Summary
Studies on the conditional life expectancy of patients with chronic myeloid leukaemia (CML) are lacking. Using data from the Netherlands Cancer Registry, we examined the life expectancy of patients with CML in the Netherlands diagnosed during 1989–2018. As of the early 2010s, the life expectancy of patients with CML who survived several years after diagnosis came narrowly close to the general population’s life expectancy, regardless of age. This finding can essentially be ascribed to the introduction and broader application of tyrosine kinase inhibitors (TKIs) and provide optimism to patients with CML who can look forward to a near‐normal life expectancy in a modern TKI era.
Keywords: chronic myeloid leukaemia, epidemiology, mathematical modelling, imatinib, leukaemia
Introduction
Around the turn of the 21st century, the management of chronic myeloid leukaemia (CML) was revolutionised with the advent of the first‐generation breakpoint cluster region‐Abelson (BCR‐ABL) tyrosine kinase inhibitor (TKI) imatinib. 1 Thereafter, subsequent generations of BCR‐ABL TKIs, that is, dasatinib, nilotinib, bosutinib, and ponatinib, have entered the therapeutic realm of CML. 2 These monumental therapeutic breakthroughs dramatically improved the population‐level survival of patients with CML. Indeed, population‐based studies mainly covering the 2000s demonstrated that the overall 5‐year relative survival rate was approximately 80%, 3 , 4 with apparent differences between age groups and geographic regions. 3 , 4 , 5 , 6
While relative survival in population‐based cancer research reflects excess mortality associated with a cancer diagnosis within a fixed period (e.g. up to 5 years after diagnosis, that is, 5‐year relative survival), 7 it does not quantify the average life expectancy after a cancer diagnosis (i.e. the average expected number of life‐years remaining). 7 A population‐based study from Sweden was the first to assess the life expectancy of patients with CML compared to the life expectancy of the general population. 8 The most striking discovery of that study, including patients diagnosed during 1973–2013, was that the life expectancy of patients with CML diagnosed in 2013 closely approximates the general population’s life expectancy, irrespective of age and sex.
In the present study, we aimed to investigate how the life expectancy and loss of life expectancy (LOLE) have evolved among patients with CML diagnosed in the Netherlands during 1989–2018. In addition to the Swedish study, we estimated the conditional LOLE to assess time trends in the average number of life‐years lost among patients with CML who survived several years after diagnosis.
Established in 1989, the nationwide Netherlands Cancer Registry (NCR) covers more than 95% of all newly diagnosed malignancies in the Netherlands. 9 The NCR builds on comprehensive case notification through the Nationwide Histopathology and Cytopathology Data Network and Archive and the National Registry of Hospital Discharges (i.e. inpatient and outpatient discharges). Information on sex and birth and diagnosis dates, as well as disease stage, topography, and morphology, is routinely recorded in the NCR via retrospective medical records review by trained NCR registrars. The Privacy Review Board of the NCR approved the use of anonymous data for this study.
We selected patients with CML aged ≥18 years and diagnosed between 1 January 1989 and 31 December 2018, with survival follow‐up through to 31 December 2020 from the NCR using the International Classification of Diseases for Oncology morphology codes 9863 and 9875. Newly diagnosed patients were included irrespective of the phase of the disease. All patients were followed for survival from the date of diagnosis to death, emigration, or end of follow‐up (31 December 2020), whichever occurred first. Patients diagnosed at autopsy were excluded.
We assessed three survival measures. The LOLE is the first measure and quantifies the difference between the life expectancy of patients and the general population, of which the latter is matched to the patients by age, sex, and calendar year. This measure reflects the average number of life‐years lost due to a cancer diagnosis. Secondly, given the reliance of LOLE on age, the LOLE can be expressed as the proportional LOLE (PLOLE). This measure is more suitable in making comparisons between groups with varying population life expectancy. The PLOLE is calculated by dividing the LOLE by the life expectancy of the general population. Lastly, the LOLE was estimated conditional on surviving a particular time after diagnosis (i.e. conditional LOLE). The conditional LOLE was estimated for patients who survived each additional year after diagnosis up to 5 years after diagnosis.
The general populations expected survival was estimated using Dutch population life tables, stratified by age, sex, and calendar year. The survival measures were modelled using restricted cubic splines within the framework of a flexible parametric relative survival model. 10 The outcomes were presented by year of diagnosis for four ages at diagnosis (i.e. 55, 65, 75, and 85 years). Full details about the statistical analyses are presented in the supplementary methods. All analyses were performed using Stata/SE version 16·1 (Stata Corp., College Station, TX, USA).
Our analytical cohort included 4702 adult patients with CML diagnosed during 1989–2018 (44% females; median age, 61 years; 55% deaths; Table SI). [Correction added on 13 January 2022, after first online publication: The word “males” in the preceding sentence was corrected to “females” in this version.]
The life expectancy of patients with CML increased between 1989 and 2018, irrespective of age and sex (Fig 1A). The increase was most pronounced for younger patients (i.e. aged 55 and 65 years) between 1989 and 2005 (Fig 1A). Thereafter, the increase was more gradual. The increase in life expectancy among older patients (i.e. aged 75 and 85 years) was not as pronounced as younger patients (Fig 1A). The life expectancy of patients with CML across all age groups largely approached that of the general population towards the end of the study period (Fig 1A).
Fig 1.
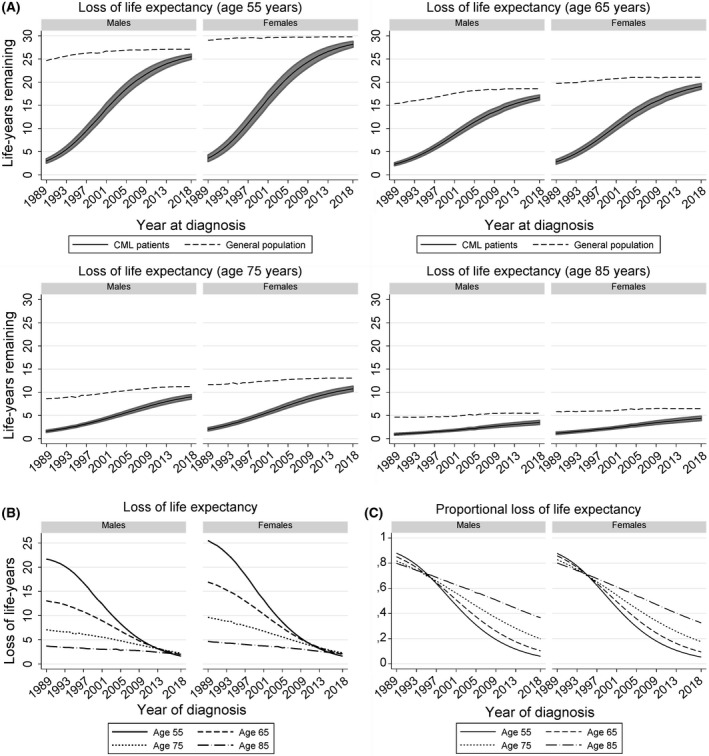
Trends in various survival measures of patients with chronic myeloid leukaemia diagnosed in the Netherlands between 1989 and 2018. (A) depicts the life expectancy of the general population (dashed lines) and patients with chronic myeloid leukaemia (CML; solid lines) by year of diagnosis for four ages, stratified by sex. The shaded area around the life expectancy of patients with CML portrays the 95% confidence interval for the point estimates, which was obtained using the Delta method. (B) presents the loss of life expectancy of patients with CML by year of diagnosis for four ages, stratified by sex. (C) presents the proportional loss of life expectancy of patients with CML by year of diagnosis for four ages, stratified by sex.
There was a substantial age differential in the LOLE among patients diagnosed in the early 1990s (Fig 1B). Due to the striking increase in CML patients’ life expectancy over time, especially among younger patients, this differential became less conspicuous since the mid‐2000s, owing to a marked decrease in the LOLE among younger patients (Fig 1B). The LOLE among older patients decreased less markedly over time because older individuals generally have fewer remaining life‐years (Fig 1B). The LOLE of patients across the adult age continuum diagnosed in 2018 ranged between 1·3 and 2·8 years, depending on age and sex (Table SII).
The PLOLE was ∼80% in 1990, irrespective of age and sex (Fig 1C; Table SII). Overall, the PLOLE decreased over time, particularly after the end‐1990s, of which the decrease was most pronounced among patients aged 55 and 65 years (Fig 1C). In 2018, patients aged 55 and 65 years lost the lowest proportion of their life expectancy (between 5·4% and 10·4%) compared to patients aged 75 and 85 years (between 17·6% and 36·6%; Fig 1C; Table SII).
Overall, the conditional LOLE decreased with each additional year survived after diagnosis, irrespective of age and sex (Fig 2). In 1990 and 2000, there was a substantial age differential in the conditional LOLE. In contrast, the conditional LOLE of patients diagnosed in 2018 was similar across the age groups and, more importantly, verges upon the general populations life expectancy (Fig 2). More specifically, the 5‐year conditional LOLE of patients across the adult age continuum diagnosed in 2018 ranged between 0·1 and 0·8 years, depending on age and sex.
Fig 2.
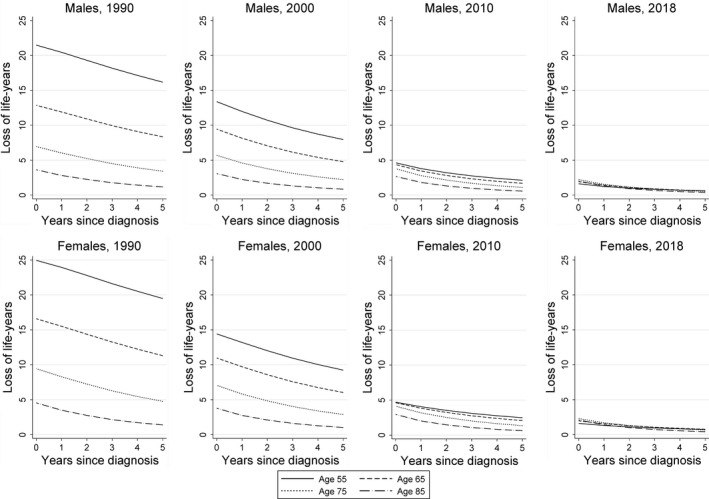
The conditional loss of life expectancy of patients with chronic myeloid leukaemia diagnosed in the Netherlands. The conditional loss of life expectancy is presented according to four age groups at diagnosis, stratified by sex and four selected calendar periods of diagnosis.
The trends in patients with CML life expectancy in the present study follow the two major trends objectified in the Swedish study. 8 Besides, the present study extends on the Swedish study by including more contemporary diagnosed patients and estimating the conditional LOLE. The first trend was that the patients with CML life expectancy improved over time, irrespective of age and sex. This increase was most pronounced between the 1990s and mid–late‐2000s among younger patients. The increase among older patients initially lagged. The improvement since the 1990s might be accounted for by the broader use of interferon‐alpha (with or without cytarabine) and stem cell transplantation (SCT) and augmented supportive care measures. 3 , 4 Of note, the application of first‐line SCT in the Netherlands was higher in the pre‐TKI era (8·9% in 1989–2000) compared to the post‐TKI era (4·8% and 2·4% in 2001–2010 and 2011–2018 respectively, Table SI). The improvement since the 2000s can fundamentally be attributed to the introduction and broader application of imatinib, followed by subsequent generations of BCR‐ABL TKIs in the mid–late 2000s. However, the broad application of BCR‐ABL TKIs following its introduction for routine use lagged in older patients, 4 , 5 which may explain the survival improvement occurring later than in younger patients.
The second trend, and perhaps the most reassuring one, was that the life expectancy of patients with CML largely approached that of the general population towards the end of the study period. As of 2010, the life expectancy of patients alive 5 years after diagnosis verges upon the life expectancy of the general population. Nevertheless, small excess mortality persisted. The overall excess mortality in contemporary patients diagnosed with CML might originate from second primary malignancies, 11 cardiovascular toxicities associated with newer TKI generations, 3 comorbidities, 12 a CML diagnosis in an advanced stage, 13 and decreased treatment adherence 2 and monitoring. 14
The improvement in the life expectancy of patients with CML occurred later in the Netherlands than in Sweden. 8 This finding was somewhat unanticipated, given that both countries provide all their residents with equal access to public healthcare services without out‐of‐pocket expenses for the patient, irrespective of their socioeconomic position. The earlier and broader uptake of BCR‐ABL TKIs in Sweden, compared to the Netherlands, might explain the lag. 4 , 5 , 13
The strength of our present study lies in the utilisation of a well‐established and long‐running nationwide population‐based registry with a relatively large number of patients that enabled us to estimate the life expectancy of patients using flexible parametric relative survival models.
Limitations mainly pertain to the lack of detailed patient (e.g. socioeconomic status and performance status), tumour (e.g. the phase of CML), and treatment characteristics (e.g. type of TKI) across most of the registry to estimate the life expectancy according to these characteristics. As information on the phase of the disease and prognostic indices was not available throughout the entire registry, we could not assess whether more recent study periods might be more enriched with patients with CML whose disease was diagnosed in an asymptomatic phase, a phenomenon referred to as lead‐time bias. However, lead‐time bias in CML, especially in light of very effective treatment strategies, might have only marginally affected the survival estimates. Further, the estimation of life expectancy is subject to extrapolation because not all deaths could be observed during the follow‐up period. However, it has been demonstrated that the estimations with comparatively short follow‐up length are accurate using flexible parametric models with the cure assumption. 15 Lastly, no details on the causes of death are known. Notwithstanding these limitations, the present study provides patients and physicians with important information about the expected remaining life‐years after a CML diagnosis from a historical and contemporary perspective.
In summary, our present study findings provide optimism for patients with CML who can look forward to a near‐normal life expectancy in an era with contemporary approaches to CML management. Concurrently, the comparatively small excess mortality emphasises a continued desideratum to optimise the use of current treatment approaches, advance therapeutic options for patients with adverse disease biology, and mitigate treatment toxicity.
Conflict of interest
The authors have no competing interests.
Author contributions
Avinash G. Dinmohamed designed the study; Carolien C.H.M. Maas analysed the data; David van Klaveren provided statistical consulting; Otto Visser was responsible for the collected data; Carolien C.H.M. Maas wrote the manuscript with contributions from all authors, who also interpreted the data, and read, commented on, and approved the final version of the manuscript.
Supporting information
Fig S1. The observed survival curves when varying the degrees of freedom to model the continuous variables.
Fig S2. The observed survival curve when varying the degrees of freedom to model the baseline hazard.
Fig S3. The observed survival curve when varying the degrees of freedom to model the time‐varying effects.
Table SI. Demographic characteristics of patients with chronic myeloid leukaemia (CML) in the Netherlands in the period 1989–2018.
Table SII. The life expectancy of the general population and patients with chronic myeloid leukaemia (CML), the loss of life expectancy of patients with CML, and the proportional loss of life expectancy of patients with CML. These survival measures, with associated 95% confidence intervals, are presented for four selected calendar years and ages at diagnosis in the Netherlands.
[Correction added on 13 January 2022, after first online publication: The word “Males” in the first column of the online Supplemental Table SI was corrected to “Females” in this version.]
Acknowledgements
The authors would like to thank the registration clerks of the Netherlands Cancer Registry (NCR) for their dedicated data collection. The nationwide population‐based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organisation (IKNL).
References
- 1. Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR‐ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. [DOI] [PubMed] [Google Scholar]
- 2. Jabbour E, Makenbaeva D, Lingohr‐Smith M, Lin J. Use of real‐world claim databases to assess prevalence of comorbid conditions relevant to the treatment of chronic myelogenous leukemia based on national comprehensive network treatment guidelines. Clin Lymphoma Myeloma Leuk. 2015;15:797–802. [DOI] [PubMed] [Google Scholar]
- 3. Björkholm M, Ohm L, Eloranta S, Derolf A, Hultcrantz M, Sjöberg J, et al. Success story of targeted therapy in chronic myeloid leukemia: a population‐based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29:2514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thielen N, Visser O, Ossenkoppele G, Janssen J. Chronic myeloid leukemia in the Netherlands: a population‐based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. Eur J Haematol. 2016;97:145–54. [DOI] [PubMed] [Google Scholar]
- 5. Ector GI, Visser O, Westerweel PE, Janssen JJ, Blijlevens NM, Dinmohamed AG. Primary therapy and relative survival among elderly patients with chronic myeloid leukemia: a population‐based study in the Netherlands, 1989–2017. Leukemia. 2020;34:3408–12. [DOI] [PubMed] [Google Scholar]
- 6. Sant M, Minicozzi P, Mounier M, Anderson LA, Brenner H, Holleczek B, et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE‐5, a population‐based study. Lancet Oncol. 2014;15:931–42. [DOI] [PubMed] [Google Scholar]
- 7. Eloranta S, Smedby KE, Dickman PW, Andersson TM. Cancer survival statistics for patients and healthcare professionals – a tutorial of real‐world data analysis. J Intern Med. 2020;289:12–28. [DOI] [PubMed] [Google Scholar]
- 8. Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7. [DOI] [PubMed] [Google Scholar]
- 9. Schouten LJ, Höppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol. 1993;22:369–76. [DOI] [PubMed] [Google Scholar]
- 10. Andersson TM, Rutherford MJ, Lambert PC. Illustration of different modelling assumptions for estimation of loss in expectation of life due to cancer. BMC Med Res Methodol. 2019;19:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sasaki K, Kantarjian HM, O’Brien S, Ravandi F, Konopleva M, Borthakur G, et al. Incidence of second malignancies in patients with chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Int J Hematol. 2019;109:545–52. [DOI] [PubMed] [Google Scholar]
- 12. Saußele S, Krauß MP, Hehlmann R, Lauseker M, Proetel U, Kalmanti L, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood. 2015;126:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Höglund M, Sandin F, Hellström K, Björeman M, Björkholm M, Brune M, et al. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population‐based Swedish CML registry. Blood. 2013;122:1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geelen IG, Thielen N, Janssen JJ, Hoogendoorn M, Roosma TJ, Willemsen SP, et al. Treatment outcome in a population‐based, 'real‐world' cohort of patients with chronic myeloid leukemia. Haematologica. 2017;102:1842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersson TM, Dickman PW, Eloranta S, Lambe M, Lambert PC. Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Stat Med. 2013;32:5286–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. The observed survival curves when varying the degrees of freedom to model the continuous variables.
Fig S2. The observed survival curve when varying the degrees of freedom to model the baseline hazard.
Fig S3. The observed survival curve when varying the degrees of freedom to model the time‐varying effects.
Table SI. Demographic characteristics of patients with chronic myeloid leukaemia (CML) in the Netherlands in the period 1989–2018.
Table SII. The life expectancy of the general population and patients with chronic myeloid leukaemia (CML), the loss of life expectancy of patients with CML, and the proportional loss of life expectancy of patients with CML. These survival measures, with associated 95% confidence intervals, are presented for four selected calendar years and ages at diagnosis in the Netherlands.
[Correction added on 13 January 2022, after first online publication: The word “Males” in the first column of the online Supplemental Table SI was corrected to “Females” in this version.]


