum across bregma. Black lines indicate simple linear regressions for separate D2R agonist-injected mice (green) and mice with seizures (red). Data are mean ± SEM. Unpaired t-test, *p < .05, **p < .01. n.s., nonsignificant
4 |. DISCUSSION
Systemic D2R agonist injection is anticonvulsant in frontal lobe focal motor to bilateral tonic–clonic seizures. D2R activation increased PV interneuron activity in the cortex and striatum ipsilateral to the seizure focus. Previous studies report increased D2 receptor density in the nucleus accumbens ipsilateral but not contralateral to the stimulating electrode in the amygdala or hippocampus.2 We hypothesize that stronger ipsilateral activation of PV interneurons is due to the stronger impact of seizures on the side of the focus, and possibly, increased number of D2Rs, compared to the contralateral side. PV interneurons express both D2 and D1 receptors.8 D2R agonists increase the excitability of fast-spiking PV interneurons8 and decrease excitatory postsynaptic potentials (EPSPs) of pyramidal cortical neurons, whereas D1 agonists do not affect pyramidal neuron EPSPs.9
We previously showed that frontal lobe focal motor to bilateral tonic–clonic seizures preferentially activate the indirect pathway of the basal ganglia.6 D2R agonists reduce activity along the indirect pathway and of the sub-stantia nigra reticulata,10 which could also potentially explain the anticonvulsant effect of D2R agonists. All D2 receptors activate G protein-regulated inwardly rectifying K+ channels and decrease Ca2+ currents.11 Thus, D2 receptor stimulation decreases the excitability of striatal medium spiny neurons by increasing K+ currents, inducing hyperpolarization. On the other hand, D2R agonists affect PV interneurons indirectly by depressing γ-aminobutyric acid (GABA) inhibitory synaptic input to them.12
Although it is uncertain whether increased ipsilateral cortical or striatal inhibition leads to decreased seizures, and although other interneurons could also play a role in increased inhibition after D2R agonist treatment, GABAergic PV interneurons are important regulators of excitatory projection pyramidal neurons, and PV interneuron dysfunction leads to seizures.13–16 Thus, enhanced activity of PV interneurons after D2 receptor activation may explain the anticonvulsant effect of D2R agonists.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (RO1 NS040337, RO1 NS044370 to J.K.) and the UVA Brain Institute.
Footnotes
CONFLICT OF INTEREST
Neither of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Turski L, Cavalheiro EA, Bortolotto ZA, Ikonomidou-Turski C, Kleinrok Z, Turski WA. Dopamine-sensitive anticonvulsant site in the rat striatum. J Neurosci. 1988;8(11):4027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahnschaffe U, Löscher W. Anticonvulsant effects of ipsilateral but not contralateral microinjections of the dopamine D2 agonist LY 171555 into the nucleus accumbens of amygdala-kindled rats. Brain Res. 1991;553(2):181–7. [DOI] [PubMed] [Google Scholar]
- 3.Turski WA, Cavalheiro EA, Ikonomidou C, Bortolotto ZA, Klockgether T, Turski L. Dopamine control of seizure propagation: intranigral dopamine D1 agonist SKF-38393 enhances susceptibility to seizures. Synapse. 1990;5(2):113–9. [DOI] [PubMed] [Google Scholar]
- 4.Koch-Stoecker S Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(2):19–24. [DOI] [PubMed] [Google Scholar]
- 5.Alper K, Schwartz KA, Kolts RL, Khan A. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345–54. [DOI] [PubMed] [Google Scholar]
- 6.Brodovskaya A, Shiono S, Kapur J. Activation of the basal ganglia and indirect pathway neurons during frontal lobe seizures. Brain. 2021. doi: 10.1093/brain/awab119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh T, Joshi S, Williamson JM, Kapur J. Neocortical injury–induced status epilepticus. Epilepsia. 2020;61(12):2811–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17(5):1235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61(10):843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–4. [DOI] [PubMed] [Google Scholar]
- 11.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct. 2004;24(3):165–205. [DOI] [PubMed] [Google Scholar]
- 12.Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, et al. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23(15):6245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto H, Tatsukawa T, Shimohata A, Yamagata T, Suzuki T, Amano K, et al. Impaired cortico-striatal excitatory transmission triggers epilepsy. Nat Commun. 2019;10:1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li KX, Lu YM, Xu ZH, Zhang J, Zhu JM, Zhang JM, et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci. 2011;15(2):267–73. [DOI] [PubMed] [Google Scholar]
- 15.Hu H, Gan J, Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345(6196):e1255263. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Lachance M, Rossignol E. Involvement of cortical fast-spiking parvalbumin-positive basket cells in epilepsy. Prog Brain Res. 2016;226:81–126. [DOI] [PMC free article] [PubMed] [Google Scholar]


