Summary
Within pregnancies occurring between 1986 and 2017 in Dutch kidney transplant recipients (KTR), we retrospectively compared short‐term maternal and foetal outcomes between patients on calcineurin inhibitor (CNI) based (CNI+) and CNI‐free immunosuppression (CNI−). We identified 129 CNI+ and 125 CNI− pregnancies in 177 KTR. Demographics differed with CNI+ having higher body mass index (P = 0.045), shorter transplant‐pregnancy interval (P < 0.01), later year of transplantation and ‐pregnancy (P < 0.01). Serum creatinine levels were numerically higher in CNI+ in all study phases, but only reached statistical significance in third trimester (127 vs. 105 µm; P < 0.01), where the percentual changes from preconceptional level also differed (+3.1% vs. −2.2% in CNI−; P = 0.05). Postpartum both groups showed 11–12% serum creatinine rise from preconceptional level. Incidence of low birth weight (LBW) tended to be higher in CNI+ (52% vs. 46%; P = 0.07). Both groups showed equal high rates of preterm delivery. Using CNIs during pregnancy lead to a rise in creatinine in the third trimester but does not negatively influence the course of graft function in the first year postpartum or direct foetal outcomes. High rates of preterm delivery and LBW in KTR, irrespective of CNI use, classify all pregnancies as high risk.
Keywords: azathioprine, calcineurin inhibitors, immunosuppression, kidney transplantation, Netherlands, pregnancy outcome, retrospective studies
A retrospective study with all Dutch pregnant kidney transplant recipients in 1986–2017, where maternal and foetal pregnancy outcomes were compared between patients using a CNI immunosuppressive regimen and patients, not using CNIs.

Introduction
Thousands of pregnancies in kidney transplant recipients (KTR) have been documented since the first successful pregnancy in 1958 [1]. Pregnancy after kidney transplantation (KT) is considered high risk because of possible foetal and maternal complications, such as preterm deliveries (43–51%), low birth weight (LBW) (42%) and pre‐eclampsia (22–32%) [2, 3]. These risks increase in case of preconceptional hypertension, poor or unstable graft function and/or relevant proteinuria [4]. Moreover, pregnancy is generally discouraged in the first year after transplantation because of the increased risk of acute rejection and infection in this period [5]. Despite the higher incidence of pregnancy complications in KTR, most pregnancies have good outcomes for mother and child. The majority of children are reported as healthy and the reported risk of graft loss or rejection after delivery is approximately 10% [2, 4, 6]. Unfortunately, most data on these pregnancies come from voluntary registries, such as the Transplant Pregnancy Registry (USA), and are subject to reporting bias and/or include small numbers of patients [2, 7, 8]. Physicians are in need of more objective data on pre‐pregnancy risk factors, pregnancy complications and outcomes in an unselected population of pregnant KTRs.
Immunosuppressive medication is one of the important aspects in (pre)pregnancy considerations, and therefore, information is needed to decide which immunosuppressive agents are preferred during pregnancy. Most medications are considered relatively safe with the exception of mycophenolate mofetil (MMF), because of its high incidence of spontaneous abortion and birth defects [7]. Although azathioprine has an old FDA pregnancy rating D whereas calcineurin inhibitors (CNIs, i.e., tacrolimus and cyclosporin) have FDA rate C, available data suggest that both drugs are safe and well tolerated during pregnancy, which will be included in the future in the more descriptive FDA Pregnancy and Lactation Labeling Rule. However, detailed information about possible negative effects of the immunosuppressive regimens during pregnancy is still lacking. Nowadays, CNIs are often the mainstay of the immunosuppressive regimen in KTR. However, CNIs have vasoconstrictive properties that are associated with nephrotoxicity and hypertension, which are absent with azathioprine [9, 10, 11]. We, therefore, hypothesize that pregnant KTR using CNIs (CNI+) are at higher risk to develop vascular complications (e.g. hypertension and increased serum creatinine) and consequently possibly also higher rates of foetal complications (LBW, preterm delivery) compared to patients using a CNI‐free regimen (CNI−). Therefore, the aim of this study was to compare maternal and foetal outcomes of pregnancies in KTR using either a CNI+ or CNI− immunosuppressive regimen.
Methods
In this retrospective cohort study, we included data of pregnancies in KTR after widespread introduction of CNIs in the mid‐eighties from all seven university medical centres in The Netherlands (Radboud University Medical Center (UMC) Nijmegen, UMC Groningen, Erasmus Medical Center Rotterdam, Amsterdam UMC (VU/AMC), Leiden UMC, Maastricht UMC and UMC Utrecht). Patients were closely monitored in one of the university medical centres during pregnancy with regular check‐ups on both the obstetric and nephrology departments. All KTR with singleton pregnancies ending in live birth in 1986–2017 were eligible for inclusion. Pregnancies ending in intrauterine foetal death (IUFD) and pregnancies with unknown immunosuppressive regimen were excluded (Fig. 1). The study was conducted retrospectively with anonymously analysed data and approval of Ethical review boards of all transplant centres was obtained (MEC‐2014‐077, MEC‐2015‐2262, MEC‐2016‐014, MEC‐2016‐021, MEC‐2016‐634).
Figure 1.
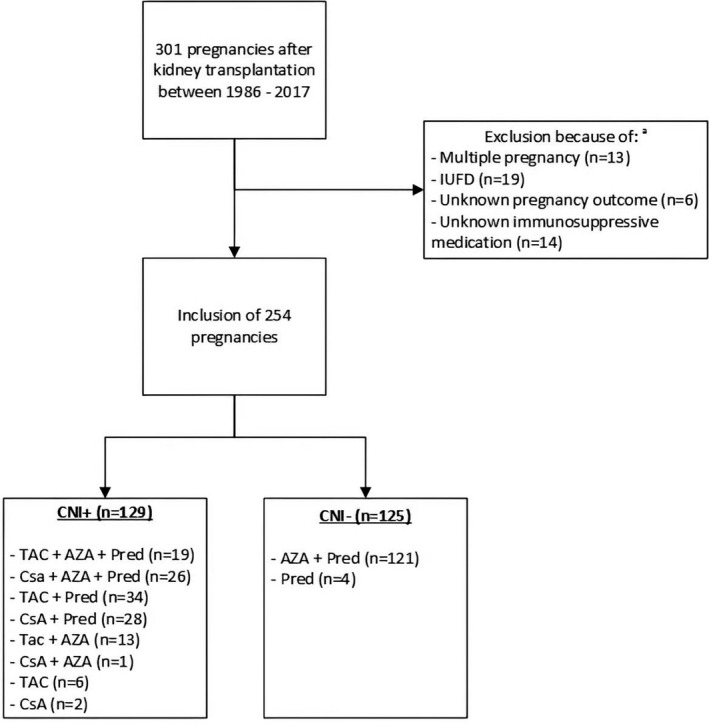
Flowchart of exclusion of Dutch pregnancies after kidney transplantation in this study. aOverlap in exclusion criteria. AZA: azathioprine, CNI+: calcineurin inhibitor use during pregnancy, CNI−: no calcineurin inhibitor use during pregnancy, CsA: cyclosporin, IUFD: Intra‐uterine foetal death, Pred: prednisolone, TAC: tacrolimus.
Data collection and management was performed using OpenClinica open‐source software. Nephrologic and obstetric characteristics were collected retrospectively by checking digital‐ and paper medical records. For maternal characteristics, we collected data on demographics, KT and pregnancy (Table 1). For every trimester of pregnancy, mean serum creatinine value and mean blood pressure level were calculated from all available values in that trimester. For our analysis on the postpartum serum creatinine value, we used the first value available between 6 and18 months postpartum, since it takes approximately 6 months for the serum creatinine level to stabilize after delivery.
Table 1.
Demographic characteristics of kidney transplant recipients with pregnancies between 1986 and 2017 in the Netherlands.
| Variable |
CNI+ Mean ± SD |
CNI− Mean ± SD |
P‐value | |
|---|---|---|---|---|
| General | Number of pregnancies (N (%)) | 129 (51) | 125 (49) | NA |
| Age at delivery, years | 32.3 ± 4.1 | 31.4 ± 4.7 | 0.088 | |
| Mean BMI, kg/m2 | 25.4 ± 4.6 | 23.8 ± 3.2 | 0.045 | |
| Underlying diagnosis, leading to ESRD | ||||
| Glomerulonephritis | 38 (30) | 52 (42) | 0.214 | |
| Tubulo‐interstitial | 24 (19) | 15 (12) | ||
| Multi‐cystic kidney diseases | 10 (8) | 6 (5) | ||
| Other congenital and hereditary | 16 (12) | 19 (15) | ||
| Renal vascular disease | 4 (3) | 8 (6) | ||
| Auto‐immune (e.g. SLE/vasculitis) | 7 (5) | 8 (6) | ||
| Diabetes mellitus | 5 (4) | 0 (0) | ||
| Urologic | 7 (5) | 4 (3) | ||
| Unknown | 18 (14) | 13 (11) | ||
| Transplant | Year of kidney transplantation | 2000 ± 9 | 1989 ± 11 | <0.01 |
| Type of transplant donor (N (% of patients with known type of donor)) | ||||
| Living donor | 58 (48) | 44 (29) | 0.161 | |
| Deceased donor | 62 (52) | 77 (71) | ||
| Unknown type of donor | 9 | 4 | ||
| Preconceptional | Last creatinine before conception, µm | 123 ± 45 | 112 ± 69 | 0.136 |
| Presence of hypertension before pregnancy (N (%)) | 70 (54) | 63 (50) | 0.823 | |
| Pregnancy | Year of pregnancy | 2006 ± 8 | 1998 ± 11 | <0.01 |
| Time between transplantation and pregnancy, months | 69 ± 49 | 104 ± 76 | <0.01 | |
| Mean number of transplants before pregnancy | 1.3 ± 0.5 | 1.2 ± 0.5 | 0.365 | |
| Nature of pregnancy (N (% of patients with known type of pregnancy)) | ||||
| Planned pregnancy | 81 (79) | 58 (72) | 0.276 | |
| Unplanned pregnancy | 21 (21) | 22 (28) | ||
| Unknown nature | 27 | 45 | ||
| Number of pregnancies after transplantation (N (%)) | ||||
| First pregnancy | 91 (71) | 83 (66) | 0.347 | |
| Second pregnancy | 37 (28) | 38 (31) | ||
| Third pregnancy | 1 (1) | 4 (3) |
BMI, Body mass index; CNI+, calcineurin inhibitor use during the first trimester of pregnancy; CNI−, no calcineurin inhibitor use during the first trimester of pregnancy; ESRD, end‐stage renal disease; N, number.
Hypertension before pregnancy was defined as a systolic blood pressure (SBP) >140 mmHg, a diastolic blood pressure (DBP) >90 mmHg and/or the use of antihypertensive medication [12]. Gestational hypertension was defined as new‐onset hypertension after 20 weeks of gestation. Diagnosis of pre‐eclampsia could unfortunately not be established unambiguously in most of our pregnant transplant patients because of the incomplete registration of proteinuria. As surrogate measure, we compared the number of patients with a diagnosis of pre‐eclampsia as registered by the responsible physician.
For neonatal outcomes, we collected data on gestational age and birth weight. Preterm delivery was defined as delivery before 37 weeks of gestation and very preterm delivery as before 34 weeks of gestation [13]. LBW was defined as a birth weight <2500 g and very low birth weight as <1500 g [14].
All patients included were divided into two groups depending on their immunosuppressive regimen in the first trimester of pregnancy: patients using CNIs (CNI+) and patients not using CNIs (CNI−).
Statistical analyses were performed with SPSS version 22 (IBM, Amornk, NY, USA). Data were described as mean (standard deviation (SD)). Categorical variables were described as frequencies with percentages. Analyses were done using one sample T‐tests and Chi‐squared tests. In all analyses, a P‐value <0.05 was considered statistically significant. Logistic regression analysis was used to correct for differences in baseline characteristics. Odds Ratios (OR) were calculated for the risk of LBW and for >20% creatinine rise during pregnancy in CNI+ compared to CNI−.
Results
In 1986–2017, we identified 301 pregnancies in KTR in the Netherlands, from which 47 pregnancies had to be excluded (Fig. 1). The excluded IUFDs (12 in CNI+ vs. 9 in CNI−; P = 0.418) and the mean gestational age of the IUFD (178 days in CNI+ vs. 213 in CNI−; P = 0.084) were equally distributed. Of the remaining 254 pregnancies, 129 were CNI+ and 125 were CNI−. In CNI+, 72 patients used a tacrolimus‐based regimen and 57 patients a cyclosporin‐based regimen. In CNI−, 121 patients used azathioprine in combination with prednisolone and four patients used prednisolone as monotherapy (Fig. 1). Three patients were using MMF in the first 4 weeks of pregnancy; however, two patients switched to CNI directly after discovering the pregnancy, one switched to azathioprine.
We included 174 first pregnancies, 75 second pregnancies and five third pregnancies, equally distributed between CNI+ and CNI− (Table 1). CNI+ and CNI− groups were comparable except for the year of KT, which was performed, on average, 11 years later in the CNI+ group, and the mean time between transplantation and pregnancy, which was 35 months shorter in CNI+. In addition, the mean body mass index (BMI) was significantly higher in CNI+ and the mean year of pregnancy was 8 years later in CNI+.
Figure 2 shows serum creatinine levels before pregnancy, during all three trimesters and postpartum. Preconceptionally, in the first and second trimester of pregnancy, creatinine levels were slightly, but not significantly higher in CNI+. In the third trimester, creatinine levels were significantly higher in CNI+ (127 ± 54 µm vs. 105 ± 42 µm in CNI−; P < 0.01). This difference in creatinine levels was no longer detected 6–18 months postpartum, but unfortunately, we were not able to obtain creatinine levels of all patients at this stage, resulting in 31% missing data (33% in CNI+ vs. 30% in CNI−). The average time between child birth and the first included postpartum serum creatinine did not differ between the two study arms (353 ± 11 days in CNI+ vs. 357 ± 11 days in CNI−; P = 0.8).
Figure 2.
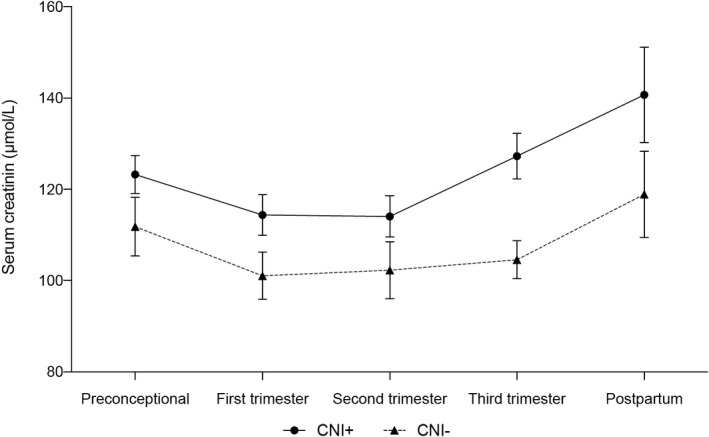
Serum creatinine levels during CNI+ and CNI− pregnancies. ∼ First, second and third trimester: mean of all values in that trimester, found in medical records. ^ Postpartum: measured at the first check‐up between 6 and 18 months postpartum. P‐values: last before conception: P = 0.14, first trimester: P = 0.05, second trimester: P = 0.14, third trimester: P < 0.01, postpartum: P = 0.12. CNI+: calcineurin inhibitor use during pregnancy, CNI−: no calcineurin inhibitor use during pregnancy.
Preconceptionally, there was no difference in the presence of hypertension between CNI+ and CNI− patients nor in the amount of antihypertensive drugs used (Table 1). Mean blood pressure levels were comparable in both groups preconceptionally and also in all trimesters of pregnancy (Table 2). During the third trimester, highest values of SBP, but not DBP tended to be higher in CNI+ patients (P = 0.08). Moreover, in CNI+ significantly more patients used antihypertensive medication during pregnancy (72% in CNI+ vs. 52% in CNI−, P = 0.04). No statistically significant differences were found in the percentage of patients with a diagnosis of pre‐eclampsia in the two groups during pregnancy (28% in CNI− vs. 40% in CNI+; P = 0.07).
Table 2.
Maternal pregnancy outcomes.
| Variable |
CNI+ N = 129 |
CNI− N = 125 |
P‐value |
|---|---|---|---|
| Antihypertensive medication use during pregnancy (N (% of patients with known history)) | |||
| Use of antihypertensive medication | 93 (80) | 65 (68) | 0.04 |
| No antihypertensive medication | 23 (20) | 31 (32) | |
| Unknown | 13 | 29 | |
| Systolic blood pressures during pregnancy (mmHg ± SEM)* | |||
| Preconceptional (N = 214) | 125 ± 1 | 122 ± 1 | 0.23 |
| First trimester, mean (N = 191) | 123 ± 1 | 124 ± 1 | 0.37 |
| Second trimester, mean (N = 197) | 123 ± 1 | 123 ± 1 | 0.87 |
| Third trimester, mean (N = 188) | 130 ± 1 | 127 ± 2 | 0.20 |
| Third trimester, highest (N = 183) | 147 ± 2 | 142 ± 2 | 0.08 |
| Diastolic blood pressures during pregnancy (mmHg ± SEM)* | |||
| Preconceptional (N = 214) | 80 ± 1 | 80 ± 1 | 0.99 |
| First trimester, mean (N = 191) | 77 ± 1 | 80 ± 1 | 0.06 |
| Second trimester, mean (N = 197) | 77 ± 1 | 76 ± 1 | 0.17 |
| Third trimester, mean (N = 188) | 83 ± 1 | 80 ± 1 | 0.05 |
| Third trimester, highest (N = 183) | 93 ± 1 | 90 ± 2 | 0.12 |
| Pre‐eclampsia during pregnancy (N (% of patients with known history)) | |||
| Pre‐eclampsia | 46 (40) | 28 (30) | 0.07 |
| No pre‐eclampsia | 69 (60) | 71 (70) | |
| Unknown pre‐eclampsia | 14 | 26 | |
| Rejection and graft loss during pregnancy & follow‐up | |||
| Rejection therapy given during pregnancy (N (%)) | 2 (1.6) | 1 (0.8) | 0.62 |
| Graft loss during pregnancy (N) | 0 | 0 | NA |
| Graft loss in the first 5 years after pregnancy (N (%)) | 10 (8) | 14 (11) | 0.81 |
| Graft loss within 10 years after pregnancy (N (%)) | 15 (12) | 21 (17) | 0.70 |
| Graft loss within 15 years after pregnancy (N (%)) | 19 (15) | 24 (19) | 0.13 |
| Graft loss within 25 years after pregnancy (N (%)) | 20 (16) | 27 (22) | 0.14 |
CNI+, calcineurin inhibitor use during the first trimester of pregnancy; CNI−, no calcineurin inhibitor use during the first trimester of pregnancy; N, number of pregnancies with available data for that period; NA, not applicable.
Missing values were equally distributed in CNI+ and CNI− arms of the study.
Three patients needed rejection therapy during pregnancy, two in the CNI+ arm (one patient received corticosteroids, in the other therapy was unknown) and one in the CNI− arm (unknown therapy). There were no cases of graft loss during pregnancy, but 24 patients lost their graft in the first 5 years after pregnancy, all due to chronic graft dysfunction (10 patients (7.8%) in CNI+ vs. 14 patients (11.2%) in CNI−; P = 0.35). The interval between delivery and graft loss was significantly shorter in CNI+ (1.2 years vs. 3.1 years in CNI−; P < 0.01) (Table 2). We provided data on graft loss within 10, 15 and 25 years, but since the total follow‐up duration differs between both arms of the study, no statistical conclusion can be taken of these data. Unfortunately, we only retrieved information of four patients that underwent biopsy, all showing interstitial fibrosis and tubular atrophy.
Table 3 describes percentual changes in creatinine levels throughout pregnancy. The percentual change in creatinine levels from preconception to the third trimester was slightly different between the two groups (+3.1 ± 19.1 in CNI+ vs. −2.2 ± 20.6 in CNI−; P = 0.05). When comparing the change from preconceptional to 6–18 months postpartum, both groups show a similar increase of around 11–12% in the approximately 2‐year interval between the measurements. Additionally, we evaluated the number of patients whose creatinine levels increased >20% from the preconceptional level during the three trimesters of pregnancy. Only in the third trimester, the number of patients with >20% increase of creatinine was slightly but not significantly higher in the CNI+ group. However, after correcting for differences in demographics, this difference was no longer observed (OR 2.06 (95% Confidence Interval (CI) 0.62–6.89)). When comparing the postpartum to the preconceptional period, the number of patients with >20% increase in serum creatinine did not differ between CNI+ and CNI−.
Table 3.
Change in creatinine levels throughout pregnancy.
| Variable |
CNI+ N = 129 |
CNI− N = 125 |
P‐value | Correction for confounders OR (95% CI) |
|---|---|---|---|---|
| Percentual change in creatinine levels (% ± SD (N)) | ||||
| Preconceptional – first trimester |
−8.5 ± 9.7 (N = 103) |
−9.9 ± 10.0 (N = 106) |
0.328 | |
| Preconceptional – second trimester |
−8.0 ± 20.9 (N = 110) |
−9.8 ± 16.7 (N = 105) |
0.468 | |
| Preconceptional – third trimester |
+3.1 ± 19.1 (N = 113) |
−2.2 ± 20.6 (N = 99) |
0.051 | |
| Preconceptional – postpartum |
+11.9 ± 44.3 (N = 81) |
+11.3 ± 33.8 (N = 83) |
0.922 | |
| Number of patients with >20% increase in creatinine levels (n/N (%)) | ||||
| Preconceptional – first trimester | 0/103 (0.0) | 1/106 (0.9) | 0.323 | NA |
| Preconceptional – second trimester | 4/110 (3.6) | 4/105 (3.8) | 0.947 | 4.52* (0.52–39.41) |
| Preconceptional – third trimester | 19/113 (16.8) | 8/99 (8.1) | 0.057 | 2.06† (0.62–6.89) |
| Preconceptional – postpartum | 19/81 (23.5) | 16/83 (19.3) | 0.514 | 1.00‡ (0.33–3.06) |
CNI+, calcineurin inhibitor use during the first trimester of pregnancy; CNI−, no calcineurin inhibitor use during the first trimester of pregnancy; N, number of pregnancies with available data for that period; NA, not applicable.
Confounders found in univariate logistic regression: BMI, year of pregnancy, year of renal transplantation, time between renal transplantation and pregnancy.
Confounders found in univariate logistic regression: BMI.
Confounders found in univariate logistic regression: BMI, time between renal transplantation and pregnancy.
Table 4 shows neonatal outcomes. Of all deliveries, approximately 50% were spontaneous or assisted vaginal deliveries and 50% were caesarean sections, all equally distributed between CNI+ and CNI−. The mean gestational age in CNI+ and CNI− was identical (253 days). The incidence of (very) preterm delivery between the two groups was comparable.
Table 4.
Neonatal outcomes in kidney transplant recipients’ offspring.
| Variable |
CNI+ N = 129 N (%) |
CNI− N = 125 N (%) |
P‐value |
|---|---|---|---|
| Mode of delivery (N (% of patients with known history) | |||
| Vaginal delivery* | 57 (47) | 60 (54) | 0.472 |
| Caesarean section | 64 (53) | 52 (46) | |
| Unknown | 8 | 13 | |
| Birth weight | |||
| Mean birth weight (grams ± SD) | 2434 ± 782 | 2541 ± 782 | 0.293 |
| Birth weight <2500 g | 64 (52) | 46 (40) | 0.072 |
| Birth weight <1500 g | 15 (12) | 12 (10) | 0.686 |
| Gestational age | |||
| Mean gestational age (days ± SD) | 253 ± 25 | 253 ± 26 | 0.956 |
| Delivery <37 weeks of gestation | 59 (49) | 53 (45) | 0.551 |
| Delivery <34 weeks of gestation | 26 (22) | 21 (18) | 0.473 |
CNI+, calcineurin inhibitor use during pregnancy; CNI−, no calcineurin inhibitor use during pregnancy; N, number.
Includes spontaneous or assisted vaginal delivery.
Mean birth weight of the offspring was similar in CNI+ and CNI−, but in CNI+ slightly more children were born with LBW (27% vs. 19% in CNI−; P = 0.07). In additional univariate analysis, none of the statistically significant differences in demographics had an influence on the difference in incidence of LBW in the two groups and, therefore, multivariate logistic regression was not possible.
The CNI+ group included both patients on a tacrolimus‐based regimen and cyclosporin‐based regimen. Therefore, we performed an additional subgroup analysis, comparing the two regimens separately with the CNI− group. This analysis could not detect any new statistically significant differences, nor did statistically significant differences disappear (data not shown).
Since this study covers a period of more than 30 years and significant changes in the fields of transplant nephrology and obstetrics have occurred in this time, we added an analysis in which we compared pregnancies in 1986–2001 with pregnancies in 2002–2017. In the latter era were more pregnancies after KT (106 in 1986–2001 vs. 148 in 2002–2017) with more living donors (13% in 1986–2001 vs. 64% in 2002–2017, P < 0.01). Mothers had more often pre‐existing hypertension (46% in 1986–2001 vs. 87% in 2002–2017, P = 0.01), higher systolic blood pressure in the first and second trimester and diastolic blood pressure in second trimester (all P = 0.01), and more often pre‐eclampsia (25% in 1986–2001 vs. 40% in 2002–2017, P = 0.02). Other maternal or neonatal outcomes did not differ between these two eras.
Discussion
In this relatively large retrospective study in unselected pregnant KTR, short‐term maternal and foetal pregnancy outcomes were compared between patients using a CNI+ immunosuppressive regimen and patients on a CNI− regimen. Maternal and foetal outcomes were compared in a unique population that included all KTR that became pregnant in an overlapping 30‐year time period after the widespread introduction of CNIs in the mid‐eighties in the Netherlands. Besides a borderline significant rise in serum creatinine in the CNI+ group in the third trimester that disappeared 6–18 months postpartum, we did not find differences in major maternal or foetal outcomes between the two groups.
The relatively short mean gestational age (36 weeks in both groups) and low mean birth weight (CNI+ 2434 vs. CNI− 2541 g) in our cohort are comparable to the 35.6 weeks and 2420 g described in a systematic review from 2011 about pregnancy after KT by Deshpande et al [4]. However, the rates of preterm birth (49% CNI+, 45% CNI−) and LBW (CNI+ 52%, CNI− 40%) are substantially lower in the general population of the Netherlands (7.2% and 6.2% respectively) [15].
When comparing postpartum serum creatinine to preconceptional levels with an inherent interval of on average 2 years, an 11–12% increase in serum creatinine was observed in our short‐term postpartum follow‐up, which is a little less than the 17–33% described in the literature [16, 17, 18]. However, in these studies, large SDs have been observed because only small numbers of patients, all using a CNI during pregnancy, were included. Without pregnancy, in a unselected population of KTR in the USRDS registry transplanted between 1987 and 1996, a general decline in eGFR of 1.66 ml/min/1.73 m2 per year from a baseline level of 49.6 ml/min/1.73 m2 was observed during a mean follow‐up of 6 years after transplantation, which indicates a decline in graft function of 3.3% per year [19]. Because the decrease in eGFR in patients with almost similar age is determined almost exclusively by the rise in serum creatinine, the decrease in eGFR in the USRDS registry can be compared to the increase of serum creatinine in our study. The 11–12% increase in serum creatinine observed in the 2‐year interval between postpartum and prepregnancy level in our study appears to be almost twice the expected 6.6% increase in creatinine from the USRDS registry, despite the fact that our cohort consists of young female patients that would be expected to have an even less decline in graft function without pregnancy. Our data, thus, might indicate that pregnancy is a risk factor for a more rapid loss of graft function in the short term, but should be confirmed by comparison of slope of creatinine decline during longer periods before and after pregnancy.
Although serum creatinine levels were somewhat higher in the third trimester in our CNI+, this difference between the two groups disappeared postpartum. Interestingly, in a study by Mohammadi et al., the risk of increased serum creatinine during pregnancy is substantially higher when preconceptional serum creatinine is 110–129 µm as compared with patients with preconceptional creatinine <110 µm (approximately 20% vs. 60% increase respectively) [20] which might indicate that the significant difference in rise of creatinine that we observed in the third trimester is not caused by the use of a CNI during pregnancy per se, but is rather a consequence of the higher preconceptional serum creatinine in the CNI+ group. In the study from Mohammadi, fitting our data of comparable postpartum creatinine levels in CNI+ and CNI− groups, the increase of creatinine 12 months postpartum between the two groups with different baseline creatinine levels was the same with approximately 20% [20].
Even though there were similar rates of preconceptional hypertension and antihypertensive drug use in both study groups, the highest SBP in the third trimester tended to be somewhat higher in CNI+ and significantly more patients needed antihypertensives during their pregnancy. This suggests that the use of a CNI during pregnancy is related to an increased risk of gestational hypertension and could also explain the slightly, but not significantly higher percentage of pre‐eclampsia diagnosis in our CNI+ patients.
Although it is well known that the use of CNIs is one of the main causes of hypertension after transplantation [11], we did not observe any differences in the percentage of patients with hypertension nor the amount of antihypertensive drugs between the CNI+ and the CNI− groups preconceptionally, which could be related to the fact that patients with difficult to treat hypertension were counselled to avoid pregnancy by their physician.
In our retrospective analyses, almost half of the patients became pregnant during azathioprine without a CNI, which represents a much higher percentage than, for example, reported in the USA, where only 24% did not use a CNI as primary immunosuppressant during pregnancy [21]. This difference likely occurs because in the Netherlands and in most other European countries, transplant physicians are less reluctant to replace a CNI by MMF or a mechanistic target of rapamycin (mTOR) inhibitor in case of decreasing transplant function due to chronic CNI toxicity, irrespective of the type of kidney donor. In patients considering pregnancy, the MMF or mTOR inhibitor will then have to be replaced by azathioprine to make pregnancy possible. Moreover, in the early years of CNI use between 1985 and 1995, in most patients, physicians performed elective conversion from cyclosporin to azathioprine 3 months after transplantation, in order to avoid chronic CNI toxicity. Hence, patients on azathioprine are a mix of patients transplanted before CNI was available, a positive selection of patients that endured elective conversion from cyclosporin to azathioprine, and a negative selection of patients where the CNI was replaced because of chronic CNI toxicity. Because of the small numbers involved, it is not possible to analyse these subgroups separately.
Previous research indicates that the risk of side effects declines with lower CNI dosage, for example, achieved by triple immunosuppressive therapy [9]. We did not include CNI dosage in our study, but in 35% of the pregnancies in the CNI+ group, the mother received triple therapy with probably lower dose of CNI. However, subgroup analysis without patients on triple therapy did not demonstrate differences between CNI+ and CNI− patients (data not shown).
Many changes have been made in transplant nephrology and obstetrics in the timespan of this study. Our subgroup analysis comparing outcomes of pregnancies between 1986–2001 and 2002–2017 only showed an increased number of pregnancies after KT, more living‐donor transplantations over time and more cases of hypertension before and during the first few months of pregnancy. All is in line of expectation, with literature already showing the increase in pregnancies after organ transplantation [22]. The benefits of living‐donor transplantations have already been proven [23] and the difference in hypertension before and during pregnancy is most likely related to development in medical knowledge as well. Over the years, we gained comprehension on the pathophysiology and treatments of hypertension during pregnancy and pre‐eclampsia, which made it possible for more KTR with hypertension to become pregnant. None of the above‐mentioned differences over time affected other maternal or neonatal outcomes. Therefore, although CNI+ and CNI− groups mainly became pregnant in different decades, it did not affect maternal and neonatal outcomes.
To the best of our knowledge, this is one of the first studies that compared the influence of a CNI−based and a CNI‐free immunosuppressive regimen on maternal and foetal outcomes in a 30‐year cohort of all pregnant KTR in the Netherlands. Because of the mandatory follow‐up of all KTR in all university medical centres in the Netherlands, we could analyse these effects in this unselected population and, hence, prevent selection bias. Even though the number of included patients is relatively high for studies on pregnancy in KTR, unfortunately sample size is still limited for firm statistical conclusions. Another main limitation of our study is its retrospective nature, leading to recall bias and missing data, especially for pregnancies from the previous century. We could not include proteinuria as variable in our analysis, because there were too many missing data. Additionally, we have no reliable information on the use of medications, other than the immunosuppressants that we studied, nor on the dose of drugs, such as prednisone, that will have changed over the past 30 years. This might have influenced our results, because pregnancies in CNI− patients occurred earlier than the pregnancies in CNI+ patients. For the variables included in our analysis, only a maximum of 13% of data per variable was missing, with the only exception of the postpartum serum creatinine, in which 31% of values were missing. Besides, since pregnancies in the CNI− group statistically occurred earlier than in the CNI+, it is reasonable to assume that the management of the immunosuppressive medication dosage changed in some of the later pregnancies. However, most of the pregnancies within CNI+ and CNI− occurred in overlapping periods of time and we have used binary logistic regression analysis to adjust our analysis on differences in serum creatinine levels for year of transplantation and year of pregnancy.
Previous research indicates that CNI use, irrespective of pregnancy, increases the risk of decline in graft function in the long term [9]. We did not find these effects during or shortly after pregnancy. However, we hypothesize that the increased vascular stress of pregnancy might accelerate or aggravate this decrease in graft function in the long term. Therefore, future research is needed to assess if pregnancy increases graft function decline in the long term and whether the use of a CNI is associated with a more rapid decline.
With respect to long‐term consequences for the offspring, previous research in humans confirmed maternal‐to‐foetal transfer of tacrolimus and cyclosporin to therapeutic levels in the offspring [24, 25, 26, 27]. Studies in both pregnant rabbits and rats demonstrated that antenatal exposure to therapeutic doses of cyclosporin in the mother, only during the phase of pregnancy wherein the kidneys develop in their offspring, led to nephron deficits, glomerular, tubular and intrarenal haemodynamic dysfunction and endothelin‐dependent systemic hypertension in their adult‐aged offspring [28, 29, 30]. Unfortunately, in humans, there are few reliable data of long‐term follow‐up of glomerular and tubular function and blood pressure until adult age in offspring of mothers exposed to CNI during their pregnancy. The only available human study in 22 children of mothers exposed to cyclosporin could not demonstrate any difference in blood and urine tests of renal function 6–72 months after delivery [31]. Recently, a study from Egerup et al. showed an increased risk of early (<1 year follow‐up) and late (1‐ to 5‐year follow‐up) infections in offspring of KTR, but unfortunately information on immunosuppressive regimen was absent [32]. Further research should, therefore, explore long‐term follow‐up up to adulthood of the offspring of KTR exposed to CNI to see if these problems also occur in humans and if they are indeed related to use of CNI.
Based on the data we retrieved, we can conclude that CNIs only temporarily induce a larger rise in serum creatinine towards the end of pregnancy, which does not seem to influence the course of serum creatinine levels 6–18 months postpartum negatively. Moreover, CNIs do not have major effects on the short‐term foetal outcomes when compared with CNI− pregnancies. Therefore, our data do not support replacement of CNI by azathioprine before pregnancy in KTR to prevent pregnancy‐related complications in mother and child. Further research is needed to reach more firm conclusions on short‐term and long‐term effects of maternal exposure to CNI on blood pressure, renal function and possible other health aspects, such as infections, in their offspring.
Authorship
LK: Data collection, Data analysis, Data interpretation, Statistics, Drafting article, Critical revision of article and Approval of article. JM: Data collection, Drafting article, Critical revision of article and Approval of article. OvdH: Concept/Design, Critical revision of article and Approval of article. AL: Concept/Design, Critical revision of article and Approval of article. MdJ: Critical revision of article and Approval of article. RvdM: Concept/Design, Critical revision of article and Approval of article. HvH: Concept/Design, Data interpretation, Critical revision of article and Approval of article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose as described by Transplant International.
Acknowledgements
The authors wish to acknowledge the services of the other members of the PARTOUT network: MC van Buren, H Groen and J van de Wetering. The authors are also pleased to acknowledge all students who participated in collection and assembly of data: L van Laar, N Paauw, B Reijtenbagh, A Schaeffers, A Schellekens, A Slob. Local investigators of the Partout network: FJ Bemelman, M de Boer, MHL Christiaans, M Groenewout, W Ganzevoort, SA Nurmohammed, FE van Reekum, J Rischen‐Vos, MEA Spaanderman, M Sueters, W Visser, APJ de Vries.
Contributor Information
Lisanne M. Koenjer, Email: Lisanne.koenjer@radboudumc.nl.
Members of the PARTOUT network*:
FJ Bemelman, M de Boer, MHL Christiaans, M Groenewout, W Ganzevoort, SA Nurmohammed, FE van Reekum, J Rischen‐Vos, MEA Spaanderman, M Sueters, W Visser, and APJ de Vries
References
- 1. Murray JE, Reid DE, Harrison JH, Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med 1963; 269: 341. [DOI] [PubMed] [Google Scholar]
- 2. Moritz MJ, Constantinescu S, Coscia LA, et al. Transplant Pregnancy Registry International: Annual Report 2018. Sept 2019.
- 3. Shah S, Venkatesan RL, Gupta A, et al. Pregnancy outcomes in women with kidney transplant: Metaanalysis and systematic review. BMC Nephrol 2019; 20: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta‐analysis. Am J Transplant 2011; 11: 2388. [DOI] [PubMed] [Google Scholar]
- 5. Vijayan M, Pavlakis M. Pregnancy and the kidney transplant recipient. Curr Opin Nephrol Hypertens 2017; 26: 494. [DOI] [PubMed] [Google Scholar]
- 6. Blume C, Pischke S, von Versen‐Hoynck F, Gunter HH, Gross MM. Pregnancies in liver and kidney transplant recipients: a review of the current literature and recommendation. Best Pract Res Clin Obstet Gynaecol 2014; 28: 1123. [DOI] [PubMed] [Google Scholar]
- 7. Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK Transplant pregnancy registry. Transplantation 2007; 83: 1301. [DOI] [PubMed] [Google Scholar]
- 8. McKay DB, Josephson MA. Pregnancy after kidney transplantation. Clin J Am Soc Nephrol 2008; 3(Suppl 2): S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malvezzi P, Rostaing L. The safety of calcineurin inhibitors for kidney‐transplant patients. Expert Opin Drug Saf 2015; 14: 1531. [DOI] [PubMed] [Google Scholar]
- 10. Rodrigues‐Diez R, Gonzalez‐Guerrero C, Ocana‐Salceda C, et al. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci Rep 2016; 6: 27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cabiddu G, Castellino S, Gernone G, et al. A best practice position statement on pregnancy in chronic kidney disease: the Italian Study Group on Kidney and Pregnancy. J Nephrol 2016; 29: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021. [DOI] [PubMed] [Google Scholar]
- 13. Quinn JA, Munoz FM, Gonik B, et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2016; 34: 6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cutland CL, Lackritz EM, Mallett‐Moore T, et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017; 35(Pt A): 6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perined (Dutch perinatal registration organisation) . Perinatal care in the Netherlands 2017. Last update year 2019. http://www.perinatreg‐data.nl/JB2017/Jaarboek2017.html. Accessed 20/12/2019.
- 16. Al‐Otaibi T, Gheith OA, Nagib AM, et al. Pregnancy after renal transplant: Single center experience from the middle east in patients using different calcineurin inhibitors. Exp Clin Transplant 2019; 17(Suppl 1): 99. [DOI] [PubMed] [Google Scholar]
- 17. Candido C, Cristelli MP, Fernandes AR, et al. Pregnancy after kidney transplantation: high rates of maternal complications. J Bras Nefrol 2016; 38: 421. [DOI] [PubMed] [Google Scholar]
- 18. van Buren MC, Schellekens A, Groenhof TKJ, et al. Long‐term graft survival and graft function following pregnancy in kidney transplant recipients: A systematic review and meta‐analysis. Transplantation 2020; 104: 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gill JS, Tonelli M, Mix CH, Pereira BJ. The change in allograft function among long‐term kidney transplant recipients. J Am Soc Nephrol 2003; 14: 1636. [DOI] [PubMed] [Google Scholar]
- 20. Mohammadi FA, Borg M, Gulyani A, McDonald SP, Jesudason S. Pregnancy outcomes and impact of pregnancy on graft function in women after kidney transplantation. Clin Transplant 2017; 31: e13089. [DOI] [PubMed] [Google Scholar]
- 21. Moritz MJ, Constantinescu S, Coscia LA, et al. Transplant Pregnancy Registry International: Annual report 2017. 2018.
- 22. Lim TY, Gonsalkorala E, Cannon MD, et al. Successful pregnancy outcomes following liver transplantation is predicted by renal function. Liver Transpl 2018; 24: 606. [DOI] [PubMed] [Google Scholar]
- 23. Naderi GH, Mehraban D, Kazemeyni SM, Darvishi M, Latif AH. Living or deceased donor kidney transplantation: a comparison of results and survival rates among Iranian patients. Transplant Proc 2009; 41: 2772. [DOI] [PubMed] [Google Scholar]
- 24. Akturk S, Sadioglu RE, Sengul S, Keven K. Acute kidney injury in an infant of a kidney allograft recipient. Clin Kidney J 2020; 13: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Resch B, Mache CJ, Windhager T, Holzer H, Leitner G, Müller W. FK 506 and successful pregnancy in a patient after renal transplantation. Transpl Proc 1998; 30: 163. [DOI] [PubMed] [Google Scholar]
- 26. Claris O, Picaud JC, Brazier JL, Salle BL. Pharmacokinetics of cyclosporin A in 16 newborn infants of renal or cardiac transplant mothers. Dev Pharmacol Ther 1993; 20: 180. [DOI] [PubMed] [Google Scholar]
- 27. Venkataramanan R, Koneru B, Wang CC, Burckart GJ, Caritis SN, Starzl TE. Cyclosporine and its metabolites in mother and baby. Transplantation 1988; 46: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shaheen FA, Al‐sulaiman MH, Al‐khader AA. Long term nephrotoxicity after exposure to cyclosporine in utero. Transplantation 1993; 56: 224. [DOI] [PubMed] [Google Scholar]
- 29. Tendron A, Decramer S, Justrabo E, Gouyon JB, Semama DS, Gilbert T. Cyclosporin A administration during pregnancy induces a permanent nephron deficit in young rabbits. J Am Soc Nephrol 2003; 14: 3188. [DOI] [PubMed] [Google Scholar]
- 30. Slabiak‐Blaz N, Adamczak M, Gut N, et al. Administration of cyclosporine A in pregnant rats ‐ the effect on blood pressure and on the glomerular number in their offspring. Kidney Blood Press Res 2015; 40: 413. [DOI] [PubMed] [Google Scholar]
- 31. Tendron‐Franzin A, Gouyon JB, Guignard JP, et al. Long‐term effects of in utero exposure to cyclosporin A on renal function in the rabbit. J Am Soc Nephrol 2004; 15: 2687. [DOI] [PubMed] [Google Scholar]
- 32. Egerup P, Carlson N, Oestergaard LB, et al. Increased risk of neonatal complications and infections in children of kidney‐transplanted women: A nationwide controlled cohort study. Am J Transplant 2020; 21: 1–8. [DOI] [PubMed] [Google Scholar]


