Abstract
Objectives
Right ventricular (RV) failure post left ventricular assist device (LVAD) implantation is associated with increased morbidity and mortality. A novel RV multi‐plane imaging method using two‐dimensional echocardiography and electronic plane rotation (MPE) was used to quantify RV function prior to LVAD implantation and to identify potential added value in this patient population.
Methods
In twenty‐five end‐stage heart failure patients (age 58.9 ± 6.8 years, 76% male), systolic function of four different RV walls (lateral, anterior, inferior and inferior coronal) were evaluated from one focussed apical view using MPE.
Results
Feasibility of tricuspid annular plane systolic excursion (TAPSE) and tricuspid annular peak systolic velocity (RV‐S') measurements were high (84–100%), with lower TAPSE values measured in the inferior (14.2 ± 4.6 mm) and inferior coronal (12.3 ± 5.0 mm) walls compared to the lateral (16.3 ± 4.5 mm) and anterior walls (16.0 ± 4.5 mm). RV wall longitudinal strain (RV‐LS) measurement was most feasible in the lateral wall (80%; mean: –12.1 ± 4.2%). TAPSE and RV‐LS values were significantly reduced in patients compared to matched healthy individuals (p = <0.001). Seven (28%) patients who developed moderate to severe RV failure (RVF) early post‐implant (≤30 days) had lower pre‐implant values across all multi‐plane parameters compared to those without significant post‐implant RVF, notably four‐wall averaged TAPSE (11.1 ± 3.4 mm vs 15.9 ± 4.0 mm; p = 0.02).
Conclusion
2D MPE was highly feasible for RV wall quantification pre‐LVAD surgery, detecting differences in regional wall function. This novel method comprehensively quantifies RV wall function and could complement current pre‐LVAD screening protocols.
Keywords: echocardiography, left ventricular assist device, multi‐plane, right ventricular failure, right ventricular strain
1. INTRODUCTION
Right ventricular failure (RVF) is recognized as a major cause of morbidity and mortality following left ventricular assist device (LVAD) implantation. 1 The prevalence of post‐LVAD RVF is variable and reported in between 13 and 44% of cases dependent on criteria applied. 2 , 3 , 4 , 5 Although several prediction models have been published to aid advanced heart failure (HF) teams, identifying patients at risk of RVF after LVAD implantation remains a significant challenge. 1 , 6 Despite being most widely used during pre‐operative screening, the assessment of RV function using two‐dimensional trans‐thoracic echocardiography (2D‐TTE) does have inherent limitations. 3 Given the structural complexity of the RV, with inlet, outlet and apical regions it is not possible to visualize the entire chamber from a single 2D‐TTE acoustic window. 7 Furthermore, current quantitative functional parameters assessed with 2D‐TTE are limited to one free wall region of the RV, namely the lateral wall. This is a limitation which may result in an over or under estimation of global RV function. 8 Whilst three‐dimensional (3D) TTE is able to overcome the geometrical assumptions made with 2D‐TTE, poor spatial resolution or artefacts arising from adjacent structures may limit measurement feasibility. 9 In order to address some of these issues, our research group previously introduced a novel imaging approach utilizing 2D multi‐plane echocardiography (MPE) performed using a 3D ultrasound transducer. Whilst maintaining a fixed RV apical transducer position, with the RV apex centered, four different RV walls based on anatomic landmarks (lateral, anterior, inferior and inferior coronal)–(Figure 1), can be imaged using electronic plane rotation. 10 Crucially, an RV centered view enables rotation through the true RV apex rather than the LV and permits optimal visualization of the entire RV free wall. This new method, also known as iRotate mode, therefore allows for a more detailed, quantitative assessment of global and regional RV wall function than presently performed with 2D‐TTE. The main aim of this study was to evaluate RV function using this multi‐plane method in a cohort of patients with end stage HF prior to LVAD implantation. A secondary aim was to identify which trends emerge amongst the multi‐plane parameters in patients who develop post‐LVAD RVF.
FIGURE 1.
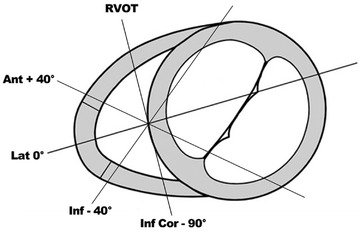
Multi‐plane imaging of the right ventricle (RV). Views obtained by electronic plane rotation around a single RV focused apical echocardiographic position. 0° rotation: lateral wall; +40°: anterior wall; ‐40°: inferior wall; ‐90°: inferior wall coronal view also visualizing the right ventricular outflow tract
2. METHODS
End‐stage HF patients undergoing echocardiographic screening prior to elective continuous flow LVAD implantation at our center between 2016 and 2019 were included in this study. These individuals underwent transthoracic echocardiography (TTE), including comprehensive 2D MPE RV assessment, right heart catheterization (RHC) and full clinical and laboratory evaluation. Emergency LVAD implantation cases were excluded. All echocardiograms were performed by DB or MS, imaging experts trained in LVAD echocardiography, using an iE33 or EPIQ7 ultrasound system (Phillips Medical Systems, Best, The Netherlands) equipped with an X5‐1 matrix array transducer. 2D/3D echocardiographic parameters for left and right ventricular size and function were collected in addition to the grading of any valvular lesions. RV basal and longitudinal linear dimensions alongside fractional area change (FAC – calculated as end‐diastolic area – end‐systolic area/end‐diastolic area x 100) were measured in the standard focused RV apical four chamber view conforming to international guidelines. 11 RHC data was included if performed within 31 days of the TTE. To be able to compare the multi‐plane RV parameters with a healthy population, we used a control group matched for age and sex. For this control group, self‐declared healthy volunteers were prospectively recruited through advertisements, the details of which have been published previously. 10 The study was carried out according to the principles of the Declaration of Helsinki and approved by the local medical ethics committee (METC) and written informed consent was obtained from all subjects.
2.1. Post‐LVAD outcomes
Early clinical outcome data (≤30 days post implantation) was collected on each subject post LVAD implantation, namely cases of death; acute kidney injury (defined by an increase in serum creatinine by ≥ .3 mg/dl [≥ 26.5 μmol/l] within 48 h or ≥ 1.5 times the recorded baseline value) 12 ; and length of ICU/hospital stay post implantation. Significant RVF post‐LVAD implantation was defined as moderate‐severe in length by a post‐operative requirement for sustained inotropic support > 7 days and/or implantation of a RV assist device (RVAD).
2.2. Right ventricular assessment by 2D multi‐plane echocardiography
2D MPE assessment of the RV has been previously demonstrated by our group and allows for multiple RV walls to be assessed from one echocardiographic position. 8 , 10 The main advantage of MPE is the ability to combine the multiplane scanning ability of 3D‐TTE whilst maintaining the temporal resolution (> 60 Hz) of 2D‐TTE at almost the same spatial resolution. To acquire the four additional RV views, a focused, non‐foreshortened RV view is required with the RV apex and inter‐ventricular septum centered along or as near to the midline of the imaging sector as possible. This allows for a full electronic rotation around the RV apex whilst maintaining a fixed probe position. The first view at 0˚ shows the lateral RV wall with the left sided landmark being the mitral valve. The second view at approximately +40˚ shows the anterior RV wall and the coronary sinus, thirdly at approximately ‐40˚ the inferior RV wall and the aortic valve and lastly at approximately ‐90˚ the inferior coronal view (CV) with the inferior wall and the right ventricular outflow tract (RVOT) (Figure 1 and supplementary Movies 1, 2, 3, 4). With correct alignment and complete RV wall visualization, it is possible to perform quantitative analysis of RV function on all walls (Figure 2). Feasibility and values of the established RV functional echo parameters, tricuspid annular plane systolic excursion (TAPSE); tissue Doppler imaging derived tricuspid annular peak systolic velocity (RV‐S’) and RV wall longitudinal strain (RV‐LS) were assessed offline by an experienced sonographer (DB) on each of the four RV walls, by averaging three to five successive measurements. TAPSE and RV‐S’ parameters were deemed feasible to measure if the respective M‐mode or tissue Doppler tracing was adequately optimized for the measurement to be performed accurately. In addition to the values from the individual RV walls, a four‐wall average of both TAPSE and RV‐S’ was calculated when measurements from all four‐walls from one individual were feasible.
VIDEO 1.
Echocardiographic movie loop of the mitral valve view demonstrating the right ventricular lateral wall (0° electronic rotation)
VIDEO 2.
Echocardiographic movie loop of the coronary sinus view demonstrating the right ventricular anterior wall (approximately +40° electronic rotation)
VIDEO 3.
Echocardiographic movie loop of the aortic valve view demonstrating the right ventricular inferior wall (approximately ‐40° electronic rotation)
VIDEO 4.
Echocardiographic movie loop of the coronal view demonstrating the right ventricular inferior wall and the right ventricular outflow tract anterior wall (approximately ‐90° electronic rotation)
FIGURE 2.
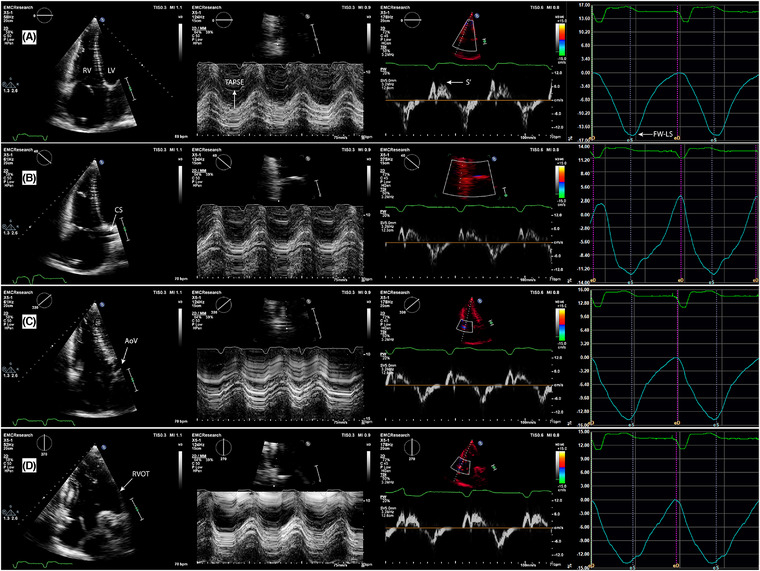
Echocardiographic images of the four multi‐plane right ventricular (RV) views (A‐D) with corresponding quantitative functional parameters of the respective free wall segments (L‐R panels). (A) – Focused four chamber view (0°), lateral wall; (B) – coronary sinus view (+40°), anterior wall; (C) – aortic view (‐40°), inferior wall; (D) – coronal view (‐90°), inferior wall and RVOT anterior wall. Second panel (center left): tricuspid annular plane systolic excursion (TAPSE); third panel, (center right): tricuspid annular peak systolic velocity (RV‐S’); fourth panel (far right): RV wall longitudinal strain (RV‐LS). LV ‐ left ventricle; CS ‐ coronary sinus; AoV ‐ aortic valve; RVOT ‐ right ventricular outflow tract
2.3. RV speckle tracking analysis
To assess RV wall peak systolic longitudinal strain (RV‐LS) an RV algorithm wall motion tracking software was used (2D CPA, Image‐Arena version 4.6; TomTec Imaging Systems, Munich, Germany) and data analysis was performed offline by one observer (DB). The endocardial border of the RV free wall and septum was manually traced at end systole and adjusted accordingly at end diastole if required. This was performed in each of the four multi‐plane views previously described. RV‐LS refers to a single segment value of the free wall, with the inter‐ventricular septum excluded. A measurement was considered feasible if all portions of the RV wall tracked accurately throughout the cardiac cycle. In cases where the automated tracking was not accurate, attempts were made to re‐adjust the endocardial border manually.
2.4. Statistical analysis
The distribution of data was assessed using histograms and the Shapiro–Wilk test. Depending on the data distribution, continuous data is presented as mean ± standard deviation (SD) or median [inter‐quartile range (IQR)], whilst categorical data is presented as frequencies and percentages. For comparison of normally distributed continuous variables the independent samples T‐test was used and in case of skewed distribution, the Mann–Whitney U‐test was applied. For comparison of frequencies the Fisher's exact test was used. All statistical analyses were performed using the Statistical Package for Social Sciences version 25.0 (SPSS, Armonk, NY, USA: IBN corp.). The statistical tests were two‐sided and a p value < 0.05 was considered statistically significant.
3. RESULTS
Twenty‐five patients with end‐stage HF (mean age 58.9 ± 6.8 years; 76.0% male) were included in the study. Detailed RV assessment by 2D MPE was feasible in all patients and performed 9 [5.0–28.5] days prior to LVAD implantation. 3D RV full volume datasets were acquired in 12 (48%) patients although only three cases were considered of sufficient quality to analyze and therefore no data was reported. Pre‐implant RHC data was available in 19 (76.0%) patients and hemodynamic values alongside other clinical, laboratory and echocardiographic data are detailed in Table 1 . Twenty‐five age and gender matched healthy subjects (mean age – 58.9 ± 7.1 years, 76.0% male), who were recruited for a separate study at our center, underwent the same echocardiographic imaging and were used as a control group.
TABLE 1.
Clinical, hemodynamic, and echocardiographic characteristics of end stage heart failure patients prior to left ventricular assist device implantation and matched healthy controls
| End stage heart failure patients (n = 25) | Healthy controls (n = 25) | p‐value | |
|---|---|---|---|
| Clinical data | |||
| Age (years) | 58.9 ± 6.8 | 58.9 ± 7.1 | 0.98 |
| Male gender (n, %) | 19 (76) | 19 (76) | 1.00 |
| Body mass index (kg/m2) | 25.9 [22.3, 28.1] | 26.0 [23.6, 27.6] | 0.73 |
| Sinus rhythm (n, %) | 15 (60) | 25 (100) | <0.001 |
| Ischemic etiology (n, %) | 10 (40) | ||
| Non‐ischemic etiology (n, %) | 16 (64) | ||
| Previous cardiac surgery (n, %) | 3 (12) | ||
| INTERMACS (n, %) | |||
| Class 1 | 1 (4) | ||
| Class 2 | 9 (36) | ||
| Class 3 | 8 (32) | ||
| Class 4 and up | 7 (28) | ||
| Indication (n, %) | |||
| Bridge to transplant | 8 (32) | ||
| Destination therapy | 12 (48) | ||
| Bridge to decision | 5 (20) | ||
| Laboratory data* | |||
| Creatinine (μmol/L) | 152.2 ± 45.1 | ||
| eGFR (ml/min) | 41 [32.5–48.5] | ||
| Total bilirubin (μmol/L) | 18.0 [10.5–29] | ||
| Albumin (g/L) | 38.3 ± 6.7 | ||
| Hb (mmol/L) | 7.7 ± 1.2 | ||
| RHC parameters (n – 19) | |||
| Right atrial pressure (mm Hg) | 11.2 ± 5.5 | ||
| Mean pulmonary artery pressure (mm Hg) | 30.4 ± 11.8 | ||
| Pulmonary capillary wedge pressure (mm Hg) | 18.1 ± 9.9 | ||
| RA/PCWP ratio | .6 [.4–.8] | ||
| Pulmonary vascular resistance (wood units) | 2.6 ± 1.3 | ||
| Trans pulmonary gradient (mm Hg) | 9.8 ± 4.0 | ||
| Pulmonary artery pressure indexed (mm Hg/m2) | 2.3 ± .9 | ||
| Diastolic pulmonary gradient (mm Hg) | 2.0 ± 4.8 | ||
| Cardiac output (l/m) | 3.7 [3.3–4.5] | ||
| Cardiac index (l/m/m2) | 1.8 [1.6–2.1] | ||
| Echocardiographic parameters | |||
| Left ventricle | |||
| LV end diastolic diameter (mm) | 73.9 ± 11.9 | 44.4 ± 4.5 | <0.001 |
| LV end systolic diameter (mm) | 68 ± 13.6 | 27.8 ± 5.5 | <0.001 |
| LV ejection fraction (%) | 19.3 ± 6.0 | 59.5 ± 4.4 | <0.001 |
| Left atrial volume index (ml/m2) | 62.7 [52.5, 90.7] | 26.4 [23.8, 32.7] | <0.001 |
| Right ventricle | |||
| RV basal dimension (mm) | 46.7 ± 8.8 | 39.4 ± 5.6 | 0.001 |
| RV mid dimension (mm) | 33.8 ± 7.6 | 30.6 ± 5.1 | 0.10 |
| RV outflow tract 1 dimension (mm) | 41.3 ± 6.0 | 32.0 ± 3.0 | <0.001 |
| RV end diastolic area (cm2) | 29.1 ± 10.3 | 25.3 ± 5.4 | 0.13 |
| RV end systolic area (cm2) | 21.1 ± 9.2 | 14.3 ± 4.1 | 0.002 |
| RV fractional area change (%) | 29.2 ± 11.7 | 44.5 ± 7.9 | <0.001 |
| RA area (cm2) | 24.2 ± 8.5 | 17.4 ± 3.6 | 0.001 |
| Valvular | |||
| ≥ Moderate mitral regurgitation (n, %) | 14 (56) | ||
| ≥ Moderate aortic regurgitation (n, %) | 2 (8) | ||
| ≥ Moderate tricuspid regurgitation (n, %) | 10 (40) | ||
| Tricuspid regurgitation velocity (m/s) | 2.8 ± .6 | ||
Data presented as mean ± SD, median [IQR] or n (%). RA/PCWP ratio ‐ right atrial/pulmonary capillary wedge pressure ratio. Cardiac output and cardiac index measured by thermodilution method.
*Laboratory data collected 2 ± 1.5 days prior to implant.
3.1. Multi‐plane parameters for RV function
The feasibility of multi‐plane TAPSE and RV‐S’ measurements was high across all RV walls (84–100%) and compared favorably with the control group (92–100%). A four‐wall average TAPSE measurement was feasible in 22 (88%) patients whilst RV S’ measurement was feasible in 21 (84%) patients. RV‐LS feasibility was 80% for the lateral wall, but much lower for the other walls (32–60%). In contrast, RV‐LS feasibility was higher across all walls in the control group (lateral wall – 92%; inferior ‐ 88%; anterior ‐ 64%; inferior CV ‐ 68%). Table 2 presents all multi‐plane values from the patient cohort in addition to comparative values from the healthy control group. The highest TAPSE/RV‐S’ values were seen in the lateral (16.3 ± 4.5 mm/10.0 ± 2.9 cm/s) and anterior walls (16.0 ± 4.5 mm/10.0 ± 2.6 cm/s), with lower values measured in the inferior (14.2 ± 4.6 mm/9.0 ± 2.9 cm/s) and inferior CV walls (12.3 ± 5.0 mm/8.7 ± 2.8 cm/s). Four‐wall averaged TAPSE was 14.6 ± 4.4 mm, whilst four‐wall averaged RV‐S’ was 9.5 ± 2.7 cm/s. Lateral wall longitudinal strain was ‐12.1% ± 4.2%. Compared to the cohort of healthy controls, all multi‐plane TAPSE and RV‐LS parameters were significantly reduced (p = < 0.001). Differences in multi‐plane RV‐S’ measurement were less pronounced and not significantly different in the inferior walls (p > 0.05).
TABLE 2.
Multi‐plane RV quantitative parameters in end stage heart failure patients compared with healthy age and gender matched controls
| End stage heart failurepatients (n = 25) | Healthy controls (n = 25) | ||||
|---|---|---|---|---|---|
| Multi‐plane echo parameters | Feasibility (%) | Values | Feasibility (%) | Values | p‐value |
| TAPSE (mm) | |||||
| Lateral wall | 100.0 | 16.3 ± 4.5 | 100.0 | 26.0 ± 5.4 | <0.001 |
| Anterior wall | 100.0 | 16.0 ± 4.5 | 100.0 | 26.6 ± 4.2 | <0.001 |
| Inferior wall | 96.0 | 14.2 ± 4.6 | 96.0 | 22.8 ± 3.5 | <0.001 |
| Inferior coronal wall | 88.0 | 12.3 ± 5.0 | 92.0 | 21.7 ± 4.1 | <0.001 |
| Four‐wall average | 88.0 | 14.6 ± 4.4 | 92.0 | 24.4 ± 3.7 | <0.001 |
| RV‐S’ (cm/s) | |||||
| Lateral wall | 96.0 | 10.0 ± 2.9 | 100.0 | 11.8 ± 2.0 | 0.014 |
| Anterior wall | 100.0 | 10.0 ± 2.6 | 100.0 | 12.0 ± 1.7 | 0.002 |
| Inferior wall | 96.0 | 9.0 ± 2.9 | 96.0 | 10.4 ± 1.6 | 0.06 |
| Inferior coronal wall | 84.0 | 8.7 ± 2.8 | 92.0 | 9.3 ± 1.8 | 0.42 |
| Four‐wall average | 84.0 | 9.5 ± 2.7 | 92.0 | 10.9 ± 1.5 | 0.047 |
| RV‐LS (‐%) | |||||
| Lateral wall | 80.0 | −12.1 ± 4.2 | 92.0 | −27.1 ± 7.0 | <0.001 |
| Anterior wall | 44.0 | −12.5 ± 6.1 | 64.0 | −24.4 ± 4.2 | <0.001 |
| Inferior wall | 60.0 | −12.6 ± 4.8 | 88.0 | −22.6 ± 3.9 | <0.001 |
| Inferior CV wall | 32.0 | −12.1 ± 4.1 | 68.0 | −19.7 ± 4.9 | 0.001 |
Data presented as mean ± SD.
Abbreviations: TAPSE, tricuspid annular plane systolic excursion; RV‐S’, tricuspid annular systolic velocity by tissue Doppler imaging; RV‐LS, right ventricular wall longitudinal strain.
3.2. Comparison with mean pulmonary artery pressure
Swan Ganz catheter derived mean pulmonary artery pressure (mPAP) was used as a clinical measurement of RV afterload of which to compare RV multi‐plane functional values against. Median mPAP was 31 [21, 40] mm Hg. Table 3 and Figure 3 present RV multi‐plane functional parameters using this value to divide the cohort. TAPSE measurement of the lateral and inferior coronal view walls, in addition to the four‐wall averaged value were significantly lower in patients with mPAP ≥31 mm Hg (lateral: 14.7 ± 3.8 mm vs 19.0 ± 4.2 mm, p = 0.03; inferior coronal view: 9.4 ± 5.3 mm vs 15.8 ± 3.7 mm, p = 0.01; average: 12.1 ± 3.9 mm vs 17.5 ± 3.8 mm, p = 0.01). Lateral RV‐LS was lower in the ≥31 mm Hg mPAP group, but this difference was not statistically significant (‐10.3 ± 2.9% vs ‐14.5 ± 4.7%; p = 0.06). There were no statistically significant differences between the groups for RV‐S’ values.
TABLE 3.
Multi‐plane RV quantitative parameters compared by mean pulmonary artery pressure as measured by right heart catheterization
| Multi‐plane echo parameters | Mean pulmonaryartery pressure <31 mm Hg (n = 9) | Mean pulmonaryartery pressure ≥31 mm Hg (n = 10) | p‐value |
|---|---|---|---|
| TAPSE (mm) | |||
| Lateral wall | 19.0 ± 4.2 | 14.7 ± 3.8 | 0.031 |
| Anterior wall | 17.5 ± 4.6 | 14.3 ± 4.4 | 0.15 |
| Inferior wall | 15.1 ± 6.0 | 11.4 ± 4.7 | 0.17 |
| Inferior coronal wall | 15.8 ± 3.7 | 9.4 ± 5.3 | 0.014 |
| Four‐wall average | 17.5 ± 3.8 | 12.1 ± 3.9 | 0.011 |
| RV‐S’ (cm/s) | |||
| Lateral wall | 10.2 ± 2.4 | 10.2 ± 3.7 | 0.97 |
| Anterior wall | 10.8 ± 2.6 | 9.1 ± 2.5 | 0.17 |
| Inferior wall | 10.4 ± 2.9 | 8.3 ± 3.2 | 0.18 |
| Inferior coronal wall | 9.4 ± 2.4 | 8.2 ± 3.7 | 0.46 |
| Four‐wall average | 10.2 ± 2.4 | 8.8 ± 3.4 | 0.32 |
| RV‐LS (‐%) | |||
| Lateral wall | −14.5 ± 4.7 | −10.3 ± 2.9 | 0.06 |
Data presented as mean ± SD. Mean pulmonary artery pressure of 31 mm Hg used to split cohort as this represented the median value of all patients.
Abbreviations: TAPSE, tricuspid annular plane systolic excursion; RV‐S’, tricuspid annular systolic velocity by tissue Doppler imaging; RV‐LS, right ventricular wall longitudinal strain.
FIGURE 3.
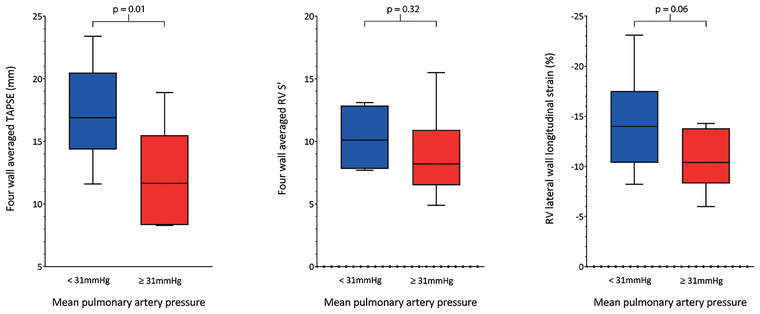
Box and whisker plots presenting comparison between multi‐plane RV echocardiographic parameters by mean pulmonary artery pressure (mPAP). A value of 31 mm Hg was used to split the cohort as this represented the median value of all patients. Left panel ‐ four‐wall averaged tricuspid annular plane systolic excursion (TAPSE); Middle panel ‐ four‐wall averaged tricuspid annular peak systolic velocity (RV‐S’); Right panel ‐ RV lateral wall longitudinal strain (FW‐LS)
3.3. Clinical outcomes post LVAD implantation
Post LVAD implantation, there were three (14.5%) deaths and twelve (48%) patients with acute kidney injury (AKI). Median length of stay in ICU and hospital was 5 (3.5–17) and 28 (21.5‐49) days respectively. Seven (28%) patients required sustained inotropic support for moderate or severe post‐operative right ventricular failure (RVF), including three (12%) RVAD implantations. Table 4 presents the values of all RV multi‐plane functional parameters when the population was split by incidence of significant post‐operative RVF. Four‐wall averaged TAPSE was the parameter most significantly reduced pre‐operatively in patients who developed RVF compared to those who did not (11.1 ± 3.4 mm vs 15.9 ± 4.0 mm; p = 0.02), in addition to the values from the lateral (13.2 ± 4.1 mm vs 17.5 ± 4.1 mm; p = 0.027), anterior (13.0 ± 3.7 mm vs 17.1 ± 4.3 mm; p = 0.037) and inferior walls (10.5 ± 4.6 mm vs 15.4 ± 4.1 mm; p = 0.020). Four‐wall averaged RV‐S’ was 7.3 [6.2‐9.7] cm/s vs 10.0 [7.8‐9.7] cm/s (p = 0.09) whilst lateral wall RV‐LS was ‐9.7 ± 2.8% compared to ‐13.1 ± 4.3% (p = 0.10). Feasibility of strain measurement for the other RV walls was considered insufficient for data analysis. Of those who developed post‐operative RVF, right heart dimensions were significantly increased pre‐operatively (RV basal dimension ‐ 53.7 ± 10.0 mm vs 43.9 ± 6.7 mm, p = 0.009; RA area – 31.2 ± 9.0 cm2 vs 21.5 ± 6.8 cm2, p = 0.008) and incidence of significant (≥ moderate) tricuspid insufficiency was also increased (71.4% vs 27.7%, p = 0.045). Additionally, this group were older (63.2 ± 5.1 vs 57.2 ± 6.7 years, p = 0.045) and there was a reduced prevalence of sinus rhythm (28.5% vs 72.2%, p = 0.05). Three (42.8%) patients died within the first 90 days post LVAD implantation in the group with RV failure, whilst acute kidney injury occurred in six patients in both groups (85.7% RVF vs 33.3% no RVF; p = 0.019). Length of ICU stay for the RVF group was 28 [10‐43] days compared to 4 [3‐9.3] days (p = 0.006), whilst length of hospital stay was 59 [22‐86] days compared to 28 [21‐39.3] days (p = 0.15).
TABLE 4.
Comparison of multi‐plane echocardiographic parameters by incidence of significant post‐operative right ventricular failure (>7 days inotropic support or RVAD implantation)
| Right ventricular failure (n = 7) | No right ventricular failure (n = 18) | p‐value | |
|---|---|---|---|
| Characteristics | |||
| Age (years) | 63.2 ± 5.1 | 57.2 ± 6.7 | 0.045 |
| Gender (male) | 6 (85.6) | 13 (72.2) | 0.48 |
| BMI (kg/m2) | 25.1 ± 3.9 | 26.0 ± 5.3 | 0.66 |
| Sinus rhythm | 2 (28.5) | 13 (72.2) | 0.05 |
| Ischemic etiology | 3 (42.8) | 7 (38.8) | 0.86 |
| Mean pulmonary artery pressure (mm Hg) | 36.5 [27.8–40.8] | 26.5 [20.3–31.8] | 0.15 |
| Multi‐plane RV echo parameters | |||
| TAPSE lateral wall (mm) | 13.2 ± 4.1 | 17.5 ± 4.1 | 0.027 |
| TAPSE anterior wall (mm) | 13.0 ± 3.7 | 17.1 ± 4.3 | 0.037 |
| TAPSE inferior wall (mm) | 10.5 ± 4.6 | 15.4 ± 4.1 | 0.020 |
| TAPSE inferior coronal wall (mm) | 9.8 ± 4.6 | 13.3 ± 5.0 | 0.15 |
| TAPSE four‐wall average wall (mm) | 11.1 ± 3.4 | 15.9 ± 4.0 | 0.016 |
| RV‐S’ lateral wall (cm/s) | 7.9 [7.7–11.0] | 9.8 [8.4–12.4] | 0.35 |
| RV‐S’ anterior wall (cm/s) | 8.8 [6.3–9.7] | 9.9 [8.4–13.2] | 0.14 |
| RV‐S’ inferior wall (cm/s) | 5.8 [5.3–9.6] | 9.8 [7.4–11.0] | 0.047 |
| RV‐S’ inferior coronal wall (cm/s) | 6.6 [5.0–9.1] | 9.8 [6.9–10.2] | 0.11 |
| RV‐S’ four‐wall average wall (cm/s) | 7.3 [6.2–9.7] | 10.0 [7.8–11.8] | 0.09 |
| RV‐LS Lateral wall | −9.7 ± 2.8 | −13.1 ± 4.3 | 0.10 |
| Right heart 2D echo parameters | |||
| RV basal dimension (mm) | 53.7 ± 10.0 | 43.9 ± 6.7 | 0.009 |
| RV mid dimension (mm) | 39.1 ± 9.5 | 31.8 ± 5.9 | 0.027 |
| RVOT1 dimension (mm) | 44.7 ± 6.6 | 40.0 ± 5.4 | 0.08 |
| FAC (%) | 21.8 ± 7.1 | 32.1 ± 11.9 | 0.045 |
| RA area (cm2) | 31.2 ± 9.0 | 21.5 ± 6.8 | 0.008 |
| ≥ Moderate TR | 5 (71.4) | 5 (27.7) | 0.045 |
| TRvel (cm/s) | 2.8 ± .2 | 2.9 ± .7 | 0.78 |
| Clinical outcomes | |||
| Deaths | 3 (42.9) | 0 (0) | 0.002 |
| Acute kidney injury | 6 (85.7) | 6 (33.3) | 0.019 |
| RVAD implantation | 3 (42.9) | 0 (0) | 0.003 |
| ICU stay (days) | 28.0 [10.0–43.0] | 4.0 [3.0–9.3] | 0.006 |
| Hospital stay (days) | 59.0 [22.0–86.0] | 28.0 [21.0–39.3] | 0.15 |
Data presented as mean ± SD, median [IQR] or n (%).
Abbreviations: TAPSE, tricuspid annular plane systolic excursion; RV‐S’, tricuspid annular systolic velocity by tissue Doppler imaging; RV‐LS, right ventricular wall longitudinal strain; RVAD, right ventricular assist device.
4. DISCUSSION
2D MPE is a relatively novel imaging technique allowing a comprehensive, quantitative assessment of four RV walls from one echocardiographic acoustic window using electronic plane rotation. This is the first study to implement this technique in a population of end stage HF patients with candidacy for LVAD implantation. This imaging approach is easily applicable in daily clinical practice and has a short‐learning curve and additional acquisition time. This single center study has demonstrated that multi‐plane TAPSE and RV S’ measurements are highly feasible in all RV wall segments. Regional differences in RV wall longitudinal shortening were evident, with lower functional values in the inferior walls compared to those of the lateral and anterior walls. Multi‐plane RV strain analysis however is limited by poor endocardial border definition and aside from the lateral wall, cannot be reliably assessed in this patient population. Patients who developed significant RVF post‐LVAD implantation had proportionally a greater combination of pre‐implant right sided impairment, with four‐wall averaged values reflective of lower global RV wall function when compared to the standard lateral wall value.
4.1. Feasibility of RV multi‐plane echocardiography
2D MPE RV assessment was first presented in our research group by McGhie et al. (2017), 8 demonstrating feasibility of this four‐view model and proposing normal values in a healthy population. The authors reported excellent measurement feasibility with higher TAPSE, RV‐S’ and RV‐LS values seen in the lateral and anterior walls compared to the inferior and inferior CV walls. 11 A similar trend was seen in the present study, namely with TAPSE and RV S’ measurements which were the most feasible. The feasibility of RV‐LS measurement was high in the lateral wall, but moderate to poor in the other three segments, limiting a direct comparison with the aforementioned study. Whilst visibility of the tricuspid annulus and basal portion of the RV was adequate, visualization of the entire free wall segment throughout the cardiac cycle in the anterior and inferior walls was more difficult, limiting the possibility to perform reliable strain analysis. 3 Significantly dilated left ventricles, highly likely in end stage HF patients, does impact on the sonographer's ability to bring the RV apex into the center of the viewing sector, therefore permitting rotation around the RV and not the LV apex. 8 A standard focused RV view 11 visualizing the entire RV lateral wall without centering the RV apex enabled good free wall visualization but did not incrementally improve the feasibility of RV lateral wall strain measurement. Strain analysis is dependent on good image quality, therefore artefacts secondary to the retro‐sternal location of the right ventricle or to concomitant respiratory disease creates an additional challenge for the sonographer. 8 Another challenge was the presence of arrhythmias in ten (40%) patients including atrial fibrillation or frequent ectopy. Beat to beat variation or tachycardia requiring higher frame rates 13 reduced the feasibility of this method, therefore only patients with stable R‐R intervals were included in the analysis (three [12%] patients were excluded from strain analysis). For these same reasons, the feasibility of RV functional assessment by 3D echocardiography was very limited and we were unable to compare 2D multi‐plane parameters to volumetric data. With the fact that end stage heart failure patients are frequently not suitable for cardiac magnetic resonance imaging (cMRI), there is added value in taking a comprehensive 2D multi‐plane approach to assess RV function in this patient population.
4.2. Multi‐plane assessment of right ventricular function
Averaging the TAPSE and RV‐S’ values across the four RV walls resulted in a reduced value compared to that of a standard lateral wall measurement, reflecting the lower values seen in the inferior segments. Longitudinal shortening accounts for a larger proportion of overall RV function hence TAPSE has been shown to have a good correlation with RV ejection fraction (RVEF) measured by cMRI and has prognostic value in HF patients. 2 , 14 Despite these advantages, several other studies have revealed important disadvantages, such as load dependency 3 and overlooking of septal contribution to RV ejection, which is important to maintain RV function after LVAD implantation. 2 , 4 The regional differences seen in RV wall function could well be explained by LV‐RV inter‐dependence by virtue of the inter‐ventricular septum and it's interlacing muscle fibres. 4 Reduced septal function could therefore impact adjacent RV segments such as the inferior wall more than the lateral wall. Moreover, in advanced left heart failure there is an alteration of right ventricular mechanics as a result of reduced LV twist. 15 Whilst conceivable, these theories all require further investigation.
4.3. RV functional parameters and mean pulmonary artery pressure
TAPSE values of the lateral and inferior CV walls and as a result the four‐wall averaged value were most affected in the cohort with higher pre‐operative mPAP, whilst lateral RV‐LS measurement was also notably reduced. RV function is well known to be sensitive to afterload both acutely and chronically and multi‐plane parameters reflect this. The importance of RV‐pulmonary circuit coupling should therefore not be underappreciated when imaging the RV. 15 Even though RV load declines early on after LVAD implantation the impact of significantly elevated RV afterload pre‐implant could have persistent and detrimental effects on RV function. 16
4.4. Right ventricular failure post LVAD implantation
Seven (28%) patients developed significant post‐operative RVF defined in this study as a requirement for sustained inotropic support (> 7 days), three of whom also were implanted with an RVAD. Although predominantly intended as a feasibility study than for predictive value comparison, some comment can be made on the trends of the multi‐plane echocardiographic findings. RVF patients had proportionally a greater combination of pre‐operative right sided impairment, notably reduced multi‐plane TAPSE, markedly reduced lateral wall RV‐LS and increased right heart 2D dimensions (demonstrated in Figure 4). The proportion of those who had greater or equal to moderate tricuspid regurgitation was also significantly higher. There was a trend for reduced pre‐operative lateral wall RV‐LS in those who developed significant post‐operative RVF (‐9.7 ± 2.8%) however RV‐LS values were also similarly reduced in those who didn't develop RVF (‐13.1 ± 4.3%). 17 Recent studies have reported reduced pre‐implant RV‐LS values to be predictive of post implant RVF 18 , 19 and comparatively to our findings, Grant et al. (2012) 20 reported that a peak cut‐off strain value of ‐9.6% predicted post‐implant RVF. In a large meta‐analysis of studies a post LVAD incidence of RVF was reported in 35% of cases. 2 The authors concluded that within these studies RV longitudinal strain showed high variability, making results only marginally significant when comparing RVF versus non‐RVF patients. TAPSE measurement, whilst showing a trend towards being lower in RVF versus No‐RVF patients, had a small, non‐significant effect size and was highly heterogeneous. With this in mind, it is debatable whether there is superiority of a sole echocardiographic functional parameter for the prediction of post‐LVAD RVF. The multi‐plane correction of TAPSE measurement may however enhance the accuracy of global RV function quantification over single plane measurements. As such, the ability to measure multi‐plane RV‐LS in cases where image quality permits could equally prove to be of better prognostic value than reported in our results. In the absence of other imaging options, taking a multi‐plane 2D echocardiographic approach to RV assessment before surgery could help to build up quantitative evidence for those patients most at risk.
FIGURE 4.
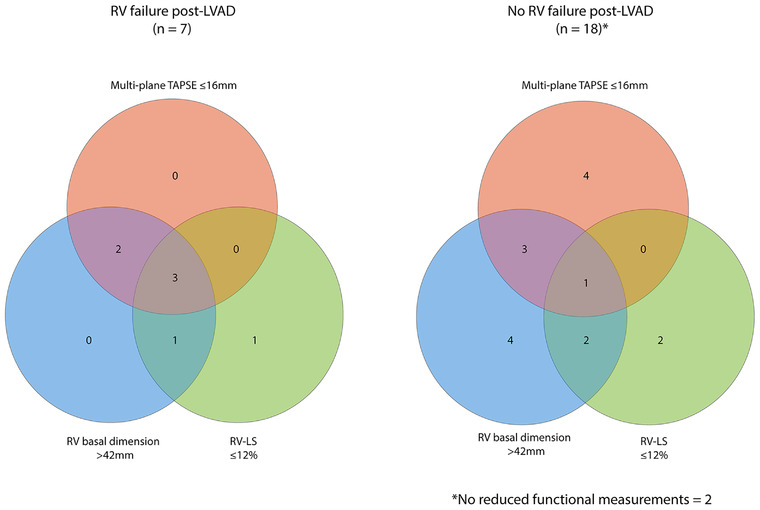
Venn diagram demonstrating incidence and combination of pre‐operative echocardiographic right sided impairment in study population. Left sided diagram represents patients with significant post‐LVAD RV failure. Right sided diagram represents no significant post‐LVAD RV failure. Cut‐off values are shown in the figure. Pink circle, multi‐plane tricuspid annular plane systolic excursion (TAPSE); blue circle, right ventricular (RV) basal dimension; green circle, right ventricular longitudinal strain (RV‐LS)
4.5. Limitations and future perspectives
This is a single center study with limited statistical power due to a relatively small sample size. The principal aim of the study was to determine the feasibility of 2D MPE RV assessment and quantification in LVAD candidates and furthermore to report any trends found in patients who develop post‐operative RVF. The next logical step is to enroll a greater number of patients in a multi‐center study, which is currently ongoing as “Serial Multiparametric Evaluation of Right Ventricular Function After Left Ventricular Assist Device Implantation (EuroEchoVAD Study)”, https://clinicaltrials.gov/ct2/show/NCT03552679 (PI's: O.I.S and K.C). This is necessary to further assess this novel echocardiographic approach alongside current post‐LVAD RVF prediction models. Echocardiograms performed in the current study were performed by experienced sonographers with expertise in advanced HF. Whilst we believe that 2D MPE assessment of the right ventricle involves a short learning curve of around 20 echocardiograms, 10 significant experience in echocardiography with attention to detail is essential to good image quality. Patients included in the study were scanned in a clinical room or bedside with a modern top of the range echo machine and a 3D ultrasound probe required for electronic rotation. Although possible in theory using manual rotation, the use of a portable bedside echo machine equipped only with a 2D probe would reduce the feasibility and reproducibility of this method. Views were attempted in each patient however not all LVAD recipients at our center were included as it was not possible to scan some patients before implantation, such as in emergency cases. Feasibility of image acquisition and quantification may therefore be lower than reported in this study if a complete pre‐LVAD population is considered.
5. CONCLUSION
This is the first study to implement 2D MPE evaluation of RV function in a population of end‐stage HF patients prior to LVAD implantation. Multi‐plane RV wall quantification was highly feasible, notably for TAPSE and RV‐S’ measurements which revealed differences in regional RV wall function. Given the limitations of RV volumetric assessment by 3D echo or cMRI in this patient population, this novel method provides potential for a more comprehensive quantification of global RV wall function. A larger multi‐center study will help to determine whether 2D MPE can aid current models for the prediction of post‐LVAD RVF, still the Achilles heel of LVAD implantation.
FUNDING
No internal or external funding was required to support this research.
CONFLICTS OF INTEREST
The authors have no relevant financial or non‐financial conflicts of interest to disclose.
ACKNOWLEDGMENT
None.
Bowen DJ, Yalcin YC, Strachinaru M, et al. Right ventricular functional assessment by 2D multi‐plane echocardiography prior to left ventricular assist device implantation. Echocardiography. 2022;39:7–19. 10.1111/echo.15191
Osama I. Soliman and Kadir Caliskan contributed equally to research design.
REFERENCES
- 1. Soliman OII, Akin S, Muslem R, et al. Derivation and validation of a novel right‐sided heart failure model after implantation of continuous flow left ventricular assist devices: the EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right‐Sided Heart Failure Risk Score. Circulation. 2018;137(9):891‐906. [DOI] [PubMed] [Google Scholar]
- 2. Bellavia D, Iacovoni A, Scardulla C, et al. Prediction of right ventricular failure after ventricular assist device implant: systematic review and meta‐analysis of observational studies. Eur J Heart Fail. 2017;19(7):926‐946. [DOI] [PubMed] [Google Scholar]
- 3. Cohen DG, Thomas JD, Freed BH, Rich JD, Sauer AJ. Echocardiography and continuous‐flow left ventricular assist devices: evidence and limitations. JACC Heart Fail. 2015;3(7):554‐564. [DOI] [PubMed] [Google Scholar]
- 4. Hayek S, Sims DB, Markham DW, Butler J, Kalogeropoulos AP. Assessment of right ventricular function in left ventricular assist device candidates. Circ Cardiovasc Imag. 2014;7(2):379‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stainback RF, Estep JD, Agler DA, et al. Echocardiography in the management of patients with left ventricular assist devices: recommendations from the American Society of echocardiography. J Am Soc Echocardiogr. 2015;28(8):853‐909. [DOI] [PubMed] [Google Scholar]
- 6. Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495‐1504. [DOI] [PubMed] [Google Scholar]
- 7. Dell'italia LJ. Anatomy and physiology of the right ventricle. Cardiol Clin. 2012;30(2):167‐187. [DOI] [PubMed] [Google Scholar]
- 8. Mcghie JS, Menting ME, Vletter WB, et al. Quantitative assessment of the entire right ventricle from one acoustic window: an attractive approach. Eur Heart J Cardiovasc Imaging. 2017;18(7):754‐762. [DOI] [PubMed] [Google Scholar]
- 9. Ostenfeld E, A Flachskampf F. Assessment of right ventricular volumes and ejection fraction by echocardiography: from geometric approximations to realistic shapes. Echo Res Pract. 2015;2(1):R1‐R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mcghie JS, Menting ME, Vletter WB, Van Den Bosch AE. A novel 13‐segment standardized model for assessment of right ventricular function using two‐dimensional iRotate echocardiography. Echocardiography. 2016;33(3):353‐361. [DOI] [PubMed] [Google Scholar]
- 11. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233‐270. [DOI] [PubMed] [Google Scholar]
- 12. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Supp. 2012;2(1):19‐36. [Google Scholar]
- 13. Voigt J‐U, Cvijic M. Cvijic M. 2‐ and 3‐dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. 2019;12(9):1849‐1863. [DOI] [PubMed] [Google Scholar]
- 14. Leite‐Moreira AF, Lourenço AP, Balligand J‐L, et al. ESC working group on myocardial function position paper: how to study the right ventricle in experimental models. Eur J Heart Fail. 2014;16(5):509‐518. [DOI] [PubMed] [Google Scholar]
- 15. Houston BA, Shah KB, Mehra MR, Tedford RJ. A new “twist” on right heart failure with left ventricular assist systems. J Heart Lung Transplant. 2017;36(7):701‐707. [DOI] [PubMed] [Google Scholar]
- 16. Muslem R, Ong CS, Tomashitis B, et al. Pulmonary arterial elastance and INTERMACS‐defined right heart failure following left ventricular assist device. Circ Heart Fail. 2019;12(8):e005923. [DOI] [PubMed] [Google Scholar]
- 17. Muraru D, Onciul S, Peluso D, et al. Sex‐ and method‐specific reference values for right ventricular strain by 2‐dimensional speckle‐tracking echocardiography. Circ Cardiovasc Imag. 2016;9(2):e003866. [DOI] [PubMed] [Google Scholar]
- 18. Charisopoulou D, Banner NR, Demetrescu C, Simon AR, Rahman Haley S. Rahman Haley S. Right atrial and ventricular echocardiographic strain analysis predicts requirement for right ventricular support after left ventricular assist device implantation. Eur Heart J Cardiovasc Imag. 2019;20(2):199‐208. [DOI] [PubMed] [Google Scholar]
- 19. Kato TS, Jiang J, Schulze PC, et al. Serial echocardiography using tissue Doppler and speckle tracking imaging to monitor right ventricular failure before and after left ventricular assist device surgery. JACC Heart Fail. 2013;1(3):216‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grant ADM, Smedira NG, Starling RC, Marwick TH. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol. 2012;60(6):521‐528. [DOI] [PubMed] [Google Scholar]


