Abstract
Background
Patients with cancer‐associated venous thromboembolism (VTE) are recommended to receive treatment with therapeutic anticoagulation for at least 3–6 months. Little data exist on extended treatment beyond 6 months.
Objective
To comprehensively summarize the best available evidence on incidence of recurrent VTE and major bleeding 6–12 months after the index event in patients with cancer‐associated VTE.
Patients/Methods
We systematically screened biomedical databases (MEDLINE, Embase, CENTRAL) to identify studies reporting recurrent VTE and/or bleeding events between 6 and 12 months after a diagnosis of cancer‐associated VTE. Based on the observed heterogeneity in study design, setting, patient cohort characteristics, anticoagulation strategies, and outcome rates, no overall quantitative estimate of outcome rates was calculated.
Results
We screened 2597 publications and identified 11 eligible studies matching predefined in‐/exclusion criteria, reporting on 3019 patients specifically during the 6‐ to 12‐month period post–index VTE. Overall rates of recurrent VTE in this timeframe varied substantially (1%–12%), with the highest risk observed in the patient subgroup with residual vein thrombosis present at 6 months randomized to receive no anticoagulation (13%–15%). Reported rates of major bleeding between 6 and 12 months were between 2% and 5%.
Conclusions
In this systematic review, we provide a comprehensive and structured summary of the best available evidence on recurrence and bleeding risk between 6 and 12 months after cancer‐associated VTE. VTE recurrence remains common beyond 6 months and continuation of different anticoagulation strategies has an acceptable safety profile indicated by lower bleeding rates. These findings support guideline recommendations to continue anticoagulation treatment beyond 6 months in patients with active cancer.
Keywords: anticoagulants, hemorrhage, neoplasms, venous thromboembolism, venous thrombosis
ESSENTIALS.
For cancer‐associated thrombosis, at least 3 to 6 months of anticoagulation therapy are recommended.
Little data exist to inform clinical management in the extended treatment period after 6 months.
Upon systematic review, heterogeneous rates of venous thromboembolism recurrence were found.
Extended anticoagulation has ongoing acceptable safety profiles, indicated by low bleeding rates.
1. INTRODUCTION
Venous thromboembolism (VTE) is a frequent complication in patients with cancer and is associated with increased mortality, morbidity, and delay in anti‐cancer treatment. 1 , 2 , 3 Therapeutic anticoagulation is recommended for treatment of cancer‐associated VTE, the risks and benefits of which are well characterized up to 6 months after the initial event. 4 Guidelines for treatment of VTE in patients with cancer recommend at least 3 to 6 months of therapeutic anticoagulation with most recommending extended therapy in patients with active cancer or based on individual risk assessment. 5 , 6 , 7 However, the continuation of treatment beyond 6 months is primarily based on expert opinion, as data characterizing the risks and benefits of extended anticoagulation for treatment of cancer‐associated VTE beyond 6 months are sparse.
The treatment of cancer‐associated VTE has recently evolved with the introduction of direct oral anticoagulants (DOACs) into clinical practice after demonstration of non‐inferiority compared to low molecular weight heparin (LMWH), the long‐standing mainstay of therapy. 4 , 8 , 9 , 10 , 11 , 12 However, management of VTE in patients with cancer remains challenging as they are at 2‐fold higher risk of major bleeding and 3‐ to 7‐fold higher risk of VTE recurrence during anticoagulation treatment compared to non‐cancer patients with VTE. 13 , 14 , 15 While the evidence for intensity and type of anticoagulation treatment of cancer‐associated VTE for the first 6 months is robust, clinical studies are currently ongoing to explore prophylactic versus therapeutic doses of anticoagulation in the extended treatment phase for VTE in cancer patients.
For clinical decision‐making, it is important to understand the risks and benefits of extended anticoagulation treatment beyond 6 months. Therefore, the aim of this systematic review was to provide a comprehensive and structured summary of available data on the risks of recurrent VTE and bleeding between 6 and 12 months in patients with cancer‐associated VTE.
2. METHODS
We conducted a systematic review of the literature to identify studies that report the rate of recurrent VTE and/or bleeding between 6 and 12 months after the index event in patients with cancer‐associated VTE. The study was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions and is presented following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA, available as Data S1 in supporting information) guidelines. 16 , 17 The study protocol for this systematic review was prepared prior to the initiation of literature research and submitted to the International Prospective Register of Systematic Reviews (PROSPERO, ID: CRD42021209847).
2.1. Literature research and study selection process
Three biomedical databases (MEDLINE, Embase, CENTRAL) were systematically screened for potentially eligible publications based on a search algorithm of predefined search terms, available in the Appendix S1 in supporting information. Titles and abstracts of records identified by the search algorithm were screened by two independent researchers (FM, MC) for eligibility based on predefined in‐ and exclusion criteria. In the second step, the corresponding full texts of studies identified during the abstract‐ and title screening were reviewed independently by the two researchers (FM, MC). Any disagreement between the two researchers conducting the literature search was resolved by discussing the respective publication with a third independent researcher (CA). Inclusion of any publication in the study was conditional on consensus between all involved researchers.
In order to be eligible for inclusion, conformity with all predefined inclusion criteria was required. Studies matching ≥1 of the predefined exclusion criteria were ineligible. Inclusion criteria for publications were defined as follows: (a) reporting results of clinical trials (controlled/single arm) or cohort studies (prospective/retrospective); (b) adult patients (≥18 years of age) with active cancer at study inclusion, as defined within the respective study; (c) diagnosis of qualifying VTE event must include pulmonary embolism (PE) and/or deep vein thrombosis (DVT), either symptomatic or incidental/asymptomatic in presentation, and could include splanchnic vein thrombosis (SVT), cerebral vein thrombosis (CVT), or central catheter–related thrombosis (CRT); (d) follow‐up and reporting of outcomes (recurrent VTE and/or bleeding events) specifically between 6 and 12 months after the index VTE event. The number of patients at risk for outcome events at 6 months post index VTE was required to obtain outcome rates between 6 and 12 months. Exclusion criteria were (a) studies with inclusion of patients <18 years of age, and (b) studies with no available data on outcomes specifically in patients with cancer (e.g., studies including a subgroup of patients with cancer with specific data not extractable for this subgroup).
2.2. Risk of bias evaluation
Generalzability and between‐study comparability of identified and eligible publications were assessed by a structured, standardized risk of bias assessment. Randomized controlled trials were assessed using the modified Cochrane Risk of Bias Tool (RoB 2), and non‐randomized studies, including cohort studies, were evaluated by applying the Newcastle‐Ottawa Scale. 18 , 19 Risk of publication bias was visualized by plotting rate of primary outcome events against its standard error within a funnel plot.
2.3. Data extraction
Data were extracted from the identified studies fulfilling the eligibility criteria by two independent researchers (FM, MC). Primary study data of included studies were not sought from the investigators and only data as extractable from published results were included. Descriptive characteristics of selected studies, including baseline demographics, cancer specifics (type, stage), and details on qualifying VTE event and anticoagulation treatment were gathered. Additionally, data on outcome events between 6 and 12 months after the initial index VTE event were obtained. Primary outcome events of this systematic review were predefined and comprise (a) the rate of recurrent VTE, as defined within the respective study, including symptomatic, incidental, or fatal PE, DVT (including CRT), splanchnic vein thrombosis, or cerebral vein thrombosis; and (b) the rate of major bleeding (MB), either defined according to the bleeding scale of the International Society on Thrombosis and Haemostasis (ISTH; i.e., fatal bleeding and/or symptomatic bleeding in a critical area or organ and/or bleeding accompanied by a fall in hemoglobin levels of ≥2 g/dl or leading to transfusion of ≥2 units of whole blood or red cell concentrates), or alternatively as defined in the respective study. 20
Secondary outcome events include (a) the rate of clinically relevant non‐major bleeding (CRNMB), defined according to the ISTH as a bleeding event that does not meet criteria of a major bleeding event, but necessitates an immediate clinical response including either hospital admission for bleeding or medical/surgical treatment for bleeding or a change in antithrombotic therapy (including interruption or discontinuation) 21 ; and (b) all‐cause mortality.
2.4. Statistics
Planned quantitative synthesis of data from identified studies was omitted due to the observed degree of underlying risk of bias and substantial between‐study heterogeneity in design, study cohorts, anticoagulation strategies, and outcome rates. Individual risk of recurrent VTE and MB are presented in Forrest plots, created with the STATA package metaprop, computing individual 95% confidence intervals by the score method. 22 Heterogeneity between included studies is reported by I2 as a measure of between‐study variance beyond expected random variation. Data extracted from included studies and analytic code are available from the corresponding author upon reasonable request. All statistical analyses were conducted in STATA v15.1 (Stata Corp.).
3. RESULTS
3.1. Study selection
In total, 2597 studies were identified after screening of the biomedical databases following predefined search algorithms on September 18, 2020 (MEDLINE via PubMed: 1134, Embase: 1068, CENTRAL: 395). After the removal of duplicates, 2210 records underwent primary eligibility screening based on titles and abstracts. Consequently, 332 publications remained for full‐text evaluation. Of those, 11 studies were identified that met the predefined eligibility criteria for this systematic review. The process of study selection is displayed in detail in a PRISMA flow diagram in Figure 1.
FIGURE 1.

Study selection flow chart. 1: Studies ineligible based on study setting (non‐cancer/not cancer specific, risk of first VTE). 2Studies not reporting primary data: (systematic‐) reviews, meta‐analyses. 3Studies ineligible based on outcomes (survival, cost effectiveness, fatal events only, thrombus resolution). RCT, randomized controlled trial; VTE, venous thromboembolism
3.2. Study characteristics
The 11 identified eligible studies varied in study design, inclusion and exclusion criteria, and characteristics of included patients. A comprehensive overview summarizing details of study characteristics is presented in Table 1, and more details on baseline characteristics of study subgroups in Table S1. Overall, three randomized controlled trials (RCT), two single‐arm interventional trials, two prospective cohort studies, two retrospective cohort studies, and two studies reporting registry data were identified. In total, these studies included 3019 patients with cancer‐associated thrombosis at 6 months after the index VTE event.
TABLE 1.
Study design of included studies
| First author, year | Study | Study design | Country | No. of patients (0 mo.) | No. of patients (6 mo.) / % of pts. at 0 mo. | Intervention / Anticoagulation | Study population | Key exclusion criteria | Primary outcome |
Rec. VTE 0–6 mo |
MB 0–6 mo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marshall et al., 2020 | SELECT‐D: 12m | RCT | UK (multi‐center) | 406 (371 during ongoing 2nd randomization) | 92 (25%, second randomization a ); PE / RVT not randomized: 107 (29%), RVT negative: 35 (9%) | RVT/index PE →Second randomization at 6 mo: Rivaroxaban b (n = 46) vs. placebo (n = 46) | Pts. with active cancer +VTE (PE/symp. prox. DVT) + RVT | ECOG >2, VTE recurrence 1–6 mo, absence of RVT at 6 mo (index DVT) | Recurrent VTE |
6.4% overall, 8.9% dalte‐parin 3.9% riva‐roxaban |
4.2% overall, 3.0% dalte‐parin, 5.4% riva‐roxaban |
| Di Nisio et al., 2019 | Hokusai VTE Cancer | RCT (post‐hoc analysis) | Multi‐national | 1046 | 567 (54%) |
Edoxaban (n = 294) vs. dalteparin (n = 273) c AC beyond 6 mo determined by treating physician |
Pts. with cancer +VTE (PE/prox. DVT) + anticoagulation beyond 6 months | ECOG >2, platelet <50G/L, CrCl <30 mL/min | Composite: Recurrent VTE & MB |
7.6% overall, 6.5% edoxaban 8.8% dalteparin |
4.4% overall, 5.6% edoxaban, 3.2% dalteparin |
| Napolitano et al., 2014 | Cancer‐DACUS | RCT | Italy (multi‐center) | n/a | 347 (242 RVT) | RVT →Randomization: Nadroparin d (n = 119) vs. placebo (n = 123); no RVT: No tx (n = 105) | Pts. with active cancer +DVT +6mo LMWH | ECOG >2, prior VTE recurrence; high risk of bleeding, PE | Recurrent VTE | n/a | n/a |
| Jara‐Palomares et al., 2017 | TiCat | Single‐arm interventional study | Spain (multi‐center) | 247 | 198 (80%) e | Tinzaparin f | Pts. with active cancer +VTE (PE/prox. DVT) | Hemodialysis, uncontrolled hypertension | MB, CRNMB | 4.5% | 2.8% |
| Francis et al., 2015 | DALTECAN | Single‐arm interventional study | Multi‐national | 334 | 185 (55%) | Dalteparin g | Pts. with active cancer +VTE (PE/symp. prox. DVT) | High risk of bleeding h , hemodialysis, uncontrolled hypertension | MB | 8.7% | 7.8% |
| Poudel et al., 2019 (Meeting Abstract) | Prospective cohort study | USA (single‐center) | n.r. | 284 | 191: AC beyond 6 mo; 93: no AC beyond 6 mo | Pts. with cancer‐associated thrombosis | n.r. | Recurrent VTE, bleeding (MB/CRNMB), OS | n.r. | n.r. | |
| Prandoni et al., 2002 | Prospective cohort study | Italy (single‐center) | 181 | 92 (51%) i | n.r. | Patients with DVT, subgroup of patients with cancer | Prior VTE | Recurrent VTE | n.r. | n.r. | |
| Mahé et al., 2020 | USCAT | Retrospective cohort study | France (multi‐center) | 719 | 432 (60%) | LMWH (n = 256), VKA (n = 56), DOAC (n = 30), no AC beyond 6 mo (n = 74) | Pts. with cancer +VTE (from 2 prospective observational studies, alive after 6 months) | n.r. | Description of anticoagulant treatment (6–12 mo) | n.r. | n.r. |
| Schmidt et al., 2020 | Retrospective cohort study | Canada (single‐center) | 524 | 322 (61%) | 222: AC beyond 6mo; 100: AC discontinuation at 6 mo | Pts. with cancer‐associated VTE | CRT | Recurrent VTE, MB, CRNMB, Mortality | n.r. | n.r. | |
| Sakamoto et al., 2019 |
COMMAND VTE Registry |
Registry | Japan (multi‐center) | 695 | 396 (57%) | n.r. | Patient with symptomatic VTE, active cancer subgroup | n.r. | Recurrent VTE, MB, Mortality | 7.8% | 9.1% |
| Yhim et al., 2013 | Registry | Korea (multi‐center) | 449 | 83 (18%) | n.r. | Pts. with recurrent/metastatic solid cancer +initiation of AC +VTE | Haematologic cancer, solid cancer not recurrent/metastatic, index: CRT or SVT, no AC after index VTE | Recurrent VTE | 11.1% | n.r. |
Abbreviations: AC, anticoagulation; CRNMB, clinically relevant non‐major bleeding; CRT, catheter‐related thrombosis; DOAC, direct oral anticoagulants; DVT, deep vein thrombosis; ECOG, Eastern Cooperative Oncology Group performance status; KM, Kaplan Meier estimate; LMWH, low molecular weight heparin; MB, major bleeding; mo., months; n.r., not reported; OS, overall survival; PE, pulmonary embolism; RCT, randomized controlled trial; RVT, residual vein thrombosis; VKA, Vitamin K antagonists; VTE, venous thromboembolism.
Sorted by design and year of publication.
Bold writing has been used to underline overall outcome rates as opposed to subgroups.
Second randomization was stopped due to futility during the ongoing trial.
Rivaroxaban 20 mg 1×/daily.
Edoxaban dose.
Nadroparin: 75% of full dose (97 IU anti–activated coagulation factor X per kilogram twice daily).
At risk for VTE 7–12 months: 184, overall n after 6 months: 198.
Tinzaparin dose.
Dalteparin dose.
For example, recent neurosurgery within 30 days, history of intracranial hemorrhage, or acute gastroduodenal ulcer.
Patients with cancer at risk 6 months after index DVT according to KM risk table.
3.2.1. Randomized controlled trials
The three identified RCTs were SELECT‐D 12m (12‐month outcomes of the study), Hokusai VTE Cancer (reporting a post hoc analysis of 6‐ to 12‐month data after index VTE), and the Cancer‐DACUS study. 23 , 24 , 25 In SELECT‐D, 406 patients with cancer‐associated VTE were randomized to either rivaroxaban (n = 203) or dalteparin (n = 203) after index VTE, with a second randomization process at 6‐month follow‐up in patients with index PE or the presence of residual vein thrombosis (RVT) on screening ultrasound at 6 months. Of 371 patients included in SELECT‐D during ongoing second randomization, 137 (38%) were not assessed for second randomization, 35 (9%) had no RVT, and 199 (54%) had index PE or RVT at 6 months. Of those, at 6 months, 92 patients (25%) underwent a second randomization to either rivaroxaban (n = 46) or placebo (n = 46), whereas 107 patients (29%) with RVT or index PE did not undergo second randomization. The trial was stopped prematurely due to low recruitment rate at the second randomization. 23 In Hokusai VTE Cancer, 1046 patients with cancer‐associated VTE were randomized after a diagnosis of cancer‐associated VTE to receive edoxaban or dalteparin for a minimum of 6 months and up to 12 months, with continuation of trial treatment after 6 months determined by the treating physician. 9 In a post hoc analysis of the trial, focusing on the 6‐ to 12‐month timeframe after the index event, 567 patients (54%) were included that were still receiving the assigned trial treatment (edoxaban: n = 294, dalteparin: n = 273). 24 Cancer‐DACUS is a study focusing on outcomes in the 6‐ to 12‐month period after DVT in patients with cancer. At 6 months after index DVT, a total of 347 patients were included. Patients with RVT (n = 242) were randomized to receive nadroparin (n = 119) or placebo (n = 123) and patients without RVT (n = 105) discontinued anticoagulation and were observed. 25
3.2.2. Single‐arm interventional studies
In the TiCAT study, an open‐label, multi‐center interventional study, 247 patients with cancer‐associated VTE received tinzaparin for a total of 12 months; 198 patients (80%) completed the follow‐up at 6 months post index VTE and were separately evaluated during the 6‐ to 12‐month period. 26 In the DALTECAN study, a multi‐national interventional study, 334 patients with cancer‐associated VTE received dalteparin for 12 months; 185 patients (55%) completed at least 6 months of follow‐up. 27
3.2.3. Cohort and registry‐based studies
Two prospective cohort studies were included. The study by Poudel et al., published as a meeting abstract, presented outcomes of patients (n = 284) 6–12 months after cancer‐associated VTE. 28 The study by Prandoni et al. observed patients with DVT, including a subgroup of patients with cancer (n = 181), and reported the number at risk for outcome events at 6 months post index DVT (n = 92, 51%). 13 The USCAT study, a multi‐center retrospective cohort study, which reported data from two prospective observational studies (n = 719), described treatment and outcomes of 432 patients (60%) beyond 6 months after index VTE. 29 Using a single‐center retrospective cohort, Schmidt et al. reported on the treatment and outcomes of 322 patients 6–12 months post index VTE. 30 Last, two registry studies were included that allow extraction of outcome data 6–12 months after cancer‐associated VTE. In the COMMAND VTE registry, outcomes of patients with symptomatic VTE, including a separately reported subgroup of patients with active cancer, are reported between 6 and 12 months (n = 396), 31 and Yhim et al. evaluated the risk of recurrent VTE in patients with recurrent/metastatic solid cancer and VTE, providing the number of patients at risk at 6 months after index VTE (n = 83) and reporting outcome rates between 6 and 12 months. 32
3.3. Risk of bias
A structured assessment of the underlying risk of bias for each study was conducted to judge the generalizability of the respective study findings, and to evaluate the possibility of aggregating the extracted outcome data of identified studies. Importantly, risk of bias assessment represents an evaluation of each included study in the framework of the research questions of the present systematic review and does not evaluate the overall scientific merit or quality of the respective study. Overall, studies varied in respect to their underlying suspected risk of bias, mostly due to the selection of study cohorts based on index VTE characteristics (e.g., DVT only, symptomatic VTE only), the presence of RVT at 6 months, or cancer‐specific restrictions (e.g., advanced disease only). Moreover, study cohorts seemed to differ beyond prespecified inclusion and exclusion criteria, as studies varied in key baseline characteristics such as cancer types and stage (Table 2). Details on the structured risk of bias assessment of all identified studies are presented in Figure 2. Based on the risk of bias assessment in combination with the observed heterogeneity in design, baseline characteristics, and outcome rates, we concluded that it was infeasible to conduct a quantitative synthesis of extracted outcome data in the framework of a meta‐analysis. No publication bias was suspected upon visual inspection of the funnel plot, plotting risk estimates against respective standard errors (Figure S1 in supporting information).
TABLE 2.
Outcomes of included studies
| First author, year | Study | No. of patients (6 mo.) | Recurrent VTE (6–12mo) | MB (6–12 mo) | CRNMB (6–12 mo) | Mortality (6–12 mo) | AC beyond 6 mo | Age, female (%) | ECOG 0–1 | Cancer types | Advanced cancer stage a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marshall et al., 2020 | SELECT‐D: 12m | Index PE / RVT: 2nd randomization: Overall (n = 92) b |
8.7% n = 8 |
2.2% n = 2 |
2.2% n = 2 |
12% n = 15 |
100% | F: 49% | 85% |
GI 39%, Breast 17% Hem 13% GU 12% Lung 8% Prostate 4% |
43% metastatic |
| Index PE / RVT at 6 mo →2nd randomization: Rivaroxaban (n = 46) |
4.3% n = 2 |
4.3% n = 2 |
4.3% n = 2 |
13% n = 6 |
100% | ||||||
| Index PE/RVT at 6 mo →2nd randomization: Placebo (n = 46) |
13% n = 6 |
0% n = 0 |
0% n = 0 |
11% n = 5 |
100% | ||||||
| Index DVT & RVT negative at 6mo (n = 35) c |
0% n = 0 |
n.r. | n.r. |
11% n = 4 |
0% | ||||||
| Di Nisio et al., 2019 | Hokusai VTE Cancer | Overall (n = 567) |
2.1% n = 12 |
1.8% n = 10 |
4.8% n = 27 |
13.8% n = 78 |
100% |
Mean (sd): 64 (11) d F: 47% |
83% |
GI 27% Breast 16% GU 22% Hem 14% Lung 11% |
41% metastatic |
| Edoxaban (n = 294) |
1.4% overall (n = 4) 0.7% on treatment (n = 2) |
2.4% overall (n = 7) 1.7% on treatment (n = 5) |
4.8% overall (n = 14) 3.7% on treatment (n = 11) |
13.3% (n = 39) e | 100% | ||||||
| Dalteparin (n = 273) |
2.9% overall (n = 8) 1.1% on treatment (n = 3) |
1.1% overall (n = 3) 1.1% on treatment (n = 3) |
4.8% overall (n = 13) 3.7% on treatment (n = 10 |
14.3% (n = 39) e | 100% | ||||||
| Napolitano et al., 2014 | Cancer‐DACUS | Overall (n = 347) |
6.9% n = 24 |
1.7% n = 6 |
2.6% n = 9 f |
12% n = 42 g |
34.3% |
Mean (sd): 61 (14) d F: 45% |
/ |
GI 27% Breast 12% GU 11% Hem 23% Lung 5% |
21% metastatic |
| RVT at 6mo →randomization: continue LMWH (n = 119) |
3.4% n = 4 |
3.4% n = 4 |
1.7% n = 2 f |
10% n = 12 g |
100% | ||||||
| RVT at 6mo →randomization: discontinue LMWH (n = 123) |
14.6% n = 18 |
0.8% n = 1 |
3.3% n = 4 f |
15% n = 19 g |
0% | ||||||
| RVT negative at 6mo (n = 105) |
1.9% n = 2 |
1.0% n = 1 |
2.9% n = 3 f |
11% n = 11 g |
0% | ||||||
| Jara‐Palomares et al., 2017 | TiCat | Tinzaparin (n = 184) h |
1.1% n=2 |
2.6% n = 5 i |
n.r. |
23% n = 23 j |
100% | Mean (sd): 62 (13). F: 55% | 83% |
GI 14% Breast 14% GU 21% Hem 8% Lung 16% Prostate 4% |
66% metastatic |
| Francis et al., 2015 | DALTECAN | Dalteparin (n = 185) |
4.1% n = 8 k |
4.3% n = 8 |
n.r. | n.r. | 100% | Mean 65, F: 47% | 84% |
GI 21% Breast 9% Hem 13% Lung 14% |
63% metastatic |
| Poudel et al., 2019 (Meeting Abstract) | Overall (n = 284) |
12.0% n = 34 |
1.8% n = 5 |
3.2% n = 9 |
n.r. | 67.3% | Mean (sd): 61 (13), F: 50% | / |
GI 17% Breast 7% GU 12% Hem 31% Lung 7% Brain 12% |
62% metastatic | |
| AC beyond 6 mo. (n = 191) |
12.0% n = 23 |
2.1% n = 4 |
4.7% n = 9 |
n.r. | 100% | ||||||
| No AC beyond 6 mo. (n = 93) |
11.8% n = 11 |
1.1% n = 1 |
0% n = 0 |
n.r. | 0% | ||||||
| Prandoni et al., 2002 | Overall (n = 92) l |
3.3% n = 3 |
n.r. | n.r. | n.r. | 100% | Mean (sd): 65 (11), F: 54% | / |
GI 20% Breast 19% GU 26% Hem 19% Lung 15% Brain 6% |
37% Stage IV („ex‐tensive“) | |
| Mahé et al., 2020 | USCAT | Overall (n = 432) m |
5.7% n = 24 |
2.7% n = 11 |
2.4% n = 10 |
22.3% n = 96 |
68.9% | Mean (sd): 67 (13), F: 52% | / |
GI 31% Breast 17% GU 26% Hem 7% Lung 20% |
74% metastatic |
| Schmidt et al., 2020 | Overall (n = 322) |
5.3% n = 17 |
4.1% CI 6–18mo CRB n | 26.3% (KM) | 68.9% | Med. (iqr): 63 (55–70), F: 52% | / |
GI 28% Breast 8% GU 18% Hem 20% Lung 13% Prostate 5% |
44% metastatic | ||
| AC beyond 6 months (n = 222) | 9.9% CI 6–18mo n | 5.1% CI 6–18mo CRB n | 25.2% (KM) | 100% | |||||||
| No AC beyond 6 months (n = 100) | 4.4% CI 6–18mo n | 1.8% CI 6–18mo CRB n | 16.2% (KM) | 0% | |||||||
| Sakamoto et al., 2019 |
COMMAND VTE Registry |
Overall (n = 396) o |
2.8% n = 11 |
4.8% n = 19 |
n.r. |
19.2 n = 82 |
79.8% | Mean (sd): 67 (12), F: 60% | / |
GI 31% Breast 4% GU 21% Hem 9% Lung 16% Prostate 5% |
49% metastatic / terminal stage |
| Yhim et al., 2013 | Overall (n = 83) p , q |
3.6% n = 3 |
n.r. | n.r. | n.r. | 100% | Med (range): 65 (27–91), F: 46% | 41% |
GI 57% Breast 5% GU 9% Lung 30% |
72% palliative chemo‐therapy | |
Abbreviations: AC, anticoagulation; CI, cumulative incidence; CRB, clinically relevant bleeding (=MB+CRNMB); CRNMB, clinically relevant non‐major bleeding; DVT, deep vein thrombosis; GI, gastrointestinal cancer; GU, genitourinary cancer; iqr, interquartile range; KM, Kaplan Meier estimate; LMWH, low molecular weight heparin; MB, major bleeding; mo., months; n.r., not reported; PE, pulmonary embolism; RVT, residual vein thrombosis; sd, standard deviation; VTE, venous thromboembolism.
If not otherwise specified, outcomes represent rates between 6 and 12 months after index VTE.
Bold writing has been used to underline overall outcome rates as opposed to subgroups.
Refers to patients with staging information available.
Overall rates for recurrent VTE and mortality comprise patients with index PE/positive RVT at 6 months who underwent second randomization (n = 92) + patients with index DVT/RVT negative at 6 months (n = 35); Overall bleeding rates comprise only patients with index PE/positive RVT at 6 months who underwent second randomization.
Index DVT/RVT negative at 6 months (n = 35): 6 patients with additional anticoagulation treatment beyond 6 months.
Combined mean and SD from subgroups.
VTE related death: 1 patient in the edoxaban group and 1 patient in the dalteparin group. No bleeding‐related death reported.
Rate of minor bleeding.
Mortality in the Cancer‐DACUS study reported for 12 months post randomization (6–18 months post index event).
Number at risk for recurrent VTE at 6 months; patient characteristics from full study cohort (n = 247).
Number at risk for major bleeding at 6 months: 189.
1 fatal recurrent PE, 2 fatal bleeding events; Mortality based on number of patients who completed 6 months follow‐up (n = 198).
Number at risk for recurrent VTE at 6 months: 194.
Patients with cancer at risk 6 months after index DVT according to KM risk table, patient characteristics from full study cohort at month 0 (n = 181).
VTE recurrence, bleeding events, and deaths were documented in 418, 415, and 430 patients, respectively.
Reported rates of recurrent VTE in subgroups and CRB represent Kaplan‐Meier cumulative incidence estimates from month 6 to month 18 after index VTE.
Baseline characteristics at baseline (Month 0) of the total active cancer subgroup (n = 695).
Baseline characteristics at baseline (Month 0) of total cohort (n = 449).
Number at risk at 6 months: 396 for VTE, 398 for MB, 428 for mortality.
FIGURE 2.
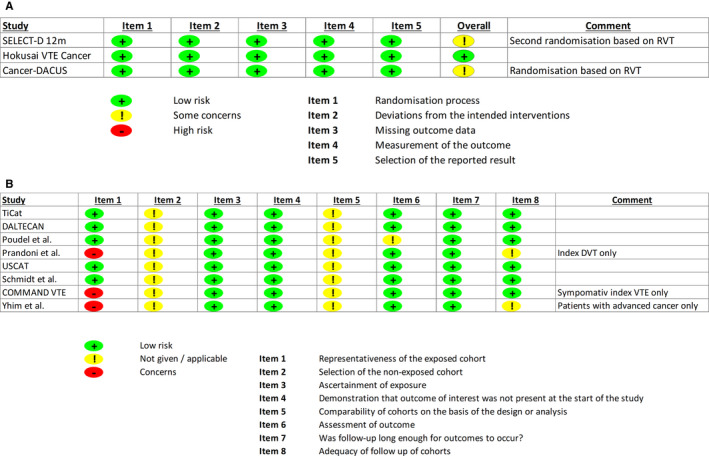
Risk of bias assessment. A, Risk of bias assessment of randomized controlled trials based on the RoB 2 assessment tool. B, Risk of bias assessment of single‐arm trials and cohort studies based on the Newcastle‐Ottawa Scale. DVT, deep vein thrombosis; RoB 2, Cochrane Risk of Bias Tool; RVT, residual vein thrombosis
3.4. Recurrent VTE between 6 and 12 months
Rates of recurrent VTE between 6 and 12 months after index VTE varied between 1.1% and 12.0% across the studies (I2: 88%). In SELECT‐D, VTE recurrence was reported in 8.7% (8/92) of the patients undergoing second randomization based on RVT/index PE (rivaroxaban: 4.3% [2/46], placebo: 13.0% [6/46]), and none of the patients (0/35) with index DVT and absence of RVT at 6 months developed VTE recurrence. In the Hokusai VTE Cancer study, 2.1% (12/567) of patients experienced VTE recurrence between 6 and 12 months; 1.4% (4/294) in the edoxaban and 2.9% (8/273) in the dalteparin arm. In Cancer‐DACUS, the overall VTE recurrence rate between 6 and 12 months was 6.9% (24/347); 3.4% (4/119) in patients with RVT randomized to continue LMWH beyond 6 months, 14.6% (18/123) in those with RVT randomized to discontinue anticoagulation, and 1.9% (2/105) in those without RVT at 6 months, all of whom discontinued anticoagulation. In the single‐arm interventional studies, recurrent VTE occurred in 1.1% (2/184) of tinzaparin‐treated patients in TiCAT and in 4.1% (8/185) of dalteparin‐treated patients in DALTECAN. Poudel et al. report a VTE recurrence rate of 12.0% (34/284); 12.0% (23/191) in those receiving anticoagulation beyond 6 months and 11.8% (11/93) in those who discontinued anticoagulation. In another prospective cohort study, Prandoni et al. observed a rate of recurrent VTE of 3.3% between 6 and 12 months in the subgroup of patients with cancer and DVT. Of the two retrospective cohort studies, the USCAT study found a recurrent VTE rate of 5.7% (24/418), and in the study by Schmidt et al., of 322 included patients, 17 (5.3%) experienced recurrent VTE events between 6 and 12 months of follow‐up. In the COMMAND VTE registry, 2.8% (11/396) of patients experienced VTE recurrence between 6 and12 months and in the registry study by Yhim et al. 3.6% (3/83) had recurrent VTE. A comprehensive summary of rates of recurrent VTE overall and in specific subgroups is presented in Figure 3 with details provided in Table 2. Further, the rates of outcome events structured by the receipt of anticoagulation therapy beyond 6 months are provided in Table S2 in supporting information.
FIGURE 3.
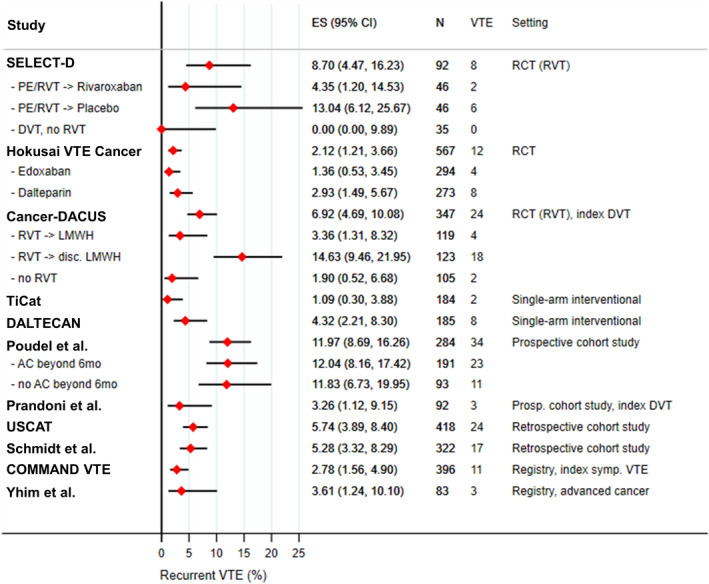
Recurrent VTE between 6 and 12 months after index VTE. AC, anticoagulation; ES (95% CI), estimated proportion and corresponding 95% confidence interval; DVT, deep vein thrombosis; LMWH, low molecular weight heparin; PE, pulmonary embolism; RCT, randomized‐controlled trial; RVT,: residual vein thrombosis; VTE, venous thromboembolism
3.5. Bleeding rates between 6 and 12 months
Overall, in the subset of studies reporting on bleeding outcomes, MB rates ranged from 1.7% to 4.8% (I 2: 30%), and CRNMB rates from 2.0% to 4.8% (I 2: 64%) between 6 and 12 months after index VTE. In SELECT‐D 12m, patients undergoing second randomization (index PE/RVT) had MB and CRNMB rates of 2.2% (2/92) and 2.2% (2/92), with all bleeding events occurring in the rivaroxaban group (4.3% MB [2/46], 4.3% CRNMB [2/46]). No bleeding outcomes were reported in the group of patients with index DVT and absence of RVT. In Hokusai VTE cancer, 1.8% (10/567) of patients experienced a MB and 4.8% (27/567) a CRNMB, 2.4% (7/294) and 4.8% (14/294) in the edoxaban group and 1.1% (3/273) and 4.8% (13/273) in the dalteparin group, respectively. In Cancer‐DACUS, overall, 1.7% of patients (6/347) experienced a MB and 2.6% (9/347) a minor bleeding event. The corresponding rates of major and minor bleeding in patients with RVT randomized to continue LMWH were 3.4% (4/119) and 1.7% (2/119), and in those with RVT randomized to discontinue LMWH 0.8% (1/123) and 3.3% (4/123). In RVT‐negative patients without LMWH, MB and minor bleeding occurred in 1.0% (1/105) and 2.9% (3/105), respectively. In the TiCAT and DALTECAN study, MB occurred in 2.6% (5/194) and 4.3% of patients (8/185), respectively. Poudel et al. reported overall MB and CRNMB rates of 1.8% (5/284) and 3.2% (9/284). In patients continuing anticoagulation beyond 6 months, rates were 2.1% (4/191) and 4.7% (9/191), compared to 1.1% (1/93%) and 0% in those with discontinued anticoagulation 6 months after index VTE. In USCAT, 2.7% (11/415) and 2.4% of patients (10/415) experienced MB and CRNMB, respectively. In the study by Schmidt et al., bleeding rates between 6 and12 months were not extractable from the manuscript. For the 6–18 months post index VTE period, cumulative incidences of clinically relevant bleeding (MB+CRNMB) were 4.1% in all patients, 5.1% in those receiving anticoagulation, and 1.8% of those without anticoagulation beyond 6 months. In the COMMAND VTE registry, MB occurred in 4.8% (19/398) of patients. Figure 4 provides an overview of reported rates of MB between 6 and 12 months after index VTE, with detailed rates provided in Table 2. Further, the rates of major bleeding events structured by the receipt of anticoagulation therapy beyond 6 months are provided in Table S3 in supporting information.
FIGURE 4.
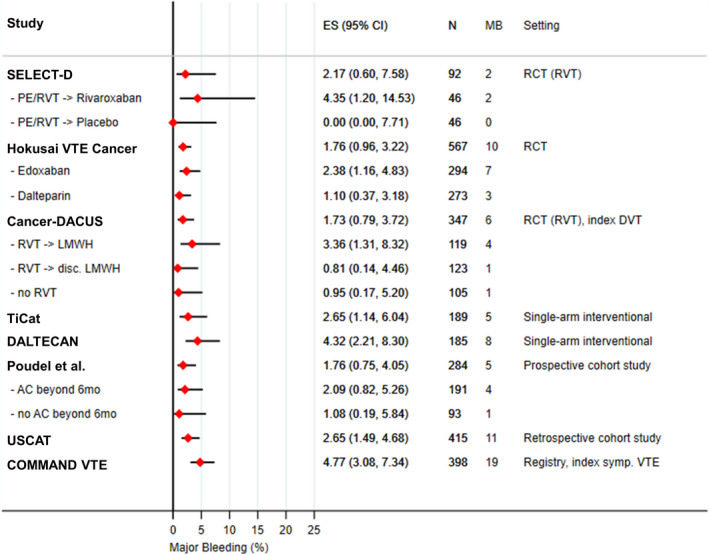
Major bleeding between 6 and 12 months after index VTE. Three studies did not report rates of MB between 6 and 12 months (Prandoni et al., Schmidt et al., Yhim et al.). Number at risk for major bleeding varies from the number at risk for recurrent VTE in TiCAT, USCAT, and COMMAND VTE. AC, anticoagulation; ES (95% CI), estimated proportion and corresponding 95% confidence interval; DVT, deep vein thrombosis; LMWH, low molecular weight heparin; MB, major bleeding; PE, pulmonary embolism; RCT, randomized‐controlled trial; RVT, residual vein thrombosis; VTE, venous thromboembolism
4. DISCUSSION
In this systematic review, we provide a comprehensive and structured summary of the published data on recurrent VTE and bleeding events specifically for the 6‐ 12‐month period after diagnosis of cancer‐associated VTE. Management of patients with cancer‐associated VTE beyond 6 months after the index event is challenging in clinical practice, due to the paucity of dedicated studies assessing extended anticoagulation treatment in this setting. This structured summary of the best available evidence can help to guide clinical decision‐making in this common and difficult clinical scenario.
Our systematic literature review identified two important issues. First, there are few studies specifically reporting rates of recurrent VTE and/or bleeding between 6 and 12 months. We found 11 studies assessing the risk of recurrent VTE and bleeding in this period. Additional studies evaluating extended anticoagulation treatment or reporting outcomes from observation periods longer than 6 months were identified (Table S4 in supporting information). However, the timeframes were heterogeneous and did not allow extraction of data for the 6‐ to 12‐month period. The timeframe between 6 and 12 months after index VTE was selected a priori because of the availability of high‐quality data up to 6 months after index VTE and to create a comparable timeframe for studies reporting outcomes beyond the initial 6 months. Second, we found that the eligible studies vary substantially in design, inclusion and exclusion criteria, characteristics of study cohorts, and anticoagulation strategies, preventing the direct comparison of reported outcome rates. Of note, several identified studies did not specifically focus on the 6‐ to 12‐month period but rather provide post hoc or subgroup analyses for this timeframe.
In general, the rate of recurrent VTE beyond 6 months was highly variable, with reported rates between 1% and 12%. Importantly, anticoagulation strategies for patients in most included studies differed and therefore, no general quantitative estimate of the baseline risk of recurrence between 6 and 12 months after cancer‐associated VTE for patients on or off anticoagulation can be made. In summary, the reported risk of recurrent VTE between 6 and 12 months in patients receiving anticoagulation therapy ranged between 1% to 4% in three randomized controlled trials and two single‐arm interventional studies, 23 , 24 , 25 , 26 , 27 with a higher risk of 12% reported in one prospective observational study. 28 This high recurrence risk reported by Poudel et al. might be explained by underlying differences in cohort characteristics, including a relatively high proportion of patients with stage IV cancers and tumor types with known elevated rates of both primary and recurrent risk of VTE. 33
Regarding patients without anticoagulation treatment, in the two RCTs using the presence of RVT at 6 months post index VTE as the qualifier for randomization, the highest rates of VTE recurrence were reported in the subgroups of patients with RVT randomized to receive placebo (13%) or to discontinue anticoagulation beyond 6 months (15%). In contrast, in patients without RVT, no recurrent VTE events in SELECT‐D (n = 35; 6 patients received anticoagulation treatment beyond 6 months), and only a 1.9% recurrence risk in Cancer‐DACUS (n = 105, no anticoagulation) was reported between 6 and 12 months. 23 , 25 These findings might suggest a very low risk of recurrent VTE in patients with index DVT and no RVT present at 6 months after the initial event. However, important limitations apply for both trials evaluating a RVT‐based strategy, with most patients having undergone cancer surgery and only a minority of patients with distant metastatic disease in Cancer‐DACUS, limiting its generalizability, and the small sample size of patients undergoing second randomization at 6 months in the SELECT‐D trial, which led to the preterm discontinuation of patient recruitment.
Overall, reported risk of recurrent VTE between 6 and 12 months is lower than the risk in the first 6 months after the index event. For example, in Hokusai VTE Cancer, recurrent VTE occurred in 7.6% of patients until month 6, compared to 2.1% between months 6 and 12 in those continuing trial treatments beyond 6 months. 9 , 24 In TiCAT, VTE recurrence occurred in 4.5% of patients between 0 and 6 months, and 1.1% of patients between 6 and 12 months. 26 In DALTECAN, the respective recurrence rates were 8.7% until month 6 and 4.1% from 6–12 months. 27 In the COMMAND VTE registry, the recurrence rate at month 6 was 7.8% and between months 6–12 it was 2.8%. Finally, Yhim et al. reported VTE recurrence rates of 11.1% at 6 months and 3.6% from 6–12 months. 31 , 32
Extended anticoagulation treatment during 6–12 months was found to have an acceptable safety profile, with rates of MB between 1% and 4% in patients receiving anticoagulation, compared to rates between 0% and 1% in those without anticoagulation. These rates are generally lower than the MB risk observed in the first 6 months after index VTE, with rates of 4.2% in SELECT‐D, 4.4% in Hokusai VTE Cancer, 2.8% in TiCAT, 7.8% in DALTECAN, and 9.1% in the COMMAND VTE Registry during this timeframe. 9 , 10 , 26 , 27 , 31
We hypothesize that survival bias and sampling bias may partially explain the observed decrease in rates of both recurrent VTE and bleeding in the 6‐ to 12‐month time period compared to the first 6 months. Patients with advanced cancer are at a higher baseline risk of a first VTE event than patients with non‐metastatic cancer and also risk of VTE recurrence, bleeding, and mortality is increased in patients with more advanced disease. 13 , 34 Therefore, patients surviving beyond the initial 6‐month period of cancer‐associated VTE might represent a lower risk population. Further, several studies only reported the first event of VTE recurrence or bleeding during follow‐up after the index VTE as outcome event and therefore, a bias toward a lower risk population observed between 6–12 months might occur.
The observed risk of VTE recurrence balanced against the risk of bleeding during extended anticoagulation supports current guideline recommendation to continue treatment beyond 6 months of the index VTE event in patients with cancer, based on an individualized risk–benefit evaluation (e.g., if the cancer is still active). 5 , 6 , 7 For instance, recent guidance by the American Society of Hematology (ASH) suggests continuing anticoagulation for secondary prevention beyond 6 months in patients with active cancer in the absence of contraindications. Discontinuation of long‐term anticoagulation after cancer‐associated VTE can be considered individually in the absence of ongoing high risk for recurrent VTE. 7 Accordingly, the American Society of Clinical Oncology (ASCO) guidelines state that continued anticoagulation beyond 6 months should be offered to patients with active cancer, including metastatic disease or ongoing chemotherapy treatment based on continued assessment of risks and benefit. 6 The International Initiative on Thrombosis and Cancer (ITAC) practice guidelines suggest using an individual risk–benefit evaluation, tolerability, patient preference, and cancer activity status to decide whether to continue or stop anticoagulation at 6 months after cancer‐associated VTE. 5 A lower threshold to continue anticoagulation beyond 6 months in patients with suspected ongoing risk for VTE recurrence is further supported by a higher case‐fatality rate of recurrent VTE compared to bleeding events in patients with cancer. 14 An overview of these guideline statements is provided in Table S5 in supporting information. However, future dedicated studies are needed to better characterize which patients with cancer continue to be at high risk for VTE recurrence and, thus, need extended anticoagulation treatment, and which patients might be candidates to discontinue anticoagulation safely at 6 months. In addition, given the efficacy of reduced dose or prophylactic dose anticoagulation at 6 months after index VTE events in selected subsets of patients, 35 , 36 comparative studies evaluating the optimal anticoagulation strategy for the long‐term secondary prophylaxis in patients after a diagnosis of cancer‐associated VTE are needed. In this regard, the ongoing randomized controlled API‐CAT study (ClinicalTrials.gov NCT03692065), comparing standard‐dose to low‐dose apixaban after 6 months post index VTE, may provide important information on net clinical benefit of reducing anticoagulation doses after 6 months. Further, the value of RVT as prognostic tool to select patients for extended anticoagulation treatment needs to be clarified.
This systematic review of the literature has some pertinent limitations. Due to the observed heterogeneity between identified studies, we were unable to provide an aggregated quantitative estimate of VTE recurrence and bleeding rates. Further, the designs of identified studies did not allow aggregated comparative analysis between different management strategies, including a comparison of continuing versus discontinuing anticoagulation, or comparing different types of anticoagulation. Additionally, by specifically focusing on the 6‐ to 12‐month timeframe post index VTE, our findings cannot be extrapolated to other timeframes. Finally, most identified studies had a non‐randomized design and therefore, subgroup comparisons of anticoagulation treatments in these studies are infeasible due to underlying indication bias.
In conclusion, in this systematic review of the literature, we found heterogeneous risk of recurrent VTE between 6 and 12 months after index cancer‐associated VTE, with the highest risk reported in patients discontinuing anticoagulation. Further, low bleeding rates in patients receiving extended anticoagulation therapy were reported. These findings support current guideline recommendations to extend anticoagulation beyond 6 months in patients with ongoing active cancer based on an individualized risk–benefit evaluation.
CONFLICTS OF INTEREST
Florian Moik: no potential conflict of interest. Meaghan Colling: no potential conflict of interest. Isabelle Mahé: grants, honoraria, and personal fees for lectures and participation on advisory boards and non‐financial support from BMS‐Pfizer, from Bayer, and Leo‐Pharma. Luis Jara‐Palomares: honoraria for presentations from Bayer Hispania, Actelion, Pfizer, Rovi, LEO Pharma, Daiichi Sankyo; grants, personal fees, and non‐financial support from LEO Pharma, Menarini, MSD, and Roche. Ingrid Pabinger: occasional honoraria for lectures and advisory board meetings from Bayer AG and Pfizer. Cihan Ay: honoraria and personal fees for lectures and participation on advisory boards from Bayer, BMS, Daiichi‐Sankyo, Pfizer, and Sanofi.
AUTHOR CONTRIBUTIONS
FM: conceptualization, literature research, data extraction, data analysis, methodology, interpretation of results, and writing of the manuscript. MC: literature research, data extraction, data analysis, and writing of the manuscript. IM: interpretation of results and writing of the manuscript. LJP: interpretation of results and writing of the manuscript. IP: conceptualization, supervision, interpretation of results, and writing of the manuscript. CA: conceptualization, supervision, interpretation of results, and writing of the manuscript.
Supporting information
Supplementary Material
Moik F, Colling M, Mahé I, Jara‐Palomares L, Pabinger I, Ay C. Extended anticoagulation treatment for cancer‐associated thrombosis—Rates of recurrence and bleeding beyond 6 months: A systematic review. J Thromb Haemost. 2022;20:619–634. doi: 10.1111/jth.15599
Manuscript handled by: Marc Carrier
Final decision: Marc Carrier, 18 November 2021
REFERENCES
- 1. Mulder FI, Horvàth‐Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2021;137(14):1959‐1969. [DOI] [PubMed] [Google Scholar]
- 2. Moik F, Chan W‐SE, Wiedemann S, et al. Incidence, risk factors and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137(12):1669‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moik F, Ay C, Pabinger I. Risk prediction for cancer‐associated thrombosis in ambulatory patients with cancer: past, present and future. Thromb Res. 2020;191:S3‐S11. [DOI] [PubMed] [Google Scholar]
- 4. Moik F, Posch F, Zielinski C, Pabinger I, Ay C. Direct oral anticoagulants compared to low‐molecular‐weight heparin for the treatment of cancer‐associated thrombosis: Updated systematic review and meta‐analysis of randomized controlled trials. Res Pract Thromb Haemostasis. 2020;4(4):550‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566‐e581. [DOI] [PubMed] [Google Scholar]
- 6. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496‐520. [DOI] [PubMed] [Google Scholar]
- 7. Lyman GH, Carrier M, Ay C, et al. American society of hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Advances. 2021;5(4):927‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moik F, Pabinger I, Ay C. How I treat cancer‐associated thrombosis. ESMO Open. 2020;5(1):e000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2018;378(7):615‐624. [DOI] [PubMed] [Google Scholar]
- 10. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36(20):2017‐2023. [DOI] [PubMed] [Google Scholar]
- 11. Agnelli G, Becattini C, Bauersachs R, et al. Apixaban versus dalteparin for the treatment of acute venous thromboembolism in patients with cancer: the caravaggio study. Thromb Haemost. 2018;118(9):1668‐1678. [DOI] [PubMed] [Google Scholar]
- 12. Moik F, Ay C. How I manage cancer‐associated thrombosis. Hamostaseologie. 2020;40(1):38‐46. [DOI] [PubMed] [Google Scholar]
- 13. Prandoni P, Lensing AWA, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484. [DOI] [PubMed] [Google Scholar]
- 14. Abdulla A, Davis WM, Ratnaweera N, Szefer E, Ballantyne Scott B, Lee AYY. A meta‐analysis of case fatality rates of recurrent venous thromboembolism and major bleeding in patients with cancer. Thromb Haemost. 2020;120(4):702‐713. [DOI] [PubMed] [Google Scholar]
- 15. Moik F, Makatsariya A, Ay C. Challenging anticoagulation cases: Cancer‐associated venous thromboembolism and chemotherapy‐induced thrombocytopenia ‐ A case‐based review of clinical management. Thromb Res. 2021;199:38‐42. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019): Cochrane. 2019.
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group atP . Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Accessed February 28, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2013
- 20. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemostasis. 2005;3(4):692‐694. [DOI] [PubMed] [Google Scholar]
- 21. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Anticoagulation tSoCo . Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119‐2126. [DOI] [PubMed] [Google Scholar]
- 22. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Archives of Public Health. 2014;72(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marshall A, Levine M, Hill C, et al. Treatment of cancer‐associated venous thromboembolism: 12‐month outcomes of the placebo versus rivaroxaban randomization of the SELECT‐D Trial (SELECT‐D: 12m). J Thromb Haemostasis. 2020;18(4):905‐915. [DOI] [PubMed] [Google Scholar]
- 24. Di Nisio M, van Es N, Carrier M, et al. Extended treatment with edoxaban in cancer patients with venous thromboembolism: A post‐hoc analysis of the Hokusai‐VTE Cancer study. J Thromb Haemostasis. 2019;17(11):1866‐1874. [DOI] [PubMed] [Google Scholar]
- 25. Napolitano M, Saccullo G, Malato A, et al. Optimal duration of low molecular weight heparin for the treatment of cancer‐related deep vein thrombosis: the Cancer‐DACUS Study. J Clin Oncol. 2014;32(32):3607‐3612. [DOI] [PubMed] [Google Scholar]
- 26. Jara‐Palomares L, Solier‐Lopez A, Elias‐Hernandez T, et al. Tinzaparin in cancer associated thrombosis beyond 6months: TiCAT study. Thromb Res. 2017;157:90‐96. [DOI] [PubMed] [Google Scholar]
- 27. Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemostasis. 2015;13(6):1028‐1035. [DOI] [PubMed] [Google Scholar]
- 28. Poudel SK, Reddy CA, Park DY, et al. Clinical outcomes of cancer‐associated thrombosis beyond 6 months of anticoagulation. Blood. 2019;134(Suppl 1):3458. [Google Scholar]
- 29. Mahé I, Plaisance L, Chapelle C, et al. Long‐term treatment of cancer‐associated thrombosis (CAT) beyond 6 months in the medical practice: USCAT, a 432‐patient retrospective non‐interventional study. Cancers. 2020;12(8):2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt RA, Al Zaki A, Desilet N, et al. Patient characteristics and long‐term outcomes beyond the first 6 months after a diagnosis of cancer‐associated venous thromboembolism. Thromb Res. 2020;188:106‐114. [DOI] [PubMed] [Google Scholar]
- 31. Sakamoto J, Yamashita Y, Morimoto T, et al. Cancer‐associated venous thromboembolism in the real world‐ from the COMMAND VTE registry. Circ J. 2019;83(11):2271‐2281. [DOI] [PubMed] [Google Scholar]
- 32. Yhim HY, Jang MJ, Kwak JY, et al. The incidence, risk factors, and prognosis of recurrent venous thromboembolism (VTE) in patients with advanced solid cancers receiving anticoagulation therapy after the diagnosis of index VTE. Thromb Res. 2013;131(4):e133‐e140. [DOI] [PubMed] [Google Scholar]
- 33. Englisch C, Moik F, Ay C. Risk assessment for recurrent venous thromboembolism in patients with cancer. Thromb Update. 2021;5:100080. [Google Scholar]
- 34. Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemostasis. 2006;4(3):529‐535. [DOI] [PubMed] [Google Scholar]
- 35. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699‐708. [DOI] [PubMed] [Google Scholar]
- 36. Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211‐1222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


