Abstract
The productive replication of human influenza viruses is almost exclusively restricted to cells in the respiratory tract. However, a key aspect of the host response to viral infection is the production of inflammatory cytokines and chemokines that are not similarly tissue restricted. As such, circulating inflammatory mediators, as well as the resulting activated immune cells, can induce damage throughout the body, particularly in individuals with underlying conditions. As a result, more holistic experimental approaches are required to fully understand the pathogenesis and scope of influenza virus-induced disease. This review summarizes what is known about some of the most well-appreciated non-respiratory tract sites of influenza virus-induced disease, including: neurological, cardiovascular, gastrointestinal, muscular, and fetal developmental phenotypes. In the context of this discussion, we describe the in vivo experimental systems currently being used to study non-respiratory symptoms. Finally, we highlight important future questions and potential models that can be used for a more complete understanding of influenza virus-induced disease.
Keywords: Influenza virus, Inflammation, Disease burden, Animal model, Mouse, Neurological disease, Cardiovascular disease, Microbiome, Muscle wasting, Fetal health
Introduction
Each year, seasonal influenza epidemics are correlated with hospitalizations for severe respiratory illnesses like pneumonia and acute respiratory distress syndrome (ARDS) [1], as well as heart attacks and strokes [2], miscarriages [3] and febrile seizures [4–6]. In an effort to prevent these well-recognized influenza-associated negative health outcomes, at-risk groups such as pregnant individuals or people with cardiovascular disease are encouraged to receive annual influenza vaccines [7,8]. However, with national vaccination rates remaining around 50% in the United States [9] and influenza-associated hospitalizations numbering in the hundreds of thousands each year [10], it is critical to understand the effects of the systemic inflammatory state that accompanies respiratory influenza virus infections.
Human influenza viral infections begin with the inhalation of viral particles that enter and replicate in the upper respiratory tract (URT) epithelium. For seasonal human influenza viral strains, entry requires the viral surface glycoprotein hemagglutinin (HA) to bind to sialic acids present on respiratory epithelial cells along with cleavage of HA by host cell proteases to facilitate membrane fusion [11]. These trypsin-like proteases are mainly expressed in airway tissues, restricting influenza viral tissue tropism to the respiratory tract [12]. Within days of exposure, the infected person will typically begin to experience respiratory symptoms such as a sore throat, sneezing, cough and congestion [13]. During most infections, these symptoms and the underlying viral replication are resolved within a week of symptom onset. However, initial influenza URT infections can progress to bronchitis or pneumonia, placing patients at higher risk for further, more serious complications. Even in cases of severe disease with seasonal strains of human influenza viruses, infectious viral particles and viral RNA are primarily detected in the respiratory tract, and productive infection is rare in non-respiratory tissues [14].
While influenza virus replication is typically restricted to human airways, some of the hallmarks of “influenza-like” illness are systemic symptoms, such as fever, chills, body aches and fatigue. These common non-respiratory manifestations of disease are recognized to be a result of circulating cytokines produced as a part of the immune response [15]. In patients with underlying conditions, or in cases where an uncontrolled inflammatory response leads to a “cytokine storm”, systemic inflammatory signaling results in significant damage to, and disease associated with, organs outside the site of infection [16,17]. Studies observing patients with confirmed influenza cases or during experimental influenza virus challenge studies have revealed an array of circulating cytokines that are consistently detected during human influenza virus infections, including interferons (IFNs), IL-6, and TNF-α [18–20]. Studies in experimental models have helped demonstrate how these individual cytokines interact with immune cells and uninfected tissues to enact an inflammatory antiviral state throughout the body [15]. Nonetheless, a complete picture of how a respiratory-restricted influenza infection leads to specific negative health outcomes in an uninfected organ requires a model system with two key characteristics: 1) The model system must support influenza viral infection in the respiratory tract; 2) The model system must recapitulate the non-respiratory influenza disease symptoms.
A wide range of animal models for the study of influenza virus disease have been used [21–23]. Despite several differences with human disease [23,24], mice have been disproportionately used to study the mechanisms of respiratory disease pathogenesis. During a typical influenza viral infection in mice, the virus primarily infects the lungs (rather than the URT), leading to pneumonia and observable evidence of infection including weight loss due to anorexia, hunching and huddling indicating discomfort, and hypothermia (rather than hyperthermia) [25,26]. However, like humans, the virus is usually cleared within 10 days of infection. A key advantage of mice is their utility for replicating the non-respiratory aspects of influenza-associated disease, particularly when studying underlying conditions. Mouse models for many neurological, cardiovascular, gastrointestinal, and muscular diseases and conditions exist [27–30]. Further, there is a growing research in modeling conditions that confer high-risk during influenza virus infections (i.e. comorbidities), such as obesity, pregnancy, aging, and chronic diseases, in mice [31]. Thus, although murine influenza infections are not entirely reflective of the human experience, studies using mice are the basis for much of what we know regarding the mechanisms of pathology and immunology related to influenza disease.
This review aims to cover previous research into the mechanisms behind well-described, non-respiratory disease associated with influenza virus infections, including neurological, cardiovascular, gastrointestinal, muscular, and fetal developmental phenotypes (Figure 1). These studies will be used to illustrate what is known about how influenza virus-induced inflammation intersects with other organ systems and underlying conditions to produce negative health outcomes. In the final section of the review, we discuss important future research directions and emerging experimental systems that may be leveraged to further illuminate the effectors of non-respiratory influenza-associated disease and lead to a more complete understanding of the scope of the disease.
Figure 1.
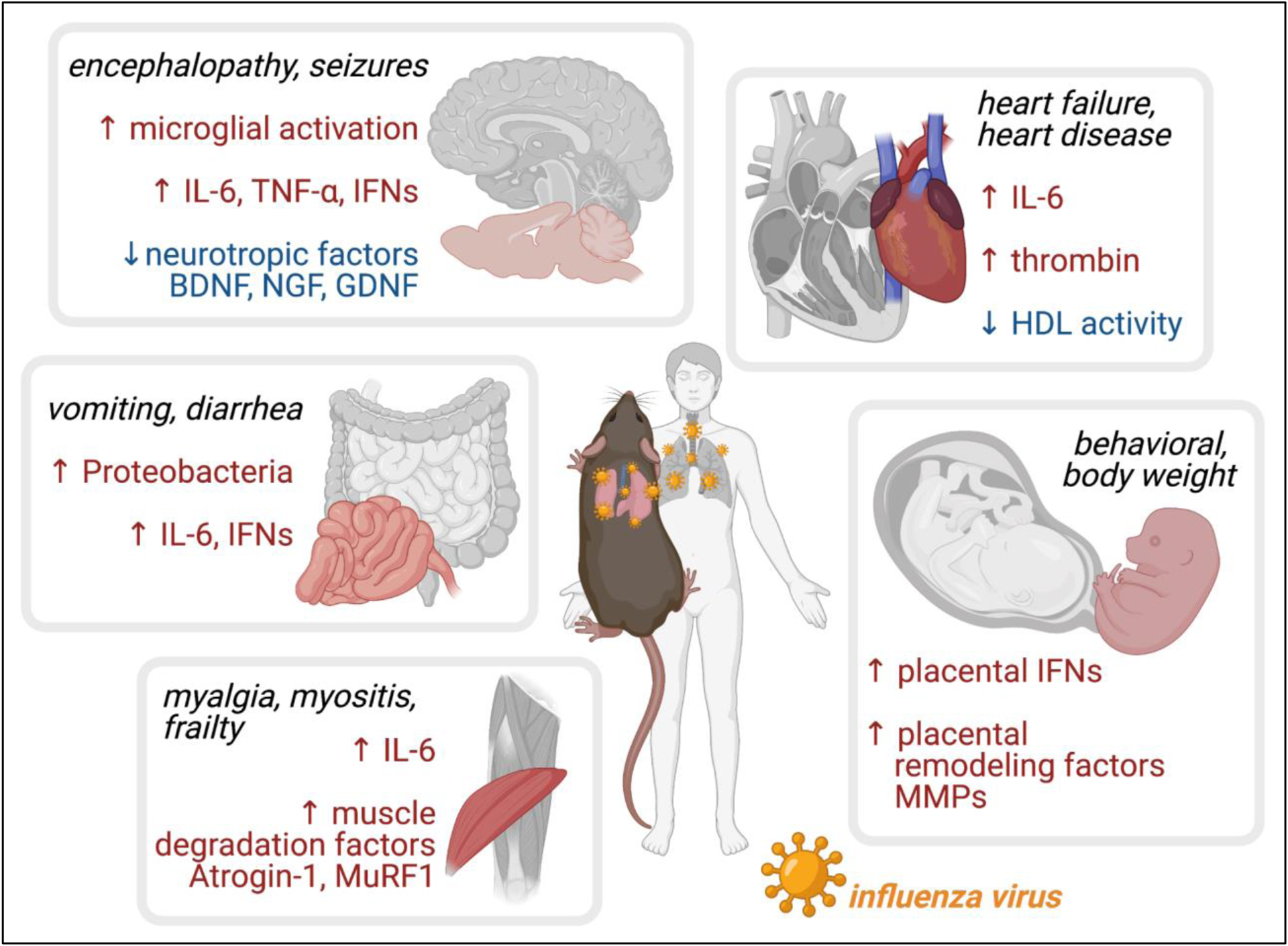
Effects of influenza viral disease outside sites of viral replication and effectors identified using animal models. Influenza virus is a respiratory infection that rarely disseminates throughout the body, while its effects can include neurological, cardiovascular, gastrointestinal, muscular, and fetal disease. Animal model studies, primarily using mice, have identified candidate inflammatory cytokines and organ-specific factors associated with damage at non-respiratory sites during influenza virus infection. Created with BioRender.com.
Neurological Effects and Effectors
Influenza A viruses (IAVs) are thought to induce most of the total influenza disease and are broadly separated into neurotropic or non-neurotropic strains. “Neurotropic” IAVs in humans, often highly pathogenic avian influenza viruses, though rare, have the ability to enter and replicate within the central nervous system (CNS) [32]. In contrast, the more common “non-neurotropic” IAVs, whether pandemic or seasonal strains, generally fail to invade the nervous system while successfully replicating throughout the respiratory tract. Despite their accepted inability to replicate and spread within the nervous system, non-neurotropic IAVs have been implicated in neurological disease.
Observations of neurological disease associated with IAV infection were first broadly recognized following the 1918 pandemic [33]. The 1918 H1N1 strain has also been controversially implicated in the contemporaneous epidemic of the neurologic disease von Economo’s encephalitis lethargica [34,35]. More recently, an increase in neurological symptoms, ranging from headaches to comas, during severe IAV disease was observed during the 2009 H1N1 pandemic [36]. However, even seasonal IAVs can result in significant neurological effects during serious infections, particularly among pediatric populations [37]. Interestingly, despite the severity of the neurological disease associated with IAVs, these so-called “non-neurotropic” viruses are only rarely detected within the central nervous systems of symptomatic patients [38], although this could be attributed to limited sample collection and time of sample collection (often post-mortem). It is also important to note that transient or extremely low levels of viral replication below the limit of detection could occur within the CNS, therefore contributing to neurological disease manifestations while virus remains “undetected” within these tissues. Whether “non-neurotropic” influenza viruses contact the CNS or not, it is well-accepted that the host immune response, whether fever or inflammation in the form of circulating cytokines or immune cells, contributes to influenza-associated neurological disease.
Influenza-associated febrile seizures and encephalopathy
Today, the most commonly observed severe neurological complications of influenza, primarily reported in young children, are febrile seizures and encephalopathy [37]. Nearly one-fifth of children hospitalized with influenza experience febrile seizures [39], which occur in young children with fevers above 38°C (100.4°F) due the vulnerability of their incompletely developed central nervous systems [40]. Influenza viral infections in mice do not result in hyperthermia, so febrile seizures alone have primarily been studied using other methods (notably the “hair dryer model” and “heated chamber model”) to raise body temperatures and induce seizures in young rodents [41]; however, these methods of study do not take into account the influence of inflammation on the development of febrile seizures. In contrast to mice, ferrets do experience increased body temperature in response to influenza viral infections [42,43], although influenza-associated seizures are predominately observed with neuroinvasive H5N1 viruses [44,45].
Influenza-associated encephalopathy is typified by altered consciousness and frequently leads to death or lasting neurological sequalae [37]. Influenza-associated encephalopathy (IAE), and its most severe form acute necrotizing encephalopathy (ANE), occurs at higher rates in East Asian populations compared to children of other ancestries, indicating a genetic link [17]. The identification of genetic predisposition for these conditions has led to clinical studies demonstrating the importance of the carnitine palmitoyl transferase II [46], ADORA2A [47], and RANBP2 [48] genes in IAE and ANE. Mouse models of IAE require lipopolysaccharide (LPS) administration in addition to influenza viral infection to replicate the hypercytokinemia (TNF-α, IL-6) and blood-brain barrier (BBB) permeability found in human patients [49,50]. In 2019, Imakita et al. used a murine model of IAE to identify a role for Caveolin-1 in regulating BBB permeability in the context of encephalopathy (Table 1) [51].
Table 1.
Key Animal Models of Neurological Disease with Respiratory Influenza Virus Infection
| Disease Model | Animal Model | Influenza Strain (and treatment) | Observed Effects | Proposed Effectors or Mechanisms | Ref |
|---|---|---|---|---|---|
| Influenza-associated encephalopathy (IAE) | Neonatal ICR mice | H3N2 A/Aichi/2/68 followed by LPS injection |
|
|
[49] |
| BALB/c mice | H1N1 A/Puerto Rico/8/34 followed by i.v. LPS |
|
|
[51] | |
| Experimental autoimmune encephalitis (EAE) model for multiple sclerosis (MS) | C57BL/6J mice | H1N1 A/Puerto Rico/8/34 followed by injection of MOG35–55 peptide |
|
|
[53] |
| 2D2 mice C57BL/6J mice |
H1N1 A/Puerto Rico/8/34 | In 2D2:
|
In BL6:
|
[55] | |
| Respiratory influenza infection | BALB/c mice | H1N1 A/Puerto Rice/8/34 |
|
|
[64] |
| C57BL/6J mice | H1N1 (pandemic) A/California/04/2009 |
|
|
[66] | |
| C57BL/6J mice | H3N2 mouse-adapted A/Hong Kong/1/68 |
|
|
[67] | |
| Ferrets | H1N1(pandemic) A/Netherlands/602/2009 |
|
|
[43] [73] |
Exacerbation of neuroimmune conditions with influenza viral infection
Influenza virus infection is also recognized to exacerbate or initiate certain neuroimmune conditions, such as Guillen-Barré syndrome, multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease. These findings are connected to a larger hypothesis that inflammation associated with repeated, acute viral diseases could in turn lead to chronic, neurodegenerative diseases [52]. Most in vivo research investigating the connection between influenza viral infections and exacerbated neuroimmune conditions have relied on mouse models of experimental autoimmune encephalitis (EAE), which is meant to replicate human multiple sclerosis (MS) flare-ups by targeting T cells to myelin oligodendrocyte glycoprotein (MOG), a component of the myelin sheath (Table 1). In 2017, Chen et al. first infected C57BL/6 mice with an H1N1 virus then allowed the mice to recover (50 days) before inducing EAE, finding mice previously exposed to influenza virus were unable to recover from EAE due to increased T cell infiltration and cytokine secretion (TNF-α, IFN-γ) into the CNS as a result of a potentially autoreactive T cells residing in the lung following infection [53]. In 2017, Blackmore et al. used the autoimmune 2D2 mouse model, transgenic mice that express T cells specific to MOG [54], to investigate the potential for influenza virus infection to induce a “flare-up” of multiple sclerosis [55]. Using this model, they found the non-neurotropic H1N1 virus in mouse lungs, but not the cerebellum or spinal cord; they did observe evidence of EAE, demyelination and inflammation in the CNS tissues and an upregulation of genes associated with glial activation and indicate a role for elevated chemokines in the CNS including CXCL5 and IFN-γ. In contrast, in 2017, Glenn et al. immunized mice against MOG [56] to induce autoimmune disease before infecting with influenza virus [57]. Using this model for MS and influenza virus co-morbidity, Glenn et al. did not find influenza virus infection caused an EAE flare-up, and instead the mortality associated with this autoimmune-infection model led to uncontrolled viral replication in the lungs due to myeloid-derived suppressor cell (MDSC) recruitment to the lungs limiting essential immune responses.
Although these three models of the interaction between MS and influenza virus utilize different experimental set ups and present somewhat conflicting results, each emphasize the increased mortality and risk respiratory viral infections pose to individuals with neuroimmune conditions. Further, and also in 2017, Sadasivan et al. tested the hypothesis that influenza infection could exacerbate neuroinflammatory damage in the context of Parkinson’s disease through systemic inflammation by infecting mice with pandemic H1N1 followed by (30 days) administration of the parkinsonian toxin (MPTP), finding a synergistic loss of dopaminergic neurons compared to mice either infected or treated with MPTP alone [58]. Similarly, in 2021, Hosseini et al. found a transgenic mouse model of Alzheimer’s disease (APP/PS1) showed more pronounced measures of cognitive impairment and amyloid plaques follow non-neurotropic H3N2 infection [59].
Microglial involvement in neurological effects of influenza
Mechanistically, animal model studies of neurological phenotypes during non-neurotropic IAV infections have nearly universally identified a role for microglial cell activation (Table 1) [60]. Microglia are macrophage-like cells in the central nervous system that mediate inflammatory responses within the brain [61]. During viral disease, microglia may be activated by pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and cytokines leading to morphological and transcriptional changes that facilitate cytokine release and other functional changes like antigen presentation [62]. In particular, the long term effects of viral infection on microglia have been implicated in brain aging and disease [63].
In 2012, Jurgens et al. infected BALB/C mice with a non-neurotropic H1N1 IAV and despite a lack of virus in the CNS, observed behavioral changes as measured using the Morris water maze, increased inflammatory cytokine expression in the hippocampus, and indicators of microglial activation [64]. The same group found these negative neurological effects as a result of IAV infection could be mediated by environmental enrichment [65]. In 2015, Sadasivan et al. infected C57BL/6J mice with a pandemic (but non-neurotropic) H1N1 virus and made similar observations about the activation of microglia, in addition to finding increases in expression of inflammatory cytokines and decreased neurotropic factors [66]. In 2018, Hosseini, et al. followed the long-term effects of influenza infection on neurological phenotypes by comparing the effects of a neurotropic H7N7 virus and a non-neurotropic H3N2 virus. They reported more extreme phenotypes with the neuroinvasive virus, but again observed similar changes in the hippocampus gene expression and microglial activation status with the non-neurotropic H3N2 virus. Further, these phenotypes fully resolved within 120 days post infection [67]. In 2021, Bantle et al. exposed young mice to manganese, a neurotoxin, and found with H1N1 infection in adulthood the exposed mice showed increased microglial activation, demonstrating how environmental factors can interact with viral infection to enhance neurological damage [68]. These studies together show that general observations about the neurological effects of non-neurotropic influenza viral infections are observable in mouse models at the level of behavioral changes, transcriptional changes within the brain, and microglial activation.
Ferrets as a model of influenza-associated neurological disease
The prominence of CNS involvement means the gold standard animal model for influenza, ferrets, have also been employed to understand how this respiratory virus impacts the brain. Influenza-induced neurological disease in ferrets has been frequently studied in the context of neuroinvasive viruses like highly pathogenic avian influenza H5N1, which readily invades and replicates within the CNS in both humans and animals [69]. In ferrets, these neuroinvasive and often systemic [70,71], H5N1 infections result in observable neurological phenotypes, while less pathogenic H1N1, pandemic H1N1 and H3N2 infections failed to induce neurological disease [43,72]. However, a follow-up study by Short et al. in 2017 measuring the induction of proinflammatory genes in the CNS of pandemic H1N1 and H5N1 infected ferrets found significant IL-8 expression in the olfactory bulb and cerebrum [73]. Further, in 2018 Sun et al. performed swine-origin H3N2 infections in ferrets and revealed inconsistent and low levels of virus within the brain and olfactory bulb, indicating these viruses were likely not neurotropic but were able to make contact with the CNS [74]. These findings show ferrets are another promising model of influenza-associated neurological effects.
Despite the general inability of non-neurotropic influenza viruses to enter the CNS, evidence from both mouse and ferret models of infection indicate the CNS is still negatively impacted by the systemic inflammation that accompanies respiratory infections. Although influenza infection alone induced behavioral changes indicative of cognitive dysfunction and neuroinflammation at the level of microglial activation, combining viral infection with additional conditions to replicate various neurological diseases resulted in extensive inflammatory damage to the CNS. Further, mouse models of influenza vaccination have also shown vaccination itself is often protective against neurological disease [75]. These findings along with epidemiological studies demonstrate the potential benefits of influenza vaccination for individuals with or at risk for ANE [76], MS [77], and other neurological conditions.
Cardiovascular Effects and Effectors
The close physical proximity and shared role in gas exchange of the respiratory and cardiovascular systems has made influenza virus infection of cardiovascular tissues an obvious area of investigation. Indeed, avian influenzas have been reported to be cardiotropic in birds but the detection of influenza virus in mammalian cardiac tissues is relatively rare [78] and mainly observed in the context of H5N1 highly pathogenic avian influenza infections [79,80]. Recently, it was found that IFITM3 is a key restriction factor preventing replication of influenza virus in the heart using an IFITM3 KO mouse model of infection, perhaps explaining why the virus only rarely accesses those tissues [81]. However, the hearts of mice fed a high fat diet had weaker antiviral responses and allowed higher viral replication in cardiac tissues during H1N1 infection compared to mice fed a low fat diet [82], identifying a potential risk factor for direct infection of the heart by influenza virus. Irrespective of the role of active cardiac infection by influenza virus, strong correlations suggest this respiratory infection leads to cardiac involvement in as many as 10% of influenza virus infections [83]. Acute influenza-associated cardiac events are especially prevalent in patients with pre-existing cardiovascular disease and include heart attacks and failure [84]. Due to strong support for the role of systemic inflammation in cardiovascular disease following respiratory viral infection [85], a number of studies have modeled these effects within mice and other animal models absent direct infection of cardiac tissues.
Cholesterol, atherosclerosis, and influenza infection
Cardiovascular disease is complex, and in animal models, influenza virus infections have been shown to exacerbate many aspects of the disease from cholesterol to plaques to coagulation (Table 2). High density lipoprotein (HDL) is well-recognized to reduce risk of coronary artery disease with roles in cholesterol transport and antioxidant and anti-inflammatory properties [86]. In 2001, Van Lenten et al. found that during H1N1 virus infection of C57BL/6 mice, while there was no evidence of viremia, HDL lost its anti-inflammatory properties and had a reduced ability to prevent low density lipoprotein (LDL) oxidation [87]; oxidized LDL is recognized to have atherogenic properties [88]. Following this work, Van Lenten et al. hypothesized treatment with a peptide mimetic of the main protein in HDL ApoA-I (D-4F) would combat the loss of HDL anti-inflammatory activity during influenza virus infection [89], particularly in LDL receptor deficient mice which develop hypercholesteremia and atherosclerotic plaques [90]. Indeed, the group found that although influenza infection led to arterial macrophage trafficking, which is associated with plaque instability, D-4F treatment was protective and reduced IL-6 lung and plasma levels.
Table 2.
Key Animal Models of Cardiovascular Disease with Respiratory Influenza Virus Infection
| Disease Model | Animal Model | Influenza Strain (and treatment) | Observed Effects | Proposed Effectors or Mechanisms | Ref |
|---|---|---|---|---|---|
| Respiratory influenza infection | C57BL/6J mice | H1N1 A/WSN/1933 |
|
|
[87] |
| C57BL/6N mice MLKL KO mice |
H1N1(pandemic) A/California/04/2009 H1N1 A/Puerto Rico/8/34 |
|
|
[98] | |
| Atherosclerosis | LDLR-null mice | H1N1 A/WSN/1933 followed by D-4F injection |
|
|
[89] |
| ApoE−/− mice | H3N2 A/Hong Kong/1/68 |
|
|
[93] | |
| Atherothrombosis | C57BL/6J mice TMpro/pro mice PAI-1−/− mice |
H1N1 A/Puerto Rico/8/34 |
|
|
[94] |
| Ferrets | H3N2 A/Netherlands/177/2008 H1N1 (pandemic) A/Netherlands/602/2009 H5N1 A/Indonesia/5/2005 |
|
|
[95] |
Other mouse models of atherosclerosis in combination with influenza viral infection have made similar conclusions, that respiratory infection resulted in systemic inflammation able to reduce atherosclerotic plaque stability, which in turn increases the risk of life-threatening thrombosis and ischemia [91]. In 2003, Naghavi et al. used the apoE−/− mouse model of atherosclerosis during influenza viral infection and found atherosclerotic plaques were subject to increased inflammation and fibrosis [92]. In 2020, Lee et al. found that in apoE−/− mice influenza infection increased MMP-13 expression from macrophages in the aorta, destabilizing atherosclerotic plaques [93].
Coagulation, atherothrombosis, and influenza infection
Research has also identified a role for influenza infection in modulating coagulation. In 2006, Keller et al. investigated the effects of influenza virus infection on thrombosis and, using a variety of mouse models, concluded influenza virus infection induced a prothrombotic state through increases in thrombin [94]. In 2014, Goeijenbier et al. used a ferret model of influenza virus infection and found similar pro-fibrotic and pro-coagulation phenotypes as observed in mouse models [95]. This association of severe influenza infection with pathogenic coagulation [96] is potentially relevant due to the prevalence of coagulation disorders in COVID-19 patients [97].
Influenza viral particles at or in cardiac tissue
Finally, influenza virus itself may play a more direct role, independent of productive cardiotropic infection, in cardiac disease. In 2021, Lin et al. infected mice with a pandemic H1N1 virus and found that although influenza viral particles were present in the heart after lung viral clearance, these particles did not appear to undergo productive replication but were able to alter the cardiac proteome [98]. They found eliminating the necroptosis pathway using MLKL KO mice reduced the harmful effects of influenza virus particles in cardiac tissues. Similarly, pandemic H1N1 influenza virus has been detected in the hearts of infected ferrets, although this did not appear to result in proinflammatory cytokine expression in cardiac tissues [43,73].
Thus, it has long been recognized that a systemic inflammatory state likely contributes to cardiovascular disease. Seasonal correlations of cardiac events with influenza viral infections build a strong epidemiological basis for this idea. Further, animal models of infection have shown that the immune response during influenza infections does create an environment conducive to atherosclerotic plaque rupture and thrombus formation. Furthermore, in 2011 Bermúdez-Fajardo & Oviedo-Orta demonstrated that vaccinating the apoE−/− mouse model of artherosclerosis against influenza virus promoted plaque stability [99]. Underlying conditions place patients at an even greater risk for cardiac complications of influenza infection. In 2021, Sinclair et al. employed a mouse model of type I diabetes and found influenza infection resulted in significant damage to the heart, without observing the same effect in healthy infected mice [100].With increasing evidence that respiratory viral infections play a role in exacerbating cardiovascular pathology, annual influenza vaccination is strongly recommended for individuals with cardiovascular and related disease [101].
Gastrointestinal and Metabolic Effects and Effectors
Forms of gastrointestinal “distress” during human influenza viral infections are relatively common [102]. Gastrointestinal symptoms of influenza virus infection like vomiting and diarrhea are not generally severe, although influenza-associated appendicitis, hemorrhagic gastritis and liver damage have been observed [17,102]. While influenza virus infections in birds are primarily gastrointestinal in nature [103], influenza viral RNA is only sometimes detected in the gastrointestinal tract of mammals and active viral replication in these tissues is controversial [43,102,104–106]. In order to understand how these alterations to the gastrointestinal tract and liver can occur without appreciable direct viral infection, a number of studies have illustrated that virally induced inflammation can alter the intestinal microbiome and overall metabolism in an infected person (Table 3) [107].
Table 3.
Key Animal Models of Gastrointestinal Disease with Respiratory Influenza Virus Infection
| Disease Model | Animal Model | Influenza Strain (and treatment) | Observed Effects | Proposed Effectors or Mechanisms | Ref |
|---|---|---|---|---|---|
| Salmonella-induced colitis | C57BL/6J mice Ifnar1−/− mice |
H1N1 A/Puerto Rico/8/34 followed by S. Typhimurium infection |
|
Lung produced type I IFNs:
|
[109] |
| C57BL/6J mice | H5N1 A/Viet Nam/1203/2004 followed by S. Typhimurium infection |
|
|
[110] | |
| C57BL/6J mice | H3N2 A/Scotland/20/1974 followed by S. Typhimurium infection |
|
|
[111] | |
| Respiratory influenza infection | C57BL/6J mice IFN-γ−/− mice Tcrd−/− mice IL-17A−/− mice |
H1N1 A/Puerto Rico/8/34 |
|
|
[112] |
| BALB/c mice | H1N1 (pandemic) A/Eng/195/2009 |
|
|
[114] | |
| BALB/c mice specific pathogen free | H1N1 (pandemic) A/Eng/195/2009 |
|
|
[115] |
Pathogenic enteric bacterial infections following respiratory influenza viral infections
It is well recognized that viral respiratory infections can lead to secondary respiratory bacterial infections; however, observations that influenza infections alter the gut microbiome have led some researchers to investigate the potential for “secondary” enteric bacterial infections [108]. In 2016, Deriu et al. infected WT and Ifnar KO mice with PR8 and observed increases in Proteobacteria (including E. coli) in the infected WT, but not Ifnar KO mice [109], indicating this effect is dependent on type I IFN. The group also observed increases in Enterobacteriaceae, and subsequently investigated whether influenza infection could impact the course of a mouse model of acute colitis associated with Salmonella infection. Infection with S. Typhimurium in previously virally infected mice led to increased bacterial colonization and further systemic dissemination. In 2018, Yildiz et al. also found influenza viral infection altered gut microbial communities, although treatment with IFN-α was not able to replicate the effect despite interferon stimulated gene expression in the intestine [110], indicating type I IFN is not sufficient to explain changes in the microbiome during viral infection. However, they did find influenza viral infection increased susceptibility to subsequent S. Typhimurium infection.
In 2021, Sencio et al. identified a role for short-chain fatty acids (SCFAs), a metabolic byproduct of gut microbiota, in protecting against S. Typhimurium secondary infections following respiratory influenza infection in mice [111]. Despite no detection of influenza viral RNA in the intestine, they observed an inflammatory gut transcriptional profile in addition to reduced SCFA levels during respiratory infection; accordingly, supplementation with SCFAs during influenza infection partially mitigated subsequent colonization by S. Typhimurium. This research indicates respiratory viral infections, like influenza, could leave patients more vulnerable to pathogenic intestinal bacteria.
Respiratory influenza infections and alterations to the gut microbiome
More general effects of respiratory influenza viral infection on the gut microbiome have also been observed. In 2014, Wang et al. infected mice with PR8 and observed intestinal changes and diarrhea independent of viral detection within the intestinal tract [112]. The group concluded these damaging effects were due to increases in E. coli within the intestine that led to Th17 cell increases. They supported a model where lung-derived T cells migrated to the intestine, secreted IFN-γ to disrupt the microbiome, subsequently inducing IL-15 secretion that led to Th17 cell polarization. In 2017, Bartley et al. explored the effects aging could have on the microbiome in the context of influenza infection [113]. Again, this group found increases in Proteobacteria during influenza infection no matter the age group. In 2018, Groves et al. also observed changes in the murine microbiome during respiratory virus infection, notably a decrease in Firmicutes, implicating increased Muc5ac levels in both the lung and gut [114].
However, in 2020, Groves et al. concluded that the main reason for the observed effects of respiratory viral infections (IAV and respiratory syncytial virus - RSV) on the microbiome were due to inappetence (reduced food consumption) of the mice during infection, with increases in TNF and CD8+ T cells during RSV infection leading to the infected mouse’s reduced food intake [115]. In further support, Sencio et al. found mice with restricted food intake mimicked the weight loss observed during an influenza viral infection and had similar gut bacterial profiles to influenza-infected mice [116]. These recent findings concerning the effect of dramatic weight loss in mice during influenza infections put to question whether mouse models of infection are appropriate models for how respiratory viral infections impact the human gut, although patient samples have similarly revealed increases in Escherichia and decreases in Firmicutes during influenza infections [117–119]. In further support, the shift in fecal microbiome observed during influenza-like illness in patients, including increased abundance of Escherichia, has been proposed as a biomarker for respiratory disease [120].
While the significance of virus-induced microbiome alterations remains incompletely understood, Zhang et al. showed changes in the murine gut microbiome following influenza infection can ultimately be protective [121]. Specifically, during H7N9 infection they observed increases in Bifidobacterium animalis in mice that survived, and when naive mice were then treated with either the fecal microbiota of survivor mice or Bifidobacterium animalis they became more resistant to viral infections. Thus, understanding the effects of virally-altered microbiome compositions during subsequent disease processes is an important area of future study.
Alterations to metabolism during influenza viral infections
As evidenced by the general impact of weight loss on the gut microbiome, the systemic effects of respiratory influenza viral infection, whether cytokine circulation or other metabolic changes, impact organs outside their source. In general, influenza infection is known to impact glucose and lipid metabolism across tissues, with the induction of interferons indicated as the main mediators in this metabolic shift [122]. In 2020, Ohno et al. found that mice infected with influenza virus had reduced tricarboxylic acid cycle substrate levels, dysregulated insulin signaling, and impaired fatty acid metabolism, implicating that the liver, and its role in metabolism, is impacted during infection [123]. IL-6 and IFNs induced during influenza infection have been shown to impact gene expression in the liver [124], and changes in the liver’s ability to respond to oxidative stress during influenza infection, independent of virus at the liver, have long been recognized [125]. Interestingly, Sencio et al. identified disruption of the intestinal barrier during respiratory influenza infection as a potential source of liver damage, due to release of bacteria, that could explain some of these large shifts in metabolism during infection [111].
It is well appreciated that our microbiomes are often reflective of our overall health [126], so it is no surprise that respiratory viral infections can throw off the balance of bacteria within the gut and leave individuals vulnerable to subsequent disease. Even further, recognition of the lung-gut axis, the concept that these microbiome-supporting organ systems are especially interconnected, has led to increasing research on the role of gut microbiota in defense against respiratory infections [127,128]. For example, the reduction in gut microbiome SCFA production during influenza viral infection was found to leave mice more vulnerable to secondary pulmonary S. pneumoniae infection [116]. Further, mice with a gut microbiome derived from wild mice, compared to laboratory mice, were protected from severe disease during respiratory influenza infection [129]. Interestingly, the studies in this review describing the impacts of viral infection on gut bacteria did not utilize any models of underlying gastrointestinal disease to understand the impact of pre-existing gut dysbiosis during respiratory viral infection. With respect to co-morbidities, it is highly recommended that patients with irritable bowel disease (IBD) are vaccinated against influenza because they are at increased risk of contracting and being hospitalized for influenza virus infections [130] in addition to the frequent use of immunomodulators to mediate IBD. There is a general understanding that an influenza infection could lead to a “flare-up” of IBD symptoms [131,132] though this connection appears mostly uninvestigated at the epidemiological or experimental level. Further work is needed to determine the exact interplay between influenza infections and gastrointestinal disease, both independent of severe inappetence and dependent on pre-existing conditions.
Muscular Effects and Effectors
One of the most recognized symptoms of “influenza-like” disease is mild muscle aches, or myalgia. Compared to some other organ systems where the role of replicating virus in disease is debated, there is limited evidence of influenza viruses actively infecting myocytes. Instead, investigations into the mechanism behind muscle complications during influenza virus infections have predominantly focused on indirect effects of the immune response. Influenza viral infections are generally associated with mild effects on the muscles; however, more detrimental effects on the musculoskeletal system have also been reported. Breakdown of muscle, or rhabdomyolysis, has been reported with influenza virus infection in more than 20 cases [17], while pediatric cases of influenza-associated-acute myositis present as calf muscle pain resulting in difficulty walking that resolves within a few days [133,134]. For elderly patients experiencing severe influenza virus complications, such as pneumonia and ARDS, there are also concerns about long term effects of muscle weakness following viral infection and hospitalization [135].
Primarily, mouse models reporting the effects of respiratory influenza associated myopathy have identified circulating IL-6 as a potential effector through increasing muscular atrogin-1 expression (Table 4). In 2019, Radigan et al. infected mice with H1N1 virus and proposed a model where increased IL-6 during infection induced FoxO3 expression, through STAT3, to further induce muscle atrophy F-box protein (atrogin-1) resulting in muscle protein degradation [136]. In 2016, Bartley et al. also found a role for IL-6 in differentiating effects of H1N1 infection in young and aged mice, focusing on impacts on mobility and muscles. The group found reductions in voluntary activity and alterations in gait during infection in both groups, with the aged mouse losses lingering longer. Similarly, increased inflammatory (Il6, Tnf) and muscle-breakdown associated gene expression (atrogin-1) was detected in both groups during infection, while in aged mice expression took longer to return to base levels and recovery of muscle growth associated genes was delayed compared to younger mice [137]. These findings appear independent of active infection within mouse muscular tissues. Treatment with an antibody that prevents IL-6 signaling mitigated the effect of influenza virus on muscle phenotypes in mice. In general agreement, in 2020 Runyan et al. compared the recovery of muscle function following influenza infection in young and old mice, and also observed increases in sera IL-6 and muscular atrogin-1 [138]. The group found activity levels recovered in young mice but not old, accompanied by an expansion of muscle progenitors in young but not old mice. During infection, they observed expansions in skeletal muscle macrophages in young but not old mice, further concluding the loss of phagocytic functions within macrophages was a driver of muscle recovery losses in infected, aged mice. These reports demonstrate how acute systemic inflammation such as during an influenza viral infection can lead to damage outside the infection site resulting in long-term reductions in mobility, particularly in elderly subjects.
Table 4.
Key Animal Models of Muscular Disease with Respiratory Influenza Virus Infection
| Disease Model | Animal Model | Influenza Strain (and treatment) | Observed Effects | Proposed Effectors or Mechanisms | Ref |
|---|---|---|---|---|---|
| Respiratory influenza infection | C57BL/6J mice Atrogin-1−/− mice |
H1N1 A/WSN/1933 |
|
|
[136] |
| Aging | C57BL/6J mice Young: 2.5–4 months Aged: 19–22 months |
H1N1 A/Puerto Rico/8/34 |
|
|
[137] |
| C57BL/6J mice Young: 9–11 weeks Aged: 18 months |
H1N1 A/WSN/1933 |
|
|
[138] | |
| C57BL/6J mice Young: 2.5–4 months Aged: 19–22 months |
PR8 NP vaccination followed by H1N1 A/Puerto Rico/8/34 |
|
|
[140] |
Interestingly, in addition to the immune effects on muscles, influenza virus may impact physical attributes of muscles during respiratory infection. In 2020, Straight et al. tested whether respiratory viral infections in mice would impact the physical properties of skeletal muscles at the cellular and molecular level, finding myosin fibers showed reduced cross sectional areas and increased myofilament stiffness, demonstrating that influenza infection causes changes within muscle properties, potentially through modulation of calcium sensitivity [139].
Although muscle aches are a highly experienced influenza symptom, most infected individuals likely expect any weakness or tenderness to fade quickly along with their fever or headaches. However, these mouse studies demonstrate the potentially lingering effects respiratory infections, and their accompanying systemic inflammation, can have on muscle weakening and regeneration, particularly in older hosts. Individuals over the age of 65 are already recognized to be an at-risk group in terms of severe influenza infections and are strongly recommended to receive annual influenza vaccinations; further, in 2020 Keilich et al. demonstrated that prior influenza vaccination prevented many of the negative muscle-associated phenotypes observed in aged mice [140]. These findings demonstrate an additional reason to highly recommend influenza vaccination in elderly populations.
Fetal Effects and Effectors
During pregnancy, infections with TORCH (Toxoplasmosis, Other – syphilis, varicella-zoster, parvovirus B19, Rubella, Cyomegalovirus, and Herpes) pathogens are well-recognized to threaten fetal health through direct infection. In contrast, influenza viruses are generally thought to remain in maternal respiratory tissues during infection of pregnant women [141], although in some cases highly pathogenic avian influenza H5N1 virus has been isolated from human fetal tissue [142]. However, despite the general lack of viral replication in fetal tissues, influenza viral infections during pregnancy still place the developing fetus at risk through activation of the maternal immune response [143]. The effects of maternal influenza disease on fetal development are well documented, including increased incidence of miscarriage, low birth weight, and preterm birth [144–146]; additionally, there is an increased risk of offspring developing schizophrenia or other neurological conditions [147–149]. Animal model studies have quantified how the state of pregnancy impacts maternal immunity, generally finding increased maternal severe infections and morbidity due to altered hormonal and immune responses (reviewed by van Riel et al. [150]). Only some of these experimental animal studies, however, have focused on investigating the impacts of maternal influenza infection on fetal health (Table 5) [151,152].
Table 5.
Key Animal Models of Fetal Disease with Respiratory Influenza Virus Infection
| Disease Model | Animal Model | Influenza Strain (and treatment) | Observed Effects | Proposed Effectors or Mechanisms | Ref |
|---|---|---|---|---|---|
| Pregnancy and Fetal Health | BALB/c mice E12 |
H1N1 A/Brisbane/59/07 |
|
|
[156] |
| C57BL/6J mice E12 |
H3N2 A/Hong Kong/1/68 |
|
|
[153] | |
| C57BL/6J mice E10 |
G15 (GPER1 inhibitor) treatment with H1N1 A/Puerto Rico/8/34 | GPER1 inhibition:
|
GPER1 inhibition:
|
[157] | |
| Pregnancy and Neurological Disease in Offspring | BALB/c mice C57BL/6J mice E9.5 |
H1N1 A/NWS/33CHINI | In offspring:
|
|
[163] [165] |
| Rhesus monkeys Week 17 (24-week pregnancy) |
H3N2 A/Sydney/5/97 | In offspring:
|
|
[166] |
Impaired fetal development with maternal influenza infection
Several recent studies have begun to describe potential mechanisms behind impaired fetal development during maternal influenza infection. In 2020, Liong et al. infected pregnant mice with influenza virus on E12 and found signs of hypoxia and altered angiogenesis responses in developing fetal brains and placentas, attributed to a maternal inflammatory “vascular storm” typified by proinflammatory leukocytes in the maternal blood vessels that were observed only in the context of pregnancy and infection [153]. They also observed reduced pup and placental weights with maternal influenza infection. Interestingly, the fetal effects of maternal influenza infection and its impact on offspring immune responses have also been reported. In 2021, Jacobsen et al. showed the offspring of pregnant mice infected on E5.5 with pandemic H1N1 were more vulnerable to early life bacterial and viral infections [154]. This vulnerability was attributed to reduced alveolar macrophage functions.
Hormones such as estrogens, which are upregulated during pregnancy, have been implicated as immunomodulatory (and specifically anti-inflammatory) compounds that potentially delay viral clearance during a maternal infection in a mouse model [155]. In 2017, Littauer et al. infected pregnant mice on E12 to closely observe impacts on maternal and fetal health and correlating hormonal and immune cytokines responses [156]. The group found infected mothers produced more stillborn pups, had dysregulated levels of pregnancy hormones (lower progesterone, higher PGF2α), showed damaged placental architecture, and experienced preterm labor. Specifically, activated matrix metalloproteinases (MMPs) were indicated as mediators of placental degradation. Our group has provided evidence consistent with the immunomodulatory hormone model, showing that estrogen-regulated GPER1 is a key suppressor of fetal type I interferon responses during maternal influenza infection [157]. In experiments where pregnant mice were infected with influenza (E10) and treated with a GPER1 inhibitor, enabling harmful IFN signaling in placental tissues, pups had lower birth weights and higher occurrences of reabsorption compared to only infected or only drug-treated dams. Treatment with an IFNAR antibody to prevent interferon signaling was able to rescue the combined effects of infection and the GPER1 inhibitor. These infection-pregnancy models emphasize the roles hormones, cytokines, and maternal changes in vasculature have in regulating infection resolution and fetal and offspring health.
Neuropsychiatric disease in offspring with maternal influenza infection
In addition to overall fetal health and neonatal viability, maternal infections, including influenza infections, are thought to have an impact on the development of autism and schizophrenia [158]. In fact, influenza infections in pregnant mice are used as a maternal immune activation (MIA) model in order to study the development of neurological conditions in offspring [159,160]. Like with more general observations of impaired fetal development with maternal influenza infections, circulating cytokines, rather than maternal-to-fetal transmission, are implicated in inducing damage to the fetal brain in clinical [161] and animal model studies [162]. In 2003, Shi et al. found that infecting pregnant mice with influenza virus on E9.5 led to offspring with behavioral phenotypes associated with schizophrenia and autism [163]. The group determined this effect was due to maternal immune responses rather than direct fetal infection by treating pregnant mice with viral mimetic poly(I:C), enacting a maternal cytokine response independent of infection, and continuing to observe these same behavioral phenotypes in offspring. Shi et al. further showed influenza viral RNA could not be detected in the placenta or fetal brain during maternal infection in mice [164]. These findings led to a follow-up in 2009 by Shi et al. where they identified a loss of Purkinje cells in the cerebellum, similar to the pathology of autism, in the offspring of influenza infected mice [165]. In 2010, Short et al. infected pregnant rhesus monkeys with H3N2 A/Sydney/5/97 and found that although offspring did not exhibit many behavioral changes, there were significant reductions in cortex gray matter and parietal cortex white matter with prenatal exposure to influenza virus [166].
Much of the work using mouse models to define the impact of maternal influenza infections on fetal neurological condition development has been performed by Fatemi et al. [162], identifying changes in fetal brain gene [167,168] and protein [169] expression, brain structural alterations [170], and reduced neurotransmitter levels [171], and further indicating placental abnormalities [172] as a potential mediator of effects on the developing CNS and eventual offspring. MIA studies using non-infectious immunogens, such as poly(I:C), have implicated maternal cytokines IL-6 and IL-17 in particular as effectors of neurological disease in offspring; however, further studies are required to determine the disease threshold that can result in schizophrenia and autism [173]. For example, in 2021, Antonson et al. found that infecting pregnant mice with a moderately pathogenic H3N2 virus resulted in maternal and placental inflammatory responses and placental damage, but failed to induce inflammation in the fetal brain or elevate circulating IL-17 as expected from non-infectious MIA models [174]. This finding demonstrates a need for use of infectious agents, like influenza, in future MIA model studies to determine the levels of disease expected to impact the outcomes of maternal infections.
As a result of the high-risk influenza viral infections pose to maternal and fetal health, influenza vaccination is highly recommended during pregnancy. The protective effect of influenza vaccination has been directly demonstrated in animal pregnancy models. For example, in 2018 Wu et al. found influenza vaccination early in pregnancy prevented autism-like phenotypes in a mouse model [175]. Further, influenza vaccination has been repeatedly shown to be associated with positive postnatal outcomes for offspring in epidemiological studies [176]. Additionally, prenatal vaccination also allows maternal antibodies against influenza to be transferred to the fetus in utero and subsequently in breast milk, thus providing protection to the immunologically vulnerable neonate [177]. These studies highlighting the impact of influenza vaccination on fetal and neonatal health again underscore the importance of understanding and preventing indirect, non-respiratory, manifestations of viral disease.
Conclusions and Future Directions
Although influenza virus tropism is predominantly restricted to cells in the respiratory tract, it is common for symptoms to occur in many organ systems independent of active replication at these sites. This review has outlined many studies that, primarily using mouse models, attempted to identify the mechanism behind these phenotypes in the cardiovascular, nervous, muscular, gastrointestinal, and fetal systems. The systems explored here are not the only non-respiratory ones impacted during influenza infection; however, they are the best examples with multiple animal studies to interrogate the mechanisms behind the influenza-associated diseases. For example, the nephrotic, hepatic, endocrine and ocular systems [17,178–180] have also been observed to be negatively impacted during respiratory influenza infections. Animal models observing the effects of respiratory influenza viral infections on these organ systems could further reveal the impacts of systemic inflammation throughout the body.
Many of the studies described above used wild-type mouse models, while other groups leveraged genetically modified mouse models for specific underlying conditions, such as LDLR−/− and apoE−/− models for atherosclerosis. In the future, it is likely other specialized models (genetic or other approaches for inducing physiological perturbations) will be important to understand how infection with influenza viruses affects non-respiratory sites. For example, murine models of irritable bowel disease [181] could be useful in defining whether influenza viral infections are indeed potential triggers for flare-ups. A newly developed mouse model for Alzheimer’s disease could also be utilized to help determine the role of viral disease in the development of neurodegenerative diseases [182]. Additionally, because obesity is recognized as a risk factor that leads to more severe influenza disease; evaluation of extrapulmonary effects of respiratory viral infection obese animal models is an important area of future research [183].
Outside of mice, ferrets (Mustela furo) are another widely used animal model of influenza that better replicate some aspects of human pathogenesis compared to the mouse model [42]. Ferrets develop fevers in response to influenza viral infections [42], perhaps providing an opportunity to study extrapulmonary influenza disease associated with raised temperatures. Virus-associated seizures, which are known to occur during CNS viral infections in ferrets [45,184,185], are one such example of a hyperthermia induced phenotype difficult to model in mice. In particular, ferrets are an appropriate model for the impact of age on the response to influenza infection [186], an issue highlighted in this review by pediatric patients’ vulnerability to neurological effects of influenza and older populations greater risk for cardiac and muscular manifestations of disease. In further support of future ferret research, tools and reagents for studies in ferrets are increasingly available [187].
Additionally, other animal models less commonly used for influenza research could be particularly applicable for different systems of interest. Guinea pigs (Cavia porcellus) are another animal model with some specific attributes that could be especially useful for studying the extrapulmonary effects of influenza virus infection. Although guinea pigs become readily infected with and transmit many influenza virus strains without requiring adaption, guinea pigs do not exhibit as severe respiratory disease phenotypes as observed with ferrets or even mice [23]. This model system may be particularly impactful in better replicating milder courses of disease in humans and the corresponding effects of influenza virus outside sites of active replication. For example, guinea pigs do not tend to lose weight during influenza infection, as observed during ferret and mouse infections [23], which could be advantageous for gastrointestinal studies to remove weight loss as a variable which frequently confounds interpretations. Additionally, guinea pigs more closely model human pregnancy based on similarities in placental structure, remodeling of the maternal spiral arteries, prolonged gestation relative to mice, and advanced maturity of the offspring [188]. Pigs (Sus domesticus) also represent an animal model with some unique advantages for study of non-respiratory impacts of influenza viral infection. Pigs are a natural host of influenza and are similar to humans in many ways, from their size and anatomy to their behaviors around sleep and eating [189]. Further, pigs have already been used to study a number of underlying conditions associated with exacerbated influenza viral disease, such as cardiovascular diseases and cystic fibrosis [190]. The similarities in anatomy (guinea pigs – placenta, pigs – size and structure) and course of disease (mild) make these animal models particularly suited to studying the impact of respiratory-restricted influenza infection on non-respiratory manifestations of disease.
Regardless of the experimental model, it is clear that many non-respiratory consequences of influenza viruses, although rare, have devastating, long term, or lethal consequences, particularly for high-risk individuals [191]. Understanding the mechanisms behind these disease effects also enables the development of therapeutic strategies to prevent specific non-respiratory tract complications influenza virus infection. As of now the only approach to prevent these aspects of disease is vaccination, which is required annually, or antiviral treatment, which is only effective early after infection. This review highlighted some common phenomena associated with negative extra-respiratory effects, such as systemic IL-6 and IFNs. Additionally, we also highlighted some system specific findings, like the ability of GPER1 to protect the developing fetus from the harmful effects of IFN exposure and that D-4F treatment can prevent the loss of the anti-inflammatory properties of HDL during influenza infection [89,157]. For individuals with underlying conditions, greater knowledge of the effectors of extrapulmonary disease could lead to more targeted therapeutic strategies to prevent negative outcomes in those at high risk.
Finally, although this review focused on the mechanisms behind extrapulmonary disease associated with respiratory influenza virus infections, other respiratory viral pathogens are also appreciated to cause significant systemic disease. These have been dramatically demonstrated recently during the SARS-CoV-2 [192] pandemic, but it is also recognized to be true for endemic pathogens such as respiratory syncytial virus (RSV) [38,193–195]. Every virus has different tropisms and a differential ability to disseminate, so mechanisms underlying disease systems outside of the primary sites of viral replication are likely different. Nonetheless, the continued study of non-respiratory effects of influenza viruses and other related infections will be important not only to more fully define the true scope of the disease, but also potentially to identify new intervention strategies capable of reducing the burden of these viruses on human health.
Acknowledgements
N.S.H. is partially supported by the National Heart, Blood and Lung institute (R01-HL142985), the National Institute of Allergy and Infectious Disease (R01-AI137031) and an award from The Hartwell Foundation.
Abbreviations
- ANE
acute necrotizing encephalopathy
- ARDS
acute respiratory distress syndrome
- BBB
blood-brain barrier
- Cal09
H1N1 A/California/04/2009
- CNS
central nervous system
- D-4F
apolipoprotein (apo)A-I mimetic peptide
- DAMP
damage-associated molecular pattern
- EAE
experimental autoimmune encephalitis
- HA
hemagglutinin
- HDL
high density lipoprotein
- IAE
influenza-associated encephalopathy
- IAV
influenza A virus
- IBD
irritable bowel disease
- IFN
interferon
- LDL
low density lipoprotein
- LPS
lipopolysaccharide
- MDSC
myeloid-derived suppressor cell
- MIA
maternal immune activation
- MMP
metalloproteinase
- MOG
myelin oligodendrocyte glycoprotein
- MPTP
parkinsonian toxin; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS
multiple sclerosis
- PAMP
pathogen-associated molecular pattern
- PR8
H1N1 A/Puerto Rico/8/1934
- RSV
respiratory syncytial virus
- SCFA
short-chain fatty acid
- TORCH
Toxoplasmosis, Other – syphilis, varicella-zoster, parvovirus B19, Rubella, Cyomegalovirus, and Herpes
- URT
upper respiratory tract
- WSN
H1N1 A/WSN/1933
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Kalil AC & Thomas PG (2019) Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care 23, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulick ER, Canning M, Parikh NS, Elkind MS V & Boehme AK (2020) Seasonality of Influenza-Like-Illness and Acute Cardiovascular Events Are Related Regardless of Vaccine Effectiveness. J Am Heart Assoc 9, e016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen IS, Mortensen LH, Krause TG & Nybo Andersen A-M (2018) The association between seasonal influenza-like illness cases and foetal death: a time series analysis. Epidemiol Infect 147, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuboi T & Okada S (1984) Seasonal variation of febrile convulsion in Japan. Acta Neurol Scand 69, 285–292. [DOI] [PubMed] [Google Scholar]
- 5.Manfredini R, Vergine G, Boari B, Faggioli R & Borgna-Pignatti C (2004) Circadian and seasonal variation of first febrile seizures. J Pediatr 145, 838–839. [DOI] [PubMed] [Google Scholar]
- 6.Sharafi R, Hassanzadeh Rad A & Aminzadeh V (2017) Circadian Rhythm and the Seasonal Variation in Childhood Febrile Seizure. Iran J child Neurol 11, 27–30. [PMC free article] [PubMed] [Google Scholar]
- 7.MacIntyre CR, Mahimbo A, Moa AM & Barnes M (2016) Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart 102, 1953–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giles ML, Krishnaswamy S, Macartney K & Cheng A (2019) The safety of inactivated influenza vaccines in pregnancy for birth outcomes: a systematic review. Hum Vaccin Immunother 15, 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flu Vaccination Coverage, United States, 2019–20 Influenza Season | FluVaxView | Seasonal Influenza (Flu) | CDC.
- 10.Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States — 2019–2020 Influenza Season | CDC.
- 11.Fukuyama S & Kawaoka Y (2011) The pathogenesis of influenza virus infections: the contributions of virus and host factors. Curr Opin Immunol 23, 481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Böttcher-Friebertshäuser E, Klenk H-D & Garten W (2013) Activation of influenza viruses by proteases from host cells and bacteria in the human airway epithelium. Pathog Dis 69, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S & Valleron A-J (2008) Time Lines of Infection and Disease in Human Influenza: A Review of Volunteer Challenge Studies. Am J Epidemiol 167, 775–785. [DOI] [PubMed] [Google Scholar]
- 14.Kalil AC & Thomas PG (2019) Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care 23, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López CB & Hermesh T (2011) Systemic responses during local viral infections: type I IFNs sound the alarm. Curr Opin Immunol 23, 495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Zhou Y & Yang Z (2016) The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol 13, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellers SA, Hagan RS, Hayden FG & Fischer WA (2017) The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respi Viruses 11, 372–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiore-Gartland A, Panoskaltsis-Mortari A, Agan AA, Mistry AJ, Thomas PG, Matthay MA, Hertz T, Randolph AG, Randolph AG, Sanders RC, Hefley G, Tellez D, Bliss C, Labell A, Liss D, Ortiz AL, Typpo K, Deschenes J, Markovitz B, Terry J, Morzov RSP, Graciano AL, Baldwin M, Anas N, Schwarz A, Onwunyi C, Osborne S, Patterson T, Vargas-Shiraishi O, Sapru A, Convery M, Lo V, Flori H, Brumfield B, Simon J, Czaja A, Mourani P, Aymami VB, Burr S, Brocato M, Huston S, Jewett E, Loyola D, Carroll C, Sala K, Thornton-Thompson S, Giuliano JSJ, Tala J, McLaughlin G, Paden M, Manghram C-C, Meisner S, Stone CL, Wardenburg JB, DeDent A, Montgomery V, Sullivan J, Evans T, Richardson K, Thomas M, Randolph A, Agan AA, Mistry AJ, Sullivan RM, Cobb S, Bembea M, White ED, Kurachek S, Doucette AA, Olson E, Hartman M, Jacobs R, Truemper E, Dawson M, Levin DL, Jarvis JD, Katyal C, Ackerman K, Daugherty LE, Baglia L, Hall MW, Greathouse K, Steele L, Thomas N, Raymond J, Spear D, Fitzgerald J, Helfaer M, Weiss S, Bush JL, Diliberto MA, Park BB, Sisko M, Barr FE, Higgerson R, Christie L, Darnell C, Johnson S, Loftis LL, Jaimon N, Kyle U, Gedeit R, Horn BE, Luther K, Murkowski K, Willson DF, Kelly RL, Jouvet PA, Fontaine A-M & Dugas M-A (2017) Cytokine Profiles of Severe Influenza Virus-Related Complications in Children. Front Immunol 8, 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClain MT, Henao R, Williams J, Nicholson B, Veldman T, Hudson L, Tsalik EL, Lambkin-Williams R, Gilbert A, Mann A, Ginsburg GS & Woods CW (2016) Differential evolution of peripheral cytokine levels in symptomatic and asymptomatic responses to experimental influenza virus challenge. Clin Exp Immunol 183, 441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser L, Fritz RS, Straus SE, Gubareva L & Hayden FG (2001) Symptom pathogenesis during acute influenza: Interleukin-6 and Other cytokine responses. J Med Virol 64, 262–268. [DOI] [PubMed] [Google Scholar]
- 21.Bouvier NM & Lowen AC (2010) Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2, 1530–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnard DL (2009) Animal models for the study of influenza pathogenesis and therapy. Antiviral Res 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thangavel RR & Bouvier NM (2014) Animal models for influenza virus pathogenesis, transmission, and immunology. J Immunol Methods 410, 60–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen T-Q, Rollon R & Choi Y-K (2021) Animal Models for Influenza Research: Strengths and Weaknesses. Viruses 2021, Vol 13, Page 1011 13, 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radigan KA, Misharin AV, Chi M & Budinger GRS (2015) Modeling human influenza infection in the laboratory. Infect Drug Resist 8, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilyushina NA, Khalenkov AM, Seiler JP, Forrest HL, Bovin NV, Marjuki H, Barman S, Webster RG & Webby RJ (2010) Adaptation of Pandemic H1N1 Influenza Viruses in Mice. J Virol 84, 8607–8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson TM, Golde TE & Lagier-Tourenne C (2018) Animal models of neurodegenerative diseases. Nat Neurosci 21, 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camacho P, Fan H, Liu Z & He J-Q (2016) Small mammalian animal models of heart disease. Am J Cardiovasc Dis 6, 70–80. [PMC free article] [PubMed] [Google Scholar]
- 29.Accarie A & Vanuytsel T (2020) Animal Models for Functional Gastrointestinal Disorders. Front Psychiatry 11, 1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romanick M, Thompson LV. & Brown-Borg HM (2013) Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle. Biochim Biophys Acta - Mol Basis Dis 1832, 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honce R, Wohlgemuth N, Meliopoulos VA, Short KR & Schultz-Cherry S (2020) Influenza in High-Risk Hosts-Lessons Learned from Animal Models. Cold Spring Harb Perspect Med 10, a038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegers JY, van de Bildt MWG, Lin Z, Leijten LM, Lavrijssen RAM, Bestebroer T, Spronken MIJ, De Zeeuw CI, Gao Z, Schrauwen EJA, Kuiken T & van Riel D (2019) Viral Factors Important for Efficient Replication of Influenza A Viruses in Cells of the Central Nervous System. J Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jelliffe SE (1919) Nervous and Mental Disturbances of Influenza. J Nerv Ment Dis 50. [Google Scholar]
- 34.Hoffman LA & Vilensky JA (2017) Encephalitis lethargica: 100 years after the epidemic. Brain 140, 2246–2251. [DOI] [PubMed] [Google Scholar]
- 35.Dickman MS (2001) von Economo Encephalitis. Arch Neurol 58, 1696. [DOI] [PubMed] [Google Scholar]
- 36.Asadi-Pooya AA, Yaghoubi E, Nikseresht A, Moghadami M & Honarvar B (2011) The Neurological Manifestations of H1N1 Influenza Infection; Diagnostic Challenges and Recommendations. Iran J Med Sci 36, 36–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Ekstrand JJ (2012) Neurologic Complications of Influenza. Semin Pediatr Neurol 19, 96–100. [DOI] [PubMed] [Google Scholar]
- 38.Bohmwald K, Gálvez NMS, Ríos M & Kalergis AM (2018) Neurologic Alterations Due to Respiratory Virus Infections. Front Cell Neurosci 12, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu SS, Tse CY, Lau YL & Peiris M (2001) Influenza A infection is an important cause of febrile seizures. Pediatrics 108, E63. [DOI] [PubMed] [Google Scholar]
- 40.Leung AK, Hon KL & Leung TN (2018) Febrile seizures: an overview. Drugs Context 7, 212536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koyama R (2013) Animal Models of Febrile Seizures. Anim Model Study Hum Dis, 889–901. [Google Scholar]
- 42.Belser JA, Eckert AM, Huynh T, Gary JM, Ritter JM, Tumpey TM & Maines TR (2020) A Guide for the Use of the Ferret Model for Influenza Virus Infection. Am J Pathol 190, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brand JMA van den, Stittelaar KJ, Amerongen G van, Reperant L, Waal L de, Osterhaus ADME & Kuiken T (2012) Comparison of Temporal and Spatial Dynamics of Seasonal H3N2, Pandemic H1N1 and Highly Pathogenic Avian Influenza H5N1 Virus Infections in Ferrets. PLoS One 7, e42343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plourde JR, Pyles JA, Layton RC, Vaughan SE, Tipper JL & Harrod KS (2012) Neurovirulence of H5N1 Infection in Ferrets Is Mediated by Multifocal Replication in Distinct Permissive Neuronal Cell Regions. PLoS One 7, 46605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long JP, Vela EM, Stark GV, Jones KJ, Miller ST & Bigger JE (2012) Early Indicators of Disease in Ferrets Infected with a High Dose of Avian Influenza H5N1. Sci Rep 2, 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Mizuguchi H, Yao D, Ide M, Kuroda Y, Shigematsu Y, Yamaguchi S, Yamaguchi M, Kinoshita M & Kido H (2005) Thermolabile phenotype of carnitine palmitoyltransferase II variations as a predisposing factor for influenza-associated encephalopathy. FEBS Lett 579, 2040–2044. [DOI] [PubMed] [Google Scholar]
- 47.Shinohara M, Saitoh M, Nishizawa D, Ikeda K, Hirose S, Takanashi J, Takita J, Kikuchi K, Kubota M, Yamanaka G, Shiihara T, Kumakura A, Kikuchi M, Toyoshima M, Goto T, Yamanouchi H & Mizuguchi M (2013) ADORA2A polymorphism predisposes children to encephalopathy with febrile status epilepticus. Neurology 80, 1571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neilson DE, Adams MD, Orr CMD, Schelling DK, Eiben RM, Kerr DS, Anderson J, Bassuk AG, Bye AM, Childs A-M, Clarke A, Crow YJ, Di Rocco M, Dohna-Schwake C, Dueckers G, Fasano AE, Gika AD, Gionnis D, Gorman MP, Grattan-Smith PJ, Hackenberg A, Kuster A, Lentschig MG, Lopez-Laso E, Marco EJ, Mastroyianni S, Perrier J, Schmitt-Mechelke T, Servidei S, Skardoutsou A, Uldall P, van der Knaap MS, Goglin KC, Tefft DL, Aubin C, de Jager P, Hafler D & Warman ML (2009) Infection-Triggered Familial or Recurrent Cases of Acute Necrotizing Encephalopathy Caused by Mutations in a Component of the Nuclear Pore, RANBP2. Am J Hum Genet 84, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka T, Sunden Y, Sakoda Y, Kida H, Ochiai K & Umemura T (2010) Lipopolysaccharide treatment and inoculation of influenza A virus results in influenza virus-associated encephalopathy-like changes in neonatal mice. J Neurovirol 16, 125–132. [DOI] [PubMed] [Google Scholar]
- 50.Kyan Y, Ueda Y, Yoshida M, Sasahara K & Shinya K (2014) Transcriptome profiling of brain edemas caused by influenza infection and lipopolysaccharide treatment. J Med Virol 86, 905–911. [DOI] [PubMed] [Google Scholar]
- 51.Imakita N, Kitabatake M, Ouji-Sageshima N, Hara A, Morita-Takemura S, Kasahara K, Matsukawa A, Wanaka A, Mikasa K & Ito T (2019) Abrogated Caveolin-1 expression via histone modification enzyme Setdb2 regulates brain edema in a mouse model of influenza-associated encephalopathy. Sci Rep 9, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deleidi M & Isacson O (2012) Viral and inflammatory triggers of neurodegenerative diseases. Sci Transl Med 4, 121ps3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Q, Liu Y, Lu A, Ni K, Xiang Z, Wen K & Tu W (2017) Influenza virus infection exacerbates experimental autoimmune encephalomyelitis disease by promoting type I T cells infiltration into central nervous system. J Autoimmun 77, 1–10. [DOI] [PubMed] [Google Scholar]
- 54.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA & Kuchroo VK (2003) Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med 197, 1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blackmore S, Hernandez J, Juda M, Ryder E, Freund GG, Johnson RW & Steelman AJ (2017) Influenza infection triggers disease in a genetic model of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 114, E6107–E6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendel I, de Rosbo NK & Ben-Nun A (1995) A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: Fine specificity and T cell receptor Vβ expression of encephalitogenic T cells. Eur J Immunol 25, 1951–1959. [DOI] [PubMed] [Google Scholar]
- 57.Glenn JD, Smith MD, Xue P, Chan-Li Y, Collins S, Calabresi PA, Horton MR & Whartenby KA (2017) CNS-targeted autoimmunity leads to increased influenza mortality in mice. J Exp Med 214, 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadasivan S, Sharp B, Schultz-Cherry S & Smeyne RJ (2017) Synergistic effects of influenza and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can be eliminated by the use of influenza therapeutics: experimental evidence for the multi-hit hypothesis. npj Park Dis 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hosseini S, Michaelsen-Preusse K, Schughart K & Korte M (2021) Long-Term Consequence of Non-neurotropic H3N2 Influenza A Virus Infection for the Progression of Alzheimer’s Disease Symptoms. Front Cell Neurosci 0, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barbosa-Silva MC, Santos LE & Rangel B (2018) The Impact of Non-Neurotropic Influenza Strains on the Brain: A Role for Microglial Priming? J Neurosci 38, 7758–7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Q & Barres BA (2018) Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18, 225–242. [DOI] [PubMed] [Google Scholar]
- 62.Lannes N, Eppler E, Etemad S, Yotovski P & Filgueira L (2017) Microglia at center stage: a comprehensive review about the versatile and unique residential macrophages of the central nervous system. Oncotarget 8, 114393–114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filgueira L, Larionov A & Lannes N (2021) The Influence of Virus Infection on Microglia and Accelerated Brain Aging. Cells 2021, Vol 10, Page 1836 10, 1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jurgens HA, Amancherla K & Johnson RW (2012) Influenza Infection Induces Neuroinflammation, Alters Hippocampal Neuron Morphology, and Impairs Cognition in Adult Mice. J Neurosci 32, 3958–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jurgens HA & Johnson RW (2012) Environmental enrichment attenuates hippocampal neuroinflammation and improves cognitive function during influenza infection. Brain Behav Immun 26, 1006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sadasivan S, Zanin M, O’Brien K, Schultz-Cherry S & Smeyne RJ (2015) Induction of Microglia Activation after Infection with the Non-Neurotropic A/CA/04/2009 H1N1 Influenza Virus. PLoS One 10, e0124047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosseini S, Wilk E, Michaelsen-Preusse K, Gerhauser I, Baumgärtner W, Geffers R, Schughart K & Korte M (2018) Long-term neuroinflammation induced by influenza a virus infection and the impact on hippocampal neuron morphology and function. J Neurosci 38, 3060–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bantle CM, French CT, Cummings JE, Sadasivan S, Tran K, Slayden RA, Smeyne RJ & Tjalkens RB (2021) Manganese exposure in juvenile C57BL/6 mice increases glial inflammatory responses in the substantia nigra following infection with H1N1 influenza virus. PLoS One 16, e0245171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peiris JSM, De Jong MD & Guan Y (2007) Avian influenza virus (H5N1): A threat to human health. Clin Microbiol Rev 20, 243–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinya K, Makino A, Tanaka H, Hatta M, Watanabe T, Le MQ, Imai H & Kawaoka Y (2011) Systemic Dissemination of H5N1 Influenza A Viruses in Ferrets and Hamsters after Direct Intragastric Inoculation. J Virol 85, 4673–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schrauwen EJA, Herfst S, Leijten LM, van Run P, Bestebroer TM, Linster M, Bodewes R, Kreijtz JHCM, Rimmelzwaan GF, Osterhaus ADME, Fouchier RAM, Kuiken T & van Riel D (2012) The Multibasic Cleavage Site in H5N1 Virus Is Critical for Systemic Spread along the Olfactory and Hematogenous Routes in Ferrets. J Virol 86, 3975–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gustin KM, Belser JA, Wadford DA, Pearce MB, Katz JM, Tumpey TM & Maines TR (2011) Influenza virus aerosol exposure and analytical system for ferrets. Proc Natl Acad Sci U S A 108, 8432–8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Short KR, Veeris R, Leijten LM, van den Brand JM, Jong VL, Stittelaar K, Osterhaus ADME, Andeweg A & van Riel D (2017) Proinflammatory Cytokine Responses in Extra-Respiratory Tissues During Severe Influenza. J Infect Dis 216, 829–833. [DOI] [PubMed] [Google Scholar]
- 74.Sun X, Pulit-Penaloza JA, Belser JA, Pappas C, Pearce MB, Brock N, Zeng H, Creager HM, Zanders N, Jang Y, Tumpey TM, Davis CT & Maines TR (2018) Pathogenesis and Transmission of Genetically Diverse Swine-Origin H3N2 Variant Influenza A Viruses from Multiple Lineages Isolated in the United States, 2011–2016. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, He Z, Xing Z, Zuo Z, Yuan L, Wu Y, Jiang M, Qi F & Yao Z (2020) Influenza vaccination in early Alzheimer’s disease rescues amyloidosis and ameliorates cognitive deficits in APP/PS1 mice by inhibiting regulatory T cells. J Neuroinflammation 17, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawai S, Nanri S, Ban E, Inokuchi M, Tanaka T, Tokumura M, Kimura K & Sugaya N (2011) Influenza vaccination of schoolchildren and influenza outbreaks in a school. Clin Infect Dis 53, 130–136. [DOI] [PubMed] [Google Scholar]
- 77.Zrzavy T, Kollaritsch H, Rommer PS, Boxberger N, Loebermann M, Wimmer I, Winkelmann A & Zettl UK (2019) Vaccination in Multiple Sclerosis: Friend or Foe? Front Immunol 10, 1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pankuweit S & Klingel K (2013) Viral myocarditis: from experimental models to molecular diagnosis in patients. Heart Fail Rev 18, 683–702. [DOI] [PubMed] [Google Scholar]
- 79.Rimmelzwaan GF, Van Riel D, Baars M, Bestebroer TM, Van Amerongen G, Fouchier RAM, Osterhaus ADME & Kuiken T (2006) Influenza A Virus (H5N1) Infection in Cats Causes Systemic Disease with Potential Novel Routes of Virus Spread within and between Hosts. Am J Pathol 168, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iwatsuki-Horimoto K, Nakajima N, Kiso M, Takahashi K, Ito M, Inoue T, Horiuchi M, Okahara N, Sasaki E, Hasegawa H & Kawaoka Y (2018) The Marmoset as an Animal Model of Influenza: Infection With A(H1N1)pdm09 and Highly Pathogenic A(H5N1) Viruses via the Conventional or Tracheal Spray Route. Front Microbiol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kenney AD, McMichael TM, Imas A, Chesarino NM, Zhang L, Dorn LE, Wu Q, Alfaour O, Amari F, Chen M, Zani A, Chemudupati M, Accornero F, Coppola V, Rajaram MVS & Yount JS (2019) IFITM3 protects the heart during influenza virus infection. Proc Natl Acad Sci 116, 18607–18612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siegers JY, Novakovic B, Hulme KD, Marshall RJ, Bloxham CJ, Thomas WG, Reichelt ME, Leijten L, van Run P, Knox K, Sokolowski KA, Tse BWC, Chew KY, Christ AN, Howe G, Bruxner TJC, Karolyi M, Pawelka E, Koch RM, Bellmann-Weiler R, Burkert F, Weiss G, Samanta RJ, Openshaw PJM, Bielefeldt-Ohmann H, van Riel D & Short KR (2020) A High-Fat Diet Increases Influenza A Virus-Associated Cardiovascular Damage. J Infect Dis 222, 820–831. [DOI] [PubMed] [Google Scholar]
- 83.Mamas MA, Fraser D & Neyses L (2008) Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol 130, 304–309. [DOI] [PubMed] [Google Scholar]
- 84.Estabragh ZR & Mamas MA (2013) The cardiovascular manifestations of influenza: A systematic review. Int J Cardiol 167, 2397–2403. [DOI] [PubMed] [Google Scholar]
- 85.Gopal R, Marinelli MA & Alcorn JF (2020) Immune Mechanisms in Cardiovascular Diseases Associated With Viral Infection. Front Immunol 11, 2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arora S, Patra SK & Saini R (2016) HDL—A molecule with a multi-faceted role in coronary artery disease. Clin Chim Acta 452, 66–81. [DOI] [PubMed] [Google Scholar]
- 87.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M & Fogelman AM (2001) High-Density Lipoprotein Loses Its Anti-Inflammatory Properties During Acute Influenza A Infection. Circulation 103, 2283–2288. [DOI] [PubMed] [Google Scholar]
- 88.Gao S & Liu J (2017) Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. Chronic Dis Transl Med 3, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, Nayak DP, Hama S, Navab M & Fogelman AM (2002) Influenza Infection Promotes Macrophage Traffic Into Arteries of Mice That Is Prevented by D-4F, an Apolipoprotein A-I Mimetic Peptide. Circulation 106, 1127–1132. [DOI] [PubMed] [Google Scholar]
- 90.Oppi S, Lüscher TF & Stein S (2019) Mouse Models for Atherosclerosis Research—Which Is My Line? Front Cardiovasc Med 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hansson GK, Libby P & Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278, 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S & Casscells W (2003) Influenza Infection Exerts Prominent Inflammatory and Thrombotic Effects on the Atherosclerotic Plaques of Apolipoprotein E–Deficient Mice. Circulation 107, 762–768. [DOI] [PubMed] [Google Scholar]
- 93.Lee HS, Noh JY, Shin OS, Song JY, Cheong HJ & Kim WJ (2020) Matrix Metalloproteinase-13 in Atherosclerotic Plaque Is Increased by Influenza A Virus Infection. J Infect Dis 221, 256–266. [DOI] [PubMed] [Google Scholar]
- 94.Keller TT, van der Sluijs KF, de Kruif MD, Gerdes VEA, Meijers JCM, Florquin S, van der Poll T, van Gorp ECM, Brandjes DPM, Büller HR & Levi M (2006) Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ Res 99, 1261–9. [DOI] [PubMed] [Google Scholar]
- 95.Goeijenbier M, van Gorp EC, Van den Brand JM, Stittelaar K, Bakhtiari K, Roelofs JJ, van Amerongen G, Kuiken T, Martina BE, Meijers JC & Osterhaus AD (2014) Activation of coagulation and tissue fibrin deposition in experimental influenza in ferrets. BMC Microbiol 14, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y & Tang H (2016) Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol 13, 432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giannis D, Ziogas IA & Gianni P (2020) Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 127, 104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Y-H, Platt MP, Gilley RP, Brown D, Dube PH, Yu Y & Gonzalez-Juarbe N (2021) Influenza Causes MLKL-Driven Cardiac Proteome Remodeling During Convalescence. Circ Res 128, 570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bermúdez-Fajardo A & Oviedo-Orta E (2011) Influenza vaccination promotes stable atherosclerotic plaques in apoE knockout mice. Atherosclerosis 217, 97–105. [DOI] [PubMed] [Google Scholar]
- 100.Sinclair JE, Bloxham CJ, Chiu H, Chew KY, Russell J, Yoshikawa Y, Bielefeldt-Ohmann H, Steele LE, Hulme KD, Verzele NA, Noye EC, Wu M, Reichelt ME, Thomas WG, Gallo LA, Redd MA & Short KR (2021) Type I Diabetes Mellitus Increases the Cardiovascular Complications of Influenza Virus Infection. Front Cell Infect Microbiol 0, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Influenza Vaccination: Proven and Effective Cardiovascular Disease Prevention - American College of Cardiology.
- 102.Minodier L, Charrel RN, Ceccaldi P-E, van der Werf S, Blanchon T, Hanslik T & Falchi A (2015) Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know? Virol J 12, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Causey D & Edwards SV (2008) Ecology of Avian Influenza Virus in Birds. J Infect Dis 197, S29–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirose R, Daidoji T, Naito Y, Watanabe Y, Arai Y, Oda T, Konishi H, Yamawaki M, Itoh Y & Nakaya T (2016) Long-term detection of seasonal influenza RNA in faeces and intestine. Clin Microbiol Infect 22, 813.e1–813.e7. [DOI] [PubMed] [Google Scholar]
- 105.Hirose R, Nakaya T, Naito Y, Daidoji T, Watanabe Y, Yasuda H, Konishi H & Itoh Y (2017) Mechanism of Human Influenza Virus RNA Persistence and Virion Survival in Feces: Mucus Protects Virions From Acid and Digestive Juices. J Infect Dis 216, 105–109. [DOI] [PubMed] [Google Scholar]
- 106.Belser JA, Jayaraman A, Raman R, Pappas C, Zeng H, Cox NJ, Katz JM, Sasisekharan R & Tumpey TM (2011) Effect of D222G Mutation in the Hemagglutinin Protein on Receptor Binding, Pathogenesis and Transmissibility of the 2009 Pandemic H1N1 Influenza Virus. PLoS One 6, e25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hanada S, Pirzadeh M, Carver KY & Deng JC (2018) Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front Immunol 9, 2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B & Delhaes L (2020) The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front Cell Infect Microbiol 10, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deriu E, Boxx GM, He X, Pan C, Benavidez SD, Cen L, Rozengurt N, Shi W & Cheng G (2016) Influenza Virus Affects Intestinal Microbiota and Secondary Salmonella Infection in the Gut through Type I Interferons. PLOS Pathog 12, e1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yildiz S, Mazel-Sanchez B, Kandasamy M, Manicassamy B & Schmolke M (2018) Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 2018 61 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sencio V, Gallerand A, Machado MG, Deruyter L, Heumel S, Soulard D, Barthelemy J, Cuinat C, Vieira AT, Barthelemy A, Tavares LP, Guinamard R, Ivanov S, Grangette C, Teixeira MM, Foligné B, Wolowczuk I, Le Goffic R, Thomas M & Trottein F (2021) Influenza virus infection impairs the gut’s barrier properties and favors secondary enteric bacterial infection through reduced production of short-chain fatty acids. Infect Immun 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang J, Li F, Wei H, Lian Z-X, Sun R & Tian Z (2014) Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell–dependent inflammation. J Exp Med 211, 2397–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bartley JM, Zhou X, Kuchel GA, Weinstock GM & Haynes L (2017) Impact of Age, Caloric Restriction, and Influenza Infection on Mouse Gut Microbiome: An Exploratory Study of the Role of Age-Related Microbiome Changes on Influenza Responses. Front Immunol 8, 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Groves HT, Cuthbertson L, James P, Moffatt MF, Cox MJ & Tregoning JS (2018) Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota. Front Immunol 9, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Groves HT, Higham SL, Moffatt MF, Cox MJ & Tregoning JS (2020) Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. MBio 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sencio V, Barthelemy A, Tavares LP, Ferreira P, Teixeira MM, Ois F & Correspondence T (2020) Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. CellReports 30, 2934–2947.e6. [DOI] [PubMed] [Google Scholar]
- 117.Qin N, Zheng B, Yao J, Guo L, Zuo J, Wu L, Zhou J, Liu L, Guo J, Ni S, Li A, Zhu Y, Liang W, Xiao Y, Ehrlich SD & Li L (2015) Influence of H7N9 virus infection and associated treatment on human gut microbiota. Sci Reports 2015 51 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H, Zheng B, Zhang J, Yan R, Zhang H, Jiang H, Xu Q, Guo J, Gong Y, Tang L & Li L (2020) Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis 71, 2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khatib HA Al, Mathew S, Smatti MK, Eltai NO, Pathan SA, Thani AA Al, Coyle PV, Maslamani MA Al & Yassine HM (2021) Profiling of Intestinal Microbiota in Patients Infected with Respiratory Influenza A and B Viruses. Pathog 2021, Vol 10, Page 761 10, 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fuentes S, Hartog G den, Nanlohy NM, Wijnands L, Ferreira JA, Nicolaie MA, Pennings JLA, Jacobi R, Wit J de, Beek J van & Baarle D van (2021) Associations of faecal microbiota with influenza-like illness in participants aged 60 years or older: an observational study. Lancet Heal Longev 2, e13–e23. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Q, Hu J, Feng J-W, Hu X-T, Wang T, Gong W-X, Huang K, Guo Y-X, Zou Z, Lin X, Zhou R, Yuan Y-Q, Zhang A-D, Wei H, Cao G, Liu C, Chen L-L & Jin M-L (2020) Influenza infection elicits an expansion of gut population of endogenous Bifidobacterium animalis which protects mice against infection. Genome Biol 2020 211 21, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keshavarz M, Solaymani-Mohammadi F, Namdari H, Arjeini Y, Mousavi MJ & Rezaei F (2020) Metabolic host response and therapeutic approaches to influenza infection. Cell Mol Biol Lett 2020 251 25, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ohno M, Sekiya T, Nomura N, Daito T ji, Shingai M & Kida H (2020) Influenza virus infection affects insulin signaling, fatty acid-metabolizing enzyme expressions, and the tricarboxylic acid cycle in mice. Sci Rep 10, 10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghoshal K, Majumder S, Zhu Q, Hunzeker J, Datta J, Shah M, Sheridan JF & Jacob ST (2001) Influenza Virus Infection Induces Metallothionein Gene Expression in the Mouse Liver and Lung by Overlapping but Distinct Molecular Mechanisms. Mol Cell Biol 21, 8301–8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hennet T, Peterhans E & Stocker R (1992) Alterations in antioxidant defences in lung and liver of mice infected with influenza A virus. J Gen Virol 73, 39–46. [DOI] [PubMed] [Google Scholar]
- 126.Wang B, Yao M, Lv L, Ling Z & Li L (2017) The Human Microbiota in Health and Disease. Engineering 3, 71–82. [Google Scholar]
- 127.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B & Delhaes L (2020) The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front Cell Infect Microbiol 0, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sencio V, Machado MG & Trottein F (2021) The lung–gut axis during viral respiratory infections: the impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol 14, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, Hickman HD, McCulloch JA, Badger JH, Ajami NJ, Trinchieri G, Villena FP-M de, Yewdell JW & Rehermann B (2017) Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell 171, 1015–1028.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tinsley A, Navabi S, Williams ED, Liu G, Kong L, Coates MD & Clarke K (2019) Increased Risk of Influenza and Influenza-Related Complications Among 140,480 Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis 25, 369–376. [DOI] [PubMed] [Google Scholar]
- 131.Ulcerative Colitis and Influenza | Everyday Health.
- 132.Crohn’s Disease and Colds and Flu | Everyday Health.
- 133.Agyeman P, Duppenthaler A, Heininger U & Aebi C (2004) Influenza-Associated Myositis in Children. Infection 32, 199–203. [DOI] [PubMed] [Google Scholar]
- 134.Ferrarini A, Lava SAG, Simonetti GD, Ramelli GP & Bianchetti MG (2014) Influenzavirus B-associated acute benign myalgia cruris: An outbreak report and review of the literature. Neuromuscul Disord 24, 342–346. [DOI] [PubMed] [Google Scholar]
- 135.Walsh CJ, Batt J, Herridge MS & Dos Santos CC (2014) Muscle wasting and early mobilization in acute respiratory distress syndrome. Clin Chest Med 35, 811–826. [DOI] [PubMed] [Google Scholar]
- 136.Radigan KA, Nicholson TT, Welch LC, Chi M, Amarelle L, Angulo M, Shigemura M, Shigemura A, Runyan CE, Morales-Nebreda L, Perlman H, Ceco E, Lecuona E, Dada LA, Misharin AV, Mutlu GM, Sznajder JI & Budinger GRS (2019) Influenza A Virus Infection Induces Muscle Wasting via IL-6 Regulation of the E3 Ubiquitin Ligase Atrogin-1. J Immunol 202, 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bartley JM, Pan SJ, Keilich SR, Hopkins JW, Al-Naggar IM, Kuchel GA & Haynes L (2016) Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging (Albany NY) 8, 620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Runyan CE, Welch LC, Lecuona E, Shigemura M, Amarelle L, Abdala‐Valencia H, Joshi N, Lu Z, Nam K, Markov NS, McQuattie‐Pimentel AC, Piseaux‐Aillon R, Politanska Y, Sichizya L, Watanabe S, Williams KJN, Budinger GRS, Sznajder JI & Misharin AV. (2020) Impaired phagocytic function in CX3CR1 + tissue‐resident skeletal muscle macrophages prevents muscle recovery after influenza A virus‐induced pneumonia in old mice. Aging Cell 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Straight CR, Ringham OR, Bartley JM, Keilich SR, Kuchel GA, Haynes L & Miller MS (2020) Influenza Infection has Fiber Type-Specific Effects on Cellular and Molecular Skeletal Muscle Function in Aged Mice. Journals Gerontol - Ser A Biol Sci Med Sci 75, 2333–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keilich SR, Lorenzo EC, Torrance BL, Harrison AG, Bartley JM & Haynes L (2020) Vaccination mitigates influenza-induced muscular declines in aged mice. GeroScience 42, 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kanmaz HG, Erdeve O, Oğz SS, Uras N, Çelen Ş, Korukluoglu G, Zergeroglu S, Kara A & Dilmen U (2011) Placental Transmission of Novel Pandemic Influenza a Virus. http://dx.doi.org/103109/155138152011572956 30, 280–285. [DOI] [PubMed] [Google Scholar]
- 142.Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, Lau LT, Lu J, Gao Z, Zhang B, McNutt MA, Lu M, Anderson VM, Gong E, Yu ACH & Lipkin WI (2007) H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet 370, 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yockey LJ & Iwasaki A (2018) Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 49, 397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang R, Yan W, Du M, Tao L & Liu J (2021) The effect of influenza virus infection on pregnancy outcomes: A systematic review and meta-analysis of cohort studies. Int J Infect Dis 105, 567–578. [DOI] [PubMed] [Google Scholar]
- 145.Rasmussen SA, Jamieson DJ & Uyeki TM (2012) Effects of influenza on pregnant women and infants. YMOB 207, S3–S8. [DOI] [PubMed] [Google Scholar]
- 146.Dawood FS, Kittikraisak W, Patel A, Hunt DR, Suntarattiwong P, Wesley MG, Thompson MG, Soto G, Mundhada S, Arriola CS, Azziz-Baumgartner E, Brummer T, Cabrera S, Chang HH, Deshmukh M, Ellison D, Florian R, Gonzales O, Kurhe K, Kaoiean S, Rawangban B, Lindstrom S, Llajaruna E, Mott JA, Saha S, Prakash A, Mohanty S, Sinthuwattanawibool C & Tinoco Y (2021) Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis 21, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kępińska AP, Iyegbe CO, Vernon AC, Yolken R, Murray RM & Pollak TA (2020) Schizophrenia and Influenza at the Centenary of the 1918–1919 Spanish Influenza Pandemic: Mechanisms of Psychosis Risk. Front Psychiatry 0, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boksa P (2008) Maternal infection during pregnancy and schizophrenia. J Psychiatry Neurosci 33, 183. [PMC free article] [PubMed] [Google Scholar]
- 149.al-Haddad BJS, Jacobsson B, Chabra S, Modzelewska D, Olson EM, Bernier R, Enquobahrie DA, Hagberg H, Östling S, Rajagopal L, Waldorf KMA & Sengpiel V (2019) Long-term Risk of Neuropsychiatric Disease After Exposure to Infection In Utero. JAMA Psychiatry 76, 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Riel D, Mittrücker H-W, Engels G, Klingel K, Markert UR & Gabriel G (2016) Influenza pathogenicity during pregnancy in women and animal models. Semin Immunopathol 38, 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim JC, Kim HM, Kang YM, Ku KB, Park EH, Yum J, Kim JA, Kang YK, Lee JS, Kim HS & Seo SH (2014) Severe pathogenesis of influenza B virus in pregnant mice. Virology 448, 74–81. [DOI] [PubMed] [Google Scholar]
- 152.Collie MH, Sweet C, Cavanagh D & Smith H (1978) Association of foetal wastage with influenza infection during ferret pregnancy. Br J Exp Pathol 59, 190–5. [PMC free article] [PubMed] [Google Scholar]
- 153.Liong S, Oseghale O, To EE, Brassington K, Erlich JR, Luong R, Liong F, Brooks R, Martin C, O’Toole S, Vinh A, O’Neill LAJ, Bozinovski S, Vlahos R, Papagianis PC, O’Leary JJ, Brooks DA & Selemidis S (2020) Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc Natl Acad Sci 117, 24964–24973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jacobsen H, Walendy-Gnirß K, Tekin-Bubenheim N, Kouassi NM, Ben-Batalla I, Berenbrok N, Wolff M, dos Reis VP, Zickler M, Scholl L, Gries A, Jania H, Kloetgen A, Düsedau A, Pilnitz-Stolze G, Jeridi A, Yildirim AÖ, Fuchs H, Gailus-Durner V, Stoeger C, de Angelis MH, Manuylova T, Klingel K, Culley FJ, Behrends J, Loges S, Schneider B, Krauss-Etschmann S, Openshaw P & Gabriel G (2021) Offspring born to influenza A virus infected pregnant mice have increased susceptibility to viral and bacterial infections in early life. Nat Commun 2021 121 12, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pazos MA, Kraus TA, Muñoz-Fontela C & Moran TM (2012) Estrogen Mediates Innate and Adaptive Immune Alterations to Influenza Infection in Pregnant Mice. PLoS One 7, e40502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Littauer EQ, Esser ES, Antao OQ, Vassilieva EV, Compans RW & Skountzou I (2017) H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLOS Pathog 13, e1006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Harding AT, Goff MA, Froggatt HM, Lim JK & Heaton NS (2021) GPER1 is required to protect fetal health from maternal inflammation. Science 371, 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP & Susser ES (2004) Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Arch Gen Psychiatry 61, 774. [DOI] [PubMed] [Google Scholar]
- 159.Meyer U (2014) Prenatal Poly(I:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems. Biol Psychiatry 75, 307–315. [DOI] [PubMed] [Google Scholar]
- 160.Lammert CR & Lukens JR (2019) Modeling Autism-Related Disorders in Mice with Maternal Immune Activation (MIA). Methods Mol Biol 1960, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang J, Luo W, Huang P, Peng L & Huang Q (2018) Maternal C-reactive protein and cytokine levels during pregnancy and the risk of selected neuropsychiatric disorders in offspring: A systematic review and meta-analysis. J Psychiatr Res 105, 86–94. [DOI] [PubMed] [Google Scholar]
- 162.Kneeland RE & Fatemi SH (2013) Viral infection, inflammation and schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry 42, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi L, Fatemi SH, Sidwell RW & Patterson PH (2003) Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shi L, Tu N & Patterson PH (2005) Maternal influenza infection is likely to alter fetal brain development indirectly: The virus is not detected in the fetus. Int J Dev Neurosci 23, 299–305. [DOI] [PubMed] [Google Scholar]
- 165.Shi L, Smith SEP, Malkova N, Tse D, Su Y & Patterson PH (2009) Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun 23, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, Gilmore JH & Coe CL (2010) Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry 67, 965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fatemi SH, Folsom TD, Reutiman TJ, Abu-Odeh D, Mori S, Huang H & Oishi K (2009) Abnormal expression of myelination genes and alterations in white matter fractional anisotropy following prenatal viral influenza infection at E16 in mice. Schizophr Res 112, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fatemi SH, Folsom TD, Reutiman TJ, Huang H, Oishi K & Mori S (2009) Prenatal viral infection of mice at E16 causes changes in gene expression in hippocampi of the offspring. Eur Neuropsychopharmacol 19, 648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S & Bailey K (1999) Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry 4, 145–154. [DOI] [PubMed] [Google Scholar]
- 170.Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L & Sidwell R (2002) Prenatal Viral Infection Leads to Pyramidal Cell Atrophy and Macrocephaly in Adulthood: Implications for Genesis of Autism and Schizophrenia. Cell Mol Neurobiol 22, 25–33. [DOI] [PubMed] [Google Scholar]
- 171.Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, Smee DF, Pearce DA, Winter C, Sohr R & Juckel G (2008) Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: Implications for genesis of neurodevelopmental disorders. Schizophr Res 99, 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fatemi SH, Folsom TD, Rooney RJ, Mori S, Kornfield TE, Reutiman TJ, Kneeland RE, Liesch SB, Hua K, Hsu J & Patel DH (2012) The viral theory of schizophrenia revisited: Abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring. Neuropharmacology 62, 1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Estes ML & McAllister AK (2016) Maternal immune activation: Implications for neuropsychiatric disorders. Science (80-) 353, 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Antonson AM, Kenney AD, Chen HJ, Corps KN, Yount JS & Gur TL (2021) Moderately pathogenic maternal influenza A virus infection disrupts placental integrity but spares the fetal brain. Brain Behav Immun 96, 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu Y, Qi F, Song D, He Z, Zuo Z, Yang Y, Liu Q, Hu S, Wang X, Zheng X, Yang J, Yuan Q, Zou J, Guo K & Yao Z (2018) Prenatal influenza vaccination rescues impairments of social behavior and lamination in a mouse model of autism. J Neuroinflammation 15, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Buchy P, Badur S, Kassianos G, Preiss S & Tam JS (2020) Vaccinating pregnant women against influenza needs to be a priority for all countries: An expert commentary. Int J Infect Dis 92, 1–12. [DOI] [PubMed] [Google Scholar]
- 177.Nunes MC & Madhi SA (2018) Prevention of influenza-related illness in young infants by maternal vaccination during pregnancy. F1000Research 7, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dalbhi S Al, Alshahrani HA, Almadi A, Busaleh H, Alotaibi M, Almutairi W & Almukhrq Z (2019) Prevalence and mortality due to acute kidney injuries in patients with influenza A (H1N1) viral infection: A systemic narrative review. Int J Health Sci (Qassim) 13, 56–62. [PMC free article] [PubMed] [Google Scholar]
- 179.Schütte A, Ciesek S, Wedemeyer H & Lange CM (2019) Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol 70, 797–799. [DOI] [PubMed] [Google Scholar]
- 180.Belser JA, Lash RR, Garg S, Tumpey TM & Maines TR (2018) The eyes have it: influenza virus infection beyond the respiratory tract. Lancet Infect Dis 18, e220–e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kiesler P, Fuss IJ & Strober W (2015) Experimental Models of Inflammatory Bowel Diseases. Cell Mol Gastroenterol Hepatol 1, 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Baglietto-Vargas D, Forner S, Cai L, Martini AC, Trujillo-Estrada L, Swarup V, Nguyen MMT, Do Huynh K, Javonillo DI, Tran KM, Phan J, Jiang S, Kramár EA, Nuñez-Diaz C, Balderrama-Gutierrez G, Garcia F, Childs J, Rodriguez-Ortiz CJ, Garcia-Leon JA, Kitazawa M, Shahnawaz M, Matheos DP, Ma X, Da Cunha C, Walls KC, Ager RR, Soto C, Gutierrez A, Moreno-Gonzalez I, Mortazavi A, Tenner AJ, MacGregor GR, Wood M, Green KN & LaFerla FM (2021) Generation of a humanized Aβ expressing mouse demonstrating aspects of Alzheimer’s disease-like pathology. Nat Commun 2021 121 12, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Honce R & Schultz-Cherry S (2019) Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front Immunol 0, 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barbeau DJ, Albe JR, Nambulli S, Tilston-Lunel NL, Hartman AL, Lakdawala SS, Klein E, Duprex WP & McElroy AK (2020) Rift Valley Fever Virus Infection Causes Acute Encephalitis in the Ferret. mSphere 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Beineke A, Baumgärtner W & Wohlsein P (2015) Cross-species transmission of canine distemper virus—an update. One Heal 1, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.M R, ME F, CL S, A G, A K & AA K (2021) The Intersection of Age and Influenza Severity: Utility of Ferrets for Dissecting the Age-Dependent Immune Responses and Relevance to Age-Specific Vaccine Development. Viruses 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Albrecht RA, Liu W-C, Sant AJ, Tompkins SM, Pekosz A, Meliopoulos V, Cherry S, Thomas PG & Schultz-Cherry S (2018) Moving Forward: Recent Developments for the Ferret Biomedical Research Model. MBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Harrell MI, Burnside K, Whidbey C, Vornhagen J, Adams Waldorf KM & Rajagopal L (2017) Exploring the Pregnant Guinea Pig as a Model for Group B Streptococcus Intrauterine Infection. J Infect Dis Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rajao DS & Vincent AL (2015) Swine as a model for influenza avirus infection and immunity. ILAR J 56, 44–52. [DOI] [PubMed] [Google Scholar]
- 190.Perleberg C, Kind A & Schnieke A (2018) Genetically engineered pigs as models for human disease. Dis Model Mech 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Uyeki TM (2020) High-risk Groups for Influenza Complications. JAMA 324, 2334. [DOI] [PubMed] [Google Scholar]
- 192.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP & Landry DW (2020) Extrapulmonary manifestations of COVID-19. Nat Med 26, 1017–1032. [DOI] [PubMed] [Google Scholar]
- 193.Harding JN, Siefker D, Vu L, You D, DeVincenzo J, Pierre J & Cormier SA (2020) Altered gut microbiota in infants is associated with respiratory syncytial virus disease severity. BMC Microbiol 20, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ivey KS, Edwards KM & Talbot HK (2018) Respiratory Syncytial Virus and Associations With Cardiovascular Disease in Adults. J Am Coll Cardiol 71, 1574–1583. [DOI] [PubMed] [Google Scholar]
- 195.Gkentzi D, Dimitriou G & Karatza A (2018) Non-pulmonary manifestations of respiratory syncytial virus infection. J Thorac Dis 10, S3815–S3818. [DOI] [PMC free article] [PubMed] [Google Scholar]


