Abstract
Objective
To investigate the safety and effectiveness of direct oral anticoagulants (DOAC) versus vitamin K antagonists (VKA) after recent stroke in patients with atrial fibrillation (AF) aged ≥85 years.
Methods
Individual patient data analysis from seven prospective stroke cohorts. We compared DOAC versus VKA treatment among patients with AF and recent stroke (<3 months) aged ≥85 versus <85 years. Primary outcome was the composite of recurrent stroke, intracranial hemorrhage (ICH) and all‐cause death. We used simple, adjusted, and weighted Cox regression to account for confounders. We calculated the net benefit of DOAC versus VKA by balancing stroke reduction against the weighted ICH risk.
Results
In total, 5,984 of 6,267 (95.5%) patients were eligible for analysis. Of those, 1,380 (23%) were aged ≥85 years and 3,688 (62%) received a DOAC. During 6,874 patient‐years follow‐up, the impact of anticoagulant type (DOAC versus VKA) on the hazard for the composite outcome did not differ between patients aged ≥85 (HR≥85y = 0.65, 95%‐CI [0.52, 0.81]) and < 85 years (HR<85y = 0.79, 95%‐CI [0.66, 0.95]) in simple (pinteraction = 0.129), adjusted (pinteraction = 0.094) or weighted (pinteraction = 0.512) models. Analyses on recurrent stroke, ICH and death separately were consistent with the primary analysis, as were sensitivity analyses using age dichotomized at 90 years and as a continuous variable. DOAC had a similar net clinical benefit in patients aged ≥85 (+1.73 to +2.66) and < 85 years (+1.90 to +3.36 events/100 patient‐years for ICH‐weights 1.5 to 3.1).
Interpretation
The favorable profile of DOAC over VKA in patients with AF and recent stroke was maintained in the oldest old. ANN NEUROL 2022;91:78–88
Atrial fibrillation (AF) becomes more prevalent with increasing age, and both are independent risk factors for ischemic stroke. 1 As the population ages, the number of patients aged 85 years and older – often termed the oldest old – suffering AF‐related ischemic stroke is growing. 2
In the current guidelines, 3 direct oral anticoagulants (DOAC) are recommended in patients with AF for recurrent stroke prevention in preference to vitamin K antagonists (VKA) based on the results of the pivotal DOAC randomized controlled trials (RCTs). 4 However, it is less clear whether this preference can be generalized to include patients: (1) aged ≥85 years, who made up less than 5% of the RCTs population 5 , 6 , 7 ; or, (2) with recent ischemic stroke, who had been excluded from the RCTs for at least some weeks after stroke. 8 , 9
Facing the paucity of randomized evidence, many physicians are reluctant to prescribe DOAC to the oldest old due to assumed safety concerns due to clinical situations particularly prevalent in the oldest old (eg, altered DOAC pharmacokinetics in the presence of unstable or declining renal function, polypharmacy, frailty, malnutrition or reduced body weight), 10 , 11 especially for fear of intracranial hemorrhage (ICH). 12 , 13 Instead, they may favor VKA, 11 or withhold oral anticoagulant (OAC) treatment, even in patients who had had an ischemic stroke. 12 To bridge this evidence gap, systematically ascertained, standardized observational data – known as “real‐world” data – may be useful. 9 , 14
With these considerations in mind, we investigated the safety and effectiveness of DOAC compared to VKA in the oldest old with AF and a recent ischemic stroke. In the absence of randomized data, we used prospectively collected, individual patient data pooled within an international collaboration of cohort studies on the use of OAC following ischemic stroke in patients with AF.
Methods
Study Design, Patient Population and Data Collection
We used prospectively collected, individual patient data pooled from an established international collaboration of investigator‐initiated cohort studies of patients with AF, recent ischemic stroke or transient ischemic attack (TIA) and OAC treatment, as described previously. 15 This included 3 single‐center (Basel, Switzerland [NOACISP‐LONGTERM; NCT03826927] 16 ; Erlangen, Germany 17 ; Verona, Italy 18 ) and four multicenter cohorts (CROMIS‐2 [NCT02513316] 19 ; RAF 20 ; RAF‐DOAC 21 ; SAMURAI‐NVAF [NCT01581502] 22 , 23 ). The number of patients contributed by each cohort, as well as the recruitment period and follow‐up duration are summarized in Table S1.
In this study, we included consecutive patients with (1) an index recent (ie, <3 months) ischemic stroke or TIA (as defined previously 15 ); (2) nonvalvular AF (either known before index event or first diagnosed thereafter); (3) treatment with DOAC [ie, apixaban, dabigatran, edoxaban, rivaroxaban] or VKA [ie, phenprocoumon, warfarin], initiated within 3 months after the index event; and (4) prospectively ascertained follow‐up data for at least 3 months after the index event for the outcomes recurrent ischemic stroke, ICH and all‐cause death, defined as reported previously. 15 , 16 We excluded patients with missing follow‐up or information on age, those with OAC initiation >3 months or unknown, and those with outcome events occurring before OAC initiation.
Data were collected as described in prior research 15 using standardized forms with predefined variables and pooled in the coordinating center in Basel, Switzerland, where the analysis was performed. We used the following baseline variables: age; sex; National Institutes of Health Stroke Scale (NIHSS) score at baseline; dichotomized type of OAC after index event (DOAC or VKA); time to OAC initiation; concomitant antiplatelet use; history of ischemic stroke or TIA before the index event; history of ICH; diabetes mellitus; hypertension or dyslipidemia; the CHA2DS2‐VASc score (congestive heart failure, hypertension, age 65–74 or ≥ 75 years, diabetes mellitus, IS or TIA, vascular disease, sex) 24 ; estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration [CKD‐EPI] equation 25 and current smoking, as described previously. 15
Follow‐up data included length of follow‐up and absence or occurrence and timing of any of the following outcome events, which were defined in line with prior research 15 , 16 : (1) recurrent ischemic stroke (defined as new neurological deficits with a corresponding finding on neuroimaging); (2) ICH (defined as new neurological deficits with detection of intracranial bleeding on neuroimaging); and (3) all‐cause death, defined as every death irrespective of the cause and regardless of whether the cause was known or not.
Outcomes
The primary outcome was the time to occurrence of the composite of recurrent ischemic stroke, ICH and all‐cause death, in accordance with prior research. 15 , 16 Secondary outcomes were the time to occurrence of each of these outcomes separately.
Statistical Analysis
We stratified patients' characteristics by dichotomized age (≥85 vs. <85 years) and type of OAC (VKA vs. DOAC). We presented categorical data using frequencies and percentages and continuous data using the median and interquartile range (IQR) or mean and standard deviation (SD) as appropriate. We compared categorical variables using the χ 2 ‐test and continuous variables using the Mann–Whitney U test or t‐test as appropriate. We calculated the annualized rate of outcome events as the total of observed events divided by patient‐years of follow‐up for each outcome.
As for the main analysis, we modelled time to primary outcome using Cox proportional hazards regression. For this, we analyzed time to first event after OAC initiation, without considering further events. To assess the effect of age on the performance of OACs, we included type of OAC (DOAC vs. VKA), dichotomized age (≥85 vs. <85 years) and an interaction term between these variables as fixed effects in the model. A significant interaction would indicate that the association between type of OAC and the composite outcome is modified by age and therefore differs in the oldest old compared to their younger counterparts. The model included the participating cohort study as a stratum.
We fitted the model three times according to the predefined analysis plan: (i) simple model including type of OAC and dichotomized age, with and without interaction term; (ii) adjusted model taking into account the known prognostic importance of sex, NIHSS at baseline and CHA2DS2‐VASc score 24 (without the age and sex components, modified as in prior research 16 ); (iii) weighted model, using the stabilized inverse probability of treatment weights (SIPTW). 26 We constructed comparable treatment groups (DOAC vs. VKA) with regard to the following potentially outcome‐modifying variables, as in previous research 15 , 16 : sex, NIHSS at baseline, diabetes mellitus, hypertension, dyslipidemia, eGFR, history of prior stroke or TIA, history of ICH, current smoking, concomitant antiplatelet use and cohort study. We calculated the SIPTW using logistic regression and used robust standard errors for the 95% confidence intervals (CI) and p‐values of the weighted analysis. We imputed missing values in the covariables used in the adjusted and weighted models with simple imputation rules (ie, using the median / mean for continuous variables and the mode [most frequent category] for categorical variables), and report the rate of missing values for all variables. For all models we report the model‐based hazard ratio (HR) estimates along with the 95%‐CI and p‐values. We present the composite outcome data stratified to type of OAC and age group in weighted Kaplan–Meier curves using SIPTW (ie., by weighting each observation by its stabilized inverse probability of treatment with DOAC vs. VKA), 27 for which we show both the crude and weighted numbers at risk.
We performed the following secondary analyses:
(1) We fitted the Cox models (i) – (iii) described above separately for the individual outcomes recurrent ischemic stroke, ICH and death. To account for competing risks, for these analyses we fitted Cox proportional cause‐specific hazards models treating competing outcomes as censored observations. 28 With this approach, the competing outcomes influence the measure of association for the outcome of interest by removing at risk patient‐years from the risk set over time.
(2) We analyzed the net clinical benefit (NCB) of DOAC over VKA in patients aged ≥85 and < 85 years. We calculated the NCB by subtracting the weighted rate of excess ICH attributable to DOAC from the rate of excess ischemic stroke prevented by DOAC according to the following formula, as in prior research 29 , 30 , 31 , 32 :
The ICH weight reflects the more severe clinical impact in terms of death and disability of ICH relative to ischemic stroke, with values ranging from 1.5 to 3.1 according to previously published weights. 29 , 30 , 31 We performed the NCB analyses for the entire range of weights according to previously used methodology. 32 We corrected the rate of ischemic stroke and ICH for baseline imbalances between DOAC‐ and VKA‐treated patients using SIPTW as described above and report the NCB in events per 100 patient‐years along with 95%‐CI, calculated based on 1,000 bootstrap replications. For the NCB analyses we considered all patients but those with death as first outcome.
As sensitivity analyses, we repeated the main analysis for the primary (composite) outcome using age as:
(1) a categorical variable, dichotomized to ≥90 vs. <90 years. For this, we refitted all Cox models (i) – (iii) as described above.
(2) a continuous variable, using cubic B‐splines to model the non‐linear association between age and log‐hazard for the composite outcome. For this, we fitted the weighted model (iii) described above twice, with and without the interaction OAC type by age, and compared the two models using a likelihood ratio test. We graphically present the predicted rate of the composite outcome by age stratified to OAC type.
Statistical analyses were performed using R version 3.6.2 (2019‐12‐20) (R Core Team, 2019).
We conducted this study in accordance with the STROBE Statement for observational studies. 33
Ethics
The NOACISP‐LONGTERM registry and the current analysis of pooled individual patient data were approved by the ethics committee in Basel, Switzerland (EKNZ 2014–027; PB_2016_00662). Patients provided written informed consent for participation in NOACISP‐LONGTERM. The requirement for additional local ethical approval and patient informed consent differed among participating studies and was acquired by the local investigators as necessary. CROMIS‐2 was approved by the National Research Ethics Committee, London Queen Square and patients with capacity gave informed written consent. When patients could not consent, written consent from a proxy was obtained as defined by relevant local legislation. The SAMURAI‐NVAF registry and the current collaboration were approved by the ethics committee in the National Cerebral and Cardiovascular Center (M23‐18‐3 and M29‐077).
Results
In total, 5,984 of 6,267 (95.5%) patients were eligible for analysis. Information on OAC type was complete. Seven patients were excluded for missing age and 125 patients for missing follow‐up data (study flowchart in Fig 1).
FIGURE 1.
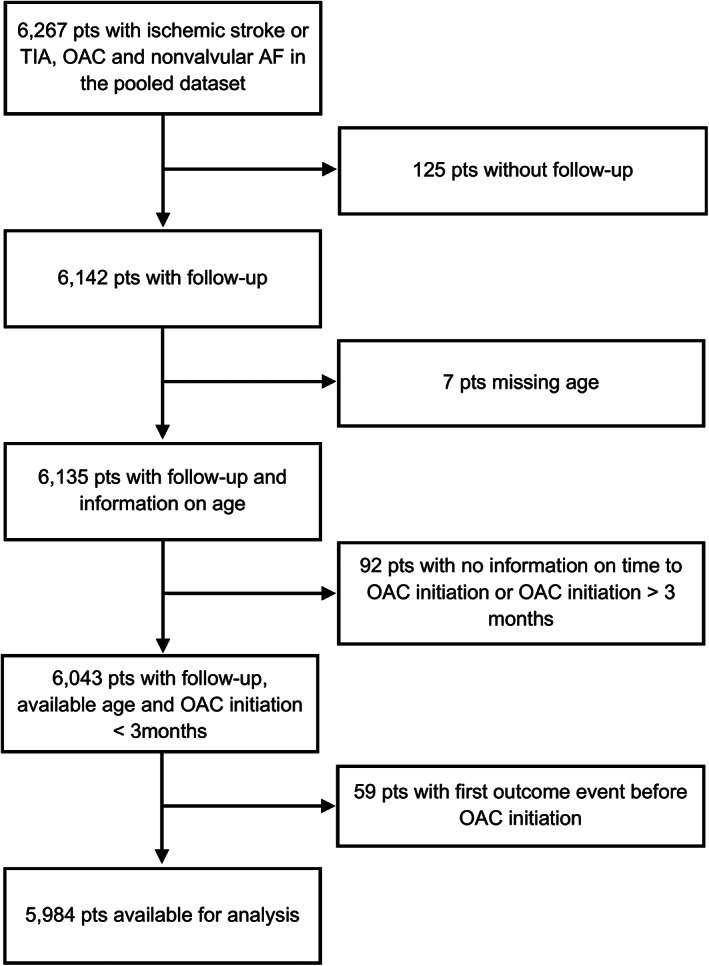
Study Flowchart
Baseline Characteristics
The index event was ischemic stroke in 5,593 patients (93.5%) and TIA in 391 (6.5%); 2,858 patients (47.8%) were female. The median age was 78 years (IQR 71–84, range 24–102); 1,380 (23.1%) patients were aged ≥85 years and 4,604 (76.9%) were aged <85 years. OAC was initiated at a median (IQR) of 5 (2–11) days after the index event with DOAC in 3,688 patients (61.6%) and VKA in 2,296 patients (38.4%).
Patients Aged ≥85 vs. <85 years
The baseline characteristics of the oldest old compared to their younger counterparts are displayed in Table 1. Patients aged ≥85 years were more commonly female and had higher NIHSS scores and lower eGFR, more often hypertension and previous ischemic stroke or TIA, as well as higher CHA2DS2‐VASc scores compared to younger patients. Diabetes mellitus was more common among younger patients, as was dyslipidemia and current smoking. Time to OAC initiation after index event was shorter in the oldest old.
TABLE 1.
Patient Characteristics Stratified to Age Group and OAC Type
| Age ≥85 years | Age <85 years | Missing values rate | Age ≥85 years | Age <85 years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOAC | VKA | DOAC | VKA | |||||||
| Patients, n (%) | 1,380 (23.1) | 4,604 (76.9) | p‐value | 865 (62.7) | 515 (37.3) | p‐value | 2,823 (61.3) | 1,781 (38.7) | p‐value | |
| Demographics | ||||||||||
| Age, years, median (IQR) | 88 (86–90) | 75 (69–80) | <0.001 | 0% | 88 (86–90) | 88.2 (86–91) | 0.001 | 75.1 (69–80) | 75.0 (69–80) | 0.119 |
| Female sex, n (%) | 881 (63.8) | 1,977 (42.9) | <0.001 | 0% | 549 (63.5) | 332 (64.5) | 0.753 | 1,249 (44.2) | 728 (40.9) | 0.027 |
| Stroke characteristics | ||||||||||
| Ischemic stroke as index event, n (%) | 1,292 (93.6) | 4,301 (93.4) | 0.836 | 0% | 794 (91.8) | 498 (96.7) | <0.001 | 2,599 (92.1) | 1,702 (95.6) | <0.001 |
| NIHSS at baseline, median (IQR) | 6 (3–12.5) | 5 (2–11) | <0.001 | 7.8% | 5 (2–11) | 8 (3–16) | <0.001 | 4 (2–10) | 6 (2–12) | <0.001 |
| Medication details | ||||||||||
| Time to OAC initiation, days, median (IQR) | 4 (2–10) | 5 (2–11) | 0.001 | 5.1% a | 4 (2‐9) | 4 (2–12) | 0.620 | 5 (2–10) | 5 (2–14) | 0.081 |
| Concomitant antiplatelet use, n (%) | 370 (30.9) | 1,255 (30.1) | 0.622 | 10.4% | 196 (27.7) | 174 (35.6) | 0.005 | 620 (25.4) | 635 (36.8) | <0.001 |
| Risk factors | ||||||||||
| Previous stroke/TIA, n (%) | 389 (28.2) | 1,110 (24.1) | 0.002 | 0.03% | 250 (28.9) | 139 (27.0) | 0.497 | 686 (24.3) | 424 (23.8) | 0.724 |
| Previous ICH, n (%) | 17 (1.5) | 37 (1.1) | 0.322 | 24.0% | 12 (1.7) | 5 (1.1) | 0.577 | 17 (0.8) | 20 (1.5) | 0.078 |
| Diabetes mellitus, n (%) | 309 (22.4) | 1,200 (26.1) | 0.006 | 0.07% | 181 (20.9) | 128 (24.9) | 0.104 | 694 (24.6) | 506 (28.4) | 0.004 |
| Hypertension, n (%) | 1,146 (83.3) | 3,474 (75.7) | <0.001 | 0.3% | 742 (85.9) | 404 (79.1) | 0.001 | 2,169 (77.0) | 1,305 (73.6) | 0.011 |
| Dyslipidemia, n (%) | 521 (43.4) | 1,908 (48.7) | 0.001 | 14.5% | 365 (51.8) | 156 (31.4) | <0.001 | 1,177 (54.5) | 731 (41.7) | <0.001 |
| CHA2DS2VASc‐Score, mean (SD) | 6.0 (1.1) | 4.9 (1.6) | <0.001 | 1.1% | 6.0 (1.2) | 5.9 (1.1) | 0.038 | 5.0 (1.6) | 4.8 (1.6) | <0.001 |
| Modified CHA2DS2VASc‐Score (without age and sex), mean (SD) | 3.3 (1.1) | 3.1 (1.2) | <0.001 | 1.1% | 3.4 (1.1) | 3.2 (1.0) | 0.018 | 3.2 (1.2) | 3.0 (1.3) | <0.001 |
| eGFR, ml/min, mean (SD) | 51.0 (24.4) | 62.3 (29.3) | <0.001 | 14.0% | 50.4 (25.6) | 51.9 (21.9) | 0.303 | 64 (48–83) | 66 (51–81) | 0.039 |
| Current smoking, n (%) | 70 (5.3) | 803 (18.0) | <0.001 | 3.4% | 49 (6.0) | 21 (4.2) | 0.205 | 501 (18.5) | 302 (17.3) | 0.331 |
DOAC = direct oral anticoagulant; eGFR = estimated glomerular filtration rate; ICH = intracranial hemorrhage; NIHSS = National Institutes of Health Stroke Scale; OAC = oral anticoagulant; VKA = Vitamin‐K‐antagonist.
exact time missing, but all <30 days.
Patients with DOAC vs. VKA
There were no substantial differences between DOAC‐ and VKA‐treated patients regarding age (median [IQR] 78 [71–84] years in both groups, p = 0.179), sex (48.8% vs. 46.2% female, p = 0.055) and time to OAC initiation (median [IQR] 5 [2–10] vs. 5 [2–14] days, p = 0.075). Compared to patients with VKA treatment, DOAC‐treated patients had less often ischemic stroke as index event (92.0% vs. 95.8%, p < 0.001), reflected in their lower NIHSS scores (median [IQR] 5 [2–10] vs. 6 [2–13], p < 0.001). They had less often concomitant antiplatelets (26.0% vs. 36.5%, p < 0.001) and diabetes (23.7% vs. 27.6%, p = 0.001), but more commonly hypertension (79.1% vs. 74.8%, p < 0.001) and dyslipidemia (53.8% vs. 39.4%, p < 0.001). DOAC‐treated patients had higher CHA2DS2‐VASc scores (mean [SD] 5.2 [1.5] vs. 5.0 [1.6], p < 0.001). This was largely consistent in the subgroups of patients aged ≥85 vs. <85 years (Table 1).
Main Analysis – Primary Composite Outcome
During a total follow‐up of 6,874 patient‐years we observed a total of 279 recurrent ischemic strokes, 69 ICH and 737 deaths. This amounted to 994 primary (composite) outcome events, a primary outcome event rate of 14.5%/year. The follow‐up time, number and crude rate of events for the primary outcome stratified to age group and OAC type are given in Table 2.
TABLE 2.
Follow‐up Time, Number and Crude Rate of Events for the Primary and Secondary Outcomes
| Patient‐years of follow‐up | Number of events (annualized rate) | |||||
|---|---|---|---|---|---|---|
| Composite outcome | Recurrent ischemic stroke | Intracranial hemorrhage | All‐cause death | |||
| All | 6,874 | 994 (14.5%) | 279 (4.1%) | 69 (1.0%) | 737 (10.7%) | |
| Stratified to age | ||||||
| ≥85 years | 1,502 | 387 (25.8%) | 72 (4.8%) | 18 (1.2%) | 337 (22.4%) | |
| <85 years | 5,372 | 607 (11.3%) | 207 (3.9%) | 51 (0.9%) | 400 (7.4%) | |
| Stratified to OAC type | ||||||
| DOAC | 3,559 | 491 (13.8%) | 150 (4.2%) | 26 (0.7%) | 351 (9.9%) | |
| VKA | 3,316 | 503 (15.2%) | 129 (3.9%) | 43 (1.3%) | 386 (11.6%) | |
| Stratified to age and OAC type | ||||||
| ≥85 years | DOAC | 779 | 181 (23.2%) | 40 (5.1%) | 8 (1.0%) | 152 (19.5%) |
| VKA | 723 | 206 (28.5%) | 32 (4.4%) | 10 (1.4%) | 185 (25.6%) | |
| <85 years | DOAC | 2,780 | 310 (11.2%) | 110 (4.0%) | 18 (0.6%) | 199 (7.2%) |
| VKA | 2,593 | 297 (11.5%) | 97 (3.7%) | 33 (1.3%) | 201 (7.8%) | |
DOAC = direct oral anticoagulant; OAC = oral anticoagulant; VKA = Vitamin‐K‐antagonist.
In the simple Cox model, age < 85 years was associated with a significantly lower hazard for the primary outcome compared to age ≥ 85 years, as indicated by a HR of 0.46 (95%‐CI [0.40, 0.52]). Likewise, DOAC‐treated patients had a lower hazard than VKA‐treated patients with a HR of 0.74 (95%‐CI [0.63, 0.86]). There was no evidence for an interaction between age group and OAC type on their impact on the composite outcome (HR DOAC vs. VKA among patients aged ≥85 years 0.65, 95% CI [0.52, 0.81]; HR DOAC vs. VKA among patients aged <85 years 0.79, 95% CI [0.66, 0.95]; pinteraction = 0.129). Consistent findings resulted from repeated analyses refined by adjustment for potential confounders (HR≥85y 0.70, 95%‐CI [0.56, 0.88] and HR<85y 0.87, 95%‐CI [0.73, 1.05]; pinteraction = 0.094) and weighting (HR≥85y 0.79, 95%‐CI [0.61, 1.01] and HR<85y 0.88, 95%‐CI [0.71, 1.09]; pinteraction = 0.512). Thus, the better performance of DOAC over VKA with regard to the composite outcome was not dependent on age and was maintained in the oldest old. The detailed results of all Cox models for the composite outcome are presented in Table 3. The weighted Kaplan–Meier estimates for the composite outcome stratified to type of OAC and age group are presented in Fig 2.
TABLE 3.
Cox Models for Time to Composite Outcome
| Model (n = 5,984) | Variable | Hazard ratio | 95% confidence interval | p‐value |
|---|---|---|---|---|
| simple model | DOAC (vs. VKA) | 0.74 | [0.63, 0.86] | <0.001 |
| Age < 85 years (vs. ≥85 years) | 0.46 | [0.40, 0.52] | <0.001 | |
| simple model with interaction term | DOAC (vs. VKA) | 0.65 | [0.52, 0.81] | <0.001 |
| Age < 85 years (vs. ≥85 years) | 0.41 | [0.34, 0.49] | <0.001 | |
| Interaction OAC by age | 1.22 | [0.94, 1.58] | 0.129 | |
| adjusted model a with interaction term | DOAC (vs. VKA) | 0.70 | [0.56, 0.88] | 0.002 |
| Age < 85 years (vs. ≥85 years) | 0.42 | [0.35, 0.50] | <0.001 | |
| Interaction OAC by age | 1.25 | [0.96, 1.61] | 0.094 | |
| weighted model b with interaction term | DOAC (vs. VKA) | 0.79 | [0.61, 1.01] | 0.060 |
| Age < 85 years (vs. ≥85 years) | 0.48 | [0.37, 0.61] | <0.001 | |
| Interaction OAC by age | 1.12 | [0.81, 1.55] | 0.512 |
DOAC = direct oral anticoagulant; OAC = oral anticoagulant; VKA = Vitamin‐K‐antagonist.
adjustment for sex, National Institutes of Health Stroke Scale score at baseline, modified CHA2DS2‐VASc score (without the age and sex components).
weighting for sex, National Institutes of Health Stroke Scale score at baseline, history of prior stroke or transient ischemic attack, history of intracranial hemorrhage, diabetes mellitus, hypertension, dyslipidemia, estimated glomerular filtration rate, current smoking, concomitant antiplatelet use, cohort study.
FIGURE 2.
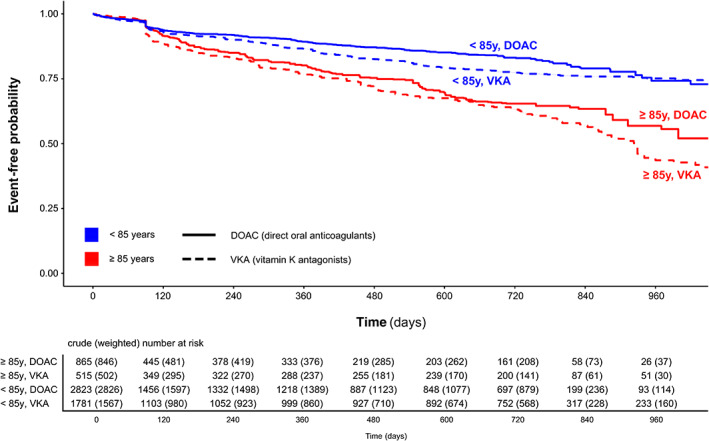
Weighted Kaplan–Meier curves for the composite outcome stratified to anticoagulant type (DOAC / VKA) and age group (≥85 / <85 years)
Secondary Analysis – Individual Outcomes Recurrent Ischemic Stroke, ICH and Death
Table 2 shows the follow‐up time, number and crude rate of events for the secondary (individual) outcomes in the entire study population and stratified to age group and OAC type. In line with the main analysis of the composite outcome, there was no evidence for an interaction between age and OAC type on the recurrence of ischemic stroke nor the occurrence of ICH in the simple, adjusted and weighted Cox proportional cause‐specific hazards models accounting for competing risks (all pinteraction > 0.05). For the outcome death, there was evidence for a weak interaction only in the simple (pinteraction = 0.054) and adjusted models (pinteraction = 0.029), indicating that the lower hazard for death among patients treated with DOAC as compared to VKA‐treated patients was even more pronounced in the oldest old than in their younger counterparts (simple model: HR≥85y 0.61, 95%‐CI [0.47, 0.79] and HR<85y 0.83, 95%‐CI [0.65, 1.05]; adjusted model: HR≥85y 0.66, 95%‐CI [0.51, 0.86] and HR<85y 0.94, 95%‐CI [0.74, 1.19]). The detailed results of all Cox cause‐specific hazards models for the individual outcomes are presented in Table S2 and Fig 3.
FIGURE 3.
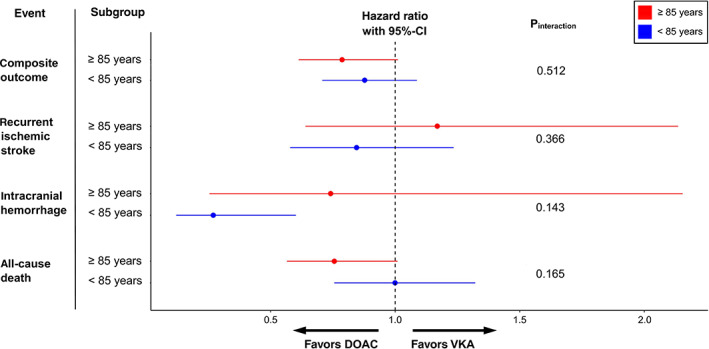
Hazard ratio estimates for the effect of DOAC vs. VKA on the primary composite outcome and all its individual components (accounting for competing risks) stratified to patients aged ≥85 versus <85 years based on the weighted model
Secondary Analysis – Net Clinical Benefit
The point estimates for the NCB of DOAC over VKA were similar in patients aged ≥85 (+1.73 to +2.66) and < 85 years (+1.90 to +3.36 events per 100 patient‐years) and remained positive over the entire range of ICH weights used (1.5 to 3.1), with wide confidence intervals crossing zero in the smaller group of patients aged ≥85 years. Fig 4 depicts the NCB for three previously published ICH weights. 29 , 30 , 31 The detailed results of the NCB analysis are presented in Table S3.
FIGURE 4.
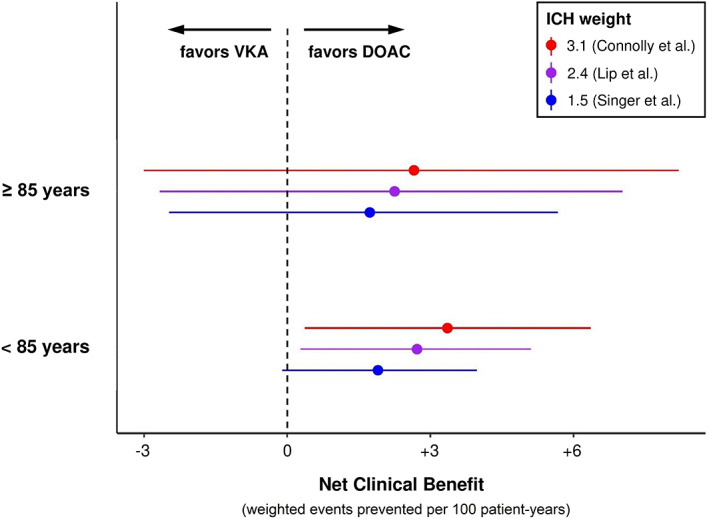
Net clinical benefit of DOAC over VKA with 95% confidence intervals stratified to age group (≥85 / <85 years), using three previously published ICH weights
Sensitivity Analyses – Age Dichotomized at 90 Years and as a Continuous Variable
The sensitivity analyses using age dichotomized to ≥90 (n = 451) vs. <90 years (n = 5,533) showed no evidence for interaction between age and OAC type on the hazard for the composite outcome in the simple (pinteraction = 0.283), adjusted (pinteraction = 0.514) and weighted (pinteraction = 0.433) models, consistent with the main analysis (Table S4).
Using age as a continuous variable, the favorable profile of DOAC over VKA regarding the composite outcome was maintained across the entire age spectrum in the weighted model (Fig 5). There was no evidence that the association between OAC type and composite outcome was modified by age upon comparison of the weighted model with vs. without interaction term (plikelyhood ratio test = 0.623). The hazard for the composite outcome continuously increased with increasing age in a non‐linear fashion.
FIGURE 5.
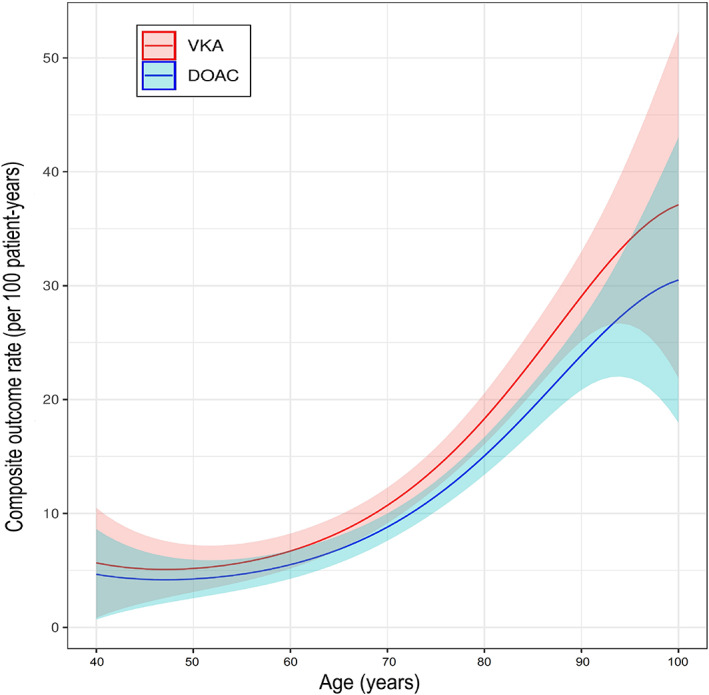
Rate of the composite outcome by age as a continuous variable stratified to type of anticoagulant (DOAC / VKA). The solid lines represent the estimates for each year of age from the weighted model without interaction term and the shaded areas the 95%‐CI.
Discussion
This study focused on the safety and effectiveness of DOAC versus VKA in the oldest‐old patients with AF and recent stroke or TIA in a real‐world setting. The key finding was that the benefits of DOAC over VKA were consistently maintained in the oldest old, even when potential confounders were accounted for.
In our study, DOAC were associated with a lower hazard than VKA for the composite outcome of recurrent ischemic stroke, ICH and all‐cause death in patients with AF and recent ischemic stroke, independent of age. The favorable profile of DOAC was maintained in the oldest old, whether defined as aged 85 or 90 years or older. This observation is highly relevant for clinical practice as it contradicts the assumptions of many clinicians who are reluctant to use DOAC in this age group, particularly in multimorbid patients. 10 , 11 In this context, it is clinically important that the beneficial effect of DOAC over VKA persisted after taking into account the high‐risk profile of the oldest old. Reassuringly, simple, adjusted, as well as weighted models which controlled for the non‐randomized treatment assignment, all yielded consistent results.
Notably, there was no signal of a safety concern regarding ICH risk among the oldest‐old DOAC‐treated patients with recent ischemic stroke, which is a widespread concern. 12 , 13 , 34 In NCB analyses balancing the benefit in stroke reduction against the weighted risk of ICH, the net benefit of DOAC over VKA in these patients was preserved, as indicated by NCB point estimates that were similar in the oldest old as in their younger counterparts, and remained consistently positive across a broad spectrum of ICH weights. Taken together, these findings provide new evidence that the overall beneficial effect of DOAC treatment following recent ischemic stroke is maintained in the oldest old.
These results are clinically important because limited randomized data exist for such patients, as patients with recent stroke within 7 days, 35 14 days, 36 , 37 or 30 days, 7 respectively, were excluded from the pivotal DOAC RCTs and the oldest old were severely underrepresented, constituting less than 5% of the RCTs population. 8 , 9 While several large observational studies later confirmed the benefits of DOAC in elderly patients with AF, 38 , 39 , 40 , 41 , 42 , 43 they did not examine patients with a recent ischemic stroke. The fact that the elderly in our study had a recent stroke matters, as such patients – compared to those without recent stroke – have a higher risk for hemorrhagic complications, including ICH 44 and hemorrhagic transformation of the ischemic infarct, 45 concomitant active small vessel disease 46 , 47 and stroke‐induced motor and cognitive deficits with an increased risk of falls. 8 , 48
For patients with recent stroke, subgroup analyses in observational studies suggested the safety of DOAC versus VKA for the age groups of ≥75 23 and > 80 years. 15 , 49 As we are not aware of any studies investigating AF patients with both (1) age over 85 years and (2) a recent ischemic stroke, our data address an important evidence gap, mitigating concerns about the applicability of the RCT findings in everyday clinical stroke practice and supporting the current guidelines for prevention of stroke recurrence. 3
Strengths and Limitations
Our study has the following strengths: (1) we used individual patient data pooled within an established collaboration of prospective observational studies from Europe and Asia; (2) the high data completeness limits the risk of spurious findings; and (3) the consistency of results both in unadjusted and in adjusted and – most importantly – weighted analyses accounting for potential confounders, as well as in net benefit analyses and in sensitivity analyses focusing on patients ≥90 years or using age as a continuous measure, underlines the robustness of our key finding.
We are aware of the following limitations: (1) as our data are observational rather than randomized, baseline imbalances in the allocation to the type of OAC that were unaccounted for might have introduced bias or confounding; (2) our study included exclusively OAC‐treated patients, so age‐matched stroke patients without OAC treatment were not available for comparison. Of note, the placebo‐controlled ELDERCARE‐AF trial suggested the benefit of anticoagulation even in very elderly patients with AF who were not appropriate candidates for standard anticoagulant treatment 10 ; (3) we did not consider extracranial bleeding or myocardial infarction in our analyses, as these outcomes were not available in all participating cohorts; (4) the follow‐up time in the participating cohorts differed, with some reporting over 2 years of follow‐up data, while others were limited to 3 months; (5) our study did not include information on adherence to oral anticoagulants, which was not systematically assessed in most cohorts, although our previous work from the single‐center NOACISP‐LONGTERM cohort indicated high rates of self‐reported adherence both in VKA‐ and DOAC‐treated patients also among the oldest old 50 ; (6) Dementia was not an explicit exclusion criterion in any of the contributing cohorts. However, as our study lacked information on the frequency of dementia, it remains unclear whether our key findings are applicable to demented patients, too.
In conclusion, our study provides new and compelling evidence indicating that the benefits of DOAC over VKA in patients with AF and recent stroke are maintained among the oldest old.
Author Contributions
All authors contributed to study design, data acquisition and analysis, and critically revised the manuscript.
Potential Conflicts of Interest
MP: speaker honoraria from Sanofi‐Aventis, Boehringer‐Ingelheim, Bayer, BMS, Daiichi‐Sankyo, Pfizer (all manufacturers of anticoagulants). MK: speaker honoraria from Bayer, Nippon Boehringer‐Ingelheim, Daiichi‐Sankyo. MC: consulting fees from Boehringer‐Ingelheim, Pfizer/BMS; advisory board Daiichi‐Sankyo. GMDM: consultant/speaker honoraria from Bayer, travel honoraria from Pfizer. ST: travel grants from BMS/Pfizer. GYHL: Consultant and speaker for BMS/Pfizer, Boehringer‐Ingelheim and Daiichi‐Sankyo; no fees are received personally. LHB: consultancy or advisory board fees or speaker's honoraria from Bayer and BMS. PAL: research grants from Bayer, travel grants from Bayer, Pfizer, advisory board compensation from Bayer, Pfizer, Daiichi‐Sankyo, BMS. KT: lecture honoraria (modest) from Daiichi‐Sankyo, Bayer Yakuhin, Nippon Boehringer‐Ingelheim, BMS. DJW: personal fees from Bayer, Portola (manufacturer of the anticoagulant reversal agent andexanet alfa). STE: research support from Pfizer, Daiichi‐Sankyo; compensation from Stago (manufacturer of coagulation testing systems) for educational material; travel/speaker honoraria from Bayer, Boehringer‐Ingelheim, BMS, Daiichi‐Sankyo; advisory board Bayer, Boehringer‐Ingelheim, BMS. The remaining authors declare no relevant conflicts.
Supporting information
Table S1 Participating cohort studies
Table S2. Cox proportional cause‐specific hazards models for time to recurrent ischemic stroke, intracranial hemorrhage and death, accounting for competing risks
Table S3. Net clinical benefit of DOAC over VKA
Table S4. Cox models for time to composite outcome using age dichotomized at 90 years
Table S5. Complete list of investigators of the cohort studies that contributed patients to this analysis
Acknowledgments
We thank all investigators from the participating cohort studies (listed in Table S5) for their contribution to data collection. This study was supported by the Science Fund Rehabilitation of the University Department of Geriatric Medicine Felix Platter Basel, the Stroke Fund and Scientific Fund of the University Hospital Basel. NOACISP‐LONGTERM was supported by grants from the Swiss Heart Foundation, Daiichi‐Sankyo AG (Switzerland) and Bayer AG (Switzerland). CROMIS‐2 was jointly funded by the Stroke Association and the British Heart Foundation and supported by researchers at the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre. UCL acted as the Sponsor for CROMIS‐2, with responsibility for the conduct and management of the study. SAMURAI‐NVAF was supported by a Grant‐in‐Aid (H23‐Junkanki‐Ippan‐010) from the Ministry of Health, Labour and Welfare, Japan and an Intramural Research Fund (H23‐4‐3 and H28‐4‐1) for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center. RAF/RAF‐DOAC has been supported by the Italian Ministry of Health Ricerca Corrente – IRCCS MultiMedica. Open access funding provided by Universitat Basel.
Contributor Information
Alexandros A. Polymeris, Email: alexandros.polymeris@usb.ch.
NOACISP‐LONGTERM, Erlangen Registry, CROMIS‐2, RAF, RAF‐DOAC, SAMURAI‐NVAF and Verona Registry Collaborators:
Stefan T Engelter, Philippe A Lyrer, Leo H Bonati, David J Seiffge, Christopher Traenka, Alexandros A Polymeris, Annaelle Zietz, Nils Peters, Gian Marco De Marchis, Sebastian Thilemann, Nikolaos S Avramiotis, Henrik Gensicke, Lisa Hert, Benjamin Wagner, Fabian Schaub, Louisa Meya, Joachim Fladt, Tolga Dittrich, Urs Fisch, Kosmas Macha, David Haupenthal, Luise Gassmann, Ruihao Wang, Svenja Stoll, Stefan Schwab, Bastian Volbers, Gabriela Siedler, Bernd Kallmünzer, Manuel Cappellari, Bruno Bonetti, Paolo Bovi, Giampaolo Tomelleri, Nicola Micheletti, Cecilia Zivelonghi, Andrea Emiliani, Adrian Parry‐Jones, Chris Patterson, Christopher Price, Abduelbaset Elmarimi, Anthea Parry, Arumug Nallasivam, Azlisham Mohd Nor, Bernard Esis, David Bruce, Biju Bhaskaran, Christine Roffe, Claire Cullen, Clare Holmes, David Cohen, David Hargroves, David Mangion, Dinesh Chadha, Djamil Vahidassr, Dulka Manawadu, Elio Giallombardo, Elizabeth Warburton, Enrico Flossman, Gunaratam Gunathilagan, Harald Proschel, Hedley Emsley, Ijaz Anwar, Ilse Burger, James Okwera, Janet Putterill, Janice O’Connell, John Bamford, John Corrigan, Jon Scott, Jonathan Birns, Karen Kee, Kari Saastamoinen, Kath Pasco, Krishna Dani, Lakshmanan Sekaran, Lillian Choy, Liz Iveson, Maam Mamun, Mahmud Sajid, Martin Cooper, Matthew Burn, Matthew Smith, Michael Power, Michelle Davis, Nigel Smyth, Roland Veltkamp, Pankaj Sharma, Paul Guyler, Paul O’Mahony, Peter Wilkinson, Prabel Datta, Prasanna Aghoram, Rachel Marsh, Robert Luder, Sanjeevikumar Meenakishundaram, Santhosh Subramonian, Simon Leach, Sissi Ispoglou, Sreeman Andole, Timothy England, Aravindakshan Manoj, Frances Harrington, Habib Rehman, Jane Sword, Julie Staals, Karim Mahawish, Kirsty Harkness, Louise Shaw, Michael McCormich, Nikola Sprigg, Syed Mansoor, Vinodh Krishnamurthy, Michela Giustozzi, Monica Acciarresi, Giancarlo Agnelli, Valeria Caso, Cecilia Becattini, Andrea Alberti, Michele Venti, Cataldo D’Amore, Maria Giulia Mosconi, Ludovica Anna Cimini, Maurizio Paciaroni, Fabio Bandini, Georgios Tsivgoulis, Chrissoula Liantinioti, Maria Chondrogianni, Shadi Yaghi, Karen L. Furie, Prasanna Tadi, Marialuisa Zedde, Azmil H Abdul‐Rahim, Kennedy R Lees, Paolo Bovi, Monica Carletti, Alberto Rigatelli, Manuel Cappellari, Jukka Putaala, Liisa Tomppo, Turgut Tatlisumak, Simona Marcheselli, Alessandro Pezzini, Loris Poli, Alessandro Padovani, Vieri Vannucchi, Luca Masotti, Sung‐Il Sohn, Gianni Lorenzini, Rossana Tassi, Francesca Guideri, Maurizio Acampa, Giuseppe Martini, George Ntaios, George Athanasakis, Konstantinos Makaritsis, Efstathia Karagkiozi, Konstantinos Vadikolias, Nicola Mumoli, Franco Galati, Simona Sacco, Cindy Tiseo, Francesco Corea, Walter Ageno, Marta Bellesini, Giovanna Colombo, Giorgio Silvestrelli, Alfonso Ciccone, Alessia Lanari, Umberto Scoditti, Licia Denti, Michelangelo Mancuso, Miriam Maccarrone, Leonardo Ulivi, Giovanni Orlandi, Nicola Giannini, Tiziana Tassinari, Maria Luisa De Lodovici, Christina Rueckert, Antonio Baldi, Danilo Toni, Federica Letteri, Alessio Pieroni, Martina Giuntini, Enrico Maria Lotti, Yuriy Flomin, Odysseas Kargiotis, Theodore Karapanayiotides, Serena Monaco, Mario Maimone Baronello, Laszló Csiba, Lilla Szabó, Alberto Chiti, Elisa Giorli, Massimo Del Sette, Davide Imberti, Dorjan Zabzuni, Boris Doronin, Vera Volodina, Patrik Michel, Peter Vanacker, Kristian Barlinn, Lars‐Peder Pallesen, Jessica Barlinn, Dirk Deleu, Gayane Melikyan, Faisal Ibrahim, Naveed Akhtar, Vanessa Gourbali, Kenichi Todo, Kazumi Kimura, Kensaku Shibazaki, Yoshiki Yagita, Eisuke Furui, Ryo Itabashi, Tadashi Terasaki, Yoshiaki Shiokawa, Teruyuki Hirano, Rieko Suzuki, Kenji Kamiyama, Jyoji Nakagawara, Shunya Takizawa, Kazunari Homma, Satoshi Okuda, Yasushi Okada, Koichiro Maeda, Tomoaki Kameda, Kazuomi Kario, Yoshinari Nagakane, Yasuhiro Hasegawa, Hisanao Akiyama, Satoshi Shibuya, Hiroshi Mochizuki, Yasuhiro Ito, Takahiro Nakashima, Hideki Matsuoka, Kazuhiro Takamatsu, Kazutoshi Nishiyama, Kanta Tanaka, Kaoru Endo, Tetsuya Miyagi, Masato Osaki, Junpei Kobayashi, Takuya Okata, Eijiro Tanaka, Yuki Sakamoto, Keisuke Tokunaga, Hotake Takizawa, Junji Takasugi, Soichiro Matsubara, Kyoko Higashida, Takayuki Matsuki, Naoto Kinoshita, Masayuki Shiozawa, Toshihiro Ide, Takeshi Yoshimoto, Daisuke Ando, Kyohei Fujita, Masaya Kumamoto, Teppei Kamimura, Muneaki Kikuno, Tadataka Mizoguchi, and Takeo Sato
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Gabet A, Guenancia C, Duloquin G, et al. Ischemic stroke with atrial fibrillation: characteristics and time trends 2006 to 2017 in the Dijon stroke registry. Stroke 2021;52:2077–2085. [DOI] [PubMed] [Google Scholar]
- 3. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021;52:e364–e467. [DOI] [PubMed] [Google Scholar]
- 4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 5. Lauw MN, Eikelboom JW, Coppens M, et al. Effects of dabigatran according to age in atrial fibrillation. Heart 2017;103:1015–1023. [DOI] [PubMed] [Google Scholar]
- 6. Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, Oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation 2014;130:138–146. [DOI] [PubMed] [Google Scholar]
- 7. Kato ET, Giugliano RP, Ruff CT, et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF‐TIMI 48 trial. J Am Heart Assoc 2016;5.e003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seiffge DJ, Polymeris AA, Fladt J, et al. Management of patients with stroke treated with direct oral anticoagulants. J Neurol 2018. Dec;265:3022–3033. [DOI] [PubMed] [Google Scholar]
- 9. Botto G, Ameri P, Cappellari M, et al. Unmet clinical needs in elderly patients receiving direct Oral anticoagulants for stroke prevention in non‐valvular atrial fibrillation. Adv Ther 2021;38:2891–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okumura K, Akao M, Yoshida T, et al. Low‐dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med 2020;383:1735–1745. [DOI] [PubMed] [Google Scholar]
- 11. Stollberger C, Brooks R, Finsterer J, Pachofszky T. Use of direct‐acting Oral anticoagulants in nonagenarians: a call for more data. Drugs Aging 2016;33:315–320. [DOI] [PubMed] [Google Scholar]
- 12. McGrath ER, Go AS, Chang Y, et al. Use of Oral anticoagulant therapy in older adults with atrial fibrillation after acute ischemic stroke. J Am Geriatr Soc 2017;65:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato ET, Goto S, Giugliano RP. Overview of oral antithrombotic treatment in elderly patients with atrial fibrillation. Ageing Res Rev 2019. Jan;49:115–124. [DOI] [PubMed] [Google Scholar]
- 14. Camm AJ, Fox KAA. Strengths and weaknesses of 'real‐world' studies involving non‐vitamin K antagonist oral anticoagulants. Open Heart 2018;5:e000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seiffge DJ, Paciaroni M, Wilson D, et al. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol 2019;85:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meya L, Polymeris AA, Schaedelin S, et al. Oral anticoagulants in atrial fibrillation patients with recent stroke who are dependent on the daily help of others. Stroke 2021;52:3472–3481. [DOI] [PubMed] [Google Scholar]
- 17. Macha K, Volbers B, Bobinger T, et al. Early initiation of anticoagulation with direct Oral anticoagulants in patients after transient ischemic attack or ischemic stroke. J Stroke Cerebrovasc Dis 2016;25:2317–2321. [DOI] [PubMed] [Google Scholar]
- 18. Cappellari M, Carletti M, Danese A, Bovi P. Early introduction of direct oral anticoagulants in cardioembolic stroke patients with non‐valvular atrial fibrillation. J Thromb Thrombolysis 2016;42:393–398. [DOI] [PubMed] [Google Scholar]
- 19. Wilson D, Ambler G, Banerjee G, et al. Early versus late anticoagulation for ischaemic stroke associated with atrial fibrillation: multicentre cohort study. J Neurol Neurosurg Psychiatry 2019;90:320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke 2015;46:2175–2182. [DOI] [PubMed] [Google Scholar]
- 21. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non‐vitamin‐K Oral anticoagulants (RAF‐NOACs) study. J Am Heart Assoc 2017;6:e007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toyoda K, Arihiro S, Todo K, et al. Trends in oral anticoagulant choice for acute stroke patients with nonvalvular atrial fibrillation in Japan: the SAMURAI‐NVAF study. Int J Stroke 2015;10:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshimura S, Koga M, Sato S, et al. Two‐year outcomes of anticoagulation for acute ischemic stroke with Nonvalvular atrial fibrillation‐ SAMURAI‐NVAF study. Circ J 2018;82:1935–1942. [DOI] [PubMed] [Google Scholar]
- 24. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie J, Liu C. Adjusted Kaplan‐Meier estimator and log‐rank test with inverse probability of treatment weighting for survival data. Stat Med 2005;24:3089–3110. [DOI] [PubMed] [Google Scholar]
- 28. Andersen PK, Borgan Ø, Hjort NL, et al. Counting process models for life history data: a review [with discussion and reply]. Scand J Stat 1985;12:97–158. [Google Scholar]
- 29. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009;151:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Connolly SJ, Eikelboom JW, Ng J, et al. Net clinical benefit of adding clopidogrel to aspirin therapy in patients with atrial fibrillation for whom vitamin K antagonists are unsuitable. Ann Intern Med 2011;155:579–586. [DOI] [PubMed] [Google Scholar]
- 31. Lip GY, Skjoth F, Nielsen PB, Larsen TB. Non‐valvular atrial fibrillation patients with none or one additional risk factor of the CHA2DS2‐VASc score. A comprehensive net clinical benefit analysis for warfarin, aspirin, or no therapy. Thromb Haemost 2015;114:826–834. [DOI] [PubMed] [Google Scholar]
- 32. Pan Y, Elm JJ, Li H, et al. Outcomes associated with Clopidogrel‐aspirin use in Minor stroke or transient ischemic attack: a pooled analysis of Clopidogrel in high‐risk patients with acute non‐disabling cerebrovascular events (CHANCE) and platelet‐oriented inhibition in new TIA and Minor ischemic stroke (POINT) trials. JAMA Neurol 2019;76:1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dodson JA, Petrone A, Gagnon DR, et al. Incidence and determinants of traumatic intracranial bleeding among older veterans receiving warfarin for atrial fibrillation. JAMA Cardiol 2016;1:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 36. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 37. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 38. Chao TF, Liu CJ, Lin YJ, et al. Oral anticoagulation in very elderly patients with atrial fibrillation: a Nationwide cohort study. Circulation 2018;138:37–47. [DOI] [PubMed] [Google Scholar]
- 39. Hohmann C, Hohnloser SH, Jacob J, et al. Non‐vitamin K Oral anticoagulants in comparison to Phenprocoumon in geriatric and non‐geriatric patients with non‐Valvular atrial fibrillation. Thromb Haemost 2019;119:971–980. [DOI] [PubMed] [Google Scholar]
- 40. Chao TF, Chiang CE, Liao JN, et al. Comparing the effectiveness and safety of nonvitamin K antagonist Oral anticoagulants and warfarin in elderly Asian patients with atrial fibrillation: a Nationwide cohort study. Chest 2020;157:1266–1277. [DOI] [PubMed] [Google Scholar]
- 41. Chao T‐F, Chiang C‐E, Chan Y‐H, et al. Oral anticoagulants in extremely‐high‐risk, very elderly (>90 years) patients with atrial fibrillation. Heart Rhythm 2021;18:871–877. [DOI] [PubMed] [Google Scholar]
- 42. Cheng WH, Chiang CE, Lin YJ, et al. Non‐vitamin K antagonist Oral anticoagulants in elderly (≥85 years) patients with newly diagnosed atrial fibrillation: changing clinical practice and outcomes for stroke prevention in a Nationwide cohort study. Mayo Clin Proc 2021;96:52–65. [DOI] [PubMed] [Google Scholar]
- 43. Grymonprez M, Steurbaut S, De Backer TL, et al. Effectiveness and safety of Oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta‐analysis. Front Pharmacol 2020;11:583311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogren J, Irewall AL, Bergstrom L, Mooe T. Intracranial hemorrhage after ischemic stroke: incidence, time trends, and predictors in a Swedish Nationwide cohort of 196 765 patients. Circ Cardiovasc Qual Outcomes 2015;8:413–420. [DOI] [PubMed] [Google Scholar]
- 45. Seiffge DJ, Werring DJ, Paciaroni M, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol 2019;18:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Du H, Wilson D, Ambler G, et al. Small vessel disease and ischemic stroke risk during anticoagulation for atrial fibrillation after cerebral ischemia. Stroke 2021;52:91–99. [DOI] [PubMed] [Google Scholar]
- 47. Seiffge DJ, Wilson D, Ambler G, et al. Small vessel disease burden and intracerebral haemorrhage in patients taking oral anticoagulants. J Neurol Neurosurg Psychiatry 2021;19:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kerse N, Parag V, Feigin VL, et al. Falls after stroke: results from the Auckland regional community stroke (ARCOS) study, 2002 to 2003. Stroke 2008;39:1890–1893. [DOI] [PubMed] [Google Scholar]
- 49. Xian Y, Xu H, O'Brien EC, et al. Clinical effectiveness of direct Oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: findings from the patient‐centered research into outcomes stroke patients prefer and effectiveness research (PROSPER) study. JAMA Neurol 2019;76:1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polymeris AA, Traenka C, Hert L, et al. Frequency and determinants of adherence to Oral anticoagulants in stroke patients with atrial fibrillation in clinical practice. Eur Neurol 2016;76:187–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Participating cohort studies
Table S2. Cox proportional cause‐specific hazards models for time to recurrent ischemic stroke, intracranial hemorrhage and death, accounting for competing risks
Table S3. Net clinical benefit of DOAC over VKA
Table S4. Cox models for time to composite outcome using age dichotomized at 90 years
Table S5. Complete list of investigators of the cohort studies that contributed patients to this analysis


