Abstract
Background and Objectives
Prostate-specific membrane antigen (PSMA) positron emission tomography (PET) combined with computed tomography (CT) is a new imaging modality to detect the extra-prostatic spread of prostate cancer. PSMA PET/CT has a higher sensitivity and specificity than conventional imaging (CT ± whole body bone scan [WBBS]). This study conducted a cost-utility analysis of PSMA PET/CT compared with conventional imaging for patients with newly diagnosed, intermediate-risk or high-risk primary prostate cancer.
Perspective
Australian healthcare perspective.
Setting
Tertiary.
Methods
A decision-analytic Markov model combined data from a variety of sources. The time horizon was 35 years. The sensitivity and specificity of PSMA PET/CT and CT alone were based on meta-analyses and the test accuracy of CT+WBBS was based on a single randomised controlled trial. Health outcomes included cases detected, life-years, and quality-adjusted life-years. Costs related to other diagnostic tests, initial treatment, adverse events, and post-disease progression were included. All costs were reported in 2021 Australian Dollars (A$).
Results
The deterministic incremental cost-effectiveness ratio of PSMA PET/CT was estimated to be A $21,147/quality-adjusted life-year gained versus CT+WBBS, and A$36,231/quality-adjusted life-year gained versus CT alone. The results were most sensitive to the time horizon, and the initial treatments received by patients diagnosed with metastatic cancer. The probability of PSMA PET/CT being cost effective was estimated to be 91% versus CT+WBBS and 89% versus CT alone, using a threshold of AU$50,000/quality-adjusted life-year gained.
Conclusions
PSMA PET/CT is likely to be more costly than CT+WBBS or CT alone in Australia; however, it is still likely to be considered cost effective compared with conventional imaging.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-022-01156-4.
Key Points for Decision Makers
| Prostate-specific membrane antigen positron emission tomography combined with computed tomography is a new and more accurate method to detect prostate cancer. |
| Previous studies estimated that prostate-specific membrane antigen positron emission tomography/computed tomography is likely to be cost saving compared with conventional imaging. |
| We found that prostate-specific membrane antigen positron emission tomography/computed tomography is likely to be more costly than computed tomography plus a whole-body bone scan or computed tomography alone in Australia; however, it is still likely to be considered cost effective for patients with newly diagnosed, intermediate-risk or high-risk primary prostate cancer. |
Introduction
Prostate cancer is the third most common cancer in men worldwide [1]. In 2018, approximately 1.3 million men were diagnosed with prostate cancer worldwide, accounting for 13.5% of all male cancers [1]. For male deaths caused by cancer, prostate cancer ranked fifth (358,989 deaths, 3.8% of all cancer deaths) after lung, liver, stomach, and colorectal cancer [1]. Prostate cancer death rates have been decreasing because of early and improved management [1]. The overall 5-year survival rate is 95.9% in Australia, but it is only 36.4% for patients with metastatic disease [2].
Patients with newly diagnosed prostate cancer often require staging with diagnostic imaging to detect the extra-prostatic spread to pelvic nodal or distant metastatic sites. Accurate cancer staging before commencing treatment helps maximise treatment efficiency, reduces unnecessary adverse events (AEs) from treatment and ultimately improves patients’ quality of life and overall survival.
Patients are stratified into low-risk, intermediate-risk1 or high risk2 groups [3]. Guidelines recommend that high-risk patients undergo conventional diagnostic imaging with computed tomography (CT) or magnetic resonance imaging (MRI) and a whole-body bone scan (WBBS) [3–5]. A WBBS is used to detect bone metastasis, while CT and MRI assess if cancer has spread to lymph nodes or other organs (e.g. liver and lungs). Guidelines recommend that some intermediate-risk patients receive a CT/MRI+WBBS or CT/MRI alone [3–5]. However, conventional imaging has low sensitivity, particularly for detecting small-volume metastatic disease [6]. Prostate-specific membrane antigen positron emission tomography (PSMA PET) combined with CT has recently emerged as an imaging tool for prostate cancer [7]. The PSMA-specific radiotracers bind selectively to PSMA protein, which is often over-expressed in prostate cancer cells. Prostate-specific membrane antigen PET/CT for attenuation correction (removal of soft-tissue artifacts from CT images) and anatomical localisation (location of tumour sites) is more accurate than conventional imaging [8, 9].
Worldwide, governments increasingly require an economic evaluation to support decisions regarding the public subsidy of medical technologies [10]. This includes the Medical Services Advisory Committee (MSAC) in Australia, which advises the Australian Government on which medical services should be listed on the Medicare Benefits Schedule (MBS) and thus publicly subsidised [11].
The MSAC recently recommended the inclusion of PSMA PET/CT for patients with primary and biochemical recurrence (BCR) prostate cancer on the MBS [12]. However, there are several issues with the economic evaluation used to inform the decision. The economic evaluation compared PSMA PET/CT to a mixed comparator of CT and/or WBBS. The results may differ by whether the comparator is CT+WBBS or CT alone. Test accuracy estimates were based on a single randomised controlled trial (RCT), the ProPSMA trial, which compared 68-Ga-PSMA-11 PET/CT with CT+WBBS (N = 302) [9]. The test accuracy of CT+WBBS may differ from CT alone. Limited information regarding the model is publicly available, although it was reported that a hybrid decision tree-Markov model was used with a 10-year time horizon. Results were reported in terms of quality-adjusted life-years (QALYs); however, the MSAC queried the appropriateness of applying disutility values for incorrect diagnoses. Prostate-specific membrane antigen PET/CT compared to conventional imaging (CT and/or WBBS) for primary staging was estimated to yield cost savings of $312 per patient and 0.11 QALYs gained. The incremental cost-effectiveness ratio (ICER) increased to $489/QALY gained with a 30-year time horizon.
This study aimed to conduct a cost-utility analysis of PSMA PET/CT in patients with recently diagnosed, intermediate-risk or high-risk prostate cancer from the Australian healthcare perspective. Two comparators were considered, CT+WBBS and CT alone, to reflect differences in guidelines and explore whether the results differed by the comparator. The test accuracy of PSMA PET/CT was based on a systematic review and meta-analysis of 36 studies [13]. Other issues with the economic evaluation considered by the MSAC were also addressed.
Methods
Model Structure
A modelled economic evaluation was conducted, combining data from various sources. The model consisted of (1) a decision tree reflecting the proportion of patients with locoregional3 versus metastatic disease,4 testing accuracy of PSMA PET/CT and conventional imaging, and initial prostate cancer treatment; followed by (2) a Markov model reflecting the progression of prostate cancer over time following initial treatment. Costs and health outcomes occurring in the first month following diagnosis were estimated using the decision tree, while the long-term effects were estimated using the Markov model. The cycle length was 1 month. The model captured the impact of an inaccurate diagnosis on unnecessary treatments and delayed appropriate treatments on resource use and health outcomes. The model structure was informed by clinical guidelines [3–5, 14] and expert opinion.
The average patient was assumed to be aged 65 years and the time horizon of the analysis was the patient’s lifetime (proxied as 100 years), consistent with guidelines [11]. Fewer than 2% of patients were alive at the end of the model. Sensitivity to the time horizon was also explored. Following Australian guidelines, the analysis was conducted from the Australian healthcare system perspective, and costs, life-years (LYs) and QALYs were discounted by 5% [11]. The analysis was conducted in Microsoft Excel for Microsoft 365 [15].
Decision Tree
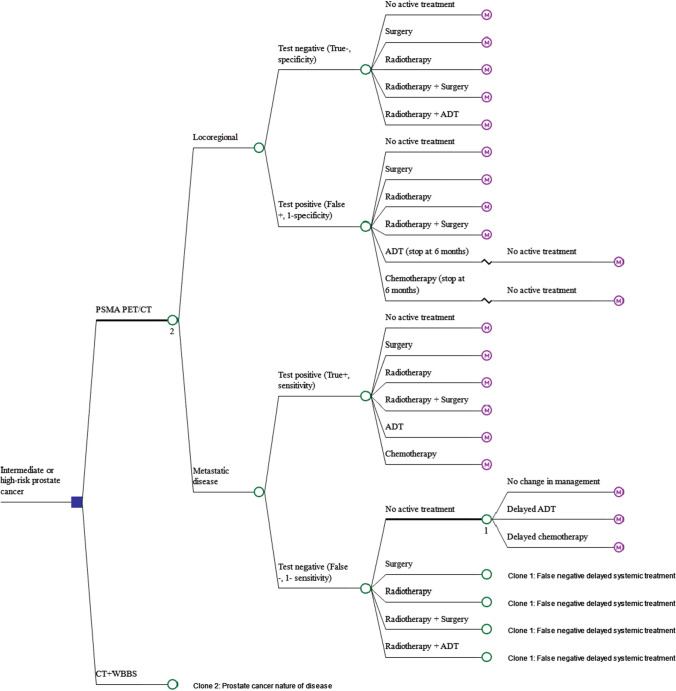
The decision-analytic model is presented in Fig. 1. It was assumed that all patients have already received prostate-specific antigen (PSA) testing and biopsy, and all patients have biopsy-proven prostate cancer. Patients then receive either PSMA PET/CT or conventional imaging. If no metastatic disease is detected (test negative), patients may receive no active treatment (e.g. active surveillance or watchful waiting) or may receive one of the following treatments: surgery; external beam radiotherapy or brachytherapy (radiotherapy); radiotherapy plus surgery; or radiotherapy plus androgen deprivation therapy (ADT). It was assumed that these patients receive no further treatment unless they are false negative, or experience BCR5 [16, 17]. If metastatic disease is detected (test positive), patients may receive no active treatment or localised treatment, including surgery, radiotherapy, radiotherapy plus surgery or systemic treatment, including ADT or chemotherapy. Patients with false-negative test results (truly metastatic disease) were assumed to receive similar treatment to those with true-negative test results and thus mainly receive localised treatment following initial diagnosis instead of systemic treatment. It was assumed that such patients would then be correctly diagnosed 24 months after the initial diagnosis because of the manifestation of symptomatic disease, after which they may receive delayed systemic treatment, including delayed ADT or delayed chemotherapy. The duration of delay was informed by studies of immediate versus delayed ADT treatment [18]. A scenario analysis was conducted to test this assumption. Patients with false-positive test results (truly locoregional disease) receive similar treatment to those with true-positive test results and thus may receive unnecessary systemic treatment following the initial diagnosis.
Fig. 1.
Decision tree model representing men with intermediate-risk/high-risk prostate cancer receiving initial diagnostic tests and initial treatments based on the cancer stages. ADT androgen deprivation therapy, CT computed tomography, PET positron emission tomography, PSMA prostate-specific membrane antigen, WBBS whole body bone scan
Markov Model
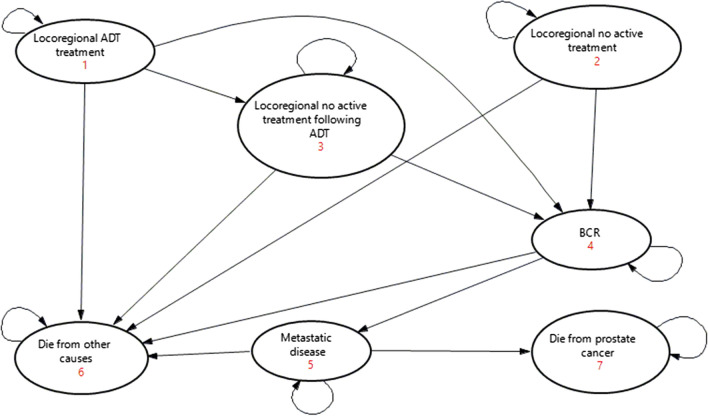
Patients enter one of 21 similarly structured Markov models (six for locoregional disease and 15 for metastatic disease) 1 month following the initial diagnosis. The Markov model structure is presented in Fig. 2. The Markov model included seven health states: (1) locoregional ADT treatment; (2) locoregional no active treatment; (3) locoregional no active treatment following ADT; (4) BCR; (5) metastatic disease; (6) died from other causes; and (7) died from prostate cancer.
Fig. 2.
Markov model representing the prostate cancer disease progression. ADT androgen deprivation therapy, BCR biochemical recurrence
Patients with locoregional disease receiving ADT in the initial treatment phase enter the locoregional ADT treatment health state (State 1). It was assumed that patients receive long-term ADT for 24 months [19, 20]. Patients then progress to the locoregional no active treatment phase following ADT (State 3). The remaining patients with locoregional disease enter the locoregional no active treatment health state (State 2). Patients remain in the above health states until they experience BCR (State 4) and then progress to metastatic disease (State 5). Patients with metastatic disease would enter the metastatic health state (State 5), even if they received a false-negative test result. Patients remain in this health state until they die from prostate cancer (State 7). Patients in all health states may also die from other causes (State 6).
Clinical Data
Diagnostic tests included PSMA PET/CT using PSMA PET/CT radiotracers (mainly 68-Ga-PSMA-11 and 18F-DCFPyL) and conventional imaging using CT+WBBS or CT alone. The test accuracies of PSMA PET/CT and CT alone were based on systematic reviews and meta-analyses of 36 and 24 studies, respectively [13, 21]. The test accuracy of CT+WBBS was based on the ProPSMA trial [9], as it was the only reliable source identified during the systematic review (see Table 1). The Australian Prostate Cancer Outcomes Registry informed the other parameters in the decision tree, including the proportion of patients with locoregional versus metastatic disease, and the initial and delayed treatments received (N = 13,336) [22, 23] (Table 1 of the Electronic Supplementary Material [ESM]). It was assumed that all patients receiving ADT for locoregional disease also receive radiotherapy, as per clinical guidelines [4, 5], and patients who do and do not receive ADT as an initial treatment would receive no active treatment and ADT when they experience BCR, respectively.
Table 1.
Test accuracy parameters
| Test accuracy | Parameter value (%) | SE (%) | Distribution | Source |
|---|---|---|---|---|
| PSMA PET/CT sensitivity | 57.0 | 3.4 | Beta | [13] |
| PSMA PET/CT specificity | 96.0 | 1.0 | Beta | [13] |
| CT+WBBS sensitivity | 38.0 | 7.2a | Beta | [9] |
| CT+WBBS specificity | 91.0 | 3.0a | Beta | [9] |
| CT sensitivity | 42.0 | 7.7 | Beta | [21] |
| CT specificity | 82.0 | 0.8 | Beta | [21] |
CT computed tomography, PET positron emission tomography, PSMA prostate-specific membrane antigen, SE standard error, WBBS whole body bone scan
aCalculated based on reported 95% confidence intervals
The transition probabilities applied in the Markov model depended on whether the patient truly had locoregional or metastatic disease and the initial treatment received. Sources were identified following a pragmatic literature review, with a preference for systematic reviews and meta-analyses, followed by RCTs and large cohort studies (Table 2 of the ESM). Monthly transition probabilities were estimated using the proportion of patients who had progressed at the follow-up using standard methods [24], which effectively assumes an exponential function. An increased risk of death was applied for false-negative patients with metastatic disease who receive delayed systemic treatment (hazard ratio = 1/0.690 = 1.449, 95% confidence interval [CI] 1/0.570, 1/0.840), based on a systematic review of early versus delayed ADT (N = 4767, median delay = 1.8–7 years, median follow-up = 5–13 years) [18]. It was assumed that delayed chemotherapy has a similar impact as delayed ADT.
AEs can lead to a poorer quality of life and increased treatment costs. False-positive patients who receive systemic treatment may experience additional unnecessary AEs. The most common AEs associated with localised treatments and ADT are urinary incontinence and bowel and erectile dysfunction [25]. The proportion of patients experiencing these AEs was based on a retrospective study (N = 2365) [25]. Other AEs included in the model were: (1) rectal bleeding due to radiotherapy [26–28]; (2) decreased bone density due to ADT [29]; and (3) grade 3 AEs with a greater than 1% occurrence with docetaxel (neutropenia, anaemia, infection, nausea, diarrhoea, and vomiting) [30] (Table 3 of the ESM). Other AEs were not explicitly modelled because of the costs being captured in the average hospitalisation cost (e.g. blood loss associated with surgery) or the low impact on costs or quality of life (e.g. hot flush, abnormal blood pressure or breast tenderness).
Table 3.
Costs experienced over 35 years per patient in the base case (discounted) [$A]
| Costs per patient over 35 years ($A) | PSMA PET/CT | Conventional imaging (CT+WBBS) | Conventional imaging (CT alone) | Difference | |
|---|---|---|---|---|---|
| PSMA PET/CT vs CT+WBBS | PSMA PET/CT vs CT alone | ||||
| No active treatment | $31 | $20 | $22 | $10 | $8 |
| Surgery | $207 | $138 | $151 | $69 | $56 |
| Radiotherapy | $334 | $222 | $243 | $112 | $91 |
| Radiotherapy + surgery | $0 | $0 | $0 | $0 | $0 |
| Radiotherapy + ADT | $957 | $636 | $694 | $321 | $263 |
| Chemotherapy | $578 | $384 | $419 | $194 | $158 |
| True-positive result (metastatic disease and test positive) | $2107 | $1400 | $1530 | $707 | $577 |
| No active treatment + no change in management | $42 | $60 | $55 | − 18 | − $14 |
| No active treatment + delayed ADT | $90 | $129 | $119 | − 39 | − 29 |
| No active treatment + delayed chemotherapy | $58 | $83 | $77 | − $25 | − 19 |
| Surgery + no change in management | $270 | $388 | $360 | − $118 | − $90 |
| Surgery + delayed ADT | $586 | $842 | $781 | − $257 | − $196 |
| Surgery + delayed chemotherapy | $365 | $524 | $487 | − $160 | − 122 |
| Radiotherapy + no change in management | $113 | $162 | $150 | − $49 | − $37 |
| Radiotherapy + delayed ADT | $242 | $347 | $322 | − $106 | − $80 |
| Radiotherapy + delayed chemotherapy | $152 | $219 | $203 | − $67 | − $51 |
| Radiotherapy + surgery + no change in management | $0 | $0 | $0 | $0 | $0 |
| Radiotherapy + surgery + delayed ADT | $0 | $0 | $0 | $0 | $0 |
| Radiotherapy + surgery + delayed chemotherapy | $0 | $0 | $0 | $0 | $0 |
| Radiotherapy +ADT | $66 | $95 | $88 | − $29 | − $22 |
| False-negative result (metastatic disease but test negative) | $1982 | $2850 | $2642 | − $868 | − $660 |
| No active treatment | $1917 | $1795 | $1549 | $123 | $368 |
| Surgery | $19,679 | $18,550 | $16,407 | $1129 | $3272 |
| Radiotherapy | $7582 | $7138 | $6287 | $444 | $1294 |
| Radiotherapy + surgery | $0 | $0 | $0 | $0 | $0 |
| Radiotherapy + ADT | $1097 | $1034 | $913 | $63 | $185 |
| True-negative result (locoregional and test negative) | $30,275 | $28,517 | $25,156 | $1758 | $5119 |
| No active treatment | $10 | $23 | $44 | − $13 | − $33 |
| Surgery | $110 | $246 | $482 | − $136 | − $373 |
| Radiotherapy | $163 | $364 | $711 | − $201 | − $548 |
| Radiotherapy + surgery | $0 | $0 | $0 | $0 | $0 |
| Radiotherapy + ADT | $473 | $1056 | $2055 | − $583 | − $1581 |
| Chemotherapy | $266 | $594 | $1155 | − $328 | − $889 |
| False-positive result (locoregional but test positive) | $1023 | $2283 | $4447 | − $1260 | − $3425 |
| Total cost per patient | $35,387 | $35,050 | $33,775 | $337 | $1612 |
A$ Australian Dollars, ADT androgen deprivation therapy, CT computed tomography, PET positron emission tomography, PSMA prostate-specific membrane antigen, WBBS whole body bone scan
Resource Use and Costs
The modelled costs included diagnostic tests, initial treatments, AEs and costs associated with disease progression. Costs were reported in 2021 Australian dollars (A$). Where required, the Australian Institute of Health and Welfare health price index was used to inflate costs to 2021 values [31] (Tables 4–6 of the ESM).
The cost per PSMA PET/CT, CT and WBBS procedure were based on the proposed or listed MBS fees [12, 32]. Radiotherapy and surgery costs were based on MBS fees and the Australian Public Hospitals Cost Report [32–35]. Drug costs were based on the Pharmaceutical Benefits Scheme [36]. Patients receiving chemotherapy were assumed to receive docetaxel for 26 weeks [30]. Costs to manage urinary incontinence and bowel and erectile dysfunction were estimated based on the published literature [37, 38], and the cost of sildenafil on the Pharmaceutical Benefits Scheme [36]. AE costs associated with hospitalisation, such as rectal bleeding and grade ≥ 3 docetaxel AEs (neutropenia, anaemia, infection, nausea, diarrhoea and vomiting), were calculated based on the Australian Public Hospitals Cost Report [35]. Costs in the disease progression phase were based on Cronin et al., which estimated the annual healthcare ongoing costs up to 9.5 years for several treatment pathways in using Australian administrative data (N = 1873) [39].
Model Inputs: Utilities
Total QALYs were estimated based on the sum of QALYs estimated in the decision tree and Markov model. QALYs were estimated depending on whether the patient truly had locoregional or metastasis disease, initial treatments and the disutilities associated with AEs.
Health-state utility values sources were selected from a published systematic review [40]. No Australian sources were identified, thus sources were selected with preferences given to studies with large sample sizes using a EuroQol 5 Dimension (EQ-5D) instrument. Utilities for prostate cancer health states (assuming no AEs) were based on a Finnish study (N = 630), which used the EuroQol 5 Dimension 3 Level (EQ-5D-3L) instrument [41]. Utilities for BCR were assumed to be equal to locoregional disease (more than 1.5 years after diagnosis). Utilities for locoregional disease were applied to patients who truly have locoregional disease, and utilities for metastatic disease were applied to patients who truly have metastatic disease, regardless of diagnosis. Disutilities for each health state were then estimated relative to the utility value for locoregional disease (Table 7 of the ESM). Disutilities for urinary, bowel and erectile dysfunction were based on a UK study (N = 316), which used the EuroQol 5 Dimension 5 Level (EQ-5D-5L) [42]. Disutilities for chemotherapy were based on a European study (N = 602) [43], which used the EQ-5D-3L (Table 7 of the ESM). Disutilities were applied for the health-state duration when the AE occurred. No additional disutilities were applied for patients undergoing the diagnostic tests, incorrect diagnoses, death from prostate cancer or death from other causes.
Analysis
The base-case ICER was calculated as cost per LY gained or QALY gained. A univariate sensitivity analysis was conducted using 95% CIs for all uncertain parameters.
Scenario analyses were conducted by selecting a parameter and changing the value to an alternative based on an alternative research study or scenario. In particular, a scenario analysis was conducted on an alternative source for test accuracy estimates. The ProPSMA trial (n = 295, PSMA PET/CT accuracy assessed in 145 patients) reported higher PSMA PET/CT sensitivity (0.85, 95% CI 0.74, 0.96) and specificity (0.98, 95% CI 0.95, 1.00) for PSMA PET/CT [9] compared with the base case used in this model (sensitivity 0.57, 95% CI 0.49, 0.64; specificity 0.96, 95% CI 0.95, 0.97), which were based on a meta-analysis of 36 studies (n = 3342 patients, including seven clinical trials) encompassing multiple clinical settings and different PSMA PET radiotracers and thus considered more generalisable [13].
Scenario analyses were also conducted on the parameters surrounding delayed systemic treatment for patients with false-negative results by (1) changing the duration of delay from 24 (base case) to six months or 48 months (assuming no change to mortality risk) and (2) assuming there is no impact on mortality risk with delayed treatment (hazard ratio = 1.00).
A scenario analysis was conducted on changing the source of the cost of PSMA PET/CT to a micro-costing study of PSMA PET/CT (A$1554.77) [44]. Finally, scenario analyses were conducted by changing the discount rate to 3% and 7% [11] and shortening the time horizon from 35 to 5 years.
A probabilistic sensitivity analysis involving 10,000 iterations was conducted to assess the overall parameter uncertainty [24]. Beta distributions were considered to represent the uncertainty in the estimates of diagnostic test sensitivity, specificity and probabilities of transitions between health states. Log-normal distributions were assumed to represent the uncertainty in the estimates of hazard rate ratios. Dirichlet and gamma distributions were assumed to represent the uncertainty in the estimates of treatment probabilities and cost parameters, respectively. 1-Gamma and -gamma distributions represented the uncertainty in the estimates of utilities and disutility, respectively. The Australian MSAC does not have an explicit threshold to assess whether investing in a new medical intervention is cost effective. Consequently, an implicit threshold of $50,000/QALY gained was used in this model to assess whether PSMA PET/CT was cost effective [45]. The results were illustrated using a cost-effectiveness acceptability curve.
Model Validation
The methods and reporting are consistent with the Consolidated Health Economic Evaluation Reporting Standards checklist [46] (see ESM). The model’s validity was assessed using the Assessment of the Validation Status of Health-Economic Decision Models Checklist [47]. Markov traces for both locoregional and metastatic cancer are presented in the ESM. Clinical experts reviewed the assumptions, model structure and results. The model was also validated against Australian prostate cancer survival data [2]. Independent checks were undertaken in the Excel sheets to detect any coding errors.
Results
Base Case
Using PSMA PET/CT to detect the extent of cancer spread for patients with newly diagnosed, primary prostate cancer compared with CT+WBBS would result in 0.011 additional true-positive cases, 0.047 true-negative cases per patient tested, 0.001 LYs (discounted) gained and 0.008 QALYs (discounted) gained and would cost A$337 more per patient over 35 years (see Tables 2 and 3). The additional costs were mainly due to a higher unit cost with PSMA PET/CT and more patients receiving a true-negative test result and thus surgery. Overall, the deterministic ICER was $21,147/QALY gained for patients who undergo PSMA PET/CT compared with CT+WBBS (see Table 4).
Table 2.
Health outcomes experienced over 35 years per patient in the base case (discounted)
| PSMA PET/CT | Conventional imaging (CT+WBBS) | Conventional imaging (CT alone) | Difference | ||
|---|---|---|---|---|---|
| PSMA PET/CT vs CT+WBBS | PSMA PET/CT vs CT alone | ||||
| True-positive cases detected (metastatic disease and test positive) | 0.034 | 0.023 | 0.025 | 0.011 | 0.009 |
| False-negative cases detected (metastatic disease but test negative) | 0.026 | 0.037 | 0.035 | − 0.011 | − 0.009 |
| True-negative cases detected (locoregional and test negative) | 0.903 | 0.856 | 0.771 | 0.047 | 0.132 |
| False-positive cases detected (locoregional but test positive) | 0.038 | 0.085 | 0.169 | − 0.047 | − 0.132 |
| Total cases | 1.000 | 1.000 | 1.000 | 0.000 | 0.000 |
| LYs experienced by patients with true-positive result | 0.118 | 0.079 | 0.087 | 0.039 | 0.031 |
| LYs experienced by patients with false-negative result | 0.095 | 0.138 | 0.129 | − 0.042 | − 0.033 |
| LYs experienced by patients with true-negative result | 10.376 | 9.836 | 8.863 | 0.540 | 1.513 |
| LYs experienced by patients with false-positive result | 0.429 | 0.966 | 1.932 | − 0.537 | − 1.503 |
| Total LYs gained over the 35 years | 11.019 | 11.018 | 11.011 | 0.001 | 0.008 |
| QALYs experienced by patients with true-positive result | 0.075 | 0.050 | 0.055 | 0.025 | 0.020 |
| QALYs experienced by patients with false-negative result | 0.057 | 0.082 | 0.076 | − 0.025 | − 0.020 |
| QALYs experienced by patients with true-negative result | 7.903 | 7.492 | 6.751 | 0.412 | 1.153 |
| QALYs experienced by patients with false-positive result | 0.317 | 0.712 | 1.425 | − 0.396 | − 1.108 |
| Total QALYs gained over the 35 years | 8.352 | 8.336 | 8.307 | 0.016 | 0.044 |
CT computed tomography, LYs life-years, PET positron emission tomography, PSMA prostate-specific membrane antigen, QALYs quality-adjusted life-years, WBBS whole body bone scan
Table 4.
Deterministic and probabilistic incremental cost-effectiveness ratios (ICERs) in the base case
| PSMA PET/CT | CT+WBBS | CT alone | Difference | ||
|---|---|---|---|---|---|
| PSMA PET/CT vs CT+WBBS | PSMA PET/CT vs CT alone | ||||
| Base-case results (deterministic) | |||||
| Total QALYs gained per patient | 8.352 | 8.336 | 8.307 | 0.016 | 0.044 |
| Total costs per patient (A$) | $35,387 | $35,050 | $33,775 | $337 | $1612 |
| ICER (A$ per QALY gained) | – | – | $21,147 | $36,231 | |
| Base-case results (probabilistic) | |||||
| Total QALYs gained per patient (95% CI) | 8.361 (7.891, 8.779) | 8.345 (7.868, 8.764) | 8.316 (7.836, 8.739) | 0.016 (− 0.002, 0.040) | 0.044 (0.029, 0.062) |
| Total costs per patient [A$] (95% CI) | $35,350 ($27,043, $48,545) | $35,008 ($26,668, $48,123) | $33,733 ($25,404, $46,890) | $342 (− $40, $918) | $1617 ($874, $2228) |
| ICER [A$ per QALY gained] (95% CI)a | – | – | – | $33,836 (− $14,280, $71,865) | $37,649 ($18,629, $59,745) |
A$ Australian Dollars, CI confidence interval, CT computed tomography, PET positron emission tomography, PSMA prostate-specific membrane antigen, QALYs quality-adjusted life-years, WBBS whole body bone scan
aThe difference in deterministic and probabilistic ICERs is because as the incremental effect approaches zero the ICER moves towards infinity, which biases the average probabilistic ICER upwards
The deterministic ICER increased when PSMA PET/CT was compared to CT alone. In this case, PSMA PET/CT would detect additional 0.09 true-positive cases and 0.132 true-negative cases, 0.008 LYs gained and 0.044 QALYs gained and would cost $1612 more than CT alone. Consequently, the ICER increased to $36,231/QALY gained. This was because the unit cost of CT alone was lower than CT+WBBS, and CT alone was estimated to have a higher sensitivity than CT+WBBS [21] (see Tables 2, 3 and 4).
Univariate Sensitivity Analysis
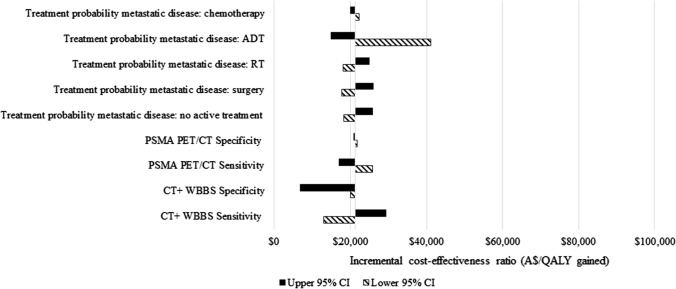
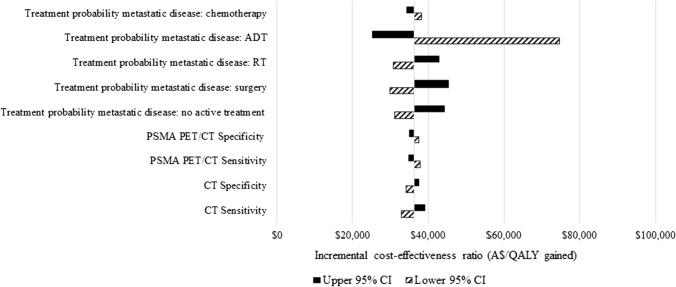
A univariate sensitivity analysis indicated that results were most sensitive to the initial treatments received by patients diagnosed with metastatic cancer, especially ADT (Figs. 3 and 4 and the ESM). This was because patients with metastatic disease who only received surgery had a higher death rate [48–50]. The results were robust to all other parameters.
Fig. 3.
Univariate sensitivity analyses of prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) versus CT + whole body bone scan (WBBS): most sensitive parameters. A$ Australian Dollars, ADT androgen deprivation therapy, CI confidence interval, QALY quality-adjusted life-year, RT radiotherapy
Fig. 4.
Univariate sensitivity analyses of prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) vs CT alone: most sensitive parameters. ADT androgen deprivation therapy, A$ Australian Dollars, CI confidence interval, QALY quality-adjusted life-year, RT radiotherapy, WBBS whole body bone scan
Scenario Analysis
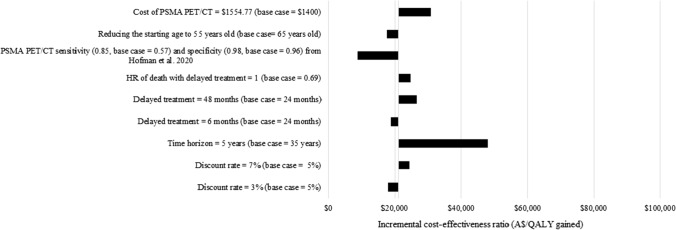
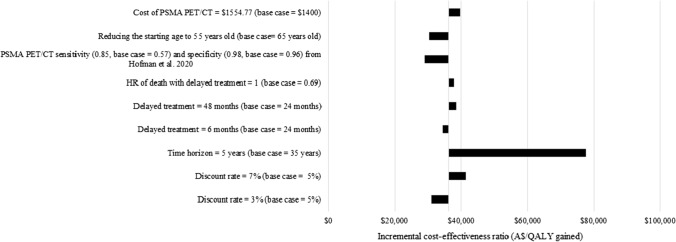
A scenario analysis found that the ICERs were most sensitive to shortening the time horizon from 35 to 5 years, resulting in the ICER increasing to $47,927/QALY gained versus CT+WBBS and $77,496/QALY gained versus CT alone (see Figs. 5 and 6). The ICERs increased to $26,535/QALY gained versus CT+WBBS and $38,541/QALY gained versus CT alone when the duration of delay of systemic treatment time frame increased from 24 to 48 months. The ICERs also increased to $30,858/QALY gained versus CT+WBBS and $39,710/QALY gained versus CT alone when the cost of PSMA PET/CT was increased to $1554.77, based on a micro-costing study [44]. Finally, the ICERs increased to $24,714/QALY gained versus CT+WBBS and $37,797/QALY gained versus CT alone if there was no additional risk of death with delayed systemic treatment. Conversely, when using estimates of test accuracy for both PSMA PET/CT and CT+WBBS from the ProPSMA trial [9], the ICER decreased to $8963/QALY gained versus CT+WBBS and $28,965/QALY gained versus CT alone. The results were not sensitive to the discount rate or the patients’ age.
Fig. 5.
Scenario analyses of prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) vs CT + whole body bone scan (WBBS). A$ Australian Dollars, ADT androgen deprivation therapy, HR hazard ratio, QALY quality-adjusted life-year
Fig. 6.
Scenario analyses of prostate-specific membrane antigen (PSMA) positron emission tomography computed tomography (PET/CT) vs CT alone. A$ Australian Dollars, ADT androgen deprivation therapy, HR hazard ratio, QALY quality-adjusted life-year
Probabilistic Sensitivity Analysis
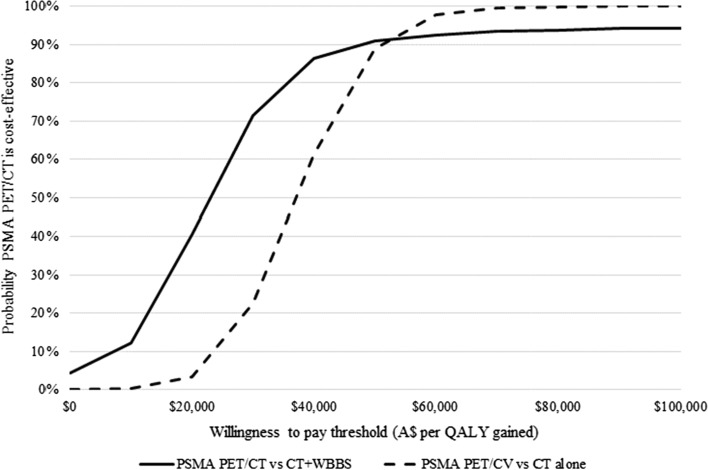
The probabilistic sensitivity analysis indicated that using PSMA PET/CT compared to CT+WBBS and CT alone for staging primary prostate cancer had an estimated 91% and 89% probability of being cost effective at a threshold of AU$50,000/QALY gained, respectively (see Fig. 7).
Fig. 7.
Incremental cost-effectiveness acceptability curve (10,000 iterations). A$ Australian Dollars, CT computed tomography, PET positron emission tomography, PSMA prostate-specific membrane antigen, QALY quality-adjusted life-year, WBBS whole body bone scan
Model Validation
In Australia, the observed 5-year overall survival rate for locoregional prostate cancer is at least 98.6% [2], similar to the results generated by the model (99.7%). The observed 5-year overall survival rate for metastatic cancer is 36.4% (95% CI 32.7, 40.2) [2], higher than the results generated by the model (31.2%). However, the former would include patients with false-positive test results.
Discussion
PSMA PET/CT would likely be considered cost effective compared with both CT+WBBS and CT alone, using an implicit cost-effectiveness threshold of $50,000/QALY gained. While the difference in sensitivity between PSMA PET/CT and CT+WBBS (57.0% vs 38.0%) was greater than the difference in specificity (96.0% vs 91.0%), true-negative cases increased more than true-positive cases using PSMA PET/CT because patients were more likely to have truly locoregional disease (94.0%). Health outcomes were mainly gained through PSMA PET/CT identifying more true-negative patients, who subsequently avoid unnecessary systemic treatments and thus AEs. Consequently, the QALYs gained through PSMA PET/CT were larger than the LYs gained. The results were most sensitive to the time horizon and the initial treatment received by patients diagnosed with metastatic cancer but were reasonably robust to all other parameters, including the estimates of the accuracy of PSMA PET/CT.
This is the first detailed published cost-utility analysis of PSMA PET/CT compared with conventional imaging (CT+WBBS or CT alone) in patients with newly diagnosed intermediate-risk or high-risk prostate cancer. Recent published economic evaluations have been conducted in patients with BCR [51, 52], comparing PSMA PET to other comparators (i.e. MRI or extended pelvic lymph node dissection, ePLND) [51, 53], or reported just costs, cost per accurate diagnosis or LY gained [51, 52, 54]. A cost-utility analysis was also considered by the MSAC when considering the inclusion of PSMA PET/CT for patients with both primary and BCR prostate cancer on the MBS. However, details regarding the inputs and methods are limited [12].
The model structure presented in this study was similar to that used in Gordon et al. and Scholte et al., which also used a decision tree with a Markov model representing prostate cancer progression [51, 53]. The Markov model health states in this study were similar to Scholte et al. [53]. Understandably, the Markov model in Gordon et al. included fewer health states as it modelled patients with BCR alone [51]. The complete structure of the model considered by the MSAC is unknown; however, the Markov model health states were similar to this study (local disease, pelvic nodal disease, distant metastases, prostate cancer death, natural death) [12].
Our study included a broader range of initial prostate cancer treatments than Gordon et al. and Scholte et al. [51] [53]. Our study also assumed that false-negative patients receive delayed systemic treatments rather than no treatment, and no disutilities were applied for incorrect diagnoses, which were more conservative assumptions. The initial prostate cancer treatments included in the model considered by the MSAC are unknown [12].
Gordon et al. estimated patients experienced 7.48–7.41 LYs, and that 68-Ga‑PSMA-11 PET/MRI resulted in 0.069 LYs gained compared with usual care [51]. Our study also estimated that patients experienced 11.001–11.019 LYs, but PSMA PET/CT resulted in only 0.001–0.008 LYs gained compared with conventional imaging. The difference in LYs experienced is likely owing to the different patient populations in the two studies, BCR versus primary disease. In contrast, Scholte et al. estimated that 68-Ga‑PSMA-11 PET/CT might result in QALYs lost compared with ePLND [53]. This is because Scholte et al. assumed that the sensitivity of ePLND was 100% and did not result in any AEs, despite noting that ePLND is invasive and is associated with up to 20% chance of complications, mostly lymphoceles [53]. The model considered by the MSAC estimated that 0.11 QALYs would be gained by PSMA PET/CT compared to conventional imaging (CT and/or WBBS), which is higher than this study (0.008–0.044 QALYs) [12]. The difference in QALYs gained may be due to the application of disutility values for incorrect diagnoses. LYs gained were not reported.
De Feria Cardet et al. conducted a within-trial analysis of the ProPSMA trial without modelling [54]. However, they did not include the impact on clinical management and thus did not estimate the impact on long-term costs or quality of life, limiting comparability with this study. Schwenck et al. also conducted a within-trial analysis of a registry database [52]. While they included the impact on clinical management and costs, they did not estimate the impact on long-term quality of life, limiting comparability with this study.
Gordon et al., Scholte et al. and de Feria Cardet et al. and the model considered by the MSAC estimated that 68-Ga-PSMA-11 PET or PSMA PET/CT more generally would be cost saving [12, 51, 53, 54], and Schwenck et al. estimated that 68-Ga-PSMA PET would be “cost effective” (i.e. cost saving) if additional costs do not exceed €3844 (US$4312) [52]. In contrast, this study estimated the cost of PSMA PET/CT to be higher than conventional imaging, although it is still likely to be considered cost effective. One reason for the difference is that our study applied a higher cost per PSMA PET/CT procedure (A$1400) than Gordon et al. (A$1000 per procedure, based on expert opinion) and de Feria Cadet et al. (A$1140 per procedure, based on one facility) [51, 54]. While our study applied the same cost per PSMA PET/CT procedure (A$1400) as the model considered by the MSAC[ 12], our study did not include the cost of repeat imaging for equivocal results, which was assumed to be higher with conventional imaging. Another reason for the difference is that our study assumed that false-negative patients receive delayed systemic treatments, minimising cost savings from treatments avoided. Finally, our study considered two comparators (CT+WBBS and CT alone) separately, rather than CT+WBBS only or a mixed comparator [12, 54].
A scenario analysis involving applying test accuracy estimates entirely from the ProPSMA trial reduced the ICER significantly (see Figs. 4 and 5). This supports the conclusion by De Feria Cardet et al. that PSMA PET/CT is likely to be cost effective when based on this trial, although it was still not cost saving.
The key strengths of this research are that more than one comparator (CT+WBBS or CT alone) was considered. This study also based estimates of PSMA PET/CT test accuracy on a comprehensive meta-analysis [13] rather than a single RCT [9, 12, 54] or a single-arm study [51]. In addition, in a scenario analysis, PSMA PET/CT costs were based on a detailed micro-costing study of several Australian facilities, using different radiotracers located in metropolitan and regional areas [44]. Finally, it was conservatively assumed that patients with a false-negative result receive delayed systemic treatment rather than applying a disutility for incorrect diagnoses.
This research is not without limitations. First, estimates of CT+WBBS test accuracy were based on a single RCT [9], and test accuracy estimates of PSMA PET/CT and CT alone were from separate meta-analyses using histopathology as the reference standard. Future research using a network meta-analysis may be informative. Second, it was assumed that patients with a false-negative result would receive delayed systemic treatment after 24 months. It was also assumed that delayed chemotherapy has a similar impact to delayed ADT. Further research is needed to confirm these assumptions. Third, it was assumed for simplicity that patients with locoregional disease only receive one initial treatment unless they are false negative and subsequently receive delayed systemic treatment, or experience BCR. Patients may receive additional treatments for other reasons (e.g. patients receiving active surveillance may change their preferences). Fourth, monthly transition probabilities were estimated assuming an exponential function, which may not be accurate. However, the results were not sensitive to any transition probabilities (see Fig. 2 of the ESM). Fifth, cost-effectiveness results were reported for PSMA PET/CT as a whole rather than for specific radiotracers. However, evidence suggests no significant difference in testing accuracy by radiotracer type [13, 55], and the micro-costing study did not report costs by radiotracer type [44]. Finally, this study was based on a modelling approach combining data from various sources to extrapolate health outcomes. The accuracy of this approach could be further validated through a longer term follow-up and collecting utility data from patients participating in an RCT of PSMA PET/CT compared to conventional imaging, such as the ProPSMA study [9]. Therefore, routine collection of utility data in future RCTs of PSMA PET/CT is recommended.
Conclusions
Economic considerations are increasingly important as healthcare resources are stretched because of increased demand and resource scarcity. Previous studies estimating that PSMA PET/CT is cost saving would thus be of particular interest to policymakers. However, this study estimated that PSMA PET/CT is likely to be more costly than conventional imaging and particularly more costly than CT alone. Thus funding PSMA PET/CT will increase the strain on the healthcare budget. That said, an economic evaluation is about ensuring healthcare services are value for money and are not about saving money. While PSMA PET/CT is unlikely to be cost saving, it is likely to be considered cost effective, regardless of the comparator and even after applying conservative assumptions. These results may be generalisable to countries with similar healthcare systems [56], and extensive details of the parameters applied in the model are presented in the ESM to maximise transferability[57].
This study focused on newly diagnosed patients with intermediate-risk or high-risk prostate cancer. Prostate-specific membrane antigen PET/CT is also reported to have a higher test accuracy for patients with prostate cancer with BCR [13]. However, further research is needed to assess the cost effectiveness of PSMA/CT in patients with BCR.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Rob Ware from Cyclotek (Aust) Pty Ltd for feedback regarding the conceptual model. We also thank the attendees at the 42nd Australian Health Economics Society Conference (21–22 September, 2021) and the anonymous reviewers for their valuable suggestions.
Declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Financial support for this study was provided by a Cooperative Research Centres Projects grant from the Australian Government. Cyclotek (Aust) Pty Ltd was a co-investigator on the project. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the report.
Conflicts of interest/Competing interests
The study authors are members of an independent evaluation group for the Medical Services Advisory Committee. The Macquarie University Centre for the Health Economy receives funding for conducting evaluations of medical services and diagnostics for the Medical Services Advisory Committee. The views expressed in the paper are not those of the Commonwealth Government of Australia.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All model inputs and model outputs are available within the main text or supplementary material. The economic model was developed in Microsoft Excel and is available upon request.
Code availability
Not applicable.
Authors’ contributions
BP conceived the idea for the study, and BP and MH managed the study. RS, BP and VJ developed the conceptual economic model. RS and BP built the model and conducted the economic analysis. All authors were involved in interpreting the results and revising the final manuscript.
Footnotes
Intermediate risk: no high-risk group features; no very high-risk group features; has one or more intermediate-risk factors: T2b–T2c; Grade Group 2 or 3; PSA 10–20 ng/mL.
High risk: has no very high-risk features and has exactly one high-risk feature: T3a or, Grade Group 4 or Grade Group 5 or PSA > 20 ng/mL. Very high risk: has at least one of the following: T3b–T4; primary Gleason pattern 5; 2 or 3 high-risk features; > 4 cores with Grade Group 4 or 5.
Clinically localised disease risk group include very low-risk, low-risk, intermediate-risk and high-risk groups, while locally advanced disease includes a very high-risk group (NCCN guidelines version 2.2021 Prostate Cancer).
Metastasis includes lymph node metastasis and distant metastasis (NCCN guidelines version 2.2021 Prostate Cancer).
A PSA increase of ≥ 0.2 ng/mL after radical prostatectomy, or ≥ 2.0 ng/mL 6 weeks after radiation therapy, on at least two consecutive occasions.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Australia. Relative survival by stage at diagnosis (prostate cancer). 2019. https://ncci.canceraustralia.gov.au/outcomes/relative-survival-rate/relative-survival-stage-diagnosis-prostate-cancer. Accessed 1 Jun 2022.
- 3.Schaeffer E, et al. NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Canc Netw. 2021;19(2):134–143. doi: 10.6004/jnccn.2021.0008. [DOI] [PubMed] [Google Scholar]
- 4.Sanda MG, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199(3):683–690. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 5.Bekelman JE, et al. Clinically localized prostate cancer: ASCO clinical practice guideline endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology guideline. J Clin Oncol. 2018;36(32):3251–3258. doi: 10.1200/JCO.18.00606. [DOI] [PubMed] [Google Scholar]
- 6.Wallitt KL, et al. Clinical PET imaging in prostate cancer. Radiographics. 2017;37(5):1512–1536. doi: 10.1148/rg.2017170035. [DOI] [PubMed] [Google Scholar]
- 7.Rayn KN, Elnabawi YA, Sheth N. Clinical implications of PET/CT in prostate cancer management. Transl Androl Urol. 2018;7(5):844–854. doi: 10.21037/tau.2018.08.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fendler WP, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5(6):856–863. doi: 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofman MS, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 10.International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world. 2018. https://www.ispor.org/PEguidelines/. Accessed 26 Jul 2018.
- 11.Medical Services Advisory Committee . Technical guidelines for preparing assessment reports for the Medical Services Advisory Committee: medical service type: investigative (version 3.0) Canberra (ACT): Australian Government Department of Health; 2017. [Google Scholar]
- 12.Medical Services Advisory Committee . Public summary document: application 1632: PSMA PET/CT imaging for informing treatment of patients with prostate cancer. Canberra (ACT): Australian Government Department of Health; 2021. [Google Scholar]
- 13.Jeet V, et al. Histopathologically validated diagnostic accuracy of PSMA PET/CT in the primary and secondary staging of prostate cancer and its impact on clinical management: a systematic review and meta-analysis. Unpublished. [DOI] [PubMed]
- 14.Sanda MG, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199(4):990–997. doi: 10.1016/j.juro.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Microsoft Corporation. Microsoft Excel (version 365). 2021. https://office.microsoft.com/excel. Accessed 1 Jun 2022.
- 16.Cookson MS, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177(2):540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 17.Roach M, 3rd, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Kunath F, et al. Early versus deferred standard androgen suppression therapy for advanced hormone-sensitive prostate cancer. Cochrane Database Syst Rev. 2019;6(6):CD003506. doi: 10.1002/14651858.CD003506.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolla M, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz EM, et al. Ten-year follow-up of radiation therapy oncology group protocol 92–02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26(15):2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 21.Hövels AM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Evans SM, et al. Prostate Cancer Outcomes Registry-Australia and New Zealand Report 2018: reporting on data 2015–2016. 2018. Melbourne: Monash University & The Movember Foundation; 2019. [Google Scholar]
- 23.Wang LL, et al. Patterns of care and outcomes for men diagnosed with prostate cancer in Victoria: an update. ANZ J Surg. 2018;88(10):1037–1042. doi: 10.1111/ans.14722. [DOI] [PubMed] [Google Scholar]
- 24.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 25.Hoffman RM, et al. Patient satisfaction with treatment decisions for clinically localized prostate carcinoma: results from the Prostate Cancer Outcomes Study. Cancer. 2003;97(7):1653–1662. doi: 10.1002/cncr.11233. [DOI] [PubMed] [Google Scholar]
- 26.Yeoh EE, et al. Evidence for efficacy without increased toxicity of hypofractionated radiotherapy for prostate carcinoma: early results of a phase III randomized trial. Int J Radiat Oncol Biol Phys. 2003;55(4):943–955. doi: 10.1016/S0360-3016(02)04146-9. [DOI] [PubMed] [Google Scholar]
- 27.D'Amico AV, et al. 6-Month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 28.Wilt TJ, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen PL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–836. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Therapeutic Goods Administration . Australian product information: docetaxel concentrated injection (docetaxel) Canberra (ACT): Therapeutic Goods Administration; 2020. [Google Scholar]
- 31.Australian Institute of Health and Welfare . Health expenditure Australia 2017–18, AIHW cat .no. HWE 67. Canberra (ACT): Australian Institute of Health and Welfare; 2020. [Google Scholar]
- 32.Australian Government Department of Health . Medicare benefits schedule book operating from 1 March 2021. Canberra (ACT): Department of Health; 2021. [Google Scholar]
- 33.Australian Government Department of Health and Ageing, Medical Services Advisory Committee. Application 1089: assessment report: review of interim funded service: brachytherapy for the treatment of prostate cancer. Canberra (ACT): Medical Services Advisory Committee Department of Health and Ageing; 2010.
- 34.Australian Government Department of Health, Medical Services Advisory Committee. Application No. 1158: robotic image-guided stereotactic precise beam radiosurgery and radiotherapy for lung cancer and prostate cancer. Canberra (ACT): Department of Health; 2012.
- 35.Independent Hospital Pricing Authority . National hospital cost data collection, public hospitals cost report, round 20, financial year 2015–16. Independent Hospital Pricing Authority; 2018. [Google Scholar]
- 36.Australian Government Department of Health . Schedule of pharmaceutical benefits, effective 1 March 2021. Canberra (ACT): Department of Health; 2021. [Google Scholar]
- 37.Deloitte Access Economics . The economic impact of incontinence in Australia. Deloitte Access Economics; 2011. [Google Scholar]
- 38.Nyrop KA, et al. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007;26(2):237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- 39.Cronin P, et al. Long-term health care costs for patients with prostate cancer: a population-wide longitudinal study in New South Wales, Australia. Asia Pac J Clin Oncol. 2017;13(3):160–171. doi: 10.1111/ajco.12582. [DOI] [PubMed] [Google Scholar]
- 40.Magnus A, et al. A systematic review and meta-analysis of prostate cancer utility values of patients and partners between 2007 and 2016. MDM Policy Pract. 2019;4(1):2381468319852332. doi: 10.1177/2381468319852332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torvinen S, et al. Health-related quality of life in prostate cancer. Acta Oncol. 2013;52(6):1094–1101. doi: 10.3109/0284186X.2012.760848. [DOI] [PubMed] [Google Scholar]
- 42.Watson E, et al. Symptoms, unmet needs, psychological well-being and health status in survivors of prostate cancer: implications for redesigning follow-up. BJU Int. 2016;117(6b):E10–E19. doi: 10.1111/bju.13122. [DOI] [PubMed] [Google Scholar]
- 43.Diels J, et al. Mapping FACT-P to EQ-5D in a large cross-sectional study of metastatic castration-resistant prostate cancer patients. Qual Life Res. 2015;24(3):591–598. doi: 10.1007/s11136-014-0794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parkinson B, et al. Micro-costing study of prostate specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT). Unpublished.
- 45.Wang S, Gum D, Merlin T. Comparing the ICERs in medicine reimbursement submissions to NICE and PBAC: dose the presence of an explicit threshold affect the ICER proposed? Value Health. 2018;21(8):938–943. doi: 10.1016/j.jval.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Husereau D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. doi: 10.1016/j.jval.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Vemer P, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–361. doi: 10.1007/s40273-015-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65(6):1058–1066. doi: 10.1016/j.eururo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Rusthoven CG, et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol. 2016;34(24):2835–2842. doi: 10.1200/JCO.2016.67.4788. [DOI] [PubMed] [Google Scholar]
- 50.Tilki D, et al. Local treatment for metastatic prostate cancer: a systematic review. Int J Urol. 2018;25(5):390–403. doi: 10.1111/iju.13535. [DOI] [PubMed] [Google Scholar]
- 51.Gordon LG, et al. Exploratory cost-effectiveness analysis of (68)Gallium-PSMA PET/MRI-based imaging in patients with biochemical recurrence of prostate cancer. Clin Exp Metastasis. 2020;37(2):305–312. doi: 10.1007/s10585-020-10027-1. [DOI] [PubMed] [Google Scholar]
- 52.Schwenck J, et al. Intention-to-treat analysis of (68)Ga-PSMA and (11)C-Choline PET/CT versus CT for prostate cancer recurrence after surgery. J Nucl Med. 2019;60(10):1359–1365. doi: 10.2967/jnumed.118.224543. [DOI] [PubMed] [Google Scholar]
- 53.Scholte M, et al. Modelling study with an interactive model assessing the cost-effectiveness of (68)Ga prostate-specific membrane antigen positron emission tomography/computed tomography and nano magnetic resonance imaging for the detection of pelvic lymph node metastases in patients with primary prostate cancer. Eur Urol Focus. 2020;6(5):967–974. doi: 10.1016/j.euf.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 54.de Feria Cardet RE, et al. Is prostate-specific membrane antigen positron emission tomography/computed tomography imaging cost-effective in prostate cancer: an analysis informed by the proPSMA Trial. Eur Urol. 2021;79(3):413–418. doi: 10.1016/j.eururo.2020.11.043. [DOI] [PubMed] [Google Scholar]
- 55.Kuten J, et al. Head-to-head comparison of (68)Ga-PSMA-11 with (18)F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J Nucl Med. 2020;61(4):527–532. doi: 10.2967/jnumed.119.234187. [DOI] [PubMed] [Google Scholar]
- 56.Boehler CE, Lord J. Mind the gap! A multilevel analysis of factors related to variation in published cost-effectiveness estimates within and between countries. Med Decis Making. 2016;36(1):31–47. doi: 10.1177/0272989X15579173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boulenger S, et al. Can economic evaluations be made more transferable? Eur J Health Econ. 2005;6(4):334–346. doi: 10.1007/s10198-005-0322-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.