Abstract
The bacterium Vibrio cholerae, the etiological agent of cholera, is often found attached to plankton, a property that is thought to contribute to its environmental persistence in aquatic habitats. The V. cholerae O1 El Tor biotype and V. cholerae O139 strains produce a surface pilus termed the mannose-sensitive hemagglutinin (MSHA), whereas V. cholerae O1 classical biotype strains do not. Although V. cholerae O1 classical does not elaborate MSHA, the gene is present and expressed at a level comparable to that of the other strains. Since V. cholerae O1 El Tor and V. cholerae O139 have displaced V. cholerae O1 classical as the major epidemic strains over the last fifteen years, we investigated the potential role of MSHA in mediating adherence to plankton. We found that mutation of mshA in V. cholerae O1 El Tor significantly diminished, but did not eliminate, adherence to exoskeletons of the planktonic crustacean Daphnia pulex. The effect of the mutation was more pronounced for V. cholerae O139, essentially eliminating adherence. Adherence of the V. cholerae O1 classical mshA mutant was unaffected. The results suggest that MSHA is a factor contributing to the ability of V. cholerae to adhere to plankton. The results also showed that both biotypes of V. cholerae O1 utilize factors in addition to MSHA for zooplankton adherence. The expression of MSHA and these additional, yet to be defined, adherence factors differ in a serogroup- and biotype-specific manner.
Throughout history, aquatic ecosystems have consistently been the focal points of cholera outbreaks (7, 11, 32, 48), and the bacillus Vibrio cholerae is now known to be endemic in aquatic environments, present even in the absence of human inputs (7, 8, 12, 16, 17, 23, 32, 33, 66). Little is known about what regulates V. cholerae abundance in aquatic systems, but outbreaks in humans are hypothesized to be correlated with seasonally high abundances (blooms) of phytoplankton or zooplankton (6, 7, 11, 33). Survival and abundance of V. cholerae in planktonic communities, and therefore cholera outbreaks in humans, are believed to depend in part on the ability of V. cholerae to attach to the surfaces of phytoplankton and zooplankton (5, 10, 24–31, 33–35, 51, 53). Bacterial attachment to planktonic detrital particles has been shown to increase bacterial productivity and is believed to be a way to escape low-nutrient conditions (see, for example, reference 47). Considerably less is known about the role of living plankton as a bacterial microhabitat, but it has been proposed for V. cholerae that attachment to plankton increases survival and growth by providing both a source of nutrition and a microenvironment that provides protection from conditions which are detrimental to V. cholerae (i.e., extremely low or high salinity) (43, 44, 62, 63, 75). Of additional medical concern, colonization of plankton surfaces may serve to spatially concentrate bacteria, making it easier for humans to consume infectious doses (7, 27).
Historically, strains of V. cholerae associated with epidemic disease have been of the O1 serogroup, which is divided into two biotypes: classical and El Tor (4, 7, 61). A distinguishing feature between these biotypes is the production of a cell-associated, mannose-sensitive hemagglutinin (MSHA) by strains of the El Tor biotype (15, 19). The hemagglutinating activity is the result of the elaboration of a type 4 pilus, for which MshA is the major subunit (37). The gene encoding the MSHA subunit, mshA, has been characterized and is located in a large gene cluster involved in biogenesis of the pilus structure (20, 38, 41, 42). Presence of pili on bacterial cells is often associated with the ability to colonize surfaces. A study with mshA mutant strains has demonstrated a role for MSHA in colonization and subsequent biofilm formation on abiotic surfaces (borosilicate glass) and biotic surfaces (cellulose) (73). Another V. cholerae type 4 pilus, the toxin-coregulated pilus (TCP), is necessary for colonization of the mammalian intestine (3, 21, 65, 68, 69). There is no apparent role for MSHA for V. cholerae colonization of the human digestive tract, as evaluated by using defined mshA null mutants in animal models and adult volunteer studies (3, 65, 69), and, vice versa, there is no known role for TCP in the colonization of nonintestinal surfaces (73, 74). Thus, MSHA might have a specific role in environmental survival for V. cholerae.
Despite the fact that O1 strains of the El Tor biotype were not associated with epidemic cholera until the beginning of the seventh pandemic in 1961, they have displaced classical biotype strains during the past 15 years to become the most prevalent epidemic strains at the present time (4, 7, 59, 61). During this time, there was also a transient outbreak caused by strains of the previously unrecognized O139 serogroup (14, 49, 59). Strains of the O139 serogroup share many characteristics with O1 El Tor strains and are thought to be derived from O1 El Tor (18, 36, 55, 71). The reasons for the predominance of El Tor and the appearance of the highly related O139 strains are probably multifactorial and likely include parameters associated with interactions of the organism with the human host as well as with the environment. Since MSHA is a factor that is expressed by O1 strains of the El Tor biotype and O139 strains and is not expressed by strains of the previously predominant O1 classical biotype (1, 19), we investigated whether MSHA might provide an advantage for environmental persistence of El Tor biotype strains by mediating the ability to colonize planktonic hosts. Specifically, we have investigated whether MSHA mediates attachment of V. cholerae to the chitinous exoskeletons of the crustacean zooplankton Daphnia pulex (V. cholerae is found on Daphnia in Bangladesh [23]) by O1 El Tor, classical, and O139 strains. For the wild-type and mutant El Tor strains, we also examined two additional factors hypothesized to increase the tendency of V. cholerae to colonize plankton: low-nutrient conditions and the presence of a mucilaginous surface on the host plankton.
MATERIALS AND METHODS
Bacterial strains and plasmids.
V. cholerae strains used in this study were serogroup O1 El Tor biotype strain C6706 str2 (69), serogroup O139 vaccine strain CVD 112 (64), and serogroup O1 classical strain O395 Sm (68) and their respective isogenic mshA mutant derivatives KHT46 (69), KHT37 (69), and JM69 (39). These strains were labeled with green fluorescent protein (GFP) by electroporation with the GFP expression plasmid, pVSP61TIR (46), followed by growth on Luria-Bertani (LB) agar plates supplemented with kanamycin (45 μg/ml) to select for acquisition of the plasmid. Bacteria were grown in either LB medium or minimal medium (M9) supplemented with 0.2% (wt/vol) glucose (45).
Daphnia source and culture methods.
Daphnia pulex (Crustacea: Cladocera) used in this study were cultured from a single clone originating from a pond located in Gunnison National Forest, Colorado. Daphnia were cultured in filtered pond water (Storrs Pond, New Hampshire) at 20°C with a 14 h–10 h light-dark cycle and were fed the alga Cryptomonas erosa. Adult Daphnia molted every 2.5 days under our culture conditions, and whole, newly molted exoskeletons (<48 h) were used as a substrate for the colonization assays.
Adherence assays.
Cultures of V. cholerae expressing GFP were grown in LB or M9 liquid medium supplemented with kanamycin (45 μg/ml) for 18 h at 37°C with aeration. Twenty milliliters of fresh LB or M9 containing three D. pulex exoskeletons was inoculated with V. cholerae from these cultures at a 1:1,000 dilution corresponding to ≈106 cells/ml, and the mixture was incubated at 23°C for 2 h. Exoskeletons were then rinsed serially three times in 20 ml of KRT buffer (128 mM NaCl, 5.1 mM KCl, 1.34 mM MgSO4, 2.7 mM CaCl2, 10 mM Tris-HCl, pH 7.5) by gentle stirring and transfer with a wide-bore pipette. Exoskeletons were slide mounted with Gel/Mount (Biomeda Corp., Foster City, Calif.), and fluorescent images were captured on Kodak Ektachrome Elite II slide film (ASA 400) utilizing a Zeiss Axiophot microscope and an HQ FITC filter set with an excitation wavelength of 490 nm and an emission wavelength of 520 nm.
Quantification of V. cholerae adherence.
For all experiments, the inoculation density of V. cholerae cells was determined by serial dilution and colony counts. Adherence to D. pulex exoskeletons by GFP-expressing V. cholerae was quantified from digitally acquired images of photographic slides with an Optimas macro (Optimas, version 6.5; Media Cybernetics) to count attached V. cholerae cells, measure the surface area examined, and then determine the number of attached cells per square millimeter. We then standardized attached-cell density among assays by multiplying actual cell density by a correction factor accounting for differences in inoculation densities, which were determined by plate counts. With one exception (noted in Results), attached bacteria were counted on the same segment of the second antennae in all assays to control for an apparent systematic variation in the number of bacteria attached to different parts of the exoskeleton. Standardized attached-cell density in different treatments was compared by analysis of variance (ANOVA), with different exoskeletons serving as replicates. Where necessary, densities were log transformed to equalize variance among treatments.
Alkaline phosphatase assays.
Expression of mshA was monitored by assaying alkaline phosphatase activity of O1 El Tor mshA-phoA fusion strain JM191 after overnight growth in either M9 or LB as previously described (41). Assays were performed on three separate occasions, and the result were averaged. The values of independent assays varied by less than 10%.
RESULTS
Effects of mshA mutation and mucilage coating on exoskeleton adherence by the V. cholerae O1 El Tor biotype.
The contribution of MSHA to planktonic adherence was examined by testing wild-type and mshA mutant strains in a D. pulex attachment assay. The results of six different assay experiments are presented in Table 1. In the first assay, we compared adherence by the V. cholerae O1 El Tor biotype strain, C6706, and isogenic mshA deletion mutant, KHT46. These strains were incubated in LB medium with “clean” Daphnia exoskeletons and with exoskeletons from Daphnia that had been completely covered with an epibiotic alga, Colacium vesiculosum. C. vesiculosum cells attach to zooplankton by using a mucilaginous polysaccharide (40, 58, 72). When the exoskeleton is molted, the epibiotic cells detach to search for a new host (40, 57, 70), leaving the exoskeleton coated with algal mucilage. Mucilage is a substance suggested to be a preferred colonization substrate for V. cholerae because of its nutritional and protective properties (22, 30–34, 53). We found that there was significantly greater colonization of the wild-type O1 El Tor strain in comparison to the mshA mutant (ANOVA, P < 0.0001) and that colonization was significantly greater on the mucilage-coated exoskeletons (ANOVA, P = 0.026). There was a larger difference between the wild type and mutant for the mucilage-coated exoskeletons (60-fold) than for the clean exoskeletons (33-fold), but this difference was not statistically significant (ANOVA, two-way interaction, P = 0.17). Mucilage-coated exoskeletons were not used in any other experiments. An additional assay with the wild type O1 El Tor and the isogenic mshA mutant strains was conducted in which a different area of the exoskeleton (side of the carapace) was examined (assay 6; Table 1). While colonization of both wild-type and mutant strains was less than that in assay 1, the reduction in colonization of the mshA mutant compared to colonization of the O1 El Tor wild type was again significant (ANOVA, P = 0.008 [Table 1]).
TABLE 1.
Results of V. cholerae attachment assays for O1 El Tor and classical biotype and O139 strains and corresponding isogenic mshA mutantsa
| Assay | Biotype | Medium | Genotype (exoskeleton type) | n | Standardized attached cells mm−2 ± 1 SD | Wild-type:mutant | ANOVA results
|
||
|---|---|---|---|---|---|---|---|---|---|
| Factor | F | P | |||||||
| 1 | O1, El Tor | LB | mshA+ (clean) | 3 | 2,750.7 ± 658.3 | 33.2 | mshA | 273.1 | <0.0001 |
| mshA (clean) | 3 | 82.9 ± 15.3 | Mucilage | 7.9 | 0.026 | ||||
| mshA+ (mucilage) | 3 | 7,462.3 ± 1,434.6 | 59.5 | ||||||
| mshA (mucilage) | 3 | 125.4 ± 73.5 | Interaction | 2.3 | 0.172 | ||||
| 2 | O139 | LB | mshA+ | 3 | 527.9 ± 159.2 | NAb | mshA | NA | NA |
| mshA | 3 | 0 | |||||||
| 3 | O1, classical | LB | mshA+ | 2 | 874.1 ± 336.5 | 1.8 | mshA | 3.4 | 0.164 |
| mshA | 3 | 494.7 ± 142.8 | |||||||
| 4 | O1, classical | LB | mshA+ | 3 | 353.4 ± 5.6 | 0.7 | mshA | 130.4 | 0.0003 |
| mshA | 3 | 534.9 ± 27.0 | |||||||
| 5 | O1, El Tor | M9 | mshA+ | 3 | 20,386.5 ± 9,363.3 | 5.1 | mshA | 24.4 | 0.0078 |
| mshA | 3 | 3,996.0 ± 743.1 | |||||||
| 6 | O1, El Tor | LB | mshA+ | 3 | 651.2 ± 107.4c | 8.9 | mshA | 23.8 | 0.0081 |
| mshA | 3 | 72.9 ± 50.0c | |||||||
The attachment substrate was exoskeletons of D. pulex, and in assay 1, attachment was also compared for “clean” exoskeletons and exoskeletons coated with mucilage from algal secretions (see text for explanation). n, number of exoskeletons examined. The medium used was LB in all assays except assay 5, which used M9, a nutrient-limited medium. ANOVA was used to compare standardized attached cells (see text). Cells were allowed to attach during a 2-h incubation period in medium with a V. cholerae concentration of ∼106 cells ml−1. Wild-type:mutant, ratio of attached cells for the two genotypes. ANOVA results shown are factor tested, F-statistic, and P value for factor tested.
NA, not applicable; statistics cannot be applied.
Different location on exoskeleton examined than in other assays.
Effect of mshA mutation on exoskeleton adherence by V. cholerae O139.
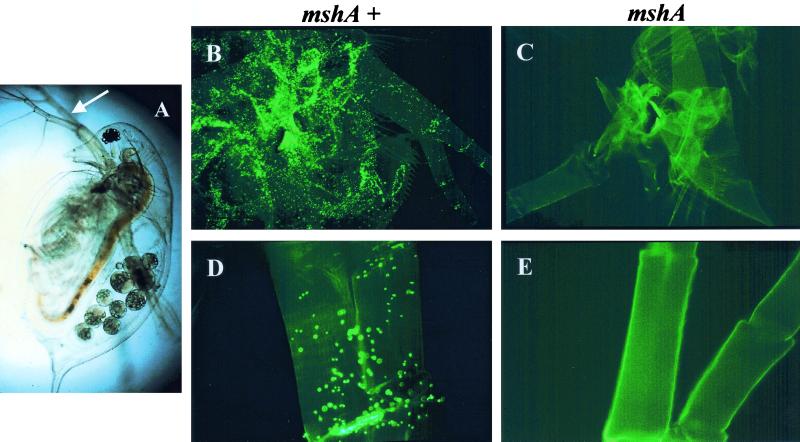
Several studies suggest that V. cholerae O139 strains are derivatives of O1 El Tor strains that have acquired properties through one or more horizontal gene transfer events that confer a different O antigen, capsule production, and antibiotic resistance (18, 36, 55, 71). We were therefore interested in determining whether the contribution of MSHA to exoskeleton adherence extended to this serogroup. In assay 2, we compared adherence by the V. cholerae O139 strain, CVD 112, and adherence by its isogenic mshA deletion mutant, KHT37, in LB medium. There was a substantial number of attached cells of the mshA+ O139 strain but essentially no attachment of the mshA mutant cells (Fig. 1). Attachment was zero on the Daphnia body part routinely examined (precluding statistical comparison), although a very few cells were observed to be attached to other areas of the exoskeletons.
FIG. 1.
Micrographs of the zooplankton attachment assay. (A) Light micrograph of adult D. pulex, ≈2.5 mm long. (B to E) Fluorescent micrographs of D. pulex exoskeletons after a 2-h attachment assay with V. cholerae O139 cells at ≈106/ml. Fluorescent micrographs on the left (B and D) have been incubated with the mshA+ strain, and those on the right (C and E) have been incubated with the mshA mutant strain. The top micrographs represent a ×75 magnification, showing most of the exoskeleton. The lower micrographs represent a higher magnification, showing the segment of the second antennae (the swimming appendage indicated by the arrow in panel A) where attachment was quantified in the assays (Table 1, assay 2). Bright green dots are individual bacteria. Bacteria often settled more heavily into joints on the exoskeletons (i.e., long, bright patch in panel D), and so these areas were not counted in our assays. Note that the mshA+ O139 bacteria thoroughly cover the exoskeletons, whereas the mshA mutant bacteria are completely absent on the second antennae and only about five attached cells can be detected when the entire exoskeleton is viewed (C).
Effect of mshA mutation on exoskeleton adherence by the V. cholerae O1 classical biotype.
V. cholerae O1 strains of the classical biotype express MshA in an extracytoplasmic location, as judged by mshA-phoA gene fusion analysis, but fail to assemble functional MSHA pili on the bacterial surface (41). Therefore, it was of interest to determine whether a classical biotype strain could adhere to D. pulex exoskeletons, assuming that expression of MshA that is not assembled into a pilus could not contribute to this process. In assays 3 and 4, we compared attachment of the O1 classical strain, O395, and its mshA mutant, JM69, in LB medium in two experiments. In assay 3, there was a 1.8-fold decrease in attachment of the mutant, but this was not statistically significant (ANOVA, P = 0.16), while in assay 4, the mshA mutant actually had a significantly higher (ANOVA, P = 0.0003) attachment density, but again by less than a twofold difference. Given the opposite results of assays 3 and 4 and the small numerical difference in attached-cell density compared with the much larger effects of the other two mutant strains, it is likely that the result of assay 4 is not biologically significant (real effect) but rather is a statistical artifact due to the unusually low variance among replicate exoskeletons for the JM69 strain (Table 1).
Effect of nutrient growth conditions on exoskeleton adherence.
In a recent study by Watnick et al. (73), V. cholerae was found to have altered attachment to various surfaces depending on the MSHA status of the bacteria and whether growth prior to the assay was in rich medium (LB) or minimal medium (M9). Assay 5 was conducted with M9 minimal medium to examine the effect of nutrient-limiting conditions on exoskeleton binding for the wild-type O1 El Tor and the mshA mutant. Again, attachment was significantly less for the mutant than for the wild-type strain (ANOVA, P = 0.0078), although the magnitude of difference between the two strains was only fivefold, much less than that seen in LB medium. For both the wild-type and mutant strains, the density of colonized cells was approximately an order of magnitude greater in the nutrient-limiting medium than was seen in LB (Table 1). This difference was not contributed to by greater MshA expression in nutrient-limiting medium since MshA expression was actually slightly decreased under this growth condition as determined by measurement of the alkaline phosphatase activity produced by mshA-phoA fusion strain JM191. The specific activity was 1,215 ± 22.6 (standard error) units in LB and 1,087 ± 28.8 units in M9 (ANOVA, P = 0.025).
DISCUSSION
Role of MSHA in crustacean exoskeleton adherence for different strains of V. cholerae.
The mshA structural subunit gene is essential for the production of MSHA type 4 surface pili present on O1 El Tor and O139 strains of V. cholerae associated with human cholera epidemics. Deletion of mshA to create mutant strains of O1 El Tor (KHT46) or O139 (KHT37) resulted in a significant decrease in adherence of V. cholerae cells on exoskeletons of the crustacean zooplankton D. pulex when assayed under a variety of conditions. These findings suggest that the MSHA type 4 pilus may be an important mediator of O1 El Tor and O139 adherence in the aquatic environment since V. cholerae is found attached to zooplankton in this setting (5, 24–31, 33–35, 50, 53). Our results with respect to the role of MSHA in mediating attachment to the chitinous exoskeleton of D. pulex differ from those reported by Watnick et al. (73), who used chitin particles on a glass support. In that study no difference was seen in the chitin binding by a wild-type O1 El Tor strain versus that by a mshA mutant. However, these results were based on a qualitative rather than a quantitative assessment and so may not have been able to distinguish between attachment levels of the wild-type and mutant strains. In addition, this discrepancy might reflect a difference between the use of a natural chitinous exoskeleton versus the use of chitin particles as the binding substrate.
V. cholerae O1 strains of the classical biotype express the MshA pilin subunit but do not assemble MSHA pili on the bacterial cell surface and do not display a MSHA-dependent hemagglutination phenotype (37, 41). As expected based on this property, the O1 classical mshA mutant strain did not show less adherence to zooplankton exoskeletons than the wild-type strain. However, the classical strain was able to attach. This finding indicates that surface factors other than MSHA pili are responsible for O1 classical attachment to chitinous exoskeletons. These factors may also be present on El Tor strains, since the O1 El Tor mshA mutant strain still showed substantial attachment to exoskeletons. An interesting aspect of the study was the greater contribution of MSHA to adherence by the O139 strain than to the highly related O1 El Tor strain. Attachment of the O139 mshA mutant was virtually undetectable. Perhaps this is due to the presence of a capsule on O139 strains. The MSHA pilus can likely protrude from the cell surface, extending out beyond the capsular material. In contrast, outer membrane factors that might participate in colonization by O1 El Tor could be shielded by the O139 carbohydrate capsule. These factors may include membrane-associated chitin binding proteins such as those that have been identified in V. alginolyticus and V. cholerae (54, 67). Our results suggest that analogous factors provide the sole mechanism for zooplankton adherence by O1 classical strains. In contrast, O139 strains appear to utilize MSHA alone, whereas O1 El Tor strains utilize a combination of MSHA and additional factors.
Mucilaginous surface and nutrient-limiting conditions enhance adherence of V. cholerae to exoskeletons.
A number of studies have found that V. cholerae is more commonly isolated from the surface of algal species that produce a mucilaginous sheath than from the surface of those that do not (30–34, 53). However, it is not clear whether this difference is due to enhanced adherence to such surfaces or to the increased growth due to the protective and nutritious microhabitat within the mucilage. In our work, the presence of a mucilaginous biofilm on the Daphnia exoskeletons significantly enhanced exoskeleton attachment by both the wild type and the mutant O1 El Tor, suggesting that the presence of mucilage on planktonic species may contribute to V. cholerae colonization in the environment. Our results suggest that both MSHA and additional undefined adherence factors have a role in this process.
We found that growth under the nutrient-limiting conditions of M9 minimal medium resulted in an order of magnitude increase in V. cholerae binding to the exoskeletons for both the wild type and the mshA mutant. Since MshA expression was actually slightly decreased under nutrient-limiting conditions, it is likely that nutrient deprivation of O1 El Tor V. cholerae resulted in increased exoskeleton binding by an MSHA-independent mechanism. Several other studies have found that harsh growth conditions such as low nutrient levels or nonoptimal salinity or pH enhance V. cholerae colonization of surfaces (22, 26, 35). The ability of some V. cholerae strains to produce chitinase and mucinase (9, 50, 51, 60) and to grow with chitin as the sole nutrient source in laboratory cultures (2) indicates a potential nutritional benefit to V. cholerae from colonization of zooplankton with chitinous exoskeletons and mucilage-sheathed phytoplankton. An additional benefit to the bacteria may be afforded by the physical protection provided by existence within a surface biofilm. Specific disadvantages of an attached lifestyle have not been demonstrated for V. cholerae, but if there is a cost of colonization, perhaps due to increased expression of colonization factors, then increased expression of colonization factors only in response to harsh conditions would be adaptive.
Key questions that emerge from this study are as follows. (i) To what degree do different plankton attachment factors contribute to persistence, growth, and survival of the various epidemiological strains in the natural aquatic environment? (ii) Does the possession of the MSHA pilus in addition to at least one additional attachment factor by the O1 El Tor strain have a role in its worldwide replacement of the classical strain in the seventh pandemic and its present epidemiological predominance over O139 in Bangladesh and India (4, 7, 11, 59, 61)? (iii) Are particular attachment factors specialized for different types of planktonic surfaces? (iv) To what degree is expression of attachment factors regulated by physical and chemical conditions in aquatic ecosystems? These are all questions which deserve further study because they may be crucial in refining the ongoing efforts to develop predictive models of cholera outbreaks based on the state of the aquatic ecosystems that V. cholerae inhabits (7, 52).
ACKNOWLEDGMENTS
We thank Jean Richardson for assistance with Optimas Image Analysis.
This work was supported by National Institutes of Health grant AI-25096 (R.K.T.). J.W.M. was the recipient of a predoctoral fellowship from the National Institutes of Health (training grant AI-07519). D.A.C. received financial support from the Dartmouth College Cramer Fund, the Dartmouth Center for Environmental Health, and the NIEHS Superfund ES07373 to C. L. Folt and S. Y. Chen. Assistance and supplies for Daphnia and C. erosa culturing were provided by the laboratory of C. L. Folt, Department of Biological Sciences.
REFERENCES
- 1.Albert M J. Vibrio cholerae O139 Bengal. J Clin Microbiol. 1994;32:2345–2349. doi: 10.1128/jcm.32.10.2345-2349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amako K, Shimodori S, Imoto T, Miake S, Umeda A. Effects of chitin and its soluble derivatives on survival of Vibrio cholerae O1 at low temperature. Appl Environ Microbiol. 1987;53:603–605. doi: 10.1128/aem.53.3.603-605.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attridge S R, Manning P A, Holmgren J, Jonson G. Relative significance of mannose-sensitive hemagglutinin and toxin-coregulated pili in colonization of infant mice by Vibrio cholerae El Tor. Infect Immun. 1996;64:3369–3373. doi: 10.1128/iai.64.8.3369-3373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barua D. History of cholera. In: Barua D, Greenbough III W B, editors. Cholera. New York, N.Y: Plenum Publishing Corp.; 1992. pp. 1–36. [Google Scholar]
- 5.Chowdhury M A R, Huq A, Xu B, Madeira F J B, Colwell R R. Effect of alum on free-living and copepod-associated Vibrio cholerae O1 and O139. Appl Environ Microbiol. 1997;63:3323–3326. doi: 10.1128/aem.63.8.3323-3326.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockburn T A, Cassenos J G. Epidemiology of epidemic cholera. Public Health Rep. 1960;75:791. [PMC free article] [PubMed] [Google Scholar]
- 7.Colwell R R. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 8.Colwell R R, Seidler R J, Kaper J, Joseph S W, Garges S, Lockman H, Maneval D, Bradford H, Roberts N, Remmers E, Huq I, Huq A. Occurrence of Vibrio cholerae serotype O1 in Maryland and Louisiana estuaries. Appl Environ Microbiol. 1981;41:555–558. doi: 10.1128/aem.41.2.555-558.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dastidir S G, Narayanaswami A. The occurrence of chitinase in vibrios. Indian J Med Res. 1968;56:654–658. [PubMed] [Google Scholar]
- 10.Dumontet, Krovacek S K, Baloda S B, Grottoli R, Pasquale V, Vanucci S. Ecological relationship between Aeromonas and Vibrio spp. and planktonic copepods in the coastal marine environment in southern Italy. Comp Immunol Microbiol Infect Dis. 1996;19:245–254. doi: 10.1016/0147-9571(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 11.Epstein P R. Algal blooms in the spread and persistence of cholera. BioSystems. 1993;31:209–221. doi: 10.1016/0303-2647(93)90050-m. [DOI] [PubMed] [Google Scholar]
- 12.Falcão D P, Lustri W R, Bauab T M. Incidence of non-O1 Vibrio cholerae and Aeromonas spp. in fresh water in Araraquara, Brazil. Curr Microbiol. 1998;37:28–31. doi: 10.1007/s002849900332. [DOI] [PubMed] [Google Scholar]
- 13.Faruque S M, Albert M J, Mekalanos J J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faruque A S, Fuchs G J, Albert M J. Changing epidemiology of cholera due to Vibrio cholerae O1 and O139 Bengal in Dhaka, Bangladesh. Epidemiol Infect. 1996;116:275–278. doi: 10.1017/s0950268800052572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelstein R A, Mukerjee S. Haemagglutination. a rapid method of the differentiating Vibrio cholerae from El Tor O1 vibrios. Proc Soc Exp Biol Med. 1963;112:355–359. [Google Scholar]
- 16.Garay E, Arnau A, Amaro C. Incidence of Vibrio cholerae and related vibrios in a coastal lagoon and seawater influenced by lake discharges along an annual cycle. Appl Environ Microbiol. 1985;50:426–430. doi: 10.1128/aem.50.2.426-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass R I, Becker S, Huq M I, Stoll B J, Khan M U, Merson M H, Lee J V, Black R E. Endemic cholera in rural Bangladesh, 1966–1980. Am J Epidemiol. 1982;116:959–970. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- 18.Hall R H, Khambaty F M, Kothary M, Keasler S P. Non-O1 Vibrio cholerae. Lancet. 1993;342:430. doi: 10.1016/0140-6736(93)92839-l. [DOI] [PubMed] [Google Scholar]
- 19.Hanne L F, Finkelstein R A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982;36:209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Häse C C, Bauer M E, Finkelstein R A. Genetic characterization of mannose-sensitive hemagglutinin (MSHA)-negative mutants of Vibrio cholerae derived by Tn5 mutagenesis. Gene. 1994;150:17–25. doi: 10.1016/0378-1119(94)90852-4. [DOI] [PubMed] [Google Scholar]
- 21.Herrington D S, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood M A, Winter P A. Attachment of Vibrio cholerae under various environmental conditions and to selected substrates. FEMS Microbiol Ecol. 1997;22:215–223. [Google Scholar]
- 23.Huq A, Colwell R R, Rahman R, Ali A, Chowdhury M A R, Parveen S, Sack D A, Russek-Cohen E. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl Environ Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huq A, Small E B, West P A, Huq M I, Rahman R, Colwell R R. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huq A, Small E B, West P A, Colwell R R. The role of planktonic copepods in the survival and multiplication of Vibrio cholerae in the environment. In: Colwell R R, editor. Vibrios in the environment. New York, N.Y: John Wiley and Sons; 1984. pp. 521–534. [Google Scholar]
- 26.Huq A, West P A, Small E B, Huq M I, Colwell R R. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosms. Appl Environ Microbiol. 1984;48:420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huq A, Xu B, Chowdhury M A R, Islam M S, Montilla R, Colwell R R. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water samples in developing countries. Appl Environ Microbiol. 1996;62:2508–2512. doi: 10.1128/aem.62.7.2508-2512.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Islam M S, Alam M J, Begum A, Rahim Z, Felsenstein A, Albert M J. Occurrence of culturable Vibrio cholerae O139 with ctx gene in various components of the aquatic environment in Bangladesh. Trans R Soc Trop Med Hyg. 1996;90:128. doi: 10.1016/s0035-9203(96)90110-8. [DOI] [PubMed] [Google Scholar]
- 29.Islam M S, Drasar B S, Bradley D J. Survival and attachment of toxigenic Vibrio cholerae O1 in association with four marine algae. Bangladesh J Microbiol. 1988;5:41–44. [Google Scholar]
- 30.Islam M S, Drasar B S, Bradley D J. Attachment of toxigenic Vibrio cholerae O1 to various freshwater plants and survival with a filamentous green alga, Rhizoclonium fontanum. J Trop Med Hyg. 1989;92:396–401. [PubMed] [Google Scholar]
- 31.Islam M S, Drasar B S, Bradley D J. Long-term persistence of toxigenic Vibrio cholerae O1 in the mucilaginous sheath of a blue-green alga, Anabaena variabilis. J Trop Med Hyg. 1990;93:133–139. [PubMed] [Google Scholar]
- 32.Islam M S, Drasar B S, Sack R B. The aquatic environment as a reservoir of Vibrio cholerae: a review. J Diarrhoeal Dis Res. 1993;11:197–206. [PubMed] [Google Scholar]
- 33.Islam M S, Drasar B S, Sack R B. The aquatic flora and fauna as reservoirs of Vibrio cholerae: a review. J Diarrhoeal Dis Res. 1994;12:87–96. [PubMed] [Google Scholar]
- 34.Islam M S, Drasar B S, Sack R B. Probable role of blue-green algae in maintaining endemicity and seasonality of cholera in Bangladesh: a hypothesis. J Diarrhoeal Dis Res. 1994;12:245–256. [PubMed] [Google Scholar]
- 35.Islam M S, Rahim Z, Alam M J, Begum S, Moniruzzaman S M, Umeda A, Amako K, Albert M J, Sack R B, Huq A, Colwell R R. Association of Vibrio cholerae O1 with the cyanobacterium, Anabaena sp., elucidated by polymerase chain reaction and transmission electron microscopy. Trans R Soc Trop Med Hyg. 1999;93:36–40. doi: 10.1016/s0035-9203(99)90171-2. [DOI] [PubMed] [Google Scholar]
- 36.Johnson J A, Salles C A, Panigrahi P, Albert M J, Wright A C, Johnson R J, Morris J G., Jr Vibrio cholerae O139 synonym Bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect Immun. 1994;62:2108–2110. doi: 10.1128/iai.62.5.2108-2110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonson G, Holmgren J, Svennerholm A M. Identification of a mannose-binding pilus on Vibrio cholerae El Tor. Microb Pathog. 1991;11:433–441. doi: 10.1016/0882-4010(91)90039-d. [DOI] [PubMed] [Google Scholar]
- 38.Jonson G, Lebens M, Holmgren J. Cloning and sequencing of Vibrio cholerae mannose-sensitive haemagglutinin pilin gene: localization of mshA within a cluster of type 4 pilin genes. Mol Microbiol. 1994;13:109–118. doi: 10.1111/j.1365-2958.1994.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 39.Jouravleva E A, McDonald G A, Marsh J W, Taylor R K, Boesman-Finkelstein M, Finkelstein R A. The Vibrio cholerae mannose-sensitive hemagglutinin is the receptor for a filamentous bacteriophage from V. cholerae O139. Infect Immun. 1998;66:2535–2539. doi: 10.1128/iai.66.6.2535-2539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Killen R P, Willey R L, Durum F A. Docking behavior of Colacium libellae (Euglenophyceae): cell-substrate adhesion and flagellar resorption. Trans Am Microsc Soc. 1984;103:67–73. [Google Scholar]
- 41.Marsh J W, Sun D, Taylor R K. Physical linkage of the Vibrio cholerae mannose-sensitive hemagglutinin secretory and structural subunit gene loci: identification of the mshG coding sequence. Infect Immun. 1996;64:460–465. doi: 10.1128/iai.64.2.460-465.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh J W, Taylor R K. Genetic and transcriptional analyses of the Vibrio cholerae mannose-sensitive hemagglutinin type 4 pilus gene locus. J Bacteriol. 1999;181:1110–1117. doi: 10.1128/jb.181.4.1110-1117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy S A. Effects of temperature and salinity on survival of toxigenic Vibrio cholerae O1 in seawater. Microb Ecol. 1996;31:167–175. doi: 10.1007/BF00167862. [DOI] [PubMed] [Google Scholar]
- 44.Miller C J, Drasar B S, Feachem R G. Response of toxigenic Vibrio cholerae O1 to physico-chemical stresses in aquatic environments. J Hyg Camb. 1984;93:475–495. doi: 10.1017/s0022172400065074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 46.Miller W G, Lindow S E. An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene. 1997;191:149–153. doi: 10.1016/s0378-1119(97)00051-6. [DOI] [PubMed] [Google Scholar]
- 47.Moeseneder M M, Winter C, Herndl G H. Horizontal and vertical complexity of attached and free-living bacteria of the eastern Mediterranean Sea, determined by 16S rDNA and 16S rRNA fingerprints. Limnol Oceanogr. 2001;46:95–107. [Google Scholar]
- 48.Mourino-Perez R R. Oceanography and the seventh cholera pandemic. Epidemiology. 1998;9:355–357. doi: 10.1097/00001648-199805000-00024. [DOI] [PubMed] [Google Scholar]
- 49.Mukhopadhyay A K, Basu A, Garg P, Bag P K, Ghosh A, Bhattacharya S K, Takeda Y, Nair G B. Molecular epidemiology of reemergent Vibrio cholerae O139 Bengal in India. J Clin Microbiol. 1998;36:2149–2152. doi: 10.1128/jcm.36.7.2149-2152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nalin D R. Cholera, copepods and chitinase. Lancet. 1976;2:958. doi: 10.1016/s0140-6736(76)90915-6. [DOI] [PubMed] [Google Scholar]
- 51.Nalin D R, Daya V, Reid A, Levine M M, Cisneros L. Adsorption and growth of Vibrio cholerae on chitin. Infect Immun. 1979;25:768–770. doi: 10.1128/iai.25.2.768-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pascual M, Rodo X, Ellner S P, Colwell R, Bouma M J. Cholera dynamics and El Nino—southern oscillation. Science. 2000;289:1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- 53.Pearl H W, Keller P E. Significance of the bacterial Anabaena (cyanophyceae) associations with respect to N2 fixation in fresh water. J Phycol. 1979;14:2. [Google Scholar]
- 54.Pruzzo C, Crippa A, Bertone S, Pane L, Carli A. Attachment of Vibrio alginolyticus to chitin mediated by chitin-binding proteins. Microbiology. 1996;142:2181–2186. doi: 10.1099/13500872-142-8-2181. [DOI] [PubMed] [Google Scholar]
- 55.Rhine J A, Taylor R K. TcpA pilin sequences and colonization requirements for O1 and O139 Vibrio cholerae. Mol Microbiol. 1994;13:1013–1020. doi: 10.1111/j.1365-2958.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 56.Rhodes J B, Smith H L, Ogg J E. Isolation of non-O1 Vibrio cholerae serovars from surface waters in western Colorado. Appl Environ Microbiol. 1986;51:1216–1219. doi: 10.1128/aem.51.6.1216-1219.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosowski J R, Willey R L. Colacium libellae sp. nov. (Euglenophyceae), a photosynthetic inhabitant of the larval damselfly rectum. J Phycol. 1975;11:310–315. [Google Scholar]
- 58.Rosowski J R, Willey R L. Development of mucilaginous surfaces in euglenoids. I. Stalk morphology of Colacium mucronatum. J Phycol. 1977;13:16–21. [Google Scholar]
- 59.Ryan E T, Dhar U, Khan W A, Salam M A, Faruque A S G, Fuchs G J, Calderwood S B, Bennish M L. Mortality, morbidity, and microbiology of endemic cholera in Dhaka, Bangladesh. Am J Trop Med Hyg. 2000;63:12–20. doi: 10.4269/ajtmh.2000.63.12. [DOI] [PubMed] [Google Scholar]
- 60.Schneider D R, Parker C D. Purification and characterization of the mucinase of Vibrio cholerae non-O1 in India and Bangladesh. Lancet. 1982;341:1347. doi: 10.1093/infdis/145.4.474. [DOI] [PubMed] [Google Scholar]
- 61.Siddique A K, Zaman K, Akram K, Mutsuddy P, Eusof A, Sack R B. Emergence of a new epidemic strain of Vibrio cholerae in Bangladesh. Trop Geogr Med. 1994;46:147–150. [PubMed] [Google Scholar]
- 62.Singleton F L, Atwell R, Jangi S, Colwell R R. Influence of salinity and organic nutrient concentration on survival and growth of Vibrio cholerae in aquatic microcosms. Appl Environ Microbiol. 1982;43:1080–1085. doi: 10.1128/aem.43.5.1080-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singleton F L, Atwell R, Jangi S, Colwell R R. Effects of temperature and salinity on Vibrio cholerae growth. Appl Environ Microbiol. 1982;44:1047–1058. doi: 10.1128/aem.44.5.1047-1058.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tacket C O, Losonsky G, Nataro J P, Comstock L, Michalski J, Edelman R, Kaper J B, Levine M M. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 65.Tacket C O, Taylor R K, Losonsky G, Lim Y, Nataro J P, Kaper J B, Levine M M. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect Immun. 1998;66:692–695. doi: 10.1128/iai.66.2.692-695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamplin M L, Gauzens A L, Huq A, Sack D A, Colwell R R. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol. 1990;56:1977–1980. doi: 10.1128/aem.56.6.1977-1980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarsi R, Pruzzo C. Role of surface proteins in Vibrio cholerae attachment to chitin. Appl Environ Microbiol. 1999;65:1348–1351. doi: 10.1128/aem.65.3.1348-1351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. The use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thelin K H, Taylor R K. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae Ol El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Threlkeld S T, Chiavelli D A, Willey R L. The organization of zooplankton epibiont communities. Trends Ecol Evol. 1993;8:317–321. doi: 10.1016/0169-5347(93)90238-K. [DOI] [PubMed] [Google Scholar]
- 71.Waldor M K, Tschape H, Mekalanos J J. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J Bacteriol. 1996;178:4157–4165. doi: 10.1128/jb.178.14.4157-4165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ward K A, Willey R L. The development of a cell-substrate attachment system in a euglenoid flagellate. J Ultrastruct Res. 1981;74:165–174. doi: 10.1016/s0022-5320(81)80074-3. [DOI] [PubMed] [Google Scholar]
- 73.Watnick P I, Fullner K J, Kolter R. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J Bacteriol. 1999;181:3606–3609. doi: 10.1128/jb.181.11.3606-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watnick P I, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu H S, Roberts N, Singleton F L, Attwell R W, Grimes D J, Colwell R R. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]