Abstract
Objective
The integration of high‐frequency oscillations (HFOs; ripples [80–250 Hz], fast ripples [250–500 Hz]) in epilepsy evaluation is hampered by physiological HFOs, which cannot be reliably differentiated from pathological HFOs. We evaluated whether defining abnormal HFO rates by statistical comparison to region‐specific physiological HFO rates observed in the healthy brain improves identification of the epileptic focus and surgical outcome prediction.
Methods
We detected HFOs in 151 consecutive patients who underwent stereo‐electroencephalography and subsequent resective epilepsy surgery at two tertiary epilepsy centers. We compared how HFOs identified the resection cavity and predicted seizure‐free outcome using two thresholds from the literature (HFO rate > 1/min; 50% of the total number of a patient's HFOs) and three thresholds based on normative rates from the Montreal Neurological Institute Open iEEG Atlas (https://mni‐open‐ieegatlas.research.mcgill.ca/): global Atlas threshold, regional Atlas threshold, and regional + 10% threshold after regional Atlas correction.
Results
Using ripples, the regional + 10% threshold performed best for focus identification (77.3% accuracy, 27% sensitivity, 97.1% specificity, 80.6% positive predictive value [PPV], 78.2% negative predictive value [NPV]) and outcome prediction (69.5% accuracy, 58.6% sensitivity, 76.3% specificity, 60.7% PPV, 74.7% NPV). This was an improvement for focus identification (+1.1% accuracy, +17.0% PPV; p < .001) and outcome prediction (+12.0% sensitivity, +1.0% PPV; p = .05) compared to the 50% threshold. The improvement was particularly marked for foci in cortex, where physiological ripples are frequent (outcome: +35.3% sensitivity, +5.3% PPV; p = .014). In these cases, the regional + 10% threshold outperformed fast ripple rate > 1/min (+3.6% accuracy, +26.5% sensitivity, +21.6% PPV; p < .001) and seizure onset zone (+13.5% accuracy, +29.4% sensitivity, +17.0% PPV; p < .05–.01) for outcome prediction. Normalization did not improve the performance of fast ripples.
Significance
Defining abnormal HFO rates by statistical comparison to rates in healthy tissue overcomes an important weakness in the clinical use of ripples. It improves focus identification and outcome prediction compared to standard HFO measures, increasing their clinical applicability.
Keywords: biomarker, epilepsy surgery, high‐frequency oscillations, interictal, normative values
Key Points.
Normalization significantly improved the ability of ripples to identify the epileptic focus and to predict seizure freedom
The regional + 10% threshold exhibited the best performance
Normalization is particularly useful in patients with a focus in cortex with high physiological ripple rates
Ripple normalization outperformed fast ripple rate > 1/min and seizure onset zone in patients with a focus in ripple‐rich cortex
Normalization did not improve the performance of fast ripples in identifying the epileptic focus or predicting seizure freedom
1. INTRODUCTION
High‐frequency oscillations (HFOs), subdivided into ripples (80–250 Hz) and fast ripples (FRs; 250–500 Hz), are a promising biomarker for epileptogenic tissue. Most studies have reported results at the group level, with higher HFO rates in epileptogenic than nonepileptogenic tissue, and a better correlation between favorable outcome and removal of tissue generating HFOs than removal of tissue generating interictal spikes or that is part of the seizure onset zone (SOZ). 1 , 2 , 3 FRs recorded after resection have been linked to seizure recurrence. 4 , 5 , 6 , 7
Prospective studies reported conflicting results on the performance of HFOs to identify the SOZ and predict good outcome at the individual patient level. 8 , 9 , 10 They showed patient examples where the performance of ripples was hindered by the detection of presumably physiological ripples. 8 , 10 That HFOs occur under physiological conditions is a challenge when assessing their validity as a biomarker for epilepsy. Physiological HFOs occur predominantly in the ripple range, at rest and linked to cognitive processes or evoked by tasks or stimuli. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Pathological and physiological HFOs largely overlap in their signal properties, and there is no reliable way to separate them. 17 , 20 , 21 The Montreal Neurological Institute (MNI) Open iEEG Atlas project (https://mni‐open‐ieegatlas.research.mcgill.ca/) 22 , 23 studied HFOs in carefully selected stereoelectroencephalographic (SEEG) channels with normal electroencephalographic (EEG) activity. 24 The rate of physiological ripples varied substantially across different regions, with the highest values in the occipital, sensorimotor, and mesiotemporal regions. Physiological FRs were rare, even in eloquent cortical areas.
This study evaluated whether "correcting" for physiological HFOs improves identification of the epileptic focus and prediction of surgical outcome. We hypothesized that using the statistical distribution of normative physiological HFO rates for each region to define rates that are too high for the physiological range, and therefore most likely to be pathological, would increase the performance of this marker. We expected the improvement to be most pronounced in patients with a focus in or close to brain areas generating high rates of physiological ripples. Because physiological FRs are rare, we expected no improvement for FRs.
2. MATERIALS AND METHODS
2.1. Patient selection and data acquisition
We screened 202 consecutive patients undergoing SEEG investigation followed by resective open epilepsy surgery at Grenoble‐Alpes University Hospital (CHUGA) or the MNI between January 2009 and January 2019. For selection criteria and flowchart of patients' inclusion see Figure 1. This study was approved by the MNI Institutional Review Board. All patients signed written informed consent.
FIGURE 1.
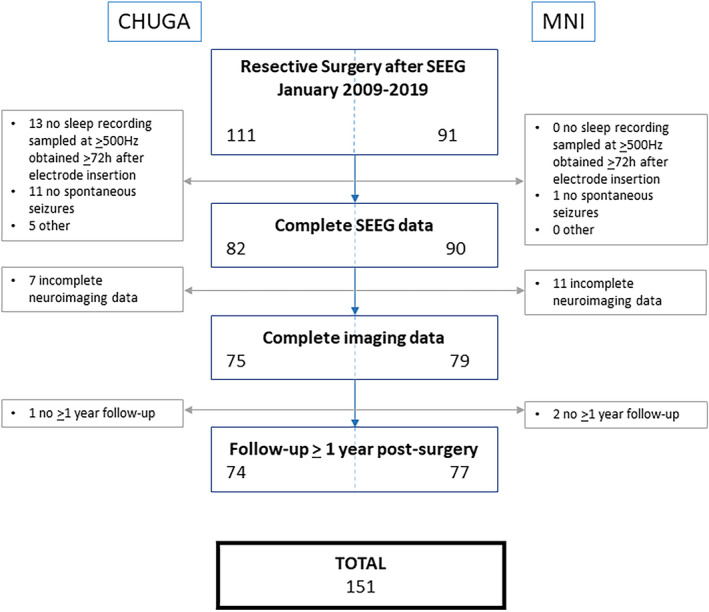
Flowchart of the patient selection process. The reasons for exclusion merged in others are no report available (n = 3), no visible interictal electroencephalographic changes at seizure onset (n = 1), and premature termination of stereoelectroencephalogram (SEEG) due to self‐removal of electrodes (n = 1). In nine of the 151 included patients, we could only select 20 min of non‐rapid eye movement (NREM) sleep containing one or more electrographic seizure. In five, we could only select 20 min of NREM sleep <2 h away from a focal seizure. In one other patient, we could only select 10 min of NREM sleep. We decided to include these patients to be as generalizable as possible. The segments with seizures themselves were excluded. CHUGA, Grenoble‐Alpes University Hospital; MNI, Montreal Neurological Institute
The MNI recordings were acquired with Harmonie (Stellate) or Nihon‐Kohden EEG amplifiers at a sampling rate of 2000 Hz, using homemade MNI or commercial DIXI electrodes. The CHUGA recordings were acquired with Micromed EEG amplifiers at sampling rates of 512, 1024, or 2048 Hz, using DIXI or, in a few occasions, ALCIS electrodes.
2.2. Data selection and HFO analysis
Analogous to the MNI Open iEEG Atlas, we automatically detected HFOs in visually selected 20‐min sections from non‐rapid eye movement (NREM) sleep stages N2/N3, 24 as this state shows the highest HFO rates 25 , 26 , 27 and best identifies the epileptic focus in the interictal EEG. 28 If possible, we chose epochs in the first sleep cycle, because it was shown to contain higher pathological HFO rates. 29 We selected epochs ≥2 h away from focal or 6 h from generalized seizures. Ripples (80–250 Hz) were analyzed in all subjects. FRs (>250 Hz) were analyzed in subjects whose recordings had a sampling frequency greater than 1000 Hz. The detector is available at https://mni‐open‐ieegatlas.research.mcgill.ca/. 12 , 29 , 30 It identifies increases in power with respect to the background in narrow frequency bands, with a duration longer than four oscillations plus the effective response time of the filters (equiripple finite impulse response filters of order 508). Results of HFO analysis were not used for clinical decision‐making.
2.3. Image coregistration and localization of electrode contacts
Registration to stereotaxic space and anatomical localization of electrode contacts and channels were performed as done previously. 22 , 23 , 24 Using MINC tools (http://www.bic.mni.mcgill.ca/ServicesSoftware/MINC) and the IBIS platform, patient‐specific peri‐implantation and postsurgical images were linearly registered to the preimplantation magnetic resonance imaging (MRI), and electrode positions were marked in the coregistered images. A nonlinear transformation from the preimplantation image to the ICBM152 2009c template was obtained and applied to the coordinates to represent them in the common MNI stereotaxic space. We used the same 17 regions as the MNI Open iEEG Atlas to correct for region‐specific physiological HFO rates. 24
2.4. Classification of channels
A bipolar channel was classified as resected if both contacts were resected on the coregistered postresection image. To account for sagging, coregistration error, and partial contact resection, contacts in or in the close vicinity (<5 mm) of the cavity were considered resected. 28 , 31 SOZ channels were identified by consensus of two neurophysiologists based on the first unequivocal visible signal changes at seizure onset independent of frequency content. 32
To determine where each bipolar channel was recording from, we modeled each contact as a sphere of 10‐mm radius and computed the percentage of each Atlas' gray matter region within this volume, assigning weights that decreased with the square of the distance from the center. We then averaged the percentages over the two contacts, and used up to three regions showing the highest percentages.
2.5. Thresholds
We compared two thresholds for automatic HFO detection frequently used in the literature with three thresholds based on the normative values of the MNI Open iEEG Atlas. 24 Studies in the literature frequently use (1) HFO rate > 1/min 5 , 6 , 7 , 10 or (2) a majority threshold relative to the total or maximum number of HFOs in a patient. 8 , 9 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 We used HFO rate > 1/min and above a threshold of 50% relative to the total number of HFOs in a patient to compare to our new HFO Atlas‐based thresholds.
We computed three thresholds using the statistical distribution of normative physiological HFO rates 24 : (1) a global Atlas threshold, (2) a regional Atlas threshold, and (3) a regional Atlas correction followed by a 10% threshold (regional + 10%). The global Atlas threshold was defined as the 90th percentile value of the distribution of normative HFO rates of all regions combined. This cutoff is commonly used in biomedical statistics to objectively set a threshold; using the 85th or 95th percentile did not change our results (data not shown). We subtracted this value from the automatically detected HFO rates. If the subtraction resulted in a negative value, it was set to zero. The regional Atlas threshold was defined as the region‐specific 90th percentile values of the normative rates. If a bipolar channel was recording from more than one region, the threshold was obtained by a weighted average of the region‐specific thresholds, the weight of each region being determined by the percentage of each region contributing to that channel. The regional + 10% threshold was calculated by removing the channels that had ≤10% of the total number of HFOs after regional correction. This was done to eliminate channels only marginally above physiological HFO levels; using a 5% or 15% cutoff did not change our results (data not shown).
Six hundred seven bipolar channels (5% total ripple channels) of 49 patients for ripples, and 299 bipolar channels (5% total FR channels) of 23 patients for FRs were also part of the MNI Open iEEG Atlas. For these patients, we recalculated the regional Atlas thresholds excluding the values from that patient, and used these corrected values for further calculation. The regional Atlas threshold for every patient included in the Atlas is therefore independent of that patient's normative values.
2.6. Surgical outcome
Outcome was determined according to the Engel classification 43 from the most recent follow‐up ≥1 year after surgery, and dichotomized into seizure‐free (Engel IA) and non‐seizure‐free (Engel ≥IB).
2.7. Statistical analyses
We tested for differences in demographic information and group‐level differences in event rates between resected and nonresected channels of seizure‐free and non‐seizure‐free patients using Mann–Whitney U (MWU), chi‐squared, or Fisher exact tests depending on the type and distribution of the variable.
To evaluate whether physiological HFO correction improved identification of the epileptic focus, we compared the HFO region to the resection cavity. We defined channels with HFOs above threshold that were or were not resected as true positive (TP) or false positive (FP), and channels without HFOs or with HFOs below threshold that were or were not resected as false negative (FN) or true negative (TN). We assessed the performance of ripples and FRs above threshold to identify the resection cavity by computing accuracy ([TP + TN]/[TP + TN + FP + FN]), sensitivity (TP/[TP + FN]), specificity (TN/[TN + FP]), positive predictive value (PPV; TP/[TP + FP]), and negative predictive value (NPV; TN/[TN + FN]). Because only in Engel IA outcome is the epileptic focus entirely inside the resection cavity, we separatly analyzed seizure‐free and non‐seizure‐free patients. We tested for differences in performance measures between thresholds, by applying a Wilcoxon signed‐rank test to the performance measures of all pairs of different thresholds within the two outcome groups and corrected for multiple comparisons (false discovery rate [FDR] < 0.05 corrected for 50 comparisons: 10 threshold‐pairs times five performance measures). We tested for differences in performance measures between seizure‐free and non‐seizure‐free patients using an MWU test (FDR < 0.05, five comparisons: five performance measures). We expected that correcting for region‐specific rates of physiological ripples would increase specificity and PPV for identification of the focus. We expected a larger increase in PPV in seizure‐free patients.
To evaluate whether physiological HFO correction improved prediction of seizure‐free outcome, we allowed residual HFOs in a maximum of 5% of the nonresected channels. We used definitions analogous to the ones described above, but now defined them at the patient level. Patients without HFOs above threshold and good or poor outcome were considered FN or FP, respectively. To test for differences in performance measures between thresholds, we applied a Cochran Q test to the proportions of true and false predictions with different thresholds. If the Cochran Q test was significant after correcting for multiple comparisons (p < .05, five comparisons: five performance measures), we performed post hoc McNemar tests to identify which pairs of thresholds were significantly different. We expected that correcting for region‐specific rates of physiological ripples would increase sensitivity and NPV at the outcome level.
We expected greater improvement in performance in patients with a focus in areas generating high rates of physiological ripples according to the MNI Open iEEG Atlas (namely occipital, sensorimotor, and mesiotemporal regions, referred to as ripple‐rich cortex) than in patients with a focus in areas generating low rates of physiological ripples (ripple‐poor cortex). 24 Therefore, we compared tissue and outcome‐level predictions between patients with a focus in ripple‐rich and ripple‐poor cortex using Cochran Q combined with McNemar and Fisher exact tests corrected for multiple comparisons.
The current gold standard to define the area to resect is the SOZ. As a last step, we compared the performance of the best corrected HFO measure to the performance of the SOZ on tissue and outcome level.
3. RESULTS
The study sample consisted of 151 patients (Table S1). Figure 2 displays the different thresholds examined and the group‐level results for ripples. It shows the ripple rates in resected and nonresected channels of seizure‐free and non‐seizure‐free patients, and indicates the data used when evaluating the different thresholds. In both seizure‐free and non‐seizure‐free patients, the majority of channels with high ripple rates were resected. In non‐seizure‐free patients there were more nonresected channels with ripples above the 50% and regional + 10% threshold than in seizure‐free patients. Table S2 provides the median values and p‐values.
FIGURE 2.
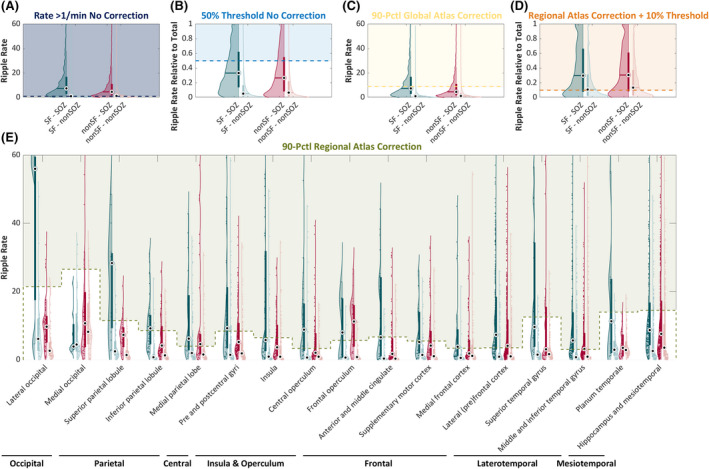
Visualization of the different thresholds examined in this study. Violin plots show the raw ripple rates (A, C, E) and ripple rates relative to a patient's total ripples (B, D) in resected (dark color) and nonresected (light color) tissue in seizure‐free (SF; teal) and non‐seizure‐free (nonSF; red) outcome patients. The colored blocks cover the data points used when evaluating the thresholds (indicated with colored dashed lines) examined in this study: raw ripple rate > 1/min (A; dark blue), 50% of the patient's total ripple rate (B; light blue), the global Atlas threshold (C; yellow), the regional Atlas threshold (E; green), and 10% of the patient's total ripple rate remaining after regional Atlas correction (D; orange). The global Atlas threshold is calculated as the 90th percentile value of the normative rates of all 17 high‐frequency oscillation (HFO) Atlas regions combined. The regional Atlas threshold is calculated as the weighted average of the region‐specific 90th percentile value of the normative rates obtained from the Montreal Neurological Institute Open iEEG Atlas Project from which an electrode channel is recording. The 17 HFO Atlas regions are indicated on the x‐axis in E. SOZ, seizure onset zone
3.1. Ripples
3.1.1. Identification of the resected tissue
The application of any threshold significantly improved accuracy, specificity, and PPV to identify the resected tissue compared to ripple rate > 1/min (Figure 3A, Table 1A). Using the regional + 10% threshold resulted in the highest accuracy, specificity, and PPV in both outcome groups (seizure‐free group: accuracy = 77.3%, specificity = 97.1%, PPV = 80.6%; non‐seizure‐free group: accuracy = 75.4%, specificity = 93.3%, PPV = 42.9%). In seizure‐free patients, this was a 1.1% increase in accuracy (p < .001), and a 17.0% increase in PPV (p < .001) compared to the 50% threshold (Table 1A). In non‐seizure‐free patients, this corresponded to a 4.6% increase in specificity (p = .047), and no difference in accuracy and PPV compared to the 50% threshold. Accuracy using ripple rate > 1/min, specificity using the 50% and regional + 10% thresholds, and PPV using all thresholds were higher in seizure‐free compared to non‐seizure‐free patients (Figure 3A).
FIGURE 3.
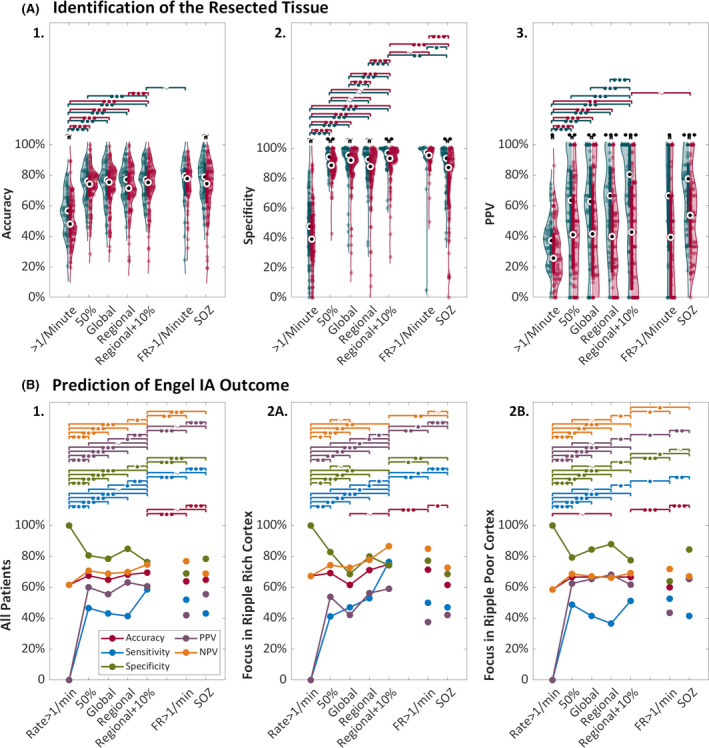
Performance of (normalized) ripples, fast ripple (FR) rate of >1/min, and the seizure onset zone (SOZ) to identify the resected tissue (A) or to predict seizure freedom (B). (A) Violin plots of the accuracy (1), specificity (2), and positive predictive value (PPV; 3) of ripples above different thresholds and the SOZ (x‐axis) to delineate the resected tissue in seizure‐free (teal) and non‐seizure‐free (red) patients. The dots represent the performance values of the individual patients. The black dots in the thicker blue or red lines show the median and interquartile range. Significant differences in performance values between the thresholds within the seizure‐free or non‐seizure‐free outcome groups are indicated with teal or red significance bars. Significant differences in performance values between seizure‐free and non‐seizure‐free patients are indicated with black significance bars. The number of filled circles indicates the level of significance: one = p < .05, two = p < .01, and three = p < .001. A tilde indicates a trend: p = .05–.1. (B) Line plot indicating the change in accuracy (red), sensitivity (blue), specificity (green), PPV (purple), and negative predictive value (NPV; orange) of ripples with increasingly sophisticated degrees of thresholding and the SOZ (x‐axis) to predict seizure‐free outcome for all patients (1), and separately for patients with a focus in ripple‐rich (2A) and ripple‐poor (2B) cortex. Significant differences in performance values between thresholds are indicated with colored significance bars. The number of filled circles indicates the level of significance: one = p < .05, two = p < .01, and three = p < .001. A tilde indicates a trend: p = .05–.1
TABLE 1.
Median values of performance measures of ripples above the different thresholds, FR rate > 1/min (B), or SOZ (C) for predicting the resected tissue in seizure‐free and non‐seizure‐free patients, overall and grouped by location of the epileptogenic focus in ripple‐rich versus ripple‐poor cortex
| Performance measure | Subgroup | Accuracy | Sensitivity | Specificity | PPV | NPV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SF | nSF | p, SF vs. nSF | SF | nSF | p, SF vs. nSF | SF | nSF | p, SF vs. nSF | SF | nSF | p, SF vs. nSF | SF | nSF | p, SF vs. nSF | ||
| Ripples above the different thresholds | ||||||||||||||||
| Rate > 1/min | All | 56.8 | 48.1 | .043 a | 85.2 | 83.3 | .714 | 47.7 | 39.2 | .077 b | 37.3 | 25.8 | .016 a | 91.1 | 89.5 | .958 |
| RRC | 54.0 | 47.8 | .187 | 65.2 | 80.0 | .907 | 52.6 | 43.0 | .317 | 37.8 | 22.7 | .007 a | 78.6 | 90.5 | .140 | |
| RPC | 57.7 | 48.7 | .042 a | 90.0 | 87.7 | .919 | 45.5 | 39.0 | .138 | 36.7 | 27.9 | .339 | 92.0 | 88.9 | .561 | |
| 50% | All | 76.2 | 74.3 | .355 | 27.3 | 24.2 | .682 | 94.3 | 88.7 | .002 a | 63.6 | 41.2 | .002 a | 77.5 | 81.0 | .958 |
| RRC | 72.5 | 76.2 | .067 b | 20.0 | 25.0 | .907 | 94.5 | 91.9 | .083 b | 60.0 | 40.0 | .023 a | 70.3 | 83.0 | .012 a | |
| RPC | 78.8 | 71.7 | .042 a | 29.4 | 23.7 | .216 | 94.1 | 86.9 | .008 a | 66.7 | 42.0 | .017 a | 82.7 | 79.4 | .622 | |
| Global | All | 77.0 | 75.5 | .355 | 25.7 | 20.0 | .099 b | 95.5 | 92.1 | .063 b | 62.8 | 41.7 | .004 a | 78.9 | 81.8 | .958 |
| RRC | 72.1 | 77.8 | .129 | 21.4 | 20.0 | .907 | 94.6 | 93.1 | .317 | 66.7 | 46.7 | .047 a | 75.8 | 83.7 | .012 a | |
| RPC | 78.0 | 72.5 | .042 a | 29.4 | 19.1 | .119 | 95.9 | 91.5 | .087 b | 60.0 | 33.3 | .023 a | 82.6 | 78.3 | .484 | |
| Regional | All | 77.3 | 71.6 | .287 | 36.1 | 27.8 | .114 | 92.6 | 87.9 | .063 b | 66.7 | 40.0 | <.001 a | 80.6 | 82.0 | .958 |
| RRC | 73.5 | 72.0 | .242 | 16.7 | 20.0 | .907 | 96.4 | 92.9 | .275 | 66.7 | 43.8 | .002 a | 74.6 | 85.0 | .021 a | |
| RPC | 81.2 | 70.0 | .042 a | 43.8 | 33.3 | .147 | 90.9 | 84.8 | .044 a | 65.2 | 40.0 | .002 a | 86.7 | 80.6 | .484 | |
| Regional + 10% | All | 77.3 | 75.4 | .355 | 27.0 | 17.1 | .079 b | 97.1 | 93.3 | .004 a | 80.6 | 42.9 | <.001 a | 78.2 | 80.0 | .958 |
| RRC | 73.0 | 77.6 | .129 | 13.0 | 16.7 | .907 | 100 | 96.6 | .083 b | 100 | 33.3 | <.001 a | 73.2 | 83.3 | .016 a | |
| RPC | 80.0 | 73.4 | .042 a | 30.0 | 23.2 | .109 | 96.6 | 92.4 | .011 a | 75.0 | 42.9 | .002 a | 84.3 | 78.4 | .484 | |
| FRs > 1/min | All | 79.6 | 77.9 | .680 | 21.4 | 10.9 | .154 | 97.4 | 95.4 | .124 | 66.7 | 39.4 | .02 a | 81.1 | 82.8 | .958 |
| RRC | 69.9 | 78.3 | .587 | 16.6 | 16.0 | .824 | 96.5 | 98.6 | .868 | 50.0 | 64.6 | .907 | 69.9 | 80.3 | .682 | |
| RPC | 79.4 | 79.4 | .903 | 11.1 | 11.1 | .756 | 96.4 | 94.4 | .551 | 40.0 | 40.0 | .891 | 81.8 | 85.7 | .889 | |
| SOZ | All | 78.9 | 74.5 | .091 b | 51.5 | 58.3 | .318 | 93.4 | 87.2 | .003 a | 77.8 | 53.8 | <.001 a | 83.2 | 87.9 | .958 |
| RRC | 71.7 | 77.6 | .822 | 30.0 | 40.0 | .178 | 94.9 | 88.5 | .167 | 80.0 | 56.3 | .056 b | 75.8 | 87.8 | .008 a | |
| RPC | 82.9 | 73.1 | .017 a | 59.1 | 69.8 | .756 | 92.6 | 81.5 | .029 a | 77.8 | 50.0 | .019 a | 89.4 | 88.6 | .889 | |
Abbreviations: FDR, false discovery rate; FR, fast ripple; NPV, negative predictive value; nSF, non‐seizure‐free; PPV, positive predictive value; RPC, ripple‐poor cortex; RRC, ripple‐rich cortex; SF, seizure‐free; SOZ, seizure onset zone.
Significant difference between the SF and nSF groups: Benjamini–Hochberg FDR‐corrected p‐value <.05.
Trend toward a significant difference between the SF and nSF groups: Benjamini–Hochberg FDR‐corrected p‐value between .05 and .1.
There was a larger increase in PPV when moving from the standard thresholds to the regional + 10% threshold in seizure‐free patients, with a focus in ripple‐rich (regional + 10% vs. >1/min: +62.2%, p = .005; regional + 10% vs. 50%: +40%, p = .015) rather than ripple‐poor cortex (regional + 10% vs. >1/min: +38.3%, p < .001; and regional + 10% vs. 50%: +8.3%, p = .014).
3.1.2. Predicting Engel IA outcome
The application of any threshold significantly increased the sensitivity, PPV, and NPV, and decreased the specificity of ripples to predict seizure freedom compared to using ripple rate > 1/min (Figure 3B1, Table S3A). Of the three normalization thresholds, the regional + 10% threshold resulted in the highest accuracy (69.5%), sensitivity (58.6%), and NPV (74.7%), and the regional Atlas threshold resulted in the highest specificity (84.9%) and PPV (63.2%). Comparing the regional + 10% to the 50% threshold showed a trend toward higher sensitivity (+12.0%, p = .05) and PPV (+1.0%, p = .05).
There was an upward trend in accuracy, sensitivity, PPV, and NPV, and a downward trend in specificity when moving from the standard to the regional + 10% threshold in patients with a focus in ripple‐rich, but not in patients with a focus in ripple‐poor cortex (Figure 3B2). In patients with a focus in ripple‐rich cortex, the sensitivity and PPV using the regional + 10% threshold were significantly higher than with the 50% threshold (sensitivity = +35.3%, p = .014; PPV = +5.3%, p = .014). In patients with a focus in ripple‐poor cortex, the 50% and regional + 10% thresholds resulted in comparable performance. Using the regional + 10% threshold, there was a trend toward higher sensitivity and NPV to predict seizure freedom in patients with a focus in ripple‐rich than ripple‐poor cortex (sensitivity = 76.5% vs. 51.2%, p = .089; NPV = 86.7% vs. 69.2%, p = .080). Figure 4 shows an example of a seizure‐free patient with an epileptic focus in ripple‐rich cortex (A), where normalization improves prediction, and a seizure‐free patient with an epileptic focus in ripple‐poor cortex (B), where the 50% and all normalization thresholds work.
FIGURE 4.
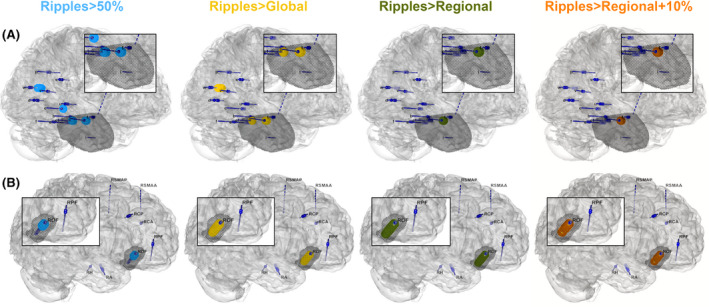
Patient examples of a case with an epileptic focus in ripple‐rich cortex where normalization improves prediction (A), and a case with an epileptic focus in ripple‐poor cortex where the 50% and all normalization thresholds work (B). Both patients had Engel IA outcome. We used Epitools 50 to map the electrodes in the patient's brain surface as dark blue cylinders and indicated which channels were above the cutoff threshold of 1 ripple/min with small dark blue spheres. The four columns indicate channels that had ripples above the 50% (light blue), global Atlas (yellow), regional Atlas (green), and regional + 10% Atlas (orange) thresholds. The black dots indicate the resection. (A) This patient benefitted from regional thresholding and shifted from false negative to true positive classification. The 50% threshold identified four channels inside, and five channels outside the resection: one in the posterior hippocampus, and four in the medial parieto‐occipital areas. The global Atlas threshold identified three channels inside, and three channels outside the resection: three in the medial parieto‐occipital areas. The regional Atlas and regional + 10% threshold identified two channels inside the resection. The residual ripples using the 50% and global Atlas thresholds surpassed the allowed 5% residual high‐frequency oscillations, hence the false negative classification. (B) This patient was classified as false negative using ripple rate > 1/min and as true positive using all other thresholds: channels with ripples above the 50%, global Atlas, regional Atlas, and regional + 10% Atlas thresholds were all inside the right orbitofrontal resection
3.1.3. Patient level classification of seizure outcome
Thirty‐four of the 58 (58.6%) seizure‐free patients were correctly classified using the regional + 10% threshold. Twelve of the 24 incorrectly classified seizure‐free patients had residual ripples in 6%–10% of the nonresected channels, which was slightly more than the allowed 5% cutoff. In all 12 patients, >50% of the channels identified by the regional + 10% threshold were included in the resection. All had the electrodes positioned relatively close together, recording from brain tissue very close to the subsequently resected tissue (Figure 5A). Ten of the 24 incorrectly classified seizure‐free patients had a separate ripple focus distant from the resection (Figure 5B). These secondary foci were predominantly located in central, parietal, and occipital areas. Eight of these 10 patients had an MRI abnormality (n = 6) or positron emission tomography (PET) hypometabolism (n = 2) in these regions. The remaining two incorrectly classified seizure‐free patients had ripples above the 50% threshold in central or occipital areas that were not resected; these ripples were below the region‐specific normalization threshold and therefore resulted in no HFOs using the regional and regional + 10% thresholds.
FIGURE 5.
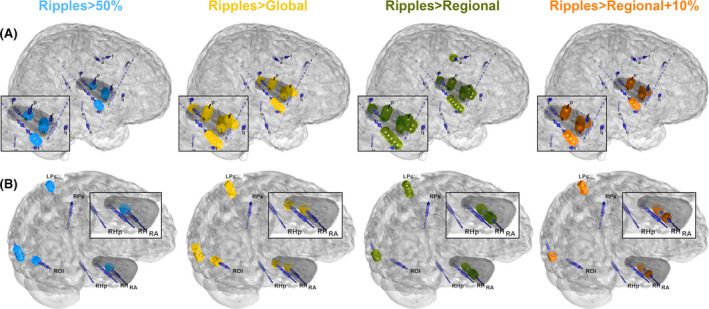
Patient examples of a case where the 5% tolerance for residual high‐frequency oscillations (HFOs) may be too strict (A), and a case where the physiological ripple correction may be insufficient (B). Both patients had Engel IA outcome. We used Epitools 50 to map the electrodes in the patient's brain surface as dark blue cylinders and indicated which channels were above the cutoff of 1 ripple/min with small dark blue spheres. The four columns indicate channels that had ripples above the 50% (light blue), global Atlas (yellow), regional Atlas (green), and regional + 10% Atlas (orange) thresholds. The black dots indicate the resection cavity. (A) All thresholds identify the same brain area, but only using the 50% threshold, this patient was classified as true positive, because there were residual HFOs in >5% of the 70 nonresected channels using the other thresholds. These channels with residual HFOs were located in the close vicinity of the resection cavity. (B) This patient was classified as false negative using all thresholds and had <50% of the channels above all thresholds resected. All thresholds correctly identified the right hippocampus, but also showed a parietocentral and occipital ripple focus. This patient's magnetic resonance imaging showed a right mesiotemporal sclerosis, a small area of gliosis and encephalomalacia/ulegyria in the inferior aspect of the left occipital pole, and a T2 signal abnormality, possibly gliosis or dysplasia, in the left centroparietal region. The parietocentral and occipital ripple foci may be secondary foci that are responsive to antiseizure medication; alternatively, these may correspond to physiological ripples, indicating an insufficient correction
3.2. Fast ripples
There was no clinically relevant difference in any performance measure of FRs to identify the epileptic focus or predict seizure freedom using FR rate > 1/min, the 50% threshold, or any of the three proposed normalization thresholds (Figures S1 and S2).
3.3. Comparing the performance of the best ripple threshold to FR rate > 1/min and the SOZ
Four of the 151 (2.6%) patients analyzed for ripples did not show ripples above the regional + 10% threshold; FRs > 1/min were not detected in eight of the 83 (9.6%) patients analyzed for FRs.
Comparing the performance of the regional + 10% threshold to that of FR rate > 1/min to identify the epileptic focus, we found no significant differences (Figure 3A, Table 1B). Comparing the regional + 10% threshold to the SOZ, we found a higher PPV in non‐seizure‐free patients (+13.8, p = .05) and lower specificity (seizure‐free, −3.7; non‐seizure‐free, −6.1; p < .001) of the SOZ. This shows that the regional + 10% threshold is a better marker for the epileptic focus than the SOZ. The higher sensitivity (seizure‐free, +24.5; non‐seizure‐free, +41.2; p < .001) and NPV (seizure‐free, +5.0; non‐seizure‐free, +7.9; p < .001) of the SOZ compared to the regional + 10% threshold is likely explained by the SOZ being used to tailor the surgical resection (Table 1C).
The regional + 10% threshold outperformed FR rate > 1/min and the SOZ for outcome prediction, especially in patients with a focus in ripple‐rich cortex (Figure 3B, Table S3).
4. DISCUSSION
In this large bicentric study of 151 patients, we demonstrated that HFO normalization based on region‐specific physiological HFO rates improves epileptic focus identification and prediction of surgical outcome. We showed that (1) normalization significantly improved the ability of ripples to identify the resected tissue and predict seizure freedom, with the regional + 10% threshold exhibiting the best performance; (2) normalization is particularly useful in patients with a focus in cortex with high physiological ripple rates; in this condition, ripple normalization even outperformed FR rate > 1/min and the SOZ, which requires many days of monitoring; and (3) normalization did not improve the performance of FRs for focus identification or oucome prediction.
4.1. Normalization improves epileptic focus identification
We found a higher accuracy, specificity, and PPV, but a lower sensitivity and NPV compared to Lachner‐Piza and colleagues and to the tissue‐level performance results from Fedele and colleagues. 9 , 42 Normalization of ripples not only performed better than standard thresholds applied to ripples alone, but also outperformed the combinations of ripples and spikes, 42 or ripples and FRs 9 reported in the literature. These latter methods are designed to differentiate pathological from physiological ripples and increase the specificity of ripples for the epileptic tissue.
Higher specificity and PPV confirmed our hypothesis that correcting for region‐specific rates of physiological ripples improves localization of the epileptic focus compared to standard thresholds applied in HFO research and compared to the SOZ as current gold standard measure. We found a larger increase in PPV in seizure‐free than non‐seizure‐free patients, and a larger increase in PPV in seizure‐free patients with a focus in ripple‐rich compared to ripple‐poor cortex. This indicates that our method corrected for physiological ripples and did not erroneously remove ripples in the nonresected part of the presumed epileptogenic region in non‐seizure‐free patients. Our results also confirm the co‐occurrence of physiological and pathological ripples in the same tissue. 19 If diseased tissue were not able to generate physiological ripples, our subtraction method would result in poorer performance in patients with a focus in ripple‐rich cortex.
The low sensitivity and NPV are likely explained by the resected tissue also containing nonpathological tissue. Reanalyzing our data using the overlap between resected and SOZ channels, for a more strict definition of the epileptogenic tissue, resulted in higher sensitivity and NPV (sensitivity = 44.9% vs. 27%, NPV = 93.9% vs. 78.2%; data not shown), values similar to those reported in the literature.
4.2. Normalization improves prediction of Engel IA outcome
We found higher accuracy, sensitivity, and NPV, but lower PPV and lower or higher specificity comparing our outcome level results to the results of the long‐term recording subgroup of Jacobs and colleagues, and the ripple results of Fedele and colleagues. 8 , 9 Higher sensitivity and NPV confirmed our hypothesis that correcting for region‐specific rates of physiological ripples improves prediction of seizure outcome compared to standard thresholds. Our results showed that all patients showed significant improvements in the performance of ripples to predict seizure freedom using any threshold compared to ripple rate > 1/min, but only patients with a focus in ripple‐rich cortex showed significant improvements in sensitivity and PPV when comparing the regional + 10% and 50% thresholds. It was also supported by the trend toward higher sensitivity and NPV in patients with a focus in ripple‐rich compared to ripple‐poor cortex when using the regional + 10% threshold. In patients with a focus in ripple‐rich cortex, the regional + 10% threshold even outperformed the SOZ. Using a similar approach, Kuroda and colleagues recently showed that the prediction of postoperative seizure outcomes can be optimized with the consideration of normalized HFOs. 44
Apart from the difference in threshold to define an HFO channel, other methodological differences were the definition of good outcome (Engel I vs. IA), and the threshold defining the number or fraction of HFOs needing to be resected for a good outcome patient to be considered TP.
4.3. Normalization does not improve FR performance
Physiological FRs are rare, even in eloquent cortical areas. 24 Also, FRs are usually recorded in only a subset of patients, 6 , 7 , 10 , 45 but when present they are very specific for epileptogenic tissue, and residual FRs after resection are tightly linked to seizure recurrence. 5 , 6 , 7 , 9 , 10 We found a high percentage of nonresected channels without FRs (specificity), a high percentage of channels with FRs that were resected (PPV) at the tissue level, and a high percentage of patients with residual FRs who had recurrent seizures (NPV) at the outcome level. As expected, because physiological FRs are rare, we found no clinically relevant changes in these performance measures upon application of the different thresholds. Interestingly, ripples above the regional + 10% threshold performed similarly to FRs in terms of focus identification, but outperformed FRs in outcome prediction.
4.4. Limitations
As prior work showed that it is not necessary to remove all channels with HFOs to achieve seizure freedom, we allowed residual HFOs in 5% of the nonresected channels. 8 , 40 , 46 We defined this threshold as a fraction of the total number of nonresected channels instead of a rate threshold to make it independent from the proportion of electrodes covering the epileptogenic tissue. By doing so, we also compensated for cases with large resections where the few identified HFO channels were resected by chance. However, our results showed that this 5% threshold may be too strict in patients with tightly grouped electrodes (Figure 5A). The nonresected electrodes close to the cavity, which showed likely pathological ripples in these seizure‐free patients, may be functionally deactivated, because they become disconnected even if not resected. 4
In addition, 10 of the 24 incorrectly classified seizure‐free patients had a clear, separate ripple focus distant from the resection cavity (Figure 5B). These secondary foci were predominantly located in central, parietal, and occipital areas, regions less densely sampled in the MNI Open iEEG Atlas; hence, the 90th percentile value may be suboptimal. However, eight of these 10 patients showed either an MRI abnormality or a PET hypometabolism, so it may be that our method is correct and seizures originating from these foci are responsive to antiepileptic drugs. Whether an extension of the atlas will eventually solve this issue awaits future research.
Lastly, we analyzed HFOs in 20‐min NREM sleep epochs. We chose this state of vigilance, and if possible the first sleep cycle, as it was shown to have the highest HFO rates, 25 , 26 , 27 with the optimal pathological to physiological HFO ratio, 29 and hence, best identifies the epileptic focus in the interictal EEG. 28 However, in contrast to what was previously suggested, 25 , 47 some recent studies demonstrated that in some patients HFO analysis from a short segment of NREM sleep might not be representative of the total HFO distribution over longer periods, and hence, analysis of prolonged durations is warranted. 28 , 48 , 49
5. CONCLUSIONS
This large bicentric study proposes a solution to one of the key problems that hamper the integration of HFOs into clinical practice: differentiating physiological from pathological HFOs. Correcting for region‐specific normative rates of physiological ripples improves epileptic focus identification and prediction of seizure freedom compared to using standard HFO measures alone. Ripple normalization is particularly useful in patients with an epileptic focus in ripple‐rich cortex. In this condition, it even outperformed FR rate > 1/min and the SOZ, the traditional gold standard for defining the epileptic focus. We found no relevant improvement using HFO normalization for the performance of FRs, which supports the general view that FRs are closely related to epileptogenicity. Future research should compare normalized ripples to other epilepsy markers in a multimarker approach on the same dataset to improve our definition of the area to be resected and ultimately epilepsy outcome.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to express their gratitude to the staff and technicians at the EEG Department of the Montreal Neurological Institute and Hospital, Lorraine Allard, Nicole Drouin, and Chantal Lessard. The authors wish also to express their gratitude to the staff and technicians at the Neurophysiopathology Laboratory of Grenoble‐Alpes University Hospital, Patricia Boschetti, and Marie‐Pierre Noto. This work was supported by the Canadian Institute of Health Research (grant FDN‐143208 to J.G.) and a donation of the Hewitt Foundation (to B.F.). B.F.'s salary was supported by a salary award (Chercheur‐boursier clinicien Junior 2) for 2018–2021 from the Fonds de Recherche du Québec–Santé. The research stay of B.F. at Dr. Kahane's laboratory at CHUGA for project planning and local data collection was supported by a CIC Brain and Mental Health Chair award from the Neurodis Foundation (to B.F.). W.J.E.M.Z. received research scholarships from the Foundations Jo Kolk Studiefonds and De Drie Lichten in the Netherlands.
Zweiphenning WJEM, von Ellenrieder N, Dubeau F, Martineau L, Minotti L, Hall JA, et al. Correcting for physiological ripples improves epileptic focus identification and outcome prediction. Epilepsia. 2022;63:483–496. 10.1111/epi.17145
Contributor Information
Willemiek J. E. M. Zweiphenning, Email: W.J.E.Zweiphenning@umcutrecht.nl.
Birgit Frauscher, Email: birgit.frauscher@mcgill.ca.
REFERENCES
- 1. Frauscher B, Bartolomei F, Kobayashi K, Cimbalnik J, van ‘t Klooster MA, Rampp S, et al. High‐frequency oscillations: the state of clinical research. Epilepsia. 2017;58(8):1316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomschewski A, Hincapié A‐S, Frauscher B. Localization of the epileptogenic zone using high frequency oscillations. Front Neurol. 2019;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engel J, Bragin A, Staba R. Nonictal EEG biomarkers for diagnosis and treatment. Epilepsia Open. 2018;3(S2):120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van ‘t Klooster MA, van Klink NEC, Leijten FSS, Zelmann R, Gebbink TA, Gosselaar PH, et al. Residual fast ripples in the intraoperative corticogram predict epilepsy surgery outcome. Neurology. 2015;85(2):120–8. [DOI] [PubMed] [Google Scholar]
- 5. van ‘t Klooster MA, van Klink NEC, Zweiphenning WJEM, Leijten FSS, Zelmann R, Ferrier CH, et al. Tailoring epilepsy surgery with fast ripples in the intraoperative electrocorticogram. Ann Neurol. 2017;81(5):664–76. [DOI] [PubMed] [Google Scholar]
- 6. Fedele T, Ramantani G, Burnos S, Hilfiker P, Curio G, Grunwald T, et al. Prediction of seizure outcome improved by fast ripples detected in low‐noise intraoperative corticogram. Clin Neurophysiol. 2017;128(7):1220–6. [DOI] [PubMed] [Google Scholar]
- 7. Weiss SA, Berry B, Chervoneva I, Waldman Z, Guba J, Bower M, et al. Visually validated semi‐automatic high‐frequency oscillation detection aides the delineation of epileptogenic regions during intra‐operative electrocorticography. Clin Neurophysiol. 2018;129(10):2089–98. [DOI] [PubMed] [Google Scholar]
- 8. Jacobs J, Wu JY, Perucca P, Zelmann R, Mader M, Dubeau F, et al. Removing high‐frequency oscillations: a prospective multicenter study on seizure outcome. Neurology. 2018;91(11):e1040–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fedele T, Burnos S, Boran E, Krayenbühl N, Hilfiker P, Grunwald T, et al. Resection of high frequency oscillations predicts seizure outcome in the individual patient. Sci Rep. 2017;7:13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roehri N, Pizzo F, Lagarde S, Lambert I, Nica A, McGonigal A, et al. High‐frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann Neurol. 2018;83(1):84–97. [DOI] [PubMed] [Google Scholar]
- 11. Nagasawa T, Juhász C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high‐frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp. 2012;33(3):569–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Ellenrieder N, Frauscher B, Dubeau F, Gotman J. Interaction with slow waves during sleep improves discrimination of physiologic and pathologic high‐frequency oscillations (80–500 Hz). Epilepsia. 2016;57(6):869–78. [DOI] [PubMed] [Google Scholar]
- 13. Kerber K, Dümpelmann M, Schelter B, le Van P, Korinthenberg R, Schulze‐Bonhage A, et al. Differentiation of specific ripple patterns helps to identify epileptogenic areas for surgical procedures. Clin Neurophysiol. 2014;125(7):1339–45. [DOI] [PubMed] [Google Scholar]
- 14. Alkawadri R, Gaspard N, Goncharova II, Spencer DD, Gerrard JL, Zaveri H, et al. The spatial and signal characteristics of physiologic high frequency oscillations. Epilepsia. 2014;55(12):1986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Girardeau G, Zugaro M. Hippocampal ripples and memory consolidation. Curr Opin Neurobiol. 2011;21(3):452–9. [DOI] [PubMed] [Google Scholar]
- 16. Wang S, Wang IZ, Bulacio JC, Mosher JC, Gonzalez‐Martinez J, Alexopoulos A, et al. Ripple classification helps to localize the seizure‐onset zone in neocortical epilepsy. Epilepsia. 2013;54(2):370–6. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto A, Brinkmann BH, Matthew Stead S, Matsumoto J, Kucewicz MT, Marsh WR, et al. Pathological and physiological high‐frequency oscillations in focal human epilepsy. J Neurophysiol. 2013;110(8):1958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malinowska U, Bergey GK, Harezlak J, Jouny CC. Identification of seizure onset zone and preictal state based on characteristics of high frequency oscillations. Clin Neurophysiol. 2015;126(8):1505–13. [DOI] [PubMed] [Google Scholar]
- 19. Liu S, Parvizi J. Cognitive refractory state caused by spontaneous epileptic high‐frequency oscillations in the human brain. Sci Transl Med. 2019;11(514):eaax8830. [DOI] [PubMed] [Google Scholar]
- 20. Burnos S, Frauscher B, Zelmann R, Haegelen C, Sarnthein J, Gotman J. The morphology of high frequency oscillations (HFO) does not improve delineating the epileptogenic zone. Clin Neurophysiol. 2016;127(4):2140–8. [DOI] [PubMed] [Google Scholar]
- 21. Cimbalnik J, Brinkmann B, Kremen V, Jurak P, Berry B, van Gompel J, et al. Physiological and pathological high frequency oscillations in focal epilepsy. Ann Clin Transl Neurol. 2018;5(9):1062–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frauscher B, von Ellenrieder N, Zelmann R, Doležalová I, Minotti L, Olivier A, et al. Atlas of the normal intracranial electroencephalogram: neurophysiological awake activity in different cortical areas. Brain. 2018;141(4):1130–44. [DOI] [PubMed] [Google Scholar]
- 23. von Ellenrieder N, Gotman J, Zelmann R, Rogers C, Nguyen DK, Kahane P, et al. How the human brain sleeps: direct cortical recordings of normal brain activity. Ann Neurol. 2020;87(2):289–301. [DOI] [PubMed] [Google Scholar]
- 24. Frauscher B, von Ellenrieder N, Zelmann R, Rogers C, Nguyen DK, Kahane P, et al. High‐frequency oscillations in the normal human brain. Ann Neurol. 2018;84(3):374–85. [DOI] [PubMed] [Google Scholar]
- 25. Bagshaw AP, Jacobs J, Levan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high‐frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50(4):617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dümpelmann M, Jacobs J, Schulze‐Bonhage A. Temporal and spatial characteristics of high frequency oscillations as a new biomarker in epilepsy. Epilepsia. 2015;56(2):197–206. [DOI] [PubMed] [Google Scholar]
- 27. Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J. High‐frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56(1):108–15. [DOI] [PubMed] [Google Scholar]
- 28. Klimes P, Cimbalnik J, Brazdil M, Hall J, Dubeau F, Gotman J, et al. NREM sleep is the state of vigilance that best identifies the epileptogenic zone in the interictal electroencephalogram. Epilepsia. 2019;60(12):2404–15. [DOI] [PubMed] [Google Scholar]
- 29. von Ellenrieder N, Dubeau F, Gotman J, Frauscher B. Physiological and pathological high‐frequency oscillations have distinct sleep‐homeostatic properties. Neuroimage Clin. 2017;14:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Ellenrieder N, Andrade‐Valença LP, Dubeau F, Gotman J. Automatic detection of fast oscillations (40–200Hz) in scalp EEG recordings. Clin Neurophysiol. 2012;123(4):670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cuello Oderiz C, von Ellenrieder N, Dubeau F, Eisenberg A, Gotman J, Hall J, et al. Association of cortical stimulation‐induced seizure with surgical outcome in patients with focal drug‐resistant epilepsy. JAMA Neurol. 2019;76(9):1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spanedda F, Cendes F, Gotman J. Relations between EEG seizure morphology, interhemispheric spread, and mesial temporal atrophy in bitemporal epilepsy. Epilepsia. 1997;38(12):1300–14. [DOI] [PubMed] [Google Scholar]
- 33. Modur PN, Zhang S, Vitaz TW. Ictal high‐frequency oscillations in neocortical epilepsy: implications for seizure localization and surgical resection. Epilepsia. 2011;52(10):1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cho JR, Koo DL, Joo EY, Seo DW, Hong SC, Jiruska P, et al. Resection of individually identified high‐rate high‐frequency oscillations region is associated with favorable outcome in neocortical epilepsy. Epilepsia. 2014;55(11):1872–83. [DOI] [PubMed] [Google Scholar]
- 35. Okanishi T, Akiyama T, Tanaka SI, Mayo E, Mitsutake A, Boelman C, et al. Interictal high frequency oscillations correlating with seizure outcome in patients with widespread epileptic networks in tuberous sclerosis complex. Epilepsia. 2014;55(10):1602–10. [DOI] [PubMed] [Google Scholar]
- 36. Leung H, Zhu CXL, Chan DTM, Poon WS, Shi L, Mok VCT, et al. Ictal high‐frequency oscillations and hyperexcitability in refractory epilepsy. Clin Neurophysiol. 2015;126(11):2049–57. [DOI] [PubMed] [Google Scholar]
- 37. Wang S, So NK, Jin B, Wang IZ, Bulacio JC, Enatsu R, et al. Interictal ripples nested in epileptiform discharge help to identify the epileptogenic zone in neocortical epilepsy. Clin Neurophysiol. 2017;128(6):945–51. [DOI] [PubMed] [Google Scholar]
- 38. Cuello‐Oderiz C, von Ellenrieder N, Sankhe R, Olivier A, Hall J, Dubeau F, et al. Value of ictal and interictal epileptiform discharges and high frequency oscillations for delineating the epileptogenic zone in patients with focal cortical dysplasia. Clin Neurophysiol. 2018;129(6):1311–9. [DOI] [PubMed] [Google Scholar]
- 39. Iimura Y, Jones K, Takada L, Shimizu I, Koyama M, Hattori K, et al. Strong coupling between slow oscillations and wide fast ripples in children with epileptic spasms: investigation of modulation index and occurrence rate. Epilepsia. 2018;59(3):544–54. [DOI] [PubMed] [Google Scholar]
- 40. Jacobs J, Zijlmans M, Zelmann R, Chatillon CÉ, Hall J, Olivier A, et al. High‐frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67(2):209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brázdil M, Pail M, Halámek J, Plešinger F, Cimbálník J, Roman R, et al. Very high‐frequency oscillations: novel biomarkers of the epileptogenic zone. Ann Neurol. 2017;82(2):299–310. [DOI] [PubMed] [Google Scholar]
- 42. Lachner‐Piza D, Jacobs J, Schulze‐Bonhage A, Stieglitz T, Dümpelmann M. Estimation of the epileptogenic‐zone with HFO sub‐groups exhibiting various levels of epileptogenicity. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:2543–6. [DOI] [PubMed] [Google Scholar]
- 43. Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical treatment of the epilepsies. New York, NY: Raven Press; 1993. p. 609–21. [Google Scholar]
- 44. Kuroda N, Sonoda M, Miyakoshi M, Nariai H, Jeong J‐W, Motoi H, et al. Objective interictal electrophysiology biomarkers optimize prediction of epilepsy surgery outcome. Brain Commun. 2021;3(2):fcab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van ‘t Klooster MA, van Klink NEC, Zweiphenning WJEM, Leijten FSS, Zelmann R, Ferrier CH, et al. Tailoring epilepsy surgery with fast ripples in the intra‐operative electrocorticogram. Ann Neurol. 2017;81(5):664–76. [DOI] [PubMed] [Google Scholar]
- 46. Haegelen C, Perucca P, Châtillon CE, Andrade‐Valença L, Zelmann R, Jacobs J, et al. High‐frequency oscillations, extent of surgical resection, and surgical outcome in drug‐resistant focal epilepsy. Epilepsia. 2013;54(5):848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zelmann R, Zijlmans M, Jacobs J, Châtillon CE, Gotman J. Improving the identification of high frequency oscillations. Clin Neurophysiol. 2009;120(8):1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gliske SV, Irwin ZT, Chestek C, Hegeman GL, Brinkmann B, Sagher O, et al. Variability in the location of high frequency oscillations during prolonged intracranial EEG recordings. Nat Commun. 2018;9(1):2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nevalainen P, von Ellenrieder N, Klimeš P, Dubeau F, Frauscher B, Gotman J. Association of fast ripples on intracranial EEG and outcomes after epilepsy surgery. Neurology. 2020;95(16):e2235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Medina Villalon S, Paz R, Roehri N, Lagarde S, Pizzo F, Colombet B, et al. EpiTools, a software suite for presurgical brain mapping in epilepsy: intracerebral EEG. J Neurosci Methods. 2018;303:7–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


