Abstract
Background and Aims
Hepatic ischemia–reperfusion injury (IRI) is the leading cause of early posttransplantation organ failure as mitochondrial respiration and ATP production are affected. A shortage of donors has extended liver donor criteria, including aged or steatotic livers, which are more susceptible to IRI. Given the lack of an effective treatment and the extensive transplantation waitlist, we aimed at characterizing the effects of an accelerated mitochondrial activity by silencing methylation‐controlled J protein (MCJ) in three preclinical models of IRI and liver regeneration, focusing on metabolically compromised animal models.
Approach and Results
Wild‐type (WT), MCJ knockout (KO), and Mcj silenced WT mice were subjected to 70% partial hepatectomy (Phx), prolonged IRI, and 70% Phx with IRI. Old and young mice with metabolic syndrome were also subjected to these procedures. Expression of MCJ, an endogenous negative regulator of mitochondrial respiration, increases in preclinical models of Phx with or without vascular occlusion and in donor livers. Mice lacking MCJ initiate liver regeneration 12 h faster than WT and show reduced ischemic injury and increased survival. MCJ knockdown enables a mitochondrial adaptation that restores the bioenergetic supply for enhanced regeneration and prevents cell death after IRI. Mechanistically, increased ATP secretion facilitates the early activation of Kupffer cells and production of TNF, IL‐6, and heparin‐binding EGF, accelerating the priming phase and the progression through G1/S transition during liver regeneration. Therapeutic silencing of MCJ in 15‐month‐old mice and in mice fed a high‐fat/high‐fructose diet for 12 weeks improves mitochondrial respiration, reduces steatosis, and overcomes regenerative limitations.
Conclusions
Boosting mitochondrial activity by silencing MCJ could pave the way for a protective approach after major liver resection or IRI, especially in metabolically compromised, IRI‐susceptible organs.
Abbreviations
- Ctrl
control
- DCD
cadaveric donors after cardiac death
- EGFR
EGF receptor
- ERK
extracellular signal–regulated kinase
- HB‐EGF
heparin‐binding EGF‐like growth factor
- HFHFD
high‐fat/high‐fructose diet
- IR
ischemia–reperfusion
- IRI
ischemia–reperfusion injury
- KO
knockout
- LT
liver transplantation
- MCJ
methylation‐controlled J protein
- OCR
oxygen consumption rate
- p‐
phosphorylated
- PCNA
proliferating cell nuclear antigen
- PET
positron emission tomography
- Phx
partial hepatectomy
- ROS
reactive oxygen species
- SDH2
succinate dehydrogenase
- si‐
small interfering
- STAT
signal transducer and activator of transcription
- WT
wild type
INTRODUCTION
Liver transplantation (LT) is the only curative treatment for acute liver failure and end‐stage liver disease. As the shortage in organ availability is the major limitation for LT, significant efforts have been made over the past decades to increase the use of extended‐criteria organs, including those from elderly donors or cadavers after cardiac death (DCD).[ 1 ] In addition, strategies aimed at improving the quality of these organs and reducing the risk of posttransplant organ malfunction are pursued, particularly new mechanical perfusion strategies.[ 2 ] However, as life expectancy and the prevalence of obesity and NAFLD have increased rapidly in the general population, the incidence of aged deceased donors or donors with steatotic livers has also increased.[ 1 , 3 ] The relationship between aged or steatotic donors and LT outcomes has been widely studied, but the results are conflicting.[ 4 , 5 ] Nevertheless, higher rates of early allograft dysfunction and postreperfusion syndrome have been consistently reported when using these marginal grafts.
Ischemia–reperfusion injury (IRI) is a contributing factor to postreperfusion syndrome. Mitochondrial dysfunction, reactive oxygen species (ROS), and ATP depletion are major processes that mediate injury after ischemia–reperfusion (IR), especially in metabolically compromised old and steatotic livers.[ 6 , 7 , 8 ] These factors, in addition to resulting in poorer tolerance to IRI, also limit proliferation during graft recovery, impeding liver regeneration.[ 9 , 10 ] Thus, strategies directed at reducing the production of ROS and liver damage and improving mitochondrial functionality and ATP resynthesis could help to expand the pool available for transplantation.[ 8 ] The benefits of this strategy could be extended to patients who undergo surgery with prolonged ischemia times, such as liver resection, a common method to treat malignant liver diseases.[ 11 ]
Mitochondria are essential biosynthetic, bioenergetic, and signaling organelles. Therefore, improving bioenergetics is expected to have an impact on cell metabolism and on the interplay among different cells that drive, carry out, or end the regenerative process. Indeed, extracellular ATP orchestrates liver homeostasis, tissue repair, and functional restoration by regulating the crosstalk between liver‐resident cells and recruited immune cells.[ 12 ]
Methylation‐controlled J protein (MCJ), also known as DnaJC15, is an endogenous negative regulator of mitochondrial respiration that inhibits complex I activity, leading to a reduction in ATP synthesis.[ 13 ] Its absence leads to increased complex I activity and ATP synthesis in the heart, immune cells, and the liver[ 13 , 14 , 15 ] and stimulates the formation of respiratory supercomplexes, thereby limiting the production of ROS.[ 15 ] MCJ seems to be dispensable under homeostatic conditions, and no altered phenotype is observed in MCJ knockout (KO) mice. We previously showed that silencing MCJ by specific N‐acetylgalactosamine (GalNAc)–small interfering (si‐) RNA molecules enhances mitochondrial activity and ATP synthesis and results in decreased ROS generation in NASH.[ 16 ] Notably, the silencing of MCJ was found to prevent hepatocyte cell damage after acetaminophen‐induced liver injury.[ 15 ] Therefore, MCJ repression constitutes a mechanism to enhance mitochondrial activity.
In this study, we aimed to determine whether silencing MCJ can reduce ischemic injury and enhance hepatic regeneration after IRI and/or major liver resection. We propose a mechanism that coordinates the hepatocyte–macrophage crosstalk during the initiation and the G1/S progression of liver regeneration based on secreted ATP levels. Using mouse models of ischemic injury, we observed that ablation of MCJ during liver regeneration accelerated mitochondrial respiration and increased ATP synthesis, enabling faster cell‐cycle entry and preventing the characteristic ATP depletion and subsequent cell death. The hepatoprotective effects were observed in three surgical interventions: 70% partial hepatectomy (Phx), prolonged IRI, and 70% Phx with IRI. Significantly, therapeutic silencing of MCJ in 15‐month‐old mice and in mice fed a high‐fat/high‐fructose diet (HFHFD) for 12 weeks improved mitochondrial respiration and ATP production, reduced steatosis, and overcame regenerative and survival limitations, further supporting the possibility of making metabolically compromised livers suitable for LT by targeting mitochondrial activity and ATP levels.
PATIENTS AND METHODS
Human samples
All studies were performed in accordance with the Declaration of Helsinki and local/national laws. The Human Ethics Committee of each hospital approved the study procedures, and written informed consent was obtained from the legal representatives of the potential donors.
Controlled donation after circulatory death was considered for patients in whom the treating team had made the decision to withdraw life‐sustaining therapy. No upper age limit was set. Only donors whose livers were ultimately transplanted were included in analyses. Functional warm ischemic time (FWIT) for abdominal grafts was defined as the time from systolic blood pressure < 60 mm Hg to the onset of normothermic regional perfusion (including a 5‐min no‐touch period). For FWIT, an upper time limit of 30 min for livers was applied. The extracorporeal membranous oxygenation device used was a Maquet Rotaflow (Maquet, Rastatt, Germany). Patient data are shown in Table S1.
Animal models
MCJ KO and MCJ wild‐type (WT) mice were bred at the CIC bioGUNE Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal facility. C57BL/6J mice were purchased from Charles River Laboratories. All animal procedures were approved by the CIC bioGUNE Institutional Animal Care and Use Committee and the Country Council of Bizkaia. Experiments in this study employed experimental models of Phx with or without 30 min of ischemia as well as 90 min of ischemia without Phx.[ 17 , 18 , 19 ]
Additional protocols used are provided in Supporting Information.
RESULTS
Expression of MCJ is increased in human liver biopsies after normothermic perfusion and in preclinical models of Phx with or without IRI
Controlled DCD is an important source of grafts for LT. However, changes during the ischemic period negatively affect postoperative outcomes.[ 20 ] To investigate whether MCJ is dysregulated under surgical ischemic conditions and, as a result, associated with susceptibility to IRI in humans, we compared its expression in liver biopsies from DCD donors 60 min after the start of the normothermic regional perfusion and in healthy control individuals. Our results show that MCJ levels were higher in livers from donor graft patients (n = 17) than in livers from healthy control individuals (n = 7) (Figure 1A).
FIGURE 1.
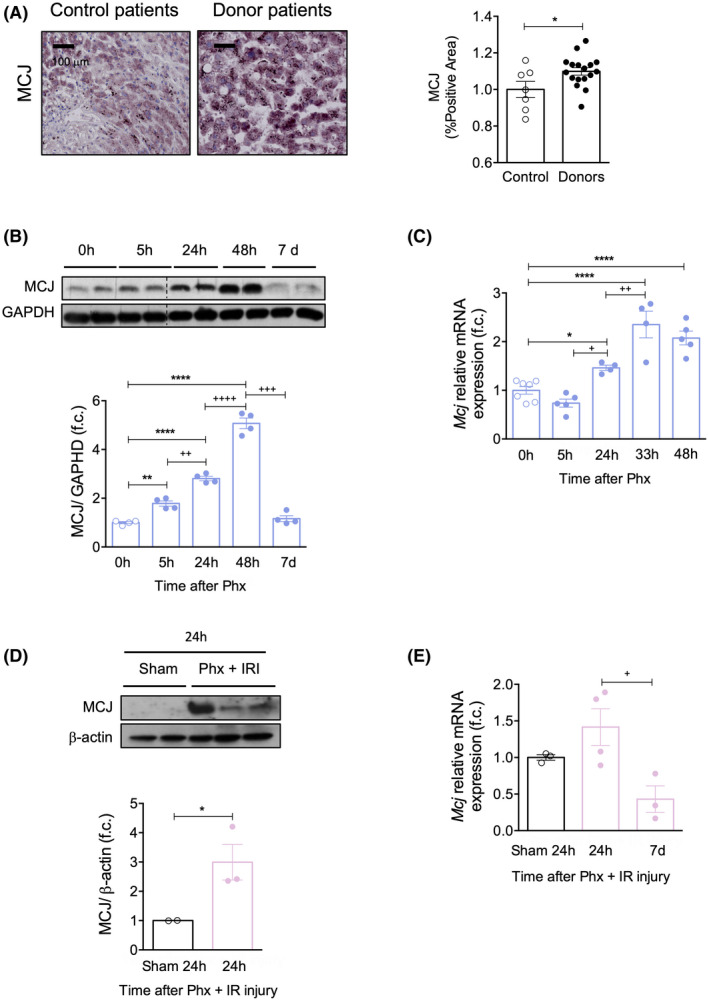
MCJ expression is increased in ischemic injury and graft regeneration. (A) Liver biopsies from transplant donors, 60 min after the start of normothermic regional perfusion (n = 17), and from healthy control individuals (n = 7) where MCJ expression was determined by immunohistochemistry (left panel) and quantified (right panel). Scale bar, 50 µm. Values are represented as median ± range. The U test was used to compare two groups. (B) MCJ levels by western blotting (upper panel) and densitometric quantification (bottom panel) in WT liver extracts at different time points after 70% Phx. Glyceraldehyde 3‐phosphate dehydrogenase was used as a loading control. (C) Mcj mRNA levels in WT liver extracts at different time points after 70% Phx. At least n = 4 were used for each experimental group. (D) MCJ levels by western blotting (upper panel) and densitometric quantification (bottom panel) in WT liver extracts at different time points after 70% Phx under IRI. ß‐Actin was used as a loading control. (E) Mcj mRNA levels in WT liver extracts at different time points after 70% Phx under IRI. n = 3 were used for sham‐operated mice, and n = 10 underwent 70% Phx under IRI. Values are represented as mean ± SEM. The Student t test was used to compare two groups, and one‐way ANOVA followed by Sidak’s posttest was used to compare multiple groups. *p < 0.05, **p < 0.01, and ****p < 0.0001 versus control and + p < 0.05, ++ p < 0.01, +++ p < 0.001, and ++++ p < 0.0001 versus the indicated time points. f.c., fold change
On the other hand, liver resection is the only curative therapy for most patients with hepatobiliary malignancies. Vascular occlusion is a common strategy to prevent loss of blood during hepatic resection. To assess the possible involvement of MCJ in liver resection that requires vascular occlusion, we examined its expression in livers from two murine models: mice subjected to (1) 70% Phx and (2) 70% hepatectomy with 30 min of ischemia (Phx with IRI). We aimed to elucidate whether alterations in MCJ occur in Phx by itself or only during Phx with vascular occlusion.
After 70% Phx, MCJ protein and mRNA levels increased progressively from 5 to 48 h before returning to baseline levels once the hepatic mass was restored after 7 days (Figure 1B,C). Similarly, in mice subjected to Phx with IRI, MCJ was up‐regulated 24 h after surgery before returning to baseline after 7 days (Figure 1D,E). These results indicate that Phx by itself induces MCJ up‐regulation. In addition, MCJ up‐regulation is maintained when vascular occlusion is applied.
In vitro Mcj silencing enhances hepatocyte proliferation following EGF treatment
We first aimed to characterize the effects of Mcj silencing and Mcj overexpression on hepatocyte proliferation. Twenty‐four hours after EGF treatment, silencing of Mcj significantly increased the mRNA and protein levels of proliferative markers compared to controls (Figure S1A,B). Conversely, MCJ up‐regulation blocked the proliferative response (Figure S1C,D). The efficiency of MCJ down‐regulation and up‐regulation was confirmed by western blot (Figure S1E).
These data show that the specific ablation of MCJ in isolated hepatocytes exclusively enhances the proliferative response.
MCJ depletion enhances liver regeneration, overcomes liver injury, and increases survival after 70% Phx with or without IRI and after prolonged IRI
In mice, the peak of regeneration (S phase) occurs 24–48 h after Phx, and liver mass is usually restored within 5–7 days.[ 21 ] Initially, we observed a faster recovery of liver weight in 3‐month‐old MCJ KO mice compared to age‐matched WT animals 48 h after Phx (Figure 2A). Importantly, no differences in the liver/body weight ratio were observed 5 days after Phx between WT and MCJ KO mice, demonstrating that the original prehepatectomy size was reached with high precision in MCJ KO mice, with no overgrowth of liver tissue. In parallel, MCJ KO mice had reduced liver damage in the postoperative period, as demonstrated by lower serum alanine aminotransferase concentrations 5 and 33 h after 70% Phx (Figure 2B). Regenerating hepatocytes in MCJ KO mice entered the cell cycle faster than those in WT mice, as reflected by an earlier increase in cyclin D1, proliferating cell nuclear antigen (PCNA), Ki67‐positive immunostaining (Figure 2C), and cyclin D1 protein levels (Figure S2A). Furthermore, the cyclin kinase inhibitor p21, a known suppressor of hepatocyte proliferation, was expressed at similar levels in both WT and MCJ KO mice 48 h after Phx (Figure S2B). Overall, these data show that the lack of MCJ accelerates liver regeneration until the “hepatostat”[ 22 ] is achieved.
FIGURE 2.
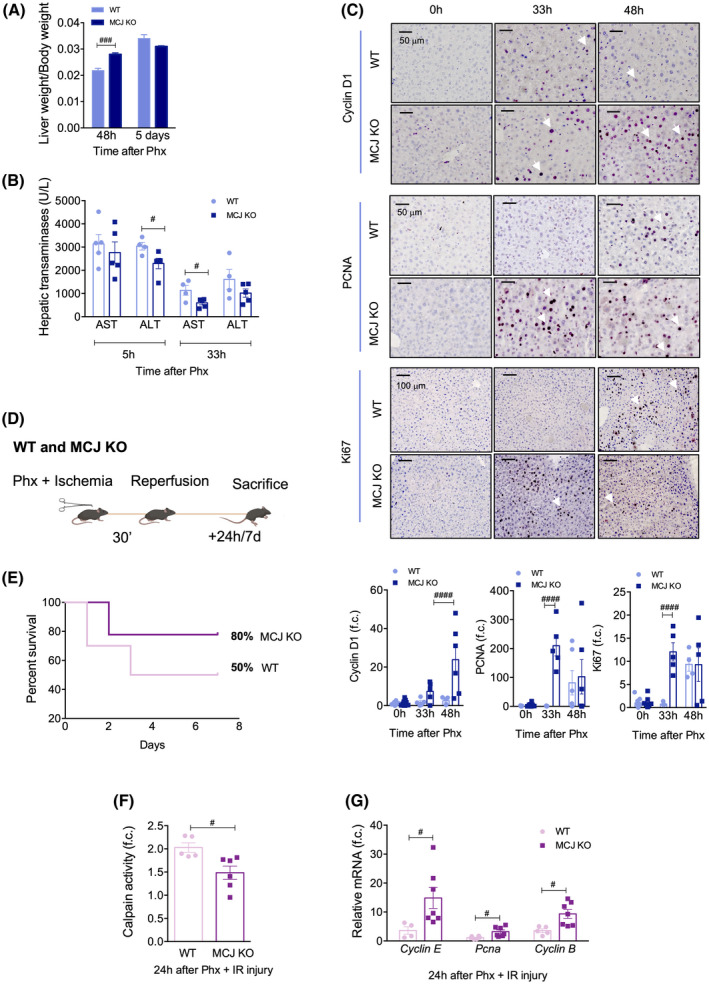
Lack of MCJ enhances graft regeneration, reduces ischemic damage, and increases survival after both 70% Phx and 70% Phx under IRI. (A) Liver weight/body weight ratio in WT and MCJ KO mice 2 and 5 days after 70% Phx. (B) Serum aspartate aminotransferase and alanine aminotransferase levels in WT and MCJ KO mice 5 and 33 h after 70% Phx. (C) Liver immunohistochemical staining and respective quantification for Cyclin D1, Ki67, and PCNA at 0, 24, 33, and 48 h after 70% Phx in WT versus MCJ KO. Scale bar, 50 µm. (D) Flowchart summarizing 70% Phx under 30 min IRI. (E) Survival percent 7 days after 70% Phx under IRI, WT (n = 10) versus MCJ KO (n = 10). (F) Hepatic calpain activity measured in WT and MCJ KO mice that underwent the procedure (n = 10) 24 h after 70% Phx under IRI, relative to sham‐operated mice (n = 2). (G) Differential expression of mRNA levels from genes involved in the cell cycle in WT and MCJ KO mice, relative to sham‐operated mice, 24 h after 70% Phx under IRI. Values are represented as mean ± SEM. Two‐way ANOVA followed by Sidak’s posttest was used to compare multiple groups. # p < 0.05, ### p < 0.001, and #### p < 0.0001 versus MCJ KO. ALT, alanine aminotransferase; AST, aspartate aminotransferase; f.c., fold change
To further demonstrate the protective role of MCJ ablation in liver regeneration, we examined the outcome of liver ischemia injury and graft regeneration after 70% Phx with vascular occlusion. We performed 70% Phx with 30 min of IRI in WT and MCJ KO mice and evaluated hepatic injury, hepatic regeneration, and survival 24 h or 7 days after the reperfusion (Figure 1D). This procedure is characterized by high mortality rates, with only about 50% of WT mice surviving[ 18 ]; we confirmed this figure in our experimental settings (Figure 2E). Interestingly, the survival rate in MCJ KO mice increased by up to 80% (Figure 2E). This was accompanied by a reduction in serum hepatic transaminases in MCJ KO mice 24 h after the procedure (Figure S2C). Diminished apoptosis and/or necrosis was also observed, as shown by the attenuated activity of calpain proteases (Figure 2F). Moreover, under these conditions, lack of MCJ resulted in significantly increased proliferative markers, including Cyclin E, Pcna, and Cyclin B (Figure 2G), compared to WT mice.
We also evaluated whether the benefits of MCJ depletion observed after 30 min of ischemia were extended after prolonged ischemic periods without hepatic resection. MCJ KO and WT mice were subjected to 90 min of ischemia (Figure S2D) and sacrificed 4 or 24 h after reperfusion, when we obtained ischemic and oxygenated lobes.[ 19 ] Serum analysis showed reduced aspartate aminotransferase and alanine aminotransferase levels in MCJ KO mice 24 h after ischemic injury (Figure S1E). This was accompanied by decreased calpain activity (Figure S2F) and reduced poly(ADP‐ribose) polymerase cleavage (Figure S2G). Regarding the regenerative response, there was a sustained increase in Cyclin D1 and Pcna in MCJ KO mice, especially in the oxygenated lobe, compared to WT and sham‐operated mice (Figure S2H).
In sum, lack of MCJ enhances liver regeneration and overcomes hepatic injury not only after Phx but also following Phx with IRI, and these effects are accompanied by an increase in posthepatectomy survival. Importantly, these protective effects are also apparent in longer ischemic periods without liver resection, resembling the conditions of LT.
MCJ absence during liver regeneration increases mitochondrial respiration and ATP synthesis
Liver regeneration is an energetically demanding process. Multiple lines of evidence have indicated that increased ATP levels after injury facilitate liver regeneration.[ 10 ] MCJ is a negative regulator of mitochondrial respiration as it inhibits complex I activity and the formation of supercomplexes, leading to a reduction in ATP synthesis.[ 13 ]
Both intracellular and extracellular ATP levels were significantly elevated in cultured hepatocytes isolated from WT and MCJ KO mice 24 h after Phx (Figure 3A). ATP can be synthesized through glycolysis in the cytosol or through oxidative phosphorylation in mitochondria. Mitochondrial respiration was evaluated in regenerative MCJ KO and WT hepatocytes in vitro 3 h after 70% Phx. Our data show a significantly increased oxygen consumption rate (OCR) and higher basal, ATP‐linked, and maximal respiration in MCJ KO hepatocytes (Figures 3B and S3A). Increased ATP production and respiration could be a consequence of an increased number of functional mitochondria in MCJ KO mice; however, electron microscopy revealed no differences in the number or morphology among basal and regenerative WT and MCJ KO mitochondria (Figure 3C and S3B). Thus, loss of MCJ during liver regeneration accelerates mitochondrial respiration and increases ATP production, inducing succinate dehydrogenase (SDH2) activity, facilitating restoration of the hepatic mass (Figure 3D). Overall, our data indicate that MCJ depletion in proliferating hepatocytes enhances mitochondrial function and ATP synthesis and secretion.
FIGURE 3.
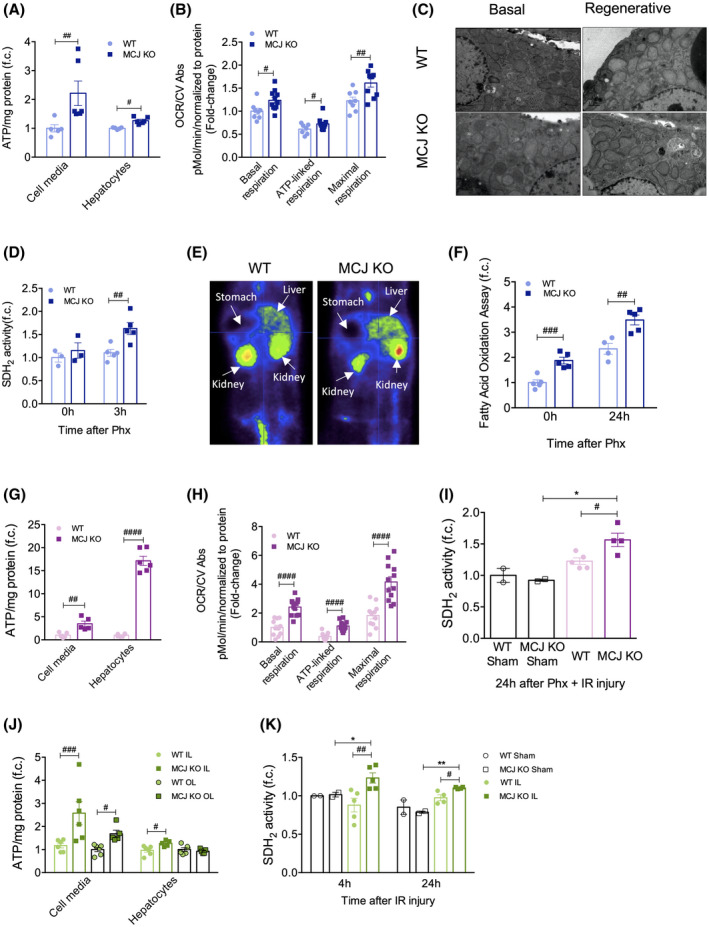
Lack of MCJ increases ATP production following 70% Phx with or without IRI and after prolonged IRI. (A) Cell media and hepatocyte ATP production in primary WT and MCJ KO hepatocytes, perfused 24 h after 70% Phx. At least quadruplicates were used for each experimental condition. (B) Basal, ATP‐linked, and maximal respirations using the Mitostress assay in primary WT and MCJ KO hepatocytes, perfused 3 h after 70% Phx. At least quadruplicates were used for each experimental condition. (C) Electron microscopy of epon‐embedded cell sections showing the number of mitochondria and mitochondrial morphology in basal conditions and 24 h after 70% Phx at ×2500 magnification (scale bar, 1 µm). (D) Hepatic SDH2 activity was measured in basal conditions and 3 h after 70% Phx in WT versus MCJ KO mice. (E) Study of the hepatic uptake of 18F fluorodeoxyglucose using a PET‐CT scan 24 h after 70% Phx in WT and MCJ KO mice. (F) Fatty acid oxidation rate was assayed in liver tissue at basal conditions and 24 h after 70% Phx. (G) Extracellular and intracellular ATP content in WT and MCJ KO primary hepatocytes, perfused 24 h after 70% Phx under IRI. (H) Basal, ATP‐linked, and maximal respirations using the Mitostress assay in primary WT and MCJ KO hepatocytes, perfused 24 h after 70% Phx under IRI. At least quadruplicates were used for each experimental condition. (I) Hepatic SDH2 activity was measured in WT and MCJ KO mice, both in sham‐operated and in those that underwent the procedure, 24 h after 70% Phx under IRI. (J) Cell media and hepatocyte ATP production in primary WT and MCJ KO hepatocytes, perfused 24 h after IRI. Hepatocytes coming from both the ischemic and the oxygenated lobes were analyzed separately. At least sextuplicates were used for each experimental condition. (K) Hepatic SDH2 activity was measured in WT and MCJ KO mice, both in sham‐operated and in those that underwent the procedure, 4 and 24 h after IRI. Values are represented as mean ± SEM. Two‐way ANOVA followed by Sidak’s posttest was used to compare multiple groups. # p < 0.05, ## p < 0.01, ### p < 0.001, and #### p < 0.0001 versus MCJ KO and *p < 0.05 versus sham‐operated. CV Abs, crystal violet absorbance; f.c., fold change; IL, ischemic lobe; OCR, oxygen consumption rate; OL, oxygenated lobe
In the early postoperative period after Phx, ATP is predominantly generated by fatty acid oxidation and, to a lesser extent, by glucose oxidation in hepatic mitochondria.[ 23 ] We observed a similar trend in the decline of blood glucose levels after Phx in both WT and MCJ KO mice (Figure S3C). Furthermore, using positron emission tomography (PET)–CT scanning, we found that glucose uptake by the liver 24 h after Phx was equal between WT and MCJ KO mice (Figures 3E and S3D). Analysis of hepatic fatty acid oxidation revealed significantly enhanced activity in MCJ KO mice, both at baseline and 24 h after Phx (Figure 3F).
The magnitude of ATP depletion during ischemia and the ability to resynthesize ATP after liver reperfusion play critical roles in graft recovery, facilitating liver regeneration. In line with our previous observations, both intracellular and extracellular ATP levels were significantly increased in MCJ KO hepatocytes 24 h after Phx with IR (Figure 3G). Mitochondrial respiration showed a significant increase in OCR; higher basal, ATP‐linked, and maximal respiration were observed in MCJ KO regenerative hepatocytes (Figures 3H and S3E). Lack of MCJ also induced an increase in SDH2 activity (Figure 3I).
Similar results were obtained following prolonged IRI. ATP production was evaluated in hepatocytes from the oxygenated and the ischemic lobes 4 h after reperfusion. ATP levels, both intracellular and in the cell culture media, were significantly higher in the ischemic lobe in the absence of MCJ (Figure 3J), along with the induction of mitochondrial SDH2 activity (Figure 3K).
In summary, MCJ depletion maintains mitochondrial function and ATP levels after Phx as well as after prolonged ischemic damage, likely preventing consequent cell death while enhancing liver regeneration after liver injury.
Lack of MCJ enhances antioxidant defenses during liver regeneration
Enhanced mitochondrial respiration is often related to increased ROS production. However, depletion of MCJ facilitates the formation of respiratory supercomplexes,[ 15 ] allowing enhanced complex I activity with a lower risk of ROS production.
Lack of MCJ prevented the hepatic production of ROS after 70% Phx, as shown by reduced levels of dihydroethidium staining in liver sections (Figure S4A) and MitoSOX in cultured hepatocytes (Figure S4B) 24 h after Phx. Reduced oxidative damage was further demonstrated by a higher glutathione/oxidized glutathione ratio in MCJ KO mice (Figure S4C). Decreased levels of MitoSOX staining were also observed in regenerative MCJ KO hepatocytes 24 h after Phx with IRI (Figure S4D).
Secreted ATP activates macrophages, enabling a faster priming phase in the absence of MCJ
Liver regeneration is a complex process involving an inflammatory response (priming phase) that is followed by the proliferation of liver cells to restore the lost mass. ATP plays a crucial role as a signaling molecule in the extracellular space; indeed, purinergic signals regulate the activation of immune cells and the subsequent cytokine production.[ 24 ] Based on these findings, we hypothesized that increased extracellular ATP levels would accelerate the activation of Kupffer cells and, therefore, enable a faster priming phase, accelerating liver regeneration, reducing liver damage, and increasing survival in mice lacking MCJ.
We initially confirmed that extracellular ATP was able to stimulate the activation of macrophages. Macrophage‐like RAW 264.7 cells, bone marrow‐derived macrophages, and hepatic Kupffer cells were treated with 3 mM ATP for 4 h; and the levels of TNF and IL‐6 were measured in the supernatants, as reported.[ 24 , 25 ] We observed significantly increased levels of both proinflammatory cytokines in ATP‐treated supernatants compared to nonstimulated cells (Figures 4A and S5A,B). Macrophage‐derived heparin‐binding EGF‐like growth factor (HB‐EGF), a ligand for the EGF receptor (EGFR), has also been identified as a mitogen for hepatocytes.[ 26 , 27 ] We observed that HB‐EGF levels were significantly up‐regulated in bone marrow–derived macrophages and Kupffer cells treated with 3 mM ATP for 4 h compared to nonstimulated cells; besides, MCJ KO showed significantly higher levels (Figures 4B and S5C).
FIGURE 4.
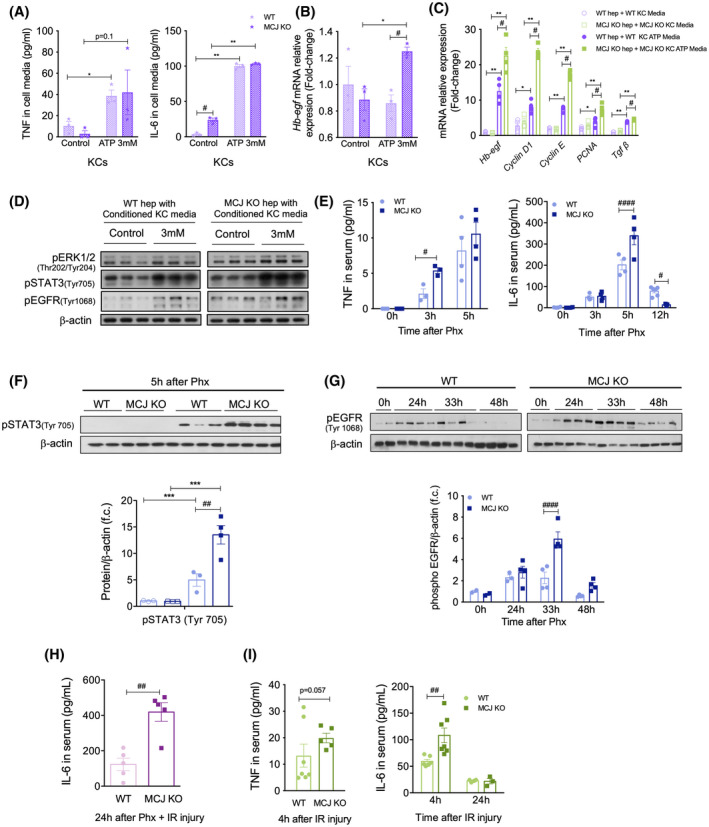
Absence of MCJ accelerates the priming phase due to increased extracellular ATP levels. (A) Cell media TNF and IL‐6 levels from stimulated and nonstimulated WT and MCJ KO hepatic Kupffer cells. Cells were stimulated with 3 mM ATP for 4 h. (B) mRNA levels of Hb‐Egf, Cyclin D1, Cyclin E, PCNA, and Tgf‐β in WT and MCJ KO hepatocytes following 24 h of treatment with stimulated and nonstimulated WT and MJC KO Kupffer cell–derived conditioned media. (C) Western blot analysis of total protein levels of phosphorylated (p‐) ERK1/2 (Thr 202/Tyr 204), pSTAT3 (Tyr 705), and pEGFR (Tyr1068) in WT and MCJ KO hepatocytes following 4 h of treatment with stimulated and nonstimulated WT and MCJ KO Kupffer cell–derived conditioned media. ß‐Actin was used as a loading control. (D) Serum TNF and IL‐6 levels, measured by ELISA, at the indicated time points after 70% Phx in WT versus MCJ KO mice. (E) Western blot analysis (upper panel) and densitometric quantification (bottom panel) of total protein levels of pSTAT3 5 h after Phx. ß‐Actin was used as a loading control. (F) Western blot analysis (upper panel) and densitometric quantification (bottom panel) of total protein levels of pEGFR at 24, 33, and 48 h after 70% Phx. ß‐Actin was used as a loading control. (G) Serum IL‐6 levels in WT and MCJ KO mice 24 h after 70% Phx under IRI. (H) Serum TNF and IL‐6 levels in WT and MCJ KO mice 24 h after IRI. Values are represented as mean ± SEM. The Student t test was used to compare two groups, and one‐way ANOVA followed by Sidak’s posttest was used to compare multiple groups. *p < 0.05, **p < 0.01, and ***p < 0.001 versus control and # p < 0.05, ## p < 0.01, and #### p < 0.0001 versus MCJ KO. f.c., fold change; KC, Kupffer cell
Kupffer cell–derived TNF and IL‐6 play essential roles in the initiation of the priming phase, and HB‐EGF promotes the G1/S transition in the hepatocyte cell cycle.[ 28 , 29 ] Thus, to confirm that ATP‐mediated Kupffer cell activation and production of inflammatory cytokines enable the initiation of hepatocyte proliferation, we performed proliferation studies with Kupffer cell–derived conditioned media. Proliferation was enhanced in both WT and MCJ KO hepatocytes stimulated with ATP‐treated Kupffer cell media; however, mRNA levels of proliferative markers Cyclin D1, Cyclin E, and Pcna were significantly higher in MCJ KO hepatocytes (Figure 4C), together with Hb‐Egf (Figure 4C). Tgfβ was also augmented following treatment with conditioned media, with significantly higher levels in MCJ KO hepatocytes, proving that not only regeneration is accelerated in these hepatocytes but also its termination (Figure 4C). Importantly, the activation of extracellular signal–regulated kinase 1/2 (ERK1/2) and signal transducer and activator of transcription 3 (STAT3) pathways, which are downstream of HB‐EGF and IL‐6 signaling, respectively, and mediators of the priming phase, along with the activation of EGFR, were increased in hepatocytes in the presence of ATP–Kupffer cell media, with this activation being significantly higher in MCJ KO hepatocytes (Figure 4D and S5D). Therefore, increased ATP levels not only enhance the priming phase but also accelerate the proliferative phase during hepatocyte proliferation.
In vivo, we found increased hepatic Tnf and Il‐6 mRNAs and serum levels in MCJ KO mice 3 h after Phx (Figures S5E and 4E), along with increased hepatic IL‐6/STAT3 signaling (Figure 4F). We also observed enhanced activation of the EGFR both by western blotting and by immunohistochemistry in MCJ KO livers 33 h after Phx (Figures 4G and S5F). Besides, the study of EGFR ligands showed increased expression of Egf, Betacellulin, and Hb‐egf in MCJ KO livers, at both early and late phases of liver regeneration (Figure S5F).
IL‐6 plays a major role in ischemia by reducing hepatic injury and promoting regeneration.[ 30 ] Notably, IL‐6 levels were significantly increased in MCJ KO mice compared to WT 24 h after 70% Phx with IRI (Figures 4H and S5G). IL‐6 levels also remained significantly higher in MCJ KO mice 4 and 24 h after prolonged IRI (Figures 4I and S5H).
Taken together, these findings confirm that increased ATP levels found in mice lacking MCJ are the driving force that enables faster entry into the cell cycle, enhancing liver regeneration.
Silencing MCJ, a therapeutic approach
MCJ‐specific siRNA (siMCJ) was evaluated after 70% Phx to assess whether it could be used as a potential therapy to accelerate liver regeneration following liver resection. Twenty‐four hours before 70% Phx, 3‐month‐old WT mice were treated by i.v. tail injection with siMCJ or si‐control (siCtrl). Thirty‐three hours after this procedure, mice were sacrificed to analyze their hepatic regenerative capacity (Figure S6A). The efficient knockdown of MCJ led to increased mRNA levels of the proliferative genes Cyclins D1, E, and A2 and Pcna (Figure 5A). This was confirmed by the increased expression of Cyclin D1 and PCNA proteins in liver sections (Figure 5B). Thus, RNA interference–mediated gene targeting of MCJ is as efficient as genetic ablation of MCJ for accelerating liver regeneration after Phx, suggesting that MCJ silencing could constitute a therapeutic alternative.
FIGURE 5.
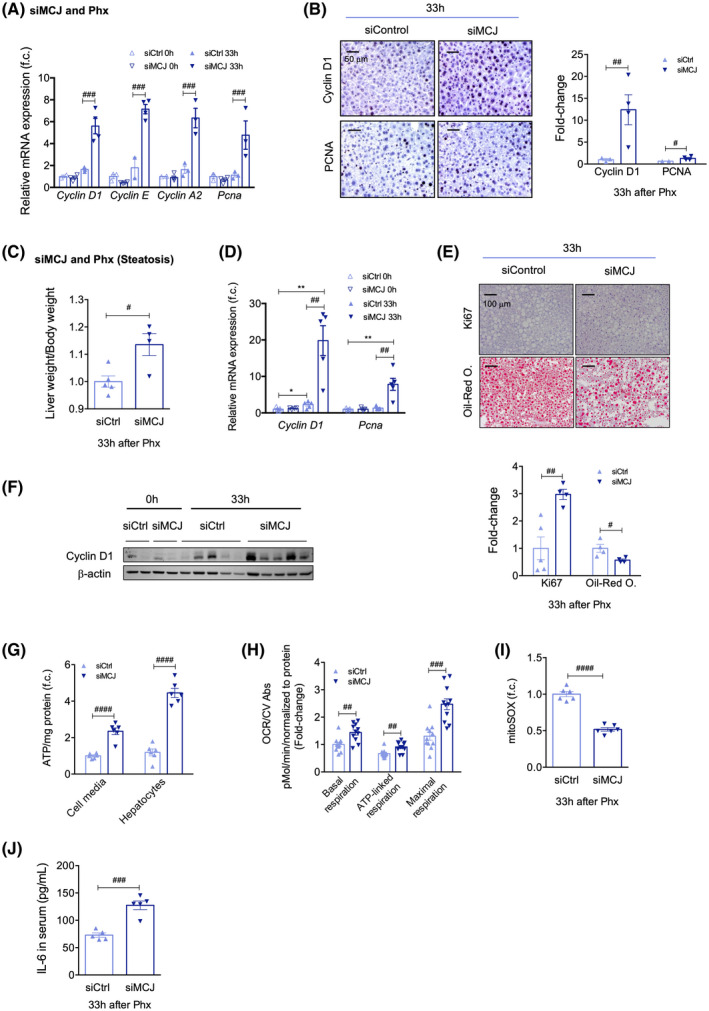
Targeting MCJ overcomes regenerative limitations associated with steatosis. (A) Differential expression of mRNA levels from genes involved in the cell cycle in siCtrl versus siMCJ WT mice, compared to basal, 33 h after 70% Phx. (B) Liver immunohistochemical staining and respective quantification for Cyclin D1 and PCNA, proliferative markers, 33 h after 70% Phx, in siCtrl versus siMCJ WT mice. Scale bar, 50 µm. (C) Liver weight/body weight ratio in siCtrl versus siMCJ WT mice, maintained on a 12‐week HFHFD, 33 h after 70% Phx. (D) Differential expression of mRNA levels from genes involved in the cell cycle in siCtrl versus siMCJ WT mice, maintained on a 12‐week HFHFD, 33 h after 70% Phx. (E) Liver immunohistochemical staining (upper panel) and respective quantification (bottom panel) for Ki67, a proliferative marker, and oil red O, a marker for hepatic steatosis, in siCtrl versus siMCJ WT mice, maintained on a 12‐week HFHFD, 33 h after 70% Phx. Scale bar, 100 µm. (F) Western blot analysis of total protein levels of Cyclin D1 33 h after 70% Phx in siCtrl versus siMCJ WT mice, maintained on a 12‐week HFHFD. ß‐Actin was used as a loading control. (G) Extracellular and intracellular ATP content in primary hepatocytes of siCtrl and siMCJ WT mice, maintained on a 12‐week HFHFD that were perfused 33 h after 70% Phx. At least sextuplicates were used for each experimental condition. (H) The OCR and basal, ATP‐linked, and maximal respirations using the Mitostress assay in primary hepatocytes of siCtrl and siMCJ WT mice, maintained on a 12‐week HFHFD, perfused 33 h after 70% Phx. At least quadruplicates were used for each experimental condition. (I) Mitochondrial ROS in primary hepatocytes of siCtrl and siMCJ WT mice, maintained on a 12‐week HFHFD, perfused 33 h after 70% Phx, using MitoSOX staining. At least sixtuplicates were used for each experimental condition. (J) Serum IL‐6 levels, measured by ELISA, 33 h after 70% Phx, in siCtrl versus siMCJ WT mice, maintained on a 12‐week HFHFD. Values are represented as mean ± SEM. The Student t test was used to compare two groups, and two‐way ANOVA followed by Sidak’s posttest was used to compare multiple groups. *p < 0.05 and **p < 0.01 versus basal and # p < 0.05, ## p < 0.01, ### p < 0.001, and #### p < 0.0001 versus MCJ KO. CV Abs, crystal violet absorbance; f.c., fold change
Targeting MCJ overcomes regenerative limitations associated with steatosis
A significant number of patients undergoing liver resection have chronic liver diseases, such as steatosis, fibrosis, or aging. These are linked to mitochondrial dysfunction and increase the risk of suffering from posthepatectomy liver failure as they severely limit liver regeneration and exacerbate the susceptibility to IRI.[ 31 ] Therefore, we examined whether MCJ silencing could reduce the susceptibility of steatotic or old livers to IRI and enhance liver regeneration.
Hepatic steatosis is a major risk factor for liver surgery, with postoperative mortality exceeding 14% after major liver resection. We used a murine model of induced steatosis, inflammation, and insulin resistance to test whether MCJ silencing could improve liver regeneration after Phx in steatotic livers. Three‐month‐old WT mice were fed the HFHFD for 12 weeks; hepatic insulin resistance development and hepatic inflammation were confirmed by an insulin tolerance test and F4/80 staining, respectively (Figure S6C,D). Mice were treated with either siMCJ or siCtrl 72 h prior to 70% Phx and euthanized 33 h after the procedure, allowing analysis of the regenerative phase (Figure S6B). MCJ‐silenced mice showed a faster recovery of liver weight, with a significantly increased liver/body weight ratio 33 h after 70% Phx (Figure 5C). These regenerating hepatocytes entered the cell cycle faster, as reflected by earlier increases in Cyclin D1 and Pcna mRNA levels (Figure 5D), Ki67‐positive immunostaining (Figures 5E and S6E), and Cyclin D1 protein levels (Figures 5F and S6F). MCJ silencing not only accelerated liver regeneration in WT mice fed a 12‐week HFHFD but also significantly reduced hepatic steatosis, as demonstrated by decreased oil‐red O staining levels (Figures 5E and S6E), confirming previous results.[ 16 ]
Both extracellular and intracellular ATP levels were measured in cultured hepatocytes isolated from siCtrl‐treated and siMCJ‐treated mice 24 h after Phx, and in line with previous results, they were significantly elevated in MCJ‐silenced hepatocytes (Figure 5G). Furthermore, mitochondrial respiration measured 24 h after 70% Phx showed a significant increase in OCR and higher basal, ATP‐linked, and maximal respiration in MCJ‐silenced hepatocytes (Figures 5H and S6G) without ROS overproduction as lower MitoSOX staining levels were observed (Figure 5I). Additionally, increased serum levels of IL‐6 were found in MCJ‐silenced mice (Figure 5J). Thus, MCJ silencing proves to be an efficient approach to accelerate mitochondrial activity and overcome the regenerative limitations that characterize insulin‐resistant and steatotic livers following a 12‐week HFHFD.
Targeting MCJ overcomes regenerative and survival limitations associated with aging
Only 8%–15% of patients undergoing hepatic resection are older than 70 years due to the increasing prevalence of comorbidities that confer a high surgical risk.[ 32 ] WT mice aged 15–17 months were treated with either siCtrl or siMCJ, and 24 h later, 70% Phx was performed. The mice were sacrificed 72 h after the procedure. MCJ silencing was equally efficient in aged (Figure S7A) and young mice. We found that hepatic steatosis was significantly corrected by MCJ silencing, as demonstrated by oil‐red O staining (Figure 6A). This was accompanied by an increase in PCNA staining, showing enhanced liver regeneration (Figure 6A).
FIGURE 6.
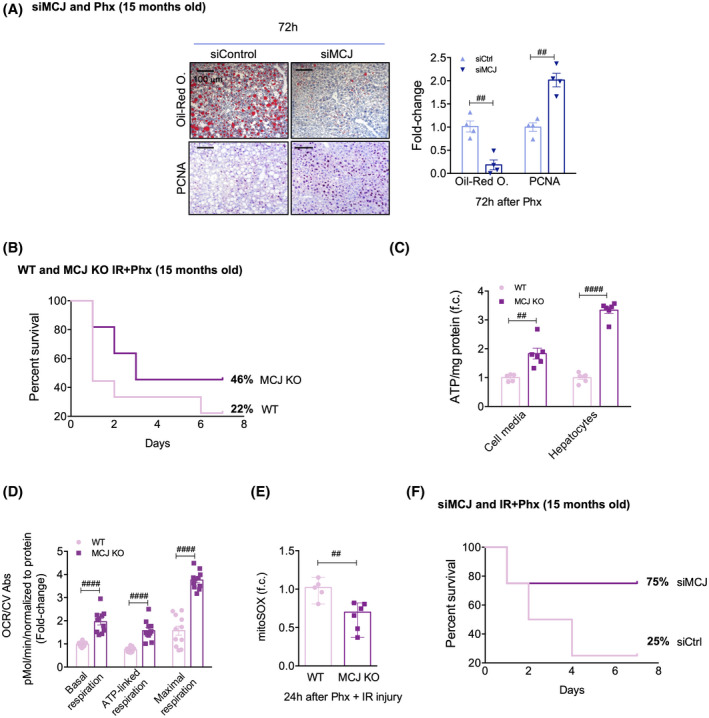
Targeting MCJ overcomes regenerative and survival limitations associated with aging. (A) Picture of the extracted livers and the liver weight/body weight ratio in siCtrl and siMCJ WT mice 72 h after 70% Phx. (B) Liver immunohistochemical staining and respective quantification for oil red O staining, a marker for hepatic steatosis, and for PCNA, a proliferative marker, 72 h after 70% Phx in siCtrl versus siMCJ WT. Scale bar, 100 µm. (C) Survival percent 7 days after 70% Phx under IRI in 15‐month‐old WT (n = 9) and MCJ KO (n = 11) mice. (D) Cell media and hepatocyte ATP production in primary hepatocytes of 15‐month‐old WT and MCJ KO mice, perfused 24 h after 70% Phx under IRI. At least sextuplicates were used for each experimental condition. (E) Basal, ATP‐linked, and maximal respirations using the Mitostress assay in primary hepatocytes of 15‐month‐old WT and MCJ KO mice perfused 24 h after 70% Phx under IRI. At least quadruplicates were used for each experimental condition. (F) Mitochondrial ROS in primary hepatocytes of 15‐month‐old WT and MCJ KO mice, perfused 24 h after 70% Phx under IRI, using MitoSOX staining. At least quadruplicates were used for each experimental condition. (G) Survival percent 7 days after 70% Phx under IRI in 15‐month‐old siCtrl (n = 4) versus siMCJ (n = 4) WT mice. Values are represented as mean ± SEM. The Student t test was used to compare two groups, and two‐way ANOVA followed by Sidak’s posttest was used to compare between multiple groups. ## p < 0.01 and #### p < 0.0001 versus MCJ KO. CV Abs, crystal violet absorbance; f.c., fold change
The survival rate following 70% Phx with IR was also studied in 15‐month‐old MCJ KO and siMCJ‐treated mice. Fifteen‐month‐old MCJ KO and WT mice were subjected to 70% Phx with 30 min of ischemia, and the survival rate was assessed 7 days after the procedure (Figure S7B). Only 22% of WT mice survived, while half of MCJ KO mice recovered from the procedure (Figure 6B). Aging is associated with a variety of diminished mitochondrial functions, and decreased mitochondrial ATP production in old livers results in lower tolerance to IRI.[ 7 ] Thus, 24 h after Phx with IRI, extracellular and intracellular ATP levels were measured in cultured hepatocytes isolated from aged WT and MCJ KO mice. ATP levels were significantly elevated in MCJ KO hepatocytes (Figure 6C). Mitochondrial respiration measured 24 h after Phx with IRI showed significantly higher basal, ATP‐linked, and maximal respiration in MCJ KO hepatocytes (Figure 6D), which was not accompanied by elevated ROS production as lower MitoSOX staining levels were detected (Figure 6E).
Furthermore, 15‐month‐old WT mice were treated with either siMCJ or siCtrl 72 h prior to 70% Phx with IR, and they were sacrificed 7 days after the procedure. Efficient MCJ silencing was confirmed (Figure S7C). Mice treated with siCtrl achieved a similar 25% survival rate when compared to 15‐month‐old WT mice. Notably, 75% of the siMCJ‐treated mice survived the procedure, confirming the positive regenerative outcomes already observed in silenced mice subjected to 70% Phx (Figure 6F). Thus, inhibiting MCJ expression reduces both hepatomegaly and steatosis and enhances hepatic regeneration, thereby improving survival in 15‐month‐old mice.
Overall, the loss of MCJ, increasing ATP production and, therefore, preventing the characteristic ATP depletion, overcomes impaired regeneration and reduces ischemic susceptibility in “marginal” organs (steatotic livers or those originating from old donors), making them suitable for hepatic surgery and LT.
DISCUSSION
LT is the only curative treatment for acute liver failure and end‐stage liver diseases. However, < 10% of the global organ transplantation needs are met at the current rates of transplantation procedures.[ 33 ] Strategies to improve the donor pool, quickly attend to patients on the waiting list, and ease the economic burden are crucial. Based on recent data showing that the lack of MCJ, a negative regulator of complex I in the mitochondrial electron transport chain, enhances mitochondrial activity without collateral ROS production in NASH in DILI and cholestatic liver injury,[ 15 , 16 , 34 ] we studied its effect after major liver resection or IRI. With these results, we demonstrate that hepatic MCJ silencing can play a unique role in reducing liver damage, accelerating regeneration, and increasing survival after Phx with or without ischemia. Our data suggest that the lack of MCJ prevents the mitochondrial dysfunction and ATP depletion that take place during the ischemic phase by improving mitochondrial activity without any additional ROS production. More importantly, the beneficial effects of MCJ silencing were observed not only in young but also in old murine models of metabolic syndrome. These results highlight MCJ as a key therapeutic target for liver regeneration and IRI. The significantly higher levels of MCJ in livers from DCD donors after ischemic damage compared to healthy controls support this conclusion.
Liver regeneration is determined by the energy status of the hepatocyte. In this setting, increased expression of MCJ, a mitochondrial complex I inhibitor, after major hepatic resection with or without vascular occlusion is surprising. We have previously shown that the expression of MCJ is regulated by the methylation of three specific CpG sites in its promotor.[ 16 ] Indeed, this epigenetic process is inversely correlated with expression, where lower DNA methylation is associated with higher levels of transcripts. In this context, S‐adenosylmethionine (SAMe) levels are known to decrease significantly during liver regeneration due to a switch between expression of methionine adenosyltransferase genes 1A and 2A (MAT1A and MAT2A)[ 35 ] along with increased S‐adenosyl homocysteine (SAH) formation. Therefore, the ratio SAMe/SAH, an index for methylation reactions, is significantly reduced, which causes the previously observed MCJ overexpression during Phx and ischemic damage.
Following liver injury, there is a need for balanced hepatocyte proliferation. However, excessive mitochondrial activity can overproduce ROS, which, instead of healing, damage the hepatic tissue. Previously, we showed that the lack of MCJ facilitates the formation of respiratory supercomplexes, improving the effectiveness of mitochondrial respiration and reducing the formation of ROS.[ 15 ] Our study confirms these results when MCJ is silenced during liver regeneration and after ischemic damage.
Mitochondrial function also correlates with posthepatectomy liver function.[ 36 ] Alexandrino et al. demonstrated that depressed oxidative phosphorylation was correlated with worse postoperative hepatic function and with increased risk of posthepatectomy liver failure in a cohort of 30 patients.[ 37 ] We observed significantly higher ATP levels in MCJ‐silenced mice in all three models (Phx, Phx + IRI, and IRI), along with accelerated entry into the cell cycle, associated with a faster recovery of liver weight after Phx and reduced liver damage after IRI. The liver weight/body weight ratio after Phx and Phx with IRI (data not shown) confirms that the lack of MCJ promotes accelerated liver regeneration until the “hepatostat” is achieved. Therefore, the lack of MCJ represents a potential mechanism for improving mitochondrial activity to rapidly meet the energy demands of liver regeneration while avoiding the harmful side effects of oxidative stress.
The initiation of liver regeneration is driven by the innate immune system and cytokine release. Kupffer cell–derived IL‐6 trans‐signaling, besides being critically involved in normal liver regeneration,[ 28 ] also plays a key protective role in chronic[ 38 ] and acute[ 39 ] liver diseases. Importantly, a recent study associated preoperative higher levels of serum IL‐6 and TNF with early liver graft regeneration following LT.[ 40 ] Ishimaru et al. showed the importance of purinergic signaling in the activation of Kupffer cells as ATP‐stimulated Kupffer cells produced significantly higher IL‐6 levels when compared to nonstimulated cells.[ 24 ] We have shown similar results in macrophage‐like RAW 264.7, bone marrow–derived monocytes/macrophages (BMMs) and hepatic Kupffer cells in the presence of ATP, not only with IL‐6 but also with TNF. Besides, ATP‐stimulated MCJ KO BMM and Kupffer cells showed significantly higher levels of HB‐EGF, an activator of the ERK1/2 pathway and a ligand for EGFR. Importantly, this mitogen has been considered the key factor for hepatocyte progression through G1/S transition during regeneration.[ 29 ]
Based on the results of our proliferative studies in primary WT and MCJ KO hepatocytes using Kupffer cell–derived conditioned media, we hypothesize that the increased ATP level found in mice lacking MCJ is the driving force that enables the accelerated regenerative response and explains the 12‐h gap between WT and MCJ KO regeneration peaks (S phase). Furthermore, the modulation of regenerative responsiveness enabled by the absence or overexpression of MCJ, in isolated primary hepatocytes after EGF treatment, suggests a synergistic mechanism based on increased extracellular and intracellular ATP levels.
In order to meet increased ATP requirements, hepatocytes undergo a series of metabolic adaptations. Phx‐induced hypoglycemia has been established to promote liver regeneration by inducing specific proregenerative signals and suppressing certain antiregenerative pathways.[ 41 ] We confirmed similar hypoglycemic levels in MCJ WT and MCJ KO mice after Phx, and by PET‐CT scanning we excluded the possibility of a more glycolytic phenotype in MCJ KO mice. Importantly, we identified that hepatic fatty acid oxidation provides the energy required for liver regeneration after major resection or ischemic damage.
Hepatic IRI is commonplace in liver surgery, particularly in hepatic transplantation, hepatic resection, and trauma. It is also the leading cause of immediate posttransplantation organ failure; the lack of oxygen during ischemia directly affects mitochondrial coupling as ATP is depleted and liver injury is further exacerbated during reperfusion.[ 42 ] Recovering ATP levels is not a new therapeutic approach; ischemic preconditioning, a brief period of portal triad occlusion and reperfusion before sustained IR, induces adenosine‐mediated tissue protection.[ 12 ] Studies proving the hepatoprotective effects of purinergic signaling agonists after IR and LT[ 43 ] highlight the need for a solution that silencing of MCJ can offer.
Moreover, LT faces a major organ shortage. Among the strategies to improve the donor pool, the use of extended‐criteria livers has been proposed. Unfortunately, the use of these marginal organs increases the incidence of allograft dysfunction and postreperfusion syndrome.[ 1 , 3 ] Therefore, we decided to mimic steatotic and aging mouse models and study the effects of MCJ silencing on hepatic damage, liver regeneration, and survival after Phx with IRI.
Recipients of organs from old donors may show increased posttransplantation morbidity and mortality due to enhanced susceptibility to IRI. Mitochondria in aged livers produce less ATP and more free radicals,[ 44 ] and because intracellular energy metabolism is considered the key mechanism in the ischemic phase, aging allografts respond poorly. Importantly, our data show a 75% survival rate in 17‐month‐old MCJ‐silenced mice subjected to Phx with IR after silencing through a single tail vein 72 h before the procedure, compared to the 25% survival rate achieved in WT mice. We also carried out 70% Phx in 15–17‐month‐old WT mice and observed enhanced liver regeneration and reduced hepatic steatosis with MCJ silencing, along with increased mitochondrial activity and ATP production. Thus, MCJ silencing reduces susceptibility to ischemic damage in old livers by improving mitochondrial activity and ATP production.
Hepatic steatosis is a major risk factor for liver surgery, with postoperative mortality exceeding 14% after major liver resection compared to 2% in patients with nonfatty livers.[ 45 ] Steatosis is associated with compromised mitochondrial function and insufficient ATP production, causing an unfavorable necrotic form of cell death when subjected to periods of ischemia.[ 46 ] Our results show increased mitochondrial activity and elevated ATP production, which exert a protective effect in fatty livers, causing accelerated liver regeneration in MCJ‐silenced mice fed an HFHFD for 12 weeks when compared to WT mice. A simple tail vein injection 72 h before the procedure was able to overcome the impaired liver regeneration associated with hepatic steatosis.
Importantly, Barbier‐Torres et al.[ 16 ] used Food and Drug Administration–approved nanoparticle‐formulated and GalNAc‐formulated siRNA to efficiently target hepatic MCJ, laying the foundation for a direct, fast, and already accessible therapeutic approach that improves the recovery and well‐being of transplanted or resected patients as MCJ silencing can be maintained until the patients are fully rehabilitated. The fact that MCJ KO mice are healthy further supports the minimal toxicity of siMCJ as a therapy. Overall, the silencing of MCJ could become a means of obtaining the long‐sought balance between the number of organs needed and the number of organs available for LT by recovering and maintaining mitochondrial activity and ATP levels, thereby enabling the successful use of extended‐criteria donor livers.
CONFLICT OF INTEREST
Dr. Martínez‐Chantar advises for Mitotherapeutix LLC. All other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Conception or design of the work: Naroa Goikoetxea‐Usandizaga, Marta Varela‐Rey, and María Luz Martínez‐Chantar. Acquisition, analysis, or interpretation of data: Naroa Goikoetxea‐Usandizaga, Marina Serrano‐Maciá, Teresa C Delgado, Jorge Simón, David Fernández Ramos, Diego Barriales, Maria E. Cornide, Mónica Jiménez, Marina Pérez‐Redondo, Sofia Lachiondo‐Ortega, Rubén Rodríguez‐Agudo, Maider Bizkarguenaga, Juan Diego Zalamea, Samuel T. Pasco, Daniel Caballero‐Díaz, Miren Bravo, Irene González‐Recio, Maria Mercado‐Gómez, Clàudia Gil‐Pitarch, Jordi Gracia‐Sancho, Leticia Abecia, Óscar Lorenzo, Paloma Martín‐Sanz, Nicola G. A. Abrescia, Guadalupe Sabio, Mercedes Rincón, Juan Anguita, Eduardo Miñambres, César Martín, Marina Berenguer, Isabel Fabregat, Marta Casado, and Carmen Peralta. Drafting the work: Naroa Goikoetxea‐Usandizaga, Marta Varela‐Rey, and María Luz Martínez‐Chantar. Substantive revision of the work: Naroa Goikoetxea‐Usandizaga, Marta Varela‐Rey, and María Luz Martínez‐Chantar.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
We thank MINECO for the Severo Ochoa Excellence Accreditation to CIC bioGUNE (SEV‐2016‐0644). We acknowledge Begoña Rodríguez Iruretagoyena for the technical support provided.
Goikoetxea‐Usandizaga N, Serrano‐Maciá M, Delgado TC, Simón J, Fernández Ramos D, Barriales D, et al. Mitochondrial bioenergetics boost macrophage activation, promoting liver regeneration in metabolically compromised animals. Hepatology. 2022;75:550–566. doi: 10.1002/hep.32149
Marta Varela‐Rey and María Luz Martínez‐Chantar are joint senior authors.
Funding information
Supported by grants from Ministerio de Ciencia, Innovación y Universidades MICINN (PID2020‐117116RB‐100, RTI2018‐096759‐A‐100, RTI2018‐095114‐B‐I00, PID2019‐108977RB‐100 and RTI2018‐095700‐B100, integrado en el Plan Estatal de Investigación Cientifica y Técnica y Innovación, cofinanciado con Fondos FEDER, to M.L.M.‐C., T.C.D., C.P., P.M.‐S., and N.G.A.A., respectively), Subprograma Retos Colaboración RTC2019‐007125‐1; Fundación Científica de la Asociación Española Contra el Cáncer (AECC Scientific Foundation) Rare Tumor Calls 2017 (to M.L.M.‐C.); Asociación Española contra el Cáncer (to T.C.D. and M.S.‐M); La Caixa Foundation Program (HR17‐00601, to M.L.M.‐C.), Proyectos Investigacion en Salud DTS20/00138 (to M.L.M.‐C.); Departamento de Industria del Gobierno Vasco (to M.L.M.‐C.); Departamento de Educación del Gobierno Vasco (to N.G.‐U. and J.S.); Acción Estratégica Ciber Emergentes 2018 (Ciberehd‐ISCIII) and Gilead Sciences International Research Scholars Program in Liver Disease (to M.V.‐R.); Ciberehd_ISCIII_MINECO is funded by the Instituto de Salud Carlos III
Contributor Information
Marta Varela‐Rey, Email: mlmartinez@cicbiogune.es.
María Luz Martínez‐Chantar, Email: mvarela.ciberehd@cicbiogune.es, Email: mlmartinez@cicbiogune.es.
REFERENCES
- 1. Trapero‐Marugán M, Little EC, Berenguer M. Stretching the boundaries for liver transplant in the 21st century. Lancet Gastroenterol Hepatol. 2018;3:803–11. [DOI] [PubMed] [Google Scholar]
- 2. Jadlowiec CC, Taner T, Von WJ. Liver transplantation current status and challenges. World J Gastroenterol. 2016;22:4438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M, et al. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidende, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 4. Vinaixa C, Selzner N, Berenguer M. Fat and liver transplantation: clinical implications. Transpl Int. 2018;31:828–37. [DOI] [PubMed] [Google Scholar]
- 5. Jaime FD, Berenguer M, Fernando A, Martorell A. Pushing the donor limits: deceased donor liver transplantation using organs from octogenarian donors. Liver Transplant. 2017;23:S22–6. [DOI] [PubMed] [Google Scholar]
- 6. Van Mierlo KMC, Schaap FG, Dejong CHC, Damink SWMO. Liver resection for cancer: new developments in prediction, prevention and management of postresectional liver failure. J Hepatol. 2016;65:1217–31. [DOI] [PubMed] [Google Scholar]
- 7. Selzner M, Selzner N, Jochum W, Graf R, Clavien P. Increased ischemic injury in old mouse liver: an ATP‐dependent mechanism. Liver Transplant. 2007;13:382–90. [DOI] [PubMed] [Google Scholar]
- 8. Martins RM, Teodoro JS, Furtado E, Rolo AP, Palmeira CM, Tralhão JG, et al. Recent insights into mitochondrial targeting strategies in liver transplantation. Int J Med Sci. 2018;15:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vajdová K, Graf R, Clavien P. ATP‐supplies in the cold‐preserved liver: a long‐neglected factor of organ viability. Hepatology. 1987;36:1543–52. [DOI] [PubMed] [Google Scholar]
- 10. Gonzales E, Julien B, Serrière‐Lanneau V, Nicou A, Doignon I, Lagoudakis L, et al. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol. 2013;52:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien P. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy. Ann Surg. 2007;245:923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang P, Jia J, Zhang D. Purinergic signalling in liver diseases: pathological functions and therapeutic opportunities. J Hepatol Rep. 2020;2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatle KM, Gummadidala P, Navasa N, Bernardo E, Dodge J, Silverstrim B, et al. MCJ/DnaJC15, an endogenous mitochondrial repressor of the respiratory chain that controls metabolic alterations. Mol Cell Biol. 2013;33:2302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Navasa N, Martín I, Iglesias‐Pedraz JM, Beraza N, Atondo E, Izadi H, et al. Regulation of oxidative stress by methylation‐controlled J protein controls macrophage responses to inflammatory insults. J Infect Dis. 2015;211:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barbier‐Torres L, Iruzubieta P, Fernández‐Ramos D, Delgado TC, Taibo D, Guitiérrez‐de‐Juan V, et al. The mitochondrial negative regulator MCJ is a therapeutic target for acetaminophen‐induced liver injury. Nat Commun. 2017;8:2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barbier‐Torres L, Fortner KA, Iruzubieta P, Delgado TC, Giddings E, Chen Y, et al. Silencing hepatic MCJ attenuates non‐alcoholic fatty liver disease (NAFLD) by increasing mitochondrial fatty acid oxidation. Nat Commun. 2020;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. López‐Luque J, Caballero‐Díaz D, Martinez‐Palacián A, Roncero C, Moreno‐Càceres J, García‐Bravo M, et al. Dissecting the role of epidermal growth factor receptor catalytic activity during liver regeneration and hepatocarcinogenesis. Hepatology. 2016;63:604–19. [DOI] [PubMed] [Google Scholar]
- 18. Bujaldon E, Cornide‐Petronio ME, Gulfo J, Rotondo F, Ávalos de León C, Negrete‐Sánchez E, et al. Relevance of VEGFA in rat livers subjected to partial hepatectomy under ischemia–reperfusion. J Mol Med. 2019;97:1299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Motiño O, Francés DE, Casanova N, Fuertes‐Agudo M, Cucarella C, Flores JM, et al. Protective role of hepatocyte cyclooxygenase‐2 expression against liver ischemia–reperfusion injury in mice. Hepatology. 2019;70:650–65. [DOI] [PubMed] [Google Scholar]
- 20. Hessheimer AJ, Coll E, Torres F, Ruíz P, Gastaca M, Rivas JI, et al. Normothermic regional perfusion vs. super‐rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol. 2019;70:658–65. [DOI] [PubMed] [Google Scholar]
- 21. Forbes SJ, Newsome PN. Liver regeneration—mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13:473–85. [DOI] [PubMed] [Google Scholar]
- 22. Michalopoulos GK. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384–92. [DOI] [PubMed] [Google Scholar]
- 23. Nakatani T, Ozawa K, Asano M. Changes in predominant energy substrates after hepatectomy. Life Sci. 1981;28:257–64. [DOI] [PubMed] [Google Scholar]
- 24. Ishimaru M, Yusuke N, Tsukimoto M, Harada H, Takenouchi T, Kitani H, et al. Purinergic signaling via P2Y receptors up‐mediates IL‐6 production by liver macrophages/Kupffer cells. J Toxicol Sci. 2014;39:413–23. [DOI] [PubMed] [Google Scholar]
- 25. Soni S, O'Dea KP, Tan YY, Cho K, Abe E, Romano R, et al. ATP redirects cytokine trafficking and promotes novel membrane TNF signaling via microvesicles. FASEB J. 2019;33:6442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ødegård J, Sondresen JE, Aasrum M, Tveteraas IH, Guren TK, Christoffersen T, et al. Differential effects of epidermal growth factor (EGF) receptor ligands on receptor binding, downstream signalling pathways and DNA synthesis in hepatocytes. Growth Factors. 2017;35:239–48. [DOI] [PubMed] [Google Scholar]
- 27. Wen HJ, Gao S, Wang Y, Ray M, Magnuson MA, Wright CVE, et al. Myeloid cell–derived HB‐EGF drives tissue recovery after pancreatitis. Cell Mol Gastroenterol Hepatol. 2019;8:173–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fazel Modares N, Polz R, Haghighi F, Lamertz L, Behnke K, Zhuang Y, et al. IL‐6 trans‐signaling controls liver regeneration after partial hepatectomy. Hepatology. 2019;70:2075–91. [DOI] [PubMed] [Google Scholar]
- 29. Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, et al. Heparin‐binding epidermal growth factor‐like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562–8. [DOI] [PubMed] [Google Scholar]
- 30. Selzner M, Camargo CA, Clavien P. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin‐6. Hepatology. 1999;30:469–75. [DOI] [PubMed] [Google Scholar]
- 31. Alexandrino H, Rolo A, Tralhão JG. Mitochondria in liver regeneration: energy metabolism and posthepatectomy liver dysfunction. In: Mitochondrial biology and experimental therapeutics; 2018. p. 127–52. https://link.springer.com/chapter/10.1007/978‐3‐319‐73344‐9_8# [Google Scholar]
- 32. Fernandes AI, Tralhão JG, Abrantes A, Hoti E, Alexandrino H, Oliveiros B, et al. Functional hepatocellular regeneration in elderly patients undergoing hepatectomy. Liver Int. 2015;35:1116–23. [DOI] [PubMed] [Google Scholar]
- 33. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–71. [DOI] [PubMed] [Google Scholar]
- 34. Iruzubieta P, Goikoetxea‐Usandizaga N, Barbier‐Torres L, Serrano‐Maciá M, Fernández‐Ramos D, Fernández‐Tussy P, et al. Boosting mitochondria activity by silencing MCJ overcomes cholestasis‐induced liver injury. JHEP Rep. 2021;3:100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen L, Zeng Y, Yang H, Lee TD, French SW, Corrales FJ, et al. Impaired liver regeneration in mice lacking methionine adenosyltransferase 1A. FASEB J. 2004;18:914–6. [DOI] [PubMed] [Google Scholar]
- 36. Alexandrino H, Rolo A, Teodoro JS, Donato H, Martins R, Serôdio M, et al. Bioenergetic adaptations of the human liver in the ALPPS procedure—how liver regeneration correlates with mitochondrial energy status. HPB (Oxford). 2017;19:1091–103. [DOI] [PubMed] [Google Scholar]
- 37. Alexandrino H, Varela AT, Teodoro JS, Martins MA, Rolo AP, Tralhão JG, et al. Mitochondrial bioenergetics and posthepatectomy liver dysfunction. Eur J Clin Invest. 2016;46:627–35. [DOI] [PubMed] [Google Scholar]
- 38. Streetz KL, Tacke F, Leifeld L, Wüstefeld T, Graw A, Klein C, et al. Interleukin 6/gp130‐dependent pathways are protective during chronic liver diseases. Hepatology. 2003;38:218–29. [DOI] [PubMed] [Google Scholar]
- 39. Gao RY, Wang M, Liu Q, Feng D, Wen Y, Xia Y, et al. Hypoxia‐inducible factor (HIF)‐2a reprograms liver macrophages to protect against acute liver injury via the production of interleukin‐6. Hepatology. 2020;71:2105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chae MS, Moon KU, Chung HS, Park CS, Lee J, Choi JH, et al. Serum interleukin‐6 and tumor necrosis factor‐α are associated with early graft regeneration after living donor liver transplantation. PLoS ONE. 2018;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang J, Schriefer AE, Cliften PF, Dietzen D, Kulkarni S, Sing S, et al. Postponing the hypoglycemic response to partial hepatectomy delays mouse liver regeneration. Am J Pathol. 2016;186:587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soares ROS, Losada DM, Jordani MC, Paulo É, Castro‐e‐Silva O. Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int J Med Sci. 2019;20:5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang LM, Zhu J‐F, Wang F, Qian J, Zhu J, Mo Q, et al. Activation of adenosine A2A receptor attenuates inflammatory response in a rat model of small‐for‐size liver transplantation. Transplant Proc. 2010;42:1915–20. [DOI] [PubMed] [Google Scholar]
- 44. Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–86. [DOI] [PubMed] [Google Scholar]
- 45. Behm KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–8. [DOI] [PubMed] [Google Scholar]
- 46. Selzner M, Clavien P. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


