Abstract
The aim of this scoping review is to summarize approaches and outcomes of clinical validation studies of clinical decision support systems (CDSSs) to support (part of) a medication review. A literature search was conducted in Embase and Medline. In total, 30 articles validating a CDSS were ultimately included. Most of the studies focused on detection of adverse drug events, potentially inappropriate medications and drug‐related problems. We categorized the included articles in three groups: studies subjectively reviewing the clinical relevance of CDSS's output (21/30 studies) resulting in a positive predictive value (PPV) for clinical relevance of 4–80%; studies determining the relationship between alerts and actual events (10/30 studies) resulting in a PPV for actual events of 5–80%; and studies comparing output of CDSSs to chart/medication reviews in the whole study population (10/30 studies) resulting in a sensitivity of 28–85% and specificity of 42–75%. We found heterogeneity in the methods used and in the outcome measures. The validation studies did not report the use of a published CDSS validation strategy. To improve the effectiveness and uptake of CDSSs supporting a medication review, future research would benefit from a more systematic and comprehensive validation strategy.
Keywords: adverse drug events, clinical decision support systems, inappropriate prescriptions, validation studies
1. INTRODUCTION
Drug‐related problems (DRP), such as adverse drug reactions (ADRs) and adverse drug events (ADEs), are common. For example, approximately 10% of the patients experience an ADR during hospital stay and approximately 20% of patients experience an ADE in outpatients settings. 1 , 2 ADRs are associated with prolonged hospital stay. 3 About 70% of the ADEs occurring in older hospitalized patients and between 16–42% of the ADEs occurring in outpatients are preventable. 2 , 4 Individualized medication reviews can prevent ADRs/ADEs. During medication reviews, prescribed medications are evaluated by reviewing their indication, interactions and appropriateness to optimize medication use and improve health outcomes. 5
Clinical decision support systems (CDSSs) can support medication reviews and detect potentially inappropriate prescribing. 6 CDSSs can, for example, identify patients in need of a medication review, identify ADRs/ADEs or offer advice on how medications should be monitored or changed. Effectiveness of previous CDSS interventions supporting (part of) a medication review for hospitalized patients varied. CDSSs were mostly effective on process‐related outcomes, but not yet on patient‐related outcomes. 7 , 8
An intervention with a CDSS is complex and must address the needs and wishes of future users. The Two‐Stream Model and the GUIDES checklist describe factors that contribute to the success of CDSSs. 9 , 10 These factors include data quality, underlying clinical knowledge and the presentation and quality of the output/advice. To ensure that the output of a CDSS is accurate, of high quality and useful for clinical practice, the system should be tested and validated. 11 According to the World Health Organization, validation includes proving and documenting that a system leads to the expected results. 12 A medication‐related CDSS validation strategy consists of four steps: (1) technical validation to check whether the CDSS functions as expected, (2) retrospective validation to review whether the output is clinically relevant, actionable and useful, (3) prospective validation before implementation in a real‐life EHR to check whether the CDSS fits in the workflow (e.g., timing and frequency), and finally (4) post‐implementation validation for continuous improvement. 13 , 14 The validation process can prevent irrelevant output for the clinical setting, alert fatigue or low user acceptance. Validation of a CDSS is, therefore, key for successful implementation. 13 , 14 To date, a systematic review was published in 2012 on the detection of ADEs, which found that the accuracy of ADE rules was reported in only 50% of the ADE detection system studies. 15 The rule accuracy was often poor and varied due to multiple factors including differences in validation methods. 15 However, this 2012 systematic review focused solely on ADE detection systems and did not focus on other ways of clinical decision support for medication review. Currently, an overview of published validation methods and outcomes, for the different CDSSs to support (part of) a medication review, is missing. A scoping review can be conducted to systematically identify literature, to clarify concepts or identify knowledge gaps. 16
Therefore, we conducted a scoping review with the aim of creating an overview of validated CDSSs supporting (part of) a medication review. Our secondary aim was to summarize and assess the methods and outcomes of the validation studies.
2. METHODS
A literature search (see Appendix 1 in the Supporting Information) combining the categories corresponding to the concepts of “CDSS”, “medication review”, and “validation” was conducted in Embase and Medline up to 9 August 2021. Search results were deduplicated in Endnote X9. 17 The references of the included articles were checked to find additional relevant articles. The study protocol was published on our departmental website (https://kik.amc.nl/KIK/reports/tech_reports.html), and the completed PRISMA checklist for scoping reviews can be found in Appendix 2 in the Supporting Information. 18
2.1. Eligibility
Articles were screened based on title and abstract by one reviewer (D.M. or B.D.) using pre‐specified eligibility criteria. Title and abstract screening was performed using the Rayyan research app. 19 Studies were included if they described the clinical validation of a CDSS (with or without a prediction model) for medication review, for adults (≥18 years), and were published in English in a peer‐reviewed journal. After title and abstract screening, the full text of the remaining articles were also screened using the eligibility criteria. The full‐text screening was performed by two researchers (D.M. and B.D.), independently. The results were compared, discussed and, if necessary, a third researcher (S.M.) was consulted. CDSS was defined as “any computer program designed to help healthcare professionals to make clinical decisions”. 20 The Pharmaceutical Care Network Europe defines medication review as “a structured evaluation of a patient's medicines with the aim of optimizing medicines use and improving health outcomes. This entails detecting drug related problems and recommending interventions”. 5 We included CDSSs supporting any part of the medication review process. CDSSs during prescribing (order‐entry pop‐ups) were excluded. For clinical validation, we included all studies assessing the quality of the output of the CDSS.
2.2. Data extraction and risk of bias assessment
Data extraction and assessment of the risk of bias were conducted by two researchers (D.M. and B.D.), independently. The results were compared, discussed and, if necessary, a third researcher (S.M.) was consulted. A template for data extraction was developed by J.d.B. and B.D. We extracted study and CDSS characteristics, validation methods and all outcomes related to clinical validation. We used these validation methods and outcomes in a narrative synthesis. The outcomes of individual studies were rated on relevance and applicability on a scale of 1–3 by two researchers (D.M. and B.D.), independently. Furthermore, we assessed the compliance of the studies to the framework of Scheepers‐Hoeks et al. 14 The references of the included studies were screened for additional articles. Risk of bias was assessed using an adapted version of the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2). 21 Originally, this tool was intended for risk of bias assessment in studies determining diagnostic accuracy against a reference standard. We adjusted the signalling questions in the QUADAS‐2 for applicability to CDSS validation studies. We did not exclude studies with a high risk of bias in the narrative analysis.
3. RESULTS
3.1. Study selection
Figure 1 shows the PRISMA flow diagram for article selection. The search in Medline and Embase identified 2434 articles, and search in additional sources identified nine further articles. After screening and eligibility assessment, 30 studies were included in this scoping review. In total, three of the included 30 articles were identified through additional sources (reference checking).
FIGURE 1.
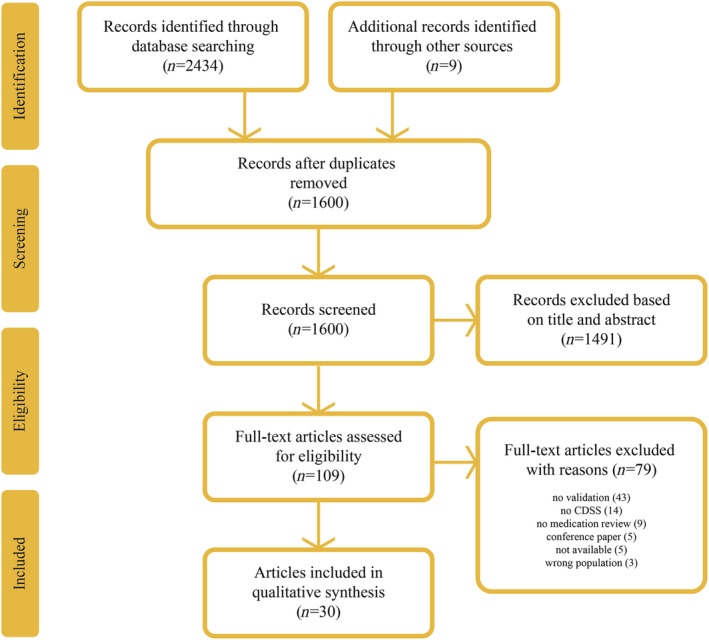
Prisma flow diagram
3.2. Characteristics
The included studies had been performed in 12 countries (Table 1): Belgium, Canada, Germany, Ireland, Israel, Korea, the Netherlands, Portugal, Spain, Sweden, Switzerland and the United States. Studies were published in the years 1998–2020. Of the 30 studies, 26 were conducted in or used data of an inpatient hospital setting, three of primary care, three of an outpatient (clinic), and two of a nursing home. The included studies used different clinical knowledge as input for their CDSSs. The alerts were based on literature, guidelines, experts' experience, prevalence and product guidelines. In two studies, the CDSS was based on machine learning algorithms, including statistical analysis and deep learning with neural networks, and in one study the CDSS used an algorithm with risk scores based on literature. 22 , 23 , 24 Five of the 30 studies described the use of a tool for potentially inappropriate prescribing such as the Beers criteria, or STOPP/START criteria. 25 , 26 , 27 , 28 , 29 Of the included studies, 11 gave support to detect ADEs, six for potentially inappropriate medications (PIMs), five for DRPs, four for ADRs, three for medication surveillance, two for medication errors and two for drug‐related hazardous conditions (DRHCs). The output of the CDSSs were presented as a list, (paper or electronic) report, database with alerts, an extra line in pharmacy module, alerts printed in the pharmacy or as a risk score.
TABLE 1.
Study characteristics and CDSS description of the included studies
| Authors | Study setting | Patients | CDSS description | CDSS input | CDSS output |
|---|---|---|---|---|---|
| Arvisais et al. (2015) 26 Canada | Hospital, all (except four) wards |
n = 182 (200 patient‐days) Mean age = 84 Female = 36% |
Computerized alert system (CAS), used by pharmacists | n = 5 geriatric explicit criteria by expert panel based on Beers criteria and prevalence | Alerts (on HTML page), daily generated by database |
| Azaz‐Livshits et al. (1998) 46 Israel | Hospital, medical ward |
n = 150 patients Mean age = NR Female = 50% |
ADR detection tool | n = 14 ADR signals from laboratory data | List of alerts |
| Buckley et al. (2018) 47 USA | Hospital, ICU and general ward |
n = 634 patients Mean age = 60 non‐ICU, 59 ICU Female = 7% non‐ICU, 42% ICU |
DRHC triggers | n = 20 unique trigger alerts | Paper and electronic reports (webpage within hospital) |
| Cossette et al. (2019) 29 Canada | Primary care |
n = 369 patients Mean age = 77 Female = 71% |
Computerized alert system (CAS) | n = 11 types of geriatric explicit criteria based on 2015 Beers and STOPP v2 criteria | List of patients with PIMs and alert reasons |
| Dalton et al. (2020) 27 Ireland | Hospital |
n = 204 patients Mean age = 77 Female = 49% |
SENATOR software, PIM CDSS for physicians | n = 111 recommendations based on STOPP/START v2 | Report per patient on paper and per email |
| DiPoto et al. (2015) 43 USA | Three hospitals, ICU and general ward |
n = 623 patients Mean age = 60 non‐ICU, 58 ICU Female = 49% non‐ICU, 39% ICU |
Automated surveillance trigger alerts for DRHCs | n = 93 logic‐based trigger rules | Alerts in centralized database |
| Dormann et al. (2000) 48 Germany | Hospital, medical ward |
n = 379 patients Age = 51 Female = 35% |
Computer‐based ADR monitoring system | n = 20 automated laboratory signals | Daily list of alerts with patient name, date of event |
| Eppenga et al. (2012) 44 Netherlands | Hospital |
n = 619 patients Mean age = 53 Female = 54% |
Two medication surveillance CDSSs: (1) Centrasys (iSOFT) (2) Pharmaps advanced CDSS |
n = NR CDSS 1 = G‐standard (Dutch drug database), CDSS 2 = G‐standard + additional expert‐based rules |
CDSS 1: Alerts at one point in time CDSS 2: Generation of alerts once a day |
| Ferrández et al. (2017) 45 Spain | Hospital |
n = 17 878 patients Mean age = 55 Female = 49% |
DRP alert in pharmacy warning system, used by pharmacists | n = 414 medications (7879 alerts), based on literature, selected by two pharmacists | Extra line in pharmacy module. Alerts include patient, dose and recommendations |
| Fritz et al. (2012) 34 Switzerland | Hospital, two general internal wards |
n = 100 patients Median age = 59 Female = 42% |
Three medication surveillance CDSSs: (1) Pharmavista (2) DrugReax (3) TheraOpt |
NR | Alerts |
| Garcia‐Caballero et al. (2018) 28 Spain | Nursing home |
n = 115 patients Mean age = 79 Female = 62% |
PIP screening (polimedication) | n = NR, STOPP criteria | Automated alerts |
| Hammar et al. (2015) 35 Sweden | Two geriatric clinics, three primary care units |
n = 254 patients Mean age = 84 Female = 64% |
Electronic expert support system (EES) for DRPs, used by physicians | n = NR, DDIs based on Swedish databank | Paper‐based reports with potential DRPs |
| Hedna et al. (2019) 24 Sweden | Data from 110 outpatient clinics, 51 primary care units, 29 departments in three hospitals |
n = 745 Mean age = 75 Female = 57% |
PHARAO, risk scores for ADE | n = 9 (p)ADE, algorithm, database of potential interactions | Low, intermediate and high risk with advice |
| Hwang et al. (2008) 49 Korea | Hospital, two ICUs, five general wards |
n = 598 patients Age = NR Female = NR |
ADE monitor, integrated in HIS | n = 46, based on previously studied CDSS in literature, modified by panel | List of alerts with report including medication, ADE, bed location |
| Ibáñez‐Garcia et al. (2019) 36 Spain | Hospital |
n = 25 449 admissions Age = NR Female = NR |
ADE CDSS (HIGEA) | n = 211 clinical rules based on bibliographic search, clinical practice, team | Real‐time list of alerts |
| Jha et al. (1998) 50 USA | Hospital, 9 units |
n = 21 964 patient‐days Age = NR Female = NR |
ADE monitor | n = 52 ADE detection rules based on literature, study team | List of alerts with report including name, bed, event, condition |
| Jha et al. (2008) 37 USA | Hospital, 5 units |
n = 2407 patients Av. age = 74 Female = 53% |
Dynamic pharmacovigilance | n = NR, rule base, using combination of laboratory, medication, patient demographic data | List of alerts |
| Levy et al. (1999) 51 Israel | Hospital, medical ward |
n = 192 patients Age = ~75% > 60 Female = 47% |
ADR detection tool | n = NR, ADR signals from laboratory data (same tool as Azaz‐Livshits et al.) | List of alerts |
| Miguel et al. (2013) 52 Portugal | Hospital |
n = 118 patients Mean age = 60 Female = 41% |
ADR detection tool, stand‐alone (patient data needs to be filled in by hand) | n = 10 ADRs, selection drugs and ADRs based on hospital formulary, INFARMED, book | Alerts with suggested ADRs and frequent ADR for prescribed drugs |
| Peterson et al. (2014) 25 USA | Hospital, general medicine, orthopaedics, urology |
n = 179 patients Mean age = 72 Female = NR |
PIM review dashboard | n = NR, triggers based on Beers and STOPP criteria, anticholinergic risk scale, formulary | List of patients with PIM(s) sorted by highest risk |
| Quintens et al. (2019) 30 Belgium | Hospital |
n = NR Age = 47–74 Female = NR |
Check of medication appropriateness (CMA) | n = 78 clinical rules for PIMs, DRPs and ADEs based on literature and guidelines, selected by team | Once a day generation of list of alerts on a worklist |
| Raschke et al. (1998) 38 USA | Hospital, non‐obstetrical patients |
n = 9306 admissions Age = NR Female = NR |
ADE system | n = 37 ADE rules defined by authors | Alerts printed in pharmacy |
| Rommers et al. (2011) 31 Netherlands | Hospital, general internal ward |
n = NR Age = NR Female = NR |
ADE alerting system (ADEAS), pharmacists | n = 121 clinical expert‐based rules | Every morning, list of alerts |
| Rommers et al. (2013) 39 Netherlands | Hospital, six wards |
n = 931 patients Age = NR Female = NR |
ADE alerting system (ADEAS), pharmacists | n = 121 clinical expert‐based rules | List of alerts for patients with possible ADE |
| Roten et al. (2010) 33 Switzerland | Hospital, internal medicine and geriatric wards |
n = 501 patients Age = NR Female = NR |
DRP screening tool | n = 6 DRP queries based on literature, experience pharmacists, another hospital | List of patients with possible DRPs |
| Schiff et al. (2017) 23 USA | Outpatients |
n = NR Age = NR Female = NR |
Medication errors, outlier detection screening (MedAware) |
n = 1706 alerts, machine learning algorithms identifying outliers |
Alerts with short explanation |
| Silverman et al. (2004) 32 USA | Hospital |
n = NR Age = NR Female = NR |
ADE detection system |
n = NR, ADE detection rules (modified version of Jha et al.) |
List of alerts |
| Segal et al. (2019) 22 Israel | Hospital, internal medicine department |
n = 3160 patients Age = NR Female = NR |
Medication errors/ADE CDSS (MedAware) | n = NR, machine learning algorithms identifying outliers | Alerts after change in clinical state patient |
| de Wit et al. (2015) 40 Netherlands | Hospital, nursing home |
n = 900 patients Age = NR Female = NR |
Medication surveillance CDSS, stand‐alone, pharmacists |
n = 39 clinical rules based on product info, known ADEs, prescribing mistakes |
Alerts |
| de Wit et al. (2016) 41 Netherlands | Hospital, geriatric ward |
n = 33 Mean age = 83 Female = 45% |
Medication review CDSS, stand‐alone, used by pharmacists | n = 469 clinical rules based on literature, guidelines, protocols, multidisciplinary team | DRP alerts with advice to prevent ADE |
ADE, adverse drug event; ADR, adverse drug reaction; CDSS, clinical decision support system; DRHC, drug‐related hazardous conditions; DRP, Drug‐related problems; ICU, intensive care unit; NR, not reported; PIM, potentially inappropriate medication; PIP, potentially inappropriate prescribing.
3.3. Validation methods
All included studies used real‐life patient data for the validation of the CDSSs. The studies included between 33 and 17 878 patients. Four studies did not report information on the number of patients or admissions. 23 , 30 , 31 , 32 Twenty‐one (70%) studies reported how many (type of) rules were included in the knowledge base of the CDSS, ranging from 5 to 7879 alert (types). As Table 2 shows, all studies reported a frequency of generated alerts either as number of alerts or as number of patients/admissions with an alert. Of the 30 articles, eight studies used retrospective data for the validation (step 2 of the validation strategy of Scheepers‐Hoeks et al.), 17 studies used prospective data (step 3), and five studies used post‐implementation data for continuous improvement (step 4). One study assessed the CDSS with a pre‐defined outcome threshold and aimed for a sensitivity of ≥80%. 33 We divided the included studies into Group A, B and/or C (Table 2). Group A assessed clinical validation by subjectively checking the clinical relevance of the alerts (21 studies). Group B checked the association of alerts/advice with actual occurrence of events (10 studies) and group C compared the CDSS's output with a chart/medication review in the whole study population (10 studies).
-
Group A
Clinical relevance of CDSS's output
In 21 of 30 studies, a reviewer checked the output of the CDSS and assessed the clinical relevance through their clinical experience. 22 , 23 , 25 , 26 , 27 , 28 , 29 , 31 , 32 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 The reviewers assessed the clinical relevance with or without checking the charts or contacting a physician/nurse. In 13 of the 21 studies, the reviewer was a pharmacist(s), in two studies a combination of a pharmacist and a physician, in one study a combination of a pharmacists and a radiology technician, in two studies a physician and in three studies the profession of the reviewer was not described.
-
Group B
CDSS's output and occurrence of actual events (in patients with alert)
In 10 of 30 studies, a reviewer checked the patient charts to find a relationship between the output of the CDSS and actual occurrence of an ADR/ADE/DRHC. 24 , 37 , 43 , 46 , 47 , 48 , 49 , 50 , 51 , 52 In eight of the 10 studies, all included patients with a CDSS alert were reviewed and in two studies, a sample of patients was reviewed.
-
Group C
CDSS's output and chart/medication review in the whole population (in patients with or without alert)
In 10 of 30 studies, the clinical validation was assessed by comparing the output of the CDSS with the output of a chart or medication review in the whole population (not only the patients with a CDSS alert). 24 , 33 , 41 , 45 , 46 , 48 , 49 , 50 , 51 , 52 In four of the 10 studies, a pharmacist conducted a medication review to find DRPs/ADEs. In four other studies, a team or clinical pharmacologist reviewed the charts for DRPs/ADEs/ADRs and two studies additionally used voluntary ADE/ADR reports. The reviewers were blinded to the output of the CDSS in four of the 10 studies. 33 , 41 , 48 , 50
TABLE 2.
Validation methods and outcomes of the included studies
| Authors | Group A/B/C and description methods | Outcomes, relevance (R), applicability (A) | Compliance framework 14 step 2, 3, 4 and outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | A | 2 | 3 | 4 | Outcomes | ||||
| Arvisais et al. (2015) 26 | A | Two junior pharmacists (independently) analysed alerts for clinical relevance |
Clinical relevance = 75% (149 with ≥1 clinically relevant alert/200 patient days) |
+ | +++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Azaz‐Livshits et al. (1998) 46 | B | Signals evaluated by expert team |
37% (78 signals related to ADR/212 signals) 29% (25 admissions ADR & signal/86 admissions with alert) |
++ | ++ | X | _ | _ |
PPV (clinical relevance) = no NPV = no Extra = yes |
| C | Clinical pharmacologist reviewed charts for ADRs, evaluated by expert team |
Sn = 66% (25 CDSS/38 admissions by expert team) Sp = 49% (56/115 admissions) |
+++ | + | |||||
| Buckley et al. (2018) 47 | B | Pharmacist reviewed alerts and patient charts to determine causality for DRHCs/ADEs using validated tools |
PPV (DRHCs) = 29% (249/870 alerts) PPV (ADEs) = 5% (47/870) |
++ | ++ | _ | _ | X |
PPV (clinical relevance) = no NPV = no Extra = yes |
| Cossette et al. (2019) 29 | A | Clinical relevance assessment by two pharmacists based on clinical experience |
Clinical relevance: 41% (34/83 alerts) 42% (27/65 patients with ≥1 alert) |
+ | +++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Dalton et al. (2020) 27 | A | Independent analysis by pharmacist and physician (6‐point scale) |
Clinical relevance = 74% (681 relevant/925 alerts) |
+ | +++ | X | _ | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| DiPoto et al. (2015) 43 | A | Pharmacists assessed alerts (clinically relevant: pharmacists proposed change) | Clinical relevance: 40% (ICU, 90/226 alerts), 45% (general ward, 235/525 alerts) | + | +++ | _ | _ | X |
PPV (clinical relevance) = yes NPV = no Extra = yes |
| B | Pharmacist assessed causality trigger and adverse events (subgroup analysis of 161 triggers of 19 rules) | PPV (DRHCs) = 71% (115/161 triggers) | ++ | ++ | |||||
| Dormann et al. (2000) 48 | B | Team of pharmacologist, clinician and pharmacists assessed alerts and patient chart for ADR (Naranjo score) | PPV (ADRs) = 13% (63/501 alerts) | ++ | ++ | _ | X | _ |
PPV (clinical relevance) = no NPV = no Extra = yes |
| C | Compare ADR detected by CDSS with ADRs from spontaneous reporting |
Relative Sn = 74% (34 ADRs CDSS/46 [all] ADRs) Relative Sp = 75% |
+ | + | |||||
| Eppenga et al. (2012) 44 | A | Two pharmacists independently assessed alerts of two CDSSs for clinical relevance (of pharmacist would take action) |
PPV (clinical relevance) CDSS 1 = 6% (150/2607 alert), PPV (clinical relevance) CDSS 2 = 17% (384/2256 alerts) |
+ | +++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Ferrández et al. (2017) 45 | A | Alerts reviewed for clinical relevance (action needed) by pharmacists | Clinical relevance = 20% (2808/13 833 alerts) | + | +++ | _ | _ | X |
PPV (clinical relevance) = yes NPV = no Extra = yes |
| C | Compare DRPs detected by CDSS with pharmacist review | 79% (2808 DRPs by CDSS/3552 [all] DRPs) | +++ | + | |||||
| Fritz et al. (2012) 34 | A | Alerts reviewed for clinical relevance (of pharmacists would take action) by pharmacists AND sensitivity to detect all 33 relevant alerts (identified by pharmacist while reviewing alerts) |
PPV (clinical relevance) = 6%, 8%, 8% (3/53, 29/364, 25/328 alerts), respectively Sn = 9%, 88%, 76% (3/33, 29/33,25/33 relevant alerts), respectively |
++ | +++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = yes |
| Garcia‐Caballero et al. (2018) 28 | A | A physician and psychiatrist reviewed alerts | Relevance = 12% (140/1155 alerts) | + | +++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Hammar et al. (2015) 35 | A | A physician reviewed alerts for clinical relevance as part of medication review | Clinical relevance = 68% (502/740 alerts) | + | +++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Hedna et al. (2019) 24 | B | For each alert, it was determined whether it was related to symptoms | PPV = 0.20–0.25 (low risk: 150/776, intermediate risk: 93/460, high risk: 53/208 alerts) | ++ | ++ | X | _ | _ |
PPV (clinical relevance) = no NPV = yes Extra = yes |
| C | Pharmacists extracted symptoms associated with medications, checked by second reviewer |
Sn = 0.12–0.37 (high–low, patients' symptom & alert/patients' symptom from review) Sp = 0.78–0.95 (low–high) NPV = 0.89–0.90 (high–low) |
++ | + | |||||
| Hwang et al. (2008) 49 | B | Pharmacist reviewed alerts and charts for association alert with ADE | PPV (ADEs) = 21% (148/718) | ++ | ++ | X | − | − |
PPV (clinical relevance) = no NPV = no Extra = yes |
| C | Pharmacist (checked by five other pharmacists) reviewed charts for patients without alert for ADEs | Sn = 79% (148/187 ADEs) | +++ | + | |||||
| Ibáñez‐Garcia et al. (2019) 36 | A | Pharmacist reviewed alerts, and advised physician | 51% (554 with advice/1086 alerts) | + | +++ | − | X | − |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Jha et al. (1998) 50 | B | Reviewer analysed alerts, charts for ADE association (checked by physician) | PPV (ADEs) = 17% (450/2620 alerts) | ++ | ++ | − | X | − |
PPV (clinical relevance) = no NPV = no Extra = yes |
| C | Reviewers (blinded to CDSS) conducted ADE detection study (three methods) | ADEs detected by CDSS = 45% (275/675 [all] ADEs) | +++ | + | |||||
| Jha et al. (2008) 37 | A | Reviewer analysed 52% alerts, and contacted physician if necessary | Clinical relevance = 11% (30 with contact/266 alerts) | + | +++ | − | X | − |
PPV (clinical relevance) = yes NPV = no Extra = yes |
| B | Chart review to identify (potential) ADEs in a sample of patients (checked by physician) |
PPV (ADEs) = 23% PPV (pADEs) = 15% |
++ | ++ | |||||
| Levy et al. (1999) 51 | B | Analyses of signals | 18% (signals related to ADR (52)/all signals [295]) | ++ | ++ | − | X | − |
PPV (clinical relevance) = no NPV = no Extra = yes |
| C | Team reviewed charts for ADRs |
Sn = 62% (40 ADR admissions by tool/65 [all] ADR admissions) Sp = 42% (79/135 admission without ADR) |
+++ | + | |||||
| Miguel et al. (2013) 52 | B | ADRs detected by CDSS reviewed for true ADRs | PPV = 80% (65 true ADRs/81 all suggested ADRs) | ++ | ++ | X | _ | _ |
PPV (clinical relevance) = no NPV = no Extra = yes |
| C | Chart review and assessment by CDSS in population | 83% (10 ADR CDSS/12 ADRs in chart review) | + | ++ | |||||
| Peterson et al. (2014) 25 | A | Pharmacist reviewed patients on dashboard and advised physician |
12% (22 with intervention/179 patients) 6% (31 with interventions/485 alerts [PIMs]) |
+ | +++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Quintens et al. (2019) 30 | A | Pharmacist checked alerts for appropriateness (clinical relevance = electronic note or phone call to physician) | Clinical relevance = 8% (3205 with action/39 481 alerts) | + | +++ | X | _ | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Raschke et al. (1998) 38 | A | Pharmacist/radiology technicians evaluated alerts and advised physician | Relevance = 71% (794 with advice/1116 alerts) | + | +++ | − | X | − |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Rommers et al. (2011) 31 | A | Hospital pharmacists reviewed true positive alerts for clinical relevance (= started intervention) | Clinical relevance = 19% (14 with intervention/72 true positive alert) | + | +++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Rommers et al. (2013) 39 | A | Pharmacists reviewed alerts, contacted and advised physician/nurse |
PPV (clinical relevance) = 8% (204 with advice/2650 alerts) |
+ | +++ | − | X | − |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Roten et al. (2010) 33 | C | Pharmacists conducted medication review (blinded to CDSS) to identify DRPs |
324 patients (65%) with alert Sn = 85% (235 patients by CDSS/276 [all] patients with DRP) Sp = 60% (136/225 [all] patients without DRP) |
+++ | + | − | X | − |
PPV (clinical relevance) = no NPV = no Extra = yes |
| Schiff et al. (2017) 23 | A | Chart of patients with an alert were reviewed for accuracy and clinical validity |
126 alerts: Accuracy = 93% (based on data) Clinical validity (clinical relevance) = 75% |
+ | ++ | X | _ | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Silverman et al. (2004) 32 | A | Pharmacists reviewed alerts, and advised physician (3× with different ADE rules) |
Rule effectiveness (clinical relevance) = 5%, 6%, 13% (169/3117, 452/7390, 792/6136 alerts), respectively |
+ | +++ | _ | _ | X |
PPV (clinical relevance) = yes NPV = no Extra = no |
| Segal et al. (2019) 22 | A | Biweekly interviews to manually review alerts |
315 alerts: Accuracy = 89% (no data issues) Clinical validity = 85% (no justification for medication) Clinical usefulness (clinical relevance) = 80% |
+ | ++ | _ | X | _ |
PPV (clinical relevance) = yes NPV = no Extra = no |
| de Wit et al. (2015) 40 | A | Pharmacists reviewed alerts for clinical relevance (= advised physician) | Efficiency (clinical relevance) = 4% (147/4065 alerts) | + | +++ | _ | _ | X |
PPV (clinical relevance) = yes NPV = no Extra = no |
| de Wit et al. (2016) 41 | A | Pharmacist and geriatrician independently checked DRPs by CDSS |
Clinical relevance = 12% (70/574 alerts) Sn = 72.9% (51 relevant alerts also classified as relevant by CDSS/70 relevant alerts) Sp = 98.6% (497 irrelevant alerts also classified as irrelevant by CDSS/504 irrelevant alerts) |
+ | ++ | X | _ | _ |
PPV (clinical relevance) = yes NPV = no Extra = yes |
| C | Geronto‐pharmacology meeting discussed DRPs from a medication review (blinded to CDSS) | 20% (44 DRPs CDSS/223 DRPs medication review) 28% (70 DRPs CDSS/249 [all] DRPs) | ++ | + | |||||
ADE, adverse drug event; ADR, adverse drug reaction; CDSS, clinical decision support system; DRHC, drug‐related hazardous conditions; DRP, drug‐related problems; Group A, studying clinical relevance of CDSS's output; Group B, CDSS's output and actual occurrence of DRPs (patients with alert); Group C, CDSS's output and chart/medication review in whole population; PIM, potentially inappropriate medication; PPV, positive predictive value; Sn, sensitivity; Sp, specificity.
3.4. Outcome measures and outcomes
-
Group A
Clinical relevance of CDSS's output
Table 2 shows that the 21 studies assessing the clinical relevance of CDSS's output used different names to indicate positive predictive value (PPV) for clinical relevance: (clinical) relevance, clinical usefulness, clinical validity, efficiency, PPV and rule effectiveness. We used the term PPV (clinical relevance) for all studies.
Twenty studies reported the PPV (clinical relevance) of alerts, using a total of 89 974 alerts ranging from 53 to 39 481 alerts (median = 833 alerts, IQR = 318–2639 alerts). A range of 4–80% of alerts were found to be clinically relevant (median = 13%, IQR = 8%–44%). One study reported the PPV (clinical relevance) in patient‐days, finding that 75% of patient‐days had ≥1 clinically relevant alert. 26
-
Group B
CDSS's output and occurrence of actual events (in patients with alerts)
All 10 studies reported the proportion of alerts related to a possible ADE/ADR/DRHC as an outcome. 24 , 37 , 43 , 46 , 47 , 48 , 49 , 50 , 51 , 52 Eight of the 10 studies referred to this outcome as the PPV (Table 2). We used the term PPV for all studies. The PPV for ADEs varied between 5 and 23% (median = 19%, IQR = 14–22%). 37 , 47 , 49 , 50 The PPV for ADRs varied between 13 and 80% (median = 28%, IQR = 17–48%). 46 , 48 , 51 , 52 The PPVs for DRHCs were 29% and 71%. 43 , 47
-
Group C
CDSS's output and chart/medication review in the whole population (in patients with or without alert)
All 10 studies reported the sensitivity (Sn), or related measure, as the proportion of the DRPs/ADRs/ADEs found by CDSSs. 24 , 37 , 43 , 46 , 47 , 48 , 49 , 50 , 51 , 52 The Sn for DRPs were 28%, 79% and 85%. 33 , 41 , 45 The methods to detect ADRs varied and the reported outcomes were therefore not comparable: Sn = 62% (40/65 admissions, ADR detection by multiple methods), Sn = 66% (25/38 admissions, by expert team), Sn = 74% (34/46 ADRs, multiple methods), Sn = 83% (10/12 ADRs, by chart review). 46 , 48 , 51 , 52 The Sn for ADEs were 45% and 79%. 49 , 50 Of the 10 studies, five reported the specificity (Sp). 24 , 33 , 46 , 48 , 51 The Sp for DRPs was 60% and the Sp for ADRs was 42–75%.
3.5. Risk of bias assessment
Most criteria of the assessment were categorized as low or unclear risk of bias for the included studies (Table 3). Twenty studies did not use a reference standard (chart/medication review for the whole population) and therefore the category was not applicable. 22 , 23 , 25 , 26 , 27 , 28 , 29 , 31 , 32 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 42 , 43 , 44 , 47 Two studies scored a high risk of bias for one category. 33 , 41 , 49
TABLE 3.
Risk of bias and applicability concerns of the included studies
| Authors | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Arvisais et al. (2015) 26 | − | − | N/A | N/A | − | − | N/A |
| Azaz‐Livshits et al. (1998) 46 | − | ? | ? | − | − | − | − |
| Buckley et al. (2018) 47 | − | − | N/A | N/A | − | − | N/A |
| Cossette et al. (2019) 29 | − | − | N/A | N/A | − | − | N/A |
| Dalton et al. (2020) 27 | − | − | N/A | N/A | − | − | N/A |
| DiPoto et al. (2015) 43 | − | − | N/A | N/A | − | − | N/A |
| Dormann et al. (2000) 48 | − | − | − | − | − | − | − |
| Eppenga et al. (2012) 44 | − | − | N/A | N/A | − | − | N/A |
| Ferrández et al. (2017) 45 | − | − | − | ? | − | − | − |
| Fritz et al. (2012) 34 | − | − | N/A | N/A | − | − | N/A |
| Garcia‐Caballero et al. (2018) 28 | ? | − | N/A | N/A | − | − | N/A |
| Hammar et al. (2015) 35 | − | − | N/A | N/A | − | − | N/A |
| Hedna et al. (2019) 24 | − | − | − | ? | − | − | − |
| Hwang et al. (2008) 49 | − | − | + | − | − | − | − |
| Ibáñez‐Garcia et al. (2019) 36 | − | − | N/A | N/A | − | − | N/A |
| Jha et al. (1998) 50 | − | − | − | − | − | − | − |
| Jha et al. (2008) 37 | − | − | N/A | N/A | − | − | N/A |
| Levy et al. (1999) 51 | − | ? | ? | − | − | − | − |
| Miguel et al. (2013) 52 | − | − | − | ? | − | − | − |
| Peterson et al. (2014) 25 | − | − | N/A | N/A | − | − | N/A |
| Quintens et al. (2019) 30 | − | − | N/A | N/A | − | − | N/A |
| Raschke et al. (1998) 38 | − | − | N/A | N/A | − | − | N/A |
| Rommers et al. (2011) 31 | − | − | N/A | N/A | − | − | N/A |
| Rommers et al. (2013) 39 | − | − | N/A | N/A | − | − | N/A |
| Roten et al. (2010) 33 | ? | − | − | − | − | − | − |
| Schiff et al. (2017) 23 | − | − | N/A | N/A | − | − | N/A |
| Silverman et al. (2004) 32 | ? | − | N/A | N/A | − | − | N/A |
| Segal et al. (2019) 22 | − | − | N/A | N/A | − | − | N/A |
| de Wit et al. (2015) 40 | − | − | N/A | N/A | − | − | N/A |
| de Wit et al. (2016) 41 | + | − | − | − | − | − | − |
− Low, + High,? Unclear.
4. DISCUSSION
We found 30 articles describing the clinical validation of a CDSS to support (part of) a medication review. Most of the studies focused on detection of ADEs, PIMs and DRPs. The methods used in these 30 articles can be categorized into three groups: (A) studies subjectively assessing the clinical relevance of CDSS's output (21/30), (B) studies describing the association between alerts and actual events (10/30), and (C) studies comparing CDSS's output against chart/medication review of the whole population (10/30).
In our scoping review, we found heterogeneity in the methods used and in the outcome measures. In alignment with our results, the systematic review from 2012 also found heterogeneity in the methods to assess the accuracy of ADE computerized detection rules. 15 We, therefore, divided the studies into three groups. The studies in Groups A and B assessed the quality of the alerts (useful/correct) for patients with an alert. Studies in Group A assessed the quality of the alerts by clinical experience of reviewers. This is a relatively easily applicable method and effective as a first step in assessing the CDSS validity. Studies in Group B assessed the quality of the alerts through determining their association with medication errors (DRHCs, ADEs, ADRs). This increased the validation efforts of the reviewers but also increased the quality and relevance of the assessment. The studies in Group C assessed the quality of the alerts by studying patients with and without an alert (coverage). The medication errors were found in the whole population through a medication review (or chart review, or spontaneous reporting) and these were compared with the output of the CDSSs. This is the most comprehensive and informative method as it gives insights into which medication errors the CDSS captured and which it missed. The outcome measures of Group A are included in the framework of Scheepers‐Hoeks et al., but the reported methods and outcomes of Groups B and C are not (yet) fully included in the framework. 14
The PPV (clinical relevance) of alerts reported in our review was 4–80% with a median of 13%. This median clinical relevance is low, when compared to the desired threshold of ≥89% mentioned in the framework of Scheepers‐Hoeks et al. 14 The PPV (clinical relevance) reported in the studies of our review were calculated using retrospective and prospective data, based on 53–39 481 generated alerts, 33–17 878 patients, 5–7879 number of rules, and different kinds of support (ADE detection, medication surveillance, PIMs, DRPs, DRHCs and medication errors). The two studies using machine learning as input (knowledge base) had a relatively high PPV (clinical relevance) of 75% and 80%. We did not find another variable or pattern that explained the difference between low vs high proportion of relevant alerts. The studies offered commentary on the reasons for low/high clinical relevance and for the impact of the clinical relevance. The clinical relevance would improve with higher data quality, by including more patient characteristics in the CDSSs and by the selection of high impact or prevalent problems. 26 , 30 , 36 , 44 , 50 In the study by Dalton et al., a higher clinical relevance of the computerized recommendations was associated with an increase in implementation of the recommendations by the users. 27 In the study by Segal et al., a new CDSS was compared to a legacy CDSS and this showed higher clinical relevance (85% vs 16%), lower alert burden (0.4% vs 37%) and more changes in practice (43% vs 5%) for the new CDSS. 22 These outcomes are in line with a systematic review on factors influencing the uptake of medication‐related CDSSs in hospitals. That review reported that the most frequent barriers were too many irrelevant alerts and information being not trustworthy. 53 However, a lower clinical relevance of alerts may be accepted when an alert prevents severe adverse events. 14
In 33% of the studies included in our review, the output of the CDSS was compared with a chart/medication review in the whole study population and therefore a CDSS coverage could be calculated (Group C). This percentage is comparable to the systematic review of Forster et al., in which nine out of 24 (38%) studies compared the accuracy of ADE detection rules to a chart review in the whole study population. 15 The reported sensitivity (28–85%) and specificity (42–75%) in our review is within or near the broad range found by Forster et al. of 40–94% (Sn) and 1.4–89% (Sp).
Most studies in our scoping review (17/30) described step 3 (prospective validation) of the validation strategy of Scheepers‐Hoeks et al. 13 , 14 We reviewed clinical validation studies and therefore step 1 (technical CDSS validation) of the strategy was not within the scope of our review. None of the included studies reported all steps (2, 3 and 4) of the validation strategy and none referred to a published CDSS validation strategy. Furthermore, only one study described the expected or desired results (threshold) beforehand. Future studies would benefit from following a more structured and comprehensive validation strategy and including a pre‐defined threshold for PPV, NPV, Sn or Sp.
A strength of our study is that we used a systematic approach with a broad search conducted in two databases and that the screening and data extraction was conducted by two researchers. Another strength is the use of a risk of bias assessment tool. We used an adapted version of the QUADAS‐2 tool and two of the included studies had one domain flagged with a high risk of bias. Validation methods and outcomes not described in the studies were not included in the narrative analysis, which may have resulted in an underrepresentation of the used methods and outcomes.
In conclusion, 30 articles validating a CDSS to support (part of) a medication review were found. These studies varied markedly in methods and outcome measures. Most included studies described the clinical relevance of the alerts and only 33% described a comparison of CDSS's output with all DRPs in the whole population. The studies did not report the use of a CDSS validation strategy. For the validation of their CDSSs, future studies would benefit from following a systematic and comprehensive approach as described in Scheepers‐Hoeks et al., in order to limit irrelevant information, limit alert fatigue and, therefore, improve the uptake of CDSSs supporting (part of) a medication review.
COMPETING INTERESTS
All authors have declared no conflicts of interest.
CONTRIBUTORS
B.D. was involved in study conception/design, data collection, analysis/interpretation of data, and drafting and revision of the manuscript. D.M. and J.B. were involved in study conception/design, data collection, analysis/interpretation of data and revision of the manuscript. J.R., A.A., S.M. and N.V. were involved in study conception/design, analysis/interpretation of data and revision of the manuscript. All authors approved the final version of the manuscript.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGEMENTS
The innovation funds of Amsterdam UMC, location AMC, supported this work. The sponsor had no role in the design, methods, data collection, analysis and preparation of this paper.
Damoiseaux‐Volman BA, Medlock S, van der Meulen DM, et al. Clinical validation of clinical decision support systems for medication review: A scoping review. Br J Clin Pharmacol. 2022;88(5):2035-2051. doi: 10.1111/bcp.15160
Funding information Innovation funds of Amsterdam UMC, location AMC
REFERENCES
- 1. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38(5):437‐453. 10.1007/s40264-015-0281-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977‐989. 10.1345/aph.1P627 [DOI] [PubMed] [Google Scholar]
- 3. Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in‐patients: a prospective analysis of 3695 patient‐episodes. PLoS ONE. 2009;4(2):e4439. 10.1371/journal.pone.0004439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klopotowska JE, Wierenga PC, Stuijt CCM, et al. Adverse drug events in older hospitalized patients: results and reliability of a comprehensive and structured identification strategy. PLoS ONE. 2013;8(8):1‐11. 10.1371/journal.pone.0071045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griese‐Mammen N, Hersberger KE, Messerli M, et al. PCNE definition of medication review: reaching agreement. Int J Clin Pharmacol. 2018;40(5):1199‐1208. 10.1007/s11096-018-0696-7 [DOI] [PubMed] [Google Scholar]
- 6. Damoiseaux‐Volman BA, Medlock S, Ploegmakers KJ, et al. Priority setting in improving hospital care for older patients using clinical decision support. J Am Med Dir Assoc. 2019;20(8):1045‐1047. 10.1016/j.jamda.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 7. Dalton K, O'Brien G, O'Mahony D, Byrne S. Computerised interventions designed to reduce potentially inappropriate prescribing in hospitalised older adults: a systematic review and meta‐analysis. Age Ageing. 2018;47(5):670‐678. 10.1093/ageing/afy086 [DOI] [PubMed] [Google Scholar]
- 8. Damoiseaux‐Volman BA, van der Velde N, Ruige S, Romijn JA, Abu‐Hanna A, Medlock S. Effect of interventions with a clinical decision support system for hospitalized older patients: a systematic review mapping implementation and design factors. JMIR Med Informatics. 2021;9(7):e28023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Medlock S, Wyatt JC, Patel VL, Shortliffe EH, Abu‐Hanna A. Modeling information flows in clinical decision support: key insights for enhancing system effectiveness. J Am Med Inform Assoc. 2016;23(5):1001‐1006. 10.1093/jamia/ocv177 [DOI] [PubMed] [Google Scholar]
- 10. Van de Velde S, Kunnamo I, Roshanov P, et al. The GUIDES checklist: development of a tool to improve the successful use of guideline‐based computerised clinical decision support. Implement Sci. 2018;13(1):86. 10.1186/s13012-018-0772-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sailors RM, East TD, Wallace CJ, et al. Testing and validation of computerized decision support systems. Proc AMIA Annu Fall Symp. 1996:234–238. [PMC free article] [PubMed]
- 12. WHO . WHO Expert Committee on Specifications for Pharmaceutical Preparations. Geneva: World Health Organization; 2019.
- 13. Kubben P, Dumontier M, Dekker A. Fundamentals of Clinical Data Science. Cham, Switzerland: Springer Open; 2018. [PubMed] [Google Scholar]
- 14. Scheepers‐Hoeks AMJ, Grouls RJ, Neef C, Ackerman EW, Korsten EH. Strategy for development and pre‐implementation validation of effective clinical decision support. Eur J Hosp Pharm. 2013;20(3):155‐160. 10.1136/ejhpharm-2012-000113 [DOI] [Google Scholar]
- 15. Forster AJ, Jennings A, Chow C, Leeder C, van Walraven C. A systematic review to evaluate the accuracy of electronic adverse drug event detection. J Am Med Inform Assoc. 2012;19(1):31‐38. 10.1136/amiajnl-2011-000454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De‐duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240‐243. 10.3163/1536-5050.104.3.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 19. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Musen MA, Shahar Y, Shirtliffe E. Clinical decision‐support systems. In: Shortliffe EH, Ciino JJ, eds. Biomedical Informatics: Computer Applications in Health Care and Biomedicine. 3rd ed. Dordrecht: Springer; 2006. [Google Scholar]
- 21. Whiting PF. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529‐536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 22. Segal G, Segev A, Brom A, Lifshitz Y, Wasserstrum Y, Zimlichman E. Reducing drug prescription errors and adverse drug events by application of a probabilistic, machine‐learning based clinical decision support system in an inpatient setting. J Am Med Inform Assoc. 2019;26(12):1560‐1565. 10.1093/jamia/ocz135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schiff GD, Volk LA, Volodarskaya M, et al. Screening for medication errors using an outlier detection system. J Am Med Inform Assoc. 2017;24(2):281‐287. 10.1093/jamia/ocw171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hedna K, Andersson ML, Gyllensten H, Hägg S, Böttiger Y. Clinical relevance of alerts from a decision support system, PHARAO, for drug safety assessment in the older adults. BMC Geriatr. 2019;19(1):164. 10.1186/s12877-019-1179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peterson JF, Kripalani S, Danciu I, et al. Electronic surveillance and pharmacist intervention for vulnerable older inpatients on high‐risk medication regimens. J Am Geriatr Soc. 2014;62(11):2148‐2152. 10.1111/jgs.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arvisais K, Bergeron‐Wolff S, Bouffard C, et al. A pharmacist–physician intervention model using a computerized alert system to reduce high‐risk medication use in elderly inpatients. Drugs Aging. 2015;32(8):663‐670. 10.1007/s40266-015-0286-5 [DOI] [PubMed] [Google Scholar]
- 27. Dalton K, Curtin D, O'Mahony D, Byrne S. Computer‐generated STOPP/START recommendations for hospitalised older adults: evaluation of the relationship between clinical relevance and rate of implementation in the SENATOR trial. Age Ageing. 2020;49(4):615‐621. 10.1093/ageing/afaa062 [DOI] [PubMed] [Google Scholar]
- 28. García‐Caballero TM, Lojo J, Menéndez C, Fernández‐Álvarez R, Mateos R, Garcia‐Caballero A. Polimedication: applicability of a computer tool to reduce polypharmacy in nursing homes. Int Psychogeriatr. 2018;30(7):1001‐1008. 10.1017/S1041610217002411 [DOI] [PubMed] [Google Scholar]
- 29. Cossette B, Taseen R, Roy‐Petit J, et al. A pharmacist‐physician intervention model using a computerized alert system to reduce high‐risk medication use in primary care. Eur J Clin Pharmacol. 2019;75(7):1017‐1023. 10.1007/s00228-019-02660-x [DOI] [PubMed] [Google Scholar]
- 30. Quintens C, De Rijdt T, Van Nieuwenhuyse T, et al. Development and implementation of “Check of Medication Appropriateness” (CMA): advanced pharmacotherapy‐related clinical rules to support medication surveillance. BMC Med Inform Decis Mak. 2019;19(1):29. 10.1186/s12911-019-0748-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rommers MK, Teepe‐Twiss IM, Guchelaar H‐J. A computerized adverse drug event alerting system using clinical rules. Drug Saf. 2011;34(3):233‐242. 10.2165/11536500-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 32. Silverman JB, Stapinski CD, Huber C, Ghandi TK, Churchill WW. Computer‐based system for preventing adverse drug events. Am J Heal Pharm. 2004;61(15):1599‐1603. 10.1093/ajhp/61.15.1599 [DOI] [PubMed] [Google Scholar]
- 33. Roten I, Marty S, Beney J. Electronic screening of medical records to detect inpatients at risk of drug‐related problems. Pharm World Sci. 2010;32(1):103‐107. 10.1007/s11096-009-9352-6 [DOI] [PubMed] [Google Scholar]
- 34. Fritz D, Ceschi A, Curkovic I, et al. Comparative evaluation of three clinical decision support systems: prospective screening for medication errors in 100 medical inpatients. Eur J Clin Pharmacol. 2012;68(8):1209‐1219. 10.1007/s00228-012-1241-6 [DOI] [PubMed] [Google Scholar]
- 35. Hammar T, Lidström B, Petersson G, Gustafson Y, Eiermann B. Potential drug‐related problems detected by electronic expert support system: physicians' views on clinical relevance. Int J Clin Pharmacol. 2015;37(5):941‐948. 10.1007/s11096-015-0146-8 [DOI] [PubMed] [Google Scholar]
- 36. Ibáñez‐Garcia S, Rodriguez‐Gonzalez C, Escudero‐Vilaplana V, et al. Development and evaluation of a clinical decision support system to improve medication safety. Appl Clin Inform. 2019;10(3):513‐520. 10.1055/s-0039-1693426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jha AK, Laguette J, Seger A, Bates DW. Can surveillance systems identify and avert adverse drug events? A prospective evaluation of a commercial application. J Am Med Inform Assoc. 2008;15(5):647‐653. 10.1197/jamia.M2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raschke RA, Gollihare B, Wunderlich TA, et al. A computer alert system to prevent injury from adverse drug events. JAMA. 1998;280(15):1317‐1320. 10.1001/jama.280.15.1317 [DOI] [PubMed] [Google Scholar]
- 39. Rommers MK, Zwaveling J, Guchelaar H‐J, Teepe‐Twiss IM. Evaluation of rule effectiveness and positive predictive value of clinical rules in a Dutch clinical decision support system in daily hospital pharmacy practice. Artif Intell Med. 2013;59(1):15‐21. 10.1016/j.artmed.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 40. de Wit HAJM, Mestres Gonzalvo C, Cardenas J, et al. Evaluation of clinical rules in a standalone pharmacy based clinical decision support system for hospitalized and nursing home patients. Int J Med Inform. 2015;84(6):396‐405. 10.1016/j.ijmedinf.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 41. de Wit HAJM, Hurkens KPGM, Mestres Gonzalvo C, et al. The support of medication reviews in hospitalised patients using a clinical decision support system. Springerplus. 2016;5(1):871. 10.1186/s40064-016-2376-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hulse RK, Clark SJ, Jackson JC, Warner HR, Gardner RM. Computer concepts. Am J Hosp Pharm. 1976;33(10):1061‐1064. [PubMed] [Google Scholar]
- 43. DiPoto JP, Buckley MS, Kane‐Gill SL. Evaluation of an automated surveillance system using trigger alerts to prevent adverse drug events in the intensive care unit and general ward. Drug Saf. 2015;38(3):311‐317. 10.1007/s40264-015-0272-1 [DOI] [PubMed] [Google Scholar]
- 44. Eppenga WL, Derijks HJ, Conemans JMH, Hermens WAJJ, Wensing M, De Smet PAGM. Comparison of a basic and an advanced pharmacotherapy‐related clinical decision support system in a hospital care setting in the Netherlands. J Am Med Inform Assoc. 2012;19(1):66‐71. 10.1136/amiajnl-2011-000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferrández O, Urbina O, Grau S, et al. Computerized pharmacy surveillance and alert system for drug‐related problems. J Clin Pharm Ther. 2017;42(2):201‐208. 10.1111/jcpt.12495 [DOI] [PubMed] [Google Scholar]
- 46. Azaz‐Livshits T, Levy M, Sadan B, Shalit M, Geisslinger G, Brune K. Computerized surveillance of adverse drug reactions in hospital: pilot study. Br J Clin Pharmacol. 1998;45(3):309‐314. 10.1046/j.1365-2125.1998.00685.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buckley MS, Rasmussen JR, Bikin DS, et al. Trigger alerts associated with laboratory abnormalities on identifying potentially preventable adverse drug events in the intensive care unit and general ward. Ther Adv Drug Saf. 2018;9(4):207‐217. 10.1177/2042098618760995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dormann H, Muth‐Selbach U, Krebs S, et al. Incidence and costs of adverse drug reactions during hospitalisation. Drug Saf. 2000;22(2):161‐169. 10.2165/00002018-200022020-00007 [DOI] [PubMed] [Google Scholar]
- 49. Hwang S‐H, Lee S, Koo H‐K, Kim Y. Evaluation of a computer‐based adverse‐drug‐event monitor. Am J Heal Pharm. 2008;65(23):2265‐2272. 10.2146/ajhp080122 [DOI] [PubMed] [Google Scholar]
- 50. Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer‐based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc. 1998;5(3):305‐314. 10.1136/jamia.1998.0050305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levy M, Azaz‐Livshits T, Sadan B, Shalit M, Geisslinger G, Brune K. Computerized surveillance of adverse drug reactions in hospital: implementation. Eur J Clin Pharmacol. 1999;54(11):887‐892. 10.1007/s002280050571 [DOI] [PubMed] [Google Scholar]
- 52. Miguel AI, Azevedo LF, Silva BM, Pereira AC. Resource‐sparing computerized tool for detection of adverse drug reactions. Int J Pharm Technol. 2013;5(1):5118‐5128. [Google Scholar]
- 53. Van Dort BA, Zheng WY, Baysari MT. Prescriber perceptions of medication‐related computerized decision support systems in hospitals: a synthesis of qualitative research. Int J Med Inform. 2019;129:285‐295. 10.1016/j.ijmedinf.2019.06.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


