Abstract
Background
The global prevalence of non-alcoholic steatohepatitis (NASH) is increasing, such that NASH is predicted to become the leading cause of liver transplantation (LT) in the US by 2025. Despite this, data on the economic burden of NASH are limited.
Objectives
This systematic literature review aimed to summarise and critically evaluate studies reporting on the economic burden of NASH and identify evidence gaps for subsequent research.
Methods
Medline, EMBASE, the Cochrane Library and EconLit were searched up to 6 January 2021 for English language articles published from January 2010 to January 2021 inclusive that reported economic outcomes of a NASH population or subpopulation. Evidence was presented and synthesised using narrative data analysis, and quality was assessed by two reviewers using an 11-item checklist developed for economic evaluations and adapted to cost of illness.
Results
Fourteen studies were included, of which five presented data on costs and resource use, four on costs only and five on resource use only. Overall, NASH is associated with a significant and increasing economic burden in terms of healthcare resource utilisation (HCRU) and direct and indirect costs. This burden was higher among NASH patients with advanced (fibrosis stage 3–4) versus early (fibrosis stage 0–2) disease, symptomatic versus asymptomatic disease and for patients with complications or comorbidities versus those without. In LT patients, those with NASH as the primary indication had greater HCRU and higher costs compared with non-NASH indications such as hepatitis B and C viruses. Considerable variability in HCRU and costs was seen across the US and Europe, with the highest costs seen in the US. The quality of the included studies was variable, and the studies themselves were heterogeneous in terms of study methodology, patient populations, comorbidities, follow-up time and outcomes measured.
Conclusions
This review highlights a general scarcity of NASH-specific economic outcomes data. Despite this, the identified studies show that NASH is associated with a significant economic burden in terms of increased HCRU, and high direct medical and non-medical costs and societal burden that increases with disease severity or when patients have complications or comorbidity. More national-level NASH prevalence data are needed to generate accurate forecasts of HCRU and costs in the coming decades.
Funding
Novo Nordisk A/S, Søborg, Denmark.
Video Abstract
The economic burden of non-alcoholic steatohepatitis: a video abstract (MP4 12242 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-022-01140-y.
Plain Language Summary
It is important to know the cost of treating different diseases because this helps to guide how healthcare resources and funds are used. Non-alcoholic steatohepatitis (NASH) is a serious liver disease that can lead to liver scarring (cirrhosis), liver transplantation and early death, and the number of people with NASH is growing around the world. Fourteen studies published over the past 10 years have investigated the costs of treating patients with NASH. Patients with NASH generally use more healthcare services with a higher cost than the general population or patients with type 2 diabetes. In people with more serious liver disease, such as liver transplant patients, NASH tends to be more expensive and use more healthcare services than other serious liver diseases such as hepatitis. Costs and use of health services are particularly high in patients with more severe NASH, or those who have other diseases or complications in addition to NASH (such as type 2 diabetes or kidney failure).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-022-01140-y.
Key Points for Decision Makers
| NASH is associated with a substantial economic burden in terms of healthcare resource utilisation, medical, non-medical and indirect costs, that increase with disease severity or when complications or comorbidity are present. |
| Available evidence on indirect costs (e.g. productivity losses, informal care) is scarce, but the limited reports suggest these may outweigh direct costs. Further research is justified to fully appreciate the impact of indirect and societal costs on the economic burden of NASH. |
| Studies evaluating economic outcomes were heterogeneous in terms of patient populations, comorbidities, follow-up time and outcomes measured, limiting comparability of studies, and varied in the quality of their analyses. More precise national-level prevalence data are needed for accurate forecasts of healthcare resource utilisation and costs in the coming decades. |
Introduction
Non-alcoholic fatty liver disease (NAFLD) comprises a spectrum of related liver disorders, ranging from non-alcoholic fatty liver (NAFL; simple steatosis) to non-alcoholic steatohepatitis (NASH) [1]. NAFLD is the hepatic manifestation of the metabolic syndrome (MetS) and is closely linked with key components of MetS including central obesity [2]. Obesity is associated with a 10-fold increase in the risk of NAFLD [3]. Fuelled by increasing rates of obesity and other key components of MetS, the prevalence of NAFLD is growing rapidly [3–7], with an estimated global prevalence of 25% [6, 8]. NASH is likely to become the leading cause of end-stage liver disease in the coming decades [2].
NASH, the most severe form of NAFLD, is defined by the presence of liver damage in the form of steatosis, hepatocyte ballooning and lobular inflammation, with or without fibrosis [9, 10]. NASH is a progressive disease, and in some patients may progress to advanced fibrosis and cirrhosis [1, 7]. NASH is also associated with an increased risk of hepatocellular carcinoma (HCC), requirement for liver transplantation (LT) and liver-related mortality [11]. The prevalence of NASH as the primary indication for LT has increased in both Europe and the US over the past two decades [12, 13]. NASH is also associated with elevated risk of other extra-hepatic complications including chronic kidney disease, malignancy and cardiovascular disease (CVD) [9, 10].
Despite its high clinical burden, there are limited data on healthcare resource utilisation (HCRU) and direct medical costs associated with NASH. The national economic burden of NAFLD in terms of direct annual medical costs has been estimated at $103 billion in the US, €27.7 billion in three European countries combined (Germany, France, Italy) and £5.24 billion in the UK [14].
Economic burden analyses are pivotal for addressing key health policy and health system management questions [15, 16]. They provide information at a microeconomic (household, employer or government agency impact) and macroeconomic (societal impact, i.e. a country’s gross domestic product [GDP]) level, and can inform decision makers about the overall magnitude of economic losses and their distribution across different categories of cost (e.g. health expenditure, labour and productivity losses). They can also be used for cost-effectiveness modelling and to guide healthcare resource allocation.
This review aimed to summarise and critically evaluate currently available evidence on the economic burden of NASH, focusing on direct medical and non-medical costs, indirect costs and HCRU, and identify evidence gaps for subsequent research.
Methods
A systematic literature review (SLR) was conducted using Medline, EMBASE, Cochrane Library and EconLit databases via the Ovid platform using pre-defined search strategies (see Supplementary Table 1 in the Electronic Supplementary Material [ESM]). The review was conducted in line with Cochrane guidelines [17] and is reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [18]. This review was not registered, and a protocol was not prepared. Further details on the review methodology are provided in Supplementary Methods in the ESM.
The primary SLR was conducted in 2020, with an update to the searches on 6 January 2021. Eligible studies were full-text English language publications of economic outcomes in NASH populations, with or without comorbidities, or a NASH subgroup within an NAFLD study, published from January 2010 to January 2021. Full eligibility criteria are described in Supplementary Table 2 in the ESM.
First-round screening of titles and abstracts was followed by second-round full-text screening of short-listed articles and data extraction of articles meeting the eligibility criteria. Both first- and second-round screening was performed by two independent researchers, and final inclusion was verified by the project lead.
Data extraction was performed using pre-designed data extraction tables by an analyst with a quality check on all publications by an independent senior analyst. Any disagreements regarding study eligibility or data extraction were referred to a third party and were resolved through discussion or inclusion of additional referees. Data extracted from each study included, but were not limited to, reference, country/region, study design, baseline characteristics (including age, sex, body mass index, method of diagnosis and comorbidities), type of intervention (including dose, duration and frequency) and outcomes reported (as shown in Supplementary Table 2 in the ESM).
Evidence was synthesised narratively, with outcomes categorised initially by type (resource use or costs), and further subdivided based on the range of components within each category. To allow comparison of like-for-like data, resource use was further categorised into hospital admissions, hospital length-of-stay (LOS) and other HCRU, and data were organised according to NASH population type (LT-related populations, cirrhosis-related populations and NASH populations with or without comorbidities). Cost data were separated into direct and indirect costs, with direct costs further subdivided into individual costs or estimates of national cost burdens.
Quality assessment was performed for all studies within the SLR. The quality of included cost of illness/economic burden studies was assessed using an 11-item checklist of questions that evaluated the methodological quality of the studies using a four-point answer scale (yes, no, partially, not specified). The checklist was developed from a model described by Drummond et al. [19] and adapted to cost of illness by Molinier et al. [20]. Questions within the checklist encompassed whether a clear definition of the illness was given; whether epidemiological sources, activity data, methodology, and cost values were appropriately described and/or assessed; whether costs were discounted, unit costs appropriately valued, and direct/indirect costs sufficiently disaggregated; whether major assumptions were tested in a sensitivity analysis; and whether presentation of the study results was consistent with the study methodology. Two reviewers independently assessed the likelihood of bias and any disagreements were resolved by discussion and/or additional referees.
Results
The electronic database search identified 4672 citations, of which 370 were identified as duplicates and excluded, 4046 were excluded based on title and abstract and a further 242 publications were excluded during full-text screening. The reasons for exclusion at first and second pass are summarised in Fig. 1. Overall, 14 unique publications were included (see Supplementary Table 3 in the ESM).
Fig. 1.
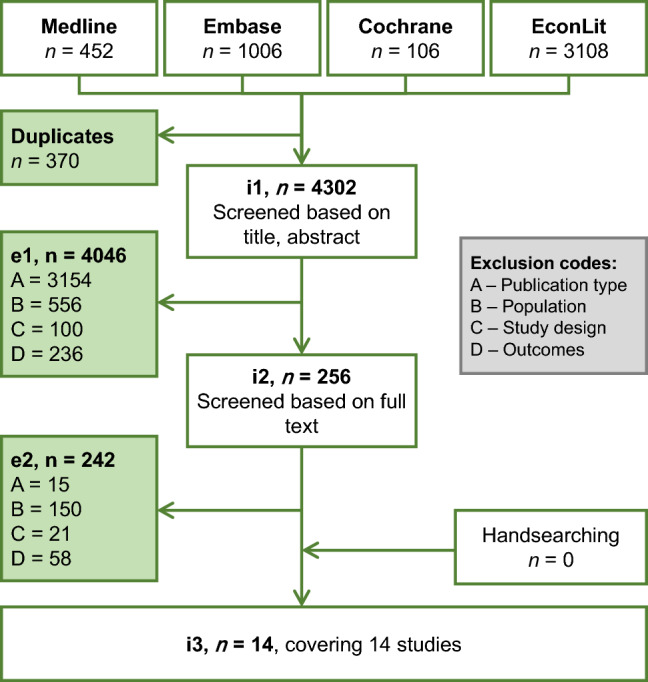
Flow diagram of publications included and excluded from systematic review. e1 excluded studies after title/abstract screening stage, e2 excluded studies after full-text review stage, i1 studies to screen at title/abstract stage, i2 studies to screen at full-text review stage, i3 included studies after full-text review stage
Study and Patient Characteristics
Overall, the included studies were heterogeneous in terms of study methodology, patient populations, comorbidities, follow-up time and outcomes measured.
Most of the identified studies were retrospective in nature, and over half of the studies were US-based (see Supplementary Table 3 in the ESM). Patient populations across the studies included general NASH populations or sub-populations, NASH with or without coexisting conditions (type 2 diabetes [T2D], sarcopenia, kidney failure), LT recipients and patients hospitalised for NASH/NASH-cirrhosis (see Supplementary Table 3 in the ESM). Approximately equal proportions of studies reported on resource use alone, costs alone or both costs and resource use. Cost perspectives were applicable for nine studies; these included patient, societal, payer and national healthcare system perspectives in seven studies, with two studies not reporting the cost perspective (see Supplementary Table 4 in the ESM).
Resource Use
Hospitalisations and Length of Stay
Ten studies reported hospitalisation (Table 1) and/or hospital LOS (Table 2) data associated with NASH, half of which reported on LT-related hospitalisations [21–25]. Most studies scored highly in all applicable categories of the quality assessment while two studies scored poorly in multiple categories (see section 3.4 for further details).
Table 1.
Hospital admissions reported across included studies
| Study | Country | Population | N | Outcome | Hospital admissions |
|---|---|---|---|---|---|
| LT-related hospitalisations | |||||
| Aby et al. [21] | US | LT recipients with NASH cirrhosis or cryptogenic cirrhosis, 2002–2015 | 1-year post-transplant hospital admissions, median (IQR) |
Sarcopenia vs no sarcopenia: 1 (0.2–2) vs 1 (0–2); p = 0.402 |
|
| With sarcopenia | 90 | ||||
| Wthout sarcopenia | 56 | ||||
| Agopian et al. [22] | US | Patients who underwent primary LT, 1993–2011 | NASH as primary indication for LT, % | 2002 vs 2011: 3 vs 19 | |
| NASH aetiology | 144 | ||||
| Non-NASH aetiology | 1150 | ||||
| HBV | 691 | ||||
| HCV | 127 | ||||
| ALD | 185 | ||||
| CC | 58 | ||||
| PBC/PSC | 89 | Pre-transplant hospitalisations, % |
NASH vs non-NASH: 62 vs 41 HCV: 37; HBV: 25; ALD: 60; CC: 67; PBC/PSC: 43 |
||
| Barbas et al. [23] | Canada | NASH patients undergoing primary LT, 2000–2014 | Pre-transplant hospitalisations, % | LDLT vs DDLT: | |
| LDLT | 48 | Home/hospital | 69.6/30.4 vs 50.8/49.2; p = 0.03 | ||
| DDLT | 128 | Home/ward/ICU | 69.6/28.3/2.2 vs 50.8/39.2/10.0; p = 0.06 | ||
| Morris et al. [24] | US | Patients who underwent LSG following LT, 2014–2018 | 15 | Post-transplant LSG ICU admissions, n (%) | 1 (6.7) |
| NASH | 14 | ||||
| Cirrhosis-related hospitalisations | |||||
| Axley et al. [27] | US | Hospital admissions for cirrhosis (2006–2014) | 1,928,764 | Proportion of cirrhosis admissions with NASH aetiology, % | 2006–8 vs 2012–14: 6 vs 12; p < 0.001 |
| NASH-related | 179,104 | NASH-related admissions: | |||
| Proportion of NASH cirrhosis hospitalisations that developed ACLF, n (%) | 2006–14: 8903 (5) | ||||
| ACLF admissions in NASH cirrhosis patients, % | 2006–8 vs 2012–14: 3.5 vs 5.7; p < 0.001 | ||||
| Frequency of ACLF admissions for NASH (proportion of total ACLF admissions in NASH cirrhosis patients), % | 2006–8/2009–11/2012–14: 12/33/55 | ||||
| CC subgroup with discharge diagnosis of NASH | 699,668 | CC subgroup: | |||
| Proportion of CC admissions with discharge diagnosis of NASH, % | 2006–8 vs 2012–14: 49 vs 54; p NR | ||||
| ACLF admissions with NASH cirrhosis, % | 2006–8 vs 2012–14: 4.5 vs 6.2; p < 0.001 | ||||
| Frequency of ACLF admissions in NASH cirrhosis (proportion of total ACLF admissions with NASH cirrhosis), % | 2006–8/2009–11/2012–14: 23/NR/43 | ||||
| Hospitalisation rates in NASH patients alone or versus general population and/or patients with T2D | |||||
| Balp et al. [26] | EU5a | Respondents to the National Health and Wellness Survey | Hospitalisations in past 6 months, mean |
NASH vs general population: 0.47 vs 0.17; p < 0.001 NASH vs T2D: 0.39 vs 0.19; p = 0.033 |
|
| NASH | 184 | ||||
| Unmatched general population | 79.267 | ||||
| Unmatched T2D | 4783 | ||||
| Carruthers et al. [28] | England | Patients with biopsy-proven NASH and diabetes (inpatient and day-case admissions to NHS hospitals 2004–2015) | 2004/05: 1303 | Number of admissions | 2004–5 vs 2014–15: 1303 vs 2341 |
| 2014/15: 2341 | Hospital admission rates from 2004–2014 (rate per 100,000 population) |
Overall: 2004: 73.8; 2005: 76.8; 2006: 73.4; 2007: 71.4; 2008: 71.9; 2009: 75.6; 2010: 79.2; 2011: 79.7; 2012: 83.5; 2013: 78; 2014: 80.4 Rate ratio: 1.01 (1.00–1.02); p = 0.04 Male: 2004: 41.5; 2005: 44; 2006: 43.9; 2007: 42.6; 2008: 43.8; 2009: 43.7; 2010: 47.9; 2011: 46.5; 2012: 48.2; 2013: 45; 2014: 47.6 Rate ratio: 1.01 (1.00–1.02); p = 0.03 Female: 2004: 32.2; 2005: 32.8; 2006: 29.5; 2007: 28.8; 2008: 28.1; 2009: 31.9; 2010: 31.3; 2011: 33.2; 2012: 35.3; 2013: 33; 2014: 32.8 Rate ratio: 1.00 (0.99–1.02); p = 0.37 17–44 years: 2004: 7.5; 2005: 8.2; 2006: 7.3; 2007: 7.5; 2008: 6.3; 2009: 7.4; 2010: 8.3; 2011: 7.3; 2012: 10.7; 2013: 7.5; 2014: 7.2 Rate ratio: 1.01 (0.98–1.03); p = 0.54 44–64 years: 2004: 33.7; 2005: 37.1; 2006: 33.6; 2007: 30.9; 2008: 33.3; 2009: 30; 2010: 31.8; 2011: 31.8; 2012: 30.9; 2013: 32.8; 2014: 29.7 Rate ratio: 0.99 (0.97–0.99); p = 0.002 65+ years: 2004: 32.5; 2005: 31.5; 2006: 32.5; 2007: 33; 2008: 32.3; 2009: 38.2; 2010: 39.1; 2011: 40.6; 2012: 41.8; 2013: 37.7; 2014: 43.5 Rate ratio: 1.03 (1.02–1.04); p < 0.001 |
|||
| Carruthers et al. [28] | England | Patients with biopsy-proven NASH without diabetes (inpatient and day-case admissions to NHS hospitals 2004–2015) | 2004/05: 12,758 | Number of admissions | 2004–5 vs 2014–15: 12,758 vs 10,988 |
| 2014/15: 10,988 | Hospital admission rates from 2004–2014 (rate per 100,000 population) |
Overall: 2004: 33.6; 2005: 35.7; 2006: 34.7; 2007: 33.5; 2008: 32.6; 2009: 31.3; 2010: 31.4; 2011: 30.7; 2012: 30.1; 2013: 28.3; 2014: 21.7 Rate ratio: 0.97 (0.96–0.98); p < 0.001 Male: 2004: 18.9; 2005: 20.1; 2006: 19.4; 2007: 18.3; 2008: 17.6; 2009: 17.3; 2010: 17.3; 2011: 16.2; 2012: 15.8; 2013: 14.3; 2014: 11 Rate ratio: 0.96 (0.95–0.97); p < 0.001 Female: 2004: 14.7; 2005: 15.6; 2006: 15.3; 2007: 15.2; 2008: 15; 2009: 14; 2010: 14.1; 2011: 14.5; 2012: 14.3; 2013: 14; 2014: 10.7 Rate ratio: 0.98 (0.97–0.99); p < 0.001 17–44 years: 2004: 10.5; 2005: 11.1; 2006: 10.7; 2007: 9.4; 2008: 9.1; 2009: 8.8; 2010: 8.5; 2011: 8.5; 2012: 8.3; 2013: 7.3; 2014: 5.4 Rate ratio: 0.95 (0.94–0.96); p < 0.001 44–64 years: 2004: 14; 2005: 15.1; 2006: 14.5; 2007: 14.3; 2008: 13.7; 2009: 12.6; 2010: 12.9; 2011: 12.5; 2012: 12.1; 2013: 10.9; 2014: 8.5 Rate ratio: 0.96 (0.95–0.97); p < 0.001 65+ years: 2004: 9.1; 2005: 9.5; 2006: 9.5; 2007: 9.8; 2008: 9.8; 2009: 9.9; 2010: 10; 2011: 9.7; 2012: 9.7; 2013: 10.1; 2014: 7.8 Rate ratio: 1.00 (0.99–1.00); p = 0.45 |
|||
| Geier et al. [29] | US, France, Germany | NASH patients (NASH-Atlas program July–November 2017) | Number of inpatient visits, mean (SD) |
Total population: 0.3 (3.9) BC vs phenotypic NASH: 0.3 (NR) vs 0.3 (NR) FR vs DE vs US: 0.5 (NR) vs 0.5 (NR) vs 0.1 (NR) |
|
| Total | 1216 | ||||
| BC NASH | 786 | ||||
| Phenotypic NASH | 430 | ||||
| French NASH population | 227 | ||||
| German NASH population | 287 | ||||
| US NASH population | 702 | ||||
aEU5 includes France, Germany, Italy, Spain and the UK
ACLF acute-on-chronic liver failure, ALD alcoholic liver disease, BC biopsy-confirmed, CC compensated cirrhosis, CI confidence interval, DDLT deceased donor liver transplant, DE German cohort, FR French cohort, HBV hepatitis B virus, HCV hepatitis C virus, ICU intensive care unit, IQR interquartile range, LDLT living donor liver transplantation, LSG laparoscopic sleeve gastrectomy, LT liver transplantation, NASH non-alcoholic steatohepatitis, NASH-Atlas Growth from Knowledge (currently Ipsos) Disease Atlas Real-World Evidence program, NHS National Health Service, NR not reported, PBC primary biliary cirrhosis, PSC primary sclerosing cholangitis, SD standard deviation, T2D type 2 diabetes, US US cohort
Table 2.
Hospital length of stay across included studies
| Study | Country | Population | N | Outcome | Length of stay |
|---|---|---|---|---|---|
| LT-related hospitalisations | |||||
| Aby et al. [21] | US | LT recipients with NASH cirrhosis or cryptogenic cirrhosis, 2002–2015 |
Post-transplant LOS, median days (IQR) Post-transplant hospital days in 1-yr, median (IQR) |
Sarcopenia vs no sarcopenia: 44.5 (23.2–81.2) vs 46 (27–69.2); p = 0.812 53 (31.2–89.8) vs 55.5 (33.5–80); p = 0.777 |
|
|
With sarcopenia Without sarcopenia |
90 56 |
||||
| Agopian et al. [22] | US | Patients who underwent primary LT, 1993–2011 | All patients: | ||
| Total LOS, days | NASH vs non-NASH: 47 vs 36 | ||||
|
NASH aetiology Non-NASH aetiology HBV HCV ALD CC PBC/PSC |
144 1150 691 127 185 58 89 |
HCV: 34; HBV: 27; ALD: 47; CC: 47; PBC/PSC: 35 | |||
| Post-transplant LOS, days | NASH vs non-NASH: 35 vs 29 | ||||
| HCV: 28; HBV: 23; ALD: 36; CC: 36; PBC/PSC: 27 | |||||
| Patients from home pre-transplant: | |||||
| Post-transplant LOS, days | NASH vs non-NASH: 28 vs 20 | ||||
| HCV: 17; HBV: 17; ALD: 21; CC: 33; PBC/PSC: 22 | |||||
| Hospitalised patients pre-transplant: | |||||
| Pre-transplant LOS, days | NASH vs non-NASH: 18 vs 18 | ||||
| HCV: 16; HBV: 16; ALD: 18; CC: 17; PBC/PSC: 18 | |||||
| Post-transplant LOS, days | NASH vs non-NASH: 40 vs 42 | ||||
| HCV: 38; HBV: 38; ALD: 46; CC: 37; PBC/PSC: 29 | |||||
| Barbas et al. [23] | Canada | NASH patients undergoing primary LT, 2000–2014 | LDLT vs DDLT: | ||
| Post-transplant LOS, median days (IQR) | 11 (8–16) vs 17 (10–31) | ||||
|
LDLT DDLT |
48 128 |
Post-transplant ICU LOS, mean days (SD) | 3.2 (9.7) vs 6.3 (14.2) | ||
| Total LOS for index admission, median days (IQR) | 12.5 (9–18) vs 19 (10–34) | ||||
| Hoehn et al. [25] | US | Patients with diabetes who underwent LT, 2007–2011 | Proportion of patients discharged home, % |
NASH vs non-NASHa: Obese: 71% vs 83%; p < 0.005 Non-obese: 79% vs 85%; p < 0.005 |
|
|
Total NASH |
2971 713 |
LOS |
NASH vs non-NASHa: Non-obese: 10 vs 9 days; p < 0.05 |
||
| Morris et al. [24] | US | Patients who underwent LSG following LT, 2014–2018 | 1 | Post-transplant LSG LOS, median days (IQR) | 2 (1–2) |
|
Total NASH |
15 14 |
||||
| Cirrhosis-related hospitalisations | |||||
| Axley et al. [27] | US | Hospital admissions for cirrhosis with ACLF (2006–2014) | LOS, mean days |
Total: 2006–8: 13; 2009–11: 13; 2012–14: 12 NASH: 2006–14: 14 |
|
|
Total NASH-related |
112,174 8903 |
||||
| NASH-related hospitalisations with or without comorbidities | |||||
| Carruthers et al. [28] | England | Patients with biopsy-proven NASH (inpatient and day-case admissions to NHS hospitals 2004–2015) | LOS, median days (IQR) |
2004–5 vs 2014–15: With diabetes: 2 (0–16) vs 0 (0–8); p < 0.001 Without diabetes: 1 (0–7) vs 0 (0–5); p < 0.001 |
|
|
With diabetes (2004–5) With diabetes (2014–15) Without diabetes (2004–5) Without diabetes (2014–15) |
1303 2341 12,758 10,988 |
||||
| Geier et al. [29] | US, France, Germany | NASH patients (NASH-Atlas program July–November 2017) | LOS,b mean (SD) |
Total population: 3.7 (7.1) BC vs phenotypic NASH: 2.7 vs 6.0; p < 0.05 FR vs DE vs US: 5.0 vs 4.3 vs 2.1 |
|
|
Total BC NASH Phenotypic NASH French NASH population German NASH population US NASH population |
1216 786 430 227 287 702 |
||||
| ICU LOS,b mean (SD) |
Total population: 0.4 (1.2) BC vs phenotypic NASH: 0.4 vs 0.4; p = ns FR vs DE vs US: 0.5 vs 0.3 vs 0.4 |
||||
| Reja et al. [31] | US | Hospitalised NASH patients with or without kidney failure, 2016 | LOS, mean days (SD) |
Total NASH: 4.8 (7.1) With vs without kidney failure: 6.4 (9.1) vs 3.1 (3.4), β/OR = 3.02 (95% CI 2.54–3.50; p < 0.0001) |
|
|
Total With kidney failure Without kidney failure |
1196 598 598 |
||||
aComparator inferred, but not explicitly stated in publication
bNights in hospital/ICU
ACLF acute-on-chronic liver failure, ALD alcoholic liver disease, BC biopsy-confirmed, CC compensated cirrhosis, CI confidence interval, DDLT deceased donor liver transplant, DE German cohort, FR French cohort, HBV hepatitis B virus, HCV hepatitis C virus, ICU intensive care unit, IQR interquartile range, LDLT living donor liver transplantation, LOS length of stay, LSG laparoscopic sleeve gastrectomy, LT liver transplantation, NASH non-alcoholic steatohepatitis, NASH-Atlas Growth from Knowledge (currently Ipsos) Disease Atlas Real-World Evidence program, NHS National Health Service, ns non-significant, OR odds ratio, PBC primary biliary cirrhosis, PSC primary sclerosing cholangitis, SD standard deviation, US US cohort, yr year
NASH was associated with a two- to three-fold higher rate of hospital admissions over 6 months compared with the general population or patients with T2D [26]. A pattern of increasing prevalence of hospitalisations due to NASH was seen across the studies, including an increase in the proportion of late-stage liver complications due to NASH relative to other liver diseases [27], an increase in the proportion of cirrhosis admissions of NASH aetiology over 8 years [27] and a five-fold increase in the frequency of NASH as primary indication for LT over 10 years [22]. The frequency of hospital admissions among patients with comorbid diabetes has increased relative to patients without diabetes over time [28].
The presence of comorbid diabetes does not appear to increase LOS among inpatient or day-case admissions (Table 2). Equivalent median LOS was reported for patients with and without diabetes in 2014–2015 following an overall decrease in median LOS in both populations over 11 years [28]. In LT recipients, one Canadian study found that patients receiving deceased donor LT had longer post-transplant and intensive care LOS than patients receiving living donor LT (LDLT), leading the study authors to conclude that LDLT for NASH facilitates transplantation of patients at a less severe stage of disease, which appears to promote a faster recovery and decreased HCRU [23].
The results of two studies suggest that LOS is longer in patients with NASH compared with other serious liver diseases (Table 2) [22, 27]. A higher rate of pre-transplant hospitalisations and longer total and post-transplant LOS were reported for NASH versus non-NASH LT recipients [22], and similarly, a longer mean LOS was reported among acute-on-chronic liver failure (ACLF) admissions of NASH-cirrhosis aetiology compared with alcohol- or viral-related cirrhosis [27].
Healthcare Resource Utilisation
Five studies reported HCRU data, which included healthcare visits (excluding inpatient admissions), use of drugs, devices, tests, procedures and non-pharmacological interventions (Table 3). All five studies scored highly in all or most relevant quality assessment categories (see section 3.4 for further details).
Table 3.
Resource use (excluding inpatient visits) across included studies
| Study | Country | Population | N | Outcome | Resource use |
|---|---|---|---|---|---|
| LT-related | |||||
| Agopian et al. [22] | US | Patients who underwent primary LT, 1993–2011 | Mechanical ventilator use before LT, % |
NASH vs non-NASH: 16 vs 17 HCV: 15; HBV: 12; ALD: 24; CC: 22; PBC/PSC: 12 |
|
|
NASH aetiology Non-NASH aetiology HBV HCV ALD CC PBC/PSC |
144 1150 691 127 185 58 89 |
Vasopressor use before LT, % |
NASH vs non-NASH: 17 vs 10* HCV: 8*; HBV: 8*; ALD: 19; CC: 16; PBC/PSC: 7 |
||
| Dialysis before LT, % |
NASH vs non-NASH: 45 vs 31* HCV: 26*; HBV: 16*; ALD: 47; CC: 37; PBC/PSC: 28* |
||||
| Operative time, mins |
NASH vs non-NASH: 402 vs 322* HCV: 323*; HBV: 308*; ALD: 330*; CC: 315*; PBC/PSC: 322* |
||||
| Intraoperative transfusion, uPRBC, % |
NASH vs non-NASH: 18 vs 14* HCV: 14*; HBV: 13*; ALD: 16; CC: 14*; PBC/PSC: 12* |
||||
| Retransplantation, % |
NASH vs non-NASH: 7 vs 7 HCV: 8; HBV: 2; ALD: 6; CC: 3; PBC/PSC: 6 |
||||
| Cirrhosis-related | |||||
| Axley et al. [27] | US | Hospital admissions for cirrhosis with ACLF (2006–2014) | NASH vs non-NASH: | ||
|
Total NASH aetiology Non-NASH |
112,174 8903 103,271b |
Endoscopic evaluation, % Dialysis use, % Ventilator use, % Long-term care, % |
5 vs 2–10; p NR 45 vs 36; p < 0.0001 78 vs 76–82; p NR 32 vs 26; p = 0.0001 |
||
| NASH-related with or without comorbidities | |||||
| Balp et al. [26] | EU5a | Respondents to the National Health and Wellness Survey | Healthcare resource use in past 6 months, adjusted mean (SE): | NASH vs matched general population: | |
|
NASH Unmatched general pop. Matched general pop. Unmatched T2D Matched T2D |
184 79,267 736 4783 368 |
General/family practitioner visits | 3.80 (0.33) vs 2.23 (0.10); p < 0.001 | ||
| Specialists (any type) visits | 6.94 (0.69) vs 3.77 (0.19); p < 0.001 | ||||
| Cardiologist visits | 0.32 (0.05) vs 0.19 (0.02); p = 0.013 | ||||
| Gastroenterologist visits | 0.28 (0.06) vs 0.07 (0.01); p < 0.001 | ||||
| Endocrinologist visits | 0.27 (0.05) vs 0.04 (0.01); p < 0.001 | ||||
| Internist visits | 0.28 (0.06) vs 0.12 (0.02); p = 0.002 | ||||
| Diabetologist visits | 0.22 (0.06) vs 0.09 (0.02); p = 0.007 | ||||
| Psychiatrist visits | 0.37 (0.12) vs 0.16 (0.04); p = 0.034 | ||||
| Hepatologist visits | 0.07 (0.02) vs 0.01 (0.00); p = 0.000 | ||||
| HCP visits | 10.73 (NR) vs 6.01 (NR); p < 0.001 | ||||
| ER visits | 0.57 (NR) vs 0.22 (NR); p < 0.001 | ||||
| Healthcare resource use in past 6 months, adjusted mean (SE): | NASH vs matched T2D: | ||||
| General/family practitioner visits | 3.68 (0.36) vs 2.81 (0.19); p = 0.033 | ||||
| Specialists (any type) visits | 7.13 (0.73) vs 5.01 (0.35); p = 0.008 | ||||
| Cardiologist visits | 0.31 (0.07) vs 0.23 (0.04); p = 0.333 | ||||
| Gastroenterologist visits | 0.28 (0.07) vs 0.08 (0.02); p = 0.001 | ||||
| Endocrinologist visits | 0.27 (0.07) vs 0.09 (0.02); p = 0.004 | ||||
| Internist visits | 0.26 (0.09) vs 0.23 (0.05); p = 0.740 | ||||
| Diabetologist visits | 0.26 (0.05) vs 0.27 (0.04); p = 0.964 | ||||
| Psychiatrist visits | 0.38 (0.11) vs 0.16 (0.04); p = 0.041 | ||||
| Hepatologist visits | 0.09 (0.03) vs 0.00 (0.00); p < 0.001 | ||||
| HCP visits | 10.85 (NR) vs 7.86 (NR); p = 0.006 | ||||
| ER visits | 0.65 (NR) vs 0.23 (NR); p = 0.009 | ||||
| Prescription treatment for T2D, % | NASH vs general population vs T2D: | ||||
| Use of a prescription | 20.1 vs 5.0 vs 82.6 | ||||
| Use of insulin prescription | 5.4 vs 1.4 vs 23.0 | ||||
| Use of non-insulin prescription | 19.6 vs 4.5 vs 75.1 | ||||
| Geier et al. [29] | US, France, Germany | NASH patients (NASH-Atlas program July–November 2017) | Annual non-routine HCRU, mean (SD) | Total population: 4.2 (3.1) | |
|
Total BC NASH Phenotypic NASH French NASH population German NASH population US NASH population F1 fibrosis F2 fibrosis F3 fibrosis F4 fibrosis |
1216 786 430 227 287 702 175 278 211 47 |
Physician visits |
BC vs phenotypic NASH: 4.5 vs 3.7 FR vs DE vs US: 4.6 vs 4.6 vs 3.9 |
||
| Outpatient visits |
Total population: 1.6 (2.0) BC vs phenotypic NASH: 1.8 vs 1.4 FR vs DE vs US: 2.3 vs 1.3 vs 1.5 |
||||
| ER visits |
Total population: 0.3 (1.0) BC vs phenotypic NASH: 0.4 vs 0.4 FR vs DE vs US: 0.4 vs 0.5 vs 0.2 |
||||
| Tests/procedures used for NASH diagnosis, % | |||||
| Liver biopsy |
Total NASH vs BC NASH: 66.0 vs 100 FR vs DE vs US: 47.1 vs 65.9** vs 72.1** |
||||
| Ultrasound |
Total NASH vs BC NASH: 62.5 vs 59.0 FR vs DE vs US: 67.4** vs 57.1 vs 63.1 |
||||
| FibroScan (transient elastography) |
Total NASH vs BC NASH: 23.2 vs 21.1 FR vs DE vs US: 54.6*** vs 23.7** vs 12.8 |
||||
| Serum transaminase (ALT, AST) |
Total NASH vs BC NASH: 65.3 vs 64.5 FR vs DE vs US: 72.7*** vs 62.0 vs 64.2 |
||||
| GGT |
Total NASH vs BC NASH: 43.8 vs 38.3 FR vs DE vs US: 70.0*** vs 58.2** vs 29.3 |
||||
| Lipid profile |
Total NASH vs BC NASH: 49.3 vs 37.3 FR vs DE vs US: 58.1** vs 57.8** vs 43.0 |
||||
| Platelet count |
Total NASH vs BC NASH: 44.4 vs 40.4 FR vs DE vs US: 59.9*** vs 43.9 vs 39.6 |
||||
| Clotting studiesd |
Total NASH vs BC NASH: 33.1 vs 27.1 FR vs DE vs US: 32.6 vs 45.6*** vs 28.2 |
||||
| ARFI imaging |
Total NASH vs BC NASH: 2.2 vs 2.4 FR vs DE vs US: 1.9 vs 1.3 vs 3.8 |
||||
| CT scan |
Total NASH vs BC NASH: 16.2 vs 17.2 FR vs DE vs US: 19.2 vs 9.3 vs 14.3 |
||||
| MRI |
Total NASH vs BC NASH: 9.5 vs 9.0 FR vs DE vs US: 7.1 vs 9.7 vs 15.0 |
||||
| MRE |
Total NASH vs BC NASH: 4.5 vs 5.0 FR vs DE vs US: 4.3 vs 3.5 vs 5.9 |
||||
| FibroTest/FibroSure |
Total NASH vs BC NASH: 19.6 vs 17.4 FR vs DE vs US: 15.7 vs 40.1 vs 12.9 |
||||
| Fibrosis-4 index |
Total NASH vs BC NASH: 6.2 vs 6.6 FR vs DE vs US: 5.7 vs 5.7 vs 7.7 |
||||
| APRI |
Total NASH vs BC NASH: 20.6 vs 21.6 FR vs DE vs US: 22.6 vs 18.5 vs 17.1 |
||||
| Steatosis, activity and fibrosis score |
Total NASH vs BC NASH: 8.1 vs 8.9 FR vs DE vs US: 8.3 vs 6.2 vs 9.1 |
||||
| NashTest |
Total NASH vs BC NASH: 9.5 vs 10.2 FR vs DE vs US: 5.3 vs 13.2 vs 17.1 |
||||
| NAFLD fibrosis score |
Total NASH vs BC NASH: 14.8 vs 15.5 FR vs DE vs US: 14.4 vs 13.2 vs 17.1 |
||||
| NAFLD activity score |
Total NASH vs BC NASH: 15.9 vs 18.7 FR vs DE vs US: 15.7 vs 9.7 vs 21.3 |
||||
| Enhanced liver fibrosis panel score |
Total NASH vs BC NASH: 3.9 vs 3.4 FR vs DE vs US: 3.3 vs 0.4 vs 8.4 |
||||
| Circulating levels of CK-18 |
Total NASH vs BC NASH: 4.4 vs 5.2 FR vs DE vs US: 2.4 vs 2.6 vs 10.5 |
||||
| Tests/procedures used for NASH monitoring, % | |||||
| Liver biopsy |
Total NASH vs BC NASH: 11.6 vs 16.7 FR vs DE vs US: 13.1 vs 2.6 vs 15.0 |
||||
| Ultrasound |
Total NASH vs BC NASH: 54.4 vs 57.3 FR vs DE vs US: 53.7 vs 48.9 vs 60.6 |
||||
| FibroScan (transient elastography) |
Total NASH vs BC NASH: 17.6 vs 18.2 FR vs DE vs US: 11.7 vs 49.3 vs 21.6 |
||||
| Serum transaminase (ALT, AST) |
Total NASH vs BC NASH: 61.8 vs 61.6 FR vs DE vs US: 64.1 vs 69.2 vs 61.7 |
||||
| GGT |
Total NASH vs BC NASH: 41.1 vs 38.8 FR vs DE vs US: 22.8 vs 62.1 vs 57.5 |
||||
| Lipid profile |
Total NASH vs BC NASH: 49.3 vs 37.3 FR vs DE vs US: 30.5 vs 31.7 vs 58.5 |
||||
| Platelet count |
Total NASH vs BC NASH: 44.4 vs 40.4 FR vs DE vs US: 37.7 vs 45.4 vs 42.9 |
||||
| Clotting studiesd |
Total NASH vs BC NASH: 33.1 vs 27.1 FR vs DE vs US: 22.9 vs 19.4 vs 43.2 |
||||
| ARFI imaging |
Total NASH vs BC NASH: 2.5 vs 3.4 FR vs DE vs US: 2.1 vs 1.3 vs 4.2 |
||||
| CT scan |
Total NASH vs BC NASH: 8.2 vs 9.5 FR vs DE vs US: 9.8 vs 1.8 vs 9.4 |
||||
| MRI |
Total NASH vs BC NASH: 5.9 vs 6.0 FR vs DE vs US: 4.8 vs 4.0 vs 10.1 |
||||
| MRE |
Total NASH vs BC NASH: 5.0 vs 3.8 FR vs DE vs US: 1.6 vs 3.1 vs 7.3 |
||||
| FibroTest/FibroSure |
Total NASH vs BC NASH: 17.4 vs 16.3 FR vs DE vs US: 12.7 vs 29.1 vs 12.9 |
||||
| Fibrosis-4 index |
Total NASH vs BC NASH: 6.6 vs 7.8 FR vs DE vs US: 6.0 vs 4.0 vs 8.7 |
||||
| APRI |
Total NASH vs BC NASH: 21.6 vs 18.6 FR vs DE vs US: 18.7 vs 10.1 vs 20.2 |
||||
| Steatosis, activity and fibrosis score |
Total NASH vs BC NASH: 8.9 vs 6.2 FR vs DE vs US: 5.7 vs 4.0 vs 8.0 |
||||
| NashTest |
Total NASH vs BC NASH: 10.2 vs 9.3 FR vs DE vs US: 5.3 vs 5.3 vs 16.4 |
||||
| NAFLD fibrosis score |
Total NASH vs BC NASH: 15.5 vs 12.9 FR vs DE vs US: 9.3 vs 9.3 vs 18.1 |
||||
| NAFLD activity score |
Total NASH vs BC NASH: 18.7 vs 15.9 FR vs DE vs US: 11.5 vs 8.4 vs 23.3 |
||||
| Enhanced liver fibrosis panel score |
Total NASH vs BC NASH: 3.4 vs 4.3 FR vs DE vs US: 3.6 vs 0.4 vs 7.7 |
||||
| Circulating levels of CK-18 |
Total NASH vs BC NASH: 5.2 vs 5.3 FR vs DE vs US: 2.8 vs 2.2 vs 8.4 |
||||
| NASH diagnosis and monitoring tests, mean number (SD) | |||||
| Liver biopsy |
Total NASH vs BC NASH: 1.2 (0.7) vs 1.2 (0.7) FR vs DE vs US: 1.2 (0.8) vs 1.1 (0.5) vs 1.2 (0.5) |
||||
| Ultrasound |
Total NASH vs BC NASH: 2.7 (2.3) vs 2.9 (2.5) FR vs DE vs US: 2.4 (2.4) vs 2.5 (1.4) vs 3.7 (2.5) |
||||
| ARFI imaging |
Total NASH vs BC NASH: 1.5 (1.2) vs 1.6 (1.2) FR vs DE vs US: 1.5 (1.0) vs 1.0 (1.2) vs 1.8 (1.3) |
||||
| CT scan |
Total NASH vs BC NASH: 1.4 (0.8) vs 1.5 (0.8) FR vs DE vs US: 1.4 (0.8) vs 1.6 (0.9) vs 1.4 (0.8) |
||||
| MRI |
Total NASH vs BC NASH: 1.5 (1.2) vs 1.7 (1.4) FR vs DE vs US: 1.6 (1.4) vs 1.5 (0.7) vs 1.6 (1.0) |
||||
| MRE |
Total NASH vs BC NASH: 1.7 (1.1) vs 1.8 (1.3) FR vs DE vs US: 1.6 (1.2) vs 1.6 (1.0) vs 1.9 (1.1) |
||||
| Fibroscan (transient elastography) |
Total NASH vs BC NASH: 2.0 (1.4) vs 2.1 (1.5) FR vs DE vs US: 2.0 (1.6) vs 2.1 (1.3) vs 2.0 (1.1) |
||||
| Serum transaminases (AST, ALT) |
Total NASH vs BC NASH: 5.0 (4.0) vs 5.0 (4.2) FR vs DE vs US: 4.7 (4.2) vs 5.3 (3.2) vs 5.4 (4.2) |
||||
| GGT |
Total NASH vs BC NASH: 4.7 (3.6) vs 5.0 (3.9) FR vs DE vs US: 3.8 (3.6) vs 5.1 (3.1) vs 5.5 (3.9) |
||||
| FibroTest/FibroSure |
Total NASH vs BC NASH: 2.0 (1.4) vs 2.2 (1.3) FR vs DE vs US: 2.0 (1.4) vs 2.3 (1.5) vs 1.7 (1.1) |
||||
| Fibrosis-4 Index |
Total NASH vs BC NASH: 2.3 (1.6) vs 2.3 (1.7) FR vs DE vs US: 2.4 (1.8) vs 2.8 (1.7) vs 1.9 (1.2) |
||||
| APRI |
Total NASH vs BC NASH: 3.5 (2.6) vs 3.4 (2.7) FR vs DE vs US: 3.6 (2.8) vs 3.1 (2.0) vs 3.3 (2.1) |
||||
| Steatosis, activity and fibrosis score |
Total NASH vs BC NASH: 2.0 (1.4) vs 1.9 (1.3) FR vs DE vs US: 1.7 (1.2) vs 3.0 (1.1) vs 2.2 (1.6) |
||||
| NashTest |
Total NASH vs BC NASH: 1.7 (1.3) vs 1.8 (1.4) FR vs DE vs US: 2.0 (1.8) vs 1.5 (0.7) vs 1.6 (0.9) |
||||
| NAFLD fibrosis score |
Total NASH vs BC NASH: 2.5 (2.1) vs 2.4 (2.2) FR vs DE vs US: 2.7 (2.4) vs 2.7 (1.6) vs 1.8 (1.2) |
||||
| NAFLD activity score |
Total NASH vs BC NASH: 2.6 (2.8) vs 2.6 (2.8) FR vs DE vs US: 2.8 (3.1) vs 2.8 (3.6) vs 2.3 (1.5) |
||||
| Enhanced liver fibrosis panel score |
Total NASH vs BC NASH: 2.0 (1.3) vs 1.9 (1.3) FR vs DE vs US: 2.1 (1.3) vs 2.0 (1.4) vs 1.8 (1.3) |
||||
| Circulating levels of CK-18 |
Total NASH vs BC NASH: 2.4 (1.5) vs 2.6 (1.6) FR vs DE vs US: 2.3 (2.0) vs 2.5 (1.2) vs 2.5 (1.3) |
||||
| Lipid profilec |
Total NASH vs BC NASH: 4.1 (3.6) vs 4.1 (3.6) FR vs DE vs US: 3.4 (3.4) vs 4.3 (3.0) vs 5.1 (4.0) |
||||
| Platelet count |
Total NASH vs BC NASH: 5.0 (4.2) vs 5.0 (4.0) FR vs DE vs US: 4.8 (4.8) vs 5.1 (3.3) vs 5.2 (3.6) |
||||
| Clotting studiesd |
Total NASH vs BC NASH: 4.4 (4.0) vs 4.4 (3.6) FR vs DE vs US: 4.0 (4.6) vs 4.4 (2.9) vs 4.8 (3.6) |
||||
| Non-invasive laboratory tests by fibrosis stage, mean number while under physician management | F1 vs F2 vs F3 vs F4: | ||||
| Platelet count | 3.9 vs 5.1 vs 5.1 vs 7.1 | ||||
| Serum transaminases | 4.1 vs 5.1 vs 5.4 vs 6.9 | ||||
| GGT | 4.4 vs 4.9 vs 4.9 vs 7.5 | ||||
| Clotting studies | 4.0 vs 4.4 vs 4.2 vs 6.9 | ||||
| Lipid profile | 3.3 vs 4.2 vs 4.3 vs 5.8 | ||||
| APRI | 3.5 vs 3.1 vs 3.4 vs 5.5 | ||||
| CK-18 | 2.3 vs 2.8 vs 2.6 vs NR | ||||
| Non-invasive procedures by fibrosis stage, mean number while under physician management | F1 vs F2 vs F3 vs F4: | ||||
| Ultrasound | 2.4 vs 3.1 vs 2.7 vs 4.0 | ||||
| Fibroscan (transient elastography) | 1.6 vs 2.2 vs 2.3 vs 2.6 | ||||
| MRE | 2.3 vs 1.8 vs 1.8 vs 0.7 | ||||
| MRI | 1.3 vs 1.9 vs 1.9 vs 1.3 | ||||
| ARFI | 1.3 vs 1.6 vs 1.4 vs 3.0 | ||||
| CT scan | 1.3 vs 1.6 vs 1.4 vs 1.7 | ||||
| Current pharmacological interventions, % of total population | |||||
| Statins | 2.5 | ||||
| Vitamin E | 23.8 | ||||
| Metformin | 20.2 | ||||
| Vitamin D | 10.4 | ||||
| Ursodeoxycholic acid | 9.3 | ||||
| Omega-3 fatty acids | 7.5 | ||||
| Thiazolidinediones | 5.5 | ||||
| Fibrates (fenofibrate) | 4.5 | ||||
| Orlistat | 3.9 | ||||
| GLP-1 analogues | 4.0 | ||||
| Pentoxifylline | 3.7 | ||||
| Betaine | 1.2 | ||||
| Current non-pharmacological interventions, % of total population | |||||
| Lifestyle modification | 64.6 | ||||
| Watchful waiting | 14.6 | ||||
| Bariatric surgery | 6.3 | ||||
| Endoscopic intervention | 5.7 | ||||
| Put on a waiting list for a liver transplant | 3.6 | ||||
| O’Hara et al. [30] | US and EU5a | NASH | Diagnostic/imaging tests, % | ||
|
Total US EU5 Spain |
3754 1221 2533 522 |
Liver biopsy | Total: NR; US vs ES: 57 vs 25 | ||
| Ultrasound imaging | Total: 68; EU5 vs US: 76 vs 51 | ||||
| Fibroscan (transient elastography) | Total: 33; EU5 vs US: 42 vs 13 | ||||
| AST/ALT ratio | Total: 23 | ||||
| NAFLD fibrosis score | Total: 9 | ||||
| BARD scoree | Total: 3 | ||||
| FIB-4 score | Total: 3 | ||||
| CK-18 | Total: 3 | ||||
aEU5 includes France, Germany, Italy, Spain and the UK
bNon-NASH total is sum of alcohol-related cirrhosis (n = 27,890), viral hepatitis cirrhosis (n = 9491) and ‘other’ cirrhosis (n = 65,890)
cCholesterol, LDL, HDL and triglycerides
dProthrombin time, international normalised ratio
eBARD score is a simple clinical score based on BMI ≥28 kg/m2, AST/ALT Ratio ≥0.8 and Diabetes mellitus
ACLF acute-on-chronic liver failure, ALD alcoholic liver disease, ALT alanine aminotransferase, APRI AST to Platelet Ratio Index, ARFI acoustic radiation force impulse, AST aspartate aminotransferase, BC biopsy-confirmed, CC compensated cirrhosis, CK-18 cytokeratin-18, CT computed tomography, DE German cohort, ER emergency room, ES Spanish cohort, F fibrosis stage, FIB-4 Fibrosis-4, FR French cohort, GGT gamma-glutamyl transferase, GLP glucagon-like peptide, HBV hepatitis B virus, HCP healthcare professional, HCRU healthcare resource utilisation, HCV hepatitis C virus, HDL high-density lipoprotein, LDL low-density lipoprotein, LT liver transplantation, MRE magnetic resonance enterography, MRI magnetic resonance imaging, NAFLD non-alcoholic fatty liver disease, NASH non-alcoholic steatohepatitis, NASH-Atlas Growth from Knowledge (currently Ipsos) Disease Atlas Real-World Evidence program, NR not reported, PBC primary biliary cirrhosis, PSC primary sclerosing cholangitis, SD standard deviation, SE standard error, T2D type 2 diabetes, uPRBC units packed red blood cells, US US cohort
*p < 0.05 versus NASH or NAFLD/NASH; **p < 0.05 versus lowest reporting country; ***p < 0.05 versus all other countries
Studies indicate a high demand for pharmacological and non-pharmacological interventions, as well as diagnosis and monitoring tests (Table 3) [22, 27, 29, 30]. Use of tests and procedures varied depending on disease severity, as shown by one multinational study that showed increased utilisation of non-invasive tests and procedures in patients with more advanced fibrosis (stage 4 [F4]) [29]. Differences in use of different diagnostic tests were also seen depending on country, with higher rates of liver biopsy, NashTest, NAFLD fibrosis score, NAFLD activity score, enhanced liver fibrosis score and less frequent use of FibroScan and some laboratory tests (e.g. GGT, lipid profile) reported in the US compared with European countries [29, 30].
Drug and device use in patients with late-stage complications often included dialysis and ventilation [22, 27]. Patients with NASH as primary indication for LT had greater device use, longer operative time and higher operative blood loss versus non-NASH LT [22], and ACLF patients with NASH aetiology required more dialysis use and long-term care versus non-NASH ACLF [27].
Two studies reporting on the frequency of healthcare provider visits in NASH populations (Table 3) showed that visits are more frequent in patients with NASH versus the general population [26], and in patients with biopsy-confirmed NASH compared with suspected NASH [29].
Costs
Direct Healthcare Costs
Direct healthcare costs were evaluated in six studies, of which three reported per-person costs [27, 30, 31] and four estimated national cost burdens [14, 30, 32, 33] (Table 4 and Supplementary Table 6 in the ESM). All six studies scored positively in all or most categories of the quality assessment (see section 3.4 for further details).
Table 4.
Direct per patient costs associated with NASH
| Study | Country | Currency, ref year |
Population | N | Total direct costs | Medical costs |
|---|---|---|---|---|---|---|
| Cirrhosis-related costs | ||||||
| Axley et al. [27] | US | USD, NR | Hospital admissions for cirrhosis with ACLF (2006–2014) | 112,174 | NR |
Total hospital charges/patient: • NASH-related: $151,196 • Alcohol-related: $97,492 • Viral hepatitis-related: $117,016 • Other aetiologies: $140,279 • Overall non-NASH-related: $134,597 (p < 0.0001 vs NASH-related) |
|
NASH-related Alcohol-related Viral hepatitis-related Other aetiologies |
8903 27,890 9491 65,890 |
|||||
| Hospital admissions for CC (2006–2014) | 1,358,275 | NR |
Median total hospital charges/admission: • NASH-related: $143,258 • Alcohol-related: $97,472 • Viral hepatitis-related: $117,016 |
|||
|
NASH-related Alcohol-related Viral hepatitis-related |
699,668 NR NR |
|||||
| NASH-related costs with or without comorbidities | ||||||
| O’Hara et al. [30] | US and EU5a | Euros, 2019 | NASH |
Total: 3754 Early (F0–2): 2604 (69%) Advanced (F3–4): 1150 (31%) Germany: 540 Spain: 522 France: 508 UK: 423 Italy: 540 US: 1221 |
Total annual costs PP, mean (SD): • Overall: €4754 (22,117) • F0–2 vs F3–4: €3883 (20,634) vs €6727 (25,052) • DE: €3629 (8795); ES: €3323 (11,258); FR: €2440 (6241); UK: €3879 (3811); IT: €2739 (8296); US: €7258 (36,704) NASH-related annual costs PP, mean (SD): • Overall: €2763 (21,216) • F0–2 vs F3–4: €2224 (19,956) vs €3983 (23,788) • DE: €1917 (7715); ES: €2162 (10,220); FR: €1357 (5427); UK: €1851 (2406); IT: €1732 (7799); US: €4881 (42,077) Non-NASH annual costs PP, mean (SD): • Overall: €1991 (4840) • F0–2 vs F3–4: €1659 (4117) vs €2743 (6107) • DE: €1712 (3698); ES: €1161 (3200); FR: €1082 (2234); UK: €2027 (2897); IT: €1006 (2397); US: €2957 (7170) |
Test/procedure annual costs PP, mean (SD): • Overall: 283 (486) • F0–2 vs F3–4: 265 (439) vs 323 (575) • DE: €183 (221); ES: €168 (180); FR: €124 (117); UK: €415 (668); IT: €220 (448); US: €435 (726) NASH treatment annual costs PP, mean (SD): • Overall: 1106 (3877) • F0–2 vs F3–4: 989 (3596) vs 1373 (4438) • DE: €655 (2218); ES: €739 (3047); FR: €502 (1292); UK: €624 (1550); IT: €431 (1718); US: €2241 (6743) Surgery annual costs PP, mean (SD): • Overall: 915 (20,512) • F0–2 vs F3–4: 653 (19,609) vs 1507 (22,420) • DE: €848 (6740); ES: €735 (8197); FR: €228 (4590); UK: €103 (1141); IT: €573 (7113); US: €1582 (34,825) Consultation annual costs PP, mean (SD): • Overall: 264 (314) • F0–2 vs F3–4: 259 (298) vs 274 (348) • DE: €89 (90); ES: €300 (312); FR: €203 (197); UK: €665 (487); IT: €275 (224); US: €210 (291) |
|
Hospitalisation annual costs PP, mean (SD): • Overall: 195 (2094) • F0–2 vs F3–4: 58 (815) vs 507 (3561) • DE: €143 (967); ES: €219 (2203); FR: €300 (1669); UK: €56 (443); IT: €233 (1232); US: €201 (3420) Non-medical annual costs PPb, mean (SD): • Overall (n=767): 2556 (7308) • F0–2 (n=546) vs F3–4 (n=221): 1704 (5785) vs 4661 (9839) • DE (n=132): €2695 (7273); ES (n=169): €4713 (10,828); FR (n=130): €213 (920); UK (n=20): €2063 (3707); IT (n=219): €1977 (5515); US (n=97): €3243 (7685) | ||||||
| Reja et al. [31] | US | USD, 2016 (Payer) | Hospitalised NASH patients with or without kidney failure, 2016 |
Total: 1196 With kidney failure: 598 Without kidney failure: 598 |
NR |
Total charge of hospitalisation, mean (SD): • Overall: $58,023.7 (116,290.8) • With vs without kidney failure: $78,022.7 (158,580.8) vs $38,024.8 (33,476.0). β/OR = 37,045.9 (95% CI 31,756.18–42,335.62; p < 0.0001) |
aEU5 includes France, Germany, Italy, Spain and the UK
bProfessional (34%) and informal (48%) caregivers account for most non-medical costs. Other costs resulted from transport, disability allowances, alternative therapies, home alteration and OTC remedies
ACLF acute-on-chronic liver failure, CC compensated cirrhosis, CI confidence interval, DE German cohort, ES Spanish cohort, F0–4 fibrosis stages 0–4, FR French cohort, IT Italian cohort, NASH non-alcoholic steatohepatitis, NR not reported, OR odds ratio, OTC over-the-counter, PP per patient, SD standard deviation, UK UK cohort, US US cohort, USD US dollars
Overall, studies showed that direct costs are correlated with disease severity and vary significantly by country, with notably high costs in the US. The GAIN study, which assessed direct costs in a real-world setting in five European countries (EU5) and the US, reported mean annual costs per patient of €4754 in total (per 2019 valuation), composed of €2763 of NASH-related and €1991 of non-NASH costs (Table 4) [30]. Greater direct medical costs and non-medical costs were incurred by patients with advanced disease (F3–4 fibrosis) compared with early-stage disease. Presence of comorbidities also led to higher costs, with two-fold higher total hospitalisation charges reported for patients with versus without kidney failure [31]. Higher per patient hospital charges were reported in patients with NASH-related versus non-NASH-ACLF [27].
Total direct costs (including NASH-related and non-NASH-related costs relating to procedures/tests, NASH treatment, surgery, consultation and hospitalisation) varied considerably by country, with two- to three-fold higher annual costs per person in the US compared with each of the EU5 countries (Table 4) [30]. Higher costs in the US were driven mainly by NASH treatment, surgery and non-medical costs. Total direct costs across the EU5 countries were similar but there was variability in terms of individual direct cost components. For example, the UK reported the highest annual costs for tests/procedures and consultation fees, but the lowest annual costs for surgery and hospitalisations. Non-medical direct costs were lowest in France and highest in Spain followed by the US [30].
Four studies predicted the national cost burden of NASH alone or NASH plus T2D (Supplementary Table 6 in the ESM). In the US and EU5, the GAIN study reported national medical costs ranging from €2.5–5 billion annually across the EU5 countries, rising to €80 billion per year in the US due to higher per person costs and its larger population compared with EU5 countries [30]. For most countries, early-stage NASH (F0–2) appears to account for most of the estimated national medical cost burden, resulting from a greater prevalence of early-stage versus advanced-stage (F3–4) cases, though in Spain and Germany, where higher per person costs were reported for advanced-stage cases compared with the other EU5 countries, the national medical cost burdens were similar for early and advanced (F3–4) NASH. Another study predicted the annual economic burden of NAFLD, broken down by different liver disease health states in the US, Germany, France, Italy and the UK [14]. Direct medical costs attributable to NASH with or without fibrosis ranged from €332 million in Germany to $7.35 billion in the US (calculated as the sum of costs for NASH with fibrosis and NASH without fibrosis), with the cost burden more than doubling when including costs attributed to later stage complications (compensated and decompensated cirrhosis, HCC, LT and post-LT).
Two studies used Markov modelling to estimate the economic burden of NASH direct medical costs in Hong Kong [32] and of NASH with T2D in the US [33] (Supplementary Table 6 in the ESM). Tampi et al. [32] estimated that the average cost of NASH per person-year in Hong Kong is USD$257, with the highest cost seen in those aged ≥80 years. This equated to a 20-year national cost burden of $1.3 billion. Direct medical costs in the US for patients with NASH plus T2D were estimated to be $7349 and $9379 per person-year in incident and prevalent patient cohorts, respectively, of which liver-related costs accounted for 25% or less of the total costs [33]. Total liver-related care costs in the US over 20 years were estimated to be $3.4 billion for the NASH incident cohort and $160 billion for the NASH prevalent cohort.
Indirect Costs
Three studies assessed the indirect costs associated with NASH (Table 5), each of which scored positively in all or most relevant categories of the quality assessment (see section 3.4 for further details). Two studies assessed productivity-related costs associated with employed patients with NASH using the Work Productivity and Activity Impairment-General Health (WPAI-GH) or WPAI-Specific Health Problem (WPAI-SHP) instruments [26, 29]. The WPAI assesses absenteeism, presenteeism, overall work impairment (for employed respondents) and overall activity impairment (for all respondents) in the previous 7 days, with WPAI-SHP completed for NASH impairment specifically. Higher WPAI scores indicate greater impairment. These studies demonstrate that patients with NASH have significantly greater productivity losses versus the general population in terms of absenteeism, presenteeism, overall work impairment (for employed persons) and activity impairment [26], and this is more apparent in symptomatic versus asymptomatic patients [29]. When comparing NASH with T2D, similar WPAI impairment was reported in both groups across all categories [26].
Table 5.
Indirect costs associated with NASH
| Study | Country | Population | N | Absenteeism | Presenteeism | Productivity impairment | Other |
|---|---|---|---|---|---|---|---|
| Balp et al. [26] | EU5a | Respondents to the National Health and Wellness Survey |
Mean WPAI-GH (SD)b: • NASH versus matched gen. population: 28.48 vs 12.36 (p = 0.003) • NASH versus matched T2D: 28.33 vs 17.42 (p = ns) |
Mean WPAI-GH (SD)b: • NASH versus matched gen. population: 33.71 vs 23.03 (p = 0.006) • NASH versus matched T2D: 37.15 vs 26.56 (p = ns) |
Overall work impairment, mean WPAI-GH (SD)b: • NASH vs matched gen. population: 49.15 vs 30.77 (p < 0.001) • NASH vs matched T2D: 50.95 vs 37.06 (p = ns) Activity impairment, mean WPAI-GH (SD): • NASH vs matched gen. population: 48.02 vs 32.64 (p < 0.001) • NASH vs matched T2D: 47.23 vs 41.12 (p = ns) |
||
| NASH | 184 | ||||||
| Unmatched general population | 79,267 | ||||||
| Unmatched T2D | 4783 | ||||||
| Geier et al. [29] | US, France and Germany | NASH patients who completed PROs (NASH-Atlas program July–November 2017) |
Total: 299 Symptomatic: 206 Asymptomatic: 93 |
Mean WPAI:SHP (SD)b: • Overall: 9.0 (22.5) • Symptomatic: 10.4 • Asymptomatic: 5.6 (p = ns vs symptomatic) |
Mean WPAI:SHP (SD)b: • Overall: 17.5 (19.9) • Symptomatic: 23.0 • Asymptomatic: 4.4 (p < 0.05 vs symptomatic) |
Overall work impairment, mean WPAI:SHP (SD)b: • Overall: 24.7 (27.4) • Symptomatic: 30.7 Asymptomatic: 9.0 (p < 0.05 vs symptomatic) Activity impairment, mean WPAI:SHP (SD)b: •Overall: 30.7 (28.5) •Symptomatic: 38.2 •Asymptomatic: 14.2 (p < 0.05 vs symptomatic) |
|
|
Biopsy-confirmed: 160 Symptomatic: 112 Asymptomatic: 48 |
Mean WPAI:SHP (SD)b: • Symptomatic: 7.4 • Asymptomatic: 6.8 (p = ns vs symptomatic) |
Mean WPAI:SHP (SD)b: • Symptomatic: 22.4 • Asymptomatic: 4.4 (p < 0.05 vs symptomatic) |
Overall work impairment, mean WPAI:SHP (SD)b: • Symptomatic: 27.7 • Asymptomatic: 10.1 (p < 0.05 vs symptomatic) Activity impairment, mean WPAI:SHP (SD)b: • Symptomatic: 40.6 • Asymptomatic: 8.5 (p < 0.05 vs symptomatic) |
||||
| O’Hara et al. [30] | US and EU5b | NASH |
Total: 3754 Early (F0–2): 2604 (69%) Advanced (F3–4): 1150 (31%) Germany: 540 Spain: 522 France: 508 UK: 423 Italy: 540 US: 1221 |
Annual indirect costs PP, mean € (SD)c: • Overall: 7871 (18,104) • F0–F2: 5939 (15,989) • F3–4: 12,642 (21,813) • Germany: 2530 (9137) • France: 11,624 (24,003) • Spain: 5451 (12,175) • UK: 9541 (18,223) • Italy: 4994 (8166) • US: 13,435 (29,493) |
aEU5 includes France, Germany, Italy, Spain and the UK
bAbsenteeism, presenteeism and overall work impairment includes only employed respondents
cMost indirect costs resulted from early retirement or stopping working (74%), followed by time off work in the previous 12 months (26%)
F0–4 fibrosis stages 0–4, Gen. general, GH general health; NASH non-alcoholic steatohepatitis, NASH-Atlas Growth from Knowledge (currently Ipsos) Disease Atlas Real-World Evidence program, ns non-significant, PP per patient, PROs patient-reported outcomes, SD standard deviation, SHP specific health problem, T2D type 2 diabetes, WPAI Work Productivity and Activity Impairment
The GAIN study calculated the monetary burden of productivity-related costs, averaging at €7871 per person annually and correlating with disease severity [30]. Costs were largely attributed to stopping work (74%), followed by time off work in the previous 12 months (26%). There was a large variability between countries, with two-to five-fold greater costs reported in France and the US compared with Germany, Italy and Spain.
Quality of Included Studies
All 14 included studies were assessed in 11 quality assessment domains (see Supplementary Table 7 in the ESM). One study scored positively in all 11 categories [33], with other high-scoring studies achieving positive scores in 10 [6, 32], nine [30], eight [27, 31] or seven [22, 23, 26, 28] categories. One study that assessed resource use in NASH cirrhosis LT recipients achieved positive scores in six categories, while four categories were not applicable to the study [21]. A multi-national real-world burden study also scored positively in six categories with the remaining four categories not specified within the publication [29]. One single-centre database study of 15 patients undergoing laparoscopic sleeve gastrectomy following LT scored poorly in six of 11 categories [24], while a multicentre database study assessing 12,442 post-LT patients scored poorly in four areas [25]. The latter two studies both failed to carefully describe and appropriately assess activity data sources, to analytically describe sources of all cost values and to appropriately value unit costs. The most frequently failed or non-evaluable questions were whether direct and indirect costs were sufficiently disaggregated, and whether costs were discounted.
Discussion
Overall, the findings of this study demonstrate that NASH places a significant demand on healthcare resources, and the increasing prevalence of NASH globally suggests that this will worsen in the future. This SLR includes results from large, well-designed database studies that indicate a high economic burden of NASH, but also highlight a general paucity of publications reporting economic outcomes data associated with NASH. This data gap, indicative of an unmet need to better understand the economic burden of NASH, is in line with other recent publications, including an SLR that discusses NASH health economic models [34]. One-third of included studies were published in 2019 or 2020, however, suggesting an increasing focus on understanding the economic impact of NASH.
The identified studies assessed a wide range of cost factors, presented data from a variety of cost perspectives, and used a variety of methods for their measurement. Furthermore, while many studies assessed similar outcomes, they reported varying measurement units for the analysis of the data. Therefore, while trends from the analyses could be assessed, it was generally not possible to make direct comparisons across studies.
There was a general trend for higher hospitalisation rates in patients with NASH versus the general population or patients with T2D [26], and the hospitalisation/LOS burden was greater among NASH populations with comorbid conditions or late-stage complications [27, 28, 31]. NASH-LT was a notable concern, with reported post-transplant LOS ranging from 10 to 46 days [21–23, 25]. Higher pre-transplant hospitalisation rates and longer LOS with NASH-LT versus non-NASH-LT patients [22, 25], coupled with a rapid increase in the proportion of LTs attributable to NASH versus non-NASH aetiology in recent years [22], demonstrate that NASH-LT will be a growing burden on healthcare services in years to come.
NASH was also associated with a high burden in terms of HCRU (drugs and devices, tests and procedures, and visits to healthcare providers). Differences in HCRU were seen between NASH subgroups, with a greater burden reported among biopsy-confirmed versus NASH-suspected patients, and in patients with more advanced [F4] versus less advanced fibrosis. Variation was also seen at a national level in terms of diagnostic testing, which may reflect differences in healthcare systems, local management practices, accessibility of procedures and NASH management guidelines [29].
Although only a limited number of studies assessed the cost burden across subpopulations of NASH patients, identified evidence indicate high direct costs incurred by patients with advanced (F3–4) NASH and those with complications [27, 30, 31]. Variability in per person hospitalisation costs between NASH subpopulations is likely linked to LOS in part, with longer LOS reported for patients with higher costs [27, 31].
National-level, comparative data on per person medical costs was scarce, but available evidence indicate substantial between-countries differences. The highest direct costs per person were reported for the US. This is not unexpected, with the US spending 17.8% of its GDP on healthcare in 2016, compared with 9.6–12.4% in other high-income countries, driven by higher administrative costs of care, pharmaceutical costs and HCP salaries [35]. Non-medical direct costs also varied considerably between countries, driven by large differences in professional and informal caregiver costs between countries. This may reflect cultural differences, but also the relatively low number of patients that returned the questionnaires covering non-medical costs (5–41%) in each country [30].
Four studies assessed the national direct cost burden of NASH or NASH plus T2D, with stark variation seen between nations and predictions that were heavily reliant on the accuracy of NASH prevalence estimates [14, 30, 32, 33]. The studies indicate higher per person costs for patients with more severe disease, although the per person cost disparity between early (F0–2) and advanced (F3–4) fibrosis was variable in different countries, ranging from four-fold greater costs for advanced- versus early-stage fibrosis in Germany to only 1.2-fold greater in the US. In Hong Kong, the predicted direct medical cost burden for NASH alone appears to be much lower than that reported for the US and EU5 countries [32]; however, any comparisons between studies should be interpreted with caution, due to differences in methodologies and prevalence estimations in each study. Two other cost-of-illness studies in the literature evaluated the economic impact of NASH in 2018 [36, 37]. Morgan et al. [36] reported UK health system costs ranging from £200 million (low prevalence estimate) to £369 million (high prevalence estimate). Per person health system costs were estimated to range from £803–828. Schattenberg et al. [37] evaluated costs in the EU5 countries, reporting average health system costs across the countries of €619–1291 and per person costs of €1244–1470. In line with the GAIN study, most economic costs in these studies were experienced in late disease stages.
Although not directly comparable, there were parallels between the national cost burden analyses, including high indirect costs in most countries. In the GAIN study, direct non-medical and indirect costs were high in all countries except France, while Younossi et al. found that the societal costs of NAFLD were consistently higher than direct medical costs in all countries evaluated [14, 30]. Similarly, the published UK and EU5 2018 cost-of-illness studies reported productivity (incurred through reduced workforce participation) and other economic costs (which included care costs, deadweight loss and other costs such as funeral costs due to premature mortality) to be 10-fold higher than health system costs in 2018, ranging from £1999–3707 million in the UK (£8285–8286 per person), and €7928–18,254 million in the EU5 (€15,083–15,212 per person) [36, 37]. There was a higher prevalence of absenteeism, presenteeism, overall work impairment and activity impairment with NASH versus the general population [26], particularly in symptomatic patients [29], indicating a high social burden. However, there was no difference between patients with NASH and those with T2D in terms of productivity, indicating similar impairment on work and daily activities [26].
Diagnosis of NASH is complex, with non-specific symptoms such as fatigue and upper abdominal pain often the only indicators of its presence. In many cases, NASH is not identified until advanced liver damage has already occurred, and despite the increasing prevalence of NASH globally, the disease remains underdiagnosed [38]. This makes it challenging to fully understand the economic and HCRU burden of the disease. In addition, in the absence of approved pharmacological therapies for NASH, current resource use is often directed at managing complications of the disease, meaning that the cost and HCRU burden associated with NASH may change in the coming years and decades as NASH-specific drugs become available. Understanding the resource utilisation and costs associated with NASH is critical to guide priorities and policy, and for society in general, especially when NASH is predicted to become the leading cause of LT in the future.
Limitations and Evidence Gaps
This SLR was limited to English language full-text publications from January 2010 to January 2021 to examine the most recent evidence. Conference abstracts were not included because there were enough full-text articles to provide a sufficient level of relevant information, but it is possible that some relevant preliminary trial results published as conference materials were omitted. Most of the studies were retrospective in nature and included heterogenous patient populations. In addition, some studies had small patient or response numbers, and some used extrapolation methodologies. Thirteen of 14 studies were conducted in Europe and/or North America, and over half were conducted in the US. Additional studies that assess costs in the rest of the world, including developing countries, are warranted to better understand the global economic burden of NASH. In many of the studies, patients had advanced fibrosis stage (including populations with cirrhosis or LT recipients), and thus average per patient costs are likely to be higher than in the overall NASH population. The burden of NASH was stratified by patient sex in only one of 14 studies, in which male patients with or without comorbid diabetes had a higher rate of hospital admissions compared with female patients [28]. Future studies may wish to consider the impact of sex on other aspects of costs and HCRU.
There was wide variation across the included studies regarding the type of cost and resource use burden reported, making it difficult to draw conclusions about specific costs and resource use associated with NASH, and, consistent with the findings of a recent appraisal of NASH health economic models [34], we found limited data on NASH-specific costs for different NASH disease stages and/or late-stage complications. Several studies included patient-reported data [26, 29, 30], which are susceptible to potential inaccuracies resulting from differences in health literacy, memory and patient condition (e.g. level of fatigue). Furthermore, some patient surveys were completed by only a small proportion of patients, which could contribute to variability seen in some outcomes.
More national-level NASH prevalence data is needed to generate accurate forecasts of HCRU and costs in the coming decades. In addition, available evidence on indirect or societal costs and caregiver burden is scarce and further research is justified to fully appreciate their impact on the economic burden of NASH. Finally, several of the included studies did not report data from patients with NASH alone but reported data from those with NASH with complications or comorbidities (e.g. T2D, kidney disease, ACLF), making it difficult to determine the cost burden driven purely by NASH and limiting the comparability of data across studies. It would be beneficial for future studies to separate costs and resource use based on comorbid conditions (e.g. cost of hospitalisation in patients with NASH only; NASH plus obesity; NASH plus obesity and T2D) to understand if HCRU increases alongside the number of comorbid conditions.
Conclusions
There is a scarcity of NASH-specific economic outcomes data, and a lack of consistency amongst available data leads to difficulties in estimating the true economic burden associated with NASH. Despite this, the studies identified in this SLR show that NASH is associated with a significant economic burden in terms of increased HCRU, direct medical costs and societal burden, that increases with disease severity or when patients have complications or comorbidity. Increasing incidence rates of NASH coupled with the propensity for disease progression, duration of hospitalisation and associated amount of resource use are major factors in the economic burden of NASH to be absorbed by healthcare providers and patients alike. This burden, in parallel with the increasing obesity and T2D epidemics, is only likely to increase over time.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This article was supported by Novo Nordisk A/S, who performed a medical accuracy review. Medical writing and editorial support were provided by Sally Humphries and Andy Bond of Aura, a division of Spirit Medical Communications Group Limited (funded by Novo Nordisk) under the direction of the authors.
Declarations
Funding
This study was funded by Novo Nordisk A/S, Søborg, Denmark.
Conflicts of Interest/Competing Interests
SIM, PJ and MA are employees of Novo Nordisk A/S or Novo Nordisk Denmark A/S, and JF was formerly an employee of Novo Nordisk A/S. SIM, PJ, MA and JF are also shareholders of Novo Nordisk A/S. MW and SN are employees of DRG Abacus (Clarivate), who were commissioned to perform this systematic literature review by Novo Nordisk A/S.
Availability of Data and Material
Not applicable to this article as no datasets were generated or analysed during the current study.
Code Availability
Not applicable to this article.
Ethics Approval
Not applicable to this article.
Consent to Participate
Not applicable to this article.
Consent for Publication
Not applicable to this article.
Authors' Contributions
PJ provided the study concept and design. JF, PA and SN performed the data extraction. All authors contributed to the analysis and interpretation of the data and contributed to the drafting of the paper, critically revised the paper for intellectual content, were involved in the final approval of the version to be published and agreed to be accountable for all aspects of the work. PJ is the overall guarantor.
References
- 1.Lindenmeyer CC, McCullough AJ. The natural history of nonalcoholic fatty liver disease-an evolving view. Clin Liver Dis. 2018;22(1):11–21. doi: 10.1016/j.cld.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flisiak-Jackiewicz M, Bobrus-Chociej A, Wasilewska N, Lebensztejn DM. From nonalcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated fatty liver disease (MAFLD)-new terminology in pediatric patients as a step in good scientific direction? J Clin Med. 2021;10(5):924. doi: 10.3390/jcm10050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–568. doi: 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.Banini BA, Sanyal AJ. Nonalcoholic fatty liver disease: epidemiology, pathogenesis, natural history, diagnosis, and current treatment options. Clin Med Insights Ther. 2016;8:75–84. doi: 10.4137/cmt.s18885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 11.Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc. 2015;90(9):1233–1246. doi: 10.1016/j.mayocp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol. 2019;71(2):313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thuluvath PJ, Hanish S, Savva Y. Waiting list mortality and transplant rates for NASH cirrhosis when compared with cryptogenic, alcoholic, or AIH cirrhosis. Transplantation. 2019;103(1):113–121. doi: 10.1097/TP.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO guide to identifying the economic consequences of disease and injury. 2009. https://apps.who.int/iris/bitstream/handle/10665/137037/9789241598293_eng.pdf?sequence=1&isAllowed=y. Accessed 19 Apr 2021.
- 16.Durand-Zaleski I. Why cost-of-illness studies are important and inform policy. Vasc Med. 2008;13(3):251–253. doi: 10.1177/1358863X08091738. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane handbook for systematic reviews of interventions version 6.0. Cochrane. 2019. http://training.cochrane.org/handbook. Accessed 11 February 2020.
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3. Oxford: Oxford University Press; 2005. [Google Scholar]
- 20.Molinier L, Bauvin E, Combescure C, Castelli C, Rebillard X, Soulié M, et al. Methodological considerations in cost of prostate cancer studies: a systematic review. Value Health. 2008;11(5):878–885. doi: 10.1111/j.1524-4733.2008.00327.x. [DOI] [PubMed] [Google Scholar]
- 21.Aby ES, Lee E, Saggi SS, Viramontes MR, Grotts JF, Agopian VG, et al. Pretransplant sarcopenia in patients with NASH cirrhosis does not impact rehospitalization or mortality. J Clin Gastroenterol. 2019;53(9):680–685. doi: 10.1097/MCG.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 22.Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, Rana A, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256(4):624–633. doi: 10.1097/SLA.0b013e31826b4b7e. [DOI] [PubMed] [Google Scholar]
- 23.Barbas AS, Goldaracena N, Dib MJ, Al-Adra DP, Aravinthan AD, Lilly LB, et al. Early intervention with live donor liver transplantation reduces resource utilization in NASH: the Toronto experience. Transplant Direct. 2017;3(6):e158. doi: 10.1097/TXD.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris MC, Jung AD, Kim Y, Lee TC, Kaiser TE, Thompson JR, et al. Delayed sleeve gastrectomy following liver transplantation: a 5-year experience. Liver Transpl. 2019;25(11):1673–1681. doi: 10.1002/lt.25637. [DOI] [PubMed] [Google Scholar]
- 25.Hoehn RS, Singhal A, Wima K, Sutton JM, Paterno F, Woodle ES, et al. Effect of pretransplant diabetes on short-term outcomes after liver transplantation: a national cohort study. Liver Int. 2015;35(7):1902–1909. doi: 10.1111/liv.12770. [DOI] [PubMed] [Google Scholar]
- 26.Balp M-M, Krieger N, Przybysz R, Way N, Cai J, Zappe D, et al. The burden of non-alcoholic steatohepatitis (NASH) among patients from Europe: a real-world patient-reported outcomes study. JHEP Rep. 2019;1(3):154–161. doi: 10.1016/j.jhepr.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axley P, Ahmed Z, Arora S, Haas A, Kuo Y-F, Kamath PS, et al. NASH is the most rapidly growing etiology for acute-on-chronic liver failure-related hospitalization and disease burden in the United States: a population-based study. Liver Transpl. 2019;25(5):695–705. doi: 10.1002/lt.25443. [DOI] [PubMed] [Google Scholar]
- 28.Carruthers JE, Bottle A, Laverty AA, Khan SA, Millett C, Vamos EP. Nation-wide trends in non-alcoholic steatohepatitis (NASH) in patients with and without diabetes between 2004-05 and 2014-15 in England. Diabetes Res Clin Pract. 2017;132:102–107. doi: 10.1016/j.diabres.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Geier A, Rinella ME, Balp M-M, McKenna SJ, Brass CA, Przybysz R, et al. Real-world burden of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2021;19(5):1020–9.e7. doi: 10.1016/j.cgh.2020.06.064. [DOI] [PubMed] [Google Scholar]
- 30.Ohara J, Finnegan A, Dhillon D, Ruiz-Casas L, Pedra G, Franks B, et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: the GAIN study. JHEP Rep. 2020;2(5):100142. doi: 10.1016/j.jhepr.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reja M, Patel R, Pioppo L, Tawadros A, Bhurwal A, Marino D, et al. Renal failure is associated with increased mortality and hospital utilization in patients admitted with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2021;55(5):433–438. doi: 10.1097/MCG.0000000000001389. [DOI] [PubMed] [Google Scholar]
- 32.Tampi RP, Wong VW-S, Wong GL-H, Shu SS-T, Chan HL-Y, Fung J, et al. Modelling the economic and clinical burden of non-alcoholic steatohepatitis in East Asia: data from Hong Kong. Hepatol Res. 2020;50(9):1024–1031. doi: 10.1111/hepr.13535. [DOI] [PubMed] [Google Scholar]
- 33.Younossi ZM, Tampi RP, Racila A, Qiu Y, Burns L, Younossi I, et al. Economic and clinical burden of nonalcoholic steatohepatitis in patients with type 2 diabetes in the U.S. Diabetes Care. 2020;43(2):283–289. doi: 10.2337/dc19-1113. [DOI] [PubMed] [Google Scholar]
- 34.Johansen P, Howard D, Bishop R, Moreno SI, Buchholtz K. Systematic literature review and critical appraisal of health economic models used in cost-effectiveness analyses in non-alcoholic steatohepatitis: potential for improvements. Pharmacoeconomics. 2020;38(5):485–497. doi: 10.1007/s40273-019-00881-7. [DOI] [PubMed] [Google Scholar]
- 35.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–1039. doi: 10.1001/jama.2018.1150. [DOI] [PubMed] [Google Scholar]
- 36.Morgan A, Hartmanis S, Tsochatzis E, Newsome PN, Ryder SD, Elliott R, et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis (NASH) in the United Kingdom (UK) in 2018. Eur J Health Econ. 2021;22(4):505–518. doi: 10.1007/s10198-020-01256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schattenberg JM, Lazarus JV, Newsome PN, Serfaty L, Aghemo A, Augustin S, et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis in five European countries in 2018: a cost-of-illness analysis. Liver Int. 2021;41(6):1227–1242. doi: 10.1111/liv.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Povsic M, Wong OY, Perry R, Bottomley J. A structured literature review of the epidemiology and disease burden of non-alcoholic steatohepatitis (NASH) Adv Ther. 2019;36(7):1574–1594. doi: 10.1007/s12325-019-00960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


