Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) is an aggressive disease worldwide. Much progress has been made in exploring mechanisms and improving the therapy of HNSCC, but only a few studies have focused on the role of ferroptosis on HNSCC progression. The current study aimed to reveal the underlining mechanisms that caveolin‐1 (CAV1)‐ROS (reactive oxygen species)‐ferroptosis axis affect the process of HNSCC and discover novo therapeutic targets or strategies.
Methods
The role of CAV1 in ferroptosis was analyzed by FerrDb, and its clinical significance was examined by TCGA dataset of HNSCC. The expressions of caveolin‐1 (CAV1) in HNSCC tissues were measured by immunohistochemistry, western blot, and real‐time PCR assay. Three siRNA sequences were designed to silence CAV1 mRNA in HNSCC cells. Cell proliferation, colony formation, wound‐healing, and transwell assays were used to examine the proliferation, migration, and invasion of cancer cells. ROS evaluation and intracellular Fe2+ content assays were performed to examine the levels of ferroptosis.
Results
Through the analysis with published data, CAV1 was found to overexpress in HNSCC than normal tissues, and was one of the vital suppressors of ferroptosis pathway. Our study showed that CAV1 was over expressed in HNSCC tissues and the high level of CAV1 predicted poorer prognosis. Further experiments indicated that CAV1 could inhibit the ferroptosis of cancer cells and promote the proliferation, migration and invasion.
Conclusions
Overexpression of CAV1 in HNSCC inhibited the process of ferroptosis, leading to aggressive phenotypes, as well as worse prognosis. The regulatory pathway of CAV1 and ferroptosis are potential targets for designing diagnostic and combined therapeutic strategies for HNSCC patients.
Keywords: caveolin‐1, ferroptosis, head and neck, squamous cell carcinoma
1. INTRODUCTION
Biological capabilities acquired during the development of cancers mainly include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. 1 Among them, cell death is essential for biological processes, such as maintaining homeostasis and healthy status. 2 Immortalization is a common feature of cancer cells, which indicates the dysbiosis between proliferation and death. 3 Cell death is classified into accidental cell death (ACD) and regulated cell death (RCD). RCD includes apoptosis, necroptosis, pyroptosis, and ferroptosis. 4 Iron is one of the most vital metal elements in human body and plays a central role in oxygen transport, DNA biosynthesis, ATP synthesis and electron transfer. 5 Ferroptosis refers to a type of regulated cell death, which is characteristic by iron‐dependent lipid peroxide accumulation. 6
Owing to the high morbidity and mortality rates, head and neck squamous cell carcinoma (HNSCC) has attracted considerable attention to its underlying mechanism and therapeutic strategies. Although studies have discovered a number of genes and pathways related to the development of HNSCC, the complexity of the disease compelled us to conduct more investigation into its progression. Caveolin‐1 (CAV1) is an integral membrane protein that involved in cell signaling and transport. CAV1 is abundantly expressed in adipocytes, endothelial cells, fibroblasts, and cancer cells. 7 Signal transduction related to CAV1 can regulate cell proliferation, invasion, cell death, and lipid metabolism. 8 , 9 By retrieving the ferroptosis database FerrDb (http://www.zhounan.org/ferrdb), it was found that CAV1 strongly inhibited ferroptosis. Research on the relationship between CAV1 and ferroptosis was rarely seen in the development of HNSCC. Therefore, it is urgent and important to explore the underlining axis among CAV1, ferroptosis and HNSCC.
In this study, we focused on the oral region of HNSCC. It was aimed to investigate the effect of CAV1 on HNSCC via suppressing ferroptosis. Through experiments and bioinformatics analysis, the results showed that CAV1 was upregulated in HNSCC and was related to poorer prognosis. The relevance of CAV1 and ferroptosis was also discussed. In addition, this study demonstrated that CAV1 could improve the proliferation and migration of cancer cells in vitro. Moreover, it was identified that inhibition of ferroptosis by CAV1 in cancer cells lead to the immortality and malignant phenotype of HNSCC, which might contribute to the progression of HNSCC.
2. METHODS
2.1. Patients and specimens
All the HNSCC patients’ cancer tissue samples were collected from the Department of Oral and Maxillofacial‐Head and Neck Oncology, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China), between 2011 and 2015. All the samples were from patients who were diagnosed with HNSCC with the region limited in tongue, gingiva, oral mucosa, etc. Subjects who previously received radiotherapy, chemotherapy were excluded. Among these specimen, 59 HNSCC cancer tissues and 5 normal tissues were chosen to perform immunohistochemical analysis. From August 2020 to March 2021, five pairs of HNSCC cancer tissues and adjacent normal tissues were collected to detect relative protein level through western blot. Moreover, 30 pairs of HNSCC cancer tissues and adjacent normal tissues were used to evaluate the relative mRNA level of CAV1 by real‐time PCR. Pathological and clinical stages were, respectively, determined according to World Health Organization Classification of Cancer and the tumor node metastasis (TNM) staging system (2010) from the Union for International Cancer Control (UICC). The Ethics Committee of Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine ratified our study. Written informed consents were provided by the participants prior to enrollment.
2.2. Cell culture
HNSCC cell lines: HN6, HN30, CAL27, SCC9, and SCC25 were used in the study. HN6, HN30, and CAL27 were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA), penicillin (100 units ml‒1), and streptomycin (100 μg ml‒1) at 37°C in a humidified 5% CO2 atmosphere. SCC9 and SCC25 were maintained in DMEM/F12 medium containing 10% FBS.
2.3. Immunohistochemical analysis
From the HNSCC patients that received surgery in Shanghai Ninth People's Hospital from 2011 to 2015, 59 patients that had complete follow‐up information were chosen. The gingival tissues from healthy donors were used as normal control, which were from 5 healthy donors. All the patients and donors were consent informed. Paraffin‐embedded slides were deparaffinized in graded xylene. After blocking the sections with 10% bovine serum albumin (BSA), the slides were incubated with primary antibodies (anti‐Caveolin‐1, 1:200) (CST, USA) at 4°C overnight. Then secondary antibody (1:300) (Yeasen, China) was incubated for 30 min at room temperature. Hematoxylin and dehydration were used to counterstain the nucleus. The images were scanned by panoramic slice scanner (Pannoramic MIDI, 3DHISTECH, Hungary) and analyzed by software (Aipathwell, Servicebio Technology, China). The scanned files or images on immunohistochemical sections were collected by tissue section digital scanner or imaging system, the tissue measurement area was automatically read by Seville image analysis system, and the positive grade was evaluated first: negative without coloring, 0 point; Weak positive light yellow, 1 point; Medium positive brownish yellow, 2 points and Strong positive sepia as 3 points. Then, analyzed and calculated the grade, the measurement area, the tissue area in the measurement area, the positive cumulative optical density IOD value and the positive area. The results were calculated to reflect the positive degree. The histochemistry score (H‐score) was used for each slide in order to evaluate the staining intensity of CAV1. H‐SCORE=∑(pi×i)=(percentage of weak intensity area×1)+(percentage of moderate intensity area×2)+(percentage of strong intensity area×3). PI represented the percentage of positive signal pixel area; I represented positive grade.
2.4. SiRNA transfection
Three siRNA sequence were designed to silence CAV1 mRNA: CAV1‐si‐1 (5'‐3’): CUUUGAAGCUGUUGGGAAATT; UUUCCCAACAGCUUCAAAGTT. CAV1‐si‐2 (5'‐3’): GCAUCAACUUGCAGAAAGATT; UCUUUCUGCAAGUUGAUGCTT. CAV1‐si‐3 (5'‐3’): GCGAGAAGCAAGUGUACGATT; UCGUACACUUGCUUCUCGCTT. HN6 and CAL27 were seeded in 6‐well plates and were transfected with targeted siRNA, when the cells were 40% confluence. The efficiency was evaluated by western blot.
2.5. RNA extraction and real‐time PCR analysis
The TRIzol Reagent (Invitrogen, USA) was used to extract RNA. The cDNA was synthesized and amplified using the SYBR Premix Ex Taq reagent kit (Takara, Japan) according to the protocol. In holding stage, it was 95°C for 30 s. Then in cycling stage, 95°C for 5 s and 60°C for 30 s, and the number of cycles was 40. The primers for CAV1(5’‐3’): AATACTGGTTTTACCGCTTGCT; CATGGTACAACTGCCCAGATG. The primers for GPX4(5’‐3’): GAGGCAAGACCGAAGTAAACTAC; CCGAACTGGTTACACGGGAA. The primers for ACSL4(5’‐3’): CATCCCTGGAGCAGATACTCT; TCACTTAGGATTTCCCTGGTCC. The primers for NOX1(5’‐3’): TTGTTTGGTTAGGGCTGAATGT; GCCAATGTTGACCCAAGGATTTT. The primers for FTH1(5’‐3’): CCCCCATTTGTGTGACTTCAT; GCCCGAGGCTTAGCTTTCATT. GAPDH (Sangon Biotech, China) was used as an internal parameter. Relative expression levels were calculated using the 2−∆∆Ct method.
2.6. Western blot analysis
The total protein of 5 pairs of HNSCC cancer tissues and adjacent normal tissues were extracted by SDS lysis buffer (Beyotime, China). Cells (HN6, HN6si, HN30, CAL27, CAL27si, SCC9, SCC25) were seeded in 6‐well plates before the experiments and were harvested by SDS lysis buffer. Pierce BCA Protein Assay kit (Thermo Scientific, USA) was used to analysis the concentrations. The mass of protein that loaded in each well was 10μg. The samples were electrophoresed and transferred to 0.45μm PVDF membranes (Merck Millipore, USA). After blocked with non‐fat milk for 1 h at room temperature, the membranes were incubated with primary antibodies (anti‐Caveolin‐1, 1:1000 and anti‐β‐actin, 1:1000), (CST, USA) overnight at 4°C. Then, the membranes were incubated with corresponding secondary antibodies (anti‐Rabbit 1:5000) (Yeasen, China) for 1 h at room temperature and visualized with ECL Ultra (New Cell and Molecular Biotech, China).
2.7. Cell proliferation assay
HNSCC cell lines, HN6 and CAL27 cells, together with cells transfected with siCAV1 were seeded in 96‐well plates in a density of 1 × 103 cells/well, respectively. CCK‐8 kit (NCM, China) was added into the wells and the OD value was measured at 450 nm after 2 h. The cells were cultured for 0, 1, 2, 3 days.
2.8. Wound‐healing assay
HN6 and CAL27 cells with or without CAV1 knockdown, respectively, were seeded in 6‐well plates. When the cells were 100% confluence, a 10‐μL pipette tip was used to scrape the cells. PBS was used to wash the cells for three times, then added serum‐free medium to culture for 12h. Pictures were taken at 0h and 12 h.
2.9. Transwell assay
Transwell assay was used for migration evaluation. HN6 and CAL27 cells with or without siCAV1 transfection were plated into upper chambers (Merck Millipore, USA), respectively, and cultured with serum‐free medium in 24‐well plates, and 600 μL DMEM containing 10% FBS in the bottom chamber. Cells were treated with 4% paraformaldehyde and stained with crystal violet after 24 h incubation at 37°C. Cells from three random non‐overlapping fields were counted at×100 original magnification.
2.10. Colony formation assay
HN6 and CAL27 cells with or without siCAV1 transfection were seeded into 6‐well plates in a density of 1 × 103 cells/well. After incubating at 37°C for 7–10 days, cells were washed with PBS and then 4% paraformaldehyde was used to fix them. After staining with 0.5% crystal violet, the colonies were photographed and counted for three times.
2.11. Reactive oxygen species (ROS) evaluation assay
The HN6 and CAL27 cells, with or without CAV1 knockdown, were seeded into 24‐well plates 24 h prior to treatment. Reactive Oxygen Species Assay Kit (Beyotime, China) was used to detect the fluorescence of 2’,7'‐dichlorofluorescein (DCF) in cell to measure the level of reactive oxygen species (ROS). The cells were washed by PBS and H2DCFDA (DCFH‐DA) was added into wells after dilution with serum‐free medium. After incubating at 37°C for 20 min, the cells were washed with PBS for three times. Pictures were taken by BioTek Cytation 5 (BioTek, USA).
2.12. Intracellular Fe2+ content assay
The level of Fe2+ in cells could measure the process of ferroptosis. HN6 and CAL27 cells, with or without CAV1 knockdown were seeded into 24‐well plates 24 h prior to treatment. FerroOrange (Dojindo, China), which could indicate the Fe2+ in cells was added into wells and incubated in 37°C for 2 h. The cells were washed with PBS, and pictures were taken by BioTek Cytation 5 (BioTek, USA).
2.13. Statistical and bioinformatics methods
SPSS V.21.0 statistical software was used to analyze the data. The image J (V 1.53k) software was applied for quantification about the result of the immunohistochemistry, immunofluorescence, colony formation, and transwell assay. All the cytological and molecular biological experiments were repeated 3 times. Student's t‐test and the Mann‐Whitney test were used to compare the means of two or more groups based on the nature of data. Kaplan‐Meier (KM) curve was adopted for survival analysis and Spearman was used for relative analysis, log‐rank test and rho coefficient were used to represent the significance, respectively. The driver and suppressors gene list were downloaded from FerrDb through the “Browse” and “Download” functions for further analysis. GSEA analysis was conducted and visualized by the clusterProfiler package of R (V4.0.5). Head and neck cancer data from TCGA were downloaded through GDC portal, the expression of driver and suppressor genes from Ferroptosis pathway were exhibited by heatmap. p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 and the exact p values are indicated in the figures.
3. RESULTS
3.1. CAV1 is associated with higher ferroptosis activity and poorer prognosis in HNSCC
Defective apoptosis in cancer cells promotes cancer aggressiveness. As a novel kind of cell death, ferroptosis is intensively related to progression of various cancers. By searching FerrDb, we found that 122 genes that driving ferroptosis, while 70 genes suppressing ferroptosis. Next, the expression of these genes in HNSCC tissues was analyzed through the TCGA datasets (Figure 1A). Among them, 25 genes in suppressor group were upregulated. Then, we screened differential 1177 genes which were higher expressed in cancer tissues than normal tissues of HNSCC (p < 0.05). Venn diagram was used to take the intersection between 25 suppressor genes and 1177 differential genes, and 3 genes were existed in both groups: CAV1, CD44 and SLC3A2 (Figure 1B). The KM analysis of these 3 genes were calculated and the log‐rank test validated that CAV1 was relative to worse prognosis in HNSCC patients. According to the literature, the higher level of ROS implies that the process of ferroptosis is activated. 6 The GSEA analysis indicated that when CAV1 was higher expressed, the expression of ROS upregulated genes was decreased, which suggested that ferroptosis was inhibited, and the expression level of CAV1 is negatively correlated with ROS (Figure 1C). Moreover, to discover the relationship between CAV1 and other ferroptosis drivers and suppressors genes, we sub‐grouped the data according to the expression level of CAV1 of HNSCC in TCGA database into a CAV1 higher group and a CAV1 lower group. The identified genes exhibited significant differences between these two groups, which indicated that these genes were closely related to CAV1. There were 15 driver genes and 8 suppressor genes aside from CAV1, and their expression in HNSCC was shown (Figure 1D). It was found that most suppressor genes of ferroptosis were overexpressed, while a number of driver genes showed low level expression. These results indicated the possibility of ferroptosis occurring in HNSCC, along with the ferroptosis suppressor function of CAV1.
FIGURE 1.
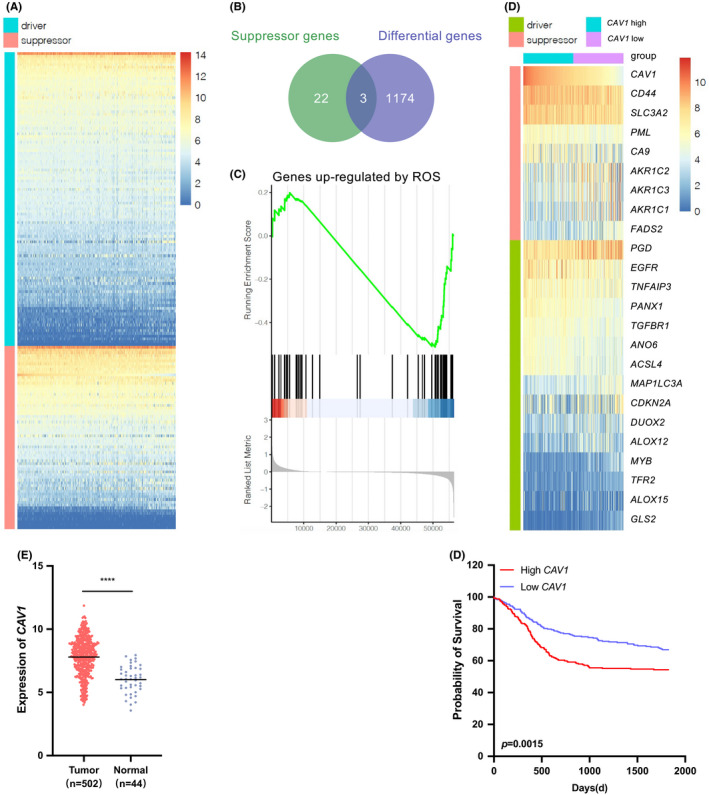
CAV1 is associated with poorer prognosis and ferroptosis in TCGA HNSCC dataset. A. Heatmap showed the expression of ferroptosis driver and suppressor genes in HNSCC. B. Venn diagram showed 3 genes both expressed in selective ferroptosis suppressor group and differential expressed genes in HNSCC from TCGA. C. The GSEA analysis showed the expression of upregulated genes mediated by ROS, on the basis of highly expression of CAV1. D. Heatmap showed the different expression of genes that were related to CAV1. E. The expression of CAV1 in HNSCC cancer and normal tissues. (p < 0.0001) F. The overall survival rate was significantly different between the high and low expression of CAV1 in HNSCC patients (p = 0.0015). Data originated from TCGA database. (ns, no significant difference, · p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001)
In addition, the TCGA dataset showed that CAV1 was highly expressed in many cancers (Figure S1A). Then we focused on HNSCC and found that the expression of CAV1 was significantly over expressed in HNSCC tissues (Figure 1E), and a high level of CAV1 was correlated with poor prognosis(p = 0.0015) (Figure 1F).
3.2. CAV1 protein is overexpressed in HNSCC tissues and related to poorer prognosis
To validate the expression of CAV1 in HNSCC, 59 HNSCC patient cancer tissues and 5 normal tissues were examined by IHC assays. The relationship between CAV1 expression levels and clinicopathological features was analyzed. The staining intensity was used to evaluate the expression of CAV1 after whole slide scanning. Although the staining intensity of CAV1 varied in different HNSCC tissues, it was significantly stronger than that in normal tissues. According to the results, weak positive, modest positive and strong positive staining intensities were used to describe the staining results and H‐SCORE was used to analyze the relationship between CAV1 expression levels and clinicopathological features (Figure 2A). The results showed that the expression level of CAV1 was not significantly associated with age, sex or lymph node metastasis (Figure S1B), but had a close relationship with cancer size (p = 0.0011), histological grade (p = 0.0452), and overall survival rate (Figure 2B‐D). Univariate analysis showed that the log‐rank test result of CAV1 is 0.059, and multivariate analysis showed that p value of CAV1 expression is 0.071, with HR of 2.9 (ranging from 0.91 to 9.1) (Table 1). In addition, 5 pairs of cancer tissues and adjacent normal tissues from HNSCC patients were collected, and western blot analysis proved that CAV1 was upregulated in cancer tissues (Figure 2E). The same result was seen for the expression of CAV1 at the mRNA level in 30 pairs of tissues from HNSCC patients (Figure 2F).
FIGURE 2.
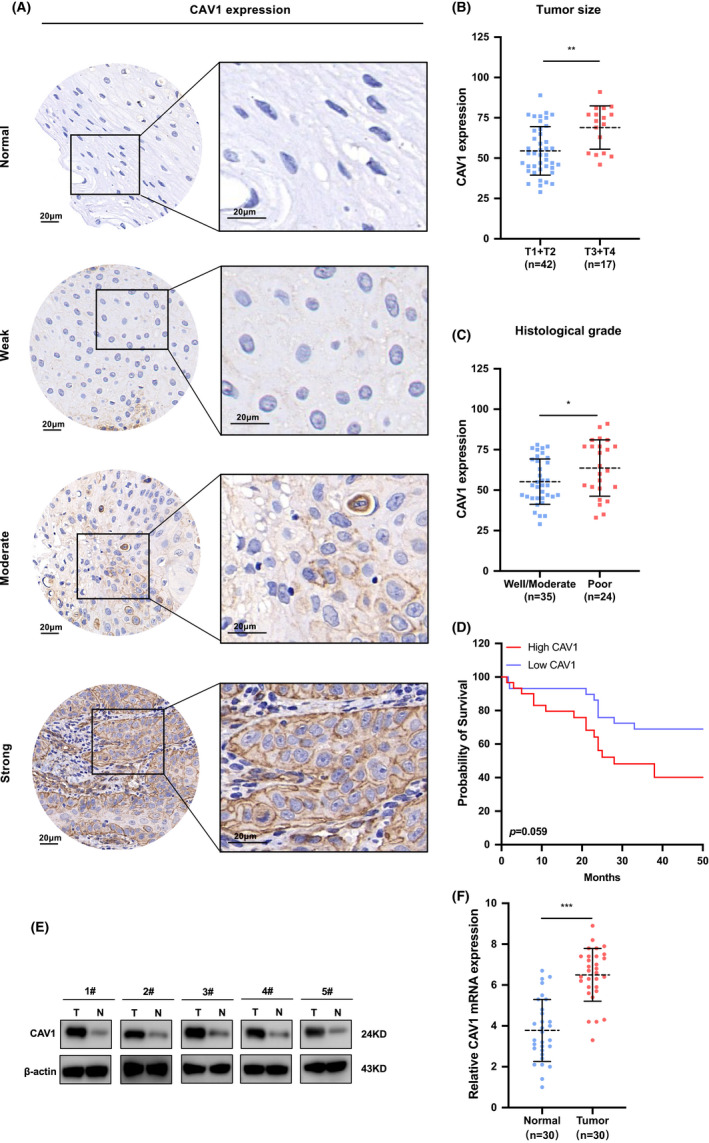
CAV1 is overexpressed in HNSCC tissues and associated with poorer prognosis. A. IHC analysis of CAV1 expression levels. Images of negative, weak, moderate, and strong CAV1 staining are shown. Scale bar: 20μm. B. CAV1 expression level was significantly associated with cancer size. (p = 0.0011) C. CAV1 expression level was significantly associated with histological grade (p = 0.0452). D. The overall survival rate was different between higher and lower expression of CAV1 in HNSCC (p = 0.059). E. Western blot measured CAV1 protein expression in 5 paired HNSCC cancer tissues and adjacent normal tissues. F. Quantitative real‐time PCR evaluated the relative CAV1 mRNA levels in 30 pairs of HNSCC cancer tissues and adjacent normal tissues (p < 0.001). (ns, no significant difference, · p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001)
TABLE 1.
The multivariate analyses of CAV1 and clinicopathologic features in HNSCC cancer tissues (n = 59)
| Beta | HR (95% CI for HR) | Wald test | p‐value | |
|---|---|---|---|---|
| Gender | −0.36 | 0.7 (0.25–1.9) | 0.48 | 0.49 |
| Age | 0.0082 | 1 (0.96–1.1) | 0.12 | 0.73 |
| Cancer size | 0.88 | 2.4 (1.2–4.8) | 6.3 | 0.012 |
| pN state | 1.3 | 3.5 (1.3–9.7) | 5.8 | 0.016 |
| CAV1 | 1.1 | 2.9 (0.91–9.1) | 3.2 | 0.071 |
| Histological grade | 0.48 | 1.6 (0.92–2.9) | 2.8 | 0.096 |
Taken together, these data indicated that CAV1 expression was upregulated in HNSCC tissues and was correlated with poor clinical outcomes in HNSCC patients (p < 0.001).
3.3. Higher expression of CAV1 inhibits ferroptosis of cancer cells in HNSCC
Among HNSCC cancer cell lines, CAL27 showed highest expression of CAV1 (Figure S1C). The siRNA knockdown assays were performed in HN6 and CAL27 cells using three different siRNA sequences. Western blot analysis demonstrated that the expression of CAV1 was significantly reduced after transfection of CAV1‐siRNA into cancer cells (Figure S1D).
To explore whether CAV1 could inhibit ferroptosis of cancer cells in HNSCC, the levels of ROS and Fe2+ in cells were detected, which could indicate the status of ferroptosis. The fluorescence of DCF was stronger in cells transfected with siCAV1 than in the control group (Figure 3A). The level of ROS was measured by fluorescence of DCF, and a decreased ROS in cells indicated the inhibition of ferroptosis. In addition, FerroOrange was used to detect the level of Fe2+ in cells, which possessed a positive correlation with ferroptosis. The results suggested that the concentration of Fe2+ was increased after CAV1 knocking down in cancer cells (Figure 3B), which indicated that ferroptosis was negatively regulated by CAV1. Ferroptosis induction is associated with the increased expression of ferroptosis marker genes such as ACSL4, NOX1, GPX4 and FTH1. When ferroptosis is induced, the expressions of GPX4 and FTH1 are reduced, and the level of ACSL4 and NOX1 increased. 10 , 11 , 12 , 13 , 14 The changes in ferroptosis‐related genes were detected by real‐time PCR assays, which meant the expression of GPX4 and FTH1 was decreased in CAV1 knockdown cells than that in the control group, while ACSL4 and NOX1 expression was higher in the siCAV1 group (Figure 3C).
FIGURE 3.
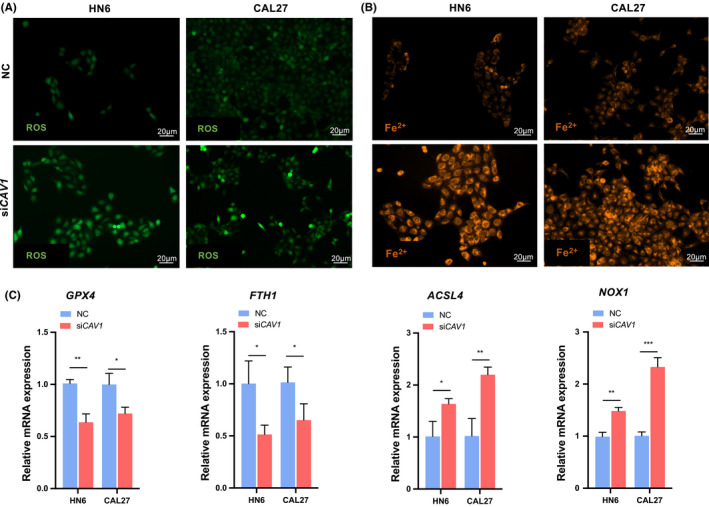
High expression of CAV1 inhibits ferroptosis of cancer cells in HNSCC. A. ROS evaluation assay was performed to detect the level of ROS in cells. DCF showed green fluorescence, and it could measure the level of ROS. Scale bar: 20 μm. B. Orange fluorescence labelled the free Fe2+ in cells. Scale bar: 20 μm. C. Quantitative real‐time PCR measured the expression of relative genes of ferroptosis: GPX4, FTH1, ACSL4 and NOX1. (ns, no significant difference, · p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001)
In conclusion, these results suggested that increased expression of CAV1 could inhibit ferroptosis of cancer cells in HNSCC.
3.4. Ferroptosis suppressor CAV1 promotes the proliferation and migration of HNSCC
To study the phenotype that caused by CAV1 upregulated in HNSCC, we performed a series of experiments in vitro. CCK‐8 assay was performed to analyze cell proliferation and the results showed a reduced growth rate of HN6 and CAL27 cells after knocking down CAV1 (Figure 4A). Compared with the control groups, fewer cancer cells migrated through the membrane in the CAV1 knockdown group compared with those of the control group (Figure 4B). In addition, the colony formation capacities of HN6 and CAL27 cells were significantly reduced after CAV1 knocking down (Figure 4C). Wound‐healing assays were performed to investigate the migration ability and we found that after knocking down CAV1, the number of migrated cells was smaller than that in the control group (Figure 4D). Taken together, we concluded that CAV1 might promote the proliferation, invasion and migration of HNSCC cells by inhibiting ferroptosis in cancer cells.
FIGURE 4.
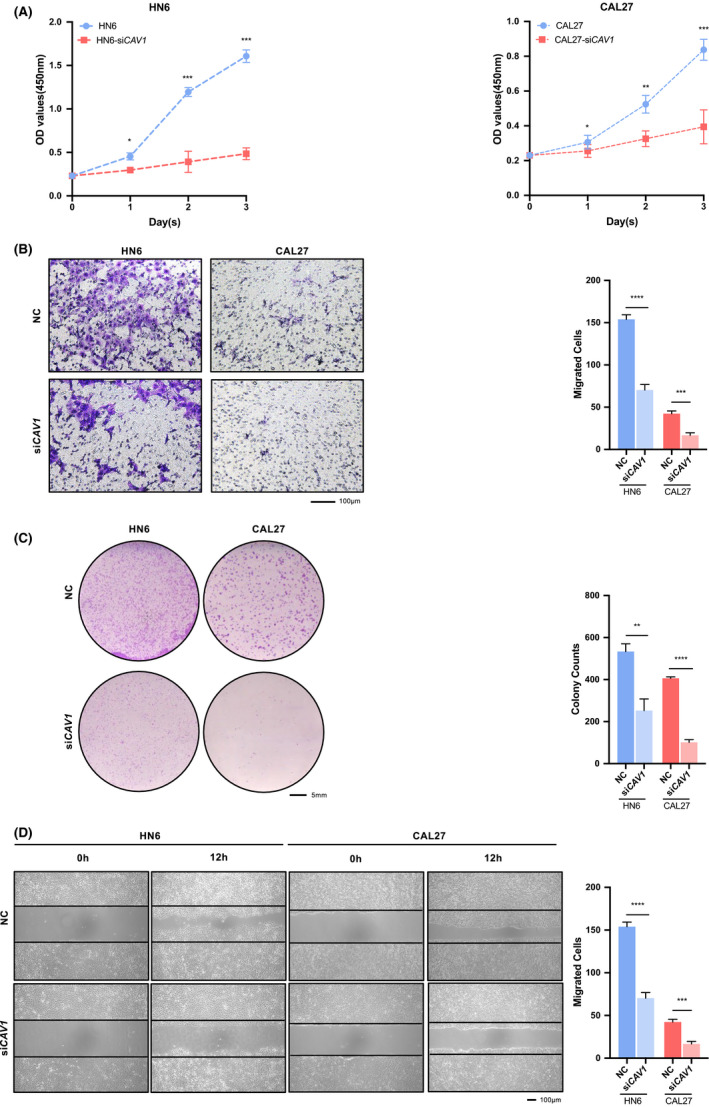
CAV1 promotes the proliferation and migration of HNSCC cells. A. CCK‐8 assay showed that proliferation was reduced in HN6 and CAL27 cell lines after being transfected with siCAV1. B. Transwell assays (Scale bar = 100μm). C. Colony formation assays (Scale bar = 5mm). D. Wound‐healing assays (Scale bar = 100μm). (ns, no significant difference, · p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001)
4. DISCUSSION
The balance of cell death is of vital importance, diseases as cancers occurs when the balance is broken. In cancer cells, the process of cell death is always suppressed. Regulated cell death includes apoptosis, necroptosis, pyroptosis and ferroptosis. 4 However, the effectiveness of apoptosis in cancers is limited due to the acquired or intrinsic resistance of cancer cells. 15 Therefore, one kind of regulated cell death, ferroptosis, is being explored as an alternative way to eradicate apoptosis‐resistant cancer cells. 16 Ferroptosis, a novel form of nonapoptotic regulated cell death, is characterized as an increase in intracellular free iron and the accumulation of lipid peroxide. 17 , 18 Many anticancer drugs provoke ferroptosis, which can eliminate cancer cells and limit the survival of drug‐resistant clones, and these findings have prompted new ideas for cancer therapy. We focused on identifying a protein that could inhibit ferroptosis and attempted to find new targets for diagnosis and therapy. The study indicated that CAV1 was highly expressed in HNSCC and could inhibit the process of ferroptosis, causing dysregulated cell death and promoting the growth of cancer cells. Therefore, we suspected that targeting CAV1 may reboot the ferroptosis of cancer cells, promote cell death and achieve better therapeutic effects.
HNSCC is an aggressive malignant cancer worldwide, and recent studies on ferroptosis have focused on exploring the related molecular mechanisms and probable therapeutic timeline. Recent study found that the regulation of the KDM5A‐MPC1 axis contributed to promoting cancer ferroptosis susceptibility. 19 GPX4 refers to glutathione peroxidase 4, which is a key protein that inhibits ferroptosis. The circKIF4A could facilitate the malignant progression of papillary thyroid cancers by sponging miR‐1231 and upregulating GPX4 expression. 20 Our research tried to explore the relationship between protein biomarkers and ferroptosis and explain the possible mechanism of cancer development in HNSCC. CAV1 is one of the major structural proteins of caveolae and is highly expressed in adipocytes, endothelial cells, pneumocytes, fibroblasts and cancer cells. 21 CAV1 acts as an oncogene in HNSCC, renal cancer, prostate cancer, etc. 22 , 23 However, studies on its function in HNSCC development are still rare. Nohata. 24 found that miR‐133a regulated CAV1 to promote cancer cell migration and invasion in HNSCC. Our results demonstrated that CAV1 was highly expressed in HNSCC, and its high level was related to poor prognosis of patients. By searching FerrDb, it was found that CAV1 was a ferroptosis suppressor protein. The following experiments proved this suppressor role in HNSCC, showing that CAV1 could inhibit the process of ferroptosis in cancer cells. In addition, the absence of CAV1 promotes ferroptosis in cancer cells and inhibits the progression of cancer development.
The balance of iron in the cancer microenvironment (TME) is associated with cancer progression. Cancer cells, immunocytes, and cancer‐associated fibroblasts (CAFs), etc. are crucial components of the TME, and the metabolism of iron in these cells affects the development of cancers. Some reports showed that autophagy‐dependent ferroptosis could drive macrophage polarization to the M2 subtype in pancreatic ductal adenocarcinoma, causing immunosuppression. 25 MiR‐522 secreted by CAFs suppresses ferroptosis and promotes chemoresistance in gastric cancer. 2 Numerous iron ions support the unlimited proliferation of cancer cells, and the disorder of ferroptosis regulation might give rise to cancer progression. Our experiments showed that upregulated CAV1in HNSCC induced the accumulation of free Fe2+ in cancer cells, which accelerated cell proliferation. Recently, a number of studies have focused on the related regulation of ferroptosis and proved that the progression of ferroptosis is related to lipid metabolism, energy metabolism, epigenetics, and molecular mechanisms. In Doll's. 12 research, ACSL4, an essential component for ferroptosis execution, could shape the cellular lipid composition and dictate ferroptosis sensitivity in breast cancer cells. lncRNA NEAT1 regulated the sensitivity of ferroptosis in non‐small‐cell lung cancer. 26 The cancer suppressor gene p53 could mediate ferroptosis of cancer cells in the ROS response. 27 Similarly, our results showed that cancer cells that expressing high levels of CAV1 had increased expressions of ACSL4 and NOX1 and reduced expressions of GPX4 and FTH1, which promoted the production of ROS and reduced sensitivity to ferroptosis. However, the study only explored the effect of CAV1 on cancer cells, but its influence on immunocytes is unknown and needs further exploration.
With the increased study of ferroptosis, the idea that inducing ferroptosis of cancer cells could serve as a therapy strategy has attracted people's attention. Currently, some agents that can induce ferroptosis have been used in the clinic. Ferroptosis‐inducing agents could enhance the efficiency of therapy in melanoma by changing ferroptosis sensitivity. 28 In addition, artesunate selectively killed head and neck cancer cells but not normal cells by inducing iron‐dependent, ROS‐accumulated ferroptosis. 29 Moreover, agents packed by nanoparticles could accurately target cancer cells. However, the complexity of the TME makes the therapy of cancers full of uncertainty, and a single therapy method cannot achieve sufficient efficiency. In this study, we demonstrated a close relationship between CAV1 and ferroptosis, together with their function in cancer progression, which might be appropriate target for HNSCC therapy. Since CAV1 could inhibit ferroptosis, the high level of CAV1 in patients’ cancer tissues might predict the high possibility of ferroptosis resistance, and the effect of ferroptosis‐inducing agents could be limited. Therefore, we hypothesize that the sensitivity of ferroptosis‐inducing agents could be analyzed by measuring the expression level of CAV1, and combined therapy of ferroptosis‐inducing agents with anti‐CAV1 treatment might achieve better efficiency. A number of chemotherapeutics have been approved by the Food and Drug Administration (FDA) to treat HNSCC, but some patients appear to be chemo‐resistant, which affects the efficiency of therapy. GPX4 is a regulator of ferroptosis, and its inhibition can render therapy‐resistant cancer cells susceptible to ferroptosis. 30 Our experiments showed that the knockdown of CAV1 could reduce the expression of GPX4. Therefore, it was suggested that the expression of CAV1 might predict the possibility of chemoresistance, and combined therapy might improve the efficiency. However, further exploration is needed to test the prognostic value of CAV1 and its potential as a therapeutic target by mediating ferroptosis. In addition, the specific relationship between CAV1 and ferroptosis and their underlying mechanisms require further investigation.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Wantao Chen, Ming Yan designed the project. Tingwei Lu and Zhen Zhang performed the experiments, data statistics and bioinformatics analysis. Xinhua Pan and Tingwei Lu collected the clinical samples. Jianjun Zhang, Xu Wang provided suggestions for the project. Miaochen Wang and Huasheng Li kindly provided support during animal experiments and cytologic assays. Tingwei Lu and Zhen Zhang wrote the manuscript. All the authors read and approved the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jop.13267.
Supporting information
Fig S1
ACKNOWLEDGMENTS
This work was supported by the Nature Science Foundation of China (81672829 and 81874126), Shanghai Municipal Science and Technology Commission Funded Project (18DZ2291500), and SJTU Trans‐med Awards Research (WF540162615).
Lu T, Zhang Z, Pan X, et al. Caveolin‐1 promotes cancer progression via inhibiting ferroptosis in head and neck squamous cell carcinoma. J Oral Pathol Med. 2022;51:52–62. doi: 10.1111/jop.13267
Tingwei Lu, Zhen Zhang contributed equally to this work.
[Correction added on 5 January 2022, after first online publication: Author Ming Yan has been added as corresponding author.]
Contributor Information
Ming Yan, Email: yanming8012@126.com.
Wantao Chen, Email: chenwantao196323@sjtu.edu.cn.
DATA AVAILABILITY STATEMENT
The transcriptome data is from TCGA HNSCC cohort.
REFERENCES
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 2. Zhang H, Deng T, Liu R. et al. CAF secreted miR‐522 suppresses ferroptosis and promotes acquired chemo‐resistance in gastric cancer. Mol Cancer. 2020;19(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reddel RR. The role of senescence and immortalization in carcinogenesis. Carcinogenesis. 2000;21(3):477‐484. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem Sci. 2016;41(3):274‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stockwell BR, Friedmann Angeli JP, Bayir H. et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ketteler J, Klein D. Caveolin‐1, cancer and therapy resistance. Int J Cancer. 2018;143(9):2092‐2104. [DOI] [PubMed] [Google Scholar]
- 8. Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin‐1 in oncogenically transformed cells abrogates anchorage‐independent growth. J Biol Chem. 1997;272(26):16374‐16381. [DOI] [PubMed] [Google Scholar]
- 9. Xu L, Qu X, Li H, et al. Src/caveolin‐1‐regulated EGFR activation antagonizes TRAIL‐induced apoptosis in gastric cancer cells. Oncol Rep. 2014;32(1):318‐324. [DOI] [PubMed] [Google Scholar]
- 10. Liang C, Zhang X, Yang M, Dong X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater. 2019;31(51):1904197. [DOI] [PubMed] [Google Scholar]
- 11. Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid Peroxidation‐Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr Top Microbiol Immunol. 2017;403:143‐170. [DOI] [PubMed] [Google Scholar]
- 12. Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Y, Zhao Y, Yang HZ, Wang YJ, Chen Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci Rep. 2021;41(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian Y, Lu J, Hao X. et al. FTH1 Inhibits Ferroptosis Through Ferritinophagy in the 6‐OHDA Model of Parkinson's Disease. Neurotherapeutics. 2020;17(4):1796‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su Z, Yang Z, Xie L, DeWitt JP, Chen Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016;23(5):748‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35(6):830‐849. [DOI] [PubMed] [Google Scholar]
- 17. Li J, Cao F, Yin HL. et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mou Y, Wang J, Wu J. et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. You JH, Lee J, Roh JL. Mitochondrial pyruvate carrier 1 regulates ferroptosis in drug‐tolerant persister head and neck cancer cells via epithelial‐mesenchymal transition. Cancer Lett. 2021;507:40‐54. [DOI] [PubMed] [Google Scholar]
- 20. Chen W, Fu J, Chen Y, et al. Circular RNA circKIF4A facilitates the malignant progression and suppresses ferroptosis by sponging miR‐1231 and upregulating GPX4 in papillary thyroid cancer. Aging (Albany NY). 2021;13(12):16500‐16512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen D, Che G. Value of caveolin‐1 in cancer progression and prognosis: Emphasis on cancer‐associated fibroblasts, human cancer cells and mechanism of caveolin‐1 expression (Review). Oncol Lett. 2014;8(4):1409‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang G, Goltsov AA, Ren C, et al. Caveolin‐1 upregulation contributes to c‐Myc‐induced high‐grade prostatic intraepithelial neoplasia and prostate cancer. Mol Cancer Res. 2012;10(2):218‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun J, Lu Y, Yu C, et al. Involvement of the TGF‐β1 pathway in caveolin‐1‐associated regulation of head and neck tumor cell metastasis. Oncol Lett. 2020;19(2):1298‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nohata N, Hanazawa T, Kikkawa N, et al. Caveolin‐1 mediates tumor cell migration and invasion and its regulation by miR‐133a in head and neck squamous cell carcinoma. Int J Oncol. 2011;38(1):209‐217. [PubMed] [Google Scholar]
- 25. Dai E, Han L, Liu J, et al. Autophagy‐dependent ferroptosis drives tumor‐associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16(11):2069‐2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu H, Liu A. Long non‐coding RNA NEAT1 regulates ferroptosis sensitivity in non‐small‐cell lung cancer. J Int Med Res. 2021; 49(3):300060521996183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang L, Kon N, Li T, et al. Ferroptosis as a p53‐mediated activity during tumor suppression. Nature. 2015;520(7545):57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsoi J, Robert L, Paraiso K, et al. Multi‐stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug‐Induced Iron‐Dependent Oxidative Stress. Cancer Cell. 2018;33(5):890‐904.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin‐resistant head and neck cancer cells to artesunate‐induced ferroptosis. Redox Biol. 2017;11:254‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor‐induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
The transcriptome data is from TCGA HNSCC cohort.


