Abstract
Purpose
This phase I/II clinical study was conducted to examine the safety, tolerability, pharmacokinetics, and efficacy of 10-min dosing of bendamustine in patients with previously untreated indolent B-cell non-Hodgkin lymphoma (iNHL) or mantle cell lymphoma (MCL) (Group 1) and patients with relapsed/refractory diffuse large B-cell lymphoma (rrDLBCL) (Group 2).
Methods
Rituximab 375 mg/m2 was administered intravenously every 28 days to Group 1 patients on day 1 and every 21 days to Group 2 patients on day 1. Bendamustine 90 mg/m2/day was administered to the former on days 1 and 2; bendamustine 120 mg/m2/day was administered to the latter on days 2 and 3. Each regimen was delivered up to six cycles for both groups. The primary endpoints were safety and tolerability in Groups 1 and 2, respectively.
Results
Among 37 enrolled patients, safety was assessed in 36. In Group 1 (n = 30), 27 patients (90%) had follicular lymphoma. Adverse events (AEs) were observed in all 30 patients in Group 1. Dose-limiting toxicities were observed in two of six patients in Group 2. Common AEs included lymphocyte count decreased (86.7%, 100%). In Group 1, overall response and complete response rates were 93.1% (95% confidence interval [CI] 77.2–99.2%) and 75.9% (95% CI 56.5–89.7%), respectively. The Cmax and AUC of bendamustine tended to be higher in Group 2 than in Group 1.
Conclusions
This study showed that bendamustine is safe, well-tolerated and effective for patients with previously untreated iNHL, MCL or rrDLBCL. Pharmacokinetic data were equivalent to those obtained outside of Japan.
Registration numbers
Registration NCT03900377; registered April 3, 2019.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00280-022-04442-2.
Keywords: Bendamustine, Rapid infusion, Non-Hodgkin lymphoma, Phase I/II, Safety, Tolerability
Introduction
Bendamustine hydrochloride (BDM), synthesized in Germany in the 1960s, is an anticancer drug with alkylating and antimetabolite properties [1–4]. BDM has shown efficacy for hematologic malignancies and solid tumors [5–9].
The original formulation of BDM (original BDM) marketed in the United States was a product for 60-min infusion that was supplied as a lyophilized powder requiring reconstitution with sterile water to a 5 mg/mL solution before further dilution into a 500-mL infusion bag of either 0.9% sodium chloride injection (normal saline) or 2.5% dextrose/0.45% sodium chloride injection [10]. The maximum plasma concentration of bendamustine was achieved at the end of intravenous infusion (~ 1 h), followed by rapid elimination in a triphasic manner [11] and with an intermediate elimination half-life (t1/2) of ~ 40 min as the effective t1/2 [12].
In a phase I, open-label, randomized, crossover study [10], Cheung et al. compared original BDM with a new 10-min rapid infusion formulation (rapid BDM) supplied as a ready-to-dilute solution of 25 mg/mL. Consequently, the authors demonstrated the bioequivalence and comparable safety of original BDM and rapid BDM, and the Food and Drug Administration approved rapid BDM for the treatment of patients with chronic lymphocytic leukemia or indolent B-cell non-Hodgkin lymphoma (iNHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen [13].
SymBio Pharmaceuticals Limited licensed rapid BDM from Eagle Pharmaceuticals, Inc. (Woodcliff Lake, NJ, USA) and gained regulatory approval for a 60-min infusion formulation not requiring reconstitution in September 2020. However, clinical data for Japanese patients were required to obtain regulatory approval to modify the dosage and administration to a 10-min infusion.
Based on the above, we conducted the present clinical phase I/II trial in Japanese B-cell lymphoma patients to examine the safety, efficacy, and tolerability of 10-min dosing of rapid BDM and the pharmacokinetics (PK) of bendamustine.
Patients and methods
Study design, endpoints, and procedures
The present clinical trial was a multicenter, open-label, phase I/II clinical study and consisted of two study groups. Group 1 comprised patients with previously untreated iNHL or mantle cell lymphoma (MCL) and Group 2 consisted of patients with relapsed/refractory diffuse large B-cell lymphoma (rrDLBCL). 375 mg/m2 of rituximab was administered intravenously on day 1 and every 28 days to Group 1 patients and every 21 days to Group 2 patients (on the day before day 1 of the first cycle for both groups), followed by bendamustine 90 mg/m2/day administered intravenously on days 1 and 2 for Group 1 and bendamustine 120 mg/m2/day on days 2 and 3 for Group 2. The primary endpoint was safety in Group 1 and tolerability in Group 2. Secondary endpoints were pharmacokinetics (PK) in Groups 1 and 2 and efficacy in Group 1.
The study period was from the time of obtaining informed consent of the patient to completion of the administration period, with follow-up monitoring once every 3 months for patients who received at least 1 dose of bendamustine through the 28-day cycles (the 29-day cycle for the first cycle only) in Group 1 and through the 21-day cycles in Group 2. The treatment period was up to 6 cycles. After cycle 2, the dose of bendamustine was reduced or study treatment postponed or discontinued on an as-needed basis according to the next cycle initiation criteria (e.g., neutrophil count: ≥ 1000/mm3) and the bendamustine dose reduction criteria in the second or subsequent cycles (e.g., grade 4 neutrophil count decreased [< 500/mm3] lasting for 1 or more weeks) based on treatment-emergent adverse events (TEAEs) found in the previous cycle and during follow-up.
Patient eligibility
Eligibility criteria were as follows: (1) 20–79 years old; (2) survival expectancy of at least 3 months; (3) Eastern Cooperative Cancer Oncology Group performance status score of 0–2 [14]; (4) major organs presenting well-conserved function; (5) patient written informed consent; and (6) A—histopathologically confirmed, CD20-positive, iNHL (small lymphocytic lymphoma, splenic marginal zone lymphoma, lymphoplasmacytic lymphoma, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue, nodal marginal zone lymphoma, and follicular lymphoma (FL, grades 1, 2, 3a) or MCL (excluding transformed lymphoma) [15]; B—a measurable lesion; C—absence of treatment history; and D—at least one of the criteria listed in GELF (excluding MCL patients) [16–18] in Group 1; and (7) A—CD20 positive, diffuse large B-cell lymphoma (excluding transformed lymphoma) [15] and B—rrDLBCL after R-CHOP (like) regimen as a first line treatment in Group 2.
The key exclusion criteria were as follows: (1) criteria common to Groups 1 and 2 were invasion into the central nervous system, patients with serious active infection requiring antibiotic, antifungal, or antiviral IV injection, and patients with serious complications, such as hepatic failure or renal failure; (2) the criterion specific to Group 1 was MCL patient ≤ 65 years of age; and (3) the criteria specific to Group 2 were a non-treatment period of < 3 weeks between the last day of previous treatment for DLBCL and enrollment and a history of allogeneic hematopoietic stem cell transplantation.
Between April 1, 2019, and September 9, 2020, the present study was conducted in Japan according to the provisions of the Declaration of Helsinki, Good Clinical Practice, and related regulations and protocols. All patients provided Institutional Review Board-approved written informed consent prior to the execution of any study-specific procedures or assessments. The present study was registered at ClinicalTrials.gov (NCT03900377).
Safety and tolerability
Safety was assessed in all patients based on TEAEs (type, incidence, and severity) that were expressed according to Medical Dictionary for Regulatory Activities-Japanese (MedDRA-J) version 23.1, with grading defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0-Japan Clinical Oncology Group, as well as on time-course changes in laboratory values. The number of patients who developed dose-limiting toxicities (DLTs) was examined only in Group 2. DLTs were defined as follows: grade 4 neutrophil count decreased [< 500/mm3] lasting for 1 or more weeks with a fever of ≥ 38 °C; decreased platelet count [< 10,000/mm3] or a bleeding tendency requiring platelet transfusion; other grade 4 hematologic toxicities excluding lymphocyte count decreased and differential white blood counts (%); and other ≥ grade 3 nonhematologic toxicities.
Blood sampling for pharmacokinetic studies
For six patients in Group 1 and all six patients in Group 2, blood sampling for PK of bendamustine was performed on day 1 of cycle 1 in Group 1 and day 2 of cycle 1 in Group 2 at the following ten timepoints: within 30 min before infusion initiation; at 5 and 10 min after infusion initiation; and at 5, 15, and 30 min and 1, 2, 4, and 6 h after infusion completion. Plasma concentrations of bendamustine were measured by high-performance liquid chromatograph–tandem mass spectrometer (LC–MS/MS) with using the analytical method validated by CMIC Pharma Science Co., Ltd. to calculate the following summary statistics according to noncompartmental model analysis using Phoenix WinNonlin version 6.4 (Certara, Princeton, NJ, USA): maximum concentration (Cmax), time of maximum observed concentration (tmax), area under the concentration–time curve from the time of dosing to the time of the last measurable (positive) concentration (AUC0-last), area under the concentration–time curve from time of dosing extrapolated to infinity (AUC0-inf), and elimination half-life (t1/2).
Efficacy
Efficacy was assessed in accordance with the following response criteria listed in the revised response criteria for malignant lymphoma (2007) [19]: complete response (CR) rate, overall response rate (ORR): CR + partial response (PR) rate, and progression-free survival (PFS).
Statistical analyses
As our previous phase II study in 69 Japanese patients with previously untreated iNHL or MCL indicated grade 3–4 TEAEs mostly at incidence rates of 10–100% [20], the target number of patients in Group 1 was set to 30 as the sample size with ≥ 95% power to detect a TEAE (incidence: ≥ 10%). The target number of patients in Group 2 was set to 6 in reference to the previously amended Japanese version of the guidelines for clinical evaluation of anticancer drugs [21]. For analysis of efficacy, the best responses were evaluated for the CR rate and the ORR with binomial probability-based 95% confidence intervals [CIs]. Kaplan–Meier estimates were obtained to analyze PFS, with 50% points and 95% CIs according to the Greenwoods formula. Safety was analyzed in the safety population comprising enrolled patients who received at least one dose of bendamustine. The data sets generated during and/or analysed during the current study are not publicly available for confidentiality reasons but certain information may be available from the corresponding author upon request.
Results
Patient characteristics
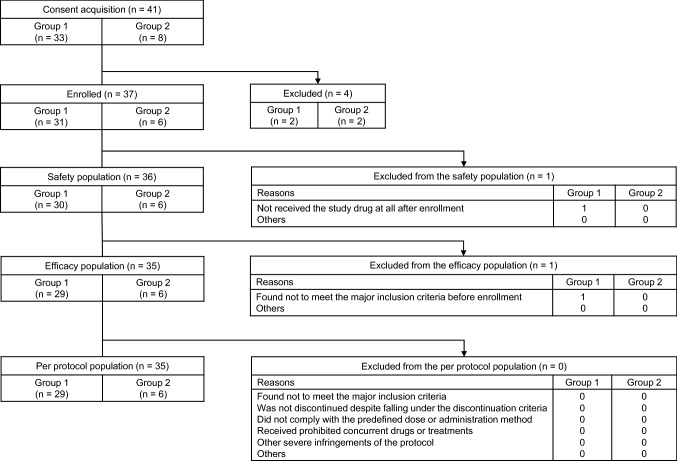
A total of 37 patients were enrolled, 36 and 35 of whom were assessed for safety and efficacy, respectively (Fig. 1). Thirty patients in Group 1 had histopathologically confirmed, CD20-positive, iNHL, and all six patients in Group 2 had rrDLBCL; the patients in this safety population had median ages of 67 years (range 43–76 years) and 73 years (range 69–78 years), respectively (Table 1). In Group 1, 27 patients (90%) had FL, 16 (53%) presented clinical stage IV, and 13 (48%) were categorized as being in the “high risk” category in the Follicular Lymphoma International Prognostic Index (FLIPI); 6 patients were assessed for pharmacokinetics as well. Four (67%) of 6 patients in Group 2 presented with clinical stage III, all 6 (100%) were responders to prior treatments, and 4 (67%) were categorized as being in the “low-intermediate risk” category in the International Prognostic Index (IPI).
Fig. 1 Patient disposition
Table 1.
Demographic and patient characteristics in the safety population
| Characteristics | Group 1 (N = 30) n (%) |
Group 2 (N = 6) n (%) |
|---|---|---|
| Sex | ||
| Male | 12 (40) | 3 (50) |
| Female | 18 (60) | 3 (50) |
| Age—median years (range) | 67 (43–76) | 73 (69–78) |
| <65 | 10 (33) | 0 (0) |
| ≥65 | 20 (67) | 6(100) |
| Diagnosis (WHO classification) | ||
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue | 1 (3) | – |
| Nodal marginal zone B-cell lymphoma | 1(3) | – |
| Follicular lymphoma | 27 (90) | – |
| Mantle cell lymphoma | 1(3) | – |
| Diffuse large B-cell lymphoma | – | 6(100) |
| Cell-of-origin | ||
| Germinal center B-cell-like (GCB) | – | 2 (33) |
| Non-GCB | – | 4 (67) |
| Clinical stage (Ann Arbor classification) | ||
| I | 2(7) | 0(0) |
| II | 6(20) | 1(17) |
| III | 6(20) | 4(67) |
| IV | 16(53) | 1(17) |
| Unknown | 0(0) | 0(0) |
| History of primer treatments | ||
| Absent | 30(100) | 0(0) |
| Present | 0(0) | 6(100) |
| Lines of prior treatments median (range) | 2(1–6) | |
| 1 regimen | – | 1(17) |
| 2 regimens | – | 4(67) |
| ≥3 regimens | – | 1(17) |
| Response to prior treatmentsa | ||
| Responder | – | 6(100) |
| Nonresponder | – | 0(0) |
| Unknown | – | 0(0) |
| Autologous hematopoietic stem cell transplantation | ||
| Absent | – | 6(100) |
| Present | – | 0(0) |
| Radiotherapy | ||
| Absent | – | 5 (83) |
| Present | – | 1(17) |
| Performance status (ECOG criteria) | ||
| 0 | 25(83) | 4(67) |
| 1 | 5(17) | 2(33) |
| 2 | 0(0) | 0(0) |
| 3 | 0(0) | 0(0) |
| 4 | 0(0) | 0(0) |
| Systemic symptoms (B symptoms) (Ann Arbor classification)b | ||
| Absent | 17(57) | 4(67) |
| Present | 13(43) | 2(33) |
| Unknown | 0(0) | 0(0) |
| Tumor diameter | ||
| <5 cm | 10(33) | – |
| ≥5 cm | 20(67) | – |
| LDH | ||
| ≤ Upper limit of normal | 22(73) | 5(83) |
| > Upper limit of normal | 8(27) | 1(17) |
| Number of nodal lesionsc | ||
| <5 | 19(63) | 6(100) |
| ≥5 | 11(37) | 0(0) |
| Number of extranodal lesionsd | ||
| <2 | 24(80) | 6(100) |
| ≥2 | 6(20) | 0(0) |
| Bone marrow infiltration | ||
| Present | 11(37) | 0(0) |
| Absent | 19(64) | 6(100) |
| Undetermined | 0(0) | 0(0) |
| Unknown | 0(0) | 0(0) |
| FLIPI risk category for FLe | 27(100) | – |
| Low (score: 0–1) | 7(26) | – |
| Intermediate (score: 2) | 7(26) | – |
| High (poor) (score: 3–5) | 13(48) | – |
| Unknown | 0(0) | – |
| IPI risk categoryf | ||
| Low (score: 0–1) | – | 1(17) |
| Low–intermediate (score: 2) | – | 4(67) |
| High–intermediate (score: 3) | – | 1(17) |
| High (score: 4–5) | – | 0(0) |
| Unknown | – | 0(0) |
–: not applicable
N number of patients, ECOG Eastern Clinical Oncology Group, LDH lactate dehydrogenase, FLIPI follicular lymphoma international prognostic index, FL follicular lymphoma, IPI international prognostic index
aThe patient, whose best response to 1 or more prior treatments was categorized as “CR or PR”, was categorized as “responder”
bSystemic symptoms (B symptoms): 1 or more tumor-related symptoms were found prior to the initiation of administration
cNumber of nodal lesions: the sum of the number of nodal target lesions and nodal non-target lesions
dNumber of extranodal lesions: the sum of the number of extranodal non-target lesions, as well as of the cases of hepatomegaly, renal enlargement, and bone marrow infiltration
eCategorized based on the number of corresponding poor prognostic factors: age, ≥ 61 years; LDH, > upper limit of normal; hemoglobin, < 12 g/dL; number of nodal lesions, ≥ 5; and clinical stage, III or IV
fCategorized based on the number of corresponding poor prognostic factors: age, ≥ 61 years; LDH, > upper limit of normal; performance status, 2–4; clinical stage, III or IV; and the number of extranodal lesions, ≥ 2
Exposure
The median number of delivered cycles in Groups 1 and 2 was 6 [range 1–6] and 2.5 [range 1–5], respectively. In Group 1, 18 patients received a maximum of 6 cycles. In Group 2, 1 patient received a maximum of 5 cycles, but none of the 6 patients completed 6 cycles. Major causes of treatment discontinuation in Groups 1 and 2 were failure to meet the next cycle initiation criteria (16.7% and 50.0%, respectively), TEAEs (6.7% and 16.7%, respectively), and other causes, including disease progression (13.3% and 33.3%, respectively).
Safety
TEAEs (incidence: ≥ 10%) in the safety population are summarized in Table 2. In Group 1, the most common hematologic TEAEs were decreased lymphocyte (87%), neutrophil and leukocyte (83% each) counts as well as decreased CD4 lymphocyte counts (77%). Grade 3/4 lymphocyte count decreased occurred in 87% of patients, while grade 3/4 neutrophil count decreased, grade 3/4 white blood cell count decreased, and grade 3/4 CD4 lymphocytes decreased occurred in 77% each of patients. Among nonhematologic TEAEs, nausea (73%), infusion-related reaction (63%), and constipation (50%) were most common. Serious TEAEs occurred in 13% of patients: 1 case each of febrile neutropenia, infection, cytomegalovirus enterocolitis, and infusion-related reaction. All these serious TEAEs were resolved or alleviated. TEAEs leading to treatment discontinuation occurred in 20% of patients, and TEAEs leading to dose reduction occurred in 13.3%. Grade 3 or greater laboratory value abnormalities occurred in 90% of patients.
Table 2.
Summary of TEAEs (incidence: ≥ 10%) in the safety population
| Patients in group 1 (n = 30), group 2 (n = 6) | |||||||
|---|---|---|---|---|---|---|---|
| All Grades, n (%) | Grade, n (%) | Grades 3–5 n (%) | |||||
| 1 | 2 | 3 | 4 | 5 | |||
| Group 1 Hematologic | |||||||
| Lymphocyte count decreased | 26 (87) | 0 (0) | 0 (0) | 2 (7) | 24 (80) | 0 (0) | 26 (87) |
| Neutrophil count decreased | 25 (83) | 0 (0) | 2 (7) | 12 (40) | 11 (37) | 0 (0) | 23 (77) |
| White blood cell count decreased | 25 (83) | 0 (0) | 2 (7) | 18 (60) | 5 (17) | 0 (0) | 23 (77) |
| CD4 lymphocytes decreased | 23 (77) | 0 (0) | 0 (0) | 9 (30) | 14 (47) | 0 (0) | 23 (77) |
| Platelet count decreased | 14 (47) | 12 (40) | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Anaemia | 10 (33) | 4 (13) | 5 (17) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Neutropenia | 3 (10) | 0 (0) | 0 (0) | 1 (3) | 2 (7) | 0 (0) | 3 (10) |
| Group 1 Nonhematologic | |||||||
| Nausea | 22 (73) | 14 (47) | 7 (23) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Infusion related reaction | 19 (63) | 4 (13) | 14 (47) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Constipation | 15 (50) | 9 (30) | 6 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Malaise | 14 (47) | 13 (43) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Decreased appetite | 11 (37) | 9 (30) | 1 (3) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| ALT increased | 9 (30) | 4 (13) | 2 (7) | 3 (10) | 0 (0) | 0 (0) | 3 (10) |
| Rash | 9 (30) | 6 (20) | 3 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| AST increased | 8 (27) | 5 (17) | 2 (7) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Gamma-glutamyltransferase increased | 8 (27) | 1 (3) | 2 (7) | 5 (17) | 0 (0) | 0 (0) | 5 (17) |
| Diarrhoea | 8 (27) | 5 (17) | 3 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood immunoglobulin M decreased | 7 (23) | 7 (23) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| C-reactive protein increased | 7 (23) | 7 (23) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hepatic function abnormal | 6 (20) | 3 (10) | 3 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 6 (20) | 5 (17) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood lactate dehydrogenase increased | 6 (20) | 6 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Headache | 6 (20) | 4 (13) | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taste disorder | 6 (20) | 6 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Phlebitis | 6 (20) | 0 (0) | 6 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pyrexia | 5 (17) | 5 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood immunoglobulin G decreased | 5 (17) | 4 (13) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood alkaline phosphatase increased | 5 (17) | 4 (13) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Insomnia | 5 (17) | 5 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood albumin decreased | 4 (13) | 2 (7) | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pruritis | 4 (13) | 2 (7) | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vascular pain | 4 (13) | 4 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Beta 2 microglobulin increased | 3 (10) | 3 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood immunoglobulin A decreased | 3 (10) | 3 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Electrocardiogram QT prolonged | 3 (10) | 2 (7) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Protein total decreased | 3 (10) | 3 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypoalbuminaemia | 3 (10) | 3 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Back pain | 3 (10) | 2 (7) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dry skin | 3 (10) | 2 (7) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Erythema | 3 (10) | 2 (7) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Group 2 Hematologic | |||||||
| Lymphocyte count decreased | 6 (100) | 0 (0) | 0 (0) | 0 (0) | 6 (100) | 0 (0) | 6 (100) |
| White blood cell decreased | 6 (100) | 0 (0) | 3 (50) | 2 (33) | 1 (17) | 0 (0) | 3 (50) |
| Neutrophil count decreased | 5 (83) | 1 (17) | 2 (33) | 1 (17) | 1 (17) | 0 (0) | 2 (33) |
| Platelet count decreased | 5 (83) | 2 (33) | 0 (0) | 3 (50) | 0 (0) | 0 (0) | 3 (50) |
| Anaemia | 3 (50) | 0 (0) | 2 (33) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| CD4 lymphocytes decreased | 3 (50) | 0 (0) | 0 (0) | 2 (33) | 1 (17) | 0 (0) | 3 (50) |
| White blood cell count increased | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Neutrophil count increased | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Red blood cell count decreased | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Group 2 Nonhematologic | |||||||
| Nausea | 5 (83) | 4 (67) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| Malaise | 4 (67) | 4 (67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Decreased appetite | 4 (67) | 3 (50) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| Stomatitis | 2 (33) | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 2 (33) | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| Pyrexia | 2 (33) | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fall | 2 (33) | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Infusion related reaction | 2 (33) | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| AST increased | 2 (33) | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Insomnia | 2 (33) | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rash | 2 (33) | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Abdominal pain | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ileus | 1 (17) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| Subileus | 1 (17) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| Folliculitis | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Oral candidiasis | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pharyngitis | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rib fracture | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ALT increased | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0)) | 0 (0) |
| Blood creatinine increased | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood immunoglobulin G decreased | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Blood immunoglobulin M decreased | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gamma-glutamyltransferase increased | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Weight decreased | 1 (17) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| Hepatitis B DNA assay positive | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Diabetes mellitus | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Disseminated intravascular coagulation | 1 (17) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| Folate deficiency | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypoalbuminaemia | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hyponatraemia | 1 (17) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (17) |
| Dizziness | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Headache | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Proteinuria | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Acute respiratory failure | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (17) |
| Cough | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Epistaxis | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hiccups | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dermatitis acneiform | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Erythema | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vascular pain | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vasculitis | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
n number of patients, TEAEs treatment-emergent adverse events, ALT alanine aminotransferase, AST aspartate aminotransferase
In Group 2, the most common hematologic TEAEs were decreased lymphocyte count and decreased white blood cell count (100% each) as well as decreased neutrophil count and decreased platelet count (83% each). Grade 3/4 lymphocyte count decreased occurred in 100% of patients. In contrast, grade 3/4 white blood cell count decreased and grade 3/4 platelet count decreased occurred in 50% each of patients, and grade 3/4 neutrophil count decreased occurred in 33% of patients. Among nonhematologic TEAEs, nausea (83%) and malaise and decreased appetite (67%) were most common. DLTs occurred in two patients: 1 case each of grade 3 vomiting and nausea. These DLTs quickly reversed, and no concerns about the tolerability of bendamustine arose, because those TEAEs were considered manageable with antiemetic prophylaxis or treatment. Seven cases of serious TEAEs occurred in 50% of patients. With the exception of decreased appetite and acute respiratory failure in one patient who died, all these events reversed.
No deaths occurred before completion of the bendamustine dosing period. Two patients with FL in Group 1 died after completion of the study: one due to pneumocystis pneumonia and the other due to primary disease progression. In addition, three patients with DLBCL in Group 2 died after study completion: 1 patient due to acute respiratory failure and two patients due to primary disease progression.
Efficacy
The best overall responses in the patients analyzed for efficacy are summarized in Table 3. In Group 1, the CR rate [19] was 75.9% (95% CI 56.5–89.7%), and the ORR was 93.1% (95% CI 77.2–99.2%). The median PFS was not reached within the median follow-up period of 275.0 days (52–481 days). ORRs were not greatly influenced by the number of delivered cycles. In Group 2, CR and OR rates were 50.0% (95% CI 11.8–88.2%) and 66.7% (95% CI 22.3–95.7%), respectively.
Table 3.
Best overall responses in patients analyzed for efficacy
| Group 1 (N = 29) n (%) |
Group 2 (N = 6) n (%) |
|
|---|---|---|
| Best overall responsea | ||
| CR | 22 (75.9) | 3 (50.0) |
| PR | 5 (17.2) | 1 (16.7) |
| SD | 1 (3.4) | 0 (0.0) |
| PD | 1 (3.4) | 2 (33.3) |
| NE | 0 (0.0) | 0 (0.0) |
| ORRb | 27 (93.1) | 4 (66.7) |
| 95% CI, %c | 77.2–99.2 | 22.3–95.7 |
| CR rate | 22 (75.9) | 3 (50.0) |
| 95% CI, %c | 56.5–89.7 | 11.8–88.2 |
N number of patients, CR complete response, PR partial response, SD stable disease, PD progressive disease, NE not evaluable, ORR overall response rate, CI confidence interval
aAssessed in accordance with the revised response criteria for malignant lymphoma (Cheson et al. J Clin Oncol. 2007;25(5):579–86)
bThe number and rate of patients who were categorized as CR or PR
cThe precise 95% confidence interval based on binominal probability
Pharmacokinetic analyses in Groups 1 and 2
The Cmax, AUC0-last, and AUC0-inf of bendamustine tended to be higher in Group 2 than in Group 1: Cmax (Group1 Mean; 9809 ng/mL, Group 2 Mean; 16256 ng/mL), AUC0-last (4707 ng·h/mL, 8242 ng·h/mL, respectively) and AUC0-inf (4708 ng·h/mL, 8244 ng·h/mL, respectively), though its tmax and t1/2 were similar in both groups (Table 4). Mean plasma concentrations of bendamustine in Groups 1 and 2 peaked at the end of the 10-min infusion (Fig. 2), followed by a rapid triphasic decline, as observed in the phase I clinical trial [3]. The pharmacokinetic parameters (Cmax, tmax, AUC0-last, AUC0-inf, and t1/2) of bendamustine 120 mg/m2 were comparable between the present study and the study conducted by Cheung et al. [10] (Supplementary Table 1).
Table 4.
Summary statistics of the major pharmacokinetic parameters of bendamustine in patients analyzed for pharmacokinetics
| Group (dose) | Cmax (ng/mL) | tmax (h) | AUC0-last (ng·h/mL) | AUC0-inf (ng·h/mL) | t1/2 (h) |
|---|---|---|---|---|---|
| Group 1 (90 mg/m2) | |||||
| N | 6 | 6 | 6 | 6 | 6 |
| Mean | 9809 | 0.18 | 4707 | 4708 | 0.43 |
| SD | 3418 | 0.03 | 1732 | 1732 | 0.11 |
| %CV | 34.8 | 18.8 | 36.8 | 36.8 | 26.0 |
| Group 2 (120 mg/m2) | |||||
| N | 6 | 6 | 6 | 6 | 6 |
| Mean | 16256 | 0.18 | 8242 | 8244 | 0.50 |
| SD | 4434 | 0.06 | 2794 | 2796 | 0.07 |
| %CV | 27.3 | 34.7 | 33.9 | 33.9 | 14.5 |
Cmax maximum concentration, tmax time of maximum observed concentration, AUC0-last area under the concentration–time curve from the time of dosing to the time of the last measurable (positive) concentration, AUC0-inf area under the concentration–time curve from the time of dosing extrapolated to infinity, t1/2 elimination half-life, N number of patients, SD standard deviation, CV coefficient of variation
Fig. 2 Time-course changes in plasma bendamustine concentration. In both Group 1 (open circles) and Group 2 (open squares), maximum plasma concentrations of bendamustine were achieved at approximately 10 min after administration, coinciding with the completion of administration. A rapid triphasic decline occurred thereafter
Discussion
This multicenter, open-label, phase I/II clinical study of rapid BDM afforded the following results. Regarding primary endpoints, bendamustine 90 mg/m2/day did not cause any new safety signals in Group 1, and bendamustine 120 mg/m2/day was well tolerated by Group 2 patients. Regarding secondary endpoints, plasma bendamustine concentrations peaked at the end of infusion in Groups 1 and 2, as described in the highlights of prescribing information on BENDEKA®, ready-to-dilute (RTD) injectable liquid formulation of bendamustine hydrochloride [13], followed by rapid elimination in a triphasic manner after the last dose, as observed in the simulation model presented by Owen et al. [11], and bendamustine 90 mg/m2/day was effective for Group 1 patients. The pharmacokinetic parameters (Cmax, tmax, and AUC0-last) of bendamustine 90 and 120 mg/m2 in the present study were similar to those obtained with the same dose of original BDM in previous studies conducted by Ogura et al. [3, 22]. Furthermore, the pharmacokinetic parameters (Cmax, AUC0-last, and AUC0-inf) tended to be higher in Group 2 than in Group 1, and exposure increased along with an increase in bendamustine dose. No major differences in pharmacokinetic parameters were found for the 10-min dosing used in the present study or in the clinical study conducted by Cheung et al. [10]. Therefore, this clinical study in Japanese patients with iNHL, MCL, or rrDLBCL provides clinical evidence about the safety and tolerability of rapid BDM, as did the clinical study of Cheung et al. [10].
Ogura et al. conducted a phase I and pharmacokinetic study of original BDM 90 and 120 mg/m2 in 8 Japanese patients with relapsed or refractory iNHL and in 1 Japanese patient with relapsed or refractory MCL [3]. The AUCs for bendamustine in their study were 8.3 ± 3.6 and 10.2 ± 5.8 μg·h⁄mL in patients receiving 90 and 120 mg⁄m2, respectively, compared to the respective AUC0-last of 4707 ± 1732 and 8242 ± 2794 ng·h/mL in the present study. Original BDM was safe for and well tolerated by the studied patients, and 120 mg/m2 was the recommended dose for a phase 2 clinical trial. The present study showed efficacy results similar to those (CR rates of 67.8% and 70.0% for iNHL and MCL, respectively) from phase 2 clinical study of original BDM 90 mg/m2/day and rituximab 375 mg/m2 in Japanese patients with treatment-naïve iNHL or MCL conducted by Ogura et al. [20] and those (CR rates of 40% and 30% for the bendamustine plus rituximab group and the R-CHOP group, respectively) from a phase 3 noninferiority study in patients with iNHL and mantle-cell lymphomas conducted by Rummel et al. [23].
The present clinical study of rapid BDM was not designed to examine the bioequivalence of original BDM and rapid BDM in relevant Japanese patients, because Cheung et al. conducted a phase 1, open-label, randomized, crossover study [10] to strictly evaluate the pharmacokinetics of these two formulations of bendamustine, demonstrating that they are bioequivalent and that rapid BDM is associated with a lower incidence of TEAEs than original BDM, except for abdominal pain, dehydration, pyrexia, and dyspnea. Nevertheless, the study exhibited a moderate gap from treatments in real-world clinical settings due to the small number of patients. The authors admitted the need to conduct additional studies to confirm the lower incidence of TEAEs observed with rapid BDM and recognized some limitations of the open-label study regarding the determination of bioequivalence due to a reduced population of evaluable patients in real-world oncology clinical care settings. The present study provides data on the long-term safety of rapid BDM that are supplementary to the above study and PK data in Japanese patients with iNHL, MCL, or rrDLBCL.
In consideration of the efficacy, safety, tolerability, and bioequivalence of original BDM and rapid BDM in the United States and based on the similar pharmacokinetic data for the 10- and 60-min infusion formulations obtained inside and outside Japan, given the similar safety profiles between original BDM and rapid BDM already in use in the US, we do not see any particular medical concern arising in the clinical use of rapid BDM to treat Japanese patients with hematologic malignancies, and we believe that the present study shows evidence of the benefits of rapid BDM for patients and clinicians.
In Japan, the original BDM is reconstituted with saline to prepare a 250-mL admixture for 60-min dosing. Rapid BDM, which allows for 10-min dosing of an admixture, shortens infusion time and causes an approximately 80% reduction in the volume of normal saline required to prepare the admixture. Thus, rapid BDM is expected to be beneficial for both patients and medical professionals, because the risk of edema or extravasation in patients is lessened and the electrolyte load of patients with impaired renal function is attenuated by a reduced volume of fluid. Furthermore, the patient’s stress is reduced, and the work of medical professionals who prepare and infuse the admixture is ameliorated by the shortened infusion time, leading to operational streamlining and cost reductions in the hospital or clinic.
In conclusion, rapid BDM shows good safety, tolerability, and efficacy in Japanese patients with previously untreated iNHL or MCL and in those with rrDLBCL. Rapid BDM has the potential to shorten the setup time for bendamustine infusion and to significantly reduce the treatment burden of patients and health care providers. Hence, rapid BDM may be a useful therapeutic alternative to the currently available 60-min dosing of bendamustine with benefits by simplifying outpatient treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This research was supported by SymBio Pharmaceuticals Limited, Sanofi, Novartis, AbbVie, Bayer, Takeda Pharmaceutical Company, Celgene, Ono Pharmaceutical, Chugai Pharmaceutical, Eisai, IQVIA, SRD, MSD K.K., Otsuka Pharmaceutical, Micron, Janssen, Kyowa Hakko Kirin, Zenyaku Kogyo, Incyte, Mundipharma, ADC, AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Genmab, HUYA, Janssen, Meiji Seika Pharma, Nippon Shinyaku, Pfizer, Solasia, Stemline, Dainippon Sumitomo Pharma, Yakult, Astellas Pharma, Amgen, Takeda, Teijin Pharma, National Hospital Organization, Chordia, Bristol-Myers, Minophagen, JIMRO, Otsuka Medical Device, Celltrion Healthcare, Verastem and DenovoBiopharma.
Declarations
Conflict of interest
K. Ishizawa reports grants from SymBio, during the conduct of the study; grants from Sanofi, grants and personal fees from Novartis, grants from AbbVie, grants from Bayer, personal fees from Takeda, personal fees from Celgene, personal fees from Ono, personal fees from Chugai, personal fees from Eisai, grants from IQVIA, personal fees from SRD, personal fees from MSD, grants from Otsuka, personal fees from Micron, personal fees from Janssen, personal fees from Kyowa Kirin, outside the submitted work; M. Yokoyama reports personal fees from Chugai, outside the submitted work; H. Kato reports personal fees and others from SymBio, during the conduct of the study; others from Zenyaku Kogyo, others from Chugai, others from Incyte, others from Mundipharma, outside the submitted work; K. Yamamoto reports grants and non-financial support from SymBio, during the conduct of the study; grants and personal fees from AbbVie, personal fees from ADC, grants and personal fees from AstraZeneca, grants from Bayer, grants and personal fees from Bristol Myers Squibb/Celgene, grants and personal fees from Chugai, personal fees from Daiichi Sankyo, grants and personal fees from Eisai, grants from IQVIA/Genmab, personal fees from IQIVA/HUYA, grants from IQIVA/Incyte, personal fees from Janssen, personal fees from Kyowa Kirin, grants and personal fees from Meiji Seika Pharma, personal fees from Micron, personal fees from MSD, grants from Mundipharma, grants and personal fees from Nippon Shinyaku, grants and personal fees from Novartis, grants and personal fees from Ono, grants and personal fees from Otsuka, personal fees from Pfizer, personal fees from Sanofi, grants from Solasia, personal fees from Stemline, personal fees from Sumitomo Dainippon, grants and personal fees from SymBio, grants and personal fees from Takeda, grants and personal fees from Yakult, grants and personal fees from Zenyaku Kogyo, outside the submitted work; K. Ando reports grants from SymBio, during the conduct of the study; grants from Chugai grants from Celgene, grants from Astellas, grants from Kyowa Kirin, grants from Takeda, outside the submitted work; Y. Ueda reports other from Otsuka, during the conduct of the study; Y. Suehiro reports grants from SymBio, during the conduct of the study; grants and personal fees from Chugai, grants from Novartis, grants from Bayer, grants and personal fees from Eisai, grants and personal fees from Ono, grants and personal fees from Otsuka, grants from Pfizer, grants from Amgen Astellas BioPharma, grants and personal fees from Celgene, grants from Takeda, grants from Incyte, grants and personal fees from Kyowa Kirin, grants from Teijin, outside the submitted work; Y. Kameoka reports personal fees from Pfizer, personal fees from Kyowa Kirin, personal fees from SymBio, personal fees from HUYA, personal fees from Celgene, personal fees from Janssen, outside the submitted work; H. Nagai reports grants from the National Hospital Organization, during the conduct of the study; grants and personal fees from Janssen, grants and personal fees from Celgene, grants and personal fees from Mundipharma, grants from Bayer, grants from Abbvie, grants and personal fees from Takeda, grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Eisai, grants and personal fees from Bristol Myers Squibb, grants and personal fees from Ono, grants and personal fees from Zenyaku Kogyo, grants from Solasia, grants and personal fees from AstraZeneca, grants and personal fees from SymBio, personal fees from Sanofi, grants from IQVIA, grants from Nippon Shinyaku, personal fees from MSD, grants and personal fees from Chugai, personal fees from Novartis, personal fees from Chordia, personal fees from Sumitomo Dainippon, outside the submitted work; N. Uoshima reports grants from SymBio, grants from Otsuka, personal fees from Eisai, outside the submitted work; M. Hidaka reports grants, personal fees and non-financial support from SymBio, during the conduct of the study; grants from Chugai, outside the submitted work; A. Utsunomiya reports personal fees from Novartis, personal fees from Kyowa Kirin, personal fees from Daiichi Sankyo, personal fees from Bristol Myers, personal fees from Celgene, personal fees from Pfizer, personal fees from Minophagen, personal fees from Jansen, personal fees from JIMRO, personal fees from Chugai, personal fees from Meiji Seika Pharma, personal fees from Otsuka Medical Devices, outside the submitted work; M. Ogura reports personal fees from SymBio, during the conduct of the study; personal fees from Celgene, personal fees from Celltrion, personal fees from Meiji Seika Pharma, personal fees from Mundipharma, personal fees from Verastem, personal fees from DenovoBiopharma, personal fees from Chugai, personal fees from Yakult, personal fees from Takeda, outside the submitted work; and K. Fukushima is an employee of SymBio. The rest of authors do not have any relationships to disclose.
Ethics approval
The present study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice and with the Declaration of Helsinki and was approved by the designated ethics committees and institutional review boards at each site.
Consent to participate
All participants provided written informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blumel S, Goodrich A, Martin C, Dang NH. Bendamustine: a novel cytotoxic agent for hematologic malignancies. Clin J Oncol Nurs. 2008;12:799–806. doi: 10.1188/08.CJON.799-806. [DOI] [PubMed] [Google Scholar]
- 2.Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, Elliott G, Niemeyer CC. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–317. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 3.Ogura M, Uchida T, Taniwaki M, Ando K, Watanabe T, Kasai M, Matsumoto Y, Shimizu D, Ogawa Y, Ohmachi K, Yokoyama H, Tobinai K, Japanese Bendamustine Lymphoma Study G Phase I and pharmacokinetic study of bendamustine hydrochloride in relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2054–2058. doi: 10.1111/j.1349-7006.2010.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson BD, Leoni L. Bendamustine: mechanism of action and clinical data. Clin Adv Hematol Oncol. 2011;9:1–11. [PubMed] [Google Scholar]
- 5.Cheson BD, Wendtner CM, Pieper A, Dreyling M, Friedberg J, Hoelzer D, Moreau P, Gribben J, Knop S, Montillo M, Rummel M. Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk. 2010;10:21–27. doi: 10.3816/CLML.2010.n.002. [DOI] [PubMed] [Google Scholar]
- 6.Elefante A, Czuczman MS. Bendamustine for the treatment of indolent non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Am J Health Syst Pharm. 2010;67:713–723. doi: 10.2146/ajhp090328. [DOI] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Chernozemsky I, Sirakova L, Chilingirov P, Souchon R, Marschner N, Kleeberg U, Tsekov C, Fritze D, Thomssen C, Stuart N, Vermorken JB, Loibl S, Merkle K, Kaufmann M. Bendamustine prolongs progression-free survival in metastatic breast cancer (MBC): a phase III prospective, randomized, multicenter trial of bendamustine hydrochloride, methotrexate and 5-fluorouracil (BMF) versus cyclophosphamide, methotrexate and 5-fluorouracil (CMF) as first-line treatment of MBC. Anticancer Drugs. 2005;16:871–877. doi: 10.1097/01.cad.0000175587.31940.19. [DOI] [PubMed] [Google Scholar]
- 8.Pirvulescu C, von Minckwitz G, Loibl S. Bendamustine in metastatic breast cancer: an old drug in new design. Breast Care (Basel) 2008;3:333–339. doi: 10.1159/000154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Höffken K, Merkle K, Schönfelder M, Anger G, Brandtner M, Ridwelski K, Seeber S. Bendamustine as salvage treatment in patients with advanced progressive breast cancer: a phase II study. J Cancer Res Clin Oncol. 1998;124:627–632. doi: 10.1007/s004320050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung EM, Edenfield WJ, Mattar B, Anthony SP, Mutch PJ, Chanas B, Smith M, Hepner A. Safety and pharmacokinetics of bendamustine rapid-infusion formulation. J Clin Pharmacol. 2017;57:1400–1408. doi: 10.1002/jcph.942. [DOI] [PubMed] [Google Scholar]
- 11.Owen JS, Melhem M, Passarell JA, D'Andrea D, Darwish M, Kahl B. Bendamustine pharmacokinetic profile and exposure-response relationships in patients with indolent non-Hodgkin's lymphoma. Cancer Chemother Pharmacol. 2010;66:1039–1049. doi: 10.1007/s00280-010-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darwish M, Bond M, Hellriegel E, Robertson P, Jr, Chovan JP. Pharmacokinetic and pharmacodynamic profile of bendamustine and its metabolites. Cancer Chemother Pharmacol. 2015;75:1143–1154. doi: 10.1007/s00280-015-2727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Full prescribing information, BENDEKA® (bendamustine hydrochloride injection), for intravenous use, Revised: 07/2018. https://www.bendeka.com/globalassets/bendeka-hcp/prescribinginformation.pdf. Accessed 1 Jun 2021]
- 14.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon: IARC Press; 2008. [Google Scholar]
- 16.Brice P, Bastion Y, Lepage E, Brousse N, Haïoun C, Moreau P, Straetmans N, Tilly H, Tabah I, Solal-Céligny P. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 1997;15:1110–1117. doi: 10.1200/jco.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- 17.Solal-Céligny P, Lepage E, Brousse N, Tendler CL, Brice P, Haïoun C, Gabarre J, Pignon B, Tertian G, Bouabdallah R, Rossi JF, Doyen C, Coiffier B. Doxorubicin-containing regimen with or without interferon alfa-2b for advanced follicular lymphomas: final analysis of survival and toxicity in the Groupe d'Etude des Lymphomes Folliculaires 86 Trial. J Clin Oncol. 1998;16:2332–2338. doi: 10.1200/jco.1998.16.7.2332. [DOI] [PubMed] [Google Scholar]
- 18.Sebban C, Mounier N, Brousse N, Belanger C, Brice P, Haioun C, Tilly H, Feugier P, Bouabdallah R, Doyen C, Salles G, Coiffier B. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF-94 randomized study from the Groupe d'Etude des Lymphomes de l'Adulte (GELA) Blood. 2006;108:2540–2544. doi: 10.1182/blood-2006-03-013193. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on L Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 20.Ogura M, Ishizawa K, Maruyama D, Uike N, Ando K, Izutsu K, Terui Y, Imaizumi Y, Tsukasaki K, Suzuki K, Izumi T, Usuki K, Kinoshita T, Taniwaki M, Uoshima N, Suzumiya J, Kurosawa M, Nagai H, Uchida T, Fukuhara N, Choi I, Ohmachi K, Yamamoto G, Tobinai K, Japanese Bendamustine Lymphoma Study G Bendamustine plus rituximab for previously untreated patients with indolent B-cell non-Hodgkin lymphoma or mantle cell lymphoma: a multicenter Phase II clinical trial in Japan. Int J Hematol. 2017;105:470–477. doi: 10.1007/s12185-016-2146-4. [DOI] [PubMed] [Google Scholar]
- 21.Minami H, Kiyota N, Kimbara S, Ando Y, Shimokata T, Ohtsu A, Fuse N, Kuboki Y, Shimizu T, Yamamoto N, Nishio K, Kawakami Y, Nihira SI, Sase K, Nonaka T, Takahashi H, Komori Y, Kiyohara K. Guidelines for clinical evaluation of anti-cancer drugs. Cancer Sci. 2021;112:2563–2577. doi: 10.1111/cas.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogura M, Ando K, Taniwaki M, Watanabe T, Uchida T, Ohmachi K, Matsumoto Y, Tobinai K, Japanese Bendamustine Lymphoma Study G Feasibility and pharmacokinetic study of bendamustine hydrochloride in combination with rituximab in relapsed or refractory aggressive B cell non-Hodgkin's lymphoma. Cancer Sci. 2011;102:1687–1692. doi: 10.1111/j.1349-7006.2011.01994.x. [DOI] [PubMed] [Google Scholar]
- 23.Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Durk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W, Study group indolent L Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.