Abstract
Background
Cases of human monkeypox are rarely seen outside of west and central Africa. There are few data regarding viral kinetics or the duration of viral shedding and no licensed treatments. Two oral drugs, brincidofovir and tecovirimat, have been approved for treatment of smallpox and have demonstrated efficacy against monkeypox in animals. Our aim was to describe the longitudinal clinical course of monkeypox in a high-income setting, coupled with viral dynamics, and any adverse events related to novel antiviral therapies.
Methods
In this retrospective observational study, we report the clinical features, longitudinal virological findings, and response to off-label antivirals in seven patients with monkeypox who were diagnosed in the UK between 2018 and 2021, identified through retrospective case-note review. This study included all patients who were managed in dedicated high consequence infectious diseases (HCID) centres in Liverpool, London, and Newcastle, coordinated via a national HCID network.
Findings
We reviewed all cases since the inception of the HCID (airborne) network between Aug 15, 2018, and Sept 10, 2021, identifying seven patients. Of the seven patients, four were men and three were women. Three acquired monkeypox in the UK: one patient was a health-care worker who acquired the virus nosocomially, and one patient who acquired the virus abroad transmitted it to an adult and child within their household cluster. Notable disease features included viraemia, prolonged monkeypox virus DNA detection in upper respiratory tract swabs, reactive low mood, and one patient had a monkeypox virus PCR-positive deep tissue abscess. Five patients spent more than 3 weeks (range 22–39 days) in isolation due to prolonged PCR positivity. Three patients were treated with brincidofovir (200 mg once a week orally), all of whom developed elevated liver enzymes resulting in cessation of therapy. One patient was treated with tecovirimat (600 mg twice daily for 2 weeks orally), experienced no adverse effects, and had a shorter duration of viral shedding and illness (10 days hospitalisation) compared with the other six patients. One patient experienced a mild relapse 6 weeks after hospital discharge.
Interpretation
Human monkeypox poses unique challenges, even to well resourced health-care systems with HCID networks. Prolonged upper respiratory tract viral DNA shedding after skin lesion resolution challenged current infection prevention and control guidance. There is an urgent need for prospective studies of antivirals for this disease.
Funding
None.
Introduction
Human monkeypox is a zoonosis caused by monkeypox virus, an orthopoxvirus and close relative of variola virus (smallpox). It was first reported in central Africa1 in 1970 and has historically affected some of the poorest and most marginalised communities in the world.2, 3 The clinical syndrome is characterised by fever, rash, and lymphadenopathy. Complications of monkeypox can include pneumonitis, encephalitis, sight-threatening keratitis, and secondary bacterial infections.3, 4, 5
Published mortality rates vary substantially and are vulnerable to case ascertainment bias.6 Case fatality rates ranging from 1% to 10% have been reported in outbreaks in the Congo Basin,5, 6 and the virus clade circulating in this region appears to be associated with higher virulence.7 The west African clade, which is responsible for recent outbreaks in Nigeria, is associated with an overall lower mortality rate consistently less than 3%.6, 8 To date, most reported deaths have occurred in young children and people with HIV.6, 8, 9
Human-to-human transmission of monkeypox is well described, including nosocomial and household transmission.3, 8 However, human-to-human chains of transmission have historically been less well recognised. A pooled estimate from a systematic review suggested a secondary attack rate of approximately 8% (range 0–11%) among household contacts who were unvaccinated against smallpox.6 Understanding of in-vivo viral kinetics and infectivity is poor,3, 7, 10 and the clinical significance of prolonged viraemia and skin shedding remains uncertain.
Research in context.
Evidence before this study
We searched PubMed for the terms “monkeypox AND (viraemia OR shedding OR pharyn* OR oropharyn* OR nasopharyn* OR tecovirimat OR brincidofovir OR treatment)” from inception up to December 11, 2021. We reviewed grey literature, including textbooks, and checked key articles for relevant supplementary references. Except for a large outbreak linked to imported rodents in the USA in 2003, monkeypox transmission has been confined to remote locations in central and west Africa. However, in the past 5 years, outbreaks in more densely populated centres have occurred, raising concern about global spread. There are no published trials or observational studies of monkeypox therapeutics in humans. In non-human primates, tecovirimat demonstrated protective efficacy against lethal smallpox and monkeypox challenge, with reduced magnitude and duration of orthopoxvirus viraemia and upper respiratory tract shedding. In animal models, brincidofovir showed a trend towards protective efficacy. People with monkeypox have traditionally been considered infectious until all the lesions have crusted. However, person-to-person transmission of monkeypox has only been recognised as a significant public health threat since the 2018 Nigerian outbreak. There are a few reports of monkeypox detection in blood and one-off upper respiratory tract swabs, but the clinical significance of viraemia is not well established and longitudinal molecular sampling of people with monkeypox is rarely possible in monkeypox-endemic settings.
Added value of this study
Defined by the UK Health Security Agency as a High Consequence Infectious Disease (HCID), our retrospective case series represents imported, nosocomial, and household transmission of monkeypox, which has not been described in the UK previously. We report the first use of antiviral agents in patients with monkeypox, with three patients receiving brincidofovir and one receiving tecovirimat. Brincidofovir was not observed to confer any convincing clinical benefit and was associated with liver function test derangement in all cases. The patient treated with tecovirimat had a shorter duration of symptoms and upper respiratory tract viral shedding than the other patients in the series, with no adverse events identified before discharge. Several of the patients experienced prolonged viraemia and upper respiratory tract viral shedding after crusting of all cutaneous lesions, leading to extended isolation in hospital.
Implications of all the available evidence
Monkeypox is an emerging global health threat, which is capable of cross-border spread and onward transmission. Although optimum infection control and treatment strategies for this potentially dangerous pathogen are not established, our first-use data suggest brincidofovir has poor efficacy; however, prospective studies of tecovirimat in human monkeypox are warranted. The infection control implications of upper respiratory tract viral shedding should be considered in future outbreaks.
Monkeypox is rarely exported from the African continent. In 2003, there was a zoonotic outbreak in the USA causing 47 confirmed or suspected cases.4, 11, 12, 13 This outbreak was linked to the importation of Gambian giant rats, squirrels, and dormice, which had transmitted the virus to prairie dogs that were then sold as pets. Only 14 patients were hospitalised and there were no confirmed cases of person-to-person transmission. Imported monkeypox infections in humans following travel have been reported in the UK,14 Israel,15 Singapore,16 and in 2021, in the USA.11
Currently there are no licensed treatments for human monkeypox; two orally bioavailable drugs, brincidofovir and tecovirimat, have been approved in the USA for the treatment of smallpox in preparation for a potential bioterrorism event.17, 18, 19 Neither drug has been studied in human efficacy trials; however, both drugs demonstrated efficacy against other orthopoxviruses (including monkeypox) in animal models. There are reports of compassionate use of tecovirimat for complicated vaccinia20, 21 and cowpox,22 with no concerning safety signals identified. An expanded access programme for tecovirimat is in preparation in the Central African Republic, where monkeypox outbreaks are common.23
In the UK, monkeypox is classified as a High Consequence Infectious Disease (HCID), and patients are managed in designated HCID treatment centres coordinated by a national network.24 Since 2018, four patients were diagnosed with travel-associated monkeypox in the UK, with onward transmission to three people, including the first reported household cluster outside Africa. The public health management of people infected with monkeypox and their contacts have been reported previously.14, 25, 26 We describe the clinical presentation, evolution, complications, and management of seven patients. We also report the viral kinetics and the use of brincidofovir and tecovirimat to treat human monkeypox.
Methods
Study design and participants
In this retrospective observational study, we did a retrospective chart review of all patients admitted to any HCID centre in the UK with confirmed monkeypox (defined as a compatible clinical illness with positive monkeypox viral PCR from any anatomical site) between Aug 15, 2018, and Sept 10, 2021. We extracted clinical data (including demographic variables, symptoms and signs at presentation, complications of illness, and any antiviral treatments received) and laboratory results (including routine biochemical tests and monkeypox virus PCR results). Our sample size was small and some patients were diagnosed later in the course of illness than others; therefore, we did not carry out formal hypothesis testing, and we present individual patient results rather than aggregate data.
Clinical sampling and documentation were conducted as part of routine patient care. All patients (or guardians of children) provided written informed consent for storage of biological samples under the International Severe Acute Respiratory and Emerging Infection Consortium protocol,27 approved by the South Central – Oxford C Research Ethics Committee in England (13/SC/0149) and the WHO Ethics Review Committee (RPC571 and RPC572). This included consent for publication of anonymised clinical details. Additional written informed consent was given for publication of clinical images.
Procedures
Virological sampling and laboratory testing were driven by clinical indications rather than a formal protocol. Monkeypox viral PCR testing was performed at the UK Rare and Imported Pathogens Laboratory (appendix p 3). Samples, including EDTA (edetic acid) blood samples, urine samples, swabs of persistent lesions or lesion fluid, and upper respiratory tract swabs, were typically taken every 48–72 h until two consecutive negative results were recorded from each anatomical site (ie, skin, blood, or respiratory tract). These negative results, coupled with desquamation of all visible lesions, no new lesions, and no active mucosal lesions, comprised the framework agreed by the HCID network for discharging patients to the community.
Serological testing was not performed given the high specificity of PCR; however, serum from four people in contact with monkeypox was tested for orthopoxvirus IgG and IgM by immunofluorescence assay at the Bundeswehr Institute of Microbiology (Munich, Germany), as outlined in the appendix (p 3).
The approach to patient care in HCID centres, including personal protective equipment (PPE), is outlined in the appendix (p 3). When novel therapeutics were available, they were offered to patients following a discussion of potential risks and benefits, therapeutic aims, supporting evidence, and clear disclosure that they remained unlicensed and of uncertain benefit for treating monkeypox. All clinical decisions, including the use of novel therapeutics, were made by the clinicians directly caring for the patients, in conjunction with the HCID Network. The use of novel therapeutics was approved by the relevant National Health Service (NHS) Trust's medicines governance group. All patients were offered outpatient appointments after discharge, but extended follow-up was not performed following full recovery.
Role of the funding source
There was no funding source for this study.
Results
Our chart review encompassed Aug 15, 2018, to Sept 10, 2021, and identified seven patients who were treated for monkeypox in HCID units in England, UK. Four patients acquired monkeypox outside of the UK (patient 1, 2, 4, and 5); three inside of the UK (patient 3, 6, and 7). Four cases occurred between 2018 and 2019, and three household cluster cases occurred in 2021.
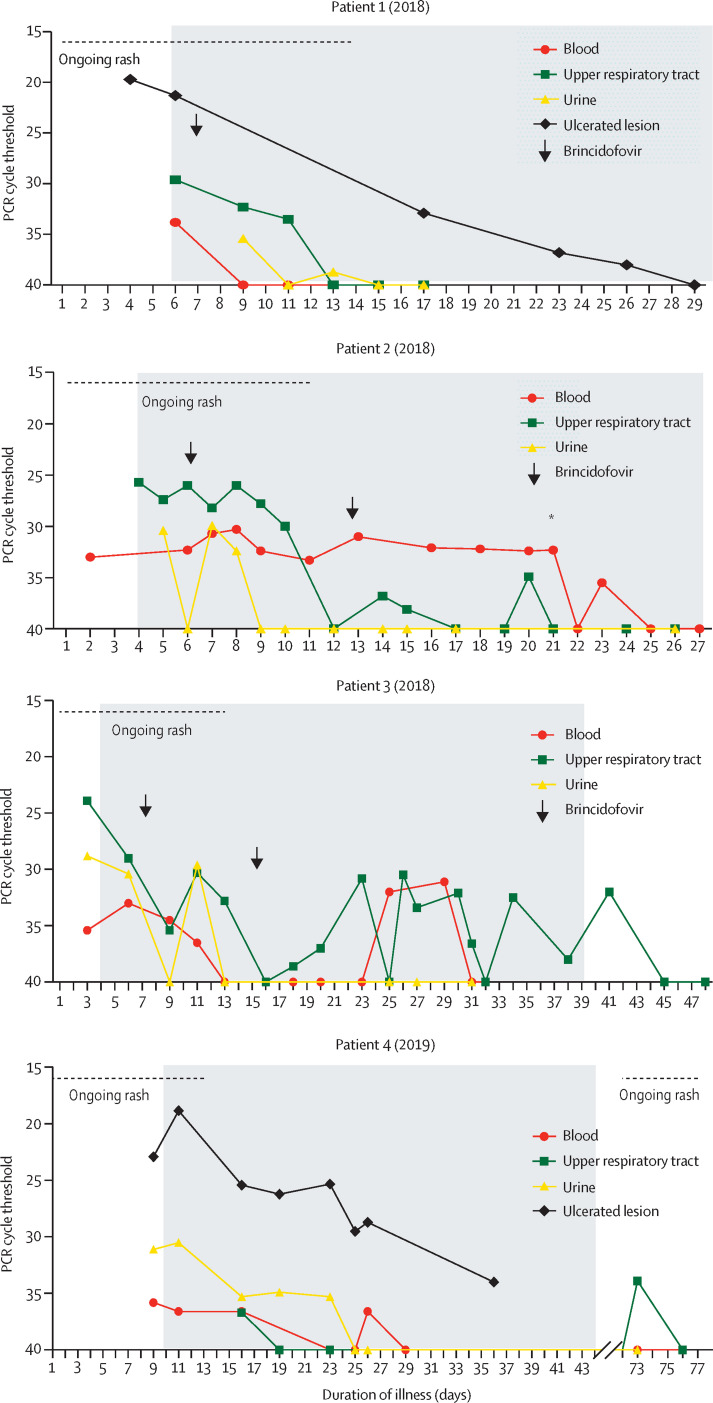
Patients 1 and 2 were diagnosed with monkeypox shortly after arriving in the UK from Nigeria. Patient 3 (2018) was a health-care worker who developed a rash, headache, and sore throat 18 days post-exposure to patient 2 (2018) without PPE, despite receiving a dose of smallpox vaccine (modified vaccinia Ankara [Imvanex, Bavarian Nordic, Denmark]) on day 6 post-exposure. The clinical and virological time courses are summarised in the table and figure 1 .
Table.
Summary of the clinical course and response to treatment in seven patients with monkeypox
|
2018 |
2019 |
2021 |
||||||
|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | ||
| Site of HCID unit | London | Liverpool | Newcastle | London | Liverpool | Liverpool | Liverpool | |
| Age range, years* | 30–40 | 30–40 | 30–40 | 40–50 | 30–40 | <2 | 30–40 | |
| Sex | Male | Male | Female | Male | Male | Female | Female | |
| Transmission rank | Isolated | Index | Secondary | Isolated | Index | Secondary | Tertiary | |
| Country of acquisition | Nigeria | Nigeria | UK | Nigeria | Nigeria | UK | UK | |
| Smallpox vaccination history | None | None | MVA six days post-exposure or 12 days pre-illness | None | None | None | None | |
| HIV, hepatitis B, and hepatitis C status | Negative | Negative | Negative | Negative | Negative | Not tested (parents negative) | Negative | |
| Prodrome | Fever and night sweats (2 days) | Fever and groin swelling (4 days) | Coryzal illness (1 day) | Fever and headache (2 days) | None | None | None | |
| Lymphadenopathy | Yes | Yes | No | Yes | Yes | Yes | No | |
| Approximate maximum number of concurrent lesions | 150 | 100 | 32 | 100 | 40 | 30 | 10 | |
| Distribution of lesions | Face, scalp, trunk, limbs, palms, glans penis, and scrotum | Face, trunk, limbs, palms, soles, and scrotum | Face, trunk, hands (including nail bed), and labia majora | Face, scalp, trunk, limbs, penile shaft, palms, and soles | Face, trunk, limbs, palms, and penile shaft | Face, trunk, arms, and legs | Face, trunk, arms, and hands | |
| Complications of illness | Low mood and emotional lability. Ulcerated inguinal lesion with delayed healing | Deep tissue abscesses, severe pain, and low mood | Conjunctivitis, painful disruption of thumbnail from subungual lesion | Ulcerated inguinal lesion with delayed healing | None | Pruritis and contact dermatitis from cleaning products | Low mood | |
| Specific management of complications | Clinical psychology input | Empiric broad-spectrum antibiotics, abscess drainage, and analgesia (including opiate and neuropathic agents) | Antibacterial eye drops | Empiric azithromycin | Nil specific | Calamine lotion and short course of antibiotics at the onset of dermatitis | Nil specific | |
| Monkeypox viral DNA detected | ||||||||
| Blood | Yes | Yes | Yes | Yes | No | Yes | Yes | |
| Nose or throat swab | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Urine | Yes | Yes | Yes | Yes | No | No | No | |
| Antivirals received | Brincidofovir 200 mg (one dose) orally | Brincidofovir 200 mg (two doses) orally | Brincidofovir 200 mg (two doses) orally | None | None | None | Tecovirimat 600 mg twice daily for 2 weeks orally | |
| Day of illness treatment commenced† | 7 | 6 | 7 | .. | .. | .. | 5 | |
| Complications of treatment | Transaminitis (peak ALT 331 U/L) | Transaminitis (peak ALT 550 U/L) | Transaminitis (peak ALT 127 U/L), nausea, and abdominal discomfort | .. | .. | .. | None | |
| Duration of hospitalisation with monkeypox, days | 26 | 27 | 35 | 39 | 13 | 22 | 10 | |
| Outcome of monkeypox infection | Full recovery | Full recovery | Full recovery | Full recovery | Full recovery | Full recovery | Full recovery | |
HCID=high consequence infectious disease. MVA=modified vaccinia Ankara. ALT=alanine transaminase.
Age ranges rather than exact ages are given for patient anonymity.
Onset of illness was defined as the first identification of skin lesions by the patient or carers.
Figure 1.
Clinical and virological timelines of seven cases of human monkeypox
Each patient's self-reported identification of a rash is taken as the first date of illness (patient 2's (2021) rash was detected by her mother). Cycle threshold denotes the number of PCR cycles required to detect monkeypox virus, with higher cycle thresholds indicating lower levels of viral DNA and a cut-off of 40 cycles indicating undetectable DNA. In most cases, the rash crusted and desquamated early in the course of illness and the duration of rash is denoted by the dashed line (ongoing rash). Two patients (patient 1 (2018) and patient 4 (2019)) had their admissions prolonged due to isolated ulcerated lesions that remained persistently positive for monkeypox virus DNA, as indicated on their respective graphs. Black arrows indicate doses of brincidofovir, whereas the blue crosses indicate doses of tecovirimat. The grey background indicates time spent admitted in a High Consequence Infectious Diseases unit: patient 7 (2021) was already in hospital caring for patient 6 (2021; her daughter) when she developed symptoms. *marks the date of drainage of a large intramuscular abscess in patient 2 (2018).
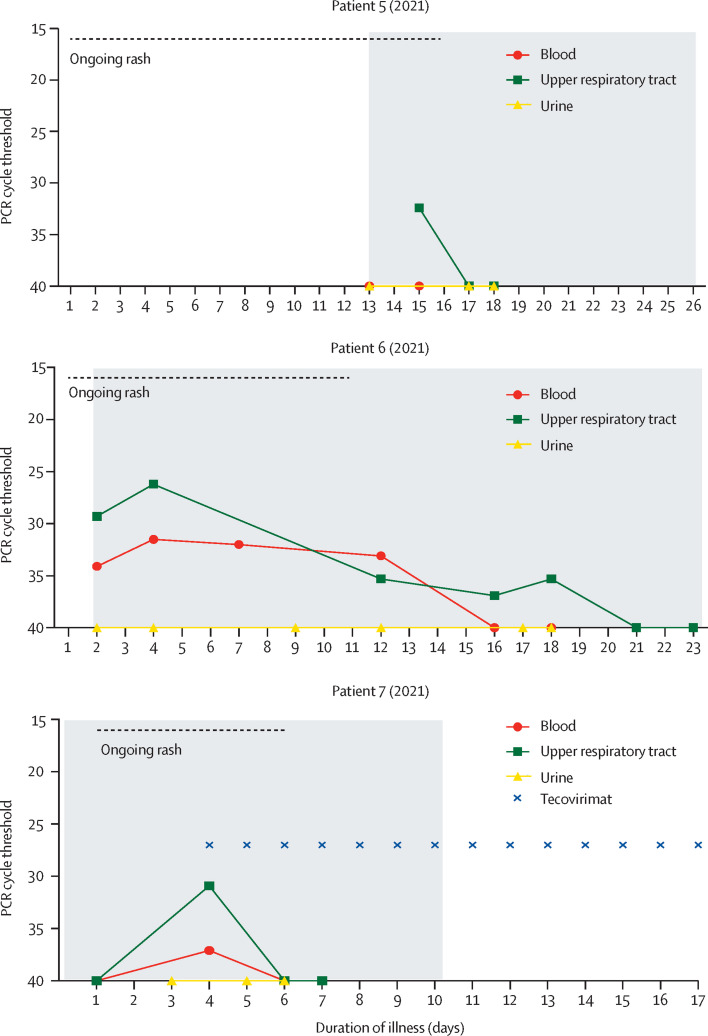
All seven patients had pleiomorphic skin lesions (including papules, vesicles, pustules, umbilicated pustules, ulcerating lesions, and scabs; figure 2 ) that were PCR positive for monkeypox virus DNA. All patients had viral DNA detectable in upper respiratory tract swabs, with DNA detectable in blood for six patients and urine for four patients.
Figure 2.
Skin and soft tissue manifestations of monkeypox
Skin and soft tissue features included: (A and D) vesicular or pustular lesions; (B and C) macular lesions involving the palms and soles; (D and E) a sub-ungual lesion; (F and G) more subtle papules and smaller vesicles; (H) and a deep abscess (arrow, image obtained during ultrasound-guided drainage).
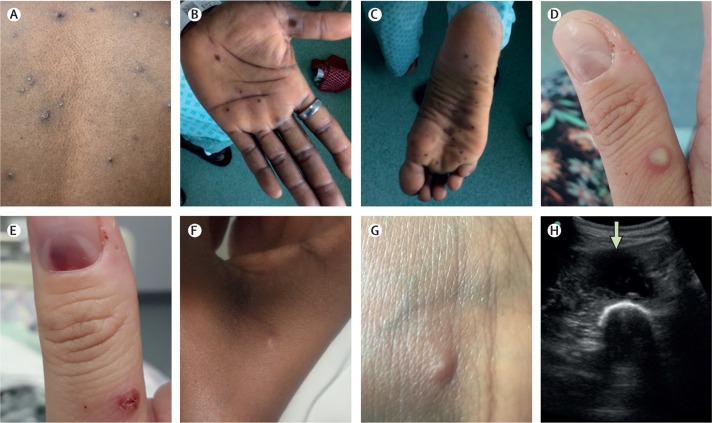
The first three patients were treated with oral brincidofovir, commenced approximately 7 days post-onset of the rash in all cases (figure 1). Following discussion with the manufacturer, the proposed treatment course was three once a week doses of 200 mg.18 All three patients developed elevated alanine transaminase (figure 3 ) and none completed the full course of treatment. No other significant biochemical or haematological disturbances were observed. There was no consistent association between doses of brincidofovir and clinical or virological parameters, although patients 2 and 3 (2018) demonstrated transient reductions in upper respiratory tract viral load around the time of their second doses; regardless of treatment received, all patients ultimately made a full recovery. Clinical complications included mood disturbance, which might have been due to monkeypox or being in an isolation facility (patient 1 [2018]), acute alcohol withdrawal, severe neuralgia requiring opiate analgesia, abscesses in the left ankle and proximal left thigh (patient 2 [2018]), and unilateral conjunctivitis (patient 3 [2018]). The ankle abscess in patient 2 (2018) was drained under ultrasound guidance at day 12 post-diagnosis, while the thigh abscess was diagnosed and drained on day 21 post-diagnosis. The ankle abscess fluid was negative for bacteria by culture and 16S ribosomal RNA sequencing; the thigh abscess fluid was negative for bacteria by culture but positive for high levels of monkeypox virus DNA (cycle threshold 13·6; figure 2). Patient 3 (2018) tested negative for monkeypox virus by PCR of an eye swab; and conjunctivitis was clinically diagnosed as bacterial rather than a result of direct toxicity by monkeypox virus, and they rapidly responded to opthalmic chloramphenicol (one drop four times a day until infection resolved).
Figure 3.
Alanine transaminase values of the three patients who received therapy with brincidofovir
Doses of brincidofovir are denoted by arrows, with the colour of the arrow corresponding with the colour of the relevant patient's alanine transaminase graph. Normal range of alanine transaminase is less than 30 U/L.
Patient 1 (2018) and patient 4 (2019) remained in hospital with ongoing ulcerating inguinoscrotal lesions that were persistently monkeypox virus PCR positive for several weeks after the clearance of viraemia and healing of all their other skin lesions (figure 1). No other pathogens were identified from the ulcerating lesions by routine bacterial culture; patient 4 (2019) was treated with azithromycin (1 g orally as a single dose) to cover the differential diagnoses of chancroid and lymphogranuloma venereum. Both patients remained in hospital until the lesions had healed completely. In the other two patients, patients 2 and 3, the rash fully resolved within 2 weeks, but viral DNA remained detectable in the blood and upper respiratory tract. Patient 2 (2018) remained viraemic until his thigh abscess was drained (figure 1). Patient 3 (2018) had persistently positive upper respiratory tract swabs; she was discharged on day 39 of her illness to self-isolate; negative results were obtained at 45 and 48 days of illness.
Patient 4 (2019) had ongoing inguinal lymphadenopathy after his rash resolved. The lymph nodes increased in size after he had sexual intercourse for the first time since his illness, approximately 6 weeks post-hospital discharge. The lymphadenopathy was associated with localised pustular and shallow ulcerating skin lesions. PCR of these lesions and upper respiratory tract swab was positive for monkeypox virus DNA (figure 1). This relapse was short and was not associated with detectable viraemia. The patient was otherwise clinically well, but he was briefly admitted to his local hospital until the lesions had crusted and a repeat upper respiratory tract swab was PCR negative.
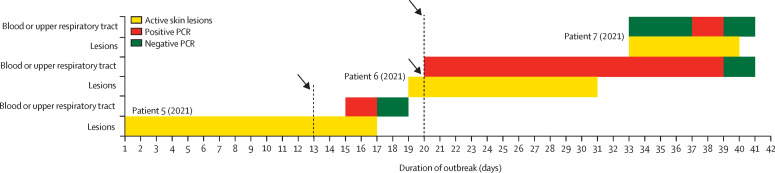
In addition to the four patients who had monkeypox between 2018 and 2019, the HCID network managed a household cluster of monkeypox in 2021. The public health aspects of this outbreak have been described,26 and are summarised in figure 4 .
Figure 4.
Timeline of the 2021 monkeypox household cluster
The duration of monkeypox infection is represented by active (uncrusted) skin lesions and positive PCR results from blood or upper respiratory tract swabs (skin lesions were typically PCR positive until crusted over). Black arrows denote hospital admission.
The family (father, mother, and four children aged younger than 10 years) had travelled from Nigeria to the UK. During their mandatory 10-day COVID-19 self-isolation period, the father (patient 5 [2021]) developed a progressive vesicular rash that he attributed to varicella. He attended a local emergency department at the end of his quarantine period and was subsequently admitted to the regional HCID unit where monkeypox was confirmed by PCR of vesicular fluid. On admission, monkeypox DNA was undetectable in blood but was detectable in upper respiratory tract swabs. Monkeypox DNA became undetectable from upper respiratory tract swabs 48 h later, which is consistent with hospital admission late in his illness (figure 1). When the youngest child subsequently developed fever and a vesicular rash, the entire family was admitted to the same HCID unit. The three older siblings were cohorted with their father, who was deemed non-infectious at that time, and all three older children and their father were discharged to complete 21 days of post-exposure isolation after a paediatric review and negative blood and upper respiratory tract swab PCR.
The mother requested to stay in hospital to continue caring for her daughter (patient 6 [2021]) after monkeypox was confirmed by PCR of a lesion swab. The child was managed in the adult HCID unit with 24 h on-site support from visiting paediatric staff. Treatment with tecovirimat was considered, but it was discounted given that it is not currently licensed for use in children, has no standardised dosing in patients less than 13 kg, and has previously only been used in a single paediatric case of vaccinia infection.20 All lesions had crusted by day 12 of illness; however, despite clearance of viraemia, monkeypox DNA remained detectable by PCR in upper respiratory tract swabs until day 20 (figure 1).
On day 14 of patient 6's (2021) illness, her mother (patient 7 [2021]) developed malaise, headache, pharyngitis, and vesicles on her thorax, which were PCR positive for monkeypox DNA. Blood and upper respiratory tract swabs were initially negative, but repeat samples obtained 4 days later were positive (figure 1). At this stage, patient 7 (2021) had been isolated at home or in hospital for 35 days; therefore, a decision was made by the treating multi-disciplinary team to offer treatment with a 2-week course of oral tecovirimat (600 mg twice daily). The therapeutic aim was to prevent complications and shorten the duration of hospital stay. Samples from blood and upper respiratory tract became PCR negative 48 h after commencing tecovirimat and remained negative at 72 h (figure 1). No new lesions developed after 24 h of tecovirimat therapy. Patient 7's (2021) haematological, renal, and liver profile remained within normal limits during the first week of therapy and she reported no adverse effects. On day 7 of tecovirimat, she was discharged to complete her second week of treatment at home; she remained clinically well and afebrile during and after finishing therapy.
The parents of the family who had the monkeypox household cluster reported that two of the three older siblings had previously had a vesicular rash illness in Nigeria, and they consented for serological testing of the three uninfected children. All three children tested negative for orthopoxvirus IgG, and the two children with a history of previous rash tested positive for varicella zoster virus IgG. Serum from patient 7 (2021) from admission (before she developed clinical symptoms) also tested negative for orthopoxvirus IgM and IgG.
Discussion
We report seven cases of patients with human monkeypox infection diagnosed in the UK. Although small, this case series provided an opportunity to describe the clinical complications, in-vivo viral kinetics, and therapeutic management of monkeypox in a non-endemic, high-income setting. Notable aspects include the first nosocomial and household transmissions to be reported outside of the African continent, surprisingly long durations of viral DNA shedding, and the use of novel direct-acting antivirals.
Brincidofovir and tecovirimat were not licensed for any indication when the first patients with monkeypox in the UK were diagnosed in 2018. Brincidofovir was selected for the patients in 2018, because it was available through approved, urgent repurposing of an existing supply from a local clinical trial. Given the small numbers involved, it is difficult to infer the relationship between treatment with brincidofovir and disease course. Although transient reductions were seen in monkeypox viral PCR cycle thresholds in a variety of sample types, these improvements were not durable or consistent between patients. We do not know whether brincidofovir administration earlier in the course of disease or at a different dosing schedule would be associated with superior clinical outcomes. In prairie dogs with established monkeypox infection, brincidofovir conferred a modest survival benefit (29% vs 14%) and reduction in end-organ viral titres.28 It is also important to note that all three patients developed deranged liver enzymes (a recognised side effect18) during treatment, resulting in precautionary decisions to curtail courses of treatment.
In patient 7 (2021), tecovirimat was offered on the basis of evidence of efficacy in animal models of orthopoxvirus infection and good tolerability in humans.17 It was hoped that treatment could prevent progression to severe disease or reduce the duration of stay in hospital. This treatment was commenced shortly after the appearance of skin lesions. There was a temporal relationship to clinical and virological responses that were more rapid than those seen in untreated patients or patients treated with brincidofovir; however, we are unable to say whether this was a result of treatment with tecovirimat. A similar reduction in lesion count and duration of PCR positivity in blood and upper respiratory tract was seen in macaques with smallpox when treated with tecovirimat versus placebo.29 We elected not to obtain further samples to demonstrate persistent viral clearance following the cessation of therapy as the patient remained clinically well and lived some distance from the treating HCID centre. Available data suggest that a 5-day course is sufficient to confer a clinical response, whereas a 2-week course allows humoral immunity to develop and durably clears the virus.17
Paediatric monkeypox infection has historically been associated with a higher likelihood of severe disease and mortality than in adults.8, 10 Patient 6 (2021) represents the first reported paediatric patient with monkeypox outside of Africa since 2003, and they are the first paediatric patient managed by the UK HCID network. The challenge of managing a young child and an adult together in isolation was met by a paediatric HCID team delivering on-site care within the adult isolation unit. The patient required careful multidisciplinary and interspecialty coordination, collaboration, and communication throughout; fortunately, the child experienced a mild course of illness.
We faced the ethical dilemma of whether to allow patient 6's (2021) mother to provide care for her. The risk of further transmission was balanced with the mother's fully informed decision, acknowledgement that extended close contact had already occurred, and the practical difficulties and associated risk of managing a small child in prolonged isolation without a parent. It was not practical for the mother to wear PPE while residing with the child 24 h/day. It was not clear at the time of admission whether the mother was already incubating monkeypox from contact with patient 5 (2021). Modified vaccinia Ankara vaccination is indicated within 4 days of exposure, although it can be considered up to a maximum of 14 days.25 Tecovirimat has not been approved for post-exposure prophylaxis, although an application to extend the license for this indication is in progress (Dennis E Hruby, personal communication). The typical incubation period of monkeypox is approximately 2 weeks, which suggests that patient 7 (2021) acquired the infection while caring for patient 6 (2021) rather than from patient 5 (2021; figure 4).30 This hypothesis was further supported by negative orthopoxvirus IgG and IgM tests on serum taken at hospital admission from patient 7 (2021), 20 days after symptom onset in patient 5 (index case) and 13 days before she became symptomatic.
In previous cases and outbreaks of monkeypox, patients have been considered infectious until all the lesions were crusted.16 There are few data available on the viral kinetics of human monkeypox infection and most cases occur in settings where regular PCR testing of blood or upper respiratory tract swabs is not available in a timely manner. We observed trajectories in blood and upper respiratory tract swab PCR positivity that were similar to those seen in non-human primate models of monkeypox and smallpox.17, 29 We identified shedding of monkeypox viral DNA in upper respiratory tract swabs for at least 3 weeks from three patients, including two treated with brincidofovir. One of these patients had received post-exposure prophylaxis with modified vaccinia Ankara, albeit outside of the 4-day window that was recommended.25 The infectivity of patients with positive upper respiratory tract swabs and crusted skin lesions remains undetermined, and it is an important area for future study with immediate practical implications for health-care resource use, patient safety and discharge, and prevention of transmission. The relapse of patient 4 (2019) was associated with a mild, short clinical illness and transient shedding of monkeypox viral DNA. We are unaware of previous reports of such relapses. The temporal association between sexual intercourse, increased inguinal lymphadenopathy, and recurrence of rash could suggest a genital reservoir of monkeypox virus, as has been reported with many other emerging viruses,31 but this theory warrants dedicated research with a larger cohort of patients. We are unaware of any reports of monkeypox virus detection in seminal fluid, and we did not collect semen samples from our patients.
All of the patients were young with no pre-existing comorbidities, and none of them had received pre-exposure smallpox vaccination. Nonetheless, most experienced a relatively mild course of illness, which is consistent with infection by the west African clade of monkeypox virus.7, 8 Low mood was common among our patients; however, their mood could have been an appropriate and predictable reaction to prolonged hospital isolation without visitors for infection control purposes. It might also have reflected a perception of stigma surrounding the diagnosis (for example, one patient's landlord attempted to evict them during their admission). Anxiety or depression requiring counselling affected more than a quarter of patients hospitalised with monkeypox in a 2018 case series from Nigeria.9
The clinical features of our patients were comparable with those seen in outbreaks of the west African clade of monkeypox virus in Nigeria and the USA,8, 13 and our patients' skin lesions exhibited a similar natural history to those described in the 2003 US outbreak.12 However, we did not identify any oral lesions; only two of seven patients reported a sore throat; pruritis was common in the 2018 Nigerian outbreak,8 but it was rare in our cohort; and gastrointestinal and respiratory symptoms were reported by a minority of patients in the 2003 US outbreak but were not reported in any of our patients.13 All of our patients were hospitalised for infection control purposes, whereas in other outbreaks the decision to admit has been made on a case-by-case basis.
None of the patients in our series experienced any of the commonly recognised severe complications of monkeypox such as pneumonitis or superimposed bacterial sepsis. Patient 2 (2018) represents what we believe to be the first report of an adult patient with a deep tissue monkeypox abscess. Neither of the patient's two abscesses communicated directly with a superficial skin lesion, and the thigh abscess was only identified using ultrasonography approximately 2 weeks into the patient's illness after diagnosis. The estimated viral loads in urine and upper respiratory tract samples reduced following two doses of brincidofovir, but the viraemia only resolved once the thigh abscess was drained. Other researchers have previously suggested that source control could be beneficial for complicated orthopoxvirus lesions.22 We are aware of one previous report of a child in the USA with monkeypox and a radiologically confirmed retropharyngeal abscess. The aetiology of the abscess was not definitively confirmed, and it resolved without drainage.32 We cannot definitively exclude a bacterial cause for our patient's abscess (he was also treated with broad spectrum antimicrobials), but the low monkeypox viral PCR cycle threshold in the abscess fluid is compelling.
The limitations of this study are its observational nature, the small number of cases, and our inability to confirm positive PCR results with viral culture assays to demonstrate ongoing shedding of viable virus.
This case series highlights the value of maintaining a collaborative network of centres on standby to manage sporadic, small numbers of patients with high consequence pathogens. The disease course of the patients we report on were challenging and resource-intensive to manage, even in the high-income setting of the UK. Monkeypox outbreaks will continue to occur in west and central Africa, and health-care workers around the world must remain vigilant to the possibility of monkeypox in travellers presenting with fever and rash. Our observations in this small series support further research into antivirals to treat this neglected tropical disease.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on May 26, 2022
Data sharing
Data from this study will not be made available to others.
Declaration of interests
JD reports an unremunerated role on a Data Safety and Monitoring Board (DSMB) for an extended access programme for tecovirimat. MGS reports non-remunerated roles in a DSMB for Pfizer's mRNA vaccine programme, the UK Scientific Advisory Group for Emergencies, and New Emerging Respiratory Virus Threats Advisory Group; and reports chairing a Scientific Advisory Board and holding stock or stock options in Integrum Scientific LLC, Greensboro, NC, USA. SHK reports grants (unrelated to the current work) from ViiV, Merck, and Gilead Sciences; advisory board membership for ViiV and Merck; honoraria from ViiV; and being an unremunerated chair of a DSMB for a Gates Foundation-funded trial.
Acknowledgments
Acknowledgments
The authors gratefully acknowledge all of the patients for their consent to publish this clinical experience, the clinical and support staff in the High Consequence Infectious Diseases (Airborne) Network isolation units, regional ambulance services, UK Health Security Agency, Public Health Wales, the Rare and Imported Pathogens Laboratory, and NHS England specialist commissioning services for their support in managing these complex cases. CJAD is funded by a Wellcome Clinical Research Career Development Fellowship (211153/Z/18/Z). TW is supported by grants from the Wellcome Trust, UK (209075/Z/17/Z), Medical Research Council (MRC; MR/V028618/1), and Joint Global Health Trials, UK (MR/V004832/1). Consent to publication was provided under the framework of the ISARIC study, which is supported by grants from: the National Institute for Health Research (NIHR; award CO-CIN-01), MRC (grant MC_PC_19059), the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections at University of Liverpool (in partnership with the UK Health Security Agency, Liverpool School of Tropical Medicine, and the University of Oxford [NIHR award 200907]), Wellcome Trust and Department for International Development (DID; 215091/Z/18/Z), the Bill and Melinda Gates Foundation (OPP1209135), and Liverpool Experimental Cancer Medicine Centre (grant reference C18616/A25153).
Contributors
HA, SG, JD, and TW contributed to conceptualisation of the Article. HA, SG, PH, LBS, WW, CFH, JCO, TR, MBJB, CJAD, JD, TEF, ERH, MJ, SHK, DP, RP, MLS, MGS, TW, and NMP contributed to investigation for the Article. MBJB, TEF, MJ, WN, LR, AT, TW, and NMP supervised the investigation. HA, SG, PH, JD, and TW contributed to writing the original draft. LBS, WW, CFH, JCO, TR, MBJB, CJAD, TEF, ERH, MJ, SHK, WN, DP, LR, MLS, MGS, and NMP contributed to reviewing and editing the manuscript. All authors had access to the data. HA, SG, CFH, and TW accessed the original data and vouch for its authenticity.
Contributor Information
NHS England High Consequence Infectious Diseases (Airborne) Network:
Mike Abouyannis, Asma Al-Balushi, Stephen Aston, Robert Ball, Nicholas J Beeching, Thomas J Blanchard, Ffion Carlin, Geraint Davies, Angela Gillespie, Scott R Hicks, Marie-Claire Hoyle, Chinenye Ilozue, Luke Mair, Suzanne Marshall, Anne Neary, Emmanuel Nsutebu, Samantha Parker, Hannah Ryan, Lance Turtle, Chris Smith, Jon van Aartsen, Naomi F Walker, Stephen Woolley, Anu Chawla, Ian Hart, Anna Smielewska, Elizabeth Joekes, Cathryn Benson, Cheryl Brindley, Urmi Das, Chin K Eyton-Chong, Claire Gnanalingham, Clare Halfhide, Beatriz Larru, Sarah Mayell, Joanna McBride, Claire Oliver, Princy Paul, Andrew Riordan, Lekha Sridhar, Megan Storey, Audrey Abdul, Jennifer Abrahamsen, Breda Athan, Sanjay Bhagani, Colin S Brown, Oliver Carpenter, Ian Cropley, Kerrie Frost, Susan Hopkins, Jessica Joyce, Lucy Lamb, Adrian Lyons, Tabitha Mahungu, Stephen Mepham, Edina Mukwaira, Alison Rodger, Caroline Taylor, Simon Warren, Alan Williams, Debbie Levitt, Denise Allen, Jill Dixon, Adam Evans, Pauline McNicholas, Brendan Payne, D Ashley Price, Uli Schwab, Allison Sykes, Yusri Taha, Margaret Ward, Marieke Emonts, Stephen Owens, Alina Botgros, Sam T Douthwaite, Anna Goodman, Akish Luintel, Eithne MacMahon, Gaia Nebbia, Geraldine O'Hara, Joseph Parsons, Ashwin Sen, Daniel Stevenson, Tadgh Sullivan, Usman Taj, Claire van Nipsen tot Pannerden, Helen Winslow, Ewa Zatyka, Ekene Alozie-Otuka, Csaba Beviz, Yusupha Ceesay, Latchmin Gargee, Morloh Kabia, Hannah Mitchell, Shona Perkins, Mingaile Sasson, Kamal Sehmbey, Federico Tabios, Neil Wigglesworth, Emma J Aarons, Tim Brooks, Matthew Dryden, Jenna Furneaux, Barry Gibney, Jennifer Small, Elizabeth Truelove, Clare E Warrell, Richard Firth, Gemma Hobson, Christopher Johnson, Alison Dewynter, Sebastian Nixon, Oliver Spence, Joachim J Bugert, and Dennis E Hruby
Supplementary Material
References
- 1.Marennikova SS, Seluhina EM, Mal'ceva NN, Cimiskjan KL, Macevic GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. 1972;46:599–611. [PMC free article] [PubMed] [Google Scholar]
- 2.McCollum AM, Nakazawa Y, Ndongala GM, et al. Human monkeypox in the kivus, a conflict region of the Democratic Republic of the Congo. Am J Trop Med Hyg. 2015;93:718–721. doi: 10.4269/ajtmh.15-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Learned LA, Reynolds MG, Wassa DW, et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73:428–434. [PubMed] [Google Scholar]
- 4.Huhn GD, Bauer AM, Yorita K, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 5.Jezek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 6.Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 8.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 10.Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824–828. doi: 10.1093/infdis/jix260. [DOI] [PubMed] [Google Scholar]
- 11.CDC Monkeypox in the United States. 2021. https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html
- 12.Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erez N, Achdout H, Milrot E, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25:980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng OT, Lee V, Marimuthu K, et al. A case of imported monkeypox in Singapore. Lancet Infect Dis. 2019;19 doi: 10.1016/S1473-3099(19)30537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44–53. doi: 10.1056/NEJMoa1705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chittick G, Morrison M, Brundage T, Nichols WG. Short-term clinical safety profile of brincidofovir: a favorable benefit-risk proposition in the treatment of smallpox. Antiviral Res. 2017;143:269–277. doi: 10.1016/j.antiviral.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 19.FDA FDA approves drug to treat smallpox. 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-drug-treat-smallpox
- 20.Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555–1561. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 21.Lindholm DA, Fisher RD, Montgomery JR, et al. Pre-emptive tecovirimat use in an active duty service member who presented with acute myeloid leukemia after smallpox vaccination. Clin Infect Dis. 2019;69:2205–2207. doi: 10.1093/cid/ciz286. [DOI] [PubMed] [Google Scholar]
- 22.Kiernan M, Koutroumanos N. Orbital Cowpox. N Engl J Med. 2021;384 doi: 10.1056/NEJMicm2033620. [DOI] [PubMed] [Google Scholar]
- 23.GlobeNewswire SIGA announces collaboration with oxford university to support expanded access protocol for use of tpoxx (tecovirimat) to treat monkeypox in Central African Republic. 2021. https://www.globenewswire.com/news-release/2021/07/29/2270930/9738/en/SIGA-Announces-Collaboration-with-Oxford-University-to-Support-Expanded-Access-Protocol-for-Use-of-TPOXX-Tecovirimat-To-Treat-Monkeypox-in-Central-African-Republic.html
- 24.UK Health Security Agency High consequence infectious diseases (HCID): guidance and information about high consequence infectious diseases and their management in England. 2018. https://www.gov.uk/guidance/high-consequence-infectious-diseases-hcid
- 25.Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, UK, October 2018. Emerg Infect Dis. 2020;26:782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hobson G, Adamson J, Adler H, et al. Family cluster of three cases of monkeypox imported from Nigeria to the UK, May 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunning JW, Merson L, Rohde GGU, et al. Open source clinical science for emerging infections. Lancet Infect Dis. 2014;14:8–9. doi: 10.1016/S1473-3099(13)70327-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutson CL, Kondas AV, Mauldin MR, et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. MSphere. 2021;6:e00927–e00930. doi: 10.1128/mSphere.00927-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mucker EM, Goff AJ, Shamblin JD, et al. Efficacy of tecovirimat (ST-246) in nonhuman primates infected with variola virus (smallpox) Antimicrob Agents Chemother. 2013;57:6246–6253. doi: 10.1128/AAC.00977-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Tortorec A, Matusali G, Mahé D, et al. From ancient to emerging infections: the odyssey of viruses in the male genital tract. Physiol Rev. 2020;100:1349–1414. doi: 10.1152/physrev.00021.2019. [DOI] [PubMed] [Google Scholar]
- 32.Anderson MG, Frenkel LD, Homann S, Guffey J. A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values. Pediatr Infect Dis J. 2003;22:1093–1096. doi: 10.1097/01.inf.0000101821.61387.a5. discussion 1096–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study will not be made available to others.