Abstract
We report a case of right upper limb ischaemia diagnosed at birth in a neonate whose mother had presented with paucisymptomatic COVID-19 four weeks previously. Typical causes were investigated and excluded. Maternal morbidity and mortality resulting from COVID-19 during pregnancy is well recognised and documented, however, foetal and neonatal complications are increasingly being reported. Our case sheds further light on the diverse nature of such complications, and in particular this type of possible association related to their delayed onset.
Keywords: COVID-19, Foetus, New-born, Thrombosis, Limb ischaemia
Background
COVID-19 during pregnancy has been reported in the literature to lead to an increased risk of acute complications including miscarriage, preeclampsia, premature delivery, and foetal death in utero [1,2]. Vertical transmission of COVID-19 from mother to foetus, and its direct consequences, has recently been described [3]. However, the indirect consequences, whether foetal or neonatal, are still poorly known, particularly medium- and long-term consequences in relation to the episode of maternal infection [4]. Some studies have demonstrated the existence of placental tissue damage associated with COVID-19 during pregnancy and upon delivery [[4], [5], [6], [7]]. Such conditions lead to hypercoagulability induced by the virus, in addition to overall inflammation due to the infection and bed confinement associated with the symptomatic phase [8,9]. The case presented here raises important concerns about possible delayed foetal and neonatal morbidity following COVID-19 in pregnant women, even in cases with a mildly symptomatic clinical course.
Case presentation
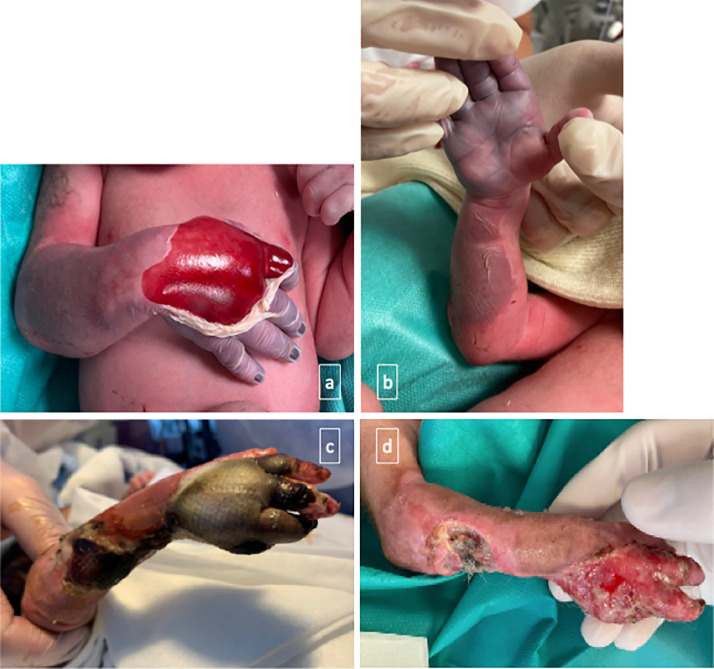
We report the case of a 29-year-old female patient (gravidity 3, parity 2) with no notable medical history. Obstetric monitoring was unremarkable for pregnancy. The patient presented with an influenza-like illness at 31 weeks and a positive COVID-19 PCR test. She was admitted at 35+1 weeks having experienced decreased active foetal movements for three days. Based on an ultrasound scan, foetal weight was estimated at 2030 g ± 10% (< 5th percentile) with a PI > 95th percentile, a positive diastole according to umbilical Doppler, and cerebro-placental inversion. It should be noted that at the second and third trimester ultrasound, the foetus was eutrophic at the 30th percentile with sustained growth. Cardiotocography (CTG) revealed a micro-oscillating and non-reactive foetal heart rate with no deceleration and no uterine contraction. A COVID-19 PCR test, performed routinely upon admission, was negative. As the foetal heart rate was non-reactive, an emergency cesarian section was performed outside of labor under general anesthesia, which resulted in the birth of a baby boy weighing 2050 gs (3rd percentile) with the following blood arterial gas analyses at birth: pH: 6.92, PCo2: 82 mm Hg, HCO3-: 16.5 mmol/l, base excess – 18.4, and lactates: 9.75 mmol/l. A neonatal examination revealed ischaemia of the right upper limb, affecting the hand and forearm to the elbow, with major skin detachment and a haemorrhagic bulla on the dorsal aspect of the hand. The thumb and thenar eminence areas were unaffected (Figs. 1 a and b). There was an absence of radial and humeral pulses, apparent pain, and spontaneous movement of the right upper limb. A neonatal blood test revealed thrombocytopenia at 61 G/L, severe prothrombin deficiency at 25%, fibrinogen at 0.96 g/L, and a protein C level of 23% (compared to the norm of 20%). Given this context, the child was transferred to a tertiary referral center for further treatment with intravenous antibiotic therapy, administration of vitamin K, and a platelet transfusion. Doppler ultrasound of the upper limbs showed good patency of the right axillary and subclavian arteries, however, a patency defect in the proximal portion of the humeral artery was identified with no anomaly in the brachial and cephalic veins. After multi-disciplinary discussion, it was decided to continue medical treatment with intravenous curative heparin therapy and daily wound care. Local improvement in the ischaemic appearance was observed in the following days (Fig. 1c). An angioscan performed when the baby was seven days old revealed the presence of a residual non-obstructive clot in the proximal part of the ulnar artery with good patency of a secondary network supplying the palmar arch, which accounted for the regression of necrosis, particularly in the thumb. Finally, after stabilization of the necrotic areas, partial amputation of the 4th and 5th rays of the right hand was performed at 24 days of age, combined with debridement of the dorsal aspect of the hand and the ulnar border of the forearm (Fig. 1d). Skin healing was achieved a fortnight after the operation and the new-born was discharged after one and a half months in hospital.
Fig. 1.
Clinical course of ischaemia: at birth (a, b), at D21 of life (c), and at D29 of life, i.e. five days after amputation (d).
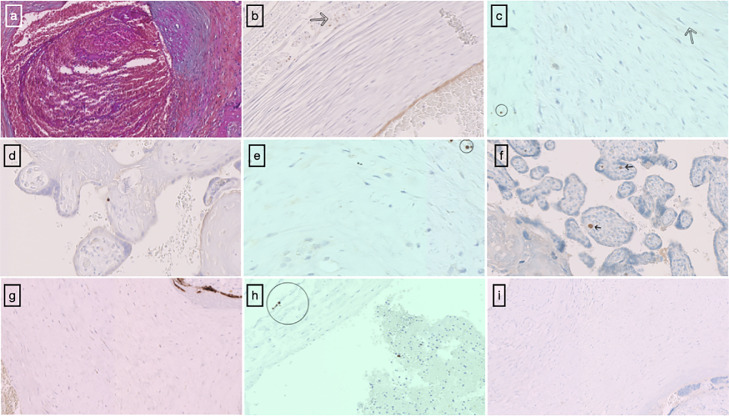
Sterile blood cultures were revealed in the mother. Thrombophilia tests (Antithrombin, Protein C, Protein S, Lupus anticoagulant, Factor V Leiden, Prothrombin gene mutation, anti-β−2-Glycoprotein-1 antibodies, and anti-Cardiolipin antibodies) performed on both parents were negative. However, such negative thrombophilia tests cannot exclude with certainty the presence of thrombophilia in the child. The baby presented with a global coagulation factor deficit, but this is usual for age and term. When rechecked at a later date, no anomalies were found. No platelet incompatibility was found between the mother and the new-born. An anatomopathological examination of the placenta revealed first and foremost a eutrophic specimen for term with lesions of foetal vascular malperfusion (FVM) (Fig. 2 a) including drastic thrombosis of the large chorionic trunks and high-grade lesions of the distal villi with large patches of avascular villi, sparse mesenchymal expression of the S1 spike protein (umbilical cord and chorionic plate vessels) (Fig. 2b and c) with a predominantly perivascular tropism and rare villous mesenchymal expression, a positive staining in rare fibroblastic-type cells in the placental villi as well as in chorionic plate (Fig. 2d and e) as well as images of chronic residual villitis on CD3 immunostaining (Fig. 2f) (References of the antibodies at Supplementary material 1). Placental specimens, the amniotic membrane, and the umbilical cord were found to be free of viral RNA based on molecular techniques (qPCR). In order to rule out any doubts over an immunohistochemistry artefact we performed a negative and a positive control of the immunostaining using similar pre-analytic conditions of formalin fixation (Fig. 2g, h, and i) (Supplementary material 2).
Fig. 2.
Microscopic examination of the placenta: Histopathological features of the placenta with thrombosis of chorionic vessels and intramural fibrin deposition (a) (b) and (c) Brown staining of mesenchymal cells of umbilical cord with SARS-CoV-2 Spike protein anti-body, arrow: vascular wall (d) and (e) Positive staining in rare fibroblastic-type cells in the placental villi as well as in chorionic plate using an antibody to SARS-CoV-2 Spike protein (x40) (f) Lymphocytic villositis with CD3 immunostaining (arrow) (x25). (g) Positive placenta control SARS-CoV-2 Spike protein with brown staining of the trophoblast layer (to right corner), fibroblastic-type cells of the chorionic plate (on the left) (x20) as well as exceptional mesenchymal cells of umbilical cord (h). (i) Negative placenta control SARS CoV-2 Spike protein.
A COVID-19 PCR test was not performed at the time of the child's birth, and the child later showed no suggestive symptoms. This test was performed when the baby was 20 days old, in preparation for surgery, and was negative. However, COVID-19 serology of the baby at one month of age revealed the presence of IgG antibodies and absence of IgM. These serological findings may reflect foetal and/or neonatal infection or result from the transplacental passage of maternal antibodies.
Discussion
In contrast to the only similar case previously published (contracting COVID-19 some days before and being positive at delivery) [10], in our case, the mother had contracted COVID-19 a month before birth, and was negative at the time of delivery. Little is known about the prothrombotic nature of this infection in utero. We report here on drastic placental vascular malperfusion lesions that may cause the fetal/neonatal complication described. Such placental abnormalities have been previously reported in patients with COVID-19 with rare cases of high-grade FVM being described [6]. However, we were unable to find any histological lesions classically associated with recent active transplacental COVID-19 infection, such as lymphohistiocytic intervillitis, intervillous thrombosis, or notable trophoblastic necrosis. In published cases of vertical transmission with severe fetal adverse effects, viral particles, proteins, and PCR were strongly positive in fetal placental tissues. Indeed, in those cases, they were acute COVID-19 infection. The delivery, examination of the placenta, COVID-19 immuno staining and molecular testing were performed grossly at the same time of the infectious event and the adverse fetal outcome [2]. We know much less about old, or sequellar, trans placental infection with COVID-19, like we supposed in this observation (viral infection occurred at 31 weeks and vascular fetal complications, delivery with examination of the placenta, immune staining, and molecular testing performed one month later). Only the expression within the placental villi was reported in those publications. Indeed, the trophoblastic expression is more obvious to visualize as we noted on the positive control comparing to mesenchymal peri vascular cells immunostaining with viral protein. These aspects have not been reported in the published cases of trans placental COVID-19 infections and virological sampling were not performed and/or not mentioned on the umbilical cord or on chorionic plate but rather on the placental tissue. No differentiated virological sampling of the chorionic plate or umbilical cord was specified in those publications nor vascular fetal malperfusion abnormalities.
2021 data obtained from umbilical cord-derived mesenchymal stem cells show that COVID-19 receptor mARN expression is absent/very low in these cells. These findings were consistent with the very low expression of viral protein on positive control as well as on the umbilical cord of the patient. Interesting fact, we observed in the positive control a lower positivity of viral protein on the umbilical cord comparing to the mesenchymal cells of chorionic plate supposing that it could be a variable expression between umbilical cord and chorionic plate COVID-19 mesenchymal cell receptor mARN. The kinetic of expression receptor mARN could also be variable according to the term of the pregnancy. In the 2021 data, obtained from umbilical cord-derived mesenchymal stem cells, the term of pregnancy were not specified as well.
The presence of rare traces of viral proteins in the villous mesenchyme, as well as stigmata of chronic villitis, may point to longstanding transplacental infection, for which we emphasize predominant tropism to perivascular and non-trophoblastic cells, which may probably cause this unusual presentation. To our knowledge, the S1 spike protein labeling sites reported here have not been reported elsewhere in the literature. Although it was not possible to demonstrate viral RNA using molecular techniques (qPCR), we cannot rule out a potential link between COVID-19 infection and this thrombotic neonatal outcome. This complication could be the consequence of an earlier infection (the interval between maternal infection and the birth of the baby was one month) due to the presence and very low expression of the S1 spike protein. Moreover, we noted some positivity of Spike Protein on rare mesenchymal cell villi in addition to the presence of residual chronic villitis on CD3 immunostaining allowed us to evoke the hypothesis of a sequellar COVID-19 trans placental infection associated to fetal vascular malperfusion. Furthermore, this immunostaining was performed on formalin-fixed, paraffin-embedded tissue and not on fresh tissue, as recommended [11], and areas of viral tropism were not targeted for molecular examination.
Typical foetal and neonatal thrombotic aetiologies, such as amniotic bridge, Kasabach-Merrit syndrome [12], and congenital coagulation factor deficiency, as well as maternal and paternal origins, were ruled out. The relationship between maternal COVID-19 and upper limb ischaemia in the case reported here is of interest, as it highlights the possibility of delayed foetal/neonatal complications.
Conclusion
We cannot conclude with certainty that the COVID-19 alone was responsible for such vascular fetal complication, but it is interesting to describe another such association and that weak perivascular protein expression at distance of infection. We highlight the need, in cases of such proven acute vertical infection, to take differentiated samples (umbilical cord, membranes and placenta) at variable terms of gestation in order to appreciate expression of viral protein and receptor viral mRNA to better interpret them in the supposed cases of possible sequellar infection.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Acknowledgments
Thanks to Dr Christine ZANDOTTI, Céline GAZIN, and to Pr Jean-Louis MEGE for their contribution to the biological evaluation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jogoh.2022.102443.
Appendix. Supplementary materials
References
- 1.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15‑27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesieur E., Torrents J., Fina F., Zandotti C., Blanc J., Collardeau-Frachon S., et al. Congenital infection of SARS-CoV-2 with intrauterine foetal death: a clinicopathological study with molecular analysis. Clin Infect Dis. 2021:ciab840. doi: 10.1093/cid/ciab840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224(1):35–53.e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baergen R.N., Heller D.S. Placental Pathology in Covid-19 Positive Mothers: preliminary Findings. Pediatr Dev Pathol. 2020;23(3):177‑80. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baral G., Shrestha O., Baral R.S. Thrombotic Pathology in Placenta of COVID Positive Pregnancy. J Nepal Health Res Counc. 2021;19(1):206‑8. doi: 10.33314/jnhrc.v19i1.3403. [DOI] [PubMed] [Google Scholar]
- 6.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental Pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23‑32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulersen M., Prasannan L., Tam Tam H., Metz C.N., Rochelson B., Meirowitz N., et al. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol MFM. 2020;2(4) doi: 10.1016/j.ajogmf.2020.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachodimitropoulou Koumoutsea E., Vivanti A.J., Shehata N., Benachi A., Le Gouez A., Desconclois C., et al. COVID-19 and acute coagulopathy in pregnancy. J Thromb Haemost. 2020;18(7):1648‑52. doi: 10.1111/jth.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadir R.A., Kobayashi T., Iba T., Erez O., Thachil J., Kazi S., et al. COVID-19 coagulopathy in pregnancy: critical review, preliminary recommendations, and ISTH registry-Communication from the ISTH SSC for Women’s Health. J Thromb Haemost. 2020;18(11):3086‑98. doi: 10.1111/jth.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ProQuest Central Neonate born with ischemic limb to a COVID-19 positive mother: management and review of literature. Case Reports in Perinatal Medicine. 2021;(1) https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-978952 |. |[Internet]. [cité 22 févr 2022]. Disponible sur: [Google Scholar]
- 11.Roberts D.J., Edlow A.G., Romero R.J., Coyne C.B., Ting D.T., Hornick J.L., et al. A standardized definition of placental infection by SARS-CoV-2, a consensus statement from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development SARS-CoV-2 Placental Infection Workshop. Am J Obstet Gynecol. 2021;225(6) doi: 10.1016/j.ajog.2021.07.029. 593.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Famularo G. Kasabach-Merritt Syndrome. Am J Med. 2020;133(12):e747. doi: 10.1016/j.amjmed.2020.02.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.