ABSTRACT
Objective
To construct reference values for fetal urinary bladder distension in pregnancy and use Z‐scores as a diagnostic tool to differentiate posterior urethral valves (PUV) from urethral atresia (UA).
Methods
This was a prospective cross‐sectional study in healthy singleton pregnancies aimed at constructing nomograms of fetal urinary bladder diameter and volume between 15 and 35 weeks' gestation. Z‐scores of longitudinal bladder diameter (LBD) were calculated and validated in a cohort of fetuses with megacystis with ascertained postnatal or postmortem diagnosis, collected from a retrospective, multicenter study. Correlations between anatomopathological findings, based on medical examination of the infant or postmortem examination, and fetal megacystis were established. The accuracy of the Z‐scores was evaluated by receiver‐operating‐characteristics (ROC)‐curve analysis.
Results
Nomograms of fetal urinary bladder diameter and volume were produced from three‐dimensional ultrasound volumes in 225 pregnant women between 15 and 35 weeks of gestation. A total of 1238 urinary bladder measurements were obtained. Z‐scores, derived from the fetal nomograms, were calculated in 106 cases with suspected lower urinary tract obstruction (LUTO), including 76 (72%) cases with PUV, 22 (21%) cases with UA, four (4%) cases with urethral stenosis and four (4%) cases with megacystis‐microcolon‐intestinal hypoperistalsis syndrome. Fetuses with PUV showed a significantly lower LBD Z‐score compared to those with UA (3.95 vs 8.83, P < 0.01). On ROC‐curve analysis, we identified 5.2 as the optimal Z‐score cut‐off to differentiate fetuses with PUV from the rest of the study population (area under the curve, 0.84 (95% CI, 0.748–0.936); P < 0.01; sensitivity, 74%; specificity, 86%).
Conclusions
Z‐scores of LBD can distinguish reliably fetuses with LUTO caused by PUV from those with other subtypes of LUTO, with an optimal cut‐off of 5.2. This information should be useful for prenatal counseling and management of LUTO. © 2021 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: congenital LUTO, fetal cystoscopy, fetal megacystis, fetal therapy, lower urinary tract obstruction, posterior urethral valves, urethral atresia, vesicoamniotic shunt
CONTRIBUTION —
What are the novel findings of this work?
Degree of bladder distension is a proxy for severity of lower urinary tract obstruction (LUTO). Z‐scores of longitudinal bladder diameter can distinguish reliably fetuses with posterior urethral valves (PUV) from those with urethral atresia or other subtypes of LUTO, with a sensitivity of 74% and specificity of 86% using the optimal Z‐score cut‐off of 5.2.
What are the clinical implications of this work?
An accurate antenatal diagnosis of PUV or urethral atresia is crucial in order to avoid unnecessary invasive procedures, to consider the option of fetal therapy only when appropriate and to counsel suitably prospective parents about the prognosis.
INTRODUCTION
The term ‘lower urinary tract obstruction’ (LUTO) covers a heterogeneous group of anatomical anomalies caused by an obstruction of the urethra 1 . The most common cause of LUTO is the presence of posterior urethral valves (PUV), which has a prevalence of 1–2 per 10 000 male live births 2 , 3 . LUTO can also be caused by a complete infravesical obstruction obliterating the most distal portion of the prostatic urethra, known as urethral atresia (UA), which represents the most severe form of LUTO 3 , 4 . These two subtypes of LUTO have different prognosis and management. In patients with PUV (Figure 1), physiological micturition can be restored by endoscopic valve ablation, which can be performed either pre‐ or postnatally 5 . Alternatively, a vesicoamniotic shunt can be placed prenatally to restore amniotic fluid and prevent lung hypoplasia. In contrast, UA (Figure 2) has a poor prognosis, is not amenable to prenatal surgical correction and has a high risk of intrauterine death. Information on UA is scarce regarding postnatal outcome, as liveborn infants are rarely reported in the literature 6 . The differential diagnosis between these two conditions prenatally is very subtle and a definitive diagnosis can be reached only after birth or at postmortem examination. So far, neither prenatal ultrasound nor urine biochemistry have been able to differentiate accurately between these two subtypes of LUTO 7 .
Figure 1.
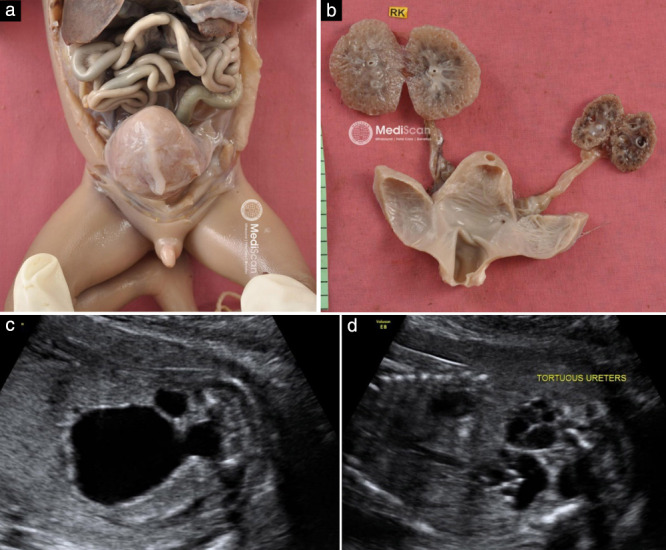
Postmortem examination (a,b) and ultrasound images (c,d) in a 20 + 6‐week fetus with posterior urethral valves, tortuous ureters and multicystic renal dysplasia. RK, right kidney.
Figure 2.

Postmortem examination (a–c) and ultrasound images (d,e) in a 23 + 2‐week fetus with urethral atresia. BLA, bladder; L, left; PU, posterior urethra; URE, ureter.
Previous studies have demonstrated a pivotal role of the degree of bladder distension in the diagnostic and prognostic assessment of megacystis and LUTO 8 , 9 , 10 . However, due to the lack of normative data, bladder distension has not yet been evaluated using an objective, reproducible and gestational‐age (GA)‐specific method. The aim of this study was to develop and validate the clinical use of nomograms of fetal bladder diameter and volume and derived Z‐scores for distinguishing fetuses with PUV from those with other subtypes of LUTO. This would enable tailored antenatal counseling and management in fetuses with congenital LUTO.
METHODS
Development of fetal bladder nomograms
This cross‐sectional prospective study was carried out from May 2016 to October 2017 at the University Medical Center Groningen. Pregnant women with a viable singleton uncomplicated pregnancy with confirmed GA were recruited from the 15th week of gestation until 35 weeks' gestation. GA was established based on a dating scan performed between 8 and 11 weeks. Exclusion criteria were: multiple pregnancy, fetal congenital abnormality detected either before or after birth, use of medication, alcohol or drugs, and maternal disease that could potentially affect fetal growth or diuresis (e.g. diabetes mellitus, smoking, hypertensive disorder). Postnatal data were collected in order to exclude neonates with abnormalities or pathological conditions at birth.
A transabdominal ultrasound examination was performed once for each patient by a trained operator (F.F.), using either a Voluson E8 or an E10 system, equipped with a 2–6‐MHz RM6C transducer (GE Healthcare, Zipf, Austria). The scan lasted 40 min and serial two‐ and three‐dimensional (2D and 3D) ultrasound images of the fetal urinary bladder were collected.
For measurement of urinary bladder volume (BV), 3D sweeps of the lower fetal abdomen were taken, stored and subsequently analyzed digitally with 4D View software (GE Healthcare). BV was calculated using two methods: automated volume calculation (SonoAVC) and manual Virtual Organ Computer‐aided AnaLysis (VOCAL), by tracing the contours of the fluid‐filled area with rotational steps of 30°. The longitudinal bladder diameter (LBD) was measured manually in a precise midsagittal plane, by placing one caliper on the inner border of the bladder wall at the upper pole (bladder dome) and the other on the inner border of the lower pole (bladder neck).
For the study design, patient selection and statistical method, the methods of Ioannou et al. 11 and Altman and Chitty 12 were followed. The measurements were modeled against GA and reference charts were constructed. Polynomial regression models were fitted to the mean and SD of each measurement as functions of GA.
The study was approved by the medical ethics committee in Groningen (dossier number: NL54636.042.15).
Validation of Z‐scores
For clinical validation of the obtained nomograms in a cohort of fetuses with LUTO, cases were collected retrospectively from both The Netherlands (2000–2015) and Mediscan Ultrasound Center in Chennai (2007–2012). The cohort included cases with megacystis referred to one of the eight fetal medicine units (FMUs) in The Netherlands and to Mediscan Ultrasound Center in Chennai. The eight FMUs act as tertiary referral centers for all anomalies suspected in peripheral hospitals in The Netherlands and, similarly, Mediscan Ultrasound Center in Chennai acts as tertiary referral center from the South Asian region for both prenatal diagnosis and fetal therapy.
For each case, the following data were collected: LBD, anteroposterior bladder diameter, transverse bladder diameter, GA at diagnosis and outcome. Ultrasound measurements were either retrieved from the local database or performed on suitable images stored in the database, by a single researcher (F.F.) using the ultrasound machine's built‐in measurement tool. The LBD was obtained from a midsagittal view of the fetus, by measuring the distance from the fetal bladder dome to the bladder neck, as was done for creation of the nomograms. BV was calculated using the formula 13 : LBD × transverse diameter × anteroposterior diameter × π/6.
Final outcome and underlying diagnosis were determined based on the postmortem examination report in cases of termination of pregnancy or perinatal death, and from medical examination or surgery reports for liveborn infants. Cases without an ascertained final diagnosis at postmortem examination or postnatal investigation were not included in this study.
Z‐scores were calculated using the formula: . Predicted LBD was calculated by the formula derived from the fetal nomogram: LBD = 1.48 × GA − 17.15 (Appendix S1).
The accuracy of the Z‐scores was evaluated by receiver‐operating‐characteristics (ROC)‐curve analysis. Antenatal characteristics were compared using the chi‐square test or Fisher's exact test for categorical variables and Student's t‐test for continuous variables. Data analysis was performed using the statistical software package SPSS Statistics 23 (IBM Corp., Armonk, NY, USA).
RESULTS
Development of fetal bladder nomograms
In total, 225 women with singleton pregnancies at different GAs between 15 and 35 weeks participated in the study (Table S1). BV and LBD were measured at 20‐min intervals. In total, 1238 measurements (619 of BV and 619 of LBD) were obtained. BV was measured using both SonoAVC and VOCAL methods.
GA‐based reference charts for the fetal urinary bladder were constructed for largest LBD (Table 1), mean LBD (Table 2), largest BV (Table 3) and mean BV (Table 4). A linear relation was observed between LBD and GA (r 2 = 0.78 for largest LBD and r 2 = 0.76 for mean LBD).
Table 1.
Fitted centiles for largest fetal longitudinal bladder diameter (LBD), according to gestational week, between 15 and 35 weeks' gestation
| Largest LBD (mm) | |||||
|---|---|---|---|---|---|
| GA (weeks) | n | 5th centile | 50th centile | 95th centile | SD |
| 15 | 12 | 3.99 | 6.08 | 8.17 | 1.27 |
| 16 | 11 | 5.43 | 7.79 | 10.15 | 1.43 |
| 17 | 13 | 6.84 | 9.50 | 12.16 | 1.62 |
| 18 | 9 | 8.21 | 11.21 | 14.21 | 1.83 |
| 19 | 10 | 9.53 | 12.92 | 16.31 | 2.06 |
| 20 | 15 | 10.82 | 14.63 | 18.44 | 2.32 |
| 21 | 17 | 12.06 | 16.34 | 20.62 | 2.60 |
| 22 | 15 | 13.25 | 18.05 | 22.85 | 2.92 |
| 23 | 19 | 14.40 | 19.76 | 25.12 | 3.26 |
| 24 | 16 | 15.49 | 21.47 | 27.45 | 3.64 |
| 25 | 12 | 16.53 | 23.18 | 29.83 | 4.04 |
| 26 | 13 | 17.51 | 24.89 | 32.27 | 4.49 |
| 27 | 7 | 18.44 | 26.60 | 34.76 | 4.96 |
| 28 | 14 | 19.30 | 28.31 | 37.32 | 5.48 |
| 29 | 9 | 20.10 | 30.02 | 39.94 | 6.03 |
| 30 | 9 | 20.84 | 31.73 | 42.62 | 6.62 |
| 31 | 6 | 21.51 | 33.44 | 45.37 | 7.25 |
| 32 | 5 | 22.11 | 35.15 | 48.19 | 7.93 |
| 33 | 5 | 22.64 | 36.86 | 51.08 | 8.64 |
| 34 | 4 | 23.10 | 38.57 | 54.04 | 9.41 |
| 35 | 4 | 23.48 | 40.28 | 57.08 | 10.22 |
GA, gestational age.
Table 2.
Fitted centiles for mean fetal longitudinal bladder diameter (LBD), according to exact gestational week, between 15 and 35 weeks' gestation
| Mean LBD (mm) | |||||
|---|---|---|---|---|---|
| GA (weeks) | n | 5th centile | 50th centile | 95th centile | SD |
| 15 | 12 | NC | 5.05 | 11.47 | 3.90 |
| 16 | 11 | NC | 6.53 | 13.32 | 4.13 |
| 17 | 13 | 0.85 | 8.01 | 15.17 | 4.36 |
| 18 | 9 | 1.95 | 9.49 | 17.03 | 4.58 |
| 19 | 10 | 3.06 | 10.97 | 18.88 | 4.81 |
| 20 | 15 | 4.17 | 12.45 | 20.73 | 5.03 |
| 21 | 17 | 5.28 | 13.93 | 22.58 | 5.26 |
| 22 | 15 | 6.38 | 15.41 | 24.44 | 5.49 |
| 23 | 19 | 7.49 | 16.89 | 26.29 | 5.71 |
| 24 | 16 | 8.60 | 18.37 | 28.14 | 5.94 |
| 25 | 12 | 9.70 | 19.85 | 30.00 | 6.17 |
| 26 | 13 | 10.81 | 21.33 | 31.85 | 6.39 |
| 27 | 7 | 11.92 | 22.81 | 33.70 | 6.62 |
| 28 | 14 | 13.03 | 24.29 | 35.55 | 6.85 |
| 29 | 9 | 14.13 | 25.77 | 37.41 | 7.07 |
| 30 | 9 | 15.24 | 27.25 | 39.26 | 7.30 |
| 31 | 6 | 16.35 | 28.73 | 41.11 | 7.53 |
| 32 | 5 | 17.46 | 30.21 | 42.96 | 7.75 |
| 33 | 5 | 18.56 | 31.69 | 44.82 | 7.98 |
| 34 | 4 | 19.67 | 33.17 | 46.67 | 8.21 |
| 35 | 4 | 20.78 | 34.65 | 48.52 | 8.43 |
GA, gestational age; NC, non‐calculable.
Table 3.
Fitted centiles for largest fetal bladder volume (BV), according to exact gestational week, between 15 and 35 weeks' gestation
| Largest BV (cm3) | |||||
|---|---|---|---|---|---|
| GA weeks | n | 5th centile | 50th centile | 95th centile | SD |
| 15 | 12 | NC | 0.85 | 2.10 | 0.64 |
| 16 | 11 | NC | 0.69 | 1.66 | 0.50 |
| 17 | 13 | NC | 0.57 | 1.33 | 0.39 |
| 18 | 9 | NC | 0.53 | 1.13 | 0.31 |
| 19 | 10 | 0.03 | 0.55 | 1.08 | 0.27 |
| 20 | 15 | 0.14 | 0.67 | 1.20 | 0.27 |
| 21 | 17 | 0.27 | 0.89 | 1.52 | 0.32 |
| 22 | 15 | 0.41 | 1.23 | 2.05 | 0.42 |
| 23 | 19 | 0.57 | 1.70 | 2.83 | 0.58 |
| 24 | 16 | 0.76 | 2.32 | 3.87 | 0.79 |
| 25 | 12 | 0.98 | 3.09 | 5.21 | 1.08 |
| 26 | 13 | 1.24 | 4.05 | 6.85 | 1.43 |
| 27 | 7 | 1.55 | 5.20 | 8.84 | 1.86 |
| 28 | 14 | 1.91 | 6.55 | 11.19 | 2.37 |
| 29 | 9 | 2.34 | 8.14 | 13.93 | 2.96 |
| 30 | 9 | 2.83 | 9.96 | 17.09 | 3.64 |
| 31 | 6 | 3.40 | 12.05 | 20.69 | 4.41 |
| 32 | 5 | 4.06 | 14.41 | 24.76 | 5.28 |
| 33 | 5 | 4.81 | 17.07 | 29.32 | 6.25 |
| 34 | 4 | 5.67 | 20.04 | 34.41 | 7.33 |
| 35 | 4 | 6.63 | 23.34 | 40.04 | 8.52 |
GA, gestational age; NC, non‐calculable.
Table 4.
Fitted centiles for mean fetal bladder volume (BV), according to exact gestational week, between 15 and 35 weeks' gestation
| Mean BV (cm3) | |||||
|---|---|---|---|---|---|
| GA (weeks) | n | 5th centile | 50th centile | 95th centile | SD |
| 15 | 12 | NC | 0.69 | 1.99 | 0.66 |
| 16 | 11 | NC | 0.55 | 1.46 | 0.46 |
| 17 | 13 | NC | 0.45 | 1.06 | 0.31 |
| 18 | 9 | NC | 0.39 | 0.79 | 0.21 |
| 19 | 10 | 0.10 | 0.39 | 0.68 | 0.15 |
| 20 | 15 | 0.18 | 0.46 | 0.73 | 0.14 |
| 21 | 17 | 0.24 | 0.60 | 0.96 | 0.18 |
| 22 | 15 | 0.29 | 0.82 | 1.36 | 0.27 |
| 23 | 19 | 0.34 | 1.15 | 1.95 | 0.41 |
| 24 | 16 | 0.40 | 1.58 | 2.75 | 0.60 |
| 25 | 12 | 0.49 | 2.13 | 3.77 | 0.84 |
| 26 | 13 | 0.62 | 2.81 | 5.01 | 1.12 |
| 27 | 7 | 0.79 | 3.64 | 6.49 | 1.45 |
| 28 | 14 | 1.02 | 4.62 | 8.22 | 1.84 |
| 29 | 9 | 1.33 | 5.77 | 10.21 | 2.27 |
| 30 | 9 | 1.72 | 7.10 | 12.48 | 2.74 |
| 31 | 6 | 2.21 | 8.62 | 15.04 | 3.27 |
| 32 | 5 | 2.81 | 10.35 | 17.90 | 3.85 |
| 33 | 5 | 3.53 | 12.30 | 21.07 | 4.47 |
| 34 | 4 | 4.40 | 14.49 | 24.58 | 5.15 |
| 35 | 4 | 5.41 | 16.92 | 28.42 | 5.87 |
GA, gestational age; NC, non‐calculable.
Validation of Z‐scores
In total, 106 cases of megacystis with suspected LUTO were included in the study. The final diagnosis was ascertained based on postmortem examination in 70 (66%) cases and postnatal reports in 36 (34%) cases. PUV was diagnosed in 76 (72%) cases, UA in 22 (21%) cases and urethral stenosis in four (4%) cases. Additionally, megacystis‐microcolon‐intestinal hypoperistalsis (MMIH) syndrome was diagnosed in four (4%) cases. Details of the study population are summarized in Table 5. Further specific characteristics of the study population collected in The Netherlands have been described previously by Fontanella et al. 8 , 10 , 14 , 15 .
Table 5.
Characteristics of retrospective cohort of 106 fetuses with suspected lower urinary tract obstruction
| Outcome | |||||
|---|---|---|---|---|---|
| Final diagnosis | GA at diagnosis (weeks) | TOP | IUFD | Survived | LBD (mm) |
| PUV (n = 76) | 24 ± 6 | 40 | 0 | 36 | 37 ± 17 |
| Urethral atresia (n = 22) | 15 ± 4 | 18 | 4 | 0 | 42 ± 28 |
| Urethral stenosis (n = 4) | 22 ± 6 | 2 | 0 | 2 | 45 ± 17 |
| MMIH (n = 4) | 19 ± 4 | 3 | 1 | 0 | 59 ± 16 |
Data are given as mean ± SD or n.
GA, gestational age; IUFD, intrauterine fetal death; LBD, longitudinal bladder diameter; MMIH, megacystis‐microcolon‐intestinal hypoperistalsis syndrome; PUV, posterior urethral valves; TOP, termination of pregnancy.
Fetuses with PUV presented with a mean LBD of 36.7 mm at a mean GA of 24 weeks. Fetuses with UA presented significantly earlier in gestation (mean, 15 weeks; P < 0.05), with a mean LBD of 42.4 mm. BV could be calculated in only 60 (57%) cases due to the absence of suitable measurements of the anteroposterior and transverse bladder diameters in the remaining cases in the study population.
Z‐scores were calculated using the predicted LBD and SD according to GA and were analyzed by t‐test (Table 6, Appendix S1). Fetuses with PUV had a significantly lower LBD Z‐score compared to those with UA (3.95 vs 8.83; P < 0.01) and also compared with the rest of the study population (3.95 vs 8.22; P < 0.01).
Table 6.
Student's t‐test for Z‐score of longitudinal bladder diameter (LBD) and bladder volume in fetuses with posterior urethral valves (PUV) vs the rest of the patient population (popn) with suspected lower urinary tract obstruction
| P for PUV vs: | ||
|---|---|---|
| Parameter | UA | Rest of popn |
| Z‐score of LBD (n = 106) | < 0.01 | < 0.01 |
| Z‐score of bladder volume (n = 60) | 0.29 | 0.34 |
Seventy‐six cases had PUV; rest of population included 22 cases of urethral atresia (UA), four of urethral stenosis and four of megacystis‐microcolon‐intestinal hypoperistalsis syndrome.
The accuracy of LBD Z‐score in discriminating fetuses with PUV from the rest of the study population was tested using ROC‐curve analysis. This identified 5.2 as the optimal cut‐off, with an area under the curve (AUC) of 0.84 (95% CI, 0.748–0.936) (P < 0.01) (Figure 3), sensitivity of 74% and specificity of 86% for LBD Z‐score < 5.2 in the prediction of PUV.
Figure 3.

Receiver‐operating‐characteristics curve for Z‐score of fetal longitudinal bladder diameter in prediction of posterior urethral valves.
The AUC for BV Z‐score in discriminating fetuses with PUV from the rest of the study population was 0.73 (95% CI, 0.599–0.900) (P = 0.39) (Figure S1). The optimal cut‐off for Z‐score of BV is not reported due to the high rate (43%) of missing data and the subsequent clinical inapplicability of this reference.
DISCUSSION
This study demonstrates that the degree of fetal bladder distension is a proxy for the severity of LUTO. We present for the first time normative data for fetal LBD and BV between 15 and 35 weeks' gestation. These nomograms were then used to develop and validate LBD Z‐scores in fetuses with megacystis. LBD Z‐scores showed good sensitivity and specificity in distinguishing fetuses with PUV from those with UA or other subtypes of LUTO (sensitivity, 74%; specificity, 86%).
Implications for clinical practice and comparison with fetal cystoscopy
The acronym LUTO encompasses a spectrum of anatomical anomalies with different pathophysiology and degree of severity and consequently there is remarkable heterogeneity in the natural history and postnatal prognosis. This makes it particularly challenging to tailor antenatal treatment as well as to evaluate objectively its effectiveness. A reliable differential diagnosis of the underlying subtype of LUTO is therefore an essential prerequisite to make an impact on the overall outcome of fetuses with LUTO.
Until now, the differential diagnosis of PUV or UA prenatally has been ascertainable only through fetal cystoscopy, an invasive and technically challenging procedure that allows direct visualization of the urethral lumen 16 . Using fetal cystoscopy, ‘membrane‐like obstructions’ can be visualized in fetuses with PUV and ablated with guidewire, hydroablation or laser fulguration 16 , 17 , 18 , 19 , 20 . Fetal cystoscopy has the potential to alter the prospective prenatal diagnosis in 25–36% of cases and improve perinatal survival compared with no intervention 16 , 21 . The use of fetal cystoscopy as an alternative to vesicoamniotic shunt placement is, however, still limited by its technical difficulty. Moreover, a considerable risk of miscarriage and spontaneous rupture of the amniotic membranes (amniorrhexis) has been reported 22 . It has been suggested that fetal cystoscopy improves postnatal renal function selectively in fetuses with PUV 18 , while its clinical role in fetuses with other subtypes of LUTO remains arguable due to the nature of the underlying conditions 19 , 20 .
To sum up, fetal cystoscopy has a clear diagnostic advantage in distinguishing the subtypes of LUTO but no clear therapeutic benefit compared with vesicoamniotic shunt placement 21 , 22 . Improvement in the diagnostic accuracy of fetal ultrasound is thus crucial in order to avoid unnecessary invasive procedures and to allow in‐utero therapy to be considered only when appropriate.
Comparison with previous studies
Fetal ultrasound has, so far, been considered of limited value in differentiating PUV from other causes of LUTO 4 , 7 , 23 . Previous studies have reported a mix of sonographic and antenatal criteria supposedly helpful in this challenging differential diagnosis, but all agree on the pivotal role of LBD 24 . In fact, LBD has demonstrated a role in guiding the differential diagnosis of fetal megacystis 8 , 14 , 25 , 26 and in predicting the prognosis of LUTO 15 , 27 , 28 .
However, all of these studies have been affected by two major limitations: the absence of an objective and reproducible definition of fetal megacystis during the second and third trimesters of pregnancy 21 and the absence of an objective stratification of the degree of bladder distension as a proxy for the severity of LUTO. Thus far, the definition of megacystis beyond the first trimester has been heterogeneous, including parameters such as a longitudinal bladder measurement > 99th centile, without referring to any normative data 29 , a bladder reaching the umbilical cord insertion, or, most commonly, a bladder failing to empty within 45 min of observation 5 . All these definitions lack an objective cut‐off to define bladder distension physiologically or pathologically, thus limiting the reproducibility and consistency among studies. Maizels et al. 29 were the first to propose a mathematical formula to calculate LBD according to GA, but this was based on only 39 normal bladder measurements between 15 and 40 weeks' gestation. The study reported a linear relationship between GA and largest LBD and the calculated formula was: LBD = GA – 5. We found a linear relationship between LBD and GA, with the following formula defining the mean physiological LBD: LBD = 1.48 × GA − 17.15. Thanks to the availability of normative data, our study enables definition of Z‐scores to guide this challenging differential diagnosis and, for the first time, reports LBD Z‐scores for PUV, UA, urethral stenosis and MMIH syndrome.
Strengths and limitations
BV can be assessed either by applying a mathematical formula to the three bladder diameters (LBD and anteroposterior and transverse diameters), measured by 2D ultrasound, or by calculating volumes directly on 3D images, taking into account the true shape of the fetal bladder. The 3D technique is the more accurate method for calculating BV 30 . We observed a decrease in BV measurements up until about 20 weeks' gestation. This counterintuitive finding might be explained by an observational bias related to the use of VOCAL for very small volumes. Paraphysiological and transient distension of the fetal bladder due to the development of the autonomic innervation of the bladder in early gestation might also explain our observation 26 , though a similar trend was not found for LBD.
For the validation of Z‐scores in our retrospective cohort, we calculated BV by multiplying the anteroposterior and transverse diameters and LBD obtained from 2D ultrasound images, rather than measuring the volume on the 3D image directly. This method carries a potential bias due to the inaccuracy of available formulae for estimating the fetal BV 30 , 31 . Moreover, due to the retrospective nature of the study, suitable images for the appropriate measurement of anteroposterior and transverse diameters could be identified in only 57% (n = 60) of our cases.
Hedriana and Moore described that, during the filling phase, the fundal portion of the bladder expands faster than does the neck area 31 . This indicates that the bladder can expand faster in the transverse plane than in the longitudinal one. Therefore, we speculate that BV measured on 3D volumes has a stronger prognostic and diagnostic value compared with that of LBD. Future studies are needed to test this hypothesis.
Strengths of this study are, first, its prospective nature and cross‐sectional design, in keeping with best practice for constructing fetal reference charts 11 , 12 , and, second, the availability of detailed postmortem examinations from a large, retrospective cohort for the clinical validation of Z‐scores.
Conclusions
Identifying the best candidates for fetal therapy is of pivotal importance to improving clinical outcome in fetuses with LUTO. We have defined a Z‐score cut‐off for LBD of 5.2 for optimizing the differential diagnosis between PUV and more severe types of LUTO prenatally. This is an important diagnostic tool for guiding management and parental counseling in fetuses with megacystis.
Supporting information
Appendix S1 Clinical example for Z‐score calculation
Figure S1 Receiver‐operating‐characteristics (ROC) curve for fetal urinary bladder volume Z‐score in the prediction of posterior urethral valves. Analysis was limited by a high rate of missing cases (43%).
Table S1 Gestational‐age distribution of our prospective cohort of healthy singleton pregnancies used to construct nomograms of fetal longitudinal bladder diameter and bladder volume between 15 and 35 weeks' gestation
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Morris RK, Kilby MD. An overview of the literature on congenital lower urinary tract obstruction and introduction to the PLUTO trial: Percutaneous shunting in lower urinary tract obstruction. Aust N Z J Obstet Gynaecol 2009; 49: 6–10. [DOI] [PubMed] [Google Scholar]
- 2. Anumba DO, Scott JE, Plant ND, Robson SC. Diagnosis and outcome of fetal lower urinary tract obstruction in the northern region of England. Prenat Diagn 2005; 25: 7–13. [DOI] [PubMed] [Google Scholar]
- 3. Malin G, Tonks AM, Morris RK, Gardosi J, Kilby MD. Congenital lower urinary tract obstruction: A population‐based epidemiological study. BJOG 2012; 119: 1455–1464. [DOI] [PubMed] [Google Scholar]
- 4. Morris RK, Kilby MD. Congenital urinary tract obstruction. Best Pract Res Clin Obstet Gynaecol 2008; 22: 97–122. [DOI] [PubMed] [Google Scholar]
- 5. Haeri S. Fetal Lower Urinary Tract Obstruction (LUTO): a practical review for providers. Matern Health Neonatol Perinatol 2015; 1: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzalez R, De Filippo R, Jednak R, Barthold J. Urethral atresia: long‐term outcome in 6 children who survived the neonatal period. J Urol 2001; 165: 2241–2244. [DOI] [PubMed] [Google Scholar]
- 7. Robyr R, Benachi A, Daikha‐Dahmane F, Martinovich J, Dumez Y, Ville Y. Correlation between ultrasound and anatomical findings in fetuses with lower urinary tract obstruction in the first half of pregnancy. Ultrasound Obstet Gynecol 2005; 25: 478–482. [DOI] [PubMed] [Google Scholar]
- 8. Fontanella F, Duin L, Adama van Scheltema PN, Cohen‐Overbeek TE, Pajkrt E, Bekker M, Willekes C, Bax CJ, Bilardo CM. Fetal megacystis: prediction of spontaneous resolution and outcome. Ultrasound Obstet Gynecol 2017; 50: 458–463. [DOI] [PubMed] [Google Scholar]
- 9. Fontanella F, Duin LK, Adama van Scheltema PN, Cohen Overbeek TE, Pajkrt E, Bekker M, Willekes C, Bax CJ, Gracchi V, Oepkes D, Bilardo CM. Prenatal diagnosis of LUTO: improving diagnostic accuracy. Ultrasound Obstet Gynecol 2018; 52: 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fontanella F, Adama van Scheltema PN, Duin L, Cohen‐Overbeek TE, Paijkrt E, Bakker MN, Willekes C, Oepkes D, Bilardo CM. Antenatal staging of congenital lower urinary tract obstruction. Ultrasound Obstet Gynecol 2019; 53: 520–524. [DOI] [PubMed] [Google Scholar]
- 11. Ioannou C, Talbot K, Ohuma E, Sarris I, Villar J, Conde‐Agudelo A, Papageorghiou AT. Systematic review of methodology used in ultrasound studies aimed at creating charts of fetal size. BJOG 2012; 119: 1425–1439. [DOI] [PubMed] [Google Scholar]
- 12. Altman DG, Chitty LS. Design and analysis of studies to derive charts of fetal size. Ultrasound Obstet Gynecol 1993; 3: 378–384. [DOI] [PubMed] [Google Scholar]
- 13. Campbell S, Wladimiroff JW, Dewhurst CJ. The antenatal measurement of fetal urine production. J Obstet Gynaecol Br Commonw 1973; 80: 680–686. [DOI] [PubMed] [Google Scholar]
- 14. Fontanella F, Maggio L, Verheij JBGM, Duin LK, Adama Van Scheltema PN, Cohen Overbeek TE, Pajkrt E, Bekker M, Willekes C, Bax CJ, Gracchi V, Oepkes D, Bilardo CM. Fetal megacystis: a lot more than LUTO. Ultrasound Obstet Gynecol 2019; 53: 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fontanella F, Duin L, Adama van Scheltema PN, Cohen Overbeek TE, Pajkrt E, Bekker M, Willekes C, Bax CJ, Oepkes D, Bilardo CM. Antenatal Workup of Early Megacystis and Selection of Candidates for Fetal Therapy. Fetal Diagn Ther 2019; 45: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruano R. Fetal surgery for severe lower urinary tract obstruction. Prenat Diagn 2011; 31: 667–674. [DOI] [PubMed] [Google Scholar]
- 17. Ruano R, Yoshizaki CT, Giron AM, Srougi M, Zugaib M. Cystoscopic placement of transurethral stent in a fetus with urethral stenosis. Ultrasound Obstet Gynecol 2014; 44: 238–240. [DOI] [PubMed] [Google Scholar]
- 18. Ruano R, Sananes N, Sangi‐Haghpeykar H, Hernandez‐Ruano S, Moog R, Becmeur F, Zaloszyc A, Giron AM, Morin B, Favre R. Fetal intervention for severe lower urinary tract obstruction: a multicenter case‐control study comparing fetal cystoscopy with vesicoamniotic shunting. Ultrasound Obstet Gynecol 2015; 45: 452–458. [DOI] [PubMed] [Google Scholar]
- 19. Morris RK, Ruano R, Kilby M. Effectiveness of fetal cystoscopy as a diagnostic andtherapeutic intervention for lower urinary tract obstruction:a systematic review. Ultrasound Obstet Gynecol 2011; 37: 629–637. [DOI] [PubMed] [Google Scholar]
- 20. Sananes N, Cruz‐Martinez R, Favre R, Ordorica‐Flores R, Moog R, Zaloszy A, Giron AM, Ruano R. Two‐year outcomes after diagnostic and therapeutic fetal cystoscopy for lower urinary tract obstruction. Prenat Diagn 2016; 36: 297–303. [DOI] [PubMed] [Google Scholar]
- 21. Morris RK, Ruano R, Kilby MD. Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynecol 2011; 37: 629–637. [DOI] [PubMed] [Google Scholar]
- 22. Kilby MD, Morris RK. Fetal therapy for the treatment of congenital bladder neck obstruction. Nat Rev Urol 2014; 11: 412–419. [DOI] [PubMed] [Google Scholar]
- 23. Morris RK, Middleton LJ, Malin GL, Quinlan‐Jones E, Daniels J, Khan KS, Deeks J, Kilby MD, PLUTO Collaborative Group . Outcome in fetal lower urinary tract obstruction: A prospective registry study. Ultrasound Obstet Gynecol 2015; 46: 424–431. [DOI] [PubMed] [Google Scholar]
- 24. Robyr R, Benachi A, Daikha‐Dahmane F, Martinovich J, Dumez Y, Ville Y. Correlation between ultrasound and anatomical findings in fetuses with lower urinary tract obstruction in the first half of pregnancy. Ultrasound Obstet Gynecol 2005; 25: 478–482. [DOI] [PubMed] [Google Scholar]
- 25. Sebire NJ, Von Kaisenberg C, Rubio C, Snijders RJM, Nicolaides KH. Fetal megacystis at 10–14 weeks of gestation. Ultrasound Obstet Gynecol 1996; 8: 387–390. [DOI] [PubMed] [Google Scholar]
- 26. Liao AW, Sebire NJ, Geerts L, Cicero S, Nicolaides KH. Megacystis at 10–14 weeks of gestation: Chromosomal defects and outcome according to bladder length. Ultrasound Obstet Gynecol 2003; 21: 338–341. [DOI] [PubMed] [Google Scholar]
- 27. Fontanella F. Antenatal diagnosis and management of fetal megacystis and lower urinary tract obstruction. PhD thesis. University of Groningen: Groningen; 2019. [Google Scholar]
- 28. Duin LK, Fontanella F, Groen H, Adama van Scheltema PN, Cohen‐Overbeek TE, Pajkrt E, Bekker M, Willekes C, Bax CJ, Oepkes D, Bilardo CM. Prediction model of postnatal renal function in fetuses with lower urinary tract obstruction (LUTO) – Development and internal validation. Prenat Diagn 2019; 39: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 29. Maizels M, Alpert SA, Houston JTB, Sabbagha RE, Parilla BV, MacGregor SN. Fetal bladder sagittal length: A simple monitor to assess normal and enlarged fetal bladder size, and forecast clinical outcome. J Urol 2004; 172: 1995–1999. [DOI] [PubMed] [Google Scholar]
- 30. Peixoto‐Filho FM, Sá RAM, Lopes LM, Velarde LGC, Marchiori E, Ville Y. Three‐dimensional ultrasound fetal urinary bladder volume measurement: Reliability of rotational (VOCALTM) technique using different steps of rotation. Arch Gynecol Obstet 2007; 276: 345–349. [DOI] [PubMed] [Google Scholar]
- 31. Hedriana HL, Moore TR. Accuracy limits of ultrasonograhic estimation of human fetal urinary flow rate. Am J Obstet Gynecol 1994; 171: 989–992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Clinical example for Z‐score calculation
Figure S1 Receiver‐operating‐characteristics (ROC) curve for fetal urinary bladder volume Z‐score in the prediction of posterior urethral valves. Analysis was limited by a high rate of missing cases (43%).
Table S1 Gestational‐age distribution of our prospective cohort of healthy singleton pregnancies used to construct nomograms of fetal longitudinal bladder diameter and bladder volume between 15 and 35 weeks' gestation
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


