Abstract
Evidence from previous studies suggests a protective effect of metformin in patients with colorectal cancer (CRC). The aim of this study was to examine the associations between metformin use and overall survival (OS) and disease-free survival (DFS) in CRC patients with type 2 diabetes mellitus (DM). We retrospectively included patients who underwent surgery for CRC at Limoges’ University Hospital between 2005 and 2019 and diagnosed with type 2 DM. Data on the characteristics of patients, CRC, comorbidities and drug exposure were collected from the electronic medical records. The exposure was the use of metformin and the outcomes were OS and DFS. We identified 290 CRC patients with type 2 DM. A total of 144 (49.7%) of them were treated with metformin. Metformin users were significantly younger, with higher body mass index and less diabetes-related complications compared to non-users. The 2-year OS was significantly higher in metformin users than in non-users (86.9 ± 2.9% vs. 71.0 ± 4.0%, p = 0.001). In multivariate analysis, metformin use was associated with better OS (adjusted hazard ratios [aHR] = 0.45 95% confidence interval [95% CI]: 0.21–0.96) and better DFS (aHR = 0.31; 95% CI: 0.18–0.54). In conclusion, the use of metformin may improve OS and DFS in CRC patients with type 2 DM.
Subject terms: Cancer, Drug discovery, Endocrinology, Gastroenterology, Oncology
Introduction
Colorectal cancer (CRC) is the second most deadly cancer worldwide (9.4%), and the third most diagnosed form of cancer globally (10.0%) for both sexes combined1. In 2020, nearly 2 million people were diagnosed with CRC, with about 935,000 CRC-related deaths2. The reference treatment of CRC is surgery. However, even after surgical removal, the recurrence rate of CRC remains high3.
The prevalence of Diabetes Mellitus (DM) in CRC patients varied between 2.8 and 14% in studies from various countries4. However, among Taiwanese patients, two studies showed higher prevalence (17% and 23.6%)4,5. Metformin, a biguanide class agent, is an antihyperglycemic drug for type 2 DM. It lowers blood glucose concentrations without causing hypoglycemia by inhibiting hepatic gluconeogenesis and by reducing peripheral insulin resistance6.
In addition to its antidiabetic effect, it has been shown that metformin has an antineoplastic effect and can inhibit cancer cell growth7. The protective effect of metformin against cancer has been reported in previous studies such as breast cancer8, lung cancer9, ovarian cancer10, renal cell carcinoma11.
The results of the association between metformin use and the risk of recurrence in CRC are not entirely consistent12. A large registry-based study was conducted in diabetic CRC patients to evaluate the effect of metformin on CRC survival. They found no association between metformin use and disease-free survival (DFS) or recurrence-free survival after adjustment for multiple confounders. However, only patients treated medically for diabetes were included and classified as diabetic12.
Another meta-analysis was performed to evaluate whether metformin could improve survival in CRC patients with type 2 diabetes. They found that metformin use was associated with increased overall survival (OS) rate and CRC-specific survival13. However, the study had several limitations, including the heterogeneity between the studies, in term of differences in CRC stage and length of follow-up, and no assessment of the impact of diabetes severity in patients on insulin13.
Given the inconsistency of the literature's results on the effect of metformin on CRC survival, which may be related to the heterogeneity of the population in terms of demographics and clinical presentation, we intended to evaluate the effect of metformin in our population.
Our objective was to examine the association between metformin use in CRC patients with type 2 DM and OS and DFS at two years after surgery for CRC.
Materials and methods
Study design
The present study was retrospective cohort including patients who underwent surgery for CRC in the department of digestive, general and endocrine surgery at Limoges University Hospital-France between January 01, 2005 and April 30, 2019 and diagnosed with type 2 DM.
Study population
Inclusion and exclusion criteria
Patients older than 18 years were eligible if they had undergone surgery for CRC and diagnosed with type 2 DM. Patients who were excluded were those with type 1 diabetes, benign colorectal tumor, non-CRC after anatomopathological examination (appendix cancer, colonic and rectal metastases from another primary cancer), non-resection of the colorectal tumor during surgery, patients under trusteeship and patients with incomplete records on anatomic pathology examination and medications.
Diabetes assessment and antidiabetic drug exposure
In our study, type 2 DM was defined as medically- or diet-treated diabetes. Patients in the "diet-treated diabetes" group were defined as patients with type 2 DM who did not receive a prescription for an antidiabetic medication (oral or injectable drugs) for their type 2 DM.
Patients in the "medically-treated diabetes" group were defined as receiving at least one antidiabetic drug within the Anatomical Therapeutic Chemical (ATC) groups: A10BA02 (metformin), A10A (insulin and its analogues), A10BB (sulfonylureas), A10BF (alpha-glucosidase inhibitor inhibitors), A10BJ (Glucagon Like Peptide-1 analogues), A10BH (Dipeptidyl peptidase-4 inhibitors) or A10BX (repaglinide).
Exposure definition
Diabetic patients were classified into two groups: metformin users and non-users. In our study, we defined the exposure to metformin as taking oral metformin for at least 90 days during the follow-up period after surgery14,15. The prescription for metformin as well as the duration of use were found in the drug prescription software of patients included in the hospital.
For the metformin group, metformin could be used alone or in combination with other antidiabetic drugs. In the metformin non-users group, patients could be treated either by diet alone or by any other antidiabetic drug than metformin.
End point: survival outcomes
Survival outcomes were compared between metformin users and non-users in patients with type 2 DM. The primary end point was overall survival (OS), defined as the time from surgery for CRC to death from any cause. Mortality was determined by the date of death using the death certificate through the deceased patient's medical record.
Disease-free survival (DFS) was analyzed as a secondary outcome and defined as the time from CRC surgery to the first documented local or distant recurrence (metastasis) of CRC, development of a new primary colorectal tumor or death from any cause within the two years after CRC surgery.
Local recurrence was defined as recurrence in the anastomosis site or in the pelvic cavity structure (vagina, bladder, and lymph nodes located in the pelvic cavity). Distant recurrence was defined as recurrence in the systemic lymph nodes, liver, lungs, peritoneum, bones, and brain16. The diagnosis of local or distant tumor recurrence of CRC was determined by computed tomography (CT) scan or magnetic resonance imaging (MRI) and verified by biopsy.
Histopathology of the biopsy was used to identify the origin of the cancer. Suspected cases were analyzed by expert (NC) in order to confirm the presence or not of recurrence of CRC. Regarding patients followed outside our hospital after CRC surgery, the presence of recurrent CRC was assessed using biological and imaging results sent to their specialists at the Limoges University Hospital.
Follow-up time
The dates of patient entry into the cohort were the dates for which each patient underwent CRC surgery; the dates of exit were the earliest date among the dates of death, recurrence of CRC or development of a new primary colorectal tumor within the two years after surgery, the date of last consultation (in case of relocation or lost to follow-up) or the date of end of the follow-up (two years after the study enrollment).
Data collection
The data collection took place over four months. It was started on January 1, 2021 and completed on April 30, 2021. The electronic medical records (EMR) of each patient were used to collect the data through the hospital computerized patient record softwares. The data were coded anonymously and entered manually into a secured database.
Variables
The following variables were collected:
Sociodemographic parameters: age at surgery, sex (male, female), body mass index (BMI), family history of cancer and CRC.
Cancer treatment: surgery alone, surgery and adjuvant chemotherapy, neoadjuvant treatment and surgery.
Clinical parameters for CRC:
Tumor site classified into colon cancer and rectal cancer. Rectal cancer includes the rectum. Colon cancer includes: the right colon (consists of caecum, ascending colon, and right hepatic flexure), transverse colon (encompasses the segment of colon between right hepatic flexure and left hepatic flexure as it is described by anatomists), left colon (consists of descending colon, left hepatic flexure and sigmoid colon) and rectosigmoid junction).
CRC TNM stage at diagnosis (in situ, I, II, III, IV) according to AJCC 8th edition17, type of CRC (Lieberkühn adenocarcinoma, mucinous adenocarcinoma, medullary carcinoma, neuroendocrine carcinoma), tumor histology (well differentiated, moderately differentiated, poorly differentiated, undifferentiated), tumor size (< 4, ≥ 4 cm), microsatellite status [microsatellite instability (MSI) or microsatellite stable tumor (MSS)], type of mutation (KRAS, BRAF, NRAF, no mutations).
Medication use: Metformin use (alone/in combination), use of other antidiabetic medications, diet-treated diabetes, comedication (antihypertensive drugs, hypolipidemic drugs), number of antidiabetics and drugs/day.
Diabetes: diabetes with complications (yes/no), and glycemic control (controlled diabetes if glycosylated hemoglobin (HbA1c) ≤ 7% and uncontrolled if HbA1c > 7%).
Charlson comorbidity index (CCI score): this score was used to assess the patients' comorbidities. It contains 19 criteria in which each disease is assigned a score according to its influence on the mortality. We classified the score into three levels (0, 1–2, > 2)12,18.
Tumor markers: included carbohydrate antigen levels (CA19-9 positive > 39 U/mL), carcinoembryonic antigen levels (CEA positive ≥ 5 ng/mL).
Statistical analysis
The normality of quantitative variables was tested using the Kolmogorov–Smirnov test.
Data were presented as mean ± standard deviation (SD) for continuous variables and median (interquartile, IQR) for skewed variables. Qualitative variables were summarized using frequency and percentages. Comparative analysis was carried out using Pearson's Chi-square test or Fisher's exact test for categorical variables and Student t test or Mann–Whitney for quantitative variables. Survival curves were obtained using the Kaplan–Meier method, and the survival curves of each group were compared by a log-rank test. Multivariate Cox proportional Hazard models were performed to estimate the adjusted hazard ratios (HR) and their corresponding 95% confidence intervals (95% CI) for the association between metformin use and the outcomes (OS, DFS).
A p-value < 0.05 was considered statistically significant and all p-values were 2-sided. All analyses were performed using Statistical Package for the Social Sciences (SPSS) version 22.0 software.
Covariates: assessment of potential confounders
The selection of the potential confounders in the multivariate analysis was based on clinical expertise and literature review. The covariates used were age at surgery, sex, BMI, CRC stage, Charlson comorbidity index, tumor site and diabetes complications.
Ethics statement
The approval for this study was obtained by the Ethics Committee of the University Hospital of Limoges (No. 479-2021-135). Informed consent was obtained from all patients. All methods were performed in accordance with the relevant guidelines and regulations (Strobe guidelines for observational studies).
Results
A flowchart outlining the process of enrollment of CRC patients is presented below in Fig. 1. After exclusion (n = 13), 290 patients operated for CRC between 2005 and 2019 at Limoges university hospital were identified with type 2 DM (Fig. 1).
Figure 1.
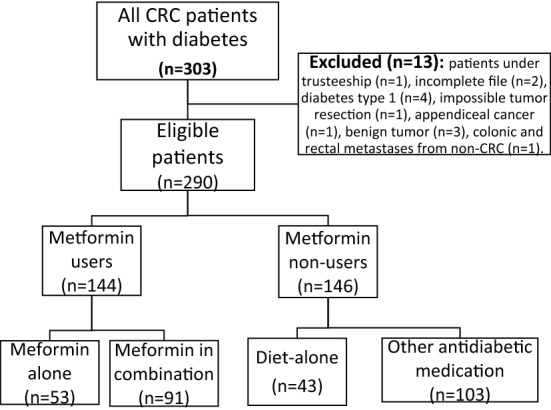
Flowchart showing the process of enrollment of colorectal cancer patients.
Patients’ characteristics
Among the 290 CRC patients with type 2 DM, 144 (49.7%) were treated with metformin: either metformin alone (36.8%) or metformin in association with other antidiabetic drugs (63.2%). Among metformin non-users (n = 146, 50.3%), 43 (29.4%) were treated by diet alone (Fig. 1).
The main demographic and clinical characteristics and medication use of patients are summarized in Tables 1 and 2. The mean age of patients who had surgery for CRC was 73.71 ± 8.70 years, 69.3% were males and 32.4% were overweight. Of all the patients in this study who have CRC, 75.2% had colon cancer and the rest had rectal cancer (24.8%). In addition, 55.8% of patients were diagnosed at stages Tis, I and II and 43.5% were diagnosed at stages III and IV (Table 2).
Table 1.
Main characteristics of CRC patients with type 2 diabetes mellitus compared by metformin status. Significant values are in bold.
| Variables | All patients (n = 290) | Metformin non-users (n = 146) | Metformin users (n = 144) | p-value |
|---|---|---|---|---|
| Age, years | 73.71 ± 8.70 | 75.05 ± 8.72 | 72.35 ± 8.53 | 0.008 |
| Male sex, n (%) | 201 (69.3) | 95 (65.1) | 106 (73.6) | 0.12 |
| BMI*, kg/m2 | 27.2 [25.0–31.9] | 26.8 [24.0–30.9] | 27.7 [25.6–32.7] | 0.02 |
| Charlson comorbidity index* | 1.0 [0.0–2.0] | 1.0 [0.0–3.0] | 1.0 [0.0–2.0] | 0.10 |
| Complications of diabetes, n (%) | 37 (12.8) | 25 (17.1) | 12 (8.3) | 0.03 |
| Glycemic control, n (%) | 0.23 | |||
| Yes (HbA1c ≤ 7%) | 136 (46.9) | 66 (56.9) | 70 (64.8) | |
| Missing | 66 (22.8) | |||
| Number of drugs/day* | 7.0 [5.0–9.0] | 7.0 [5.0–9.3] | 7.0 [5.0–9.0] | 0.82 |
| Number of antidiabetics/day* | 1.0 [1.0–2.0] | 1.0 [0.0–1.0] | 2.0 [1.0–2.0] | < 0.001 |
| Metformin use, n (%) | 144 (49.7) | |||
| Medications, n (%) | ||||
| Insulin use | 78 (26.9) | 50 (34.2) | 28 (19.4) | 0.004 |
| Sulfonylureas use | 87 (30.0) | 43 (29.5) | 44 (30.6) | 0.84 |
| GLP-1 analogues use | 7 (2.4) | 2 (1.4) | 5 (3.5) | 0.28 |
| AGIs inhibitors use | 16 (5.5) | 5 (3.4) | 11 (7.6) | 0.12 |
| DDP-4 inhibitors use | 44 (15.2) | 14 (9.6) | 30 (20.8) | 0.008 |
| Other glucose-lowering drugs use | 18 (6.2) | 11 (7.5) | 7 (4.9) | 0.35 |
| Diet-alone | 43 (14.8) | |||
| Antihypertensive drugs | 234 (80.7) | 117 (80.1) | 117 (81.3) | 0.81 |
| Hypolipidemic drugs | 147 (50.7) | 65 (44.5) | 82 (56.9) | 0.03 |
| Chemotherapy | 103 (35.5) | 48 (32.9) | 55 (38.2) | 0.34 |
| Neoadjuvant treatment | 37 (12.8) | 16 (11.0) | 21 (14.6) | 0.36 |
*Indicates variables that are not normally distributed.
Quantitative variables are reported as mean ± SD or median [IQR].
Table 2.
Main characteristics of CRC patients with type 2 diabetes mellitus compared by metformin status (continued).
| Variables | All patients (n = 290) | Metformin non-users (n = 146) | Metformin users (n = 144) | p-value |
|---|---|---|---|---|
| Tumor site, n (%) | 0.69 | |||
| Right colon | 120 (41.4) | 63 (43.2) | 57 (39.6) | |
| Colon transverse | 12 (4.1) | 7 (4.8) | 5 (3.5) | |
| Left colon | 62 (21.4) | 32 (21.9) | 30 (20.8) | |
| Rectum | 72 (24.8) | 35 (24.0) | 37 (25.7) | |
| Rectosigmoid junction | 24 (8.3) | 9 (6.2) | 15 (10.4) | |
| CRC stage, n (%) | 0.19 | |||
| In situ | 11 (3.8) | 8 (5.5) | 3 (2.1) | |
| I | 52 (17.9) | 24 (16.6) | 28 (19.6) | |
| II | 99 (34.1) | 47 (32.4) | 52 (36.4) | |
| III | 95 (32.8) | 54 (37.2) | 41 (28.7) | |
| IV | 31 (10.7) | 12 (8.3) | 19 (13.3) | |
| Missing | 2 (0.7) | |||
| Type of cancer, n (%) | 0.99 | |||
| Lieberkühn adenocarcinoma | 271 (93.4) | 136 (93.2) | 135 (93.8) | |
| Mucinous adenocarcinoma | 15 (5.2) | 8 (5.6) | 7 (4.9) | |
| Medullary carcinoma | 3 (1.0) | 1 (0.7) | 2 (1.4) | |
| Neuroendocrine carcinoma | 1 (0.3) | 1 (0.7) | 0 (0.0) | |
| Family history of cancer, n (%) | 45 (15.5) | 22 (15.1) | 23 (16.0) | 0.83 |
| Family history of CRC, n (%) | 25 (8.6) | 15 (10.3) | 10 (6.9) | 0.31 |
| Tumor size, cm | 0.40 | |||
| ≥ 4 | 160 (55.2) | 76 (55.9) | 84 (60.9) | |
| Missing | 16 (5.5) | |||
| Histological type, n (%) | 0.71 | |||
| Well differentiated | 49 (16.9) | 28 (23.0) | 21 (16.9) | |
| Moderately differentiated | 181 (62.4) | 87 (71.3) | 94 (75.8) | |
| Poorly differentiated | 14 (4.8) | 6 (4.9) | 8 (6.5) | |
| Undifferentiated | 2 (0.7) | 1 (0.8) | 1 (0.8) | |
| Missing | 44 (15.2) | |||
| Type of mutation, n (%) | 0.84 | |||
| KRAS | 26 (9.0) | 12 (34.3) | 14 (35.9) | |
| BRAF | 9 (3.1) | 4 (11.4) | 5 (12.8) | |
| NRAS | 4 (1.4) | 1 (2.9) | 3 (7.7) | |
| No mutations | 35 (12.1) | 18 (51.4) | 17 (43.6) | |
| Missing | 216 (74.5) | |||
| Microsatellite status, n (%) | 0.84 | |||
| MSI | 12 (4.1) | 6 (21.4) | 6 (19.4) | |
| MSS | 47 (16.2) | 22 (78.6) | 25 (80.6) | |
| Missing | 231 (79.7) | |||
| CEA positive, ≥ 5 ng/mL, n (%) | 76 (26.2) | 41 (39.4) | 35 (30.7) | 0.18 |
| CA 19-9 positive, > 39 U/mL, n (%) | 28 (9.7) | 11 (12.5) | 17 (16.2) | 0.47 |
Comparison between metformin users and non-users
Metformin non-users were significantly older than users (p = 0.008), had lower BMI (p = 0.02) and more diabetes complications (p = 0.03). In addition, there was a significant higher proportion of insulin use within the metformin non-users group (p = 0.004, Table 1).
Outcomes
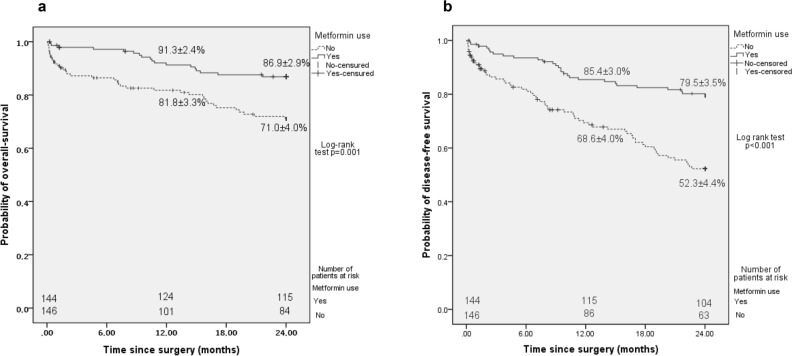
During a mean follow-up of 18.94 ± 8.62 months, 56 (19.3%) death and 54 (18.6%) recurrence of CRC, resulting in a 2-year OS and DFS of 79.2 ± 2.5% and 66.3.8 ± 2.9% respectively. As compared to metformin non-users (including patients with diet-treated diabetes), patients receiving metformin depicted significant higher 2-year OS (71.0 ± 4.0% vs. 86.9 ± 2.9%, p = 0.001, Fig. 2a). In addition, metformin users had significantly improved 2-year DFS than metformin non-users (79.5 ± 3.5% vs. 52.3 ± 4.4%, p < 0.001, Fig. 2b).
Figure 2.
Survival curves comparing metformin users and non-users. (a) Overall survival, (b) disease-free survival.
Multivariate analyses
On multivariate analysis, after adjustment for confounding factors, Cox modeling showed that metformin was associated with better OS (adjusted Hazard Ratio [aHR] = 0.45; 95% Confidence Interval [95% CI]: 0.21–0.96; p = 0.04, Table 3). Others independent determinants of OS were age (aHR = 1.10; 95% CI: 1.05–1.16), Stages III, IV CRC (aHR = 4.64; 95% CI: 2.11–10.22, Table 3).
Table 3.
Overall survival assessed by the Cox regression model. Significant values are in bold.
| Variables | Overall-survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | aHRa | 95% CI | p-value | |
| Age at surgery, years | 1.11 | 1.07–1.15 | < 0.001 | 1.10 | 1.05–1.16 | 0.001 |
| Female sex | 1.14 | 0.65–1.99 | 0.66 | |||
| BMI, kg/m2 | 0.95 | 0.89–1.02 | 0.14 | |||
| Tumor site | ||||||
| Colon (reference) | ||||||
| Rectum | 0.52 | 0.25–1.06 | 0.07 | |||
| CRC stage | ||||||
| In situ, I, II (reference) | ||||||
| III, IV | 3.64 | 2.04–6.51 | < 0.001 | 4.64 | 2.11–10.22 | 0.001 |
| Charlson comorbidity index | 1.21 | 1.04–1.40 | 0.01 | |||
| Complications of diabetes | 2.45 | 1.32–4.55 | 0.005 | |||
| Metformin use | 0.40 | 0.23–0.71 | 0.001 | 0.45 | 0.21–0.96 | 0.04 |
HR, hazard ratio; CI, confidence interval.
aAdjusted for age at surgery, sex, BMI, tumor site, CRC stage, Charlson comorbidity index, diabetes complication.
In addition, metformin use was associated with better DFS (aHR = 0.31; 95% CI: 0.18–0.54, Table 4). The independent determinants of DFS were age at surgery (aHR = 1.03; 95% CI: 1.00–1.07), female sex (aHR = 0.48; 95% CI: 0.23–0.98), and stages III, IV CRC (aHR = 3.15, 95% CI: 1.85–5.36).
Table 4.
Disease-free survival assessed by the Cox regression model. Significant values are in bold.
| Variables | Disease-free survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | aHRa | 95% CI | p-value | |
| Age at surgery, years | 1.05 | 1.02–1.07 | 0.001 | 1.03 | 1.00–1.07 | 0.04 |
| Female sex | 0.87 | 0.54–1.38 | 0.55 | 0.48 | 0.23–0.98 | 0.04 |
| BMI, kg/m2 | 0.96 | 0.91–1.00 | 0.06 | |||
| Tumor site | ||||||
| Colon (reference) | ||||||
| Rectum | 0.61 | 0.36–1.04 | 0.07 | |||
| CRC stage | ||||||
| In situ, I, II (reference) | ||||||
| III, IV | 3.23 | 2.08–5.01 | < 0.001 | 3.15 | 1.85–5.36 | < 0.001 |
| Charlson comorbidity index | 1.21 | 1.08–1.36 | 0.001 | |||
| Complications of diabetes | 2.31 | 1.39–3.84 | 0.001 | |||
| Metformin use | 0.36 | 0.23–0.56 | < 0.001 | 0.31 | 0.18–0.54 | < 0.001 |
HR, hazard ratio; CI, confidence interval.
aAdjusted for age at surgery, sex, BMI, tumor site, CRC stage, Charlson comorbidity index, diabetes complications.
Subgroup analyses
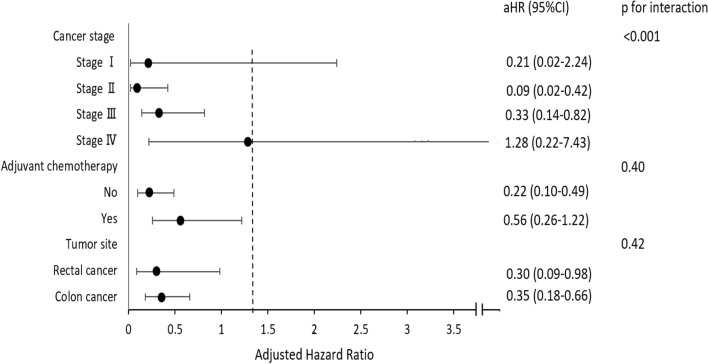
Figure 3 summarized the effect of metformin on DFS in different subgroups in multivariate analysis. The use of metformin was associated with better DFS in stage II CRC (aHR = 0.09; 95% CI: 0.02–0.42), stage III (aHR = 0.33; 95% CI: 0.14–0.82), colon cancer (aHR = 0.35; 95% CI: 0.18–0.66) and rectal cancer subgroup (aHR = 0.30; 95% CI: 0.09–0.98, Fig. 3). Furthermore, among metformin users, having a BMI ≥ 25 had no effect on DFS compared with a normal BMI (aHR = 0.43; 95% CI: 0.17–1.09). In multivariate analysis, the use of metformin in combination with other antidiabetic drugs was not associated with OS (aHR = 2.17, 95% CI: 0.49–9.59) nor DFS (aHR = 0.68, 95% CI: 0.23–2.01) compared with metformin users alone.
Figure 3.
Forest plot for subgroup analysis: effect of metformin on disease-free survival.
Comparison of the survival of metformin users according to their microsatellite status
Among patients with MSS, metformin users had an improved DFS compared to non-users (62.3 ± 10.0% vs. 22.2 ± 9.8%; p = 0.03). However, there was no significant differences between metformin users and non-users in patients with MSI (83.3 ± 15.2% vs. 80.0 ± 17.9%; p = 0.95).
Among metformin users with colon cancer and MSS, there was no significant difference in DFS between patients who received adjuvant chemotherapy and those who did not (50.0 ± 15.8% vs. 72.9 ± 16.5%; p = 0.31). However, among metformin users with MSI and colon cancer, there was a significant difference in DFS between patients who received adjuvant chemotherapy and those who did not (p = 0.05). In addition, among patients with rectal cancer and MSS who used metformin, there was no significant difference in DFS between those who received neoadjuvant treatment and those who did not (p = 0.09).
Stratified analysis
In stratified analysis by age of 74 years, metformin users have a better DFS compared to non-users in both age categories; younger and older than 74 years separately (aHR = 0.40; 95% CI: 0.18–0.88 and aHR = 0.30; 95% CI: 0.13–0.69 respectively).
Discussion
This study evaluated the association between metformin use and survival of CRC patients with type 2 DM. Our results showed that metformin-users have better OS and DFS with a 55% decrease in all-cause mortality at 2-year after surgery for CRC. Our findings were consistent with previous results conducted by Ramjeesingh et al. in 2016 at the Southeastern Ontario Cancer Center (2-year OS: 80.5% for the metformin group vs. 67.4% for the non-metformin group, p = 0.01)19.
Previous studies have attempted to identify the effect of metformin on OS and DFS in CRC patients. However, the results were considerably different. In a register-based observational study of 1 116 diabetic CRC patients in Denmark, they found no association between metformin use and DFS or recurrence-free survival in patients who underwent surgery for CRC after adjusting for confounders12. However, in this study, only patients medically treated for diabetes were classified as diabetic. Indeed, there are about 2 million diabetic patients in France, and more than 200,000 to 300,000 are treated by diet alone20.
One of the antineoplastic action of metformin is based on the epithelial-mesenchymal transition (EMT) activity21. EMT occurs when epithelial cells lose their epithelial characteristics, including polarity and adhesion, and develop migratory properties by transformation into mesenchymal cells. In cancer, invasion, recurrence and metastasis are associated with EMT22. E-cadherin is an inter-cellular adhesion marker. Its loss demonstrates activation of EMT, thus witnessing tumor aggressiveness in CRC3. An ex-vivo study showed an increase of E-cadherin expression in CRC tissues in diabetic patients with CRC using metformin in comparison to metformin non-users, and better OS and DFS for metformin users23.
Our study was able to highlight further interesting elements. In subgroup analysis, we found that the use of metformin in combination with other antidiabetic drugs was not associated with better DFS compared with metformin users alone. This may be due to a possible antagonistic effect between metformin and the other antidiabetic drugs24.
We also performed a subgroup analysis according to cancer location. We found that the protective effect of metformin was detected among patients with colon and rectal cancer subgroups. Results obtained by Lee et al. showed that survival benefits of metformin use were present for rectal cancer, but not for colon cancer25. CRC has different clinical characteristics and tumorigenic pathways depending on tumor location, including different molecular pathways, microsatellite stability, and prognosis26. Therefore, more studies targeting cancer localization and CRC survival of metformin users should be conducted.
While DFS was significantly higher in metformin users regardless of CRC stage, subgroup analysis revealed that metformin use was protective in stages II and III CRC.A previous study demonstrated an attenuated association between metformin use and CRC–specific survival after including patients with stage IV CRC in their study27. These results suggest that metformin has a protective effect in patients with early-stage CRC. However, this analysis was limited by a small simple size.
Among metformin users, having a BMI ≥ 25 was not associated with DFS compared with a normal BMI (18–24). This suggests that metformin acts through a glucose-independent pathway, as it was shown in an in vitro study focusing on metformin and stomach cancer28. This finding is of high importance and may query about the potential therapeutic effect of metformin not only on diabetic patients but also in non-diabetic ones. However, further in vivo evidence on CRC should be conducted.
To explore the effect of microsatellite instability on the relation between CRC survival and metformin, we examined the association between DFS and metformin use in MSI and MSS subgroups. The protective effect of metformin was detected among subgroup of MSS patients and not MSI. A study conducted on patients with resected stage III colon cancer found no difference in DFS, OS and time to recurrence between metformin users and non-users for both defective DNA mismatch repair (dMMR) and proficient DNA mismatch repair (pMMR) tumors29. Because of the low frequency of MSI in CRC, few studies on the use of metformin and MSI have been reported in CRC. Hence, further studies are needed to detect any significant effects’ difference of this medication regarding MMR status.
Despite a large number of CRC patients with type 2 DM, the number of patients was small in some subgroups, which limited the ability to detect small differences between metformin users and non-users within some subgroups. Consequently, subgroup results should be interpreted with caution.
This study has several strengths, including the availability of a large French cohort operated for CRC over a long period of 14 years. In addition, patients were operated for CRC including all stages (from I to IV). The fact that it was a monocentric study implies same environment and same oncological management avoiding thus some biases13. The demographic, clinical, drug use and laboratory findings were available allowing to control for multiple potential confounders in the multivariate analysis and the effect of metformin on OS and DFS has remained significant.
Because metformin is the first-line pharmacological treatment for type 2 DM, and is therefore used in patients with early diabetes, we may see better survival among metformin users who are considered healthier than non-users of metformin (healthy user bias 38). Furthermore, complications associated with diabetes may also lead to an increased risk of mortality. In our study, this confounding bias was reduced by adjusting for diabetes complications. In addition, because metformin users were younger than non-users, we stratified the data by age of 74 years, to confirm that metformin use is the main cause of the better survival and rule out the effect of age. In stratified and multivariate analysis, metformin users still have a better DFS in both age categories; younger and older than 74 years. This means that the better survival observed in metformin users is not due to the younger age of metformin-users given its protective effect detected in both younger and older patients separately. However, the study presents some limits. Information bias may have occurred due to the retrospective nature of the study. The analysis was limited by the lack of information on the effect of time-dependent exposure and the duration of diabetes, therefore, their effects could not be examined which increased the effects of time-related biases, such as the immortal time and time lag bias, which were also present in some previous studies30,31.
Although we focused on OS in our study, we recognize that cancer-specific survival would have been more relevant. However, the cause of death was unavailable due to limitations in death certificate information. In addition, the source of mortality was limited to the death certificate available in the hospital's cross-crossway system, this could underestimate the events that occurred.
A possible selection bias may be present, because only patients admitted to Limoges University hospital were included which may have more advanced stages and more comorbidities compared to the patients in other centers but to reduce these effects, we adjusted to the CRC stage and Charlson comorbidity index in our multivariate analysis.
The improved survival observed in metformin-users needs to be confirmed in larger prospective cohorts of people with diabetes over an extended follow-up period, considering time-related drug-exposure and applying the propensity score method. Randomized controlled trials are also needed to further evaluate the survival benefit of metformin use.
Conclusion and perspectives
Our data suggest that the use of metformin may improve OS and DFS in CRC patients with type 2 DM. Interesting findings have been underlined especially the beneficial role of metformin for early CRC stages and MSS status.
Author contributions
Z.T. and K.M.: prepared the main manuscript text, tables, figures, formal analysis, Methodology and writing the original draft. N.C. and J.J.: Conceptualization; investigation; Methodology; Project administration; Supervision; Validation; Writing—review and editing. J.M. and M.M.: Validation; Writing—review and editing All authors reviewed the manuscript.
Data availability
The data presented in this study are available on request from the corresponding author (Z.T.).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Cancer Observatory, all cancers. Globocan 2020. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (2020).
- 2.Global Cancer Observatory, Colorectal cancer. Globocan 2020. https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (2020).
- 3.Christou N, et al. The extracellular domain of E cadherin linked to invasiveness in colorectal cancer: A new resistance and relapses monitoring serum-bio marker? J. Cancer Res. Clin. Oncol. 2017;143:1177–1190. doi: 10.1007/s00432-017-2382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C-W, et al. The impact on clinical outcome of high prevalence of diabetes mellitus in Taiwanese patients with colorectal cancer. World J. Surg. Oncol. 2012;10:76. doi: 10.1186/1477-7819-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y-C, et al. Diabetes mellitus negatively impacts survival of patients with colon cancer, particularly in stage II disease. J. Cancer Res. Clin. Oncol. 2011;137:211–220. doi: 10.1007/s00432-010-0879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999;58(Suppl 1):31–39. doi: 10.2165/00003495-199958001-00009. [DOI] [PubMed] [Google Scholar]
- 7.Del Barco S, et al. Metformin: Multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 9.Gagnon B, et al. Protective effect of metformin in lung cancer patients. JCO. 2009;27:e22063. doi: 10.1200/jco.2009.27.15_suppl.e22063. [DOI] [Google Scholar]
- 10.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: A case–control analysis. Gynecol. Oncol. 2011;123:200–204. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, et al. Metformin inhibits renal cell carcinoma in vitro and in vivo xenograft. Urol. Oncol. Semin. Orig. Investig. 2013;31:264–270. doi: 10.1016/j.urolonc.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Fransgaard T, Thygesen LC, Gögenur I. Association between metformin use after surgery for colorectal cancer and oncological outcomes: A nationwide register-based study. Int. J. Cancer. 2018;143:63–72. doi: 10.1002/ijc.31305. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, et al. For colorectal cancer patients with type II diabetes, could metformin improve the survival rate? A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2020;44:73–81. doi: 10.1016/j.clinre.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Kuan Y-C, Huang K-W, Lin C-L, Hu C-J, Kao C-H. Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;79:77–83. doi: 10.1016/j.pnpbp.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Oh TK, Song I-A. Metformin use and the risk of cancer in patients with diabetes: A nationwide sample cohort study. Cancer Prev. Res. 2020;13:195–202. doi: 10.1158/1940-6207.CAPR-19-0427. [DOI] [PubMed] [Google Scholar]
- 16.Zafar SN, et al. Predicting risk of recurrence after colorectal cancer surgery in the united states: An analysis of a special commission on cancer national study. Ann. Surg. Oncol. 2020;27:2740–2749. doi: 10.1245/s10434-020-08238-7. [DOI] [PubMed] [Google Scholar]
- 17.Weiser MR. AJCC 8th edition: Colorectal cancer. Ann. Surg. Oncol. 2018;25:1454–1455. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Ramjeesingh R, Orr C, Bricks CS, Hopman WM, Hammad N. A retrospective study on the role of diabetes and metformin in colorectal cancer disease survival. Curr. Oncol. 2016;23:e116–122. doi: 10.3747/co.23.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SFEndocrino. http://sfendocrino.org/article/393/item-233-b-ndash-diabete-sucre-de-type-2
- 21.Song Y, Ye M, Zhou J, Wang Z, Zhu X. Targeting E-cadherin expression with small molecules for digestive cancer treatment. Am. J. Transl. Res. 2019;11:3932–3944. [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Wu Z, Hu L. Epithelial-mesenchymal transition phenotype, metformin, and survival for colorectal cancer patients with diabetes mellitus II. Gastroenterol. Res. Pract. 2017;2017:2520581. doi: 10.1155/2017/2520581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: Response to Farooki and Schneider. Diabetes Care. 2006;29:1990–1991. doi: 10.2337/dc06-0997. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int. J. Cancer. 2012;131:752–759. doi: 10.1002/ijc.26421. [DOI] [PubMed] [Google Scholar]
- 26.Elsaleh H, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 27.Spillane S, Bennett K, Sharp L, Barron TI. A cohort study of metformin exposure and survival in patients with stage I-III colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2013;22:1364–1373. doi: 10.1158/1055-9965.EPI-13-0347. [DOI] [PubMed] [Google Scholar]
- 28.Valaee S, Yaghoobi MM, Shamsara M. Metformin inhibits gastric cancer cells metastatic traits through suppression of epithelial–mesenchymal transition in a glucose-independent manner. PLoS One. 2017;12:e0174486. doi: 10.1371/journal.pone.0174486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh P, et al. Relationship between metformin use and recurrence and survival in patients with resected stage III colon cancer receiving adjuvant chemotherapy: Results from North Central Cancer Treatment Group N0147 (Alliance) Oncologist. 2016;21:1509–1521. doi: 10.1634/theoncologist.2016-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lega IC, et al. The effect of metformin on mortality following cancer among patients with diabetes. Cancer Epidemiol. Biomark. Prev. 2014;23:1974–1984. doi: 10.1158/1055-9965.EPI-14-0327. [DOI] [PubMed] [Google Scholar]
- 31.Paulus JK, Williams CD, Cossor FI, Kelley MJ, Martell RE. Metformin, diabetes, and survival among US veterans with colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2016;25:1418–1425. doi: 10.1158/1055-9965.EPI-16-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (Z.T.).