Abstract
Ferroptosis is an iron-dependent form of programmed cell death and an important type of biological catabolism. Through the action of divalent iron or ester oxygenase, ferroptosis can induce lipid peroxidation and cell death, regulating a variety of physiological processes. The role of ferroptosis in the modulation of bone homeostasis is a significant topic of interest. Herein, we review and discuss recent studies exploring the mechanisms and functions of ferroptosis in different bone-related cells, including mesenchymal stem cells, osteoblasts, osteoclasts, and osteocytes. The association between ferroptosis and disorders of bone homeostasis is also explored in this review. Overall, we aim to provide a detailed overview of ferroptosis, summarizing recent understanding on its role in regulation of bone physiology and bone disease pathogenesis.
1. Introduction
Ferroptosis is a form of cell death that was only recently defined by [1]. who proposed the concept in 2012 to describe a nonapoptotic type of cell death which is iron-dependent and is characterized by an accumulation of reactive oxygen species (ROS). Ferroptosis is closely related to a variety of metabolic disorders, tumors, and injuries [2–4]. During ferroptosis, the most susceptible lipids to peroxidation are polyunsaturated fatty acids (PUFAs).
In cell physiology, an increase of polyunsaturated fatty acids (PUFAs) on the cell membrane enhances the fluidity of the cell membrane, which indirectly increases the migratory ability of the cell [5]. Therefore, the increase in PUFAs is an important hallmark in the process of cell evolution. However, introduction of PUFAs also endangers cell survival. Hydrogen molecules produced during the dissociation of PUFAs can react with oxide and ferrous ions in the surrounding environment, resulting in the accumulation of peroxide and subsequent cell damage [6]. Normally, cells use PUFAs efficiently without causing cell damage by employing glutathione peroxidase 4 (GPX4) signaling which partially decreases the levels of PUFAs [7]. Mechanistically, GPX4 uses its catalytic activity to weaken the toxicity of lipid peroxides and maintain the homeostasis of lipid bilayer. Recent evidence identified the regulatory role of GPX4 and ferroptosis in multiple pathological processes. Currently, ferroptosis has attracted accumulative focus in studies on a wide range of diseases. Plenty of evidences have demonstrated that the pathological process of ferroptosis involves the excessive production of ROS, followed by abnormal activation of lipid peroxidation in an iron-dependent manner, accompanied with a marked elevated uptake of PUFAs into the cellular membrane. The unique characteristics of ferroptosis make it complicated related to several biological processes.
Bone homeostasis is a physiological process regulated by bone related-stem cells, osteoblasts, osteoblasts, and osteocytes [8]. During bone remodeling, osteocytes, osteoblasts, and osteoclasts interact with one another in a paracrine manner and regulate angiogenesis in the bone marrow to maintain bone homeostasis [9]. Research has demonstrated the crucial role of ferroptosis in regulating the survival of bone-related cells and identified oxidative stress as an important factor in cell death [10, 11]. However, the exact mechanism of ferroptosis in bone homeostasis regulation remains largely unknown, and it is yet unclear whether ferroptosis is a driver or a passenger event in bone homeostasis.
Herein, we aim to review the recent literature on the subject to explore the underlying mechanisms of ferroptosis and its roles in different bone-related cells, including mesenchymal stem cells, osteoblasts, osteoclasts, and osteocytes. We summarize the recent findings on the role of ferroptosis in regulation of bone physiology and osteopathogenesis.
2. Ferroptosis
As previously mentioned, ferroptosis was first proposed in 2012 but was redefined as a mode of programmed cell death closely related to cell oxidative disturbance by the Nomenclature Committee on Cell Death in 2018 [12]. Compared to other classic forms of cell death, ferroptosis is characterized by the accumulation of iron-dependent lipid ROS. Ferroptosis occurs following a depletion of glutathione (GSH), subsequent decrease in the activity of glutathione peroxidase 4 (GPx4), and inhibition of lipid oxide metabolism as this is a GPX4-dependent reaction. Following this, divalent iron ions oxidize the lipid to produce ROS leading to ferroptosis [13].
Susceptibility to ferroptosis is closely related to multiple biological processes, including iron and PUFA metabolism and biosynthesis of GSH, phospholipid, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), and coenzyme Q10 [13]. It has also been linked to the pathological cell death seen in mammalian degenerative diseases, such as tumors, stroke, cerebral hemorrhage, traumatic brain injury, and renal failure [14, 15].
2.1. The Characteristics of Ferroptosis
During ferroptosis, a large number of iron ions are deposited in the dead cells, lipid peroxidation occurs intracellularly, ROS levels increase significantly, and proteins that regulate iron homeostasis and lipid peroxidation metabolism are altered [16]. Microscopically, the mitochondrial membrane shrinks, the mitochondrial crest decreases or disappears, and the outer membrane is broken, although the morphological changes of the nucleus are not as obvious (Table 1).
Table 1.
The comparative characteristics among ferroptosis, apoptosis, and autophagy.
| RCD | Ferroptosis | Apoptosis | Autophagy |
|---|---|---|---|
| Hallmarks | Mitochondrial crest disappeared; mitochondrial outer membrane rupture and shrinkage; mitochondria are deeply stained | Condensation and fragmentation of chromatin; nucleoli disappeared; nuclear pyknosis and fragmentation | Autophagy lysosome formation |
| Other characteristics | No nuclear rupture; cell membrane rupture | Cell shrinkage; the outflow of the cytoplasm and vacuolation of membrane | No changes in nuclear and cell membrane |
| Biomarkers | Upregulated: ROS, PTGS2; downregulated: NADPH | Cytochrome C releases caspase-activated intracellular calcium increases | Transformation from LC3-I into LC3-II |
| Positive regulators | Erastin, RSL3, RAS, Sorafenib, p53 | P53, Bax, Bak, TGF-B, radiation | ATG family, Beclin1 |
| Negative regulators | GPX4, FSP1, SLC7A11, NRF2, Ferrostatin-1, Liproxstatin-1, DFO | Bcl-2, Bcd-XL, Z-VAD-FMK | 3-Methyladenine, Wortmannin, Spautin1 |
RCD: regulated cell death; PTGS2: prostaglandin endoperoxide synthase 2; FSP1: fibroblast-specific protein 1; NRF2: nuclear factor erythroid 2-related factor 2; DFO: desferrioxamine.
2.2. Underlying Mechanisms of Ferroptosis
Investigations on the regulation of ferroptosis have mainly focused on system Xc− and GSH metabolism, regulation of GPX4 activity, and ROS production (Figure 1). System Xc−, which comprises of SLC3A2 and SLC7A11 dimers, has been reported in various cells as a promising target for ferroptosis induction [17–19]. System Xc− is embedded into the cell membrane and as an effective cystine/glutamate antiporter system regulates the transport of cysteine and glutamate [20]. Glutamate is transferred outside the cell, while simultaneously, cystine is imported into the cell where it participates in the generation of GSH and thereby prevents ferroptosis [21]. A recent study reported that IFN-γ was capable of suppressing the expression of SLC3A2 and SLC7A11 via activation of JAK/STAT signaling, and repression of system Xc− could induce ferroptosis in hepatocellular carcinoma cells [22]. Similarly, as a tumor suppressor gene, p53 was demonstrated to inhibit cystine uptake by downregulating the expression of SLC7A11, which could decrease the activity of GPX4 and reduce the antioxidant ability of the cells ultimately inducing ferroptosis [23].
Figure 1.
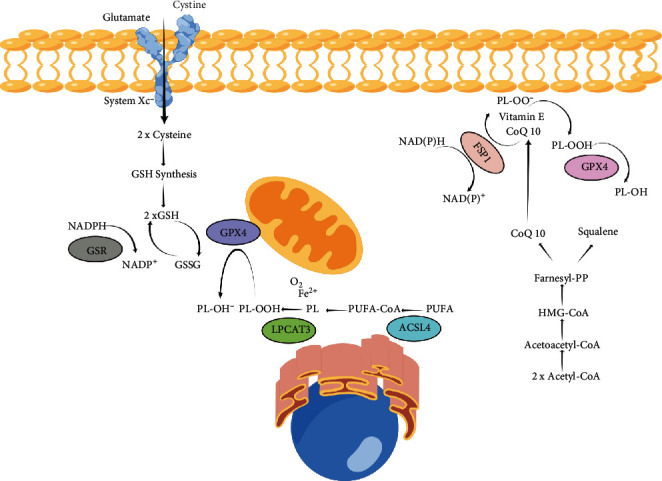
The molecular mechanism of ferroptosis.
Furthermore, GPX4 is considered a crucial molecule in the regulation of ferroptosis [24]. The basis of ferroptosis is the presence of free iron in the cells. The Fenton reaction between iron ions and ROS leads to peroxidation of PUFAs and formation of lipid peroxides, resulting in damage to the cell membrane [25]. GPX4 is able to ameliorate the toxicity of lipid peroxides via its catalytic activity and maintain the homeostasis of the lipid bilayer. Prior studies have shown that RSL3, an inhibitor of GPX4, can covalently bind to GPX4 and inactivate it, ultimately leading to the accumulation of intracellular peroxide and induction of ferroptosis [26].
In addition, the ROS-mediated pathway is a critical mechanism of ferroptosis. As induction of ferroptosis leads to the increase of intracellular lipid ROS, theoretically, lipid antioxidants may be promising antiferroptosis agents [27]. Mitochondria, an organelle with abundant iron and ROS production, are considered to be an important location of the occurrence of ferroptosis.
2.3. Mitochondrial Dysfunction Regulates Ferroptosis
Given the important role of mitochondria in ROS generation, their function is critical in ferroptosis [28]. Prior research revealed that complete inhibition of mitochondrial function could significantly decrease cell sensitivity to ferroptosis under cysteine-deprivation conditions [29]. Furthermore, Gaschler et al. [30] reported that partially decreased functioning of mitochondria could restore cell sensitivity to ferroptosis, findings which highlight mitochondria's significant function in initiating ferroptosis. Suppression of the tricarboxylic acid (TCA) cycle and electron transport chain (ETC) was also demonstrated to inhibit ferroptosis, which is consistent with the role of mitochondria in ROS generation [29, 30]. Several enzymes in the TCA cycle are critical for inducing ferroptosis [31]. For instance, a recent study showed that deprivation of fumarate hydratase in renal cancer cells could increase cell tolerance to ferroptosis [32]. Moreover, disruption of the TCA cycle was capable of suppressing lipid peroxidation and ferroptosis [33]. Consistent with the significant role of this process in mediating ferroptosis, suppression of the ETC was found to inhibit ROS accumulation and the induction of ferroptosis in response to either cysteine deprivation or erastin (a ferroptosis inducer) treatment [29].
3. Ferroptosis and Bone Homeostasis
Homeostasis is a complicated balance that is crucial for cells to maintain their normal physiological functions [30]. A tight balance between energy input and consumption is important for cell homeostasis. During metabolism, cells continuously consume energy and nutrients, while producing new energy and nutrients [31]. Similarly, our skeletal system has a continuous remodeling cycle, and an appropriate balance between anabolism and catabolism is needed to maintain the strength and healthy microstructure of bone tissue [32]. Bone remodeling is accomplished through the coordinated efforts of four key cells: bone marrow mesenchymal stem cells (MSCs) which are the source of osteoblasts (OBs) and exert regulatory functions throughout remodeling; OBs are located on the bone surface and secrete bone matrix; matrix-embedded OBs further differentiate into persistent osteocytes (OCT), which form a mechanosensory network in bone and play a crucial role in paracrine signaling. At the same time, osteoclasts (OC) continuously degrade and absorb the surrounding bone base [33]. The dynamic balance between bone formation and bone resorption is continuously coordinated. As ferroptosis is an important mode of regulated cell death, its relationship with skeletal cells, including MSCs, OBs, OCs, and OCTs, has attracted attention in recent decades [34].
3.1. Ferroptosis and the MSCs
Recent research in the study of bone tissue repair and regeneration has paid particular attention to MSCs. MSCs have the potential of multidirectional differentiation with low immunogenicity and wide availability. They can migrate to damaged tissues and organs to reconstruct these through direct differentiation or secretion of exosomes, growth factors, and cytokines [35, 36]. Furthermore, the regulatory role of MSCs in ameliorating cell ferroptosis has been well-documented [37]. For example, it was recently shown that MSCs are capable of inhibiting the production of lipid peroxidation and alleviating ferroptosis both in vitro and in vivo. The authors also demonstrated that MSC-derived exosomes are involved in the underlying mechanisms of the effect of MSCs on ferroptosis, which could significantly downregulate the expression of prostaglandin-endoperoxide synthase 2 and promote SLC7A11 expression [38]. Similarly, the suppressive effect of MSCs on ferroptosis was seen in neuronal cells [39]. In an acute spinal cord injury mouse model, researchers demonstrated that MSCs and their exosomes could ameliorate spinal cord injury through promotion of the expression of ferroptosis inhibitor (FSP1) [39]. In addition to the discovery of the antiferroptotic effect of MSCs, the underlying mechanism of ferroptosis in MSCs was also investigated. It is well-documented that NOP2/Sun RNA methyltransferase 5 (NSUN5) posttranscriptionally can mediate ferroptosis in MSCs through RNA methylation [40]. A recent study further found that NSUN5 is downregulated in erastin-induced ferroptosis in MSCs, while NSUN5 is capable of suppressing ferritin heavy chain/light-chain (FTH1/FTL) activity. In the NSUN5 depletion experiments, they found an accumulation of intracellular iron and a marked decrease of GPX4, suggesting that the NSUN5-FTH1/FTL pathway mediates ferroptosis in MSCs and that therapeutic targeting of components of this pathway may promote resistance to ferroptosis and improve the survival of MSCs [40].
3.2. Ferroptosis and OBs
The integrity of bone is maintained through an appropriate balance between osteogenic and osteoclastic activities, and the bone remodeling process is a continuous cycle. OBs are mainly involved in bone reconstruction, including formation, mineralization, and construction of osteocytes [41]. A variety of studies have focused on the potential mechanisms and agents regulating OB ferroptosis [42]. Advanced glycation end products were recently found to induce OB ferroptosis and promote osteoporosis [43]. Inversely, melatonin, a hormone secreted by the pineal gland, was shown to ameliorate OB ferroptosis and enhance the osteogenic capacity of OB via activation of Nrf-2/HO-1 signaling [44]. Mechanistically, mitochondrial ferritin (FtMt) was reported to exert a critical role in regulating cell ferroptosis via storing iron ions and intercepting toxic ferrous ions in mitochondria [45]. The researchers found that activation of FtMt could ameliorate OB ferroptosis while inhibition of FtMt could induce mitophagy through ROS/PINK1/Parkin signaling [45]. Moreover, increased ferroptosis in OBs could be seen after activating mitophagy, with the findings suggesting that FtMt can effectively suppress ferroptosis in OBs [45]. Interestingly, exosomes, extracellular vesicles containing active regulatory factors, have been shown to participate in the regulation of ferroptosis in OB. For example, one recent study reported that vascular endothelial cells could effectively prevent osteoblastic ferroptosis through exosome release which could further suppress ferritinophagy and limit ferroptosis of OBs [46]. Similarly, using an osteoporotic murine model, it was reported that exosomes from endothelial progenitor cells could inhibit steroid-induced osteoporosis through suppression of the ferroptotic pathway [47].
3.3. Ferroptosis and OCs
Iron ions is capable to induce OC differentiation and bone resorption through the production of ROS [48, 49]. Zoledronic acid (ZA), a bisphosphonate, has been reported to inhibit OC growth via induction of ferroptosis of the OC [49]. The role of ZA in regulating osteoclast function was evaluated using a RANKL-induced cell model, which indicated that ZA treatment suppressed the cell viability of osteoclasts and facilitated osteoclast ferroptosis with an increase in iron ions and ROS and decrease in the GPX4 and GSH level [49]. Similarly, ferroptosis was reported involved in OC function during RANKL-induced differentiation and is induced by iron starvation response and ferritin phagocytosis [50]. Mechanically, subsequent RANKL stimulation can lead to iron droop due to iron starvation response (increased transferrin receptor 1 and decreased ferritin) under normoxic but not hypoxic conditions, due to downregulation of aconitase activity [50]. Based on these results, it can be assumed that ferroptosis of OCs can limit bone resorption, while inducing ferroptosis in OCs could be an alternative treatment of disorders of bone formation.
3.4. Ferroptosis and OCTs
OCTs, the most prevalent cells in mineralized bone tissue, communicate with other bone cells, such as OBs and OCs, via the lacunar-canalicular system and through various secreted hormones [51]. Decreased activity and death of OCTs induced by internal and external factors can lead to bone loss and destruction of bone microstructure. Therefore, effective promotion of OCT survival is a promising therapeutic strategy for maintenance of bone homeostasis. It has been reported that ferroptosis is an important form of OCT death, which can be reversed by targeting the inhibition of ferroptosis signaling pathways [52]. Yang et al. [53] found that a hyperglycemic microenvironment is capable of promoting lipid peroxidation and iron overload, thereby inducing osteocyte ferroptosis. Furthermore, RNA sequencing results indicated that heme oxygenase-1 (HO-1) is overexpressed in ferroptotic osteocytes, suggesting that HO-1 is essential for osteocyte ferroptosis. Similarly, a recent study reported that dexamethasone could notably induce ferroptosis in MC3T3-E1 cells (a type of OCT precursor cell) via downregulation of the p53/SLC7A11/GPX4 signaling pathway, providing a potential mechanism for the effect of ferroptosis on osteocytes in steroid- (glucocorticoid) induced osteonecrosis of the femoral head [54]. These findings highlight a potential therapeutic target for the treatment of skeletal disorders.
4. Ferroptosis and Bone Degenerative Disorders
Ferroptosis differs from apoptosis, autophagy, necrosis, and pyrodeath, in that it mainly involves iron metabolism and lipid peroxidation. Ferroptosis plays an important role in malignant tumors, cardiovascular diseases, and neural system diseases [55, 56]. Iron overload is closely related to cellular ferroptosis, and iron overload and lipid peroxide accumulation jointly mediate bone destruction, ultimately leading to bone disorders.
4.1. Ferroptosis and Osteoporosis
Osteoporosis is a systemic bone disease characterized by a reduction in bone mass and a degeneration of the fibrous structure of bone tissue, which results in increased bone brittleness and risk of fracture [57]. Its pathological features include the following [58]: (1) decreased bone mass, including a reduction in the proportion of bone minerals and other substrates; (2) degeneration of bone microstructure, caused by absorption and imbalance of bone tissue homeostasis, manifesting as destruction, deformation, and fracture of bone trabecular structure; and (3) increased bone brittleness and decreased bone strength, increased fracture deformation, decreased load bearing force, and more frequent microfracture or complete fracturing. Iron is a strong oxidant that can promote the production of ROS radicals, and iron metabolism can directly or indirectly affect the occurrence and development of type 2 diabetes [59, 60]. Ferroptosis results in the production of abundant ROS through the Fenton reaction, inducing accumulation of lipid peroxides and cell damage [61]. It was well-documented that hyperglycemia can induce ferroptosis in the bone tissue of an osteoporotic rat model by production of ROS/lipid peroxidation. Melatonin was shown to ameliorate the level of ferroptosis through activation of Nrf2/HO-1 signaling and promotion of the osteogenic differentiation of MC3T3-E1 cells [44]. Similarly, in a murine model of diabetic osteoporosis (DOP), the researchers verified the important role of ferroptosis in DOP-induced OCT death. Mechanistically, activation of Nrf2/HO-1 signaling could lead to lipid peroxidation and cell ferroptosis, suggesting that targeting inhibition of OCT ferroptosis may be a potential therapeutic strategy for DOP treatment [53].
Furthermore, the relationship between ferroptosis and glucocorticoid-induced osteoporosis (GIOP) has been well investigated [62]. For example, a recent study [47] reported that high-dose dexamethasone (10 μM) can induce ferroptosis of OB by inhibiting the expression of GPX4 and system Xc−. To investigate the underlying mechanisms, extracellular vesicles were extracted from bone marrow-derived endothelial progenitor cells (EPC-EVs), which were seen to suppress ferroptosis by restoring the activity of GPX4 and system Xc−. Significantly, EPC-EVs were capable of reversing dexamethasone-induced changes in cysteine and oxidative damage markers and improved skeletal parameters in mice. These results suggest that EPC-EVs reverse murine GIOP through inhibition of OB ferroptosis.
4.2. Ferroptosis and Osteoarthritis (OA)
OA is a degenerative disease characterized by the pathological alteration of the function and morphology of an entire joint, as well as articular cartilage destruction and damage to other joint components [63–66]. Generally, OA occurs due to chronic heavy loading and biomechanical damage; however, pathological progress at the molecular level has also been proposed in the development of OA. Therefore, maintaining chondrocytes in a healthy state is considered to be an effective strategy for preserving the integrity of the entire cartilage [67–70]. It can be, therefore, assumed that ferroptosis may be involved in the progression of OA. In a recent study [71], researchers used interleukin-1 beta (IL-1β) to construct an in vitro iron-overload model. The authors found that IL-1β could induce both ROS and lipid ROS accumulations and saw ferroptosis-related protein expression changes in the chondrocytes. Furthermore, increased MMP13 expression and decreased collagen II expression were seen in the ferroptotic chondrocytes (Figure 2). In a murine OA model, intra-articular injection of a ferroptosis inhibitor was seen to prevent OA progression. These findings highlight the contribution of ferroptosis in chondrocytes to the progression of OA. Studies have also been conducted to identify a feasible treatment for chondrocyte degeneration with a focus on cell ferroptosis [72]. Deferoxamine (DFO) [73] and D-mannose were recently demonstrated to alleviate OA progression by inhibiting of chondrocyte ferroptosis. DFO was found to both effectively ameliorate chondrocyte ferroptosis and induce activation of the Nrf2 antioxidant system, which is crucial for chondrocyte protection [73]. The efficacy of injection of DFO in OA mice was also demonstrated in vivo [73]. Similarly, Zhou et al. [74] investigated whether D-mannose mediates chondrocyte ferroptosis during OA cartilage degeneration in vitro and in vivo. They found that D-mannose could exert a chondroprotective effect by attenuating the sensitivity of chondrocytes to ferroptosis and could alleviate OA progression. Furthermore, HIF-2α was identified as a central mediator in the D-mannose-induced resistance of chondrocytes to ferroptosis. These findings provide potential therapeutic strategies for ferroptosis-related bone diseases.
Figure 2.
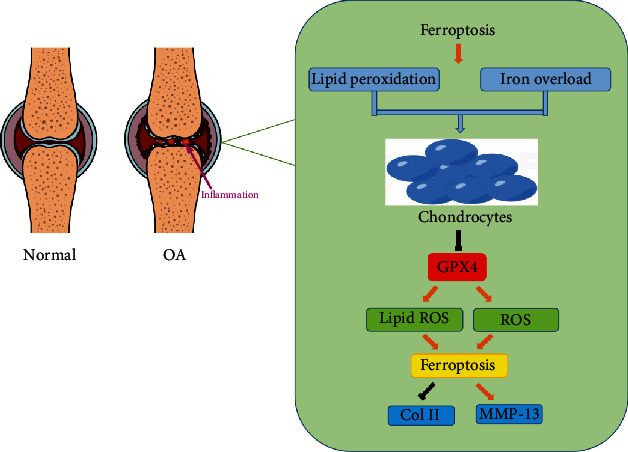
The potential mechanism of ferroptotic chondrocytes.
5. Conclusion
During the past decades, there has been an accumulative research focus on the relationship between ferroptosis and diseases [75, 76]. The significance of ferroptosis in cell survival and differentiation is widely accepted, and its regulatory role in the modulation and treatment of diverse disease has been gradually uncovered [77, 78]. However, there are still some academic problems yet to be resolved. For example, the relationship between ferroptosis and the other forms of regulated cell death in the regulation of skeletal disorders should be further revealed. Furthermore, the detailed molecular mechanisms that activate ferroptosis are still unascertained. In addition, plenty of emerging evidences have demonstrated that exosomes are involved in the modulation of ferroptosis and skeletal disease [79, 80]. In-depth knowledge of this exosome-mediated effect should be achieved by performing more researches regarding the crosstalk between exosome and ferroptosis.
The recent continuous efforts in the research of ferroptosis have shed light on the interaction between ferroptosis and bone homeostasis. Technical limitations currently restrict the in-depth understanding of the mechanisms underlying ferroptotic regulation. Specifically, lack of an effective and specific ferroptotic blocker precludes the observation of the effect of blockade on the physiological functions of ferroptosis in in vivo models. Furthermore, lack of defined and specific ferroptotic signaling pathways or biomarkers hinders the verification of ferroptosis in physiological or pathological conditions. In addition, given the limitations of existing experimental techniques, we do not yet have a visual reporting method of in vivo ferroptosis detection. In future studies of ferroptosis, research should focus on in-depth study of the molecular mechanisms underlying ferroptosis and screening and identifying specific signaling pathways. Furthermore, efforts should be invested in developing a feasible detectable tool for measuring ferroptosis in vivo. Finally, the regulatory role of ferroptosis in the process of bone aging should be elucidated.
Acknowledgments
The authors would like to thank Figdraw software (http://www.figdraw.com) for its picture source assistance during the preparation of this manuscript. This work was supported by the National Natural Science Foundation of China (No. 82002313) and Hubei Province Key Laboratory of Oral and Maxillofacial Development and Regeneration (Nos. 2020kqhm008, 2021kqhm002).
Contributor Information
Bobin Mi, Email: mibobin@hust.edu.cn.
Guohui Liu, Email: liuguohui@hust.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
YX, BM, and GL conceived the ideas. YX, LC, ACP, and ZL wrote the manuscript. ACP edited the manuscript. YH, WZ, YS, FC, GL, GD, and RZ participated in the discussions. Yuan Xiong, Ze Lin, and Lang Chen contributed equally to this work.
References
- 1.Dixon S. J., Lemberg K. M., Lamprecht M. R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell . 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: the role of ferroptosis in cancer. Nature Reviews. Clinical Oncology . 2021;18(5):280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H., Hu B., Li Z., et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nature Cell Biology . 2022;24(1):88–98. doi: 10.1038/s41556-021-00818-3. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X., Stockwell B. R., Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nature Reviews. Molecular Cell Biology . 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Research . 2021;31(2):107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng J., Conrad M. The metabolic underpinnings of ferroptosis. Cell Metabolism . 2020;32(6):920–937. doi: 10.1016/j.cmet.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Lei G., Zhang Y., Koppula P., et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Research . 2020;30(2):146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salhotra A., Shah H. N., Levi B., Longaker M. T. Mechanisms of bone development and repair. Nature Reviews. Molecular Cell Biology . 2020;21(11):696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chande S., Bergwitz C. Role of phosphate sensing in bone and mineral metabolism. Nature Reviews. Endocrinology . 2018;14(11):637–655. doi: 10.1038/s41574-018-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang T., Chen J., Xu G., et al. Ferroptosis-related gene SOCS1, a marker for tuberculosis diagnosis and treatment, involves in macrophage polarization and facilitates bone destruction in tuberculosis. Tuberculosis . 2022;132:p. 102140. doi: 10.1016/j.tube.2021.102140. [DOI] [PubMed] [Google Scholar]
- 11.Lei T., Qian H., Lei P., Hu Y. Ferroptosis-related gene signature associates with immunity and predicts prognosis accurately in patients with osteosarcoma. Cancer Science . 2021;112(11):4785–4798. doi: 10.1111/cas.15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye L., Jin F., Kumar S. K., Dai Y. The mechanisms and therapeutic targets of ferroptosis in cancer. Expert Opinion on Therapeutic Targets . 2021;25(11):965–986. doi: 10.1080/14728222.2021.2011206. [DOI] [PubMed] [Google Scholar]
- 13.Kuang F., Liu J., Xie Y., Tang D., Kang R. MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chemical Biology . 2021;28(6):765–775.e5. doi: 10.1016/j.chembiol.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Deng F., Zhao B., Yang X., et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes . 2021;13(1):1–21. doi: 10.1080/19490976.2021.1902719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byun J., Lee S., Kang G. W., et al. Macropinocytosis is an alternative pathway of cysteine acquisition and mitigates sorafenib-induced ferroptosis in hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research . 2022;41(1):p. 98. doi: 10.1186/s13046-022-02296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei G., Mao C., Yan Y., Zhuang L., Gan B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein & Cell . 2021;12(11):836–857. doi: 10.1007/s13238-021-00841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H., Ye D., Ren M., Zhang H., Bi F. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends in Molecular Medicine . 2021;27(9):856–867. doi: 10.1016/j.molmed.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Ajoolabady A., Aslkhodapasandhokmabad H., Libby P., et al. Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends in Endocrinology and Metabolism . 2021;32(7):444–462. doi: 10.1016/j.tem.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Koppula P., Zhuang L., Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein & Cell . 2021;12(8):599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen P., Wu Q., Feng J., et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduction and Targeted Therapy . 2020;5(1):p. 51. doi: 10.1038/s41392-020-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou A., Fang T., Chen K., Xu Y., Chen Z., Ning X. Biomimetic activator of sonodynamic ferroptosis amplifies inherent peroxidation for improving the treatment of breast cancer. Small . 2022;18(12, article e2106568) doi: 10.1002/smll.202106568. [DOI] [PubMed] [Google Scholar]
- 22.Kong R., Wang N., Han W., Bao W., Lu J. IFNγ-mediated repression of system xc− drives vulnerability to induced ferroptosis in hepatocellular carcinoma cells. Journal of Leukocyte Biology . 2021;110(2):301–314. doi: 10.1002/JLB.3MA1220-815RRR. [DOI] [PubMed] [Google Scholar]
- 23.Shao M., Jiang Q., Shen C., Liu Z., Qiu L. Sinapine induced ferroptosis in non-small cell lung cancer cells by upregulating transferrin/transferrin receptor and downregulating SLC7A11. Gene . 2022;827:p. 146460. doi: 10.1016/j.gene.2022.146460. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Li J., Li Z., et al. Homeostasis imbalance of YY2 and YY1 promotes tumor growth by manipulating ferroptosis. Advancement of Science . 2022;13(article e2104836) doi: 10.1002/advs.202104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kan X., Yin Y., Song C., et al. Newcastle-disease-virus-induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells. iScience . 2021;24(8):p. 102837. doi: 10.1016/j.isci.2021.102837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng W., Ouyang Y., Wang S., et al. L-F001, a multifunctional fasudil-lipoic acid dimer prevents RSL3-induced Ferroptosis via maintaining iron homeostasis and inhibiting JNK in HT22 cells. Frontiers in Cellular Neuroscience . 2022;16:p. 774297. doi: 10.3389/fncel.2022.774297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang F., Jiang X., Cao L., Gu Q., Teng X., He L. Diverse sesquiterpenoids from the roots of Croton crassifolius and their inhibitory effects on ferroptosis. Chemistry & Biodiversity . 2022;19(4, article e202101028) doi: 10.1002/cbdv.202101028. [DOI] [PubMed] [Google Scholar]
- 28.Tadokoro T., Ikeda M., Ide T., et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. Insight . 2020;5(9) doi: 10.1172/jci.insight.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basit F., van Oppen L. M. P. E., Schöckel L., et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death & Disease . 2017;8(3):p. e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaschler M. M., Hu F., Feng H., Linkermann A., Min W., Stockwell B. R. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chemical Biology . 2018;13(4):1013–1020. doi: 10.1021/acschembio.8b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgir S., Bastami M., Ilkhani K., Safi A., Seif F., Alivand M. R. The pathways related to glutamine metabolism, glutamine inhibitors and their implication for improving the efficiency of chemotherapy in triple-negative breast cancer. Mutation Research, Reviews in Mutation Research . 2021;787:p. 108366. doi: 10.1016/j.mrrev.2021.108366. [DOI] [PubMed] [Google Scholar]
- 32.Ooi A. Advances in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) research. Seminars in Cancer Biology . 2020;61:158–166. doi: 10.1016/j.semcancer.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao M., Yi J., Zhu J., et al. Role of mitochondria in ferroptosis. Molecular Cell . 2019;73(2):354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh B. M., Lee S., Park G. L., et al. Erastin inhibits septic shock and inflammatory gene expression via suppression of the NF-κB pathway. Journal of Clinical Medicine . 2019;8(12):p. 2210. doi: 10.3390/jcm8122210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhai Q., Ren Y. Q., Ni Q. S., Song Z. H., Ge K. L., Guo Y. L. Transplantation of human umbilical cord mesenchymal stem cells-derived neural stem cells pretreated with Neuregulin1β ameliorate cerebral ischemic reperfusion injury in rats. Biomolecules . 2022;12(3):p. 428. doi: 10.3390/biom12030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Zhang X., Liu Y., et al. Exosomes derived from mesenchyml stem cells ameliorate oxygen-glucose deprivation/reoxygenation-induced neuronal injury via transferring microRNA-194 and targeting Bach1. Tissue & Cell . 2021;73:p. 101651. doi: 10.1016/j.tice.2021.101651. [DOI] [PubMed] [Google Scholar]
- 37.Xu J., Zhang M., Liu F., et al. Mesenchymal stem cells alleviate post-resuscitation cardiac and cerebral injuries by inhibiting cell pyroptosis and ferroptosis in a swine model of cardiac arrest. Frontiers in Pharmacology . 2021;12:p. 793829. doi: 10.3389/fphar.2021.793829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin F., Chen W., Zhou J., et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death & Disease . 2022;13(3):p. 271. doi: 10.1038/s41419-022-04708-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao C., Chen Y., Yang T., Zhao H., Li D. Mesenchymal stem cell derived exosomes suppress neuronal cell ferroptosis via lncGm36569/miR-5627-5p/FSP1 axis in acute spinal cord injury. Stem Cell Reviews and Reports . 2022;18(3):1127–1142. doi: 10.1007/s12015-022-10327-x. [DOI] [PubMed] [Google Scholar]
- 40.Liu J., Ren Z., Yang L., et al. The NSUN5-FTH1/FTL pathway mediates ferroptosis in bone marrow-derived mesenchymal stem cells. Cell Death Discovery . 2022;8(1):p. 99. doi: 10.1038/s41420-022-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L., Xiong Y., Yan C., et al. LncRNA KCNQ1OT1 accelerates fracture healing via modulating miR-701-3p/FGFR3 axis. The FASEB Journal . 2020;34(4):5208–5222. doi: 10.1096/fj.201901864RR. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J. The osteoprotective effects of artemisinin compounds and the possible mechanisms associated with intracellular iron: a review of in vivo and in vitro studies. Environmental Toxicology and Pharmacology . 2020;76:p. 103358. doi: 10.1016/j.etap.2020.103358. [DOI] [PubMed] [Google Scholar]
- 43.Ge W., Jie J., Yao J., Li W., Cheng Y., Lu W. Advanced glycation end products promote osteoporosis by inducing ferroptosis in osteoblasts. Molecular Medicine Reports . 2022;25(4) doi: 10.3892/mmr.2022.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma H., Wang X., Zhang W., et al. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxidative Medicine and Cellular Longevity . 2020;2020:18. doi: 10.1155/2020/9067610.9067610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X., Ma H., Sun J., et al. Mitochondrial ferritin deficiency promotes osteoblastic ferroptosis via mitophagy in type 2 diabetic osteoporosis. Biological Trace Element Research . 2022;200(1):298–307. doi: 10.1007/s12011-021-02627-z. [DOI] [PubMed] [Google Scholar]
- 46.Yang R., Xu W., Zheng H., et al. Exosomes derived from vascular endothelial cells antagonize glucocorticoid- induced osteoporosis by inhibiting ferritinophagy with resultant limited ferroptosis of osteoblasts. Journal of Cellular Physiology . 2021;236(9):6691–6705. doi: 10.1002/jcp.30331. [DOI] [PubMed] [Google Scholar]
- 47.Lu J., Yang J., Zheng Y., Chen X., Fang S. Extracellular vesicles from endothelial progenitor cells prevent steroid- induced osteoporosis by suppressing the ferroptotic pathway in mouse osteoblasts based on bioinformatics evidence. Scientific Reports . 2019;9(1):p. 16130. doi: 10.1038/s41598-019-52513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong Y., Chen L., Yan C., Endo Y., Mi B., Liu G. The lncRNA Rhno1/miR-6979-5p/BMP2 axis modulates osteoblast differentiation. International Journal of Biological Sciences . 2020;16(9):1604–1615. doi: 10.7150/ijbs.38930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu X., Sun Z., Wang Y., Ong H. S. Zoledronic acid promotes osteoclasts ferroptosis by inhibiting FBXO9-mediated p 53 ubiquitination and degradation. Peer J . 2021;9, article e12510 doi: 10.7717/peerj.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ni S., Yuan Y., Qian Z., et al. Hypoxia inhibits RANKL-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radical Biology & Medicine . 2021;169:271–282. doi: 10.1016/j.freeradbiomed.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 51.Xiong Y., Yan C., Chen L., et al. IL-10 induces MC3T3-E1 cells differentiation towards osteoblastic fate in murine model. Journal of Cellular and Molecular Medicine . 2020;24(1):1076–1086. doi: 10.1111/jcmm.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun F., Zhou J. L., Liu Z. L., Jiang Z., Peng H. Dexamethasone induces ferroptosis via P53/SLC7A11/GPX4 pathway in glucocorticoid-induced osteonecrosis of the femoral head. Biochemical and Biophysical Research Communications . 2022;602:149–155. doi: 10.1016/j.bbrc.2022.02.112. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y., Lin Y., Wang M., et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Research . 2022;10(1):p. 26. doi: 10.1038/s41413-022-00198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J., Lou C., Zhen C., Wang Y., Shang P., Lv H. Iron plays a role in sulfasalazine-induced ferroptosis with autophagic flux blockage in K7M2 osteosarcoma cells. Metallomics . 2022;14(5) doi: 10.1093/mtomcs/mfac027. [DOI] [PubMed] [Google Scholar]
- 55.Qiu C., Liu T., Luo D., Luan D., Cheng L., Wang S. Novel therapeutic savior for osteosarcoma: the endorsement of ferroptosis. Frontiers in Oncology . 2022;12:p. 746030. doi: 10.3389/fonc.2022.746030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Y., Xiong Y., Yan C., et al. Downregulation of microRNA-16-5p accelerates fracture healing by promoting proliferation and inhibiting apoptosis of osteoblasts in patients with traumatic brain injury. American Journal of Translational Research . 2019;11(8):4746–4760. [PMC free article] [PubMed] [Google Scholar]
- 57.Yu T., Wang Z., You X., et al. Resveratrol promotes osteogenesis and alleviates osteoporosis by inhibiting p 53. Aging . 2020;12(11):10359–10369. doi: 10.18632/aging.103262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu T., You X., Zhou H., et al. MiR-16-5p regulates postmenopausal osteoporosis by directly targeting VEGFA. Aging . 2020;12(10):9500–9514. doi: 10.18632/aging.103223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krümmel B., von Hanstein A., Plötz T., Lenzen S., Mehmeti I. Differential effects of saturated and unsaturated free fatty acids on ferroptosis in rat β-cells. The Journal of Nutritional Biochemistry . 2022;106:p. 109013. doi: 10.1016/j.jnutbio.2022.109013. [DOI] [PubMed] [Google Scholar]
- 60.He J., Li Z., Xia P., et al. Ferroptosis and ferritinophagy in diabetes complications. Molecular Metabolism . 2022;60:p. 101470. doi: 10.1016/j.molmet.2022.101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong L., Bin Zhou, Young J. L., Wintergerst K., Cai L. Exposure to low-dose cadmium induces testicular ferroptosis. Ecotoxicology and Environmental Safety . 2022;234:p. 113373. doi: 10.1016/j.ecoenv.2022.113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Y., Shen X., Ke Y., et al. Activation of osteoblast ferroptosis via the METTL3/ASK1-p 38 signaling pathway in high glucose and high fat (HGHF)-induced diabetic bone loss. The FASEB Journal . 2022;36(3, article e22147) doi: 10.1096/fj.202101610R. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y., You X., Cohen J. D., et al. Sex differences in osteoarthritis pathogenesis: a comprehensive study based on bioinformatics. Medical Science Monitor . 2020;26, article e923331 doi: 10.12659/MSM.923331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Q., Luo X., Xiong Y., et al. Platelet-rich plasma versus hyaluronic acid in knee osteoarthritis: a meta-analysis with the consistent ratio of injection. Journal of Orthopaedic Surgery (Hong Kong) . 2020;28(1):p. 230949901988766. doi: 10.1177/2309499019887660. [DOI] [PubMed] [Google Scholar]
- 65.Xiong Y., Mi B., Liu M., Xue H., Wu Q. P., Liu G. H. Bioinformatics analysis and identification of genes and molecular pathways involved in synovial inflammation in rheumatoid arthritis. Medical Science Monitor . 2019;25:2246–2256. doi: 10.12659/MSM.915451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Mi B., Lv H., et al. Shared KEGG pathways of icariin-targeted genes and osteoarthritis. Journal of Cellular Biochemistry . 2019;120(5):7741–7750. doi: 10.1002/jcb.28048. [DOI] [PubMed] [Google Scholar]
- 67.Chen J., Chen S., Cai D., Wang Q., Qin J. The role of Sirt 6 in osteoarthritis and its effect on macrophage polarization. Bioengineered . 2022;13(4):9677–9689. doi: 10.1080/21655979.2022.2059610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kokai L., Chen J., Wang D., et al. Comparison of clinically relevant adipose preparations on articular chondrocyte phenotype in a novel in vitro co-culture model. Stem Cells and Development . 2022;12 doi: 10.1089/scd.2021.0355. [DOI] [PubMed] [Google Scholar]
- 69.Lv M., Cai Y., Hou W., et al. The RNA-binding protein SND1 promotes the degradation of GPX4 by destabilizing the HSPA5 mRNA and suppressing HSPA5 expression, promoting ferroptosis in osteoarthritis chondrocytes. Inflammation Research . 2022;71(4):461–472. doi: 10.1007/s00011-022-01547-5. [DOI] [PubMed] [Google Scholar]
- 70.Mo Z., Xu P., Li H. Stigmasterol alleviates interleukin-1beta-induced chondrocyte injury by down-regulatingsterol regulatory element binding transcription factor 2 to regulateferroptosis. Bioengineered . 2021;12(2):9332–9340. doi: 10.1080/21655979.2021.2000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao X., Sun K., Yu S., et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. Journal of Orthopaedic Translation . 2021;27:33–43. doi: 10.1016/j.jot.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun K., Guo Z., Hou L., et al. Iron homeostasis in arthropathies: from pathogenesis to therapeutic potential. Ageing Research Reviews . 2021;72:p. 101481. doi: 10.1016/j.arr.2021.101481. [DOI] [PubMed] [Google Scholar]
- 73.Guo Z., Lin J., Sun K., et al. Deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf 2 pathway. Frontiers in Pharmacology . 2022;13, article 791376 doi: 10.3389/fphar.2022.791376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou X., Zheng Y., Sun W., et al. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2α-dependent manner. Cell Proliferation . 2021;54(11, article e13134) doi: 10.1111/cpr.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duan J., Lin X., Xu F., et al. Ferroptosis and its potential role in metabolic diseases: a curse or revitalization? Frontiers in Cell and Development Biology . 2021;9:p. 701788. doi: 10.3389/fcell.2021.701788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simão M., Cancela M. L. Musculoskeletal complications associated with pathological iron toxicity and its molecular mechanisms. Biochemical Society Transactions . 2021;49(2):747–759. doi: 10.1042/BST20200672. [DOI] [PubMed] [Google Scholar]
- 77.Wang F., Lv H., Zhao B., et al. Iron and leukemia: new insights for future treatments. Journal of Experimental & Clinical Cancer Research . 2019;38(1):p. 406. doi: 10.1186/s13046-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu L., Huang X., Lou Y., Xie W., Zhao H. Regulation of apoptosis, autophagy and ferroptosis by non-coding RNAs in metastatic non-small cell lung cancer (review) Experimental and Therapeutic Medicine . 2022;23(5):p. 352. doi: 10.3892/etm.2022.11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mi B., Yan C., Xue H., et al. Inhibition of circulating miR-194-5p reverses osteoporosis through Wnt5a/β-catenin-dependent induction of osteogenic differentiation. Molecular Therapy--Nucleic Acids . 2020;21:814–823. doi: 10.1016/j.omtn.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mi B., Chen L., Xiong Y., et al. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. Journal of Nanobiotechnology . 2020;18(1):p. 68. doi: 10.1186/s12951-020-00624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


