Abstract
Background and Aims
Research has shown that alcohol use and common mental disorders (CMDs) co‐occur; however, little is known about how the global prevalence of alcohol use compares across different CMDs. We aimed to (i) report global associations of alcohol use (alcohol use disorder (AUD), binge drinking and consumption) comparing those with and without a CMD, (ii) examine how this differed among those with and without specific types of CMDs and (iii) examine how results may differ by study characteristics.
Methods
We used a systematic review and meta‐analysis. Cross‐sectional, cohort, prospective, longitudinal and case–control studies reporting the prevalence of alcohol use among those with and without a CMD in the general population were identified using PsycINFO, MEDLINE, PsyARTICLES, PubMed, Scopus and Web of Science until March 2020. Depression, anxiety and phobia were included as a CMD. Studies were included if they used a standardized measure of alcohol use. A random‐effects meta‐analysis was conducted to generate pooled prevalence and associations of AUD with CMD with 95% confidence intervals (CI). A narrative review is provided for binge drinking and alcohol consumption
Results
A total of 512 full‐texts were reviewed, 51 included in our final review and 17 in our meta‐analyses (n = 382 201). Individuals with a CMD had a twofold increase in the odds of reporting an AUD [odds ratio (OR) = 2.02, 95% CI = 1.72–2.36]. The odds of having an AUD were similar when stratified by the type of CMD (mood disorder: OR = 2.00, 95% CI = 1.62–2.47; anxiety/phobic disorder: OR = 1.94, 95% CI = 1.35–2.78). An analysis of study characteristics did not reveal any clear explanations for between‐study heterogeneity (I 2 > 80%). There were no clear patterns for associations between having a CMD and binge drinking or alcohol consumption, respectively.
Conclusions
People with common mental disorders (depression, anxiety, phobia) are twice as likely to report an alcohol use disorder than people without common mental disorders.
Keywords: Alcohol, anxiety disorders, associations, comorbidity, epidemiology, mood disorders
INTRODUCTION
It is estimated that 32.5% of the global population consume alcohol [1]. While there are differences between countries [2], approximately 18.4% of adults report binge drinking [3] and 5.1% have an alcohol use disorder (AUD) [2], including harmful and dependent drinking. Despite differences between countries, alcohol use was ranked the seventh leading risk factor for premature death and disability. Alcohol use has also led to 1.6 and 6% of disability‐adjusted life‐years for females and males, respectively [1]. Meanwhile, depressive and anxiety disorders (known as common mental disorders; CMD) are also prevalent in the general population globally, with 4.4 and 3.6% reporting a depressive or anxiety disorder, respectively [4].
Drinking alcohol can be harmful to an individual's mental health, particularly if they meet criteria for an AUD (symptoms include an impaired ability to control alcohol use [5]), binge drinking (generally consuming more than 5 units of alcohol in a certain period [6]) or drinking excessively (drinking excessive amounts of alcohol on most days or weeks [7]). Among the general population, research has found associations between CMD with binge drinking [8, 9, 10] and AUD [11]. Research has also shown that those with co‐occurring panic disorder and AUD or depression and AUD are at an increased risk of mortality compared to those without such disorders [12, 13]. Elsewhere, a narrative review found evidence to suggest that anxiety and depressive episodes are related to binge drinking which can subsequently lead to injury [14]. Other research also found that college students with co‐occurring anxiety and depressive symptoms reported increased weekly alcohol use, more hazardous use and negative alcohol consequences compared to those without symptoms [15]. Nineteen per cent of all alcohol‐related hospital admissions have been attributed to mental health problems that resulted from alcohol use [16], and those with co‐occurring alcohol and mental health problems may have difficulties accessing treatment compared to those with only one of these problems [17]. These findings indicate that having a CMD is associated with a range of alcohol outcomes which have negative health implications on health; however, previous research has focused specifically on associations with AUD.
There is evidence for an association between worsening mental health and increased alcohol use [18]. Motivational models argue that individuals may be motivated to use alcohol to cope with stress [19], where benefits outweigh the cost [20]. Such models suggest that alcohol may be used to cope with symptoms of poor mental health, and used specifically due to its rapid onset of action [21]. This might be the case among those with a CMD, as drinking alcohol may be perceived to alleviate symptoms of a disorder [21].
Genome‐wide studies have shown a causal relationship between CMDs, such as major depression and alcohol dependence, while the reverse association has not been found [22]. However, associations between alcohol use and mental health comorbidity may be more complex and vary based upon the specific type of CMD [23, 24]. Among the general population, research has shown that those with major depressive disorder (MDD) were more likely to report life‐time moderate/severe AUD compared to those without MDD [25], whereas those with generalized anxiety disorder (GAD) were more likely to report mild or severe AUD compared to those without GAD [25]. Elsewhere, a significant association with alcohol dependence among those meeting criteria for alcohol abuse was reported among those with dysthymia but not MDD compared to those without the respective disorder [26], while a review across observational studies showed differences in associations with AUD with specific types of anxiety disorders, such as panic disorder [27]. Differences in associations have also been found for other patterns of alcohol use. For example, in Portugal a positive association of binge drinking with anxiety disorder was found among individuals attending primary care, while a negative association with binge drinking was found for major depression compared to those without the respective disorders [10].
Previous systematic reviews have explored alcohol misuse and CMD in both directions; for example, the prevalence of CMD among those misusing alcohol [28] and the prevalence of alcohol misuse among those with a CMD [11]. The latter was most recently reported by Lai and colleagues, where those with an anxiety disorder or major depression were approximately 1.5 times more likely to report alcohol abuse and 2.5 and three times more likely to report dependence, respectively [11]. This indicates that those with a CMD are more likely to use alcohol at harmful levels and that there may be differences based upon the type of CMD. However, this review included bipolar disorder in their definition of CMD, which UK health guidelines on CMD exclude, together with other psychotic and related disorders [29, 30, 31]. This review also did not include post‐traumatic stress disorder (PTSD), despite its inclusion as a CMD in UK health guidelines [32].
To date, there has not been a systematic review or meta‐analysis reporting the prevalence of other types of alcohol use, such as binge drinking, among those with and without a CMD in the adult general population, and by specific CMD diagnoses. The current systematic review and meta‐analysis aimed to (i) estimate the pooled prevalence of alcohol use (AUD, binge drinking and alcohol consumption) in those with and without a CMD, (ii) evaluate associations between CMD and patterns of alcohol use, (iii) examine how prevalence and associations differed across specific types of CMDs and (iv) examine how results may differ by study characteristics.
METHODS
This study is pre‐registered on PROSPERO (ref. CRD42019126770) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines [33] (see PRISMA diagram in Figure 1 and checklist in Supporting information), and in line with the condition, context, population (CoCoPop) framework [34]. The CoCoPop framework is a quality appraisal tool suitable for systematic reviews and meta‐analyses which aim to examine the prevalence of a condition, and therefore require specific information concerning groups that may not be required using other frameworks [35].
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram
Inclusion and exclusion criteria
We included peer‐reviewed observational studies, comprising cross‐sectional, national surveys, cohort, prospective, longitudinal and case–control studies published in English. Where the same data set was used by multiple studies and reported the same outcome, we used the study which reported information on more CMDs. If two or more studies reported the same information, the more recent study was chosen. Reviews and intervention studies were excluded.
Studies which measured the prevalence of life‐time or 12‐month AUD, binge drinking or alcohol use, comparing those with and without a CMD and used a standardized measure of alcohol use, alcohol use disorder and CMD—for example, the Diagnostic and Statistical Manual (DSM) diagnostic instruments—were included. The authors note that definitions of binge drinking may vary among countries and details of standardized measures of alcohol use and CMD are reported in Table 1. CMDs were defined in this review as MDD, dysthymia, GAD, panic disorder, phobias, PTSD, obsessive–compulsive disorder (OCD) or social anxiety disorder (SAD) [36]. Studies were excluded if they did not report the prevalence of alcohol use in those with and without a CMD.
TABLE 1.
Study characteristics
| Study | Year(s) conducted | Country | Data set | Waves used | Sample size (response rate) | Gender and age | Type of CMD studied (and measure and criteria used to assess presence of CMD) | Type of alcohol use studied (and measure and criteria used) | Duration of AUD | Risk of bias score (max. score of 9) |
|---|---|---|---|---|---|---|---|---|---|---|
|
2005 | Canada | Canadian Community Health Survey | Cycle 3.1 | 17 524 (response rate not stated) |
Gender Female = 8587 (49.0%) Male = 8937 (51.0%) Age range 15–24 |
Depressive symptoms (derived depression scale, cut‐off score of 5 or more) | Binge drinking (item on alcohol use, 5 or more drinks once a month or more) | 12 months | 7 |
|
Baseline = 1996, time 1 = 1997. time 2 = 1999 | Netherlands | Netherlands Mental Health Survey and Incidence Study (NEMESIS) | Baseline |
5571 (no overall response rate) |
Gender Female = 2896 (51.9%) Male = 2675 (48.1%) Age range not clear |
Panic disorder (composite international diagnostic interview, DSM‐III‐R) | Alcohol dependence (composite international diagnostic interview, DSM‐III‐R) | 12 months | 6 |
|
2001–02 | United States | National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) | Wave 1 | 43 093 (81%) |
Gender Female = 21 662 (51.43%) Male = 19 598 (48.57%) Age range 18+ |
Chronic MDD Dysthymic disorder (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) |
Alcohol abuse Alcohol dependence (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) |
12 months Life‐time |
9 |
|
1997 | Australia | National Survey of Mental Health and Well Being (NSMH&WB) | Wave 1 | 10 641 (78%) |
Gender Female = 5452 (51.2%) Male = 5189 (48.8%) Age range 18+ |
Depression Dysthymia Bipolar disorder Panic disorder Social phobia OCD PTSD GAD Agoraphobia (composite international diagnostic interview, DSM‐IV) |
Alcohol abuse Alcohol dependence Alcohol use disorder (Composite International Diagnostic Interview, DSM‐IV) |
12 months | 7 |
|
1995 | Mexico | – | – | 1932 (60.4%) |
Gender Female = not stated Male = not stated Age range 18+ |
OCD (composite international diagnostic interview, ICD‐10) |
Alcohol abuse Alcohol dependence (composite international diagnostic interview, ICD‐10) |
12 months Life‐time |
5 |
|
2007–08 | Brazil | – | – | 3744 (81 and 95%) |
Gender Female = 1584 Male = 2160 Age range 15–75 |
PTSD (composite international diagnostic interview 2.1, ICD‐10 and DSM‐IV) |
Hazardous alcohol use Alcohol dependence (composite international diagnostic interview 2.1, ICD‐10 and DSM‐IV) |
12 months | 9 |
|
2009 | Singapore | Singapore Mental Health Study (SMHS) | – | 6616 (76%) |
Gender Female = 3317 (50.1%) Male = 3299 (49.9%) Age range 18+ |
MDD Dysthymia GAD OCD (World Mental Health composite international diagnostic interview, DSM‐IV) |
Alcohol abuse Alcohol dependence (World Mental Health composite international diagnostic interview, DSM‐IV) |
Life‐time | 8 |
|
NESARC, 2001–02; KECA, 2000 | Korea and United States |
NESARC and Korean Epidemiologic Catchment Area (KECA) |
NESARC wave 1 KECA wave 1 |
NESARC = 35 336 (81%) KECA = 6253 (79.8%) |
NESARC: Gender Female = 23 227 (65.7%) Male = 15 619 (44.2%) Age range 18–65 KECA: Gender Female = 3510 (56.1%) Male = 2743 (43.9%) Age range 18–65 |
MDD Dysthymia Panic disorder Social phobia GAD (NESARC: associated disabilities interview schedule‐DSM‐IV version, DSM‐IV; KECA: Korean version of composite international diagnostic interview 2.1, DSM‐IV) |
Alcohol abuse Alcohol dependence (NESARC: associated disabilities interview schedule‐DSM‐IV version, DSM‐IV; KECA: Korean version of composite international diagnostic interview 2.1, DSM‐IV) |
12 months | 9 |
|
2009–11 | Ireland | The Irish Longitudinal Study on Ageing (TILDA) | Wave 1 | 8175 (62%) |
Gender Female = 2041 (53.4%) Male = 1774 (46.6%) Age range 60–99 |
Depression (Center for Epidemiologic Studies Depression Scale, score of 16 or more) |
Problem drinker (CAGE, score of 2 or more) | 6 months | 7 |
|
1993–96 | United States | Baltimore Epidemiologic Catchment Area (ECA) follow–up | Wave 2 | 2633 (73%) |
Gender Female: 1644 (63.2%) Male: 989 (36.8%) Age range 31–99 |
MDD (diagnostic interview schedule, DSM‐III‐R) | Alcohol dependence (diagnostic interview schedule, DSM‐III‐R) | Life‐time | 7 |
|
2002 | Canada | Canadian Community Health Survey (CCHS) | Cycle 1.2 | 36 984 (77%) |
Gender Female = not stated Male = not stated Age range 15+ |
MDE (Canadian World Mental Health composite international diagnostic interview, DSM‐IV) |
Harmful alcohol use (ICD‐10) Alcohol dependence (DSM‐IV) (composite international diagnostic interview short form) |
12 months | 7 |
|
2000–01 | Norway | Oslo Health Study (HUBRO) | – | 2676: 446 = SPAS groups and 2230 controls (response rate not stated) |
Gender Female = 1558 (58%) Male = 1118 (42%) Age range 30–45 |
Social phobia and anxiety symptoms (MINI–social phobia inventory, score of 8 or more) |
Alcohol frequency (self‐report item, more than 1 time per week) Alcohol problems (self‐report item on impairment in job due to alcohol) |
1 week and 5 years | 3 |
|
2017 | United Kingdom | UK Biobank | – | 157 366 (46%) |
Gender Female = 89 101 (56%) Male = 68 265 (44%) Age range 45–82 |
Depression (composite international diagnostic interview short form) Anxiety disorder (composite international diagnostic Interview short form) PTSD (post‐traumatic stress disorder checklist‐6, score of 14 or more) |
Harmful alcohol use (alcohol use disorder identification test score of 16 or more) | 12 months | 4 |
|
2013–15 | Portugal | EpiDoC 2 (CoReumaPt) study | Wave 2 | 1680 (response rate not stated) |
Gender Female = 908 (54%) Male = 772 (46%) Age range 65+ |
Depression and anxiety symptoms (hospital and anxiety disorder scale, score of 11 or more) | Alcohol intake (self‐report of frequency of alcohol intake and categorized as daily, occasionally, never, cut‐offs not stated) | Not known | 5 |
|
2012–13 | United States | NESARC | Wave 3 | 36 309 (60.1%) |
Gender Female = 10 940 (55.5%) Male = 8765 (44.5%) Age range 18+ |
PTSD (alcohol use disorder and associated disabilities interview schedule‐5, DSM‐5) | Alcohol use disorder (alcohol use disorder and associated disabilities interview schedule‐5, DSM‐5) |
12 months |
5 |
|
2006 | Italy | The Faenza Community Aging Study | – | 366 (65.8%) |
Gender Female = 184 (50.3%) Male = 182 (49.7%) Age range 70+ |
Anxiety symptoms (geriatric anxiety inventory short form, cut‐off of 3 or more) | Alcohol consumption (quantity of alcoholic drink converted to units per day, defined as alcoholic unit as a glass of wine (125 ml), a can of beer (330 ml) and a small glass of hard liquor (40 ml), cut‐off of more than 2 alcohol units per day) | Per day | 5 |
|
2008 | Japan | Nihon University Sleep and Mental Health Epidemiology Project (NUSMEP) | – | 2559 (54%) |
Gender Female = 1396 (52.53%) Male = 1163 (47.47%) Age range 20+ |
Depressive symptoms (Center for Epidemiologic studies depression scale, score of 16 or more) |
Alcohol consumption (self‐report item on drinking more than one glass of sake three times per week), defined as a glass of sake is equal to a 500–ml bottle of beer, 80 ml of distilled spirit, 60 ml of whiskey, or two glasses of wine (240 ml), cut‐off yes) | 1 week | 5 |
|
1992 | United States | National Longitudinal Alcohol Epidemiologic Survey (NLAES) | – | 42 862 (household response rate = 91.9%, sample person = 97.4%) |
Gender Not stated without depression Age range 18+ |
MDD (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) |
Alcohol abuse Alcohol dependence (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) |
12 months Life‐time |
7 |
|
2001–02 | United States | NESARC | Wave 1 | 43 093 (81%) |
Gender Female = 25 575 (57%) Male = 18 518 (43%) Age range 18+ |
MDD Dysthymia GAD Panic disorder Phobia (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) |
Alcohol abuse Alcohol dependence (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) |
12 months | 8 |
|
2005–14 | United States | National Survey on Drug Use and Health (NSDUH) | 10 waves (2005–14) | 61 240 (71.2–76%) |
Gender Female = 32 825 (53.6%) Male = 28 415 (46.4%) Age range 50+ |
Depressive episode Anxiety (self‐report item, cut‐off not stated) |
Binge drinking (Self‐report idem, five more drinks on same occasion) Alcohol use disorder (measure not stated, DSM‐IV) |
Binge drinking‐30 days Alcohol abuse/dependence‐12 months |
7 |
|
2003–04 | Singapore | Singapore Longitudinal Aging Study (SLAS) | Wave 1 | 1070 (72.4%) |
Gender Female = 585 (53.75%) Male = 485 (46.25%) Age range 18+ |
Depressive symptoms (geriatric mental state examination, DSM‐IV) | Alcohol consumption (measure not stated, more than one drink per week) | 1 week | 5 |
|
2005 | France | – | – | 17 237 (62.7%) |
Gender Female = 10 262 (59.5%) Male = 6975 (40.5%) Age range 18–99 |
MDD GAD Panic disorder PTSD OCD Specific phobia Social phobia (composite international diagnostic interview short form, DSM‐IV) |
Alcohol abuse Alcohol dependence (Composite International Diagnostic Interview short form, DSM‐IV) |
12 months | 7 |
|
1997–99 | Japan | – | – | 1029 (56.9%) |
Gender Female = 578 (56.2%) Male = 451 (43.8%) Age range 20+ |
MDD Mania Dysthymia GAD Panic disorder (World Mental Health University of Michigan composite international diagnostic interview, DSM‐III‐R) |
Alcohol use disorder (World Mental Health University of Michigan composite international diagnostic interview, DSM‐III‐R) |
6 months Life‐time |
6 |
|
1990–92 | United States | National Comorbidity Survey (NCS) | Wave 1 | 8098 (82.6%) |
Gender Female = 4263 (49.26%) Male = 3835 (50.74%) Age range 15–54 |
MDD Dysthymia Panic disorder Social phobia Simple phobia GAD (World Mental Health composite international diagnostic interview, DSM‐III‐R) |
Alcohol abuse Alcohol dependence (World Mental Health Composite International Diagnostic Interview, DSM‐III‐R) |
Life‐time | 6 |
|
2002–03 | Canada | Canadian Community Health Survey – Mental Health and Well–Being | Cycle 1.2 | 28 541 (77.0%) |
Gender Female = 15 074 (52.82%) Male = 13 467 (47.18%) Age range 15+ |
Panic disorder (World Mental Health composite international diagnostic interview, DSM‐IV) |
Alcohol dependence (World Mental Health composite international diagnostic interview short form, DSM‐III‐R) | 12 months | 8 |
|
2006–08 | Estonia | Estonian Health Interview Survey (EHIS) | – | 6105 (60.2%) |
Gender Female = 3177 (52.04%) Male = 2928 (47.95%) Age range 18–85 |
MDD (mini‐international neuropsychiatric interview, DSM‐IV) | Binge drinking (self‐report item partly based on the European Health Determinant Module, defined as five bottles of beer, five glasses of wine, or five glasses of vodka at a time, 5 or more) | 12 months | 7 |
|
2011 | Slovenia | – | – | 1002 (response rate not reported) |
Gender Female = 512 (51.1%) Male = 490 (48.9%) Age range not clear |
Anxiety/depression (Gothenburg quality of life instrument, answering yes to mood and anxiety items) | ‘Risky’ drinker (Slovenian version of the alcohol use disorder identification test‐consumption, score of 6 or more for men or 5 or more for women) | ‐ | 6 |
|
2002–04 | 47 countries | World Health Survey (WHS) | – | 201 279 (98.5%, range = 63%–99%) |
Gender Female = 102 279 (50.8%) Male = 99 058 (49.2%) Age range 18+ |
Depressive symptoms (self‐report items to four mandatory questions and two additional ones, DSM‐IV) |
Alcohol consumption (self report, defined as how many alcoholic drinks they had on each day in the past 7 days, cut‐off 4 or 5 drinks on at least 3 days) | 7 days | 7 |
|
1999–2003 | France | Mental Health in General Population (MHGP) survey | French data set | 36 105 (response rate not stated) |
Gender Female = 19 458 (53.9%) Male = 16 647 (46.1%) Age range 18+ |
GAD Panic disorder Social phobia PTSD (mini international neuropsychiatric interview, ICD‐10) |
Alcohol abuse (mini international neuropsychiatric interview, ICD 10) | 6 months | 6 |
|
2007 | Finland | National FINRISK 2007 Study | – | 10 000 but used a random subsample (n = 2086, 51.9%) |
Gender Female = 1140 (54.7%) Male = 946 (45.3%) Age range 25–74 |
Depressive symptoms (modified Beck depression inventory, score of 8 or more) | Heavy drinking occasion (time‐line‐follow‐back reporting frequency and quantity of consumption of standard alcoholic drink, defined as 12 g of absolute alcohol, cut‐off of seven, or five or more for men and women, respectively) | 28 days | 6 |
|
2000–01 and 2011 | Finland | Health 2000 Survey and Health 2011 Survey | Waves 1 and 2 |
Wave 1 = 6005 (75%) Wave 2 = 4620 (80.6%) |
Gender Female = 3257 (54.2%) Male = 2748 (45.8%) Age range 30+ |
Depressive symptoms (Munich composite international diagnostic interview, DSM‐IV) | Alcohol use disorder (Munich composite international diagnostic interview, DSM‐IV) | 12 months | 7 |
|
Baseline = 1996, time 1 = 1997, time 2 = 1999 | Netherlands | Netherlands Mental Health Survey and Incidence Study (NEMESIS) | All waves |
Baseline = 7076 (69.7%), time 1 = 5618 (79.4%) time 2 = 4796 (67.8%) |
Gender Female = 3777 (53.4%) Male = 3299 (46.6%) Age range 18–64 |
GAD Panic disorder Social phobia Agoraphobia OCD (composite international diagnostic interview version 1.1, DSM‐III‐R) |
Alcohol dependence (composite international diagnostic interview version 1.1, DSM‐III‐R) | Life‐time and 1 month | 8 |
|
2000 | Brazil | – | – | 1260 (7%) |
Gender Female = 679 (53.9%) Male = 581 (46.1%) Age range 15+ |
Minor psychiatric disorder (depression or anxiety; self‐report questionnaire‐20, cut‐off score of 6 for men and 7 for women) | Probable alcohol use disorder (alcohol use disorder identification test, score of 8 or more) | 12 months | 7 |
|
1996–97 | Germany | Transitional in Alcohol Consumption and Smoking (TACOS) | – | 4048 (70.2%) |
Gender Female = 2019 (61.1%) Male = 2029 (63.3%) Age range 18–64 |
MDD Dysthymia Phobia PTSD OCD (Munich composite international diagnostic interview, DSM‐IV) |
Alcohol abuse Alcohol dependence (Munich composite international diagnostic interview, DSM‐IV) |
Life‐time and 12 months | 6 |
|
2004–08 | Switzerland | PsyCoLaus | Wave 2 | 3694 (67.0%) |
Gender Female = 1958 (53.0%) Male = 1736 (47.0%) Age range 35–75 |
PTSD (French version of diagnostic interview for genetic studies, DSM‐IV) |
Alcohol use disorder (French version of diagnostic interview for genetic studies, DSM‐IV) |
12 months | 7 |
|
2011–12 | Spain | COURAGE | – | 4569 (69.9%) |
Gender Female = 2498 (50.6%) Male = 2071 (49.4%) Age range 18+ |
Panic disorder (adapted version of the composite international diagnostic interview, DSM‐5) | Alcohol consumption (frequent drinkers: consumed alcohol in either last 30 days and 7 days or 1–2 days per week with 5/4 standard drinks in last 7 days or 3 or more days per week with 5/4 standard drinks in the last 7 days) | 30 days | 6 |
|
2012 | Canada | Canadian Community Health Survey–Mental Health (CCHS–Mental Health) | Wave 2 | 25 097 (68.9%) |
Gender Female = not stated Male = not stated Age range 15+ |
OCD (self‐reported diagnosis) |
Alcohol abuse Alcohol dependence (composite international diagnostic interview short form, DSM‐IV) |
12 months Life‐time |
3 |
|
2001–02 | United States | NESARC | Wave 1 | 43 093 (81.0%) |
Gender Female = 25 575 (57%) Male = 18 518 (43%) Age range 18+ |
GAD Panic disorder Social phobia Specific phobia (alcohol use disorders and associated disabilities interview schedule, DSM‐IV) |
Alcohol dependence (alcohol use disorders and associated disabilities interview schedule, DSM‐IV) | Life‐time | 7 |
|
2001 | Korea | Korean Epidemiologic Catchment Area–Replication (KECA–R) | Wave 2 | 6510 (81.7%) |
Gender Female = 3229 (49.6%) Male = 3281 (50.4%) Age range 18–64 |
Specific phobia (Korean version of composite international diagnostic interview, DSM‐IV) |
Alcohol abuse Alcohol dependence (Korean version of composite international diagnostic interview, DSM‐IV) |
Life‐time | 9 |
|
1994–95 | United Kingdom | Surveys of psychiatric morbidity in Great Britain | Wave 1 | 8564 (67.2%) |
Gender Female = not reported Male = not reported Age range 16–64 |
Social phobia (clinical interview schedule‐revised, ICD‐10) | Alcohol dependence (self‐completion questionnaire, cut‐off score of four or more) | 12 months | 6 |
|
2015 | Canada |
Canadian Community Health Survey–Mental Health (CCHS–Mental Health) |
Wave 2 | 25 113 (68.9%) |
Gender Female = 12 883 (50.7%) Male = 12 230 (49.3%) Age range 15+ |
MDE MDD (World Mental Health composite international diagnostic interview, DSM‐IV) |
Alcohol abuse Alcohol dependence (World Mental Health composite international diagnostic interview, DSM‐IV) |
12 months Life‐time |
9 |
|
2000–01 | Finland | Health 2000 Survey | Wave 1 | 6005 (75.0%) |
Gender Female = 3257 (54.2%) Male = 2748 (45.8%) Age range 30+ |
MDD Dysthymia GAD Panic disorder Social phobia Agoraphobia (Finnish version of Munich composite international diagnostic interview, DSM‐IV) |
Alcohol use disorder (Finnish version of Munich composite international diagnostic interview, DSM‐IV) | 12 months | 9 |
|
– | Poland | WOBASZ | – | 13 545 (response rate not reported) |
Gender Female = 7153 (52.8%) Male = 6392 (47.2%) Age range 20–74 |
Depressive symptoms (Beck depression inventory, cut‐off score of 10 or more) | Alcohol consumption (self‐reporting consumption of three times per week) | 7 days | 6 |
|
1999–2003 | France | Mental Health in General Population (MHGP) survey | French data set | 38 600 (not stated) |
Gender Female = 20 342 (52.7%) Male = 18 258 (47.3%) Age range 18+ |
MDD Panic disorder Social phobia GAD PTSD (mini international neuropsychiatric interview version 5.0, ICD‐10) |
Alcohol use disorder (mini international neuropsychiatric interview version 5.0, ICD‐10) | Life‐time | 7 |
|
2000 | United Kingdom | British National Psychiatric Morbidity Survey 2000 | Wave 2 | 8580 (69.5%) |
Gender Female = 4300 (50.1%) Male = 4280 (49.9%) Age range 16+ |
OCD (clinical interview schedule‐revised, ICD‐10) |
Hazardous use Alcohol dependence Problem drinking (alcohol use disorder identification test and severity of alcohol dependence questionnaire, cut offs not stated) |
12 months | 7 |
|
1993–94 | United States | Chinese American Psychiatric Epidemiology Study (CAPES) | – | 1735 (82%) |
Gender Female = 876 (50.5%) Male = 859 (49.5%) Age range 18–65 |
PTSD (diagnostic interview schedule, DSM‐III‐R) | Heavy alcohol use (two items from composite international diagnostic interview, frequency: 1–3 times per month and quantity‐5+ and 4+ per day) |
12 months |
7 |
|
2002 | Canada | – | – | 2991 (68.1%) |
Gender Female = 1811 (51.5%) Male = 1180 (48.5%) Age range 18+ |
PTSD [revised version of composite international diagnostic interview (CIDI), DSM‐IV] | Alcohol use disorder (mini international neuropsychiatric interview) |
3 days Life‐time |
8 |
|
Not stated | Netherlands | Longitudinal Aging Study Amsterdam (LASA) | ‐ | 3056 (86.0%). Used a subsample (n = 659) of this restricted to those aged between 55 and 84 |
Gender Female = 354 (54.0%) Male = 305 (46.0%) Age range 55+ |
GAD OCD Phobia (diagnostic interview schedule, DSM‐III) |
Heavy/excessive alcohol intake (Garretsen scale, cut‐off score of 4) | 6 months | 9 |
|
2002–05 | Netherlands | Rotterdam study | Wave 3 | 5019 (85.4%) |
Gender Female = 2848 (56.7%) Male = 2171 (43.3%) Age range Range 58–100 |
MDD Dysthymia GAD Panic disorder Specific phobia Social phobia (depression = schedules for clinical assessment in neuropsychiatry, DSM‐IV‐TR; Anxiety = Munich version of the composite international diagnostic interview) |
Excessive alcohol use (self‐reported question, more than 21 alcoholic drinks per week) | 7 days | 5 |
|
1992–93 and 1998–99 | Netherlands | Longitudinal Aging Study Amsterdam (LASA) | Waves 1 and 2 | 1280 (response rate not reported) |
Gender Female = 698 (54.5%) Male = 582 (45.5%) Age range 55–85 |
Depressive symptoms (Center of Epidemiologic Studies Depression, cut off score of 16 or more) | Alcohol consumption (health interview questionnaire, three or more drinks per day) | Daily | 7 |
|
2012–13 | China | China National Health and Wellness Survey (NHWS) | Waves 3 and 4 | 36 806 (response rate not reported) |
Gender Female = 16 698 (45.4%) Male = 20 108 (54.6%) Age range 18+ |
GAD (generalized anxiety disorder‐7, cut off score 10 or more) | Alcohol use (measure not stated, excessive) | Not stated | 5 |
As this review aimed to report the global prevalence of alcohol use among those with and without a CMD within the adult general population, studies that focused upon treatment‐seeking individuals were excluded. Studies which examined the prevalence of alcohol use in those with and without a CMD within a population who experienced a specific traumatic event (e.g. military) or with a specific health condition, such as epilepsy, were also excluded (see Supporting information, Table S1 for a full list of criteria).
Search strategy
PsycINFO, MEDLINE, PsycARTICLES, PubMed, Scopus and Web of Science were searched using Boolean methods. Key terms were chosen using databases’ own ‘MeSH’ terms or subject headings and broad enough to cover possible synonyms for alcohol use (e.g. alcohol*), CMDs (e.g. depression), comorbidity (e.g. comorbid*) and prevalence (e.g. prevalence) (see Supporting information, Table S2 for full search terms). Titles, abstracts and keywords were searched. A manual search of reference lists of studies which met the inclusion criteria was also conducted. The search was conducted from inception until March 2020.
A second researcher (P.I.) reviewed a random sample of 10% of titles, abstracts and full texts and checked against the first author's screening to establish reliability for inclusion. A kappa score of 0.62 was confirmed between researchers, indicating moderate agreement in study inclusion [37].
Assessment of methodological quality
The Joanna Briggs Critical Appraisal Checklist for Studies Reporting Prevalence Data was used to assess the methodological quality of each study [34]. This checklist consists of nine items (scored 0 if no or unclear evidence or 1 if evidence was present) which covers different methodological aspects, such as the sampling frame, appropriateness of the analysis conducted and response rate. The maximum possible score was nine.
Data extraction
In accordance with the Joanna Briggs Institute Data Extraction Form for Prevalence Studies, the following study characteristics were extracted: name and date of study, author, titles, journal, year survey was conducted, sample size, use of methods for establishing the diagnosis of CMD and AUD, use of methods to measure socio‐economic status (SES), study population, country, description of main results and reviewer comments. We contacted authors for additional information if any key information was missing.
Synthesis of data
Statistical analyses
Our meta‐analysis focuses on the prevalence and associations of AUD among those with and without a CMD; other alcohol outcomes were not included due to variance in the measures and cut‐offs used. In light of changes to the diagnostic criteria of AUD, we categorized AUD as mild, moderate or severe [5]. Studies that used earlier definitions of AUD, such as DSM‐IV abuse and dependence, were re‐categorized whereby abuse was considered mild and dependence as moderate or severe, given that previous research indicates that there may be differences in those meeting criteria for alcohol abuse and moderate AUD [38]. Due to the small number of studies examining the prevalence among those with and without a specific CMD (e.g. GAD), we grouped CMDs into two broad categories: mood disorder (dysthymia and MDD) and anxiety/phobic disorder (GAD, OCD, PTSD, panic disorder, social phobia, simple phobia and specific phobia). The comparison group was not meeting criteria for any CMD.
A random‐effects meta‐analysis was conducted to examine the global associations of AUD (e.g. mild, moderate or severe AUD) and any CMD. To consider both within‐ and between‐study variability [39], we then conducted an a priori random‐effects meta‐analysis to examine the global prevalence and associations of any AUD stratified by type of CMD (e.g. mood disorder), and then two post‐hoc random‐effects meta‐analyses by (i) severity of AUD (e.g. mild AUD versus no AUD excluding moderate/severe AUD and moderate/severe AUD versus no AUD excluding mild AUD) and (ii) severity of AUD by type of CMD.
For all analyses, studies which reported the total number of participants meeting criteria for a mood, anxiety/phobic disorder or no disorder were included. Studies which tested multiple CMDs within the same sample, over multiple time‐frames in the same sample (e.g. 12‐month AUD and life‐time AUD) or did not state the cut‐off used to determine AUD severity were excluded. Stratified analyses, such as severity of AUD by type of CMD, were not conducted where there were fewer than three sources of data within a group.
The metaprop command with Freeman–Tukey transformation was used to pool proportions of those with and without a CMD who reported AUD [40] using the numbers of those with a CMD who reported having an AUD and those with a CMD who did not report having an AUD, and this was repeated among those without a CMD for each study. The pooled proportions were then converted to an odds ratio (OR) using the metan command with the DerSimonian & Laird mode in Stata version 16 [39]. Forest plots and tables were generated to present the pooled prevalence, ORs and 95% confidence intervals (CIs). We conducted a sensitivity analysis by removing studies with the largest and smallest ORs to test the effect on the overall odds of having any AUD among those with a CMD, and publication bias was assessed using the Egger's test [41] and funnel plot. A planned a priori subgroup analysis by decade of data collected and continent was conducted. It was not possible to conduct other subgroup analyses due to a lack of reporting of demographic characteristics stratified by those with and without a CMD. Heterogeneity was assessed using I 2 and funnel plots using the metafunnel command [42].
Narrative synthesis
Due to a small number of studies reporting the prevalence of binge drinking, of which one study had a much larger sample size than others, it was not appropriate to conduct a meta‐analysis. Further, due to variances in the measures and cut‐offs used to measure alcohol consumption, we were unable to conduct a meta‐analysis of alcohol consumption. Instead, a narrative synthesis is provided for these alcohol outcomes.
The current systematic review and meta‐analysis had planned to examine the prevalence of alcohol use among those with and without a CMD from different SES backgrounds; however, studies included in this review did not report adequate information. Instead, studies generally reported the overall SES characteristics of the total sample and did not provide the required data stratified by SES.
RESULTS
Study selection
Our initial search yielded 2862 results, after removing duplications with 512 full texts reviewed after screening titles and abstracts. Fifty‐one studies were included in our final review and 17 in our meta‐analyses (n = 382 201; see PRISMA diagram in Figure 1). Of the 51 studies included, 33 reported the prevalence of mild, moderate or severe AUD (including earlier diagnostic classifications), five of binge drinking and 12 of alcohol consumption. Studies were conducted in 24 countries, with the majority in the United States (n = 10), and used data from 33 surveys. Bias scores ranged from 3 to 9 with a median of 7, indicating medium to low bias (see Table 1).
Study characteristics
Of the 51 studies identified in the systematic review, 34 examined the prevalence of alcohol use among those meeting criteria for an anxiety/phobic disorder and 31 for mood disorder. The type of CMD most commonly studied was MDD (39%). None of the included studies examined alcohol use among those with and without SAD. Of the 33 studies reporting the prevalence of AUD among those with and without a CMD, 16 were not included in the meta‐analysis (see reasons in Figure 1).
Primary analysis
Prevalence and associations of any AUD among those with and without a CMD
The pooled prevalence of having any AUD among those with a CMD was higher than those without (K = 17, 15% versus 8%, see Table 2), with those with a CMD being twice as likely to report any AUD (OR = 2.02, 95% CI = 1.72–2.36, I 2 = 90.70%, see Table 2). When stratified by 12‐month and life‐time AUD, the prevalence remained higher for life‐time AUD among those with a CMD (12‐month: K = 9, 10%, life‐time: K = 8, 21%, see Table 2) compared to those without (12‐month: 5%, life‐time: 12%, see Table 2). Our meta‐analysis found that associations for both 12‐month and life‐time AUD were approximately twofold among those with a CMD compared to those without (12‐month: OR = 2.14, 95% CI = 1.75–2.62, I 2 = 78.90%; life‐time: OR = 1.91, 95% CI = 1.45–2.52, I 2 = 94.70%, see Table 2 and Figure 2).
TABLE 2.
Prevalence and associations of having any AUD among those with and without a CMD (n = 382 201)
| Any AUD | Prevalence of those with a CMD (%) | 95% CI Lower (%) | 95% CI Upper (%) | Weight | Heterogeneity (I 2) | P | Prevalence of those without a CMD (%) | 95% CI Lower (%) | 95% CI Upper (%) | Weight | Heterogeneity (I 2) | P | OR | 95% CI Lower | 95% CI Upper | Weight | Heterogeneity (I 2) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12‐month | ||||||||||||||||||
| Batelaan et al. 2012 | 8.00 | 5.00 | 14.00 | 10.79 | 3.00 | 2.00 | 3.00 | 11.09 | 2.89 | 1.53 | 5.45 | 6.60 | ||||||
| De Castro Longo et al. 2020 | 8.00 | 6.00 | 12.00 | 11.52 | 2.00 | 2.00 | 3.00 | 11.06 | 3.39 | 2.15 | 5.34 | 9.75 | ||||||
| Currie et al. 2005 | 17.00 | 15.00 | 19.00 | 12.00 | 10.00 | 10.00 | 11.00 | 11.15 | 1.66 | 1.44 | 1.90 | 17.87 | ||||||
| Davis et al. 2020 | 4.00 | 4.00 | 4.00 | 12.14 | 2.00 | 2.00 | 2.00 | 11.15 | 1.70 | 1.60 | 1.81 | 19.16 | ||||||
| Husky et al. 2018 | 7.00 | 4.00 | 12.00 | 10.93 | 4.00 | 4.00 | 4.00 | 11.14 | 1.72 | 0.93 | 3.18 | 6.88 | ||||||
| Kinley et al. 2009 | 8.00 | 6.00 | 10.00 | 11.82 | 2.00 | 2.00 | 2.00 | 11.14 | 3.63 | 2.66 | 4.94 | 13.36 | ||||||
| Markkula et al. 2016 | 9.00 | 6.00 | 12.00 | 11.64 | 4.00 | 3.00 | 4.00 | 11.10 | 2.42 | 1.67 | 3.51 | 11.68 | ||||||
| Muller et al. 2014 | 21.00 | 15.00 | 28.00 | 10.88 | 11.00 | 10.00 | 12.00 | 11.06 | 1.90 | 1.27 | 2.84 | 10.97 | ||||||
| Patel et al. 2002 | 14.00 | 6.00 | 29.00 | 8.28 | 15.00 | 14.00 | 15.00 | 11.12 | 0.94 | 0.37 | 2.41 | 3.72 | ||||||
| Subtotal | 10.00 | 5.00 | 16.00 | 100.00 | 97.87 | 0.01 | 5.00 | 3.00 | 8.00 | 100.00 | 99.84 | 0.01 | 2.14 | 1.75 | 2.62 | 100.00 | 78.90% | 0.01 |
| Life‐time | ||||||||||||||||||
| Chong et al. 2012 | 10.00 | 8.00 | 13.00 | 12.51 | 3.00 | 3.00 | 4.00 | 12.51 | 3.47 | 2.61 | 4.60 | 11.95 | ||||||
| Crum et al. 2005 | 22.00 | 19.00 | 27.00 | 12.41 | 11.00 | 10.00 | 13.00 | 12.48 | 1.98 | 1.50 | 2.62 | 11.99 | ||||||
| Kessler et al. 1997 | 35.00 | 33.00 | 37.00 | 12.62 | 19.00 | 18.00 | 20.00 | 12.51 | 1.81 | 1.64 | 2.00 | 13.41 | ||||||
| Marquenie et al. 2007 | 9.00 | 7.00 | 11.00 | 12.57 | 4.00 | 4.00 | 5.00 | 12.51 | 2.08 | 1.64 | 2.64 | 12.38 | ||||||
| Pacek et al. 2013 | 40.00 | 37.00 | 44.00 | 12.50 | 42.00 | 41.00 | 43.00 | 12.52 | 0.97 | 0.83 | 1.13 | 13.11 | ||||||
| Park et al. 2013 | 23.00 | 18.00 | 28.00 | 12.26 | 16.00 | 15.00 | 17.00 | 12.51 | 1.42 | 1.05 | 1.91 | 11.80 | ||||||
| Tebeka et al. 2018 | 9.00 | 8.00 | 10.00 | 12.63 | 3.00 | 3.00 | 4.00 | 12.52 | 2.68 | 2.38 | 3.01 | 13.32 | ||||||
| Van Ameringan et al. 2008 | 28.00 | 24.00 | 31.00 | 12.50 | 14.00 | 12.00 | 17.00 | 12.42 | 1.92 | 1.46 | 2.53 | 12.05 | ||||||
| Subtotal | 21.00 | 12.00 | 32.00 | 100.00 | 99.35 | 0.01 | 12.00 | 4.00 | 24.00 | 100.00 | 99.92 | 0.01 | 1.91 | 1.45 | 2.52 | 100.00 | 94.70 | 0.01 |
| Overall | 15.00 | 9.00 | 22.00 | 100.00 | 99.50 | 0.01 | 8.00 | 5.00 | 12.00 | 100.00 | 99.90 | 0.01 | 2.02 | 1.72 | 2.36 | 100.00 | 90.70 | 0.01 |
AUD = alcohol use disorder; CMD = common mental disorders; OR = odds ratio; CO = confidence interval.
FIGURE 2.
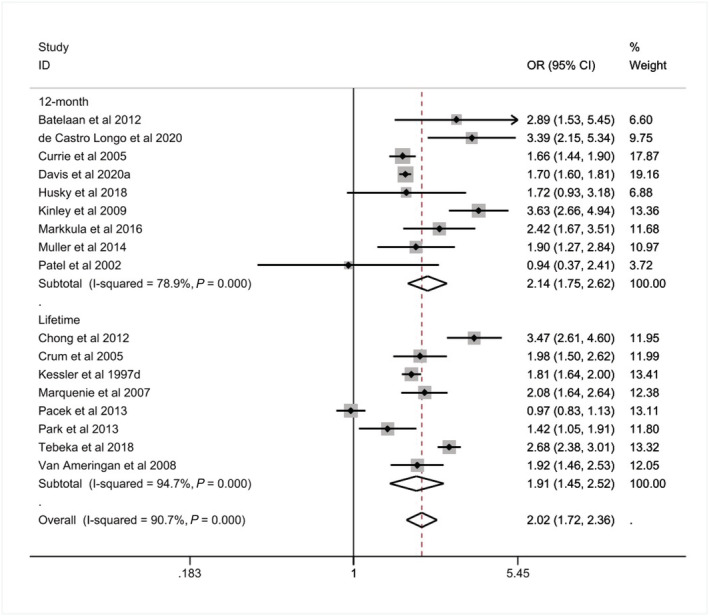
12‐month and life‐time associations of alcohol use disorder (AUD) among those with a common mental disorder (CMD) compared to those without (n = 382 201)
The pooled prevalence and associations of any AUD by the type of CMD, regardless of duration, among those with an anxiety/phobic disorder was 17% (K = 9 compared to 10% for those without, see Table 3) and 11% for mood disorder (K = 6 compared to 5% for those without). Associations of having any AUD were similar for those with a mood or anxiety/phobic disorder (mood: OR = 2.00, 95% CI = 1.62–2.47, I 2 = 90.00%; anxiety/phobic: OR = 1.94, 95% CI = 1.35–2.78, I 2 = 91.40%, see Table 3 and Figure 3).
TABLE 3.
Prevalence and associations of any AUD stratified by type of CMD (n = 367 487)
| Type of CMD | Prevalence among those with the specific CMD (%) | 95% CI Lower (%) | 95% Upper (%) | Weight | Heterogeneity (I 2) | P | Prevalence among those without the specific CMD (%) | 95% CI Lower (%) | 95% Upper (%) | Weight | Heterogeneity (I 2) | P | OR | 95% CI Lower | 95% CI Upper | Weight | Heterogeneity (I 2) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anxiety/phobic disorder | ||||||||||||||||||
| Batelaan et al. 2012 | 8.00 | 5.00 | 14.00 | 10.95 | 3.00 | 2.00 | 3.00 | 11.12 | 2.89 | 1.53 | 5.45 | 9.32 | ||||||
| De Castro Longo et al. 2020 | 8.00 | 6.00 | 12.00 | 11.34 | 2.00 | 2.00 | 3.00 | 11.11 | 3.39 | 2.15 | 5.34 | 10.86 | ||||||
| Kinley et al. 2009 | 8.00 | 6.00 | 10.00 | 11.49 | 2.00 | 2.00 | 2.00 | 11.13 | 3.63 | 2.66 | 4.94 | 11.99 | ||||||
| Marquenie et al. 2007 | 9.00 | 7.00 | 11.00 | 11.57 | 4.00 | 4.00 | 5.00 | 11.12 | 2.08 | 1.64 | 2.64 | 12.43 | ||||||
| Muller et al. 2014 | 21.00 | 15.00 | 28.00 | 11.00 | 11.00 | 10.00 | 12.00 | 11.11 | 1.90 | 1.27 | 2.84 | 11.30 | ||||||
| Pacek et al. 2013 | 40.00 | 37.00 | 44.00 | 11.49 | 42.00 | 41.00 | 43.00 | 11.12 | 0.97 | 0.83 | 1.13 | 12.85 | ||||||
| Park et al. 2013 | 23.00 | 18.00 | 28.00 | 11.26 | 16.00 | 15.00 | 17.00 | 11.12 | 1.42 | 1.05 | 1.91 | 12.07 | ||||||
| Patel et al. 2002 | 14.00 | 6.00 | 29.00 | 9.39 | 15.00 | 14.00 | 15.00 | 11.12 | 0.94 | 0.37 | 2.41 | 6.95 | ||||||
| Van Ameringan et al. 2008 | 28.00 | 24.00 | 31.00 | 11.50 | 14.00 | 12.00 | 17.00 | 11.04 | 1.92 | 1.46 | 2.53 | 12.23 | ||||||
| Subtotal | 17.00 | 9.00 | 26.00 | 100.00 | 97.86 | 0.01 | 10.00 | 3.00 | 20.00 | 100.00 | 99.92 | 0.01 | 1.94 | 1.35 | 2.78 | 100.00 | 91.40 | 0.01 |
| Mood disorder | ||||||||||||||||||
| Crum et al. 2005 | 22.00 | 19.00 | 27.00 | 16.47 | 11.00 | 10.00 | 13.00 | 16.37 | 1.98 | 1.50 | 2.62 | 16.05 | ||||||
| Currie et al. 2005 | 17.00 | 15.00 | 19.00 | 17.15 | 10.00 | 10.00 | 11.00 | 16.75 | 1.66 | 1.44 | 1.90 | 20.34 | ||||||
| Davis et al. 2020 | 4.00 | 4.00 | 4.00 | 17.41 | 2.00 | 2.00 | 2.00 | 16.76 | 1.70 | 1.60 | 1.81 | 21.83 | ||||||
| Husky et al. 2018 | 7.00 | 4.00 | 12.00 | 15.18 | 4.00 | 4.00 | 4.00 | 16.73 | 1.72 | 0.93 | 3.18 | 7.74 | ||||||
| Markkula et al. 2016 | 9.00 | 6.00 | 12.00 | 16.47 | 4.00 | 3.00 | 4.00 | 16.65 | 2.42 | 1.67 | 3.51 | 13.20 | ||||||
| Tebeka et al. 2018 | 9.00 | 8.00 | 10.00 | 17.34 | 3.00 | 3.00 | 4.00 | 16.74 | 2.68 | 2.38 | 3.01 | 20.84 | ||||||
| Subtotal | 11.00 | 6.00 | 17.00 | 100.00 | 99.15 | 0.01 | 5.00 | 3.00 | 9.00 | 100.00 | 99.85 | 0.01 | 2.00 | 1.62 | 2.47 | 100.00 | 90.00 | 0.01 |
AUD = alcohol use disorder; CMD = common mental disorders; OR = odds ratio; CO = confidence interval.
FIGURE 3.
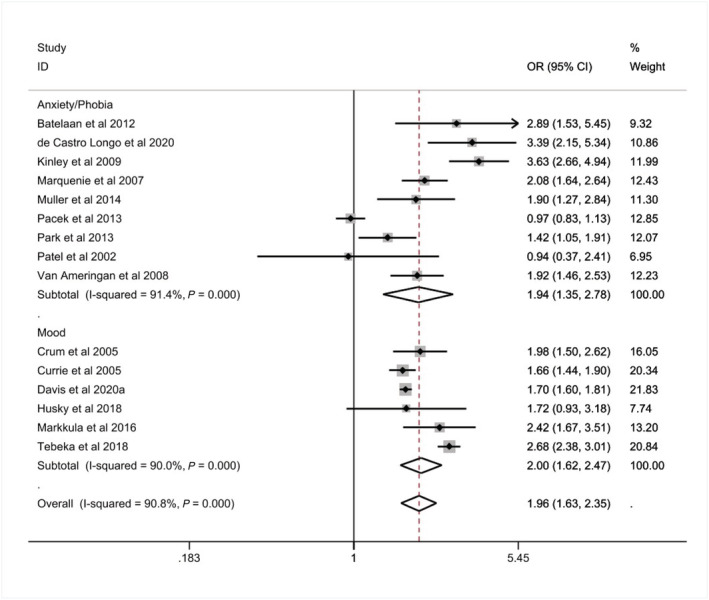
Associations of any alcohol use disorder (AUD) with common mental disorder (CMD), stratified by anxiety/phobic and mood disorders (n = 367 483)
A sensitivity analysis removing studies with the largest [43] and smallest [44] OR resulted in only a small change in the total and life‐time effect size (see Supporting information, Figures S5 and S6). In light of changes to the categorization of mental disorders whereby PTSD and OCD are now two distinct diagnosis classifications (‘trauma‐ and stressor‐related disorders’ and ‘obsessive‐compulsive and related disorders’ [5]), a sensitivity analysis examining differences in associations of any AUD among those with PTSD compared to other anxiety/phobic disorder (without OCD) was conducted and showed a twofold increase in associations among those with PTSD, while associations with other anxiety/phobic disorders were non‐significant (see Supporting information, Table S4). We were unable to conduct a sensitivity analysis of OCD due to an insufficient number of studies.
Exploratory analysis
When stratified by the decade (e.g. 1990s) and continent (e.g. Europe) in which the study was conducted, respectively, we found similar strengths of associations (see Supporting information, Tables S5 and S6).
Heterogeneity
There was substantial heterogeneity between studies when conducting each meta‐analysis, as illustrated in the forest plots (see Figures 1, 2, 3, 4) where I 2 percentages were greater than 80%, which was further confirmed by our overall funnel plot (see Supporting information, Figure S1). An Egger's test was non‐significant (P = 0.86) and a funnel plot showed that studies remained close to the overall effect size, indicating limited evidence of bias (see Supporting information, Figure S7). We also explored sources of heterogeneity by conducting a subgroup analysis according to the decade during which data was collected, the continent in which the studies were conducted and bias score (see Supporting information, Figures S2–S4), but these did not substantially reduce heterogeneity estimates. We were unable to explore heterogeneity according to group characteristics due to a lack of reporting among those with and without a CMD; however, there were differences in the diagnostic criteria used to assess both AUD and CMD which may explain some of the heterogeneity.
FIGURE 4.
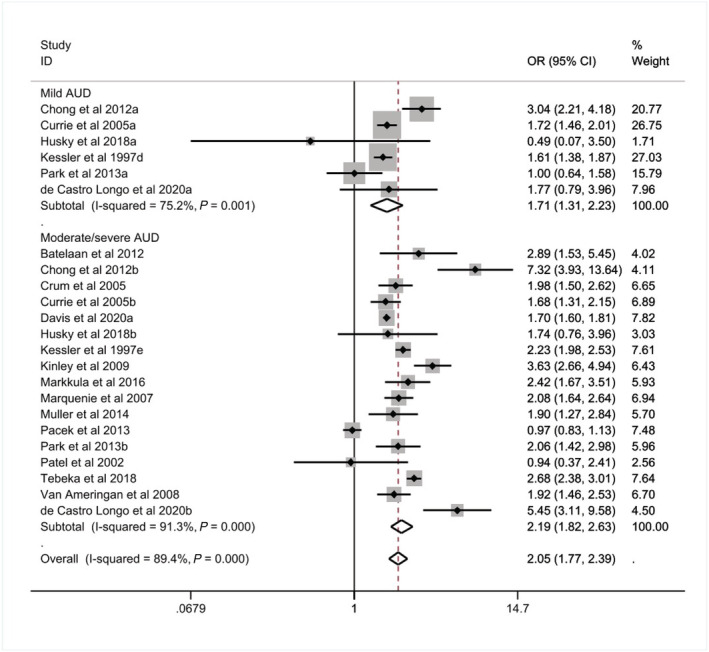
Associations of alcohol use disorder (AUD) among those with a common mental disorder (CMD), stratified by AUD severity (n = 382 201)
Secondary analyses
Prevalence and associations of mild and moderate/severe AUD among those with and without a CMD
The pooled prevalence of mild AUD was higher among those with a CMD compared to those without (K = 6, 7 versus 5%, see Table 4). Those with a CMD were more likely to report mild AUD compared to those without a CMD (OR = 1.71, 95% CI = 1.31–2.23, I 2 = 75.20%, see Table 4 and Figure 4). We found that 12% of those with a CMD reported moderate/severe AUD compared to 6% of those without a CMD (K = 17, see Table 4) and those with a CMD were twice as likely to report moderate/severe AUD (OR = 2.19, 95% CI = 1.82–2.63, I 2 = 91.30%, see Table 4 and Figure 4).
TABLE 4.
Prevalence and associations of mild and moderate/severe AUD among those with and without a CMD (n = 382 201)
| AUD severity | Prevalence among those with a CMD (%) | 95% CI Lower (%) | 95% CI upper (%) | Weight | Heterogeneity (I 2) | P | Prevalence among those without a CMD (%) | 95% CI Lower (%) | 95% CI Upper (%) | Weight | Heterogeneity (I 2) | P | OR | 95% CI Lower | 95% CI Upper | Weight | Heterogeneity (I 2) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild AUD | ||||||||||||||||||
| Chong et al. 2012 | 8.00 | 6.00 | 10.00 | 17.31 | 3.00 | 2.00 | 3.00 | 16.66 | 3.04 | 2.21 | 4.18 | 20.77 | ||||||
| Currie et al. 2005 | 13.00 | 11.00 | 15.00 | 17.72 | 8.00 | 7.00 | 8.00 | 16.73 | 1.72 | 1.46 | 2.01 | 26.75 | ||||||
| Husky et al. 2018 | 1.00 | 0.00 | 4.00 | 14.90 | 1.00 | 1.00 | 2.00 | 16.71 | 0.49 | 0.07 | 3.50 | 1.71 | ||||||
| Kessler et al. 1997 | 15.00 | 14.00 | 17.00 | 17.86 | 10.00 | 9.00 | 10.00 | 16.64 | 1.61 | 1.38 | 1.87 | 27.03 | ||||||
| Park et al. 2013 | 10.00 | 7.00 | 15.00 | 15.80 | 10.00 | 9.00 | 11.00 | 16.66 | 1.00 | 0.64 | 1.58 | 15.79 | ||||||
| De Castro Longo et al. 2020 | 2.00 | 1.00 | 5.00 | 16.41 | 1.00 | 1.00 | 2.00 | 16.60 | 1.77 | 0.79 | 3.96 | 7.96 | ||||||
| Subtotal | 7.00 | 4.00 | 12.00 | 100.00 | 95.66 | 0.01 | 5.00 | 2.00 | 8.00 | 100.00 | 99.71 | 0.01 | 1.71 | 1.31 | 2.23 | 100.00 | 75.20 | 0.01 |
| Moderate/severe AUD | ||||||||||||||||||
| Batelaan et al. 2012 | 8.00 | 5.00 | 14.00 | 5.66 | 3.00 | 2.00 | 3.00 | 5.89 | 2.89 | 1.53 | 5.45 | 4.02 | ||||||
| Chong et al. 2012 | 3.00 | 2.00 | 4.00 | 6.02 | 0.00 | 0.00 | 1.00 | 5.89 | 7.32 | 3.93 | 13.64 | 4.11 | ||||||
| Crum et al. 2005 | 22.00 | 19.00 | 27.00 | 5.96 | 11.00 | 10.00 | 13.00 | 5.84 | 1.98 | 1.50 | 2.62 | 6.65 | ||||||
| Currie et al. 2005 | 5.00 | 4.00 | 7.00 | 6.08 | 3.00 | 3.00 | 3.00 | 5.90 | 1.68 | 1.31 | 2.15 | 6.89 | ||||||
| Davis et al. 2020 | 4.00 | 4.00 | 4.00 | 6.13 | 2.00 | 2.00 | 2.00 | 5.91 | 1.70 | 1.60 | 1.81 | 7.82 | ||||||
| Husky et al. 2018 | 4.00 | 2.00 | 9.00 | 5.70 | 2.00 | 2.00 | 3.00 | 5.90 | 1.74 | 0.76 | 3.96 | 3.03 | ||||||
| Kessler et al. 1997 | 26.00 | 24.00 | 28.00 | 6.10 | 12.00 | 11.00 | 13.00 | 5.89 | 2.23 | 1.98 | 2.53 | 7.61 | ||||||
| Kinley et al. 2009 | 8.00 | 6.00 | 10.00 | 6.02 | 2.00 | 2.00 | 2.00 | 5.90 | 3.63 | 2.66 | 4.94 | 6.43 | ||||||
| Markkula et al. 2016 | 9.00 | 6.00 | 12.00 | 5.96 | 4.00 | 3.00 | 4.00 | 5.88 | 2.42 | 1.67 | 3.51 | 5.93 | ||||||
| Marquenie et al. 2007 | 9.00 | 7.00 | 11.00 | 6.07 | 4.00 | 4.00 | 5.00 | 5.89 | 2.08 | 1.64 | 2.64 | 6.94 | ||||||
| Muller et al. 2014 | 21.00 | 15.00 | 28.00 | 5.69 | 11.00 | 10.00 | 12.00 | 5.88 | 1.90 | 1.27 | 2.84 | 5.70 | ||||||
| Pacek et al. 2013 | 40.00 | 37.00 | 44.00 | 6.02 | 42.00 | 41.00 | 43.00 | 5.90 | 0.97 | 0.83 | 1.13 | 7.48 | ||||||
| Park et al. 2013 | 15.00 | 11.00 | 21.00 | 5.84 | 8.00 | 7.00 | 8.00 | 5.89 | 2.06 | 1.42 | 2.98 | 5.96 | ||||||
| Patel et al. 2002 | 14.00 | 6.00 | 29.00 | 4.68 | 15.00 | 14.00 | 15.00 | 5.89 | 0.94 | 0.37 | 2.41 | 2.56 | ||||||
| Tebeka et al. 2018 | 9.00 | 8.00 | 10.00 | 6.12 | 3.00 | 3.00 | 4.00 | 5.90 | 2.68 | 2.38 | 3.01 | 7.64 | ||||||
| Van Ameringan et al. 2008 | 28.00 | 24.00 | 31.00 | 6.03 | 14.00 | 12.00 | 17.00 | 5.76 | 1.92 | 1.46 | 2.53 | 6.70 | ||||||
| De Castro Longo et al. 2020 | 6.00 | 4.00 | 9.00 | 5.92 | 1.00 | 1.00 | 2.00 | 5.88 | 5.45 | 3.111 | 9.58 | 4.52 | ||||||
| Subtotal | 12.00 | 8.00 | 17.00 | 100.00 | 99.26 | 0.01 | 6.00 | 4.00 | 10.00 | 100.00 | 99.88 | 0.01 | 2.19 | 1.82 | 2.63 | 100.00 | 91.30 | 0.01 |
AUD = alcohol use disorder; CMD = common mental disorder; OR = odds ratio; CO = confidence interval.
Due to the small number of studies examining the prevalence of mild AUD (n = 6) it was not possible to conduct a subgroup analysis of mild AUD by the type of CMD, although this was possible for moderate/severe AUD. We found those with a mood or anxiety/phobic disorder were approximately twice as likely to report moderate/severe AUD (mood: K = 6, OR = 2.02, 95% CI = 1.60–2.57, I 2 = 89.60%; anxiety/phobic: K = 9, OR = 2.12, 95% CI = 1.43–3.14, I 2 = 92.20%, see Supporting information, Table S3).
Narrative synthesis
Binge drinking among those with and without a CMD
Five studies reported the prevalence of binge drinking among those with and without a CMD, although there was variation in the cut‐offs used to assess this and the duration of binge drinking (see Table 5). Of the five studies, four examined the prevalence of binge drinking among those with and without depression, one with anxiety and one with PTSD. Four of the five studies reported a higher prevalence of binge drinking among those with a CMD (3.70–35.03%, see Table 5) compared to those without (1.01–31.62%). One reported a lower prevalence (12.60 versus 15.10%, see Table 5); this may have been due to the study measuring depressive episode or having any anxiety, whereas other studies examined specific types of CMDs or depressive symptoms.
TABLE 5.
Overview of findings of studies examining the prevalence of binge drinking among those with and without a CMD
| Study | Type of CMD assessed | Outcome | Duration | Summary of findings | Demographic characteristics |
|---|---|---|---|---|---|
|
Depression (cut‐off score > 5 or more, derived depression scale) | Binge drinking (5 or more drinks on one occasion once a month or more) | 12 months |
Binge drinker and depressed: 420/1199 (35.03%) Binge drinker and not depressed: 5161/16324 (31.62%) |
Gender: Binge drinker/depressed: Male: 188/375 (50.00%) Female: 232/824 (28.13%) Binge drinker/not depressed: Male: 3351/8561 (39.14%) Female: 1310/7763 (23.31%) |
|
Major depressive episode (yes/no) Anxiety (yes/no) |
Binge drinking (5 or more drinks in last 30 days) | 30 days |
With depressive episode: 2013–2014 = 169/1339 (12.60%) Without depressive episode: 2013–2014 = 2142/13963 (15.10%) With anxiety: 2013/2014 = 123/931 (13.20%) Without anxiety: 2206/14371 (15.00%) |
|
|
MDD (ICD‐10) | Binge drinking (5 or more drinks, never, some times per year, 1–3 times per month, at least once a week*) | 12 months |
With MDD: 26/342 (7.50%) Without MDD: 265/5763 (4.60%) |
|
|
Depressive symptoms (BDI cut‐off score >8) | Heavy drinking occasion (7 or more drinks for men or 5 or more for women) | 28 days |
With depression: 25/321 (7.79%) Without depression: 86/1765 (4.87%) |
Gender and depression Female = 4/198 (2.02%) Male = 21/123 (17.07%) Gender and no depression Female = 36/942 (3.82%) Male = 50/823 (6.08%) |
|
PTSD (DSM‐III‐R) | Heavy alcohol use (frequency 1–3 times per month and quantity 5 + drinks per day for men and 4 + drinks per day for women, respectively) | 12 months |
With PTSD: 1/27 (3.70%) Without PTSD: 17/1691 (1.01%) |
CMD = common mental disorder; MDD = major depressive disorder; BDI = Beck depression inventory; PTSD = post‐traumatic stress disorder.
Alcohol consumption among those with and without a CMD
Twelve studies reported the prevalence of alcohol consumption among those with and without a CMD, although there was variation in the type of alcohol consumption and CMD assessed and cut‐off scores used (see Table 6). Three studies reported a higher prevalence of alcohol consumption among those with a CMD (1.66–24.29%) compared to those without (0.92–7.94%), six reported a lower prevalence among those with a CMD (0.00–42.00%) and three reported both higher and lower prevalence depending on the type of CMD and alcohol consumption outcome (0.00–14.81%, see Table 6).
TABLE 6.
Overview of findings of studies examining the prevalence of alcohol consumption among those with and without a CMD
| Study | Type of CMD assessed | Outcome | Duration | Summary of findings |
|---|---|---|---|---|
|
Social phobia and anxiety (MINI‐SPIN cut‐off score 8 >) |
Frequency of alcohol use (> 1 times per week) Alcohol problems (one or more periods in the last 5 years affected job) |
Alcohol frequency = 1 week Alcohol problems = 5 years |
With SPAS: Weekly drinker = 182/446 (40.80%) Alcohol problems = 54/446 (11.21%) Without SPAS: Weekly drinker = 1124/2230 (50.40%) Alcohol problems = 121/2230 (5.43%) |
|
Depression (HADS‐D cut‐off score 11 >) Anxiety (HAD‐A cut‐off score 11 >) |
Alcohol frequency (daily,* occasionally, never; cut‐offs not stated) |
Not stated |
With depression: Daily drinker = 58/241 (24.07%) Without depression: Daily drinker = 553/1439 (38.43%) With anxiety: Daily drinker = 40/176 (22.73%) Without anxiety: Daily drinker = 571/1504 (37.97%) |
|
Anxiety symptoms (GAI‐SF cut‐off score of 3 >) |
Alcohol consumption (< 1, 1, 2, 2 >* units per day) |
Not stated |
With anxiety: 7/77 (9.09%) Without anxiety: 14/289 (4.84%) |
|
Depressive symptoms (CES‐D cut‐off score 16 >) |
Alcohol frequency (3 times per week) |
1 week |
With depression: 32/159 (20.13%) Without depression: 533/2175 (24.51%) |
|
Depression (GSM, answering yes to questions 1 and 2) |
Alcohol frequency (> 1 drink per week) |
1 week |
With depression: 0/54 (0.00%) Without depression: 14/915 (1.53%) |
|
Depressive symptoms (yes to 4 mandatory questions and 2 additional) |
Alcohol consumption (life‐time abstainers, non‐heavy drinkers, infrequent heavy drinkers, frequent heavy drinkers;* 4 or 5 drinks for women and men on at least 3 days) |
7 days |
With depression: Frequent heavy drinker = 64/12886 (0.50%) Without depression: Frequent heavy drinker = 1558/155835 (1.00%) |
|
Panic disorder (DSM‐5) |
Alcohol consumption (frequent drinkers: consumed alcohol in either last 30 days and 7 days or 1–2 days per week with 5/4 standard drinks in last 7 days or 3 or more days per week with 5/4 standard drinks in the last 7 days) |
12 months |
With panic disorder: 25/96 (31.30%) Without panic disorder: 1680/4176 (42.00%) |
|
Depressive symptoms (BDI cut‐off score > 10) |
Alcohol consumption (> = 3 times per week) |
Not stated |
With depression: 63/3800 (1.66%) Without depression: 85/9279 (0.92%) |
|
GAD OCD Phobia (DSM‐III) |
Heavy/excessive alcohol intake (cut‐off score of 4 on Garretsen scale) | 6 months |
With phobic disorder: 1/21 (4.76%) With panic disorder: 0/6 (0.00%) With GAD: 3/47 (6.38%) Without an anxiety disorder: 1/27 (3.70%) |
|
MDD Dysthymia GAD Panic disorder Specific phobia Social phobia (DSM‐IV‐TR) |
Excessive alcohol use (more than 21 drinks per week) |
Alcohol use = 7 days |
With MDD: 9/96 (9.38%) With Dysthymia: 0/19 (0.00%) With GAD: 7/103 (6.80%) With panic disorder: 4/27 (14.81%) With specific phobia: 6/77 (7.79%) With social phobia: 5/56 (8.93%) Without a CMD: 488/4499 (10.85%) |
|
Depressive symptoms (CES‐D cut‐off score 16 >) |
Alcohol consumption (no alcohol intake, moderate, excessive: 3 or more drinks per day) |
Not stated |
With depression: 7/176 (4.00%) Without depression: 45/1104 (4.10%) |
|
GAD (GAD‐7 cut‐off score 10 >) |
Alcohol consumption (do not drink, moderate, excessivea) |
Not stated |
With GAD: 43/177 (24.29%) Without GAD: 2769/34854 (7.94%) |
CMD = common mental disorder; GAD = generalized anxiety disorder; MDD = major depressive disorder; BDI = Beck depression inventory; HAD = Hospital Anxiety and Depression Scale.
DISCUSSION
Key findings
Our systematic review and meta‐analysis aimed to examine the prevalence and associations of AUD, binge drinking and alcohol consumption among those with and without a CMD, respectively. We found that those with a CMD were twice as likely to report an AUD compared to those without, and these associations were similar among types of CMD throughout decades and continents. Based on the ORs, associations between CMD and AUD were stronger for moderate/severe AUD compared to mild AUD. In addition, our narrative review identified both positive and negative associations for CMD with binge drinking and alcohol consumption, indicating that more research using similar methods is required.
Our findings identified that those with a CMD were more likely to report severe levels of AUD and that most studies focused upon associations with a specific type of CMD, such as MDD. We were unable to identify any studies examining associations with SAD. In addition, much of the research has focused upon AUD as opposed to other problematic drinking patterns, such as binge drinking, despite the high prevalence in the general population [3] and the known negative health impacts [6, 14].
Models of comorbidity and comparisons to previous research
Models of comorbidity have debated whether alcohol worsens mental health or vice versa [18] and previous longitudinal research assessing both pathways indicate stronger support for the notion that poor mental health increases alcohol use [45]; however, there is likely to be a bidirectional association. Psychological models, such as the stress–coping and incentive–motivation models, hypothesize that individuals may be motivated to use alcohol to cope with stress and enhance positive affect [19], and that benefits of drinking outweigh the consequences of not drinking [20]. Considering that symptoms of a CMD include low mood and irritability [32], alcohol may be used to cope with symptoms initially, increasing alcohol use [46]. The self‐medication model argues further that alcohol may be used specifically because of its rapid onset of action and differs according to the individuals’ symptoms [21]. Our findings are based on cross‐sectional research, therefore we cannot infer causality. We found associations between AUD and CMD regardless of the type of CMD and severity of AUD. It may be that individuals with a CMD may use alcohol to enhance positive affect and cope with symptoms of poor mental health. Further qualitative and longitudinal research is required to understand the reasons why those with a CMD use alcohol.
Our narrative review of associations between binge drinking and CMDs and consumption, respectively, showed mixed evidence. Studies included in this review suggest that alcohol use and CMD comorbidity may be more complex, as some studies reported increases in binge drinking or consumption while others did not. This may have been due to the range of CMDs measured or the measures used to assess alcohol use and CMDs. However, previous research suggests that this may also be explained by additional factors such as gender [10, 15], age [14, 47] and specific CMD diagnoses [9]. Future research should consider such characteristics when examining associations between alcohol use and CMD. In addition, further research is required on associations of CMDs with other alcohol outcomes, given that they are more prevalent in the general population compared to AUD [3] and are known to have implications on health [6].
A previous systematic review reported a twofold increase in the odds of reporting any AUD among those with an anxiety disorder and 2.5‐fold increase for those with major depression, in addition to a 2.3‐fold and threefold increase in the odds of reporting alcohol dependence for any anxiety disorder and major depression, respectively. We found slightly weaker associations, with a twofold increase in the odds of any AUD (and the same for moderate/severe AUD) for any anxiety or mood disorder, respectively. This difference could be explained by the types of CMDs included in our review in which we included MDD, dysthymia, GAD, panic disorder, phobias, PTSD, OCD or SAD, whereas Lai and colleagues [11] included agoraphobia, GAD, panic disorder, social phobia, bipolar disorder, dysthymia and MDD. Our sensitivity analysis also showed a twofold increase in the odds of having any AUD among those with PTSD, while a non‐significant association was found among those with any other anxiety disorder, excluding OCD.
Other psychological models suggest that comorbid alcohol and mental health problems are due to shared vulnerabilities, such as SES factors [23, 48, 49, 50]. We attempted to explore this by reviewing evidence examining the prevalence of alcohol use among those with and without a CMD based on SES characteristics; however, studies included in this review did not report this and thus we cannot support or reject these suggestions.
Strengths and limitations
With regard to the studies included in this review, the majority of studies used large sample sizes representative of the general population and standardized criteria to assess alcohol use and CMD, particularly those reporting the prevalence of AUD. There are some limitations to note. First, the majority of studies focused upon the prevalence of alcohol use among those with and without types of CMDs, namely MDD, rather than other disorders such as SAD. Therefore, we were unable to explore associations beyond broad mood and anxiety/phobic disorders, including more specific disorders. Secondly, we were unable to conduct a meta‐analysis on the prevalence and association of binge drinking or alcohol consumption due to variations in the measures and cut‐offs used; therefore, we cannot conclude whether those with a CMD are more likely to report different patterns of alcohol use compared to those without beyond AUD.
With regard to our review, we conducted an extensive search of the literature across multiple databases and included a range of CMDs and types of alcohol use, with large sample sizes. There are also some limitations to note. First, there was substantial heterogeneity between studies. While the majority of studies used diagnostic criteria to establish the presence of CMD and AUD, different versions of criteria were used between studies. There was also limited reporting of group characteristics among those with and without a CMD, which may explain some of the heterogeneity. We overcame this by exploring differences in associations between the severity of AUD and type of CMD, as well as the continent and decade in which the study was conducted. Secondly, we included published research, therefore we may have missed some grey literature. However, given that multiple databases and references were searched, we believe our review was inclusive. Thirdly, some of the associations may have been driven by specific types of CMD, as found in previous research [25]; we conducted a sensitivity analysis with PTSD but were unable to conduct further analyses to due to insufficient numbers. Fourthly, the stratified prevalence by AUD severity would equal the overall any AUD prevalence for studies that provided these stratified data; however, some studies reported moderate/severe AUD only. For those studies which reported the stratified prevalence by AUD severity, the sum of the mild and moderate/severe prevalence would then equal the overall prevalence, but some studies only reported the prevalence for moderate/severe AUD and, in these cases, this was the same as the numbers included in the overall meta‐analysis. Finally, while studies included in this review generally included individuals aged 18 years and over, in some cases studies had a minimum age in adolescence (e.g. 15 years and over). Due to the way in which data were presented in these studies, it was not possible to exclude these participants and restrict the prevalence estimates to those aged 18 years and over. However, in large population studies the numbers aged under 18 years would be in the minority, and this should not impact upon the prevalence reported.
CONCLUSIONS
Our review and meta‐analysis show that having a CMD is associated with increased odds of having an AUD, particularly moderate/severe AUD. There was little difference in associations based on the type of CMD. There is a need to ensure that alcohol and mental health problems are treated in parallel, while more research is required to investigate group characteristics and differences beyond broad CMD classifications. Additional research examining associations between having a CMD with other alcohol outcomes is required to provide a more holistic understanding of drinking patterns among individuals with a CMD.
DECLARATION OF INTERESTS
J.P. is funded as part of a PhD Studentship by the Society for the Study of Addiction.
AUTHOR CONTRIBUTIONS
Jo‐Anne Puddephatt: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; validation. Patricia Irizar: Data curation; investigation; resources; validation. Andrew Jones: Conceptualization; formal analysis; methodology; supervision. Suzanne Gage: Conceptualization; formal analysis; methodology; supervision. Laura Goodwin: Conceptualization; formal analysis; funding acquisition; methodology; supervision.
Supporting information
Table S1: Study Inclusion and Exclusion Criteria
Table S2: Full Search Terms
Table S3: Associations of Moderate/Severe AUD Among Those With an Anxiety or Mood Disorder Compared to Those Without (N = 210 121)
Table S4: Associations of Any AUD Among Those With Any Anxiety Disorder (excluding PTSD and OCD) and Among Those with PTSD (N = 137 916)
Table S5: Associations of Any AUD Among Those With a CMD Compared to Those Without Stratified by Continent (N = 365 331)
Table S6: Associations of Any AUD Among Those With a CMD Compared to Those Without Stratified by the Decade Data was Collected (N = 224 835)
Figure S1: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) (N = 382 201)
Figure S2: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by the decade in which the study was conducted (N = 224 835)
Figure S3: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by the continent in which the study was conducted in (N = 382 201)
Figure S4: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by each study's bias score (N = 382 201)
Figure S5: 12‐month and life‐time associations of alcohol use disorder (AUD) among those with a common mental disorder (CMD), compared to those without, after removing Kinley et al. 2009 study (N = 353 660)
Figure S6: 12‐month and life‐time associations of any alcohol use disorder (AUD) stratified by type of common mental disorder (CMD), compared to those without, after removing Patel et al. 2002 study (N = 373 637)
Figure S7: Figure S7: Funnel plot to explore publication bias
ACKNOWLEDGEMENTS
This work was supported as part of a PhD Studentship by the Society for the Study of Addiction.
Puddephatt J‐A, Irizar P, Jones A, Gage SH, Goodwin L. Associations of common mental disorder with alcohol use in the adult general population: a systematic review and meta‐analysis. Addiction. 2022;117:1543–1572. 10.1111/add.15735
Funding information Society for the Study of Addiction
REFERENCES
- 1. Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2018;392:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Global status report on alcohol and health 2018. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 3. Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Addiction. 2018;113:1905–26. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) . Depression and Other Common Mental Disorders: Global Health Estimates. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 5. American Psychiatric Association (APA) . Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). Washington, DC: APA Publishing; 2013. [Google Scholar]
- 6. Wilsnack RW, Wilsnack SC, Gmel G, Kantor LW. Gender differences in binge drinking: prevalence, predictors, and consequences. Alcohol Res. 2018;39:57–76. [PMC free article] [PubMed] [Google Scholar]
- 7. National Health Service Alcohol misuse. 2018. Available at: https://www.nhs.uk/conditions/alcohol-misuse/. Accessed 3 November 2021.
- 8. Paljärvi T, Koskenvuo M, Poikolainen K, Kauhanen J, Sillanmäki L, Mäkelä P. Binge drinking and depressive symptoms: a 5‐year population‐based cohort study. Addiction. 2009;104:1168–78. [DOI] [PubMed] [Google Scholar]
- 9. Lee YY, Wang P, Abdin E, Chang S, Shafie S, Sambasivam R, et al. Prevalence of binge drinking and its association with mental health conditions and quality of life in Singapore. Addict Behav. 2020;100:106114. [DOI] [PubMed] [Google Scholar]
- 10. Nazareth I, Walker C, Ridolfi A, Aluoja A, Bellon J, Geerlings M, et al. Heavy episodic drinking in Europe: a cross section study in primary care in six European countries. Alcohol Alcohol. 2011;46:600–6. [DOI] [PubMed] [Google Scholar]
- 11. Lai HMX, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: a systematic review and meta‐analysis. Drug Alcohol Depend. 2015;154:1–13. [DOI] [PubMed] [Google Scholar]
- 12. Kingsbury M, Sucha E, Horton NJ, Sampasa‐Kanyinga H, Murphy JM, Gilman SE, et al. Life‐time experience of multiple common mental disorders and 19‐year mortality: results from a Canadian population‐based cohort. Epidemiol Psychiatr Sci. 2020;29:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hjorthøj C, Østergaard MLD, Benros ME, Toftdahl NG, Erlangsen A, Andersen JT, et al. Association between alcohol and substance use disorders and all‐cause and cause‐specific mortality in schizophrenia, bipolar disorder, and unipolar depression: a nationwide, prospective, register‐based study. Lancet Psychiatry. 2015;2:801–8. [DOI] [PubMed] [Google Scholar]
- 14. Kuntsche E, Kuntsche S, Thrul J, Gmel G. Binge drinking: health impact, prevalence, correlates and interventions. Psychol Health. 2017;32:976–1017. [DOI] [PubMed] [Google Scholar]
- 15. Austin MA, Villarosa‐Hurlocker MC. Drinking patterns of college students with comorbid depression and anxiety symptoms: the moderating role of gender. J Subst Abuse. 2021. 10.1080/14659891.2021.1879291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Institute for Alcohol Studies . Alcohol and Mental Health: Policy and Practice in England. 2018. Available at: http://www.ias.org.uk/uploads/pdf/IAS%20reports/rp31042018.pdf. Accessed 3 November 2021. [Google Scholar]
- 17. Alsuhaibani R, Smith DC, Lowrie R, Aljhani S, Paudyal V. Scope, quality and inclusivity of international clinical guidelines on mental health and substance abuse in relation to dual diagnosis, social and community outcomes: a systematic review. BMC Psychiatry. 2021;21:209–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jane‐Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: a review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug Alcohol Rev. 2006;25:515–36. [DOI] [PubMed] [Google Scholar]
- 19. Wills TA, Shiffman S. Coping and substance use: a conceptual framework. In: Coping Subst Use. 3; 1985. p. 24. [Google Scholar]
- 20. Cox WM, Klinger E. A motivational model of alcohol use: determinants of use and change. In: Handbook of Motivational Counseling: Goal‐based Approaches to Assessment and Intervention With Addiction and Other Problems. 2nd ed. Oxford, UK: Wiley Blackwell; 2011. p. 131–58. [Google Scholar]
- 21. Khantzian E. The self‐medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–44. [DOI] [PubMed] [Google Scholar]
- 22. Polimanti R, Peterson RE, Ong J‐S, MacGregor S, Edwards AC, Clarke T‐K, et al. Evidence of causal effect of major depression on alcohol dependence: findings from the psychiatric genomics consortium. Psychol Med. 2019;49:1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castillo‐Carniglia A, Keyes KM, Hasin DS, Cerdá M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry. 2019;6:1068–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puddephatt J, Jones A, Gage S, Fear N, Field M, McManus S, et al. Associations of alcohol use and mental health in England: findings from a representative population survey. Drug Alcohol Depend. 2020. 10.1016/j.drugalcdep.2020.108463 [DOI] [PubMed] [Google Scholar]
- 25. Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM‐5 alcohol use disorder: results from the National Epidemiologic Survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR, et al. Mental disorders as risk factors for substance use, abuse and dependence: results from the 10‐year follow‐up of the National Comorbidity Survey. Addiction. 2010;105:1117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith JP, Randall CL. Anxiety and alcohol use disorders: comorbidity and treatment considerations. Alcohol Res. 2012;34:414–31. [PMC free article] [PubMed] [Google Scholar]
- 28. Oliveira LM, Bermudez MB, de Amorim Macedo MJ, Passos IC. Comorbid social anxiety disorder in patients with alcohol use disorder: a systematic review. J Psychiatr Res. 2018;106:8–14. [DOI] [PubMed] [Google Scholar]
- 29. National Health Service England . General Medical Services (GMS) contract Quality and Outcomes Framework (QOF), Guidance for GMS contract 2019/20 in England London, UK: British Medical Association; 2019. p. 96–106. [Google Scholar]
- 30. Hardoon S, Hayes JF, Blackburn R, Petersen I, Walters K, Nazareth I, et al. Recording of severe mental illness in United Kingdom primary care, 2000–2010. PLOS ONE. 2013;8:e82365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pilling S, Whittington C, Taylor C, Kendrick T. Identification and care pathways for common mental health disorders: summary of NICE guidance. BMJ. 2011;342:d2868. [DOI] [PubMed] [Google Scholar]
- 32. National Collaborating Centre for Mental Health, National Institute for Health, Clinical Excellence, British Psychological Society, Royal College of Psychiatrists . Common Mental Health Disorders: Identification and Pathways to Care. London, UK: RCPsych Publications; 2011. [Google Scholar]
- 33. Moher D, Liberati A, Tetzlaff J, Altman DG. the PRISMA group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. PLOS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–53. [DOI] [PubMed] [Google Scholar]
- 35. Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. National Institute for Health and Care Excellence (NICE) Common mental disorders in primary care overview. London, UK: NICE; 2018. Available at: https://pathways.nice.org.uk/pathways/common-mental-health-disorders-in-primary-care. Accessed 3 November 2021.
- 37. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 38. Dawson DA, Goldstein RB, Grant BF. Differences in the profiles of DSM‐IV and DSM‐5 alcohol use disorders: implications for clinicians. Alcohol Clin Exp Res. 2013;37:E305–E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JA. Metan: fixed‐and random‐effects meta‐analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 40. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014;72:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sterne JA, Harbord RM. Funnel plots in meta‐analysis. Stata J. 2004;4:127–41. [Google Scholar]
- 43. Kinley DJ, Cox BJ, Clara I, Goodwin RD, Sareen J. Panic attacks and their relation to psychological and physical functioning in Canadians: Results from a nationally representative sample. Can J Psychiatry. 2009;54:113–22. [DOI] [PubMed] [Google Scholar]
- 44. Patel A, Knapp M, Henderson J, Baldwin D. The economic consequences of social phobia. J Affect Disord. 2002;68:221–33. [DOI] [PubMed] [Google Scholar]
- 45. Bell S, Britton A. An exploration of the dynamic longitudinal relationship between mental health and alcohol consumption: a prospective cohort study. BMC Med. 2014;12:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. [DOI] [PubMed] [Google Scholar]
- 47. Keyes KM, Hamilton A, Patrick ME, Schulenberg J. Diverging trends in the relationship between binge drinking and depressive symptoms among adolescents in the U.S. from 1991 through 2018. J Adolesc Health. 2020;66:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mueser KT, Drake RE, Wallach MA. Dual diagnosis: a review of etiological theories. Addict Behav. 1998;23:717–34. [PubMed] [Google Scholar]
- 49. Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. Am J Hum Genet. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- 50. McHugh RK, Weiss RD. Alcohol use disorder and depressive disorders. Alcohol Res. 2019;40:e1–e8. 10.35946/arcr.v40.1.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Study Inclusion and Exclusion Criteria
Table S2: Full Search Terms
Table S3: Associations of Moderate/Severe AUD Among Those With an Anxiety or Mood Disorder Compared to Those Without (N = 210 121)
Table S4: Associations of Any AUD Among Those With Any Anxiety Disorder (excluding PTSD and OCD) and Among Those with PTSD (N = 137 916)
Table S5: Associations of Any AUD Among Those With a CMD Compared to Those Without Stratified by Continent (N = 365 331)
Table S6: Associations of Any AUD Among Those With a CMD Compared to Those Without Stratified by the Decade Data was Collected (N = 224 835)
Figure S1: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) (N = 382 201)
Figure S2: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by the decade in which the study was conducted (N = 224 835)
Figure S3: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by the continent in which the study was conducted in (N = 382 201)
Figure S4: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by each study's bias score (N = 382 201)
Figure S5: 12‐month and life‐time associations of alcohol use disorder (AUD) among those with a common mental disorder (CMD), compared to those without, after removing Kinley et al. 2009 study (N = 353 660)
Figure S6: 12‐month and life‐time associations of any alcohol use disorder (AUD) stratified by type of common mental disorder (CMD), compared to those without, after removing Patel et al. 2002 study (N = 373 637)
Figure S7: Figure S7: Funnel plot to explore publication bias


