Abstract
The prognostic performance of PREDICT in patients with HER2-positive early breast cancer (EBC) treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies is unclear. Therefore, we investigated its prognostic performance using data extracted from ALTTO, a phase III trial evaluating adjuvant lapatinib ± trastuzumab vs. trastuzumab alone in patients with HER2-positive EBC. Our analysis included 2794 patients. After a median follow-up of 6.0 years (IQR, 5.8–6.7), 182 deaths were observed. Overall, PREDICT underestimated 5-year OS by 6.7% (95% CI, 5.8–7.6): observed 5-year OS was 94.7% vs. predicted 88.0%. The underestimation was consistent across all subgroups, including those according to the type of anti HER2-therapy. The highest absolute differences were observed for patients with hormone receptor negative-disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively). AUC under the ROC curve was 73.7% (95% CI 69.7–77.8) in the overall population, ranging between 61.7% and 77.7% across the analyzed subgroups. In conclusion, our analysis showed that PREDICT highly underestimated OS in HER2-positive EBC. Hence, it should be used with caution to give prognostic estimation to HER2-positive EBC patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.
Subject terms: Breast cancer, Targeted therapies
Introduction
The addition of trastuzumab to adjuvant chemotherapy has dramatically improved the outcomes of patients with HER2-positive early breast cancer, reducing the risk of mortality by more than 30%1. Despite the undoubted benefit of adjuvant therapy, several clinical questions remain open. Approximately 25% of patients still experience recurrence up to 10 years from diagnosis, and further research efforts are needed to better refine patient selection for adopting escalation or de-escalation treatment strategies2,3.
PREDICT (www.predict.nhs.uk) is a publicly available, online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery. It uses traditional clinical-pathological factors, and it is aimed to support clinical decision making in the adjuvant setting. The original version of PREDICT (v.1.0) was derived from cancer registry information on 5,694 women treated in East Anglia from 1999–2003, and was subsequently validated in several datasets of patients with breast cancer4,5. In 2011, the model was updated to include HER2 status. Estimates for the prognostic effect of HER2 status were based on an analysis of 10,179 cases collected by the Breast Cancer Association Consortium (BCAC), none of which had been diagnosed after 2004, to ensure that patients did not receive trastuzumab6. A subsequent validation was done in 2012 in a British Columbia Canadian cohort7. This study demonstrated that the inclusion of the HER2 status allowed the model to perform better than the previous PREDICT version and Adjuvant! Online in estimating overall and breast-cancer-specific survival7.
Although the use of PREDICT is recommended to aid decision making in the adjuvant setting8, its prognostic role in HER2-positive early breast cancer patients treated with modern chemotherapy and anti-HER2 therapies remains unclear. We aimed to investigate the prognostic performance of PREDICT in patients with HER2-positive early breast cancer who received trastuzumab-based therapy started concurrently with chemotherapy within the ALTTO trial. The ALTTO trial is the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer and, including at least 5-year follow-up data from all patients9, represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease.
Results
Out of 8381 patients included in the ALTTO trial, 2836 were treated with chemotherapy and concurrent trastuzumab-based therapy and were potentially eligible for the present analysis. In 42 patients, the PREDICT algorithm was not evaluable (due to age of the patient <25 years old [n = 7], missing tumor size [n = 13], or missing lymph nodes status [n = 22]). Therefore, 2794 patients were included in the present analysis (Fig. 1).
Fig. 1. STROBE flow-chart.
This figure illustrates the patient selection process.
Most patients (71%) were aged between 41 and 64 years (Table 1). Twenty-five percent of patients had negative nodal status, 45% had a tumor size ≤2 cm and 58% had hormone receptor-positive disease. Regarding administered treatments, 88% underwent an anthracycline-based chemotherapy regimen (design 2). The majority of patients with hormone receptor-positive disease (45%) received a selective estrogen receptor modulator (SERM) (tamoxifen).
Table 1.
Characteristics of the patients (overall and per randomization arm).
| All patients | Trastuzumab + lapatinib | Trastuzumab alone | Trastuzumab followed by lapatinib | |
|---|---|---|---|---|
| N (%) | 2794 (100.0) | 925 (100.0) | 936 (100.0) | 933 (100.0) |
| Age at randomization | ||||
| ≤40 | 495 (17.7) | 161 (17.4) | 160 (17.1) | 174 (18.7) |
| 41–64 | 1989 (71.2) | 667 (72.1) | 667 (71.3) | 655 (70.2) |
| ≥65 | 310 (11.1) | 97 (10.5) | 109 (11.7) | 104 (11.2) |
| Ethnicity | ||||
| Asian | 606 (21.7) | 201 (21.7) | 200 (21.4) | 205 (22.0) |
| Black | 59 (2.1) | 28 (3.0) | 14 (1.5) | 17 (1.8) |
| White | 2001 (71.6) | 657 (71.0) | 677 (72.3) | 667 (71.5) |
| Other/missing | 128 (4.6) | 39 (4.2) | 45 (4.8) | 44 (4.7) |
| Histology | ||||
| Ductal | 2605 (93.2) | 868 (93.8) | 867 (92.6) | 870 (93.2) |
| Lobular | 101 (3.6) | 34 (3.7) | 38 (4.1) | 29 (3.1) |
| Others | 119 (4.3) | 35 (3.8) | 43 (4.6) | 41 (4.4) |
| Central HR status | ||||
| Negative | 1185 (42.4) | 393 (42.5) | 398 (42.5) | 394 (42.2) |
| Positive | 1609 (57.6) | 532 (57.5) | 538 (57.5) | 539 (57.8) |
| Number of positive lymph nodes | ||||
| 0 | 567 (25.5) | 180 (24.7) | 200 (26.5) | 187 (25.3) |
| 1–3 | 945 (42.6) | 319 (43.8) | 314 (41.6) | 312 (42.3) |
| ≥4 | 709 (31.9) | 230 (31.6) | 240 (31.8) | 239 (32.4) |
| Tumor size (mm) | ||||
| ≤20 mm | 1248 (44.7) | 397 (42.9) | 436 (46.6) | 415 (44.5) |
| 21–50 mm | 1356 (48.5) | 466 (50.4) | 439 (46.9) | 451 (48.3) |
| >50 mm | 190 (6.8) | 62 (6.7) | 61 (6.5) | 67 (7.2) |
| Tumor grade | ||||
| 1 | 79 (2.8) | 26 (2.8) | 22 (2.4) | 31 (3.3) |
| 2 | 936 (33.6) | 310 (33.6) | 295 (31.6) | 331 (35.6) |
| 3 | 1698 (60.9) | 561 (60.9) | 589 (63.0) | 548 (58.9) |
| X (differentiation cannot be assessed) | 75 (2.7) | 25 (2.7) | 29 (3.1) | 21 (2.3) |
| Surgery | ||||
| BCS | 1226 (43.9) | 399 (43.1) | 408 (43.6) | 419 (44.9) |
| Mastecomy | 1538 (56.1) | 526 (56.9) | 529 (56.4) | 514 (55.1) |
| Type of CT | ||||
| Non-anthracycline based | 322 (11.5) | 103 (11.1) | 109 (11.7) | 110 (11.8) |
| Anthracycline-based | 2472 (88.5) | 822 (88.9) | 827 (88.4) | 823 (88.2) |
| Type of endocrine therapy | ||||
| AI | 581 (39.4) | 192 (39.4) | 197 (40.3) | 192 (38.5) |
| AI & SERM | 212 (14.4) | 68 (14.0) | 77 (15.8) | 67 (13.4) |
| LHRH | 11 (0.8) | 4 (0.8) | 5 (1.0) | 2 (0.4) |
| SERM | 671 (45.5) | 223 (45.8) | 210 (42.9) | 238 (47.7) |
HR hormone receptors, BCS breast conserving surgery, CT chemotherapy, AI aromatase inhibitors, SERM selective estrogen receptor modulators, LHRH luteinizing hormone-releasing hormone.
Median follow-up of included patients was 6.0 years (interquartile range: 5.8–6.7). Overall, 182 deaths were observed.
Calibration
Median predicted and observed 5-year overall survival (OS) were 88.0% and 94.7%, respectively (standard error 0.0044, difference −6.7%, 95% Confidence Intervals [CI] −7.5 to −5.8), thus indicating an underestimation of OS by PREDICT score (Table 2, Fig. 2).
Table 2.
Median predicted probability of 5-year overall survival and observed 5-year overall survival rate in the study population.
| % 5 years OS | |||||
|---|---|---|---|---|---|
| Predicted | Observed | s. e. | Difference (95% CI) | ||
| All patients | 2794 | 88.00 | 94.69 | 0.44 | −6.69 (−7.55 to–5.83) |
| Type of anti-HER2 therapy | |||||
| Lapatinib + trastuzumab | 925 | 87.90 | 94.88 | 0.75 | −6.98 (−8.45–5.51) |
| Trastuzumab alone | 936 | 87.90 | 94.18 | 0.79 | −6.28 (−7.83–4.73) |
| Trastuzumab followed by lapatinib | 933 | 88.20 | 95.02 | 0.73 | −6.82 (−8.25–5.39) |
| Type of chemotherapy | |||||
| Non-anthracycline-based | 322 | 88.15 | 96.22 | 1.12 | −8.07 (−10.27–5.87) |
| Anthracycline-based | 2472 | 87.95 | 94.51 | 0.47 | −6.56 (−7.48–5.64) |
| Age at randomization | |||||
| ≤40 | 495 | 90.40 | 95.64 | 0.95 | −5.24 (−7.10–3.38) |
| 41–64 | 1989 | 88.20 | 94.91 | 0.51 | −6.71 (−7.71–5.71) |
| ≥65 | 310 | 82.05 | 91.78 | 1.61 | −9.73 (−12.89–6.57) |
| Central HR status | |||||
| Negative | 1185 | 80.20 | 93.15 | 0.76 | −12.95 (−14.44–11.46) |
| Positive | 1609 | 93.10 | 95.82 | 0.52 | −2.72 (−3.74–1.70) |
| Number of positive lymph nodes | |||||
| 0 | 567 | 91.80 | 97.93 | 0.62 | −6.13 (−7.35–4.91) |
| 1–3 | 945 | 87.40 | 96.40 | 0.63 | −9.00 (−10.23–7.77) |
| ≥4 | 709 | 71.80 | 87.61 | 1.27 | −15.81 (−18.30–13.32) |
| Tumor size (mm) | |||||
| ≤20 mm | 1248 | 91.25 | 97.43 | 0.46 | −6.18 (−7.08–5.28) |
| 21–50 mm | 1356 | 86.00 | 93.33 | 0.70 | −7.33 (−8.70–5.96) |
| >50 mm | 190 | 71.05 | 86.37 | 2.59 | −15.32 (−20.40–10.24) |
OS overall survival, s.e. standard error, CI confidence interval, CT chemotherapy, HR hormone receptors.
Fig. 2. Calibration plot showing observed versus predicted 5-year overall survival: for each decile of the predicted 5-year overall survival, the mean observed 5-year overall survival is presented, with error bars presenting the standard error.
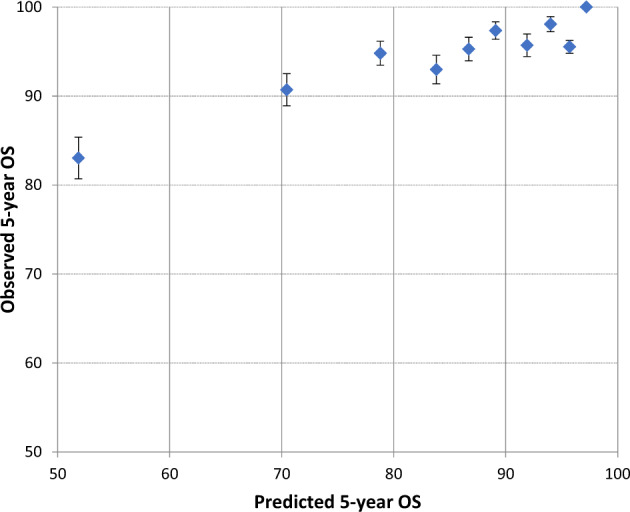
OS overall survival.
This finding was consistent across all subgroups, with a difference ranging from 2.7% (in the hormone receptor-positive subgroup) to 15.8% (in patients with ≥4 positive lymph nodes) (Table 2). The underestimation of survival by PREDICT was consistent and similar in all analyzed subgroups, including among patients treated with lapatinib and trastuzumab (Predicted—observed 5-year OS: −6.98), trastuzumab alone (Predicted – observed 5-year OS: −6.28), or trastuzumab followed by lapatinib (Predicted—observed 5-year OS: −6.82).
The highest absolute differences were observed for patients with hormone receptor-negative disease (13.0%), larger tumor size (>50 mm) (15.3%), and high number of nodes (≥4 positive lymph nodes) (15.8%).
Discrimination
AUC under the ROC curve was 73.7% (95% CI 69.7–77.8) in the overall population (Fig. 3).
Fig. 3. Discriminatory accuracy of PREDICT represented by the area under the receiver-operator characteristic (ROC) curve at the 5-year timepoint in the overall population.
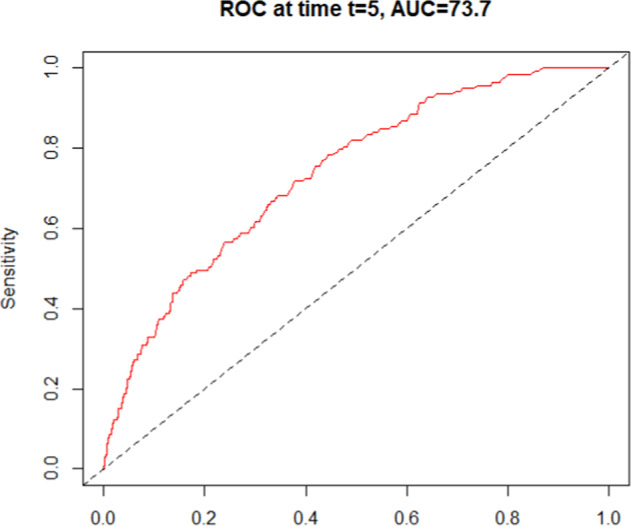
ROC receiver-operator characteristic, AUC area under curve.
This finding of suboptimal discriminatory accuracy was consistent across all subgroups, ranging from 61.7% (in patients with ≥4 positive lymph nodes) to 77.7% (in patients receiving trastuzumab alone as anti-HER2 therapy) (Table 3). The lowest discriminatory accuracy was observed for patients with high number of nodes (≥4 and 1–3 positive lymph nodes) (Supplementary Figs 1 and 2), and for patients receiving a non-anthracycline-based chemotherapy (61.7%, 64.8%, and 65.2%, respectively). The highest discriminatory accuracy was observed for patients with negative lymph nodes (Supplementary Fig. 3) and for patients receiving trastuzumab alone as anti-HER2 therapy (77.3% and 77.7%, respectively).
Table 3.
Discriminatory accuracy of PREDICT in the overall population and in subgroups.
| AUC for time-point 5 years (95% CI) | ||
|---|---|---|
| All patients | 2794 | 73.75 (69.73–77.76) |
| Type of anti-HER2 therapy | ||
| Lapatinib + trastuzumab | 925 | 72.37 (64.31–80.42) |
| Trastuzumab alone | 936 | 77.67 (72.02–83.32) |
| Trastuzumab followed by lapatinib | 933 | 70.64 (63.51–77.78) |
| Type of chemotherapy | ||
| Non-anthracycline based | 322 | 65.18 (50.36–80.00) |
| Anthracycline based | 2472 | 74.44 (70.32–78.57) |
| Age at randomization | ||
| ≤40 | 495 | 76.09 (66.20–85.97) |
| 41–64 | 1989 | 73.69 (68.75–78.62) |
| ≥65 | 310 | 67.42 (56.95–77.89) |
| HR status | ||
| Negative | 1185 | 71.87 (65.79–77.96) |
| Positive | 1609 | 76.81 (71.58–82.04) |
| Number of positive lymph nodes | ||
| 0 | 567 | 77.25 (65.5–89.01) |
| 1–3 | 945 | 64.76 (54.58–74.96) |
| ≥4 | 709 | 61.74 (55.05–68.43) |
| Tumor size (mm) | ||
| ≤20 mm | 1248 | 70.63 (61.83–79.44) |
| 21–50 mm | 1356 | 68.61 (63.27–73.94) |
| >50 mm | 190 | 72.97 (63.09–82.84) |
AUC area under the curve, CI confidence interval, CT chemotherapy, HR hormone receptors.
Discussion
To the best of our knowledge, PREDICT is the only publicly available, free, online tool developed to predict individual prognosis in the specific population of patients with HER2-positive early breast cancer based on traditional and easily retrieved clinical-pathological factors including HER2. In our ALTTO analysis, PREDICT highly underestimated patients’ OS; this finding was consistent across all patient subgroups, with highest absolute differences for patients with hormone receptor-negative disease, nodal involvement, and large tumor size. In terms of discrimination, the accuracy of PREDICT was overall low, with the lowest discriminatory accuracy observed in patients with nodal involvement (≥4 and 1–3 positive lymph nodes), and in patients receiving non-anthracycline-based chemotherapy.
The low performance of this tool raises several questions about the reliability of PREDICT to give prognostic estimation in HER2-positive early breast cancer patients. To potentially explain the reasons for the underestimation of patients’ OS, we can speculate whether the population used to validate this prognostic tool accurately mirrors the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. The prognostic effect of HER2 status was evaluated and incorporated in the PREDICT tool for the first time in October 2011, based on data from the Breast Cancer Association Consortium (BCAC)6 consisting in 10,179 cases not exposed to anti-HER2 treatment (Supplementary Table 1). The subsequently developed model (called PREDICT Plus) was then validated in the original British Columbia dataset, a cohort including 203 HER2-positive breast cancer patients7. In this latter cohort, PREDICT demonstrated an improved ability to estimate breast cancer-specific and overall survival in HER2-positive patients, compared to other prognostication tools such as PREDICT and Adjuvant! Online7. In the HER2-positive cohort of the British Columbia dataset, the observed 10-year OS was 44.3%, and none of the included patients had received trastuzumab7. A further step forward, was the inclusion in PREDICT of the estimates of benefit from adjuvant trastuzumab, with its proportional reduction of 31% in the mortality rate up to five years. These estimates were based on the results of four clinical trials: FinHER10, HERA11, B31/N983112,13, and BCIRG00614 (Supplementary Table 2).
Patients with HER2-positive early breast cancer are experiencing a consistent shift towards better survival across the years, mainly due to the increasingly effective local and systemic therapies available in this setting. This change might not be reflected by a prognostic tool developed and validated 10 years ago. In particular, newer drugs like pertuzumab and T-DM1 have become available for many patients developing disease progression after treatment in the ALTTO trial. These two drugs improve OS in metastatic patients and may contribute to the “better-than-predicted” OS15,16. Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study, including neoadjuvant therapy with pertuzumab, adjustment of adjuvant therapy based on pathological response to neoadjuvant therapy (i.e., T-DM1 for patients who do not achieve pathological complete response) and considering extended adjuvant anti-HER2 therapy with neratinib and endocrine therapy for patients with hormone receptor-positive disease. As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore, our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.
It should be also considered that at least part of the discordance observed between the observed and predicted 5-yr OS by PREDICT could be due to the differences existing between a highly selected population enrolled in a clinical trial and the real-world patient population, which might have slightly different prognosis17,18. Clinical trials have a strong internal validity, but their external validity could be weaker, particularly in the case of narrow inclusion criteria. For this reason, findings from clinical trials might overestimate outcomes as compared to real-world practice. Due to differences in the distribution of age, comorbidity status, and overall health, differences between predicted and observed OS in a clinical trial sample as compared to real-world data are expected. Consistently with our findings, an independent validation of PREDICT on data from real-world patients led by Gray and colleagues showed a general pattern of overestimation of mortality (expected and observed 5-year mortality: 15.3% and 14.5%, respectively), although not focusing specifically on HER2-positive disease19.
Additionally, prognostication estimates of PREDICT are provided as OS rates. Although OS is an important endpoint, being free from any ambiguity in its definition, it could be influenced by several variables (competing risks) not strictly related to breast cancer and not considered in PREDICT, such as comorbidities and performance status20. Non-cancer deaths may not entirely reflect tumor biology, aggressiveness, and responsiveness to therapy20. On the other hand, the more aggressive the disease, the higher the relevance of OS. Indeed, HER2-positive breast cancer tend to develop more early recurrences compared to hormone receptor positive/HER2-negative disease, thus having an undoubtedly more relevant impact on OS21.
In our analysis, the highest absolute differences between observed and predicted OS were observed for patients with hormone-receptor negative disease, larger tumor size, and high number of nodes (≥4 positive lymph nodes), namely those patients traditionally considered at higher risk of relapse. Further investigations are urgently needed to better predict prognosis of these patients. Of note, despite the traditional stigma of poor prognosis for patients with high-risk HER2-positive breast cancer, recent clinical trials have shown good outcomes also for this high-risk subset of patients22.
The prediction of prognosis in patients with early breast cancer is an issue of paramount importance, not only in hormone receptor-positive/HER2-negative disease, where prognostication may settle whether adjuvant chemotherapy should be administered or not, but also in HER2-positive disease. Indeed, although in HER2-positive breast cancer almost all patients deserve chemotherapy as per standard of care, a reliable prognostic estimation has several implications, from the planning of premenopausal patients’ reproductive life (e.g. affecting the choice of having or not a pregnancy later on23), to a therapeutic perspective (adoption of escalation or de-escalation treatment strategies, including type of chemotherapy to be administered together with anti-HER2 treatment and use of extended adjuvant endocrine therapy in hormone receptor-positive disease24).
Several molecular assays are now available for hormone receptor-positive/HER2-negative breast cancer25, and, recently, some molecular assays have been also developed for HER2-positive disease26.
It is likely that these assays will refine prognostication beyond what can be provided by clinical prognostic models like PREDICT27,28, and their increasing use, as a consequence, will reduce reliance on tools like PREDICT. Nevertheless, one strength of PREDICT is the fact that it is “free” and easy to use in everyday clinical practice, and its integration with molecular assay could provide a more complete prognostic evaluation of each single patient. Recently, Prat et al. developed a new prognostic score, HER2DX, based on the combination of clinical-pathological and molecular characteristics of the tumor (nodal and tumor stage, the number of stromal tumor-infiltrating lymphocytes, PAM50 subtypes, and expression of 13 genes relating to proliferation and underlying subtype-related biology)26,29. This was the first attempt to build a combined prognostic score based on clinicopathological and genomic variables in early-stage HER2-positive breast cancer, using tumor samples from the phase 3 Short-HER trial30. However, the HER2DX prognostic model is still immature to be used as biomarker, and future clinical validations are warranted in order to establish its use in different scenarios, especially in the neoadjuvant setting.
Our study has some limitations that should be acknowledged. First, this is an unplanned exploratory analysis. Second, some information (including prognostic factors like the proliferation index Ki67 and type of method for breast cancer detection) were not available in the ALTTO database and could not be included in the model. Third, PREDICT did not allow for estimates of dual-targeted anti-HER2 therapy efficacy, and, in particular, does not provide estimates for lapatinib use. However, our subgroup analysis confirmed that PREDICT still underperforms for patients treated with trastuzumab alone. Additionally, PREDICT tool does not consider the presence of comorbidities and/or the patient performance status, thus further limiting the possibility to compare predicted vs. observed outcomes using a clinical trial sample. Finally, only the point estimates by PREDICT, without its range, were included in the present analysis.
On the other hand, our study has several strengths. Our results derive from a large cohort (n = 2794) of patients enrolled in the largest, randomized adjuvant trial ever conducted in the field of HER2-positive breast cancer. We included only patients receiving adjuvant trastuzumab-based therapy started concurrently with modern chemotherapy. Trial sample size allowed the exploration of relevant patient subgroups. All data used for the analyses were prospectively collected during the trial conduction, as detailed in the study protocol.
In conclusion, in patients with HER2-positive early breast cancer enrolled in the ALTTO trial and treated with modern chemotherapy and trastuzumab-based therapies, the PREDICT score highly underestimated OS. The suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size. Our results suggest that the current version of PREDICT should be used with caution to give prognostic estimation in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. The further improvement of therapeutic strategies expected in the next future will likely increase the survival of patients with HER2-positive early breast cancer, thus requiring the current version of PREDICT to be updated to provide reliable prognostic estimation in these patients.
Methods
Study design and patients
Details of the ALTTO trial study design were previously published31. Shortly, the ALTTO trial (Breast International Group [BIG] 2-06/EGF106708 and North Central Cancer Treatment Group [Alliance] N063D) was an international, open-label, randomized phase III study testing the use of trastuzumab and/or lapatinib as adjuvant anti-HER2 therapy in patients with HER2-positive early breast cancer.
Primary tumor samples from all patients were centrally tested to assess HER232 and hormone receptor status33.
Eligible patients were randomized to one of four anti-HER2 treatment arms: trastuzumab alone, lapatinib alone, sequential treatment with trastuzumab for 12 weeks followed by a 6-week washout period before other 34 weeks of lapatinib, and dual anti-HER2 blockade with trastuzumab plus lapatinib. The CONSORT diagram of the ALTTO study is reported in the ALTTO primary analysis paper.
Anti-HER2 treatment could be administered as per physician’s choice following chemotherapy completion (design 1), or concomitantly, either with a taxane after anthracycline-based chemotherapy (design 2) or with 6 cycles of docetaxel and carboplatin in an anthracycline-free regimen (design 2B). In all treatment arms, adjuvant anti-HER2 therapy was administered for 1 year.
In 2011, after the first interim analysis, the lapatinib arm was closed and patients were offered adjuvant commercial trastuzumab31.
In the present analysis, in order to reflect current clinical practice in this setting, only patients who received concurrent chemotherapy (design 2 and design 2B) and who received trastuzumab-based anti-HER2 therapy (i.e. trastuzumab alone arm, trastuzumab followed by lapatinib arm and trastuzumab plus lapatinib arm) were included. All patients originally assigned to the lapatinib alone arm, and those who received anti-HER2 therapies at the completion of all chemotherapy (sequential treatment, design 1) were excluded.
Ethics section
All patients signed a written informed consent prior to enrollment in ALTTO. The project proposal of the present exploratory analysis was submitted and approved by the ALTTO Steering Committee.
Study objectives
The primary objective of the current analysis was to investigate the prognostic performance of PREDICT in breast cancer patients with early-stage HER2-positive disease treated with modern chemotherapy and concurrent trastuzumab-based anti-HER2 therapy.
Secondary objectives were to investigate the prognostic performance of PREDICT according to the type of anti-HER2 treatment received (trastuzumab alone, trastuzumab followed by lapatinib and, trastuzumab plus lapatinib), type of chemotherapy regimen received (anthracycline-based chemotherapy regimens vs. non-anthracycline-based chemotherapy regimens), age of patients at the time of diagnosis (age ≤ 40 years vs. age 41–64 vs. age ≥65 years), central hormone receptor status (hormone receptor -positive vs. negative), pathological nodal status (node-negative vs. node-positive disease [1–3 positive nodes] vs. node-positive disease [≥4 positive nodes]), and pathological tumor size (small [≤2 cm] vs. medium [2–5 cm] vs. large [>5 cm] disease).
Data extraction
PREDICT estimates for each patient were calculated by one investigator blinded to patient outcomes. Patient and tumor characteristics, as well as administered adjuvant anticancer treatments, were entered in the PREDICT v.2.2 program to calculate the predicted 5-year OS for each patient. Detection modality and Ki67 status were considered “unknown” for all patients (as these variables were not collected as part of the ALTTO trial).
The most updated ALTTO database was used for this analysis9, which corresponds to at least 5-year follow-up for every single patient.
Statistical analysis
The present analysis should be considered as exploratory, since it was not preplanned in the study protocol and the power of the statistical analyses performed was not pre-specified.
The prognostic performance of PREDICT was evaluated by assessing the following endpoints: i) calibration, defined as the agreement between the predicted and observed survival rates, and ii) discriminatory accuracy, defined as the ability of distinguishing individuals who will survive 5 years compared to those who will not (i.e. the ability to discern patients with good outcomes from those with poor outcomes at the individual patient level).
The observation time for each patient was defined as the time between the date of diagnosis and an event. OS event was defined as death from any cause.
The median predicted 5-year OS was calculated from individual predicted outcomes by PREDICT v. 2.2.
For assessing calibration, the median predicted 5-year survival probabilities (by PREDICT) were compared with the observed 5-year survival rates (as obtained by Kaplan-Meier curves). We had to use the median 5-year prediction instead of the mean 5-year prediction, due to the skewness in the distribution, i.e. mean 5-year prediction was 83.6% while median 5-year prediction was 88.0%, and thus the mean predicted 5-year survival probability underestimated the center of the distribution. Therefore, we used the median as a robust estimator of the center of the distribution. Using the standard error as obtained by the Kaplan-Meier curve, we calculated 95% CI for the difference in predicted vs. observed 5-year survival. Calibration plots for PREDICT were constructed by visualizing mean predicted vs. observed survival outcomes by deciles of predicted outcomes.
For assessing discriminatory accuracy, the area under the receiver-operator characteristic curve (AUC under the ROC) and corresponding 95% CI for 5-year predicted OS were calculated. The AUC translates into the probability that the predicted outcome of a randomly selected patient who indeed had that outcome is higher than that of a patient who did not; the higher the AUC, the better the tool is at identifying patients with a better survival.
Subgroup analyses were performed to investigate the prognostic performance of PREDICT according to the type of anti-HER2 treatment and chemotherapy received, age at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.
Statistical analysis was performed by L.A. using SAS 9.4 statistical software (SAS Institute, Cary, NC) and R.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by Les Amis de l’Institut Bordet (Grant No. 2018-09). The ALTTO trial received financial support from GlaxoSmithKline (until January 2015), Novartis Pharma AG (as of January 2015) and the National Cancer Institute of the National Institutes of Health (NCI-NIH; Grant No. U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology and Grant No. CA025224 to the legacy North Central Cancer Treatment Group). Matteo Lambertini acknowledges the support from the Italian Association for Cancer Research (“Associazione Italiana per la Ricerca sul Cancro”, AIRC; MFAG 2020 ID 24698) and the Italian Ministry of Health (5 × 1000 funds 2017). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the present manuscript. All authors agreed and share the final responsibility for the provided interpretation of the study results and for the decision to submit for publication. The abstract of the present work was presented at the American Society of Clinical Oncology (ASCO) Annual Meeting 2021 and was awarded an ASCO Merit Award.
Author contributions
M.L. conceived and designed the study. E.A. and E.d.A. were involved in data acquisition. L.A. analyzed the data. All authors were involved in data interpretation. E.A., E.d.A., and M.L. wrote the first draft of the paper. LA and EA produced the manuscript figures and tables. All authors were involved in reviewing and editing drafts of the paper and approving the manuscript.
Data availability
Data can be made available upon reasonable request. Data and results from the Data Centre at Institut Jules Bordet in Brussels (Belgium) can be made available upon approval of a research proposal.
Competing interests
The authors have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Elisa Agostinetto: Consultancy/honoraria from Eli Lilly, Sandoz. Support to attend medical conferences (travel accommodations/expenses) from Novartis, Roche, Eli Lilly, Genetic, Istituto Gentili (outside the submitted work). Samuel Martel: Honoraria and advisory board for Novartis, Pfizer, Knight Therapeutics, Exact Science, Taiho, Apobiologix (outside the submitted work). Philippe Aftimos: Consulting: Boehringer Ingelheim, Macrogenics, Roche, Novartis, Amcure, Servier, G1 Therapeutics, Radius, Deloitte, Menarini; Honoraria: Synthon, Amgen, Novartis, Gilead; Travel grants: Amgen, MSD, Pfizer, Roche; Research funding to my institution: Roche (outside the submitted work). Noam Pondé: Honoraria and advisory board for Lilly, Novartis, Roche/Genentech, AstraZeneca and Pfizer (outside the submitted work). Christian Maurer: Travel grants: Mundipharma, Amgen, Servier Deutschland GmbH, Abbvie. Honoraria: Abbvie. Advisory board: Celgene/ BMS and Pfizer (outside the submitted work). Sarra El-Abed: grants from Novartis during the conduct of the ALTTO study, and grants from Roche/Genentech and Pfizer (outside of the submitted work). Yingbo Wang: Employee of Novartis Pharmaceuticals and holds stock in the company (outside the submitted work). Saranya Chumsri: Research funding from Merck and Pfizer (outside the submitted work). Judith Bliss: Research Funding to her Institution: AstraZeneca, Merck Sharp & Dohme, Medivation, Puma Biotechnology, Clovis Oncology, Pfizer, Janssen-Cilag, Roche, Novartis (previously GSK), Eli Lilly (outside the submitted work). Judith Kroep: Honoraria and advisory board: AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pfizer, GSK. Research grant: Amgen, Astra Zeneca, Novartis, Philips (outside the submitted work). Marco Colleoni: Research grant to his Institution: Roche (outside the submitted work). Lucia Del Mastro: Honoraria: Roche, Novartis, Eli Lilly, MSD, Pfizer, Ipsen. Honoraria to her Institution: Novartis. Consulting or Advisory role: Roche, Eli Lilly, Novartis, MSD, Genomic Health, Pierre Fabre, Daiichi Sankyo, Seagen, Astrazeneca, Eisai, Gilead, Exact Science. Travel, accomodation, expenses: Celgene, Roche, Pfizer, Daiichi Sankyo (outside the submitted work). Alvaro Moreno Aspitia: Institutional research funding from GlaxoSmithKline, Genentech, Daiichi Sankyo, Novartis, Merck, Pfizer, AbbVie and Eli Lilly (outside the submitted work). Martine Piccart: Board Member (Scientific Board): Oncolytics. Consultant (honoraria): AstraZeneca, Camel-IDS, Immunomedics, Lilly, Menarini, MSD, Novartis, Odonate, Pfizer, Roche-Genentech, Seattle Genetics, Immutep, Seagen, NBE Therapeutics. Research grants to her Institute: AstraZeneca, Immunomedics, Lilly, Menarini, MSD, Novartis, Pfizer, Radius, Roche-Genentech, Servier, Synthon (outside the submitted work). Evandro de Azambuja: honoraria and advisory board: Roche/GNE, Novartis, Seattle Genetics, Zodiacs, Libbs and Pierre Fabre; travel grants: Roche/GNE, GSK/Novartis. Research grant for his Institute: Roche/GNE, AstraZeneca, Novartis, and Servier (outside the submitted work). Matteo Lambertini acted as a consultant for Roche, Lilly, AstraZeneca, Exact Sciences, Seagen, Gilead, Pfizer, MSD and Novartis, and received honoraria from Sandoz, Takeda, Roche, Lilly, Pfizer, Ipsen, Libbs, Knight and Novartis (outside the submitted work). Lieveke Ameye, Marianne Paesmans, Malou Vicente, and Fausto Petrelli have no conflict of interests to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-022-00452-8.
References
- 1.Bradley R, et al. Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13,864 women in seven randomised trials. Lancet Oncol. 2021;22:1139–1150. doi: 10.1016/S1470-2045(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackisch, C. et al. Risk-based decision-making in the treatment of HER2-positive early breast cancer: recommendations based on the current state of knowledge. Cancer Treat. Rev. 99, 10222 (2021). [DOI] [PubMed]
- 3.Pernas S, Tolaney SM. Management of early-stage human epidermal growth factor receptor 2-positive breast cancer. JCO Oncol. Pract. 2021;17:320–330. doi: 10.1200/OP.21.00020. [DOI] [PubMed] [Google Scholar]
- 4.Wishart GC, et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. 2010;12:R1. doi: 10.1186/bcr2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wishart GC, et al. A population-based validation of the prognostic model PREDICT for early breast cancer. Eur. J. Surg. Oncol. 2011;37:411–417. doi: 10.1016/j.ejso.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 6.The Breast Cancer Association Consortium (BCAC). http://bcac.ccge.medschl.cam.ac.uk/.
- 7.Wishart GC, et al. PREDICT Plus: development and validation of a prognostic model for early breast cancer that includes HER2. Br. J. Cancer. 2012;107:800–807. doi: 10.1038/bjc.2012.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NICE. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer. https://www.nice.org.uk/guidance/dg34/ (2018). [DOI] [PMC free article] [PubMed]
- 9.Moreno-Aspitia A, et al. Updated results from the international phase III ALTTO trial (BIG 2-06/Alliance N063D) Eur. J. Cancer. 2021;148:287–296. doi: 10.1016/j.ejca.2021.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joensuu H, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J. Clin. Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 11.Smith I, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 13.Perez EA, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slamon D, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swain SM, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 16.Diéras V, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saturni S, et al. Randomized controlled trials and real life studies. Approaches and methodologies: a clinical point of view. Pulm. Pharmacol. Ther. 2014;27:129–138. doi: 10.1016/j.pupt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 19.Gray E, et al. Independent validation of the PREDICT breast cancer prognosis prediction tool in 45,789 patients using Scottish Cancer Registry data. Br. J. Cancer. 2018;119:808–814. doi: 10.1038/s41416-018-0256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudis CA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J. Clin. Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 21.van Maaren MC, et al. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: a large population-based study. Int. J. Cancer. 2019;144:263–272. doi: 10.1002/ijc.31914. [DOI] [PubMed] [Google Scholar]
- 22.Piccart M, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J. Clin. Oncol. 2021;39:1448–1457. doi: 10.1200/JCO.20.01204. [DOI] [PubMed] [Google Scholar]
- 23.Lambertini M, et al. Pregnancies during and after trastuzumab and/or lapatinib in patients with human epidermal growth factor receptor 2–positive early breast cancer: analysis from the NeoALTTO (BIG 1-06) and ALTTO (BIG 2-06) trials. Cancer. 2019;125:307–316. doi: 10.1002/cncr.31784. [DOI] [PubMed] [Google Scholar]
- 24.Burstein HJ, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: asco clinical practice guideline focused update. J. Clin. Oncol. 2018;37:423–438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 25.Puppe J, et al. Genomic signatures in luminal breast cancer. Breast Care. 2020;15:355–365. doi: 10.1159/000509846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prat A, et al. A multivariable prognostic score to guide systemic therapy in early-stage HER2-positive breast cancer: a retrospective study with an external evaluation. Lancet Oncol. 2020;21:1455–1464. doi: 10.1016/S1470-2045(20)30450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardoso F, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N. Engl. J. Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso F, et al. MINDACT: Long-term results of the large prospective trial testing the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy in breast cancer patients. J. Clin. Oncol. 2020;38:506. doi: 10.1200/JCO.2020.38.15_suppl.506. [DOI] [Google Scholar]
- 29.Hurvitz SA. HER2DX: a tool that might inform treatment choices for HER2-positive breast cancer. Lancet Oncol. 2020;21:1392–1393. doi: 10.1016/S1470-2045(20)30552-0. [DOI] [PubMed] [Google Scholar]
- 30.Conte P, et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER study‡. Ann. Oncol. 2018;29:2328–2333. doi: 10.1093/annonc/mdy414. [DOI] [PubMed] [Google Scholar]
- 31.Piccart-Gebhart M, et al. Adjuvant Lapatinib and Trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J. Clin. Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff AC, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 33.Lambertini M, et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast Cancer Res. Treat. 2019;177:103–114. doi: 10.1007/s10549-019-05284-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be made available upon reasonable request. Data and results from the Data Centre at Institut Jules Bordet in Brussels (Belgium) can be made available upon approval of a research proposal.


