Abstract
Background
The potential decrease in daily physical activity associated with the COVID-19 pandemic lockdowns may have a negative impact on people living with dementia. Given the limited literature around the effects of home confinement in people living with dementia, this study investigated changes in physical exercise levels of participants in the intervention arm of the Promoting Activity, Independence and Stability in Early Dementia (PrAISED) Randomised Controlled Trial during the first COVID-19 national lockdown. It hypothesised that participants would maintain physical exercise levels.
Methods
A repeated measure (three time points) study involving 30 participants (mean age = 78.0 years, 15 male and 15 female, 22 (73.0%) living with their primary caregiver), from four regions in England receiving the PrAISED intervention. PrAISED is an individually tailored intervention of physical exercises and functional activities. Trained therapists deliver therapy sessions over a period of 52 weeks. Study participants received therapy sessions via phone or video calling during the COVID-19 lockdown. This study investigated self-reported minutes of physical exercise recorded on study calendars for the months of February (i.e., baseline – pre-lockdown), May (i.e., T1 – during lockdown), and August (i.e., T2—post-lockdown) 2020.
Results
Participants reported a statistically significant increase in activity levels between February and May (Wilcoxon Z = -2.013, p = 0.044) and a statistically significant decrease between May and August (Wilcoxon Z = -2.726, p = 0.004). No significant difference was found in the physical activity levels from pre- to post-lockdown (Wilcoxon Z = 0.485, p = 0.620).
Conclusion
Despite concerns that the restrictions associated with the COVID-19 pandemic might lead to reductions in physical exercise, participants in receipt of the PrAISED intervention increased their amount of physical exercise during lockdown. Our findings support the potential of remote support for people living with dementia to help them maintain physical exercise levels in circumstances where face-to-face service provision is not possible.
Trial registration
The PrAISED trial and process evaluation have received ethical approval number 18/YH/0059 from the Bradford/Leeds Ethics Committee.
The Clinical Trial Identifier for PrAISED is: ISRCTN15320670 (https://doi.org/10.1186/ISRCTN15320670). Registration was made on 04/09/2018.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-03239-5.
Keywords: Sports Medicine, Telemedicine, PrAISED, Neurodegenerative disease, SARS-CoV-2
Background
Dementia presents with a cluster of symptoms, including impairment in motor skills [1–4]. Research has found that being physically active generates numerous benefits in older adults living with dementia, including the maintenance of physical abilities, independence, and quality of life [5].
To achieve these benefits, the World Health Organisation (WHO) recommend at least 150 min of moderate-intensity aerobic physical activity or 75 or more minutes of vigorous-intensity aerobic physical activity per week, or an equivalent combination of the two [6]. Older adults who cannot exercise due to health conditions should engage in physical activity which is commensurate to their abilities [6]. The United Kingdom Chief Medical Officers' Physical Activity Guidelines argue that even minimal level of physical activity (e.g., standing) generates some health benefits, compared to being inactive [7].
The Promoting Activity, Independence and Stability in Early Dementia (PrAISED) Randomised controlled Trial (RCT) is evaluating the clinical and cost-effectiveness of an intervention promoting physical exercise in people living with dementia [8]. Participants in the intervention arm receive a programme of physical exercises that are designed to be at least of moderate intensity, based on to each participant’s individual ability, and functional activities (i.e., activities of daily living with an element of physical activity, such as going out for food shopping). It is delivered through up to 50 therapy sessions over a period of 52 weeks by trained physiotherapists, occupational therapists and rehabilitation support workers in the participants’ homes.
In March 2020, due to the rapid global spread of the Coronavirus disease 2019 (COVID-19) outbreak, the United Kingdom (UK) government mandated a national lockdown and social distancing measures, which required people to stay at home and allowed them to receive only strictly necessary in-home medical care, [9] making home visits from the PrAISED therapists impossible. Studies have found that lockdowns and social distancing measures impede individuals' mobility, daily activities, [10, 11] and social interactions [12]. As a result, an increase in the prevalence of psychological distress and disorder symptoms (e.g., depression, anxiety, negative feelings, emotional exhaustion, somatic symptoms, panic disorder) has been widely reported [10, 11]. To mitigate these risks and continue with the RCT, it was decided to deliver the PrAISED therapy sessions remotely using video calls and telephone. The PrAISED research team provided the therapists with plans and guidance on how to deliver PrAISED remotely (Additional file 1: Appendices 1, 2 and 3).
Studies on older adults have reported a decrease in physical activity during the COVID-19 lockdown [13]. Yet, there are currently few studies that investigate the effects of home confinement in people living with early dementia, and the potential associated decrease in daily physical activity levels. In a previous study published on BMC Geriatrics, [14] we reported preliminary qualitative evidence that, provided the support offered by the PrAISED team, some participants seemed to have been able to keep physically active during lockdown. The aim of this follow up study was to empirically investigate quantitative data around changes in the levels of physical exercise of participants who were in receipt of PrAISED through video/telephone during the first wave of the COVID-19 pandemic lockdown.
The research question was: Did the physical exercise levels of participants in receipt of PrAISED change throughout (i.e., before, during and after) the first wave of COVID-19 lockdown? Based on our previous preliminary study, [14] we hypothesised that through the remote support of the PrAISED team, the participants maintained their physical exercise levels throughout lockdown.
Methods
This was a repeated measure study, using secondary data from the PrAISED RCT, [8] (but not reporting results from the RCT, which is ongoing). It made use of self-reported minutes of physical exercise compiled by all participants in the intervention arm of the PrAISED RCT on monthly calendars during the COVID-19 pandemic first lockdown in England.
The Clinical Trial Identifier for PrAISED is: ISRCTN15320670 (https://doi.org/10.1186/ISRCTN15320670). Registration was made on 04/09/2018.
Sample
Inclusion criteria were:
Aged 65 years or over
Having a diagnosis of mild cognitive impairment or dementia
Scoring 13–25 (out of 30) in the Montreal Cognitive Assessment (MoCA) [15]
Being able to walk without human help, communicate in English, see andhear, perform neuropsychological tests, and give consent to participate
Taking part in the PrAISED RCT in the intervention arm during the COVID-19 pandemic first wave national lockdown in England (Fig. 1)
Agreeing to continue to take part in PrAISED when converted from in-home face-to-face to remote support using video calls and/or telephone.
Fig. 1.
Timeline of the first national lockdown during the COVID-19 pandemic in England [16]
Setting
The participants were supported either through the telephone or video calling, based on their preference. For phone appointments, a suitable date and time for the therapy session was agreed between the therapist and the participant. On the agreed date, the therapist called the participant and held the session. If the participant needed support, the phone was put in “on speaker” mode, so that the caregiver (where present) could participate. For video calling appointment, the therapy sessions took place on Q Health (https://qhealth.io/), a National Health Service (NHS) Digital and NHS England-approved video patient consultation solution. Q Health allowed therapists and participants to set up and attend digital appointments. The system required access to technology (i.e., internet connection and a computer, tablet or smart phone) and the ability to download the Q Health application, to book the digital appointments and connect for the therapy session. The therapy team provided the participants with a set of instructions on how to operate the system and offered guidance via phone, if needed. Once installed, the participants were provided with a password to grant secure access, to select an appointment time and to start the video therapy session.
Data collection
All participants signed informed consent prior to their inclusion in the trial. Demographic information about participants including age, gender, ethnicity, years of education, residency status, and location were collected at the time of involvement in the trial.
As part of the trial, the participants were asked to complete at home a diary of their PrAISED exercises in the form of a calendar (Additional file 2: Appendix 4). A previous study showed that return rates of 63–90% of these calendars are accurate reflections of activity levels in people living with dementia [17]. Instructions were provided to participants on how to fill in the calendar (Additional file 3: Appendix 5).
Minutes of PrAISED exercise were recorded by the participants each day, and at the end of each month, the calendar sheet (in paper format) listing all daily minutes of physical activity (i.e., one entry for each day of the month) was sent to the PrAISED team. Caregivers could support the participants to fill in the calendars, if needed. Using the participant ID on the calendar, the data were added to the trial database [8].
Data analysis
Descriptive statistics and return rates were calculated. To investigate physical exercise levels of participants during lockdown, data from the following months were analysed:
February 2020, used as proxy for pre-lockdown period in England – participants received face-to-face in-home visits only;
May 2020, used as proxy for lockdown period in England – participants received remote support via phone/videoconferencing only;
August 2020, used as proxy for post-lockdown period in England – participants received a mix of face-to-face in-home visits and remote support via phone/videoconferencing.
These months were selected based on government policy on social distancing in England [16].
To convert data to minutes of exercise per week, the daily minutes of exercise for each participant were added to obtain monthly figures. These were divided by the number of recordings in the month, and the result multiplied by seven. To test whether any differences in exercise levels were statistically significant across months, Wilcoxon signed-rank tests were conducted, comparing February and May, May and August, and August and February. Level of significance was set at p < 0.05. The analysis was completed on IBM SPSS [18].
Patient and Public Involvement (PPI)
The PrAISED RCT includes two Patient and Public Involvement (PPI) contributors who have lived experience of caring for someone living with dementia. The PPI contributors were involved in the development of the RCT from its conception. Although they did not actively contribute to this manuscript, their feedback on the relevance of findings and implication for people living with dementia were sought and integrated in this study.
Results
Thirty participants were included, with a mean age of 78.0 years (SD = 6.0; Range 66–88). Male and female participants were equally represented (n = 15; 50.0%). Most participants were white (n = 29; 97.0%) and lived with their primary caregiver (n = 22; 73.0%). The mean score at the MoCA [15] was 21.3 (SD = 3.3). Participants’ characteristics are reported in Table 1.
Table 1.
Participants’ characteristics
| n | % | Mean | Standard Deviation | Range | ||
|---|---|---|---|---|---|---|
| Age | 77.6 | 6.0 | 66—88 | |||
| Gender | Male | 15 | 50.0 | |||
| Female | 15 | 50.0 | ||||
| Ethnicity | White | 29 | 96.7 | |||
| Black | 1 | 3.3 | ||||
| Years of education | 13.1 | 3.9 | 8–25 | |||
| Residency status | Living with caregiver | 22 | 73.3 | |||
| Living alone | 7 | 26.7 | ||||
| Location | Nottinghamshire | 9 | 30.0 | |||
| Derbyshire | 10 | 33.3 | ||||
| Lincolnshire | 7 | 23.3 | ||||
| Somerset | 4 | 13.3 | ||||
| MoCAa score | 21.3 | 3.3 | 13–25 | |||
aMoCA Montreal Cognitive Assessment [15]
One hundred and ninety-six therapy sessions were delivered to the participants in the three months, with an average of 2.2 sessions per month and a mean duration of 49.0 min per session (SD = 33.1) (Table 2). There was no significant difference in the duration of sessions between phone and video calls. The mean return rate of calendars was 76.0%, which is within range to reflect accurate activity levels [11].
Table 2.
Information on sessions
| Month | Sessions delivered (n) | Duration of session in minutes—mean (STD) | Frequency of sessions/month—mean | Mode of delivery – n (%) | ||
|---|---|---|---|---|---|---|
| Face-to-face delivery in participants’ homes | Remote delivery—phone | Remote delivery—videoconferencing | ||||
| Feb 2020 | 84 | 76.0 (24.0) | 2.8 | 84 (100.0) | - | - |
| May 2020 | 78 | 35.0 (20.0) | 2.6 | - | 67 (86.0) | 11 (14.0) |
| Aug 2020 | 34 | 36.0 (20.0) | 1.1 | 1 (3.0) | 25 (74.2) | 8 (22.8) |
| Total | 196 | 49.0 (33.1) | 2.2 | 85 (-) | 102 (-) | 19 (-) |
In February 2020 (pre-lockdown), participants reported an average of 101.0 min per week of physical exercise; in May 2020 (during lockdown), they reported an average of 132.0 min per week; in August 2020 (post-lockdown), they reported an average of 103.0 min per week (Fig. 2). A Wilcoxon signed-rank test showed a statistically significant increase in activity levels between February 2020 and May 2020 (Z = -2.013, p = 0.040) and a statistically significant decrease in activity levels between May 2020 and August 2020 (Z = -2.726, p = 0.004). No statistically significant differences were found between February 2020 and August 2020 (Z = -0.485, p = 0.620).
Fig. 2.
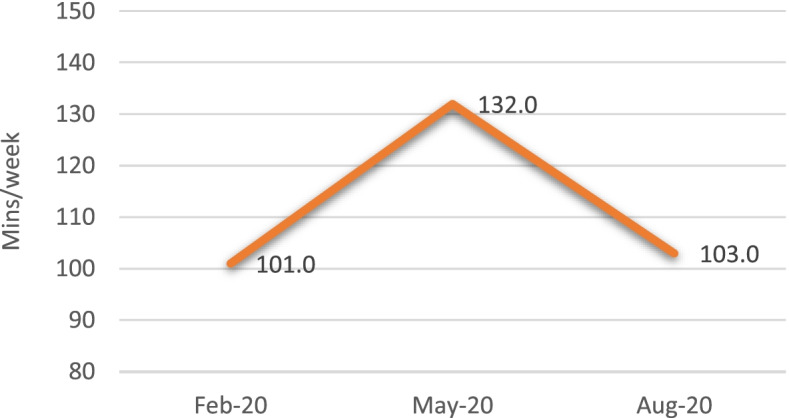
Average physical exercise time (minutes per week), self-reported by participants in the monthly calendars
Discussion
This study found that before the lockdown, the participants undertook an average of 101.0 min per week of exercise (67% of the 150 min of weekly activity minutes recommended by the WHO [6]). Yet during the lockdown, participants increased the amount of physical exercise they undertook to an average of 132.0 min per week (88% of the WHO recommended amount). Once the lockdown restrictions were lifted, their levels of exercise returned close to pre-lockdown levels.
Our results are in contrast with existing research around physical activity levels during the COVID-19 pandemic, which seems to suggest that the overall population has experienced an increase in sedentariness [19–22]. A study with people with Mild Cognitive Impairment (MCI) also found a similar trend, with more than a third of one hundred and twenty-six participants reporting a reduction in physical activity and 70% reporting an increase in idle time [22]. The main difference with the previous studies is that our participants were consistently (i.e., up to one remote therapy session per week) supported throughout the lockdown.
In line with other programmes successfully increasing physical fitness in people living with dementia, [23, 24] and with findings from our companion paper, [14] we can speculate that PrAISED might have provided a resource that participants could make use of during lockdown to boost activity levels. The increase in activity levels might have been compounded by a lack of alternative activities (e.g., going out for walks) during lockdown, which may have made the participants more willing to “pass time” at home by doing something beneficial. In-home caregivers might also have spent more time at home during lockdown and might have been instrumental in encouraging this virtuous trend. In the absence of scientific literature comparing lockdown with post-lockdown activity levels, we can only speculate that with the return to “normality” post-lockdown, the participants reverted back to their pre-lockdown routines (and exercise trends).
Results from this study must be interpreted with caution, owing to a number of limitations. External validity is limited by the fact that the analysis focused on a small sample, a sub-set of the PrAISED study participants (i.e., those who were in the intervention group in PrAISED during the lockdown). Given the carefully designed nature of the PrAISED intervention, our results should not be assumed to be generalisable to all forms of activity support to people living with dementia, let alone to people without dementia. Additionally, because the PrAISED participants were supported during lockdown, findings about the increase in activity levels emerging from this study should not be generalized to people living with dementia who remained unsupported during lockdown. Another potential limitation pertains to the reliability of self-reported data on exercise levels in the calendars, which may have been affected by social desirability bias on the part of participants. However, it is assumed that, if occurring, social desirability bias would be consistent across the three timepoints, thus not impacting on changes of reported exercise level by the same participant. Finally, the PrAISED RCT has not ended, and so we are yet unable to determine the effect of increased activity, if any, upon health outcomes and well-being.
Nevertheless, results from this study present some important practical implications. The finding that the efficacy of the PrAISED intervention to increase activity was enhanced when the intervention delivery mechanism switched from face-to-face to remote during the lockdown raises the possibility that future service implementation could usefully include some amount of remote clinician-client contact. In the context of PrAISED, unfortunately, remote contact meant primarily telephone (84.3% of sessions were delivered through the phone in the lockdown months of May and August 2020), as most participants living with dementia experience a digital divide that made videoconferencing an option for the least cognitively deteriorated, most tech savvy and / or with a caregiver who can fully support them to navigate digital systems [25].
However, there is accumulating evidence on the benefits of health services via electronic information and telecommunication technologies in people living with dementia. Provided adequate infrastructure and support, digital interventions including exergaming, [26–31] computerised cognitive training, [32–34] assistive technology, [35] teleassistance, [36] and dyadic (i.e., person living with dementia and caregiver) care support, [37] have been linked to improvements in executive functioning, maintenance of physical abilities, reduction of frailty, delayed admission to care and service optimisation (reduction of staff travel time).
Conclusion
Despite the limitations of this small, self-reported study, study finding suggest the need to further implementation of effective and inclusive individualised remote support for people living with dementia to help them increase their level of activity towards levels necessary to maintain their health in any circumstance where face-to-face support is unavailable. Investment in addressing the current challenges with telehealth should be prioritised, so that this model can be more widely implemented and successfully used in circumstances of social distancing or to ensure equitability of services with clients living with dementia who risk being underserved (e.g., rural areas).
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- PrAISED
Promoting Activity, Independence and Stability in Early Dementia
- SD
Standard Deviation
- WHO
World Health Organisation
- RCT
Randomised controlled Trial
- MoCA
Montreal Cognitive Assessment
- NHS
National Health Service
- PPI
Patient and Public Involvement
- MCI
Mild Cognitive Impairment
Authors’ contributions
CDL contributed to the conception and design of the study, data cleaning, data analysis, and the write up of the manuscript. VvdW contributed to the conception and design of the study and provided feedback on the manuscript. ROB contributed to data validation and writing the manuscript. JG contributed to the interpretation of results and provided feedback on the manuscript. TM contributed to the conception of the study and provided feedback on the manuscript. RHH contributed to the conception and design of the study and provided feedback and final approval of the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Reference Number RP-PG-0614- 20007). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available to safeguard anonymity of participants, but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted according to the Declaration of Helsinki and The University of Nottingham’s Code of Research Conduct and Research Ethics. The PrAISED RCT has received ethical approval number 18/YH/0059 from the Bradford/Leeds Ethics Committee. At the beginning of their involvement in PrAISED, participants were asked to sign a form consenting to the data collected during the RCT to be used in research outputs.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martyr A, Clare L. Executive function and activities of daily living in Alzheimer’s disease: a correlational meta-analysis. Dement Geriatr Cogn Disord. 2012;33(2–3):189–203. doi: 10.1159/000338233. [DOI] [PubMed] [Google Scholar]
- 2.Giebel CM, Sutcliffe C, Stolt M, Karlsson S, Renom-Guiteras A, Soto M, Verbeek H, Zabalegui A, Challis D. Deterioration of basic activities of daily living and their impact on quality of life across different cognitive stages of dementia: a European study. Int Psychogeriatr. 2014;26(8):1283–1293. doi: 10.1017/S1041610214000775. [DOI] [PubMed] [Google Scholar]
- 3.Giebel CM, Sutcliffe C, Challis D. Activities of daily living and quality of life across different stages of dementia: a UK study. Aging Ment Health. 2015;19(1):63–71. doi: 10.1080/13607863.2014.915920. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Research UK. About Dementia. Available online: https://www.alzheimersresearchuk.org/about-dementia/?gclid=Cj0KCQjw3JXtBRC8ARIsAEBHg4myuYeahFMLCcSGLr-flQxznmdB-dObW2gXc5MUN9o_dfNw5wwI5EwaAvH6EALw_wcB . Accessed 26 Mar 2020.
- 5.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126. [PMC free article] [PubMed] [Google Scholar]
- 6.Lam FM, Huang MZ, Liao LR, Chung RC, Kwok TC, Pang MY. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: a systematic review. J Physiother. 2018;64(1):4–15. doi: 10.1016/j.jphys.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Global recommendations on physical activity for health. World Health Organization; 2010. [PubMed]
- 8.Gibson-Moore H. UK Chief Medical Officers’ physical activity guidelines 2019: What’s new and how can we get people more active? Nutr Bull. 2019;44(4):320–328. doi: 10.1111/nbu.12409. [DOI] [Google Scholar]
- 9.Bajwa RK, Goldberg SE, Van der Wardt V, Burgon C, Di Lorito C, Godfrey M, Dunlop M, Logan P, Masud T, Gladman J, Smith H. A randomised controlled trial of an exercise intervention promoting activity, independence and stability in older adults with mild cognitive impairment and early dementia (PrAISED)-A Protocol. Trials. 2019;20(1):1–1. doi: 10.1186/s13063-019-3871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health England. Guidance on social distancing for everyone in the UK. Available at: https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults Accessed on 24 Mar 2020.
- 11.Trabelsi K, Ammar A, Masmoudi L, Boukhris O, Chtourou H, Bouaziz B, Brach M, Bentlage E, How D, Ahmed M, Mueller P. Sleep quality and physical activity as predictors of mental wellbeing variance in older adults during COVID-19 lockdown: ECLB COVID-19 international online survey. Int J Environ Res Public Health. 2021;18(8):4329. doi: 10.3390/ijerph18084329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trabelsi K, Ammar A, Masmoudi L, Boukhris O, Chtourou H, Bouaziz B, Brach M, Bentlage E, How D, Ahmed M, Mueller P. Globally altered sleep patterns and physical activity levels by confinement in 5056 individuals: ECLB COVID-19 international online survey. Biol Sport. 2021;38(4):495. doi: 10.5114/biolsport.2021.101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EP, Man RE, Gan TL, Fenwick EK, Aravindhan A, Ho KC, et al. The longitudinal psychological, physical activity, and financial impact of a COVID‐19 lockdown on older adults in Singapore: The PIONEER‐COVID population‐based study. Int J Geriatr Psychiatry. 2022;37(1):1–10. [DOI] [PMC free article] [PubMed]
- 14.Di Lorito C, Masud T, Gladman J, Godfrey M, Dunlop M, Bosco A, Harwood RH. Deconditioning in people living with dementia during the COVID-19 pandemic: qualitative study from the Promoting Activity, Independence and Stability in Early Dementia (PrAISED) process evaluation. BMC Geriatr. 2021;21(1):1. doi: 10.1186/s12877-021-02451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 16.Dunn P, Allen L, Cameron G, Alderwick H. Covid-19 policy tracker: a timeline of national policy and health system responses to covid-19 in England. 2020. https://www.health.org.uk/news-and-comment/charts-and-infographics/covid-19-policy-tracker Accessed on 30 Nov 2020.
- 17.van der Wardt V, Hancox JE, Burgon C, Bajwa R, Goldberg S, Harwood RH. Measuring physical activity levels in people with mild cognitive impairment or mild dementia. J Aging Phys Act. 2020;1(1):1–7. doi: 10.1123/japa.2019-0234. [DOI] [PubMed] [Google Scholar]
- 18.IBM SPSS® . Statistics for Windows, Version 25.0. Armonk: IBM Corp; 2017. [Google Scholar]
- 19.Castañeda-Babarro A, Arbillaga-Etxarri A, Gutiérrez-Santamaría B, Coca A. Physical activity change during COVID-19 confinement. Int J Environ Res Public Health. 2020;17(18):6878. doi: 10.3390/ijerph17186878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer J, McDowell C, Lansing J, Brower C, Smith L, Tully M, Herring M. Changes in physical activity and sedentary behavior in response to COVID-19 and their associations with mental health in 3052 US adults. Int J Environ Res Public Health. 2020;17(18):6469. doi: 10.3390/ijerph17186469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tison GH, Avram R, Kuhar P, Abreau S, Marcus GM, Pletcher MJ, Olgin JE. Worldwide effect of COVID-19 on physical activity: a descriptive study. Ann Intern Med. 2020;173(9):767–770. doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Santo SG, Franchini F, Filiputti B, Martone A, Sannino S. The effects of COVID-19 and quarantine measures on the lifestyles and mental health of people over 60 at increased risk of dementia. Front Psychiatry. 2020;11. [DOI] [PMC free article] [PubMed]
- 23.Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, Dosanjh S, Slowther AM, Khan I, Petrou S, Lall R. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:k1675. [DOI] [PMC free article] [PubMed]
- 24.Pitkälä KH, Pöysti MM, Laakkonen ML, Tilvis RS, Savikko N, Kautiainen H, Strandberg TE. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med. 2013;173(10):894–901. doi: 10.1001/jamainternmed.2013.359. [DOI] [PubMed] [Google Scholar]
- 25.Di Lorito C, Duff C, Rogers C, Tuxworth J, Bell J, Fothergill R, Wilkinson L, Bosco A, Howe L, O’brien R, Godfrey M. Tele-Rehabilitation for people with dementia during the COVID-19 Pandemic: a case-study from England. Int J Environ Res Public Health. 2021;18(4):1717. doi: 10.3390/ijerph18041717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson-Hanley C, Barcelos NM, Zimmerman EA, Gillen RW, Dunnam M, Cohen BD, Yerokhin V, Miller KE, Hayes DJ, Arciero PJ, Maloney M. The aerobic and cognitive exercise study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front Aging Neuroscience. 2018;4(10):76. doi: 10.3389/fnagi.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh CC, Lin PS, Wei YT, Huang YC. Tai chi-based exergaming program for older adults at risk of cognitive impairment. Physiotherapy. 2015;1(101):e592. doi: 10.1016/j.physio.2015.03.3418. [DOI] [Google Scholar]
- 28.Karssemeijer EG, Bossers WJ, Aaronson JA, Sanders LM, Kessels RP, Rikkert MG. Exergaming as a physical exercise strategy reduces frailty in people with dementia: a randomized controlled trial. J Am Med Dir Assoc. 2019;20(12):1502–1508. doi: 10.1016/j.jamda.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Padala KP, Padala PR, Lensing SY, Dennis RA, Bopp MM, Roberson PK, Sullivan DH. Home-based exercise program improves balance and fear of falling in community-dwelling older adults with mild Alzheimer’s disease: a pilot study. J Alzheimers Dis. 2017;59(2):565–574. doi: 10.3233/JAD-170120. [DOI] [PubMed] [Google Scholar]
- 30.Swinnen N, Vandenbulcke M, de Bruin ED, Akkerman R, Stubbs B, Firth J, Vancampfort D. The efficacy of exergaming in people with major neurocognitive disorder residing in long-term care facilities: a pilot randomized controlled trial. Alzheimer's Res Therapy. 2021;13(1):1–3. doi: 10.1186/s13195-021-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert P, Albrengues C, Fabre R, Derreumaux A, Pancrazi MP, Luporsi I, Dubois B, Epelbaum S, Mercier G, Foulon P, Bremond F. Efficacy of serious exergames in improving neuropsychiatric symptoms in neurocognitive disorders: Results of the X-TORP cluster randomized trial. Alzheimer's Dementia: Trans Res Clin Interv. 2021;7(1):e12149. doi: 10.1002/trc2.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahar-Fuchs A, Webb S, Bartsch L, Clare L, Rebok G, Cherbuin N, Anstey KJ. Tailored and adaptive computerized cognitive training in older adults at risk for dementia: a randomized controlled trial. J Alzheimers Dis. 2017;60(3):889–911. doi: 10.3233/JAD-170404. [DOI] [PubMed] [Google Scholar]
- 33.Manenti R, Gobbi E, Baglio F, Macis A, Ferrari C, Pagnoni I, Rossetto F, Di Tella S, Alemanno F, Cimino V, Binetti G. Effectiveness of an innovative cognitive treatment and telerehabilitation on subjects with mild cognitive impairment: a multicenter, randomized, active-controlled study. Front Aging Neuroscience. 2020;16(12):400. doi: 10.3389/fnagi.2020.585988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira J, Gamito P, Souto T, Conde R, Ferreira M, Corotnean T, Fernandes A, Silva H, Neto T. Virtual reality-based cognitive stimulation on people with mild to moderate dementia due to Alzheimer’s disease: a pilot randomized controlled trial. Int J Environ Res Public Health. 2021;18(10):5290. doi: 10.3390/ijerph18105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard R, Gathercole R, Bradley R, Harper E, Davis L, Pank L, Lam N, Talbot E, Hooper E, Winson R, Scutt B. The effectiveness and cost-effectiveness of assistive technology and telecare for independent living in dementia: a randomised controlled trial. Age Ageing. 2021;50(3):882–890. doi: 10.1093/ageing/afaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tchalla AE, Lachal F, Cardinaud N, Saulnier I, Rialle V, Preux PM, Dantoine T. Preventing and managing indoor falls with home-based technologies in mild and moderate Alzheimer's disease patients: pilot study in a community dwelling. Dement Geriatr Cogn Disord. 2013;36(3–4):251–261. doi: 10.1159/000351863. [DOI] [PubMed] [Google Scholar]
- 37.Laver K, Liu E, Clemson L, Davies O, Gray L, Gitlin LN, Crotty M. Does telehealth delivery of a dyadic dementia care program provide a noninferior alternative to face-to-face delivery of the same program? A randomized, controlled trial. Am J Geriatr Psychiatry. 2020;28(6):673–682. doi: 10.1016/j.jagp.2020.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available to safeguard anonymity of participants, but are available from the corresponding author on reasonable request.



