Abstract
Mycoplasma arginini, M. fermentans, M. hyorhinis, M. orale, and Acholeplasma laidlawii are the members of the class Mollicutes most commonly found in contaminated cell cultures. Previous studies have shown that the published PCR primer pairs designed to detect mollicutes in cell cultures are not entirely specific. The 16S rRNA gene, the 16S-23S rRNA intergenic spacer region, and the 5′ end of the 23S rRNA gene, as a whole, are promising targets for design of mollicute species-specific primer pairs. We analyzed the 16S rRNA genes, the 16S-23S rRNA intergenic spacer regions, and the 5′ end of the 23S rRNA genes of these mollicutes and developed PCR methods for species identification based on these regions. Using high melting temperatures, we developed a rapid-cycle PCR for detection and identification of contaminant mollicutes. Previously published, putative mollicute-specific primers amplified DNA from 73 contaminated cell lines, but the presence of mollicutes was confirmed by species-specific PCR in only 60. Sequences of the remaining 13 amplicons were identified as those of gram-positive bacterial species. Species-specific PCR primers are needed to confirm the presence of mollicutes in specimens and for identification, if required.
Contamination of biological materials by members of the class Mollicutes (including Mycoplasma and Acholeplasma species) can lead to unreliable experimental results and unsafe biological products (4, 15, 38). Mycoplasma arginini, M. fermentans, M. hyorhinis, M. orale, and Acholeplasma laidlawii are the species found most commonly in cell cultures (8, 25, 33). Many methods of detecting contaminant mollicutes have been described, and each has advantages and disadvantages with respect to cost, time, reliability, specificity, and sensitivity (4, 14, 25, 35). These methods include culture, immunological (5, 29), and DNA staining techniques (25, 27, 34); transmission electron microscopy (2); nucleic acid hybridization (23, 24); and PCR (8, 10, 33).
Recently, PCR methods have been used to detect contaminant mollicutes in cell culture (8, 36, 37, 41). They are rapid and sensitive (8, 10, 33), especially if nested PCR is used, although care is required to avoid contamination (14). Similar methods have been used to investigate a possible etiological role for mycoplasmas in some chronic diseases (3, 6, 9, 30, 39). However, previous studies have shown that none of the published “mycoplasma-specific” primer pairs is entirely specific for Mycoplasma species or other members of the class Mollicutes (8, 9, 17, 33, 36, 37). Species-specific PCR primers are needed to confirm the presence of mollicutes in specimens and for identification. However, only a limited number of species-specific primers have been described (8, 36), and their specificity and sensitivity need to be evaluated by other methods, including the use of other primers, before they can be widely accepted for routine use.
During the development of species-specific PCR for identification of Ureaplasma parvum and U. urealyticum, we found that the 16S-23S rRNA intergenic spacer regions contained more species-specific target sequences than the 16S rRNA genes themselves (20). Others have suggested that the 5′ end of the 23S rRNA gene should contain species-specific regions (22), but the relevant sequences are available in GenBank for only a limited number of mollicutes (11, 33). To supplement available information and to design species-specific primers for identification, we sequenced the 5′ end of the 23S rRNA genes for the most common contaminant mollicutes. In a previous study, we successfully developed rapid-cycle PCR for detection and typing of M. pneumoniae (21); in the present study, we used similar methods to develop fast, practical methods for detection and identification of contaminant mollicutes.
MATERIALS AND METHODS
Mollicute strains.
M. hyorhinis ATCC 17981, M. fermentans ATCC 19989, A. laidlawii ATCC 23206, M. pneumoniae ATCC 29342 (M129) and ATCC 15531 (FH), M. genitalium ATCC 33530, and 14 serovars of U. parvum and U. urealyticum ATCC reference strains were obtained directly from the American Type Culture Collection (ATCC). Human mycoplasmas that are rarely, if ever, detected as contaminants in cell culture were also included in this study to help confirm primer specificity.
Seven strains of M. hominis that had been isolated in our laboratory were identified by sequencing of their 16S-23S rRNA intergenic spacer regions (1, 10). One strain each of M. arginini and M. orale were obtained from Capital Paediatric Institute in Beijing, People's Republic of China, and identified by sequencing the 16S-23S rRNA intergenic spacer regions (1, 10).
Contaminant mollicutes in cell cultures.
Cell cultures were received for mycoplasma-mollicute screening from over 30 clinical and research laboratories in Sydney, Australia. On receipt, one aliquot was frozen and kept as an archive specimen for retesting, if necessary; another aliquot was tested by PCR for mollicute contamination (see below) using primers GPO-3 and MGSO as described by van Kuppeveld et al. (37). DNA samples from 73 cell cultures that gave positive results were stored at −20°C for further study.
Oligonucleotide primers.
The oligonucleotide primers used, including modifications to previously published primers, primer specificities, and expected amplicon lengths are shown in Tables 1 and 2. Primers GPO-1 and 23SA1 were used as outer primers for nested PCR in this study.
TABLE 1.
Positions and sequences of oligonucleotide primers used to target 16S rRNA genes, 16S-23S rRNA intergenic spacer regions, and 5′ ends of 23S rRNA genes of several mollicutesa
| Target and primer name | Tm (°C)b | Primer sequencec |
|---|---|---|
| 16S rRNA genes | ||
| HYR+d | 71.5/96 | 182CATGATGAGTAATA GAA AGG AGC TTC ACA GCT TC216 |
| FER+d | 74.4/82 | 183TTACGGAA AAG AAG CGT TTC TTC GCT GG215 |
| ARG+d | 72.4/86 | 183GATTCCGTT GTG AAA GGA GCC CTT AAA GC210 |
| ORA5d | 75.5/84 | 189TTGTGAAA GGA GCG TTT CGT CCG CTA AG216 |
| ACH3d | 73.1/92 | 265GATGAA(/T)G(/T)CGT AGC CGG ACT GAG AGG TCT AC294 |
| GPO−1e | 76.2/96 | 326ACGGCCCAG(/A/T) ACT C(/T)CT ACG GG(/A)A GGC AGC AGT A356 |
| GPO−3f | 73.1/98 | 762GAAAGC(/T)GTG GG GAG CAA AT(/C)A GGA TTA GAT ACC CT795 |
| UNI−d | 70.5/88 | 797CC(/T)AGGGTATC TAA TCC TG(/A)TT TG CTC CCC AC768 |
| ARG−d | 75.2/84 | 853GCGTTAG CTG CGT CAG TGA ACT CTC CA828 |
| MGSOe | 71.8/86 | 1049CCA(/G) TGC ACC AT(/C)C TGT CA(/T)C(/A/T) T(/A/C)C(/A/T)T(/G/C) GT(/A)T(/A) AAC CTC1020 |
| RNA3d | 76.6/84 | 1068CGA(/G)C ACG AG(/A)C TGA CGA CAA CCA(/G) TGC AC1042 |
| ACHS | 75.7/94 | 791TGA GAA CTA AGT GTT GGG CAA AAG GTC AGT GC822 |
| MOS | 76.1/98 | 798CGC TGT AAA CGA TGA TCA TTA GTC GGT GGA AAA C831 |
| MHS | 73.4/92 | 803CGT AAA CGA TGA TCA TTA GTC GGT GGA GAATC834 |
| MAS | 75.8/100 | 803CCG TAA ACG ATG ATC ATT AGT CGG TGG AGA GTTC836 |
| MFS | 75.2/94 | 803CCC TAA ACG ATG ATC ATT AGC TGA TGG GGA A C833 |
| MHYS | 71.5/94 | 808CGT AAA CGA TGA TCA TTA GTT GGT GGA ATA ATT TC841 |
| MCGpF11g | 47.6/56 | 1383AAA CTA TGG GAG CTG GTA AT1402 |
| 16S–23S rRNA intergenic spacer regions | ||
| ACHA | 70.0/124 | 1564CTA CTT ACT AGT AGT CAT CTT GTG CTA AGT GTT AGT TAG CCT TTC1520 |
| MHA1 | 72.1/106 | 1567CAA AACT GGA TAC AAA TAT TTT TTT TCC ATA ACG TAG GTT G1529 |
| MHYA | 70.0/92 | 1585TAA AGC TCT CAA AAC TAG ACA CGA ATC GAT TAT G1553 |
| MOA | 72.4/92 | 1590CTC AAA ACT GGA TAC GAA TAT TGG GCC ATT AAC1566 |
| MHA2 | 73.3/106 | 1610AAT GTC GAT ATT CAG TTT TCA AAG AAC CGA GAG ATA AAT C1571 |
| MAA | 72.2/104 | 1613CGA TAT TCA GTT TTC AAA GAA CAA ATT GAG AGA TAG GTC1575 |
| MFA | 72.1/118 | 1634CAA AAC TAG ATA TAA AGC TTT AGA CCC ATA AAA AAG CCA CAT AAC1590 |
| SPMHYS | 70.2/114 | 1633GAT TAA CTT CAT ATT TAT TAT TTC AAC GAT CTT TTT TAT AAC CGA G1678 |
| SPMHS | 69.0/122 | 1639GAT CAA CCA TAG AAT ATT TAT ATT TTA TAA GAC AAA CAA TAG GTC ATA C1687 |
| SPACHS | 71.8/116 | 1640GAG ATC TTT GAA AAG TAG ATA AAT GAT GTC TGA AAA GAA ATA AGG1684 |
| SPMAS | 67.7/128 | 1655CAA CCT ATA GAA TAT ATC ATA CAA TAG ACA AAC AAT AGG TCT TAT ACT AC1704 |
| SPMOS | 71.0/114 | 1692GAG ACA AAT ACA AAA ACA AAA CAA TAG GTC AAA AAT ACT TAT ACG1736 |
| SPMFS | 70.8/118 | 1772CAT TGA AAT GTC TTA AAA TAC ACA TCA TAA CAA ACT ATA ACA ATA GG1818 |
| 5′-end of 23S rRNA genes | ||
| 23SS | 63.6/72 | 1820CTA AGA GCT TAT GGT GA(/G)A TGC CTT G1844 |
| R23-1Rg | 54.7/62 | 1854CTC CTA GTG CCA AGG CAT C(/T)C1835 |
| MO23A | 77.9/88 | 1954TTC AAT TCA CAG CGT GTC TCA TCA TTC GGC1924 |
| MHY23A | 74.9/88 | 1979GTT TCA ATT CGC AAC GTG TCT CGC TAA TTT G1949 |
| ACH23A | 76.5/86 | 2001CTT CGA CCG ATT TTC CCA CAT CGT TCA TC1973 |
| MH23A | 77.3/92 | 2009GTC GCA GTC CTA CAA CCC CAA CAA CAT GTG1980 |
| MA23A | 76.7/100 | 2021CTCTAA AAT GTA GTC CTA TAA CCC CAA CAA CGA ATG1986 |
| MF23A | 75.3/88 | 2132TGT AGA TCG TCC TAC AAC CCC CAT TGC TG2104 |
| 23SA1 | 75.5/80 | 2240ACA/G GA/GT TC/TC ACG TGC/T CCC GCC CTA CT2214 |
| 23SA2 | 75.1/110 | 2323CTTTTCACC TTT CCA/C TCA CG/TG TAC TA/GG TTC ACT ATC GGT2286 |
| 23SA3 | 65.7/66 | 2336CCA TCT CTG GGT TCT TTT CAC C2315 |
Specificities of primer pairs are shown in Table 2.
The first primer Tm value is that provided by the primer supplier (Sigma-Aldrich), and the second is that calculated by the formula Tm = [4 × numbers of (G+C)] + [2 × numbers of (A+T)].
Boldface numbers represent the numbered base positions at which primer sequences start and finish (position 1 corresponds to the starting point of U. parvum genome 16S rRNA genes [GenBank accession no. AE002112 and AE002127]). Underlined sequences show bases added to modify previously published primers. Letters in parentheses indicate alternative nucleotides in different Mollicutes species.
Dussurget and Roulland-Dussoix (8).
van Kuppeveld et al. (36).
van Kuppeveld et al. (37).
Harasawa et al. (10).
TABLE 2.
Specificities and expected lengths of amplicons of different oligonucleotide primer pairs
| Primer paira | Specificity | Length of amplicon (bp) |
|---|---|---|
| GPO-1–MGSO | Mycoplasmas-mollicutes | 724 |
| GPO-1–UNI− | Mycoplasmas-mollicutes | 472 |
| GPO-3–MGSO | Mycoplasmas-mollicutes | 288 |
| GPO-3–RNA3 | Mycoplasmas-mollicutes | 307 |
| MHS–MHA1 | M. hominis | 765 |
| MHS–MHA2 | M. hominis | 808 |
| SPMHS–MH23A | M. hominis | 371 |
| HYR+–UNI− | M. hyorhinis | 616 |
| MHYS–MHYA | M. hyorhinis | 778 |
| SPMHYS–MHY23A | M. hyorhinis | 347 |
| FER+–UNI− | M. fermentans | 610 |
| MFS–MFA | M. fermentans | 832 |
| SPMFS–MF23A | M. fermentans | 361 |
| ARG+–ARG− | M. arginini | 671 |
| MAS–MAAb | M. arginini | 811 |
| SPMAS–MA23A | M. arginini | 367 |
| ORA5–UNI− | M. orale | 609 |
| MOS–MOA | M. orale | 793 |
| SPMOS–MO23A | M. orale | 263 |
| ACH3–UNI− | A. laidlawii | 533 |
| ACHS–ACHA | A. laidlawii | 774 |
| SPACHS–ACH23A | A. laidlawii | 362 |
DNA preparation and PCR.
DNA preparation and PCR mixtures were as previously described (18, 19). In each PCR, the positive and negative controls were processed in parallel with the tested samples to identify possible false-negative results and contamination. Thermal profiles for modified putative mycoplasma (or mollicute)-specific primers were as follows: for single-step PCR, the denaturation, annealing, and elongation temperatures and times used were 96°C for 1 s, 68°C for 1 s, and 74°C for 10 s, respectively, for 40 cycles, using a Perkin-Elmer Thermal Cycler 9600. For nested PCR, the first-step denaturation, annealing, and elongation temperatures and times used were 96°C for 1 s, 70°C for 1 s, and 74°C for 30 s, respectively, for 25 cycles. For the second step, the denaturation, annealing, and elongation temperatures and times used were 96°C for 1 s, 68 to 70°C (according to their melting temperature [Tm] values) for 1 s, and 74°C for 10 s, respectively, for 30 cycles.
Twelve microliters of PCR product was analyzed by gel electrophoresis using 1.5% agarose; gels were stained with 0.5 g of ethidium bromide per liter, and visible bands with appropriate size on a UV transilluminator were read as positive results.
Detection of mollicutes by several putative mycoplasma-specific primer pairs.
Specimens that were positive using the GPO-3–MGSO primer pair, but in which the presence of mollicutes was not subsequently confirmed using species-specific PCR (presumed false-positive specimens), were retested using four other published, putative mycoplasma-specific primer pairs: GPO-1–MGSO, GPO-1–UNI−, GPO-3–RNA3, and MCGpF11–R23-1R. We used PCR conditions as previously described (8, 36, 37) as well as more stringent conditions, namely, higher annealing temperatures (55 to 70°C, according to Tm values of the primers), shorter annealing and extension times (1 and 10 s, respectively), and a lower primer concentration (10 pmol).
Culture and identification of bacteria.
Archived aliquots of the (presumed) false-positive specimens were cultured for bacteria on horse blood agar, which was incubated at 37°C in 5% CO2 and examined for growth daily for 5 days. Bacterial isolates were identified at least to genus level by appropriate conventional methods, including Gram staining; determination of catalase, oxidase, and coagulase activities; esculin test; and determination of bacitracin sensitivity.
Identification of mollicutes and bacteria by directly sequencing and sequence searching.
All of the ATCC and other control strains, and 10 individual cell line contaminant mycoplasmas that had been confirmed by species-specific PCR, were identified by sequencing of amplified fragments of the 16S-23S rRNA intergenic spacer regions, using the primer pair MCGpF11–R23-1R. In addition, amplicons of 13 cell line contaminants that were not identified by species-specific PCR were also sequenced, using primers GPO-3–RNA3 and GPO-3 for amplification and sequencing, respectively. Sequencing was performed using an ABI 373A sequencing machine with Applied Biosystems Taq DyeDeoxy Terminator Cycle-Sequencing Ready Reaction kits according to the manufacturer's instructions. The sequence search was performed with the FastA program in the SeqSearch program group, provided in WebANGIS, ANGIS (3rd version; Australian National Genomic Information Service).
Sequencing the 5′ end of 23S rRNA genes.
After comparing the sequences of all mollicute 23S rRNA genes available in GenBank (see Table 3), we designed primers 23SS, 23SA3, and 23SA2 for sequencing the 5′ end of the 23S rRNA genes, which were located in the conserved regions of the 5′ end of the 23S rRNA genes.
TABLE 3.
Summary of GenBank sequence accession numbers of the 16S rRNA genes, 16S-23S rRNA intergenic spacer regions, and 23S rRNA genes used as reference strains
| Species | GenBank sequence accession no. of:
|
||
|---|---|---|---|
| 16S rRNA gene | 16S-23S rRNA intergenic spacer region | 23S rRNA gene | |
| M. arginini | M24579, U15794, AF125581 | X58560 | |
| M. fermentans | M24289, AF031374 | X58553 | |
| M. hyorhinis | M24658, AF121891, AF121890 | X58555 | |
| M. orale | M24659 | X58556 | |
| A. laidlawii | M23932, U14905 | D13259, D13260 | |
| M. hominis | M24473, M96660, AJ002265-AJ002270 | X58559 | |
| M. pneumoniae | D14528, AF132740, AF132741 | X68422, U00089 | |
| M. genitalium | D14526 | U39694 | |
| M. capricolum | X00921, X00922 | ||
| M. hyopneumoniae | X68421 | ||
| M. gallisepticum | AF036708, L08897 | ||
| M. flocculare | L22210 | ||
| U. parvum | AE002112, AE002127 | ||
Multiple-sequence alignments.
Multiple-sequence alignments were performed with the Pileup and Pretty programs in the Multiple Sequence Analysis program group, provided in WebANGIS, ANGIS (3rd version; Australian National Genomic Information Service).
Nucleotide sequence accession numbers.
The 16S-23S rRNA intergenic spacer regions and the 5′ ends of the 23S rRNA genes that we sequenced will appear in the GenBank nucleotide sequence databases with the following accession numbers: AF294989 (M. hominis, clinical isolate 1), AF294990 (M. hominis clinical isolate 2, one A deletion), AF294991 (M. hominis clinical isolate 3, two A deletions), AF294992 (M. fermentans), AF294993 (M. hyorhinis), AF294994 (M. arginini), AF294995 (M. orale), and AF294996 (A. laidlawii). They will also appear in the EMBL nucleotide sequence databases in Europe and the DDBJ in Japan.
The GenBank references, for sequence data of 16S rRNA genes, 16S-23S rRNA intergenic spacer regions, and 23S rRNA genes used in this study, are shown in Table 3.
RESULTS
Specific identification of mollicutes by sequencing the 16S–23S rRNA intergenic spacer regions.
Our sequencing results showed that the 16S-23S rRNA intergenic spacer regions of M. arginini, M. hyorhinis, M. orale, A. laidlawii, M. pneumoniae, and M. genitalium were identical to the corresponding sequences in GenBank (Table 3). There was an additional C for M. fermentans in position 182, compared with the corresponding sequences in GenBank (X58553), which was confirmed by sequencing several times. Two of seven strains of M. hominis were identical to those in GenBank (X58559); the other strains had deletions of one (four strains) or two (one strain) A nucleotides in a polyadenine region (8A; positions 176 to 183) located at the 5′ end of the 16S-23S rRNA intergenic spacer regions. The results were confirmed by resequencing each strain twice. By sequencing the 16S-23S rRNA intergenic spacer regions, we identified DNA amplified from 10 contaminated cell lines as M. hyorhinis (five), M. fermentans (four), and M. arginini (one).
Sequencing the 5′ end of 23S rRNA genes.
We amplified approximately 500-bp DNA sequences of the 5′ end of the 23S rRNA genes of M. hyorhinis, M. fermentans, M. arginini, M. orale, M. hominis, and A. laidlawii. We designed the antisense outer primer 23SA1 for nested PCR from the conserved region at the 5′ end of the 23S rRNA gene and antisense species-specific primers (MO23A, MHY23A, ACH23A, MA23A, and MF23A) from variable sites. Forward-species-specific primers (SPMOS, SPMHYS, SPACHS, SPMAS, and SPMFS) originated at the 3′ end of the 16S-23S rRNA intergenic spacer regions to complete the primer pairs for the most common contaminant mollicutes (see Tables 1 and 2 for sequences and their specificities, respectively).
Specificity of PCR using species-specific primer pairs.
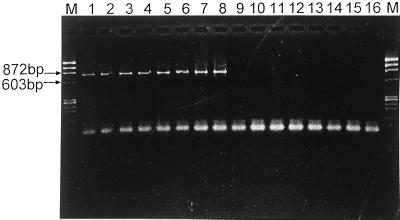
The lengths of amplicons, obtained using different primer pairs, are shown in Table 2, and a representative example is shown in Fig. 1. We evaluated primer pair specificity (based on nested PCR) in three ways. (i) Using reference strains, all the species-specific primer pairs produced the expected amplicon length from six corresponding mollicute species but from none of the control species. (ii) Retesting of the 73 presumed mollicute-contaminated cell lines by PCR, using our newly designed species-specific primers and another published set of species-specific primer pairs (8), gave similar results, confirming the specificity of our primer pairs. (iii) For 10 contaminant mycoplasmas from cell lines (5 M. hyorhinis, 4 M. fermentans, and 1 M. arginini), identification by sequencing the 16S-23S rRNA intergenic spacer regions corresponded with the species-specific PCR result. This further confirmed the specificity of three species-specific primer pairs (M. hyorhinis, M. fermentans, and M. arginini).
FIG. 1.
Results of PCR amplification using primers MAS and MAA. Lanes M, molecular weight markers ΦX174 DNA/HaeIII; lanes 9 to 16, U. urealyticum serovar 8 ATCC 27618, M. hyorhinis ATCC 17981, M. pneumoniae ATCC 15531 (FH), M. genitalium ATCC 33530, A. laidlawii ATCC 23206, M. fermentans ATCC 19989, M. orale, and M. hominis reference strains, respectively; lane 8, M. arginini reference strain; lanes 1 to 7, seven M. arginini-positive specimens.
Sensitivity of PCR.
We did not evaluate the sensitivity of individual primer pairs quantitatively. However, our species-specific primer pairs formed amplicons when DNA extracts of the corresponding reference strains were diluted to 10−3 to 10−4 for one-step PCR and 10−4 to 10−5 for nested PCR. Because of its 10- to 100-fold-greater sensitivity, nested PCR was used in this study. In addition, as indicated above, similar results were obtained for species-specific nested PCR testing of the 73 presumed mycoplasma-contaminated cell lines using either our newly designed species-specific primers or a previously published set (8), suggesting that they have comparable sensitivities when applied to specimens.
Species identification in mollicute-contaminated cell lines.
The presence of one or more Mycoplasma species was confirmed in 60 of 73 presumed mycoplasma- or other mollicute-contaminated cell lines using species-specific PCR assays. Twenty-five cell lines contained M. hyorhinis only, 12 contained M. fermentans only, 8 contained M. arginini only, 7 contained M. hyorhinis and M. arginini, 3 contained M. fermentans and M. hyorhinis, 3 contained M. fermentans and M. arginini, and 2 contained M. fermentans, M. orale, and M. arginini. Neither A. laidlawii nor M. hominis was detected by PCR.
PCR using different putative mycoplasma-specific primers.
PCR assays of all 13 remaining cell lines were also positive when additional putative mycoplasma-specific primers, GPO-1–MGSO, GPO-1–UNI−, and GPO-3–RNA3, were used, even under highly stringent conditions. A PCR product was obtained from 6 of the 13 samples when the primers MCGpF11 and R23-1R were used.
Identification of putative mycoplasma-specific PCR-positive but species-specific PCR-negative organisms.
Despite positive results in several PCR assays with putative mycoplasma-specific primers, the presence of mollicutes was not confirmed by species-specific PCR in 13 of 73 cell lines. Therefore, portions of the 16S rRNA genes were amplified using GPO-3–RNA3 (RNA3 located 22 bp downstream of the primer MGSO) and sequenced. None of these 13 contaminant organisms was identified as a mollicute, based on comparisons with 16S rRNA gene sequences available in GenBank.
Matching of sequences amplified from these cell lines with those available in GenBank showed that all were identical or closely related to those of various bacterial species. Bacterial cultures of 6 of these 13 cell lines were also positive. Five cell lines contained sequences closely related to Staphylococcus spp. (GenBank accession no. L37601, X66101, and D83366), and Staphylococcus spp. were isolated from three of the five. Four cell lines contained sequences closely related to Enterococcus spp. (GenBank accession no. Y18341 and Y18293), but culture produced no growth. Two cell lines contained sequences related to Bacillus spp. (GenBank accession no. Z99107 and Z99104), and Bacillus spp. were isolated from both, mixed with a Micrococcus spp. in one and a Streptococcus spp. in the other. Two specimens contained sequences related to Streptococcus spp. (GenBank accession no. AF003929), and Streptococcus spp. were isolated from one of these.
DISCUSSION
Sequence analysis is, increasingly, the basis of improved bacterial detection methods based on PCR (1). The 16S rRNA genes, 16S-23S rRNA intergenic spacer regions, and 23S rRNA genes have been widely used as targets to detect and identify many different types of bacteria (1, 7, 12, 22, 24). After comparing intra- and interspecies sequence homology and diversity of the 16S rRNA genes, 16S-23S rRNA intergenic spacer regions, and 23S rRNA genes individually, we studied these three regions as a whole (28, 40). This approach makes it much easier to design species-specific primers than when using the 16S rRNA genes, the 16S-23S rRNA intergenic spacer regions, and the 23S rRNA genes separately (1, 20).
In this study, we designed two sets of species-specific primer pairs. Forward primers were located at the 3′ end of the 16S rRNA gene and at the 3′ end of the 16S-23S rRNA intergenic spacer region; antisense primers were located at the 5′ end of the 16S-23S rRNA intergenic spacer region and the 5′ end of the 23S rRNA gene. As in a previous study (21), primers were designed with high Tm values; after optimizing the thermal profiles (13, 32), the fast-cycle nested PCR assays could be completed within 1.5 h.
Using several different mollicute species-specific primer pairs, we detected mollicute DNA in 60 of 73 cell lines that had given positive results using GPO-3–MGSO. GPO-3–RNA3-amplified sequences from other presumed mollicute-specific PCR-positive cell lines most closely matched those of various bacterial species. These results indicate that PCR is more sensitive than culture for detecting contamination but not necessarily mycoplasma-specific contamination, unless combined with sequencing. Similar findings have been reported previously (8, 16). The MGSO primer sequence was not found in amplicons from these 13 specimens (16), and we assumed that the false-positive results were due to mismatch at the 3′ end of the primer, where GC content is high (26, 31). Further evaluation showed that the putative mycoplasma-specific primers GPO-1–MGSO, GPO-1–UNI−, GPO-3–RNA3, and MCGpF11–R23-1R also formed amplicons from some or all of the 13 false-positive specimens, confirming their lack of specificity (8).
These results indicate that until more accurate class-specific primers are available, species-specific PCR primers are needed to confirm the presence of contaminant mollicutes in cell cultures reliably, although this makes screening more complicated and expensive (33). Cell cultures with positive mycoplasma-mollicute PCR results not confirmed by species-specific PCR cannot be assumed to contain other less common contaminant species. For practical use, only one set of species-specific primer pairs is needed for identification of common contaminant mollicutes.
In conclusion, we designed two sets of species-specific primer pairs, based on the combined sequences of the 16S rRNA genes, the 16S-23S rRNA intergenic spacer regions, and the 5′ end of the 23S rRNA genes, to detect the mollicutes most commonly found in contaminated cell cultures. Primers were designed with high Tm values, that enabled us to develop fast-cycle PCR, which was able to significantly shorten the PCR time. Evaluation of the primary fast-cycle PCR, using ATCC and cell culture specimens or isolates from them, showed that our new primers were highly specific.
ACKNOWLEDGMENT
We thank Mark Wheeler for help in sequencing.
REFERENCES
- 1.Barry T, Colleran G, Glennon M, Dunican L K, Gannon F. The 16s/23s ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1991;1:51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Barth O M, Majerowicz S. Rapid detection by transmission electron microscopy of mycoplasma contamination in sera and cell cultures. Mem Inst Oswaldo Cruz. 1988;83:63–66. doi: 10.1590/s0074-02761988000100009. [DOI] [PubMed] [Google Scholar]
- 3.Baseman J B, Tully J G. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis. 1997;3:21–32. doi: 10.3201/eid0301.970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benisheva T, Sovova V, Ivanov I, Opalchenova G. Comparison of methods used for detection of mycoplasma contamination in cell cultures, sera, and live-virus vaccines. Folia Biol (Praha) 1993;39:270–276. [PubMed] [Google Scholar]
- 5.Blazek R, Schmitt K, Krafft U, Hadding U. Fast and simple procedure for the detection of cell culture mycoplasmas using a single monoclonal antibody. J Immunol Methods. 1990;131:203–212. doi: 10.1016/0022-1759(90)90191-w. [DOI] [PubMed] [Google Scholar]
- 6.Chan P J, Seraj I M, Kalugdan T H, King A. Prevalence of mycoplasma conserved DNA in malignant ovarian cancer detected using sensitive PCR-ELISA. Gynecol Oncol. 1996;63:258–260. doi: 10.1006/gyno.1996.0316. [DOI] [PubMed] [Google Scholar]
- 7.Clayton R A, Sutton G, Hinkle P S, Jr, Bult C, Fields C. Intraspecific variation in small-subunit rRNA sequences in GenBank: why single sequences may not adequately represent prokaryotic taxa. Int J Syst Bacteriol. 1995;45:595–599. doi: 10.1099/00207713-45-3-595. [DOI] [PubMed] [Google Scholar]
- 8.Dussurget O, Roulland-Dussoix D. Rapid, sensitive PCR-based detection of mycoplasmas in simulated samples of animal sera. Appl Environ Microbiol. 1994;60:953–959. doi: 10.1128/aem.60.3.953-959.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haier J, Nasralla M, Franco A R, Nicolson G L. Detection of mycoplasmal infections in blood of patients with rheumatoid arthritis. Rheumatology (Oxford) 1999;38:504–509. doi: 10.1093/rheumatology/38.6.504. [DOI] [PubMed] [Google Scholar]
- 10.Harasawa R, Mizusawa H, Nozawa K, Nakagawa T, Asada K, Kato I. Detection and tentative identification of dominant mycoplasma species in cell cultures by restriction analysis of the 16S–23S rRNA intergenic spacer regions. Res Microbiol. 1993;144:489–493. doi: 10.1016/0923-2508(93)90057-9. [DOI] [PubMed] [Google Scholar]
- 11.Harasawa R. Genetic relationships among mycoplasmas based on the 16S–23S rRNA spacer sequence. Microbiol Immunol. 1999;3:127–132. doi: 10.1111/j.1348-0421.1999.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 12.Harasawa R, Kanamoto Y. Differentiation of two biovars of Ureaplasma urealyticum based on the 16S–23S rRNA intergenic spacer region. J Clin Microbiol. 1999;37:4135–4138. doi: 10.1128/jcm.37.12.4135-4138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris S, Jones D B. Optimisation of the polymerase chain reaction. Br J Biomed Sci. 1997;54:166–173. [PubMed] [Google Scholar]
- 14.Hopert A, Uphoff C C, Wirth M, Hauser H, Drexler H G. Specificity and sensitivity of polymerase chain reaction (PCR) in comparison with other methods for the detection of mycoplasma contamination in cell lines. J Immunol Methods. 1993;164:91–100. doi: 10.1016/0022-1759(93)90279-g. [DOI] [PubMed] [Google Scholar]
- 15.Hu M, Buck C, Jacobs D, Paulino G, Khouri H. Application of PCR for detection and identification of mycoplasma contamination in virus stocks. In Vitro Cell Dev Biol Anim. 1995;31:710–715. doi: 10.1007/BF02634093. [DOI] [PubMed] [Google Scholar]
- 16.Jensen J S, Bruun B, Gahrn-Hansen B. Unexpected cross-reaction with Fusobacterium necrophorum in a PCR for detection of mycoplasmas. J Clin Microbiol. 1999;37:828–829. doi: 10.1128/jcm.37.3.828-829.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidder M, Chan P J, Seraj I M, Patton W C, King A. Assessment of archived paraffin-embedded cervical condyloma tissues for mycoplasma-conserved DNA using sensitive PCR-ELISA. Gynecol Oncol. 1998;71:254–257. doi: 10.1006/gyno.1998.5177. [DOI] [PubMed] [Google Scholar]
- 18.Kong F, Zhu X, Wang W, Zhou X, Gordon S, Gilbert G L. Comparative analysis and serovar-specific identification of multiple-banded antigen genes of Ureaplasma urealyticum biovar 1. J Clin Microbiol. 1999;37:538–543. doi: 10.1128/jcm.37.3.538-543.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong F, James G, Ma Z, Gordon S, Wang B, Gilbert G L. Phylogenetic analysis of Ureaplasma urealyticum—support for the establishment of a new species, Ureaplasma parvum. Int J Syst Bacteriol. 1999;49:1879–1889. doi: 10.1099/00207713-49-4-1879. [DOI] [PubMed] [Google Scholar]
- 20.Kong F, Ma Z, James G, Gordon S, Gilbert G L. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol. 2000;38:1175–1179. doi: 10.1128/jcm.38.3.1175-1179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong F, Gordon S, Gilbert G L. Rapid-cycle PCR for detection and typing of Mycoplasma pneumoniae in clinical specimens. J Clin Microbiol. 2000;38:4256–4259. doi: 10.1128/jcm.38.11.4256-4259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwig W, Schleifer K H. Bacterial phylogeny based on 16S and 23S rRNA sequence analysis. FEMS Microbiol Rev. 1994;15:155–173. doi: 10.1111/j.1574-6976.1994.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 23.Mattsson J G, Johansson K E. Oligonucleotide probes complementary to 16S rRNA for rapid detection of mycoplasma contamination in cell cultures. FEMS Microbiol Lett. 1993;107:139–144. doi: 10.1111/j.1574-6968.1993.tb06020.x. [DOI] [PubMed] [Google Scholar]
- 24.McGarrity G J, Kotani H. Detection of cell culture mycoplasmas by a genetic probe. Exp Cell Res. 1986;163:273–278. doi: 10.1016/0014-4827(86)90581-1. [DOI] [PubMed] [Google Scholar]
- 25.McGarrity G J, Kotani H, Carson D. Comparative studies to determine the efficiency of 6 methylpurine deoxyriboside to detect cell culture mycoplasmas. In Vitro Cell Dev Biol. 1986;22:301–304. doi: 10.1007/BF02623401. [DOI] [PubMed] [Google Scholar]
- 26.Okano K, Uematsu C, Matsunaga H, Kambara H. Characteristics of selective polymerase chain reaction (PCR) using two-base anchored primers and improvement of its specificity. Electrophoresis. 1998;19:3071–3078. doi: 10.1002/elps.1150191805. [DOI] [PubMed] [Google Scholar]
- 27.Payment P, Corbeil M, Chagnon A. Detection of Mycoplasma hominis and Mycoplasma orale in cell cultures by immunofluorescence. Can J Microbiol. 1978;24:689–692. doi: 10.1139/m78-116. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson B, Tully J G, Bolske G, Johansson K E. Updated phylogenetic description of the Mycoplasma hominis cluster (Weisburg et al., 1989) based on 16S rDNA sequences. Int J Syst Evol Microbiol. 2000;50:291–301. doi: 10.1099/00207713-50-1-291. [DOI] [PubMed] [Google Scholar]
- 29.Radka S F, Hester D M, Polak-Vogelzang A A, Bolhuis R L. Detection of mycoplasma contamination lymphoblastoid cell lines by monoclonal antibodies. Hum Immunol. 1984;9:111–116. doi: 10.1016/0198-8859(84)90033-8. [DOI] [PubMed] [Google Scholar]
- 30.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rychlik W. Priming efficiency in PCR. BioTechniques. 1995;18:84–86. , 88–90. [PubMed] [Google Scholar]
- 32.Swerdlow H, Dew-Jager K, Gesteland R F. Rapid cycle sequencing in an air thermal cycler. BioTechniques. 1993;15:512–519. [PubMed] [Google Scholar]
- 33.Tang J, Hu M, Lee S, Roblin R. A polymerase chain reaction based method for detecting Mycoplasma/Acholeplasma contaminants in cell culture. J Microbiol Methods. 2000;39:121–126. doi: 10.1016/s0167-7012(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 34.Thangavelu M, Erno H, Therkelsen A J. Detection of mycoplasma contamination in tissue cultures by fluorescence microscopy. Hum Genet. 1979;47:199–202. doi: 10.1007/BF00273202. [DOI] [PubMed] [Google Scholar]
- 35.Uphoff C C, Brauer S, Grunicke D, Gignac S M, MacLeod R A, Quentmeier H, Steube K, Tummler M, Voges M, Wagner B, et al. Sensitivity and specificity of five different mycoplasma detection assays. Leukemia. 1992;6:335–341. [PubMed] [Google Scholar]
- 36.van Kuppeveld F J M, van der Logt J T M, Angulo A F, van Zoest M J, Quint W G V, Niesters H G M, Galama J M D, Melchers W J G. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl Environ Microbiol. 1992;58:2606–2615. doi: 10.1128/aem.58.8.2606-2615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Kuppeveld F J M, Johansson K-E, Galama J M D, Kissing J, Bölske G, van der Logt J T M, Melchers W J G. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Appl Environ Microbiol. 1994;60:149–152. doi: 10.1128/aem.60.1.149-152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verkooyen R P, Sijmons M, Fries E, van Belkum A, Verbrugh H A. Widely used, commercially available Chlamydia pneumoniae antigen contaminated with mycoplasma. J Med Microbiol. 1997;46:419–424. doi: 10.1099/00222615-46-5-419. [DOI] [PubMed] [Google Scholar]
- 39.Vojdani A, Choppa P C, Tagle C, Andrin R, Samimi B, Lapp C W. Detection of Mycoplasma genus and Mycoplasma fermentans by PCR in patients with Chronic Fatigue Syndrome. FEMS Immunol Med Microbiol. 1998;22:355–365. doi: 10.1111/j.1574-695X.1998.tb01226.x. [DOI] [PubMed] [Google Scholar]
- 40.Weisburg W G, Tully J G, Rose D L, Petzel J P, Oyaizu H, Yang D, Mandelco L, Sechrest J, Lawrence T G, van Etten J, Maniloff J, Woese C R. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989;171:6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirth M, Berthold E, Grashoff M, Pfutzner H, Schubert U, Hauser H. Detection of mycoplasma contaminations by the polymerase chain reaction. Cytotechnology. 1994;16:67–77. doi: 10.1007/BF00754609. [DOI] [PubMed] [Google Scholar]