Abstract
Background
Multiple types of vaccinations are associated with lower risk for dementia, but it is not known if receiving more than one vaccination type is associated with a greater decrease in incident dementia as compared with receiving only one type. We determined if dementia risk is lowest in patients who receive both herpes zoster (HZ) and tetanus, diphtheria, pertussis (Tdap) vaccinations as compared with receipt of only one or the other type of vaccination.
Methods
Primary analysis in a Veterans Health Administration (VA) cohort was replicated in private sector medical claims data. Eligible patients were ≥65 years of age and free of dementia for 2 years prior to baseline (VHA n = 80,070; MarketScan n = 129,200). At index, patients either had both HZ and Tdap, only HZ, only Tdap, or neither vaccination. Confounding was controlled with generalized boosted propensity scores and inverse probability of treatment weighting. Competing risk (VHA) and Cox proportional hazard (MarketScan) models estimated the association between vaccination status and incident dementia.
Results
VHA patients' mean age was 76.8 ± 7.6 years, 4.4% were female and 90.9% were White, and MarketScan patients' mean age was 70.5 ± 5.9 and 65.4% were female. In both cohorts, having both HZ and Tdap vaccinations compared with no vaccination was significantly associated with lower dementia risk (VHA HR = 0.50; 95% CI: 0.43–0.59; MarketScan HR = 0.58; 95% CI: 0.38–0.89). In both cohorts, compared with neither vaccination, patients with only one or the other vaccination types had a significantly lower risk for dementia. Incident dementia was lower in patients with both vaccinations versus only one vaccination type.
Conclusions and Relevance
Receiving two types of vaccinations versus one type was associated with lower dementia risk. Vaccinations may have non‐specific associations with incident dementia. Low cost and accessible, common adult vaccinations may be an overlooked intervention for reducing dementia risk.
Keywords: cohort, dementia, epidemiology, infectious disease, vaccination
Key points
Is incidence of dementia different between older adults who receive both herpes zoster (HZ) and a tetanus, diphtheria, pertussis (Tdap) vaccine versus those who receive only one or the other vaccine?
In two patient cohorts, (Veterans Health Administration data and MarketScan claims data) receipt of HZ alone or Tdap alone, compared with no vaccination, was associated with a statistically significant 25% and 18% lower risk for dementia, respectively.
Results were robust and conclusions unchanged after accounting for potential healthy adherer bias, socioeconomic status, and results from a negative outcome control analysis.
Why does this paper matter?
Receipt of both vaccinations was associated with a lower dementia incidence compared with receiving only one type of vaccination and no vaccination, which suggests vaccinations have a common mechanism in the link with incident dementia.
INTRODUCTION
Animal and human studies have provided support for infectious etiologies of Alzheimer's disease (AD) and dementia. 1 Viral infection may exacerbate inflammation and oxidation in the aging brain and precipitate cerebrovascular damage leading to dementia. 2 , 3 , 4 , 5 , 6 , 7 , 8 Treatment and/or prevention of viral infection may reduce neurocognitive impairment through this mechanism. 4 , 5 With respect to prevention, a range of vaccines, including influenza, herpes zoster (HZ) and tetanus, diphtheria and pertussis (Tdap), are associated with a reduced dementia risk. 9 , 10 , 11 , 12 , 13
Training of the immune system with a lifetime of vaccinations may lower dementia risk by decreasing neuroinflammation. This may explain the dose–response relationship observed in an analysis of Taiwanese National Health data. Compared with unvaccinated patients, the risk of dementia decreased with increasing number of annual influenza vaccinations. 13 Our own analysis of Veterans Health Administration (VHA) data suggests reduced dementia risk requires ≥6 annual influenza vaccines as compared with none. 14 However, accurate measurement of influenza vaccination status in the United States is a challenge because many persons obtain vaccinations outside their healthcare system, 15 and we lack a national immunization registry. 16 Overall, the dose–response relationship between vaccination and dementia has not been well described.
The present study was designed to first determine if there is a dose–response relationship between the number of different adult vaccinations received and incident dementia among patients ≥65 years of age. We determined if patients who had both the HZ and Tdap vaccinations had a lower risk for dementia than those with HZ alone, Tdap alone, or neither. Second, we employed an active comparator design to test whether dementia risk differed between patients who received only HZ versus only Tdap. Third, we conducted analysis in a VHA patient cohort with replication in a private sector MarketScan claims database.
METHODS
Design
This was a secondary analysis of de‐identified data and was deemed non‐human subjects research by the Saint Louis University Institutional Review Board. This retrospective cohort study followed STROBE reporting guidelines (Table S1).
Data sources
National VHA administrative medical record data included observation time from fiscal years (FY) FY09–FY19 (October 1, 2008–September 30, 2019). VHA data included ICD‐9 and ICD‐10 diagnostic codes, medication dispensing, current procedural terminology (CPT) codes, vital signs, laboratory results, type of clinic visit (e.g., primary care,), and demographic measures. VHA maintains Medicare claims and Part‐D pharmacy claims linked to administrative medical record data. Medicare claims and Part‐D pharmacy claims were used to obtain diagnoses codes, laboratory values, and prescription fills.
IBM® MarketScan® Commercial Claims and Medicare Supplemental databases covered January 1, 2009–December 12, 2018 (calendar year [CY]2009–CY2018) and contained the same measures available in VHA data, unless otherwise stated. The IBM® MarketScan® database included healthcare claims from ambulatory and hospital care, academic and nonacademic health systems, and patients had either private health insurance or Medicaid/Medicare.
Eligibility
Receipt of vaccinations and use of preventive health care are correlated 17 and may contribute to dementia. To control for this bias, we first limited the VHA and MarketScan samples to patients with at least three well‐visits in the observation period. As reported below, we also controlled for the number of well‐visits in the 2 years prior to index.
In the VHA, index date or baseline was October 1, 2011 (FY2012), by which time patients must have been ≥65 years of age. In the 2 years prior to the index date, we excluded patients who did not have VHA encounters. Patients with prevalent dementia or dementia‐related medications (e.g., donepezil, rivastigmine, galantamine, memantine) or those with conditions associated with memory impairment such as Creutzfeldt–Jakob disease in the 2 years prior to index were excluded. Minimum follow‐up time was 91 days after index to allow for a biologically plausible association between vaccination and incident dementia. Dementia cases or non‐cases censored in the first 90 days of follow‐up were excluded. There were 195,375 eligible VHA patients at index date. Sample selection was similar in the MarketScan cohort, which resulted in 193,259 eligible patients at index date (January 1, 2012; CY2012).
We employed a per‐protocol approach; therefore, patients' Tdap and HZ vaccination status was measured as receiving or not receiving a vaccine by index date, and those without a vaccination at index were required to remain without vaccination during follow‐up. Also, the Tdap only group had to remain free of HZ vaccination in follow‐up and vice versa. After removing patients with missing demographic measures, the VHA analytic sample contained 80,070 patients, and the MarketScan sample contained 129,200 patients. The sampling process is shown in Figure S1A,B.
Variable definitions
Detailed definitions of all variables are shown in Table S2.
Exposure
For persons ≥65 years of age, Tdap vaccination was defined by CPT codes 90701 or 90715 and by product names Adacel and Boostrix, and HZ vaccination was defined by CPT codes: 90710, 90716, 90736, 90750, and product names: Proquad, Varivax, Zostavax, and Shingrix. Vaccinations were measured using the CPT codes or pharmacy product name. Vaccination exposure was classified into (1) no vaccination, (2) Tdap vaccination only, (3) HZ vaccination only, and (4) Tdap and HZ vaccination. This approach to defining vaccinations has been shown to have excellent agreement with manual chart abstraction. 18
Outcome
Incident dementia in follow‐up was defined by ICD‐9 and or ICD‐10 diagnostic codes on two separate days in any 12‐month period. The first of the two codes were used to date the diagnosis. This definition has good agreement with Mini Mental State Exam and the Saint Louis University Mental Status Examination scores indicating mild or worse dementia. 19 We have applied this method to define dementia in prior studies of metformin and incident dementia 20 , 21 , 22 and in studies of Tdap vaccination, 11 influenza vaccination, 14 and incident dementia.
Follow‐up time
Follow‐up time was defined as months from index date to dementia or censoring. Censoring in the VHA occurred at last available VHA encounter or Medicare claim, or death. Mortality data were not available in MarketScan, therefore censoring in MarketScan occurred at last available inpatient or outpatient claim.
Covariates
To compute propensity scores, covariates were measured in the 2 years prior to index date. Covariates included sociodemographics (age, gender, race‐VHA only, and marital status‐VHA only), insurance status (VHA only vs. VHA plus other source of insurance), geographic region, overall health care utilization, and use of well‐visits in the 2 years prior to index. Thus, we both sampled on 3 or more well‐visits and controlled for the number of well‐visits 2 years before index. Comorbid conditions included cancer, type 2 diabetes, obesity, hypertension, stroke, ischemic heart disease, congestive heart failure, atrial fibrillation, asthma, chronic obstructive pulmonary disease, traumatic brain injury, vitamin B12 deficiency, depression, any anxiety disorder, nicotine dependence, and alcohol and drug abuse/dependence. We controlled for sustained use of medications that may impact cognitive function. Sustained use was defined as two prescriptions within any 6‐month period in the 2 years prior to index. Medications included antidepressants, benzodiazepines, anticholinergics, NSAIDS, antihypertensives, statins, steroids, antivirals, metformin, and sulfonylurea.
Analytic approach
Receipt of vaccination is not random. Factors that are associated with vaccination, such as use of preventive health care, may also be linked to a lower dementia risk. To control for this and other sources of confounding, we computed propensity scores (PS) and inverse probability of treatment weighting (IPTW). Detailed PS and IPTW methods are reported in Appendix S1.
All primary analyses were performed with SAS v9.4 (SAS Institute, Cary, NC) at a two‐tailed alpha = 0.05. Bivariate comparisons between covariates and vaccine group were assessed using chi‐square tests and maximum ASMD% before and after IPTW. Cox proportional hazard models before and after weighting calculated hazard ratios and 95% confidence intervals for the association of vaccine group and incident dementia. Since mortality information was available in the VHA, competing‐risk survival models were used to control for bias associated with a competing event (e.g., death). 23 Weighted models used robust, sandwich‐type variance estimators for confidence intervals and p values. 24 The proportional hazard assumption was tested in all models with a time‐dependent interaction term of vaccine group and log (follow‐up time); the assumption was met for all models (p > 0.05).
To determine if unmeasured confounding could explain the current results, the e‐values for the hazard ratio were calculated. 25 The e‐value is the minimum strength of association that is needed for an unmeasured confounder to have with both the exposure and outcome to completely explain observed associations. Since there were four exposure groups and multiple significant pairwise comparisons, the e‐value for the largest significant point estimates was given for each cohort. This e‐value would show the strength of association a confounder would need to explain all significant associations. We also employed a negative outcome control by modeling the association between vaccination and incident back pain in patients without back pain at index. 26
Sensitivity analyses
To account for healthy adherer bias, we selected a subset of patients with hypertension at index (in VHA only, MarketScan sample size was insufficient) and expanded the final weighted model by adjusting for anti‐hypertensive medication adherence, defined as ≥80% of proportion of days covered, in follow‐up. Last, we adjusted for neighborhood socioeconomic index (nSES) measured at index date. Higher SES has been associated with a lower risk of dementia 27 and is positively associated with vaccination. 28 , 29 , 30 , 31 The nSES is a validated index 32 created using 5‐year census data from the American Community Survey and linked to zip codes (only available in VHA).
To determine if the association between vaccination and incident dementia was partly explained by tetanus, diptheria or pertussis, or HZ infection, we expanded weighted survival models to include two separate time‐dependent variables for Tdap infection and HZ infection that could occur between index and end of follow‐up.
RESULTS
As shown in Table 1, VHA patients were 76.8 ± 7.6 years of age, 4.4% were female, and 90.9% were White, most were married (69.5%), and 16.5% had access to only VHA health insurance. MarketScan patients were 70.5 ± 5.9 years of age. With exceptions for asthma and traumatic brain injury, comorbid conditions were more prevalent in VHA patients relative to MarketScan. VHA and MarketScan samples had similar prevalences of anticholinergic, NSAID, antidepressants, benzodiazepines, and antiviral use. Other medications measured were more prevalent among VHA patients.
TABLE 1.
Baseline patient characteristics (%) of ≥65 years old patients in VHA (n = 80,070) and MarketScan (n = 129,200)
| Covariates | VHA (n = 80,070) | MarketScan (n = 129,200) |
|---|---|---|
| Sociodemographic‐related | ||
| Age, mean (±SD) | 76.8 (±7.6) | 70.5 (±5.9) |
| Age category | ||
| 65–69 | 18,814 (23.5) | 73,448 (56.9) |
| 70–74 | 13,548 (16.9) | 27,592 (21.4) |
| ≥75 | 47,708 (59.6) | 28,160 (21.8) |
| Female gender | 3540 (4.4) | 84,480 (65.4) |
| Race: White | 72,761 (90.9) | — |
| Black | 6404 (8.0) | — |
| Other | 905 (1.1) | — |
| Married | 55,673 (69.5) | — |
| VHA only insurance | 13,216 (16.5) | — |
| Region | ||
| Northeast | 9353 (11.7) | 51,329 (39.7) |
| Northcentral | 32,711 (40.8) | 22,591 (17.5) |
| South | 28,846 (36.0) | 32,879 (25.5) |
| West | 9160 (11.4) | 22,401 (17.3) |
| High healthcare utilization a | 20,004 (25.0) | 32,351 (25.0) |
| # well‐visits 2 years prior to index, mean (±SD) b | 1.0 (±2.1) | 1.5 (±0.8) |
| # well‐visits 2 years prior to index, category b | ||
| 0 | 45,262 (56.5) | 14,186 (11.0) |
| 1–2 | 26,124 (32.6) | 104,921 (81.2) |
| ≥3 | 8684 (10.9) | 10,093 (7.8) |
| Comorbidities | ||
| Cancer | 26,644 (33.3) | 26,119 (20.2) |
| Type II diabetes | 26,594 (33.2) | 20,424 (15.8) |
| Obesity | 26,070 (32.6) | 7703 (6.0) |
| Hypertension | 66,803 (83.4) | 78,550 (60.8) |
| Stroke | 3932 (4.9) | 2350 (1.8) |
| Ischemic heart disease | 35,085 (43.8) | 19,747 (15.3) |
| Congestive heart failure | 13,386 (16.7) | 4426 (3.4) |
| Atrial fibrillation | 14,878 (18.6) | 7847 (6.1) |
| Asthma | 6118 (7.6) | 9697 (7.5) |
| chronic obstructive pulmonary disease | 18,094 (22.6) | 8372 (6.5) |
| Traumatic brain injury | 2298 (2.9) | 2316 (1.8) |
| Vitamin B12 deficiency | 4935 (6.2) | 3600 (2.8) |
| Depression | 7143 (8.9) | 4762 (3.7) |
| Anxiety disorder c | 6457 (8.1) | 4153 (3.2) |
| Nicotine dependence | 20,488 (25.6) | 6341 (4.9) |
| Alcohol abuse/dependence | 3173 (4.0) | 570 (0.4) |
| Drug abuse/dependence | 895 (1.1) | 198 (0.2) |
| Medications d | ||
| Anticholinergics | 9325 (11.7) | 14,532 (11.3) |
| NSAIDS | 9098 (11.4) | 12,032 (9.3) |
| Antihypertensives | 56,107 (70.1) | 64,578 (50.0) |
| Statins | 45,003 (56.2) | 49,736 (38.5) |
| Steroids | 8261 (10.3) | 7293 (5.6) |
| Antivirals | 903 (1.1) | 2479 (1.9) |
| Metformin | 9757 (12.2) | 9274 (7.2) |
| Sulfonylurea | 9412 (11.8) | 4574 (3.5) |
| Antidepressants | 11,736 (14.7) | 16,345 (12.7) |
| Benzodiazepines | 4348 (5.4) | 7850 (6.1) |
Abbreviation: VHA, Veterans Health Administration.
High healthcare utilization = Average number of outpatient clinic visits per month, calculated as total visits divided by number of months. Total visits is the total number of visits in the 2 years prior to index. Number of months followed is the difference between the first and last visit in 2 years prior to index. The distribution of the mean is then dichotomized into high utilizer, >75th percentile versus low utilizer, ≤75th percentile.
Well‐visits measured in the in the 2 years prior to index, not well‐visits used to sample cohort.
Anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS.
Medications = sustained use prior to index (at least two fills in a 6‐month period).
Patient characteristics by vaccination status are shown in Table 2. Among VHA patients, 78.7% (n = 63,021) had no Tdap or HZ vaccination, 4.8% had Tdap only, 14.3% had HZ only, and 2.1% had both the Tdap and HZ vaccination by index. Among MarketScan patients, 78.8% had no Tdap or HZ vaccination, 7.6% had Tdap only, 10.7% had HZ only, and 2.9% had both the Tdap and HZ vaccination at index.
TABLE 2.
Baseline patient characteristics (%) of ≥65 years old patients by vaccine status, VHA (n = 80,070) and MarketScan (n = 129,200)
| VHA patient cohort | MarketScan patient cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No vaccine n = 63,021 | Tdap only n = 3874 | HZ only n = 11,434 | Tdap + HZ n = 1741 | ASMD% | No vaccine n = 101,819 | Tdap only n = 9816 | HZ only n = 13,774 | Tdap + HZ n = 3791 | ASMD% | |
| Covariates | ||||||||||
| Age category | ||||||||||
| 65–69 | 14,169 (22.5) | 1571 (40.6) | 2320 (20.3) | 754 (43.3) | 51.0 | 55,759 (54.8) | 6785 (69.1) | 8060 (58.5) | 2844 (75.0) | 43.4 |
| 70–74 | 10,328 (16.4) | 611 (15.8) | 2307 (20.2) | 302 (17.4) | 11.5 | 22,228 (21.8) | 1711 (17.4) | 3104 (22.5) | 549 (14.5) | 20.9 |
| ≥75 | 38,524 (61.1) | 1692 (43.7) | 6807 (59.5) | 685 (39.4) | 44.6 | 23,832 (23.4) | 1320 (13.5) | 2610 (18.9) | 398 (10.5) | 34.9 |
| Female gender | 2711 (4.3) | 207 (5.3) | 514 (4.5) | 108 (6.2) | 8.5 | 67,395 (66.2) | 5957 (60.7) | 8816 (64.0) | 2312 (61.0) | 11.5 |
| Race | ||||||||||
| White | 56,616 (89.8) | 3442 (88.8) | 11,061 (96.7) | 1642 (94.3) | 30.9 | – | – | – | – | – |
| Black | 5681 (9.0) | 385 (9.9) | 267 (2.3) | 71 (4.1) | 32.1 | – | – | – | – | – |
| Other | 724 (1.2) | 47 (1.2) | 106 (0.9) | 28 (1.6) | 6.1 | – | – | – | – | – |
| Married | 42,977 (68.2) | 2447 (63.2) | 8998 (78.7) | 1251 (71.9) | 34.7 | – | – | – | – | – |
| VHA only insurance | 11,020 (17.5) | 862 (22.3) | 1043 (9.1) | 291 (16.7) | 36.7 | – | – | – | – | – |
| Region | ||||||||||
| Northeast | 7260 (11.5) | 512 (13.2) | 1260 (11.0) | 321 (18.4) | 21.1 | 43,355 (42.6) | 2868 (29.2) | 4227 (30.7) | 879 (23.2) | 42.2 |
| Northcentral | 23,604 (37.5) | 1432 (37.0) | 6832 (59.8) | 843 (48.4) | 46.8 | 16,786 (16.5) | 1938 (19.7) | 3037 (22.1) | 830 (21.9) | 14.1 |
| South | 25,199 (40.0) | 1285 (33.2) | 2161 (18.9) | 201 (11.6) | 68.8 | 26,741 (26.3) | 2092 (21.3) | 3327 (24.2) | 719 (19.0) | 17.5 |
| West | 6958 (11.0) | 645 (16.6) | 1181 (10.3) | 376 (21.6) | 31.1 | 14,937 (14.7) | 2918 (29.7) | 3183 (23.1) | 1363 (35.9) | 50.5 |
| High healthcare utilization | 15,496 (24.6) | 1553 (40.1) | 2310 (20.2) | 645 (37.1) | 44.4 | 25,555 (25.1) | 2395 (24.4) | 3502 (25.4) | 899 (23.7) | 4.0 |
| # well‐visits 2 years prior to index | ||||||||||
| 0 | 35,698 (56.6) | 2318 (59.8) | 6301 (55.1) | 945 (54.3) | 11.2 | 12,116 (11.9) | 716 (7.3) | 1125 (8.2) | 229 (6.0) | 20.6 |
| 1–2 | 20,380 (32.3) | 1131 (29.2) | 4075 (35.6) | 538 (30.9) | 13.8 | 82,197 (80.7) | 8173 (83.3) | 11,362 (82.5) | 3189 (84.1) | 8.9 |
| ≥3 | 6943 (11.0) | 425 (11.0) | 1058 (9.3) | 258 (14.8) | 17.2 | 7506 (7.4) | 927 (9.4) | 1287 (9.3) | 373 (9.8) | 8.8 |
| Comorbid conditions | ||||||||||
| Cancer | 21,374 (33.9) | 1130 (29.2) | 3696 (32.3) | 444 (25.5) | 18.5 | 20,458 (20.1) | 1944 (19.8) | 2954 (21.5) | 763 (20.1) | 4.1 |
| Type II diabetes | 21,333 (33.8) | 1353 (34.9) | 3416 (29.9) | 492 (28.3) | 14.4 | 16,599 (16.3) | 1392 (14.2) | 1954 (14.2) | 479 (12.6) | 10.4 |
| Obesity | 19,736 (31.3) | 1552 (40.1) | 4047 (35.4) | 735 (42.2) | 22.8 | 6059 (5.9) | 628 (6.4) | 753 (5.5) | 263 (6.9) | 6.1 |
| Hypertension | 52,786 (83.8) | 3244 (83.7) | 9396 (82.2) | 1377 (79.1) | 12.0 | 63,047 (61.9) | 5552 (56.6) | 7920 (57.5) | 2031 (53.6) | 17.0 |
| Stroke | 3288 (5.2) | 172 (4.4) | 427 (3.7) | 45 (2.6) | 13.6 | 1901 (1.9) | 172 (1.8) | 214 (1.6) | 63 (1.7) | 2.4 |
| Ischemic heart disease | 28,311 (44.9) | 1519 (39.2) | 4687 (41.0) | 568 (32.6) | 25.4 | 16,229 (15.9) | 1255 (12.8) | 1850 (13.4) | 413 (10.9) | 14.8 |
| Congestive heart failure | 11,284 (17.9) | 582 (15.0) | 1397 (12.2) | 123 (7.1) | 33.2 | 3758 (3.7) | 250 (2.6) | 343 (2.5) | 75 (2.0) | 10.3 |
| Atrial fibrillation | 12,187 (19.3) | 588 (15.2) | 1907 (16.7) | 196 (11.3) | 22.6 | 6374 (6.3) | 520 (5.3) | 742 (5.4) | 211 (5.6) | 4.1 |
| Asthma | 4788 (7.6) | 280 (7.2) | 922 (8.1) | 128 (7.4) | 3.2 | 7581 (7.5) | 811 (8.3) | 1014 (7.4) | 291 (7.7) | 3.4 |
| Chronic obstructive pulmonary disease | 14,894 (23.6) | 954 (24.6) | 1982 (17.3) | 264 (15.2) | 23.9 | 6971 (6.9) | 508 (5.2) | 718 (5.2) | 175 (4.6) | 9.6 |
| Traumatic brain injury | 1921 (3.1) | 95 (2.5) | 250 (2.2) | 32 (1.8) | 7.8 | 1789 (1.8) | 201 (2.1) | 248 (1.8) | 78 (2.1) | 2.2 |
| Vitamin B12 deficiency | 4061 (6.4) | 193 (5.0) | 582 (5.1) | 99 (5.7) | 6.3 | 2970 (2.9) | 205 (2.1) | 335 (2.4) | 90 (2.4) | 5.3 |
| Depression | 5626 (8.9) | 454 (11.7) | 894 (7.8) | 169 (9.7) | 13.2 | 3591 (3.5) | 47 (4.5) | 559 (4.1) | 175 (4.6) | 5.5 |
| Anxiety disorder a | 5031 (8.0) | 433 (11.2) | 816 (7.1) | 177 (10.2) | 14.0 | 3230 (3.2) | 323 (3.3) | 473 (3.4) | 127 (3.4) | 1.5 |
| Nicotine dependence | 16,602 (26.3) | 1176 (30.4) | 2344 (20.5) | 366 (21.0) | 22.8 | 5080 (5.0) | 445 (4.5) | 638 (4.6) | 178 (4.7) | 2.1 |
| Alcohol abuse/dependence | 2566 (4.1) | 190 (4.9) | 329 (2.9) | 88 (5.1) | 11.2 | 419 (0.4) | 59 (0.6) | 70 (0.5) | 22 (0.6) | 2.7 |
| Drug abuse/dependence | 772 (1.2) | 51 (1.3) | 56 (0.5) | 16 (0.9) | 8.8 | 145 (0.1) | 18 (0.2) | 23 (0.2) | 12 (0.3) | 3.6 |
| Medications b | ||||||||||
| Anti‐cholinergics | 7202 (11.4) | 617 (15.9) | 1269 (11.1) | 237 (13.6) | 14.2 | 11,258 (11.1) | 1026 (10.5) | 1789 (13.0) | 459 (12.1) | 7.9 |
| NSAIDS | 6806 (10.8) | 606 (15.6) | 1403 (12.3) | 283 (16.3) | 16.0 | 9312 (9.2) | 895 (9.1) | 1450 (10.5) | 375 (9.9) | 4.7 |
| Anti‐hyper‐tensives | 43,676 (69.3) | 2862 (73.9) | 8310 (72.7) | 1259 (72.3) | 10.2 | 50,996 (50.1) | 4552 (46.4) | 7150 (51.9) | 1880 (49.6) | 11.1 |
| Statins | 34,285 (54.4) | 2319 (59.9) | 7312 (64.0) | 1087 (62.4) | 19.5 | 38,446 (37.8) | 3723 (37.9) | 5997 (43.5) | 1570 (41.4) | 11.8 |
| Steroids | 6458 (10.3) | 441 (11.4) | 1183 (10.4) | 179 (10.3) | 3.7 | 5745 (5.6) | 529 (5.4) | 834 (6.1) | 185 (4.9) | 5.2 |
| Antivirals | 675 (1.1) | 73 (1.9) | 121 (1.1) | 34 (2.0) | 7.4 | 1760 (1.7) | 273 (2.8) | 327 (2.4) | 119 (3.1) | 9.2 |
| Metformin | 7411 (11.8) | 609 (15.7) | 1482 (13.0) | 255 (14.7) | 11.5 | 7425 (7.3) | 637 (6.5) | 959 (7.0) | 253 (6.7) | 3.2 |
| Sulfonylurea | 7517 (11.9) | 545 (14.1) | 1164 (10.2) | 186 (10.7) | 11.9 | 3779 (3.7) | 280 (2.9) | 406 (2.9) | 109 (2.9) | 4.8 |
| Anti‐depressants | 8984 (14.3) | 783 (20.2) | 1651 (14.4) | 318 (18.3) | 15.8 | 12,280 (12.1) | 1350 (13.8) | 2092 (15.2) | 623 (16.4) | 12.5 |
| Benzodiazepines | 3364 (5.3) | 251 (6.5) | 629 (5.5) | 104 (6.0) | 4.8 | 6130 (6.0) | 549 (5.6) | 937 (6.8) | 234 (6.2) | 5.0 |
Abbreviation: ASMD%, maximum absolute standardized mean difference percent.
Anxiety disorders = panic disorder, OCD, social phobia, GAD, Anxiety NOS.
Medications = sustained use prior to index (at least two fills in a 6‐month period).
In the VHA cohort, the distribution of most patient characteristics differed by vaccination status (ASMD% ≥10). Exceptions were gender, “other” race, diagnoses of asthma, traumatic brain injury, vitamin B12 deficiency, drug abuse/dependence and sustained use of steroids, antivirals, or benzodiazepines. In the MarketScan cohort, the majority of patient characteristics did not differ by vaccination status (ASMD% <10). Exceptions were age groups, geographic regions, absence of well‐visits in the 2 years prior to index date, type 2 diabetes, hypertension, ischemic heart disease, congestive heart failure, and sustained use of antihypertensives, statins, or antidepressants.
The meaningful differences in the distribution of covariates by vaccination status were successfully balanced (see Table S3 for covariate distributions and ASMD% after weighting). After weighting data, there were no patient characteristics associated (all ASMD% <10) with vaccine status in the VHA or MarketScan cohort.
In the VHA cohort, the overall median follow‐up time was 85 (IQR = 43–94) months, and median follow‐up time by vaccine status was: 79 (IQR = 39–94) months for patients without Tdap or HZ vaccination, 87 (IQR = 50–95) for Tdap only, 91 (IQR = 65–95) for HZ only, and 95 (IQR = 87–96) months for those with both Tdap and HZ vaccination. In the MarketScan cohort, the overall median follow‐up time was 35 (IQR = 21–48) months, and the median follow‐up time by vaccine status was: 35 (IQR = 21–48) months in those without Tdap or HZ vaccination, 35 (IQR = 21–51) for Tdap only, 35 (IQR = 21–55) for HZ only, and 36 (IQR = 23–60) months for those with both Tdap and HZ vaccination.
Among patients who developed dementia, the median follow‐up time (months) to incident dementia was: 45 (IQR = 24–64) in VHA and 33 (IQR = 18–53) in MarketScan. Among patients without either vaccination, median follow‐up time was 44 (IQR = 23–63) in VHA and 33 (IQR = 18–53) in MarketScan. Among patients with Tdap vaccination alone, median follow‐up time was 47 (IQR = 30–66) in VHA and 34 (IQR = 18–58) in MarketScan. Among HZ vaccination alone, median follow‐up time was 51 (IQR = 29–68) in VHA and 36 (IQR = 20–56) in MarketScan. Median follow‐up time to incident dementia in patients with both Tdap and HZ vaccination was 54.5 (IQR = 36–70) in VHA and 41 (IQR = 17–65) in MarketScan.
Table 3 shows crude observed cumulative incidence and rate of dementia. Cumulative incidence curves are shown in Figure S2A,B. The overall incidence rate of dementia was 314.0/10,000 person years (PY) in the VHA cohort and 56.2/10,000 PY in the MarketScan cohort. Among VHA and MarketScan patients, the cumulative dementia incidence was largest in those without either vaccination (18.9% VHA and 1.9% MarketScan) and was smallest in those with both Tdap and HZ vaccination (9.1% VHA and 0.9% MarketScan).
TABLE 3.
Dementia events, cumulative incidence %, and incidence rate per 10,000 person‐years (PY), patients ≥65 years old
| VHA | Total n | Dementia events | Person‐years | Cumulative incidence % | Incidence rate per 10,000PY |
|---|---|---|---|---|---|
| VHA | |||||
| Overall | 80,070 | 14,141 | 450,911.25 | 17.7% | 314.0/10,000PY |
| Four group | |||||
| No vaccine | 63,021 | 11,895 | 342,580.47 | 18.9% | 347.2/10,000PY |
| Tdap only | 3874 | 542 | 23,155.81 | 14.0% | 234.1/10,000PY |
| HZ only | 11,434 | 1546 | 73,017.78 | 13.5% | 211.7/10,000PY |
| Tdap + Hz | 1741 | 158 | 12,157.19 | 9.1% | 130.0/10,000PY |
| MarketScan | |||||
| Overall | 129,200 | 2268 | 403,141.07 | 1.8% | 56.2/10,000PY |
| Four group | |||||
| No vaccine | 101,819 | 1928 | 314,691.87 | 1.9% | 61.3/10,000PY |
| Tdap only | 9816 | 114 | 30,960.01 | 1.6% | 36.8/10,000PY |
| HZ only | 13,774 | 191 | 44,305.23 | 1.4% | 43.1/10,000PY |
| Tdap + Hz | 3791 | 35 | 13,183.96 | 0.9% | 26.5/10,000PY |
The results from competing risk survival models and Cox proportional hazard models are shown in Figure 1. In weighted analyses, as compared with no Tdap and HZ vaccination, the receipt of both Tdap and HZ vaccination was significantly associated with a 50% lower risk of dementia (HR = 0.50; 95% CI: 0.43–0.59). Compared with no Tdap or HZ vaccination, Tdap only (HR = 0.82;95% CI: 0.76–0.89) and HZ only (HR = 0.75; 95% CI: 0.71–0.79) vaccination were significantly associated with lower risk for dementia. Patients with both vaccinations compared with those with only Tdap (HR = 0.61; 95% CI: 0.52–0.73) or only HZ vaccination (HR = 0.68; 95% CI: 0.58–0.79) were significantly less likely to develop dementia. Dementia risk did not significantly differ between patients who received only Tdap compared with those who received only HZ vaccination.
FIGURE 1.
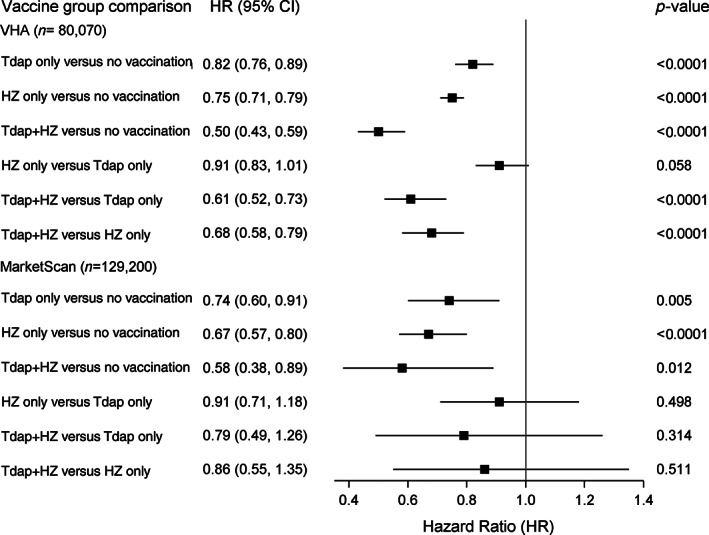
Results from weighted competing risk (VHA) and Cox proportional hazard (MarketScan) models estimating the association (HR [95% CI]) of Tdap, HZ, and Tdap + HZ vaccinations and incident dementia
In the MarketScan cohort, compared with no vaccination, those who had both Tdap and HZ vaccinations had a significantly lower risk for dementia (HR = 0.58; 95% CI: 0.38–0.89). Compared with no vaccination, patients with Tdap only (HR = 0.74; 95% CI: 0.60–0.91) and HZ only (HR = 0.67; 95% CI: 0.57–0.80) vaccination were significantly less likely to develop dementia. The risk of dementia did not differ significantly between those who received both vaccinations versus those who received only Tdap or only HZ vaccination. Dementia risk did not significantly differ between only Tdap compared with only HZ vaccination.
In the VHA, the e‐value was 3.41, whereas in MarketScan it was 2.84. In addition, the results from the negative outcome control revealed no association between vaccination and incident back pain (Table S4). The results from sensitivity analyses are shown in Appendix S1. The hazard ratios shown in Table S5 were nearly the same after adjusting for anti‐hypertensive adherence among patients with hypertension and after controlling for nSES (Table S6). Analysis accounting for new infections after index revealed that 71 (0.01%) and 40 (0.03%) developed tetanus, pertussis, or diphtheria in follow‐up in the VHA and MarketScan cohorts, respectively. In the VHA, 5199 (6.5%) and in MarketScan 4777 (3.7%) developed HZ infection after index and before end of follow‐up. The results remained largely unchanged after adjusting for tetanus, pertussis, diphtheria, or HZ infection between index and end of follow‐up (Table S7).
DISCUSSION
In a large cohort of VHA patients ≥65 years of age, receipt of either Tdap or HZ vaccination was associated with a significantly lower risk of incident dementia. Patients who received both vaccinations compared with none had a 50% lower risk for dementia and were significantly less likely to develop dementia as compared with those who received only Tdap or only HZ vaccination. These results were largely replicated in MarketScan data; however, in this patient sample, we did not observe a lower risk for dementia in patients with both vaccinations compared with only Tdap or only HZ vaccination, which is at least partly explained by fewer dementia cases and less precise hazard ratios in the younger MarketScan patients.
Our results are consistent with prior studies of adult vaccination and lower dementia risk. 9 , 10 , 11 , 12 , 13 , 33 To our knowledge, our results from VHA data provide the first evidence that receipt of two different types of vaccinations is associated with a greater reduction in dementia risk compared with receiving only Tdap or only HZ. This finding is partly consistent with Luo et al.'s observation of a trend for greater decrease in dementia risk as the number of annual influenza vaccinations increased. The results are also consistent with our prior study of influenza vaccination, which suggests that the number of vaccinations may reach a threshold at which association with incident dementia declines. 14
Importantly, our design included robust control for healthy adherer effects and results did not change after controlling for receipt of preventive healthcare, that is, well‐visits, anti‐hypertensive medication adherence. Thus, it appears that the relationship between vaccination and decreased dementia risk is not explained by a correlation between adhering to vaccinations and utilizing more preventive health care or adoption of health behaviors linked to lower dementia risk. Further, the associations between Tdap and HZ vaccinations and incident dementia did not change after adjusting for low nSES. Thus, it is unlikely that our results are explained by the correlation between higher SES and lower HZ vaccine hesitancy 34 or by the link between higher SES and lower dementia incidence. 27 , 28 , 29 , 30 , 31
There are several other possible reasons vaccinations are associated with lower dementia incidence. Herpes viruses and some bacteria are thought to be AD triggers, 1 contributing to deposits of Amyloid‐β. 1 Infections contribute to cytokine release and neuroinflammation. Vaccination may block this process. HZ infection is associated with elevated risk for dementia. 4 , 5 HZ vaccination may have a specific effect by preventing HZ infection and subsequent cognitive decline. In the current study, we found no attenuation of the association between vaccinations and incident dementia after adjusting for tetanus, diptheria, pertussis, or HZ infection that could have onset between index and end of follow‐up. This is consistent with our previous research indicating post‐vaccination HZ infection does not moderate the association between HZ vaccination and incident dementia. 35 The current results, taken together with the number of different vaccinations linked to lower dementia incidence and findings related to HZ vaccination, lead us to suspect a nonspecific mechanism. Although speculative, repeated vaccinations may train the immune system and lower the risk for chronic inflammation, increasing appropriate immune responses and the body's ability to resist bacterial and viral threats. 36
Limitations
Our study is vulnerable to misclassification of exposure and outcome. If we misclassified patients who received vaccinations as unvaccinated or misclassified dementia cases as being free of dementia, our point estimates would be biased toward the null. Therefore, we may have underestimated the association between vaccinations and incident dementia. Although we adjusted for marital status in the VHA cohort, we did not have measures of social support or current relationship status, which could contribute to greater vaccination and early detection of dementia. Unmeasured confounding could bias our analysis. However, the e‐values were large: 3.41 in VHA and 2.84 in MarketScan. An unmeasured confounder would require an association of this magnitude with vaccination status and incident dementia to explain all our significant results. We are unable to conceive of such an unmeasured confounder. In addition, results from the negative control analysis further reduce concern regarding unmeasured confounding and bias. The results may not generalize to other regions of the world. We lacked sufficient new cases of dementia to compare results between age groups (65–69, 70–74, and >75 years of age). In prior studies, we did not observe an age by vaccination interaction in the association between Tdap vaccination and incident dementia. 11 Although some evidence indicates age group differences in the relationship between HZ vaccination and risk for dementia, current findings are inconsistent across data sets. 35
Further research is needed to confirm these findings and overcome limitations of our retrospective cohort design. Although a randomized controlled trial (RCT) could be informative, it may be too difficult to detect incident dementia using sample sizes and follow‐up time in typical RCTs. A prospective cohort study that recruited thousands of persons 65 years of age and older and cognitively intact could overcome existing limitations. By building the cohort from a network of healthcare systems, and obtaining medical record release, vaccination status can be confirmed at baseline and during follow‐up. Repeated assessments could measure cognitive decline, not just onset of dementia. Neurophysiological testing and immunological evaluation could identify mechanisms for the association between vaccination and incident dementia. Surveys could assess social support, cognitive reserve, and orientation toward health to confidently disentangle potential residual confounding factors from vaccination status.
CONCLUSION
HZ and Tdap vaccinations in adults 65 years of age and older are associated with substantial reduction in dementia risk. Receiving both vaccinations was associated with lower dementia risk as compared with receiving only one, suggesting a common pathway may underly the association between vaccines and dementia risk.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors met the criteria for authorship including contributions to: Data analysis: Joanne Salas. Design: All authors. Interpretation of results: All authors. Drafting and completing the final manuscript for submission: All authors).
SPONSOR'S ROLE
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Appendix S1. Supporting Information.
Table S1. STROBE Statement—Checklist of items that should be included in reports of cohort studies.
Table S2. Variable definitions.
Table S3. Weighted baseline characteristics, %, and absolute maximum standardized mean difference percent (ASMD%), after inverse probability of treatment weighting.
Table S4. Results from negative outcome control analyses: Vaccination status and Incident backpain among patients 65 and older who are free of backpain at baseline.
Table S5. VHA – sensitivity analysis adding antihypertensive medication adherence among 66,803 patients with hypertension at baseline/index to weighted models.
Table S6. VHA – sensitivity analysis adding nSES to weighted models.
Table S7. Survival models adjusted for HZ and Tdap infection that could occur between vaccination and end of follow‐up.
Figure S1. (A) VHA sampling. (B) MarketScan sampling.
Figure S2. (A) Cumulative incidence curve, VHA vaccination group and incident dementia. (B) Cumulative incidence curve, MarketScan vaccination group and incident dementia.
ACKNOWLEDGMENTS
Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR02‐237 and 98‐004). This material is the result of work supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans' Hospital. Data sharing statement: Data are available with appropriate IRB approval and fees paid to the vendor of commercial claims data. Programming code available upon request.
Wiemken TL, Salas J, Morley JE, Hoft DF, Jacobs C, Scherrer JF. Comparison of rates of dementia among older adult recipients of two, one, or no vaccinations. J Am Geriatr Soc. 2022;70(4):1157‐1168. doi: 10.1111/jgs.17606
The views expressed do not necessarily reflect those of the Veterans Health Administration.
Funding information Benter Foundation, Grant/Award Number: 2020‐01
REFERENCES
- 1. Abbott A. Are infections seeding some cases of Alzheimer's disease? Nature. 2020;587(7832):22‐25. [DOI] [PubMed] [Google Scholar]
- 2. Rubin K, Glazer S. The pertussis hypothesis: Bordetella pertussis colonization in the pathogenesis of Alzheimer's disease. Immunobiology. 2017;222(2):228‐240. [DOI] [PubMed] [Google Scholar]
- 3. Tsai MC, Cheng WL, Sheu JJ, et al. Increased risk of dementia following herpes zoster ophthalmicus. PLoS One. 2017;12(11):e0188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bae S, Yun SC, Kim MC, et al. Association of herpes zoster with dementia and effect of antiviral therapy on dementia: a population‐based cohort study. Eur Arch Psychiatry Clin Neurosci. 2020;271:987‐997. [DOI] [PubMed] [Google Scholar]
- 5. Chen VC, Wu SI, Huang KY, et al. Herpes zoster and dementia: a nationwide population‐based cohort study. J Clin Psychiatry. 2018;79(1):16m11312. [DOI] [PubMed] [Google Scholar]
- 6. Honjo K, van Reekum R, Verhoeff NP. Alzheimer's disease and infection: do infectious agents contribute to progression of Alzheimer's disease? Alzheimers Dement. 2009;5(4):348‐360. [DOI] [PubMed] [Google Scholar]
- 7. Lövheim H, Gilthorpe J, Johansson A, Eriksson S, Hallmans G, Elgh F. Herpes simplex infection and the risk of Alzheimer's disease: a nested case‐control study. Alzheimers Dement. 2015;11(6):587‐592. [DOI] [PubMed] [Google Scholar]
- 8. Sipila PN, Heikkila N, Lindbohm JV, et al. Hospital‐treated infectious diseases and the risk of dementia: a large, multicohort, observational study with a replication cohort. Lancet Infect Dis. 2021;21:1557‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verreault R, Laurin D, Lindsay J, Serres GD. Past exposure to vaccines and subsequent risk of Alzheimer's disease. CMAJ. 2001;165(11):1495‐1498. [PMC free article] [PubMed] [Google Scholar]
- 10. Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer's disease: a population‐based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30(3):590‐597. [DOI] [PubMed] [Google Scholar]
- 11. Scherrer JF, Salas J, Wiemken TL, Jacobs C, Morley JE, Hoft DF. Lower risk for dementia following adult tetanus, diphtheria and pertussis (Tdap) vaccination. J Gerontol A Biol Sci Med Sci. 2021;76(8):1436‐1443. [DOI] [PubMed] [Google Scholar]
- 12. Liu JC, Hsu YP, Kao PF, et al. Influenza vaccination reduces dementia risk in chronic kidney disease patients: a population‐based cohort study. Medicine (Baltimore). 2016;95(9):e2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luo CS, Chi CC, Fang YA, Liu JC, Lee KY. Influenza vaccination reduces dementia in patients with chronic obstructive pulmonary disease: a nationwide cohort study. J Investig Med. 2020;68(4):838‐845. [DOI] [PubMed] [Google Scholar]
- 14. Wiemken TL, Salas J, Hoft DF, Jacobs C, Morley JE, Scherrer JF. Dementia risk following influenza vaccination in a large veteran cohort running head: influenza vaccination and dementia. Vaccine. 2021;39:5524‐5531. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control . National and State‐Level Place of Flu Vaccination among Vaccinated Adults in the United States, 2014–15 Flu Season. Accessed August 4, 2021. https://www.cdc.gov/flu/fluvaxview/place-vaccination-2014-15.htm
- 16. Rutschman AS, Wiemken TL. Re‐examining vaccine staggering within hesitancy frameworks. Front Immunol. 2021;12:662814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hostetter J, Schwarz N, Klug M, Wynne J, Basson MD. Primary care visits increase utilization of evidence‐based preventative health measures. BMC Fam Pract. 2020;21(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hachem CY, Kramer JR, Kanwal F, El‐Serag HB. Hepatitis vaccination in patients with hepatitis C: practice and validation of codes at a large Veterans Administration Medical Centre. Aliment Pharmacol Ther. 2008;28(9):1078‐1087. [DOI] [PubMed] [Google Scholar]
- 19. Harding BN, Floyd JS, Scherrer JF, et al. Methods to identify dementia in the electronic health record: comparing cognitive test scores with dementia algorithms. Healthc (Amst). 2020;8(2):100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scherrer JF, Morley JE, Salas J, Floyd JS, Farr SA, Dublin S. Association between metformin initiation and incident dementia among African American and white Veterans Health Administration patients. Ann Fam Med. 2019;17(4):352‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scherrer JF, Salas J, Floyd JS, Farr SA, Morley JE, Dublin S. Metformin and sulfonylurea use and risk of incident dementia. Mayo Clin Proc. 2019;94(8):1444‐1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salas J, Morley JE, Scherrer JF, et al. Risk of incident dementia following metformin initiation compared with noninitiation or delay of antidiabetic medication therapy. Pharmacoepidemiol Drug Saf. 2020;29(6):623‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496‐509. [Google Scholar]
- 24. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661‐3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haneuse S, VanderWeele TJ, Arterburn D. Using the E‐value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602‐603. [DOI] [PubMed] [Google Scholar]
- 26. Lipsitch M, Tchetgen ET, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;3:383‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Almeida‐Meza P, Steptoe A, Cadar D. Markers of cognitive reserve and dementia incidence in the English Longitudinal Study of Ageing. Br J Psychiatry. 2020;1‐9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ackerson B, Qian L, Sy LS, et al. Completion of the two‐dose recombinant zoster vaccine series in adults 50 years and older. Vaccine. 2021;39(6):926‐932. [DOI] [PubMed] [Google Scholar]
- 29. Jain A, van Hoek AJ, Boccia D, Thomas SL. Lower vaccine uptake amongst older individuals living alone: a systematic review and meta‐analysis of social determinants of vaccine uptake. Vaccine. 2017;35(18):2315‐2328. [DOI] [PubMed] [Google Scholar]
- 30. Fogelberg S, Lamb F, Gronlund O, et al. Differential uptake of herpes zoster vaccination associated with socioeconomic status: a population‐based study in Stockholm County, Sweden. Pharmacoepidemiol Drug Saf. 2018;27(11):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 31. Lu PJ, Hung MC, Srivastav A, Williams WW, Dooling KL. Shingles vaccination of U.S. adults aged 50‐59 years and ≥ 60 years before recommendations for use of recombinant zoster vaccine. Am J Prev Med. 2020;59(1):21‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roblin DW. Validation of a neighborhood SES index in a managed care organization. Med Care. 2013;51(1):e1‐e8. [DOI] [PubMed] [Google Scholar]
- 33. Wiemken TL, Salas J, Hoft DF, Jacobs C, Morley JE, Scherrer JF. Dementia risk following influenza vaccination in a large veteran cohort. Vaccine. 2021;39:5524‐5531. [DOI] [PubMed] [Google Scholar]
- 34. Shuvo S, Hagemann T, Hohmeier K, Chiu CY, Ramachandran S, Gatwood J. The role of social determinants in timely herpes zoster vaccination among older American adults. Hum Vaccin Immunother. 2021;17(7):2043‐2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scherrer JF, Salas J, Wiemken TL, Hoft DL, Jacobs C, Morley JE. Impact of herpes zoster vaccination on incident dementia: a retrospective study in two patient cohorts. PLoS One. Forthcoming. 2021;16(11):e0257405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benn CS, Netea MG, Selin LK, Aaby P. A small jab ‐ a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431‐439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Table S1. STROBE Statement—Checklist of items that should be included in reports of cohort studies.
Table S2. Variable definitions.
Table S3. Weighted baseline characteristics, %, and absolute maximum standardized mean difference percent (ASMD%), after inverse probability of treatment weighting.
Table S4. Results from negative outcome control analyses: Vaccination status and Incident backpain among patients 65 and older who are free of backpain at baseline.
Table S5. VHA – sensitivity analysis adding antihypertensive medication adherence among 66,803 patients with hypertension at baseline/index to weighted models.
Table S6. VHA – sensitivity analysis adding nSES to weighted models.
Table S7. Survival models adjusted for HZ and Tdap infection that could occur between vaccination and end of follow‐up.
Figure S1. (A) VHA sampling. (B) MarketScan sampling.
Figure S2. (A) Cumulative incidence curve, VHA vaccination group and incident dementia. (B) Cumulative incidence curve, MarketScan vaccination group and incident dementia.


