Summary
The phase I/II AU‐003 study in patients with treatment‐naïve (TN) or relapsed/refractory (R/R) chronic lymphocytic leukaemia/small lymphocytic lymphoma demonstrated that zanubrutinib therapy results in clinically meaningful and durable responses with acceptable safety and tolerability. We report updated safety and efficacy data for 123 patients with a median follow‐up of 47·2 months. Patients received zanubrutinib 160 mg twice daily (81 patients), 320 mg once daily (40), or 160 mg once daily (two). Discontinuations due to adverse events or disease progression were uncommon. The overall response rate (ORR) was 95·9% (TN, 100%; R/R, 95%) with 18·7% achieving complete response (CR). Ongoing response at 3 years was reported in 85·7%. The ORR in patients with del(17p)/tumour protein p53 mutation was 87·5% (CR 16·7%). The 2‐ and 3‐year progression‐free survival estimates were 90% (TN, 90%; R/R, 91%) and 83% (TN, 81%; R/R, 83%) respectively. The most reported Grade ≥3 adverse events were neutropenia (15·4%), pneumonia (9·8%), hypertension (8·9%) and anaemia (6·5%). The annual incidence of atrial fibrillation, major haemorrhage, Grade ≥3 neutropenia and Grade ≥3 infection decreased over time. With a median follow‐up of ~4 years, responses remain clinically meaningful and durable and long‐term tolerability to zanubrutinib therapy continues.
Keywords: zanubrutinib, Bruton tyrosine kinase, chromosome 17p deletion, chronic lymphocytic leukaemia, small lymphocytic lymphoma
Introduction
Inhibitors of Bruton tyrosine kinase (BTK) are a standard of care for patients with chronic lymphocytic leukaemia (CLL), with several studies showing superior outcomes in comparison to chemoimmunotherapy. Long‐term follow‐up studies of the first‐generation BTK inhibitor, ibrutinib, show efficacy in patients with treatment‐naïve (TN) and relapsed or refractory (R/R) CLL/small lymphocytic lymphoma (SLL). 1 , 2 However, discontinuation of ibrutinib due to adverse events (AEs) or disease progression can limit utility in some patients. Discontinuation rates of 54–72% have been reported at a median follow‐up of 3·7–5 years, with AEs accounting for 12–21%. 2 , 3 A real‐world analysis in a large cohort of ibrutinib‐treated patients found an estimated discontinuation rate of 41% at a relatively short median follow‐up of 17 months, and, notably, ibrutinib toxicity was the most common reason for discontinuation. 4
Zanubrutinib is a highly selective next‐generation BTK inhibitor. In kinase inhibition and cell‐based assays, it was more selective than ibrutinib for BTK inhibition, exhibiting less off‐target activity against epidermal growth factor receptor, interleukin 2‐inducible T‐cell kinase (ITK), TEC, and other tyrosine kinases, which may account for observed toxicities. 5 Zanubrutinib also has favourable pharmacokinetic/pharmacodynamic properties that result in a more complete and sustained BTK inhibition compared with ibrutinib. At the recommended phase II dose (RP2D) of 160 mg twice daily, BTK exposure levels were ~eightfold higher than those observed for ibrutinib at 560 mg daily in blood and lymph node compartments. 6 , 7
In the first‐in‐human phase I/II AU‐003 study (NCT02343120) in a cohort of 94 patients with CLL/SLL at a median follow‐up of 13·7 months, zanubrutinib demonstrated high overall response rates (ORR; TN, 100%; R/R, 94·6%) and a tolerable safety profile. 7 In the present study, we report long‐term efficacy and safety outcomes in 123 patients with TN or R/R CLL/SLL in the AU‐003 study.
Methods
Study design and treatment
The AU‐003 study design has been previously reported. 7 Briefly, AU‐003 is a phase I/II, open‐label study of zanubrutinib in patients with various B‐cell malignancies, including TN and R/R CLL/SLL. Patient enrolment began in September 2014; the last patient enrolled received the first dose of zanubrutinib in November 2018.
Patients
Patients were eligible if they had CLL/SLL according to World Health Organization classifications and required treatment per the International Workshop on CLL. 8 Additional eligibility criteria included age ≥18 years, Eastern Cooperative Oncology Group performance status of 0−2; adequate haematological (neutrophil and platelet counts >1·0 × 109/l and ≥50 × 109/l respectively), renal (creatine clearance ≥30 ml/min), and liver [transaminase levels three‐times or less the upper limit of normal (ULN); total bilirubin ≤1·5‐times ULN] function. Key exclusion criteria included prior BTK inhibitor treatment, central nervous system involvement, significant cardiac disease and prior allogenic stem cell transplantation 6 months or less before study entry. Patients requiring concurrent strong cytochrome P450 family 3 subfamily A (CYP3A) inhibitors/inducers or QT‐prolonging medications were excluded; aspirin and other anticoagulants, including warfarin, were allowed.
The recommended phase II dose was determined in the 3 + 3 dose escalation cohort that included four patients with CLL/SLL who received doses of 160 mg once (two) or twice daily (two). No dose‐limiting toxicity was encountered, and thus, no maximally tolerated dose was identified. Based on pharmacokinetic and pharmacodynamic data, both 320 mg once‐daily and 160 mg twice‐daily schedules were selected for further study in phase II dose expansion. 7 Phase II enrolled patients with B‐cell malignancies in disease‐specific cohorts, including R/R or TN mantle cell lymphoma, CLL/SLL, and Waldenström macroglobulinaemia. In part II, zanubrutinib was administered orally at 320 mg once daily or 160 mg twice daily in 28‐day cycles until unacceptable toxicity or progression. Part II enrolled 121 patients with CLL/SLL who received an initial dose of 160 mg twice daily (81) or 320 mg once daily (40). In all, 28 patients receiving an initial 320 mg once‐daily dose switched to 160 mg twice daily. A total of 125 patients with CLL/SLL were enrolled in parts I and II of the study. Data presented herein are for 123 patients with CLL/SLL who received zanubrutinib; one patient with prior BTK inhibitor exposure and one with prior Richter transformation were excluded.
At the closure of the AU‐003 study in March 2021, patients who remained on zanubrutinib and who investigators determined were benefitting from treatment had the option to cross over to a long‐term extension study that assessed longer‐term safety and efficacy.
All patients provided written informed consent. The study was conducted according to the principles of the Declaration of Helsinki and International Conference on Harmonisation guidelines. The protocol was approved by the institutional review boards/independent ethics committees at each site.
Statistical analysis
Analyses included all patients with CLL/SLL receiving one or more doses of zanubrutinib. Efficacy end‐points included ORR [complete response (CR) and partial response (PR), including PR with lymphocytosis], duration of response (DOR), progression‐free survival (PFS), and overall survival (OS). Response assessments were per the International Workshop in Chronic Lymphocytic Leukemia 2008 criteria including PR with lymphocytosis. 8 , 9
Subgroup analyses were performed using prespecified baseline subgroups. The ORR was summarised for each category with 95% confidence intervals (CIs). The DOR was defined as the time from the first qualifying response until disease progression or any‐cause death. The PFS was defined as time from treatment initiation to disease progression or any‐cause death. OS was defined as time from treatment initiation until death. Estimation of PFS and DOR, including event‐free rates at landmark time‐points, were estimated using Kaplan–Meier methodology with corresponding 95% CIs. The reverse Kaplan–Meier method was used to estimate follow‐up times for PFS and DOR. Descriptive statistics were used to summarise AE data.
Results
Baseline demographics and disease characteristics
The study enrolled 118 patients with CLL and five with SLL between September 2014 and March 2021. Of these, 22 patients (18·9%) were TN and 101 (82·1%) were R/R. The median (range) age at study entry for the overall population was 67 (24–87) years, with 17·1% aged ≥75 years. Most patients were male (75%). The median (range) number of prior therapies for R/R patients was two (one–10). Prior therapies included any anti‐CD20 antibody (93·1%), any alkylator (other than bendamustine; 90·1%), any chemo‐immunotherapy (89·1%), any purine analogue (excluding bendamustine; 73·3%), CVP (cyclophosphamide, vincristine, prednisone)‐ or CHOP [cyclophosphamide, doxorubicin, vincristine (Oncovin) and prednisone]‐containing regimen (16·8%), bendamustine (13·9%), any anthracycline (10·9%), and anti‐B‐cell lymphoma 2 (Bcl‐2) therapy (5%; Table SI). The median time since first CLL/SLL diagnosis to enrolment was 97·2 months (TN, 45·4 months; R/R, 101·2 months). Of the patients with available results, 16 of 99 (16·2%; TN, three; R/R, 13) had 17p deletion, 14 of 43 (32·6%; TN, 3; R/R, 11) had tumour protein p53 (TP53) mutation, 23 of 98 (23·5%; all R/R) had 11q deletion, and six of 101 had both 17p deletion and TP53 mutation (5·9%; TN, one; R/R, five). Additional characteristics are summarised in Table I. Mutation testing data were limited because assessments were optional.
Table I.
Summary of baseline demographics and disease characteristics.
| Characteristic |
TN (n = 22) |
R/R (n = 101) |
|---|---|---|
| Demographics | ||
| Age, years, median (range) | 69·5 (48–87) | 66·0 (24–87) |
| ≥75 years, n (%) | 4 (18·2) | 17 (16·8) |
| Male sex, n (%) | 18 (81·8) | 74 (73·3) |
| ECOG performance status, n (%) | ||
| 0 | 9 (40·9) | 48 (47·5) |
| 1 | 11 (50·0) | 50 (49·5) |
| 2 | 2 (9·1) | 3 (3·0) |
| Prior no. of anti‐cancer therapies, median (range) | 0 (0) | 2 (1–10) |
| Disease characteristics | ||
| SLL, n (%) | 2 (9·1) | 3 (3·0) |
| Rai stage for CLL, n (%) | ||
| Low risk (Stage 0) | 2 (9·1) | 9 (8·9) |
| Intermediate risk (Stages I–II) | 10 (45·5) | 31 (30·7) |
| High risk (Stages ≥III) | 7 (31·8) | 42 (41·6) |
| No staging | 1 (4·5) | 16 (15·8) |
| Bulky disease, n (%) | ||
| Any TL LDi ≥5 cm | 7 (31·8) | 40 (39·6) |
| Any TL LDi ≥10 cm | 1 (4·5) | 4 (4·0) |
| Mutational status, n/N (%) | ||
| del(13q) | 8/18 (44·4) | 37/80 (46·3) |
| del(11q) | 0/18 (0) | 23/80 (22·8) |
| del(17p) | 3/18 (16·7) | 13/81 (16·0) |
| Trisomy 12 | 3/18 (16·7) | 12/79 (15·2) |
| TP53 mutated | 3/18 (37·5) | 11/35 (31·4) |
| del(17p) and TP53 mutated | 1/18 (5·6) | 5/83 (6·0) |
| IGHV unmutated | 2/5 (40·0) | 27/37 (73·0) |
| ALC, × 109/l, median | 76·9 | 26·4 |
| Haemoglobin, g/l, median | 122·5 | 124·0 |
| Platelet count, × 109/l, median | 136·0 | 124·0 |
ALC, absolute lymphocyte count; CLL, chronic lymphocytic lymphoma; ECOG, Eastern Cooperative Oncology Group; IGHV, immunoglobulin heavy chain variable; TL, target lesion; LDi, diameter of largest lymph node; R/R, relapsed/refractory; SLL, small lymphocytic leukaemia; TN, treatment‐naïve; TP53, tumour protein p53.
Patient disposition
The median study follow‐up was 54·1 months for TN and 43·7 months for R/R patients. Overall the median treatment duration was 43 months. The median relative dose intensity for the 320 mg once‐daily dose was 97·0% and for the 160 mg twice‐daily dose was 96·3%.
At time of data cut‐off, 46 patients (37·4%) discontinued therapy: 26 patients (21·1%) due to progressive disease (TN, four; R/R, 22), 12 (9·8%) due to AEs, three (2·4%) due to investigator’s discretion, three (2·4%) due to withdrawal by subject, one (0·8%) due to stem cell transplantation, and one (0·8%) due to death. A total of 77 patients (62·6%) are continuing therapy in a long‐term extension study. Of the patients who discontinued due to progression, two experienced Richter transformation and one had disease that transformed into Hodgkin lymphoma.
Efficacy
The ORRs were 100% (95% CI 84·6–100·0) in TN patients and 95·0% (95% CI 88·8–98·4) in R/R patients (Table II). The CR rates were 22·7% in TN patients and 17·8% in R/R patients. The ORR rate in patients with del(17p)/TP53 mutations was 87·5% [95% CI 67·6–97·3; TN, 100% (95% CI 47·8–100·0); R/R, 84·2% (95% CI 60·4–96·6)]. Responses deepened over time; the CR rate in the overall population was 5·7% at 12 months, 10·6% at 24 months, 17·1% at 36 months, and 18·7% at 48 months (Figure S1). The ORR was high regardless of age, stage at study entry, del(17p)/TP53 mutation status, immunoglobulin heavy chain variable (IGHV) mutation status, prior lines of therapy, and other disease characteristics (Fig 1). ORR was 100% in TN and R/R patients treated with 320 mg once daily and 93·8% in TN and R/R patients treated with 160 mg twice daily (Table SII).
Table II.
Best overall response.
|
TN (n = 22) |
R/R (n = 101) |
|
|---|---|---|
|
ORR: PR‐L or better, n (%) [95% CI] |
22 (100) [84·6–100·0] |
96 (95·0) [88·8–98·4] |
| CR, n (%) | 5 (22·7) | 16 (15·8) |
| CRi, n (%) | 0 (0) | 2 (2·0) |
| nPR, n (%) | 0 (0) | 2 (2·0) |
| PR, n (%) | 17 (77·3) | 72 (71·3) |
| PR‐L, n (%) | 0 (0) | 4 (4·0) |
| SD, n (%) | 0 (0) | 4 (4·0) |
| Discontinued prior to first assessment | 0 (0) | 1 (1·0) |
| DOR event rate, % (95% CI) | ||
| 12 months | 95·2 (70·7–99·3) | 97·8 (91·6–99·5) |
| 24 months | 85·7 (62·0–95·2) | 89·4 (80·6–94·4) |
| 36 months | 81·0 (56·9–92·4) | 86·8 (77·4–92·5) |
| 48 months | 75·2 (50·0–88·9) | 67·5 (50·4–80·3) |
| Follow‐up for DOR, months, median (range) | 44·6 (8·2+–59·7+) | 36·9 (0+–65·1+) |
CI, confidence interval; CR, complete response; CRi, CR with incomplete bone marrow recovery; DOR, duration of response; nPR, nodular partial response; ORR, overall response rate; PR, partial response; PR‐L, PR with lymphocytosis; R/R, relapsed/refractory; SD, stable disease; TN, treatment‐naïve; +, censored patient.
Fig 1.

Forest plot of ORR. CLL, chronic lymphocytic leukaemia; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; LDi, longest transverse diameter; ORR, overall response rate; SLL, small lymphocytic leukaemia. *Stage at study entry: high risk includes (Rai) high risk of CLL, and (Ann Arbor) Stage III–IV for SLL; intermediate risk includes intermediate risk of CLL and Stage I–II for SLL; low risk includes low risk of CLL only. †Includes patients without baseline target lesion. ‡Cytopenia is defined as haemoglobin ≤110 g/l or platelet count ≤100 × 109/l or absolute neutrophil count ≤1·5 × 109/l.
Responses were durable: 97·3% of responders continued to respond at 12 months, 88·7% continued to respond at 24 months, and 85·7% continued to respond at 36 months (Fig 2A, Figure S2).
Fig 2.
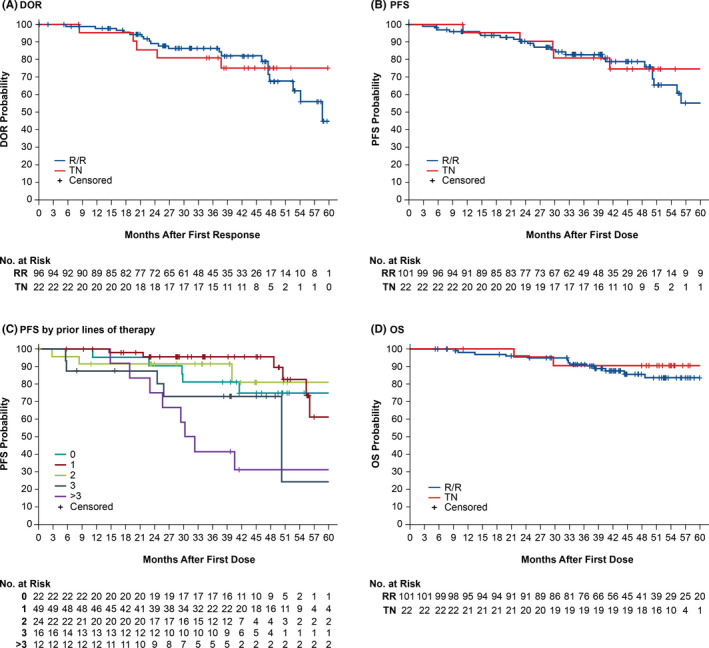
Kaplan–Meier estimates of DOR (A), PFS (B), PFS by prior lines of therapy (C), and OS (D) in TN or R/R patients. Number of events: (A) five TN, 20 RR; (B) five TN, 24 R/R; (C) five, six, four, six, eight events for zero, one, two, three and >3 lines respectively; (D) two TN, 14 R/R. DOR, duration of response; PFS, progression‐free survival; OS, overall survival; RR, relapsed/refractory; TN, treatment‐naïve. [Colour figure can be viewed at wileyonlinelibrary.com]
Progression‐free survival and overall survival
The median PFS was not reached for TN patients [95% CI 41·4 months–not evaluable (NE)] and was estimated to be 61·4 months (95% CI 50·4–NE) for R/R patients (Fig 2B). Estimated PFS rates for all patients were 96% (95% CI 90–98) at 12 months, 90% (95% CI 83–95) at 24 months, and 83% (95% CI 74–89) at 36 months. Although limited in size (n = 24), estimated PFS rate for patients with del(17p)/TP53 mutations was 91% (95% CI 69–98) at 12 months and 82% (95% CI 58–93) at 24 months (Figure S3). Longer PFS was observed in patients with fewer lines of therapy (Fig 2C). OS was 98% (95% CI 93·5–99·6) at 12 months, 96% (95% CI 90·1–98·2) at 24 months, and 91% (95% CI 84·4–95·2) at 36 months (Fig 2D).
Haematological recovery
Levels of haemoglobin and platelets improved over time. The median (interquartile range, IQR) haemoglobin at baseline was 124 (110–138) g/l and by Week 49 increased to 137·5 (150–136·9) g/l (Figure S4 and Table SIII). The median (IQR) platelets at baseline were 125 (95–176) × 109/l and by Week 49 increased to 158 (124–203) × 109/l (Figure S5 and Table SIV).
Safety
All patients reported at least one AE, 73·2% reported at least one Grade ≥3 AE, and 62·6% reported a serious AE. In all, 11 patients (8·9%) had dose reductions and 54 (43·9%) had dose interruptions due to AEs. The most common AEs (Table III) were infections (86·2%), including upper respiratory tract infections (48·8%), urinary tract infections (23·6%), sinusitis (19·5%) and pneumonia (19·5%).
Table III.
Treatment emergent adverse events occurring in ≥10% of patients by preferred term and maximum severity.
| AE, n (%) | Any grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Any | 123 (100) | 1 (0·8) | 32 (26·0) | 62 (50·4) | 24 (19·5) | 4 (3·3) |
| Contusion | 64 (52·0) | 58 (47·2) | 6 (4·9) | 0 | 0 | 0 |
| URTI | 60 (48·8) | 3 (2·4) | 56 (45·5) | 1 (0·8) | 0 | 0 |
| Cough | 44 (35·8) | 30 (24·4) | 14 (11·4) | 0 | 0 | 0 |
| Diarrhoea | 44 (35·8) | 29 (23·6) | 14 (11·4) | 1 (0·8) | 0 | 0 |
| Headache | 33 (26·8) | 24 (19·5) | 9 (7·3) | 0 | 0 | 0 |
| Constipation | 31 (25·2) | 23 (18·7) | 6 (4·9) | 2 (1·6) | 0 | 0 |
| UTI | 29 (23·6) | 1 (0·8) | 22 (17·9) | 6 (4·9) | 0 | 0 |
| Rash | 29 (23·6) | 19 (15·4) | 9 (7·3) | 1 (0·8) | 0 | 0 |
| Fatigue | 28 (22·8) | 20 (16·3) | 7 (5·7) | 1 (0·8) | 0 | 0 |
| Arthralgia | 27 (22·0) | 13 (10·6) | 11 (8·9) | 3 (2·4) | 0 | 0 |
| Nausea | 26 (21·1) | 15 (12·2) | 11 (8·9) | 0 | 0 | 0 |
| Back pain | 25 (20·3) | 15 (12·2) | 9 (7·3) | 1 (0·8) | 0 | 0 |
| Pneumonia | 24 (19·5) | 0 | 12 (9·8) | 12 (9·8) | 0 | 0 |
| Hypertension | 24 (19·5) | 1 (0·8) | 12 (9·8) | 11 (8·9) | 0 | 0 |
| Sinusitis | 24 (19·5) | 0 | 23 (18·7) | 1 (0·8) | 0 | 0 |
| Neutropenia | 22 (17·9) | 1 (0·8) | 2 (1·6) | 6 (4·9) | 13 (10·6) | 0 |
| LRTI | 21 (17·1) | 2 (1·6) | 16 (13·0) | 2 (1·6) | 1 (0·8) | 0 |
| Haematuria | 20 (16·3) | 18 (14·6) | 2 (1·6) | 0 | 0 | 0 |
| Fall | 17 (13·8) | 6 (4·9) | 7 (5·7) | 4 (3·3) | 0 | 0 |
| Muscle spasms | 16 (13·0) | 11 (8·9) | 5 (4·1) | 0 | 0 | 0 |
| Dizziness | 16 (13·0) | 15 (12·2) | 1 (0·8) | 0 | 0 | 0 |
| Cellulitis | 14 (11·4) | 0 | 8 (6·5) | 6 (4·9) | 0 | 0 |
| GERD | 14 (11·4) | 8 (6·5) | 6 (4·9) | 0 | 0 | 0 |
| Anaemia | 14 (11·4) | 2 (1·6) | 4 (3·3) | 7 (5·7) | 1 (0·8) | 0 |
| Vomiting | 14 (11·4) | 10 (8·1) | 4 (3·3) | 0 | 0 | 0 |
AE, adverse event; GERD, gastro‐oesophageal reflux disease; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection; UTI, urinary tract infection.
There were 12 patients with AEs resulting in zanubrutinib discontinuation (summarised in Table SV). These included muscular weakness in the context of degenerative joint disease and bilateral shoulder and bicep rupture, cryptococcal pneumonia, pleural effusion, progressive squamous cell carcinoma (SCC) of the right periauricular area and wide complex tachycardia in the context of atrial fibrillation and cardiac ablation.
Fatal AEs occurred in four patients (3·3%): coronavirus disease 2019 (COVID‐19), progressive SCC of the right periauricular area, subdural haematoma, and one patient with influenza‐related pneumonia and multiple organ dysfunction syndrome. The patient with progressive SCC had a history of multiple skin cancers and intra‐auricular skin cancer prior to enrolment. As of the data cut‐off date, 16 patients (13·0%) had died, including nine (7·3%) from disease progression, one (0·8%) from an unknown reason and two (1·6%) from other causes (advanced CLL or deteriorating comorbidity).
The most commonly reported AE of interest was bruising, which was reported in 55·3% of patients (Table IV) and included contusion (Grade 1, 47·2%; Grade 2, 4·9%), purpura (7·3%) and ecchymosis (0·8%). Minor bleeding was reported in 38·2% of patients, of which the most reported were haematuria (16·3%) and epistaxis (8·1%). Four patients had Grade ≥3 haemorrhage events: Grade 3 haemarthrosis, purpura and traumatic haematoma, and one fatal subdural haematoma. Diarrhoea, mostly Grade 1 (23·6%) and Grade 2 (11·4%), occurred in 35·8% of patients; one patient had Grade 3 diarrhoea. The median duration of any grade diarrhoea events was 6 days.
Table IV.
Adverse events of interest by maximum severity at 24‐month follow‐up.
| AEI, n (%) | Any grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Any | 123 (100) | 7 (5·7) | 41 (33·3) | 51 (41·5) | 20 (16·3) | 4 (3·3) |
| Bruising* | 68 (55·3) | 60 (48·8) | 7 (5·7) | 1 (0·8) | 0 | 0 |
| Minor bleeding† | 47 (38·2) | 41 (33·3) | 6 (4·9) | 0 | 0 | 0 |
| Diarrhoea | 44 (35·8) | 29 (23·6) | 14 (11·4) | 1 (0·8) | 0 | 0 |
| Headache | 33 (26·8) | 24 (19·5) | 9 (7·3) | 0 | 0 | 0 |
| SPM | 32 (26·0) | 4 (3·3) | 14 (11·4) | 12 (9·8) | 1 (0·8) | 1 (0·8) |
| Fatigue | 28 (22·8) | 20 (16·3) | 7 (5·7) | 1 (0·8) | 0 | 0 |
| Neutropenia‡ | 28 (22·8) | 1 (0·8) | 4 (3·3) | 7 (5·7) | 16 (13·0) | 0 |
| Arthralgia | 27 (22·0) | 13 (10·6) | 11 (8·9) | 3 (2·4) | 0 | 0 |
| Nausea | 26 (21·1) | 15 (12·2) | 11 (8·9) | 0 | 0 | 0 |
| Hypertension | 24 (19·5) | 1 (0·8) | 12 (9·8) | 11 (8·9) | 0 | 0 |
| Thrombocytopenia§ | 14 (11·4) | 5 (4·1) | 5 (4·1) | 4 (3·3) | 0 | 0 |
| Anaemia | 14 (11·4) | 2 (1·6) | 4 (3·3) | 7 (5·7) | 1 (0·8) | 0 |
| Myalgia | 12 (9·8) | 10 (8·1) | 1 (0·8) | 1 (0·8) | 0 | 0 |
| Petechiae | 10 (8·1) | 9 (7·3) | 1 (0·8) | 0 | 0 | 0 |
| Opportunistic infections | 9 (7·3) | 0 | 3 (2·4) | 3 (2·4) | 3 (2·4) | 0 |
| Atrial fibrillation/flutter | 6 (4·9) | 0 | 2 (1·6) | 4 (3·3)¶ | 0 | 0 |
| Major bleeding# | 4 (3·3) | 0 | 0 | 3 (2·4) | 0 | 1 (0·8) |
AEI, adverse event of interest; SPM, second primary malignancy.
Purpura, contusion, or ecchymosis.
Pooled term of bleeding, not including bruising, petechiae, or major bleeding.
Neutropenia, neutrophil count decreased, or febrile neutropenia.
Thrombocytopenia or platelet count decreased.
Three atrial fibrillation and one atrial flutter.
Grade ≥3 haemorrhage.
Atrial fibrillation or flutter was reported in six patients (4·9%), with three patients reporting Grade 3 atrial fibrillation (2·4%; Table SVI). Two of the six patients had a prior history of atrial fibrillation. Dose was interrupted for both patients and recommenced at full dose; one patient later discontinued study drug due to wide complex tachycardia attributed to underlying atrial fibrillation and underwent a cardiac ablation procedure. For the patient experiencing Grade 2 atrial fibrillation (patient number 4, Table SVI), the dose was interrupted and recommenced at 80 mg once daily. For one of the patients with Grade 3 atrial fibrillation (patient number 5, Table SVI), a pacemaker was inserted after development of bradycardia due to rate control therapy, and no dose interruptions or reductions were reported.
No tumour lysis syndrome was reported. Opportunistic infections occurred in nine patients (7·3%). Cryptococcal infections were reported in three R/R patients: one with Grade 2 cryptococcal pneumonia and one with Grade 4 cryptococcal meningitis, both successfully treated with antimicrobial agents and/or corticosteroids. Another patient had both Grade 4 cryptococcal pneumonia and cryptococcal meningitis (Cryptococcus gattii), this patient discontinued treatment due to the pneumonia infection. All three patients had previously received fludarabine, cyclophosphamide, and rituximab chemotherapy. Additional details are provided in Table SVII. The other opportunistic infections (one patient each) were herpes zoster oticus (Grade 3), mycobacterium ulceron (Grade 2), disseminated zoster/zoster meningoencephalitis (Grade 3), fungal pneumonia (Grade 2), disseminated varicella zoster aggravation (Grade 3), and listeria sepsis (Grade 4). No opportunistic infection resulted in zanubrutinib discontinuation.
The incidence of atrial fibrillation, Grade ≥3 hypertension (cumulative incidence 8·9%), major haemorrhage, Grade ≥3 neutropenia, and Grade ≥3 infection decreased over time (Table V). Grade ≥3 neutropenia decreased from 15·4% in year 1 to 1·8% in year 2 to 1·0% in year 3. A single incidence of Grade ≥3 haemorrhage was reported in year 3. Atrial fibrillation rates remained consistently low (0·9% to 2·4%) in years 1–4 of the study, with a cumulative incidence of 4·1% (any grade).
Table V.
Incidence of treatment emergent adverse events of interest over time.
| Category, % |
TEAEs 0–12 months (n = 123) |
TEAEs 12–24 months (n = 114) |
TEAEs 24–36 months (n = 104) |
TEAEs 36–48 months (n = 82) |
|---|---|---|---|---|
| Gr ≥3 infection* | 15·4 | 9·6 | 9·6 | 3·7 |
| Gr ≥3 neutropenia† | 15·4 | 1·8 | 1·0 | 1·2 |
| Gr ≥3 anaemia | 4·9 | 0·9 | 0·0 | 1·2 |
| Gr ≥3 hypertension | 4·1 | 3·5 | 0·0 | 2·4 |
| Opportunistic infection | 4·1 | 1·8 | 0·0 | 1·2 |
| Atrial fibrillation/flutter | 2·4 | 0·9 | 1·0 | 1·2 |
| Gr ≥3 major haemorrhage‡ | 1·6 | 0·0 | 1·0 | 0·0 |
| Gr ≥3 arthralgia | 1·6 | 0·0 | 1·0 | 0·0 |
| Gr ≥3 thrombocytopenia§ | 0·8 | 0·0 | 1·9 | 1·2 |
Gr, grade; TEAE, treatment emergent adverse event.
All infection terms pooled. Only Grade ≥3 infections are reported. Opportunistic infections and pneumonia/lung infections are a subgroup of all pooled infections.
Neutropenia, neutrophil count decreased, or febrile neutropenia.
Grade ≥3 haemorrhage.
Thrombocytopenia or platelet count decreased.
Discussion
Zanubrutinib is highly effective therapy for patients with TN or R/R CLL/SLL. At a median follow‐up of 47·2 months, the ORR was 95·9% with an 18·7% CR rate. The depth of response increased over time and the responses were durable: after 2 and 3 years, 88·7% and 85·7% of patients continued to respond to treatment respectively. Estimated 2‐ and 3‐year PFS was 90% (TN, 90%; R/R, 91%) and 83% (TN, 81%; R/R, 83%).
The responses to zanubrutinib were seen in patients with poor prognostic factors, including del(17p)/TP53 mutation, IGHV unmutated, those with three or more previous therapies, and those with bulky disease. Patients with del(17p)/TP53‐mutated CLL/SLL have particularly bad outcomes and poor response to standard chemoimmunotherapy. In this study, ORR in the subset of patients with del(17p)/TP53 was 87·5%. The estimated probability of responding for >36 months was 84·2%, indicating durable responses. These findings are consistent with the SEQUOIA study in TN patients with CLL/SLL and del(17p), reporting a 94·5% response rate in 109 patients with del(17p) and an estimated 12‐month PFS rate of 94·5%. 10
Results of the present analysis are also consistent with those of other studies on zanubrutinib in patients with R/R CLL/SLL. A single‐arm study in China (NCT03206918) reported an ORR of 84·6% (CR 3·3%) in 91 patients at a median 15·1 months, with 87·2% of patients alive and progression‐free at 12·9 months. 11 The ORRs were similar in patients with and without the TP53 mutation (85·0% and 84·5% respectively). 11 In the first interim report of the phase III ALPINE study of zanubrutinib versus ibrutinib in R/R CLL/SLL (NCT03734016), 12 the investigator‐assessed ORR (CR + PR + PR‐L) was 88·4% (CR 1·9%) in the zanubrutinib arm at a median follow‐up of 15·3 months. 13 The ORR defined as CR + PR was slightly higher among the 24 patients with del(17p) than in the full study population (N = 207; 83·3% vs. 78·3%). The PFS and OS rates at 1 year were 94·9% and 97·0% respectively, in the zanubrutinib arm. 13 Although differences in patient populations and other factors make cross‐trial comparisons problematic, favourable response rates and survival in patients treated with zanubrutinib are a common feature of these various studies.
In the present study, zanubrutinib was well tolerated. No new safety signals emerged with prolonged treatment, and AEs were generally consistent with previous reports. 7 , 11 , 13 , 14 The annual incidence of major haemorrhage, Grade ≥3 neutropenia, and Grade ≥3 infection decreased over time, and the rate of atrial fibrillation remained consistently low. The present study findings were consistent with results from the randomised ALPINE study, which reported significantly lower rates of atrial fibrillation/flutter in the zanubrutinib arm compared with the ibrutinib arm (2·5% vs. 10·1%, P = 0·0014) at a 15·3‐month follow‐up. 13 Similarly, a lower incidence of atrial fibrillation for zanubrutinib compared with ibrutinib was demonstrated in patients with Waldenström macroglobulinaemia in ASPEN (median follow‐up 19·4 months). 15 The cumulative rate of Grade ≥3 hypertension in the present study (8·9%) was higher than the 6% rate reported in the zanubrutinib arm of ASPEN, but lower than the corresponding 11% rate in its ibrutinib arm. 15
Chronic lymphocytic leukaemia commonly occurs in older patients who often have comorbidities; therefore, long‐term tolerability is important. Discontinuations or dose reductions due to toxicities with zanubrutinib were relatively uncommon and only 9·8% of patients discontinued due to AEs. This is consistent with data from other studies having shorter (~15 months) median follow‐up, which have reported 7·7% 13 and 8·8% 11 rates of zanubrutinib discontinuation due to AEs. These low discontinuation rates suggests that zanubrutinib has a favourable safety profile and is suitable for long‐term use as a single agent.
In conclusion, the AU‐003 study includes a large number of patients with TN and R/R CLL/SLL being treated with single‐agent zanubrutinib over ~4 years and provides valuable evidence supporting deeper and durable responses with prolonged treatment. The combination of sustained efficacy with acceptable tolerability with prolonged single‐agent zanubrutinib in patients with CLL/SLL suggest long‐term use is feasible, even for those with unfavourable disease characteristics.
Author contributions
All the investigators and their research teams collected data. The sponsor confirmed the accuracy of the data and compiled the data for analysis. All the authors contributed to data interpretation, reviewed the manuscript, and made the decision to submit it for publication and vouch for the accuracy and completeness of the data and analyses and adherence to the trial protocol. Together with BeiGene authors (Kenneth Wu, William Novotny, and Jane Huang), AU‐003 safety monitoring committee members (Constantine S. Tam and Stephen Opat) were responsible for the study design, and the authors Gavin Cull, Jan A. Burger, David Gottlieb, Emma Verner, Judith Trotman, Paula Marlton, Javier Munoz, Patrick Johnston, David Simpson, and Jennifer C. Stern further contributed to data interpretation and analysis.
Conflict of interests
Gavin Cull has received research funding from BeiGene, Acerta, and Glycomimetics; travel, accommodations, and expenses from Glycomimetics. Jan A. Burger has received research funding from Pharmacyclics, Gilead, and BeiGene; and received honoraria from Janssen and AstraZeneca. Stephen Opat has received honoraria from AbbVie, Roche, AstraZeneca, Merck, Gilead, Janssen, and Novartis; has a consulting or advisory role for AbbVie, Roche, AstraZeneca, and Merck; received research funding from BeiGene, Roche, AstraZeneca, Janssen, Merck, Amgen, and Epizyme; and received travel, accommodations, and expenses from Roche. David Gottlieb has equity in Indee; has received honoraria from AbbVie, Link Health Care, Gilead, Merck, Novartis, and Pfizer; and received research funding from Haemaologix. Emma Verner has received research funding from Janssen‐Cilag Pty Ltd. Judith Trotman has received research funding from BeiGene, Roche, Pharmacyclics, Celgene, a Bristol‐Myers Squibb Company, and Takeda. Paula Marlton has had an advisory role for BeiGene, Janssen, AstraZeneca, AbbVie, Roche, Astellas, Novartis, and Gilead. Javier Munoz has received honoraria from Kyowa and Seattle Genetics; has a consulting or advisory role with Pharmacyclics, Bayer, Gilead, Kite Pharma, Pfizer, Janssen, Juno, Celgene, a Bristol‐Myers Squibb Company; Kyowa, Alexion, BeiGene, Fosunkite, Innovent, and Seattle Genetics; has participated in speakers’ bureaus for Gilead, Kite Pharma Kyowa, Bayer, Pharmacyclics, Janssen, Seattle Genetics, Acrotech, BeiGene, Verastem, AstraZeneca, Celgene, a Bristol‐Myers Squibb Company, Genentech, and AbbVie; received research funding from Kite Pharma, Celgene, a Bristol‐Myers Squibb Company, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, and Millennium. Patrick Johnston has nothing to disclose. David Simpson has employment and equity at BeiGene; received honoraria from AbbVie, Janssen, and Roche; received research funding from AbbVie, Amgen, BeiGene, Celgene, a Bristol‐Myers Squibb Company, MSD, Acerta, Pharmacyclics, Sanofi, and GSK; and received funding for travel, accommodations, and expenses from AbbVie. Jennifer C. Stern, Radha Prathikanti, Kenneth Wu and William Novotny have employment and equity at BeiGene. Jane Huang has employment, equity, and leadership at BeiGene. Constantine S. Tam has received research funding from Janssen, AbbVie, BeiGene, Pharmacyclics, and TG Therapeutics.
Supporting information
Table SI. Prior anti‐cancer therapies in relapsed/refractory patients
Table SII. Best response by dose.
Table SIII. Haemoglobin levels (g/l).
Table SIV. Platelet levels (109/l).
Table SV. Adverse events leading to treatment discontinuation.
Table SVI. Patients with atrial fibrillation/flutter.
Table SVII. Vignettes of patients with cryptococcal infections.
Fig S1. Cumulative best response over time.
Fig S2. Kaplan–Meier estimates of duration of response (DOR) (A), progression‐free survival (PFS) (B), and overall survival (OS) (C) in the study population.
Fig S3. Progression‐free survival in relapsed/refractory patients with del(17p)/TP53 mutation.
Fig S4. Box plot representation of the haemoglobin levels.
Fig S5. Box plot representation of platelet levels.
Acknowledgements
We thank study patients, their supporters, and investigators and clinical research staff at study centres. We thank Siminder Kaur Atwal for helping with conducting the study and with data interpretation. The study was funded by BeiGene USA, Inc. Editorial assistance was provided by Alessandra Richardson, PhD, of Bio Connections, LLC, (Chicago, IL), and funded by BeiGene USA, Inc.
Cull G, Burger JA, Opat S, Gottlieb D, Verner E, Trotman J, et al. Zanubrutinib for treatment‐naïve and relapsed/refractory chronic lymphocytic leukaemia: long‐term follow‐up of the phase I/II AU‐003 study. Br J Haematol.2022;196:1209–1218. 10.1111/bjh.17994
References
- 1. Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum K, et al. Ibrutinib treatment for first‐line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC‐1102 study. Clin Cancer Res. 2020;26:3918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrd JC, Hillmen P, O’Brien S, Barrientos JC, Reddy NM, Coutre S, et al. Long‐term follow‐up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133:2031–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K, et al. Single‐agent ibrutinib in treatment‐naive and relapsed/refractory chronic lymphocytic leukemia: a 5‐year experience. Blood. 2018;131:1910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 ibrutinib‐treated patients in the United States: a real‐world analysis. Haematologica. 2018;103:874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li N, Sun Z, Liu Y, Guo M, Zhang Y, Zhou D, et al. BGB‐3111 is a novel and highly selective Bruton's tyrosine kinase (BTK) inhibitor. Cancer Res. 2015;75(15 Suppl):Abstract 2597. [Google Scholar]
- 6. Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI‐32765) has significant activity in patients with relapsed/refractory B‐cell malignancies. J Clin Oncol. 2013;31:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of selective BTK inhibitor zanubrutinib in B‐cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hallek M, Cheson BD, Catovsky D, Caligaris‐Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute‐Working Group 1996 guidelines. Blood. 2008;111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hallek M, Cheson BD, Catovsky D, Caligaris‐Cappio F, Dighiero G, Dohner H. Response assessment in chronic lymphocytic leukemia treated with novel agents causing an increase of peripheral blood lymphocytes. Blood. 2012;119:5348. [Google Scholar]
- 10. Tam CS, Robak T, Ghia P, Kahl BS, Walker P, Janowski W, et al. Zanubrutinib monotherapy for patients with treatment naive chronic lymphocytic leukemia and 17p deletion. Haematologica. 2020;106:2354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single‐arm, multicenter study. J Hematol Oncol. 2020;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hillmen P, Brown JR, Eichhorst BF, Lamanna N, O’Brien SM, Qiu L, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16:517–23. [DOI] [PubMed] [Google Scholar]
- 13. Hillmen P, Eichhorst B, Brown JR, Lamanna N, O’Brien S, Tam CS, et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinb vs. ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. European Hematology Association 2021 Virtual Meeting; June 11, 2021. Available at: https://library.ehaweb.org/eha/2021/eha2021‐virtual‐congress/330170/peter.hillmen.first.interim.analysis.of.alpine.study.results.of.a.phase.3.html. Accessed December 2021.
- 14. Tam CS, Opat S, Zhu J, Cull G, Gottlieb D, Li J, et al. Pooled analysis of safety data from monotherapy studies of the Bruton tyrosine kinase (BTK) inhibitor, zanubrutinib (BGB‐3111) in B‐cell malignancies. 24th European Hematology Association Congress; June 13–16, 2019. Available at: https://library.ehaweb.org/eha/2019/24th/266776/constantine.s.tam.pooled.analysis.of.safety.data.from.monotherapy.studies.of.html. Accessed December 2021.
- 15. Tam CS, Opat S, D'Sa S, Jurczak W, Lee HP, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood. 2020;136:2038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Prior anti‐cancer therapies in relapsed/refractory patients
Table SII. Best response by dose.
Table SIII. Haemoglobin levels (g/l).
Table SIV. Platelet levels (109/l).
Table SV. Adverse events leading to treatment discontinuation.
Table SVI. Patients with atrial fibrillation/flutter.
Table SVII. Vignettes of patients with cryptococcal infections.
Fig S1. Cumulative best response over time.
Fig S2. Kaplan–Meier estimates of duration of response (DOR) (A), progression‐free survival (PFS) (B), and overall survival (OS) (C) in the study population.
Fig S3. Progression‐free survival in relapsed/refractory patients with del(17p)/TP53 mutation.
Fig S4. Box plot representation of the haemoglobin levels.
Fig S5. Box plot representation of platelet levels.


