FIGURE 3.
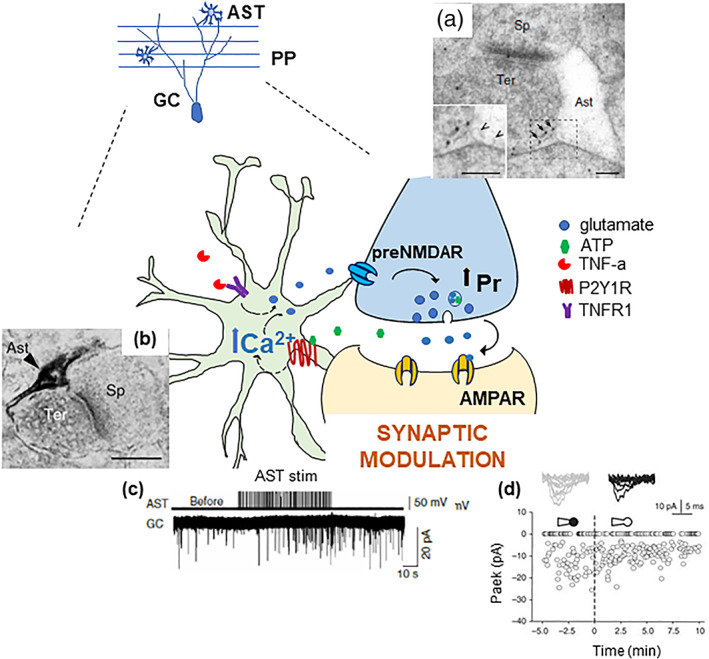
Molecular and functional evidence for a specific presynaptic astrocyte control on dentate perforant path‐granule cell synapses. Schematic representation of the pathways underlying the bidirectional communication between astrocytes (AST) and perforant path (PP) granule cell (GC) synapses in the dentate gyrus. The axonal terminals of PP fibers originating in the entorhinal cortex express pre‐synaptic NMDA receptors (preNMDAR) containing the GluN2B (electron microscopy image in [a]) and the GluN3A subunit (Savtchouk et al., 2019). Notice their extra‐synaptic location in PP terminals (arrows), facing astrocyte membrane displaying small clear vesicles (arrowheads). Intracellular Ca2+ elevations in astrocytes are induced by purinergic P2Y1R stimulation (P2Y1R immunoreactivity selectively in a perisynaptic astrocytic process at PP‐GC synapses is shown in the electron micrograph in [b]). Such elevations cause glutamate release onto pre‐NMDARs facing the astrocytic membranes. This event increases the probability of synaptic release (Pr) and results in the positive modulation of synaptic activity in GCs. We revealed the astrocyte modulation in several ways, for example, by either astrocytic electrical stimulation ([c], increased mEPSC frequency) or by perfusion of astrocytes with a BAPTA‐containing intracellular solution via a whole‐cell pipette ([d], increased synaptic failures). Endogenous ATP, possibly co‐released from glutamatergic terminals expressing the ATP vesicular transporter, VNUT (Larsson et al., 2012), could be the stimulus activating the astrocyte modulatory effect. Constitutive TNFα, via TNF receptor type 1, gates the glutamate release process from the astrocyte (Santello et al., 2011). Excerpt from Jourdain et al., 2007 (a, b and c) and Di Castro et al., 2011 (d). The authors hold the copyright to reproduce panels from Jourdain et al., Nature Neuroscience 2007 and Di Castro et al., 2011
