Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the major diseases of chronic liver damage caused by oxidative stress. In this study, we investigated hepatoprotective effect of methyl gallate (MG) against t-BHP induced oxidative stress. Our results revealed that MG possessed strong antioxidant activity and lipid peroxidation inhibitory activity. In addition, MG inhibited t-BHP induced cell cytotoxicity, ROS production and sub-G1 phase cells in Chang liver cells. MG attenuated also activated signal p38 and decreased mitochondrial-mediated cell death by regulating pro- and anti- apoptotic proteins. Our results indicate that MG could be potentially used as protective agent in NAFLD therapy by modulating oxidative stress.
Keywords: Methyl gallate, Protective effect, t-BHP, Oxidative stress
Introduction
The main detoxifying organ in the body is liver, which has a high metabolic rate. The liver is subjected to many damages caused by high oxidative stress. Therefore, hepatic antioxidant defense system plays a vital role to maintain reactive oxygen species (ROS) homeostasis for health (Alía et al., 2003). Liver diseases have been recognized as significant health problems. It is a very well-proven fact that free radicals can damage cells through mechanisms such as lipid peroxidation and covalent binding, resulting in tissue injury (Babu et al., 2001).
Non-alcoholic fatty liver disease (NAFLD) is one kind of fatty liver diseases that occurs when liver accumulates fat exceeding 5% of hepatocytes in the absence of significant alcohol consumption, viral infection, or any other known causes of liver diseases (Gambino et al., 2011). NAFLD disease spectrum ranges from simple steatosis to steatohepatitis, liver fibrosis, cirrhosis, and ultimately hepatocellular carcinoma (Koek et al., 2011). NAFLD has emerged as one of the major causes of chronic liver damage in relation to increasing incidence of obesity, type 2 diabetes mellitus (T2DM), and other components of metabolic syndrome. It has been described as the hepatic manifestation of metabolic syndrome (Narasimhan et al., 2010). NAFLD has started to grow in the world in combination with increased prevalence of obesity, diabetes, and hyperlipidemia. Although NAFLD is known to be the predominant cause of chronic liver disorders, mechanisms of its development or progression remain incompletely understood (Spahis et al., 2016). However, growing evidence have revealed that the pathogenesis of NAFLD is closely associated with hepatic oxidative stress. Lipid peroxidation of polyunsaturated fatty acids in membranes by free radicals can lead to membrane disruption, production of reactive aldehydes, and depletion of cellular storage (Kučera et al., 2014).
Reactive oxygen species (ROS) contain an unpaired peripheral electron. Therefore, they are unstable and very reactive. They are produced during normal intracellular metabolism and are also produced by exogenous substances (Koek et al., 2011). Excessive accumulation of ROS is involved in several human pathologies. Oxidative stress is developed as a result of an imbalance between systems generating and scavenging ROS (Lee et al., 2014). To induce oxidative stress, tert-Butyl hydroperoxide (t-BHP), a short chain organic hydroperoxide, can be used as an exogenous substance. It has been found that t-BHP is an analogue of lipoperoxidation products produced in the event of oxidative stress. It may induce oxidative stress involved in different human diseases (Kučera et al., 2014). Cytochrome P450 in hepatocytes or hemoglobin in erythrocytes can metabolize t-BHP into free radical intermediates that can initiate lipid peroxidation, modify cell integrity, and make covalent bonds with cellular molecules, leading to cell injury (Hwang et al., 2002). t-BHP is a popular oxidant often employed in in vitro and in vivo models to induce acute oxidative stress (Kim et al., 2015). It is also used as an exogenous inducer to lead to NAFLD (Kučera et al., 2014).
Methyl gallate (MG) is a plant-derived phenolic compound known to possess numerous pharmacological properties (Baek et al., 2017). MG is widely distributed in medicinal and edible plants such as Rosa rugosa, Schinus terebinthifolius, Givotia rottleriformis, Terminalia chebula, Bergenia ligulata, and Paeonia suffruticosa (Acharyya et al., 2015; Cho et al., 2004; Kamatham et al., 2015; Park et al., 2019; Rosas et al., 2015; Sharanya et al., 2018). It has been found that MG exhibits a broad range of biological activities, including anti-oxidant, anti-apoptotic, anti-inflammatory, anti-tumor, anti-microbial, anti-platelet activity, defense against DNA damage and lung injury resulted from oxidative stress, and reducing diabetic oxidative stress (Rahman et al., 2016). However, hepatoprotective effect of MG has not been published yet. Therefore, the objective of this study was to investigate antioxidant activity and hepatoprotective effect of MG in vitro using t-BHP induced oxidative stress models.
Materials and methods
Materials
tert-Butyl hydroperoxide (t-BHP), methyl gallate, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), ferrous and ferric chloride, trichloroacetic acid (TCA), thiobarbituric acid (TBA), linoleic acid, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), N-Acetyl-L-cysteine (NAC) and 2′,7-dichlorodihydrofluorescin diacetate (DCFH-DA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies against Bax and Bcl-2 were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Antibodies against p-P38, t-P38, cleaved-caspase- 3,7,9, PARP/cleaved PARP, and β-actin were purchased from Cell Signaling Technology (Danvers, MA, USA). All other reagents were of the highest grade available commercially.
ABTS radical scavenging activity
ABTS assay was performed using procedures described by Erkan et al. (2008) with minor modifications. Briefly, stock solutions of 7.4 mM ABTS˙ and 2.6 mM potassium persulfate were used in this assay. Working solution was prepared by mixing these two stock solutions in equal quantities and allowing them to react for 15 h at room temperature (RT) in the dark. The mixture was diluted to obtain absorbance in the range of 1.5 ~ 2.0 at wavelength of 734 nm. To determine scavenging activity, 1.0 mL of ABTS reagent was mixed with 0.1 mL of 1 mM MG. After 10 min reaction time at RT, the absorbance was measured at 734 nm using a spectrophotometer (SpectraMax M2/M2e, CA, USA). The antioxidant activity was expressed in trolox equivalent of antioxidant capacity (TEAC) as mM Trolox equivalents/1 mM compound.
Ferric reducing antioxidant power (FRAP) assay
FRAP assay was carried out using the method of Benzie and Strain (1996) with minor modifications. This method was based on the reduction of a ferric 2,4,6-tripyridyl-s-triazine complex (Fe3+-TPTZ) to ferrous form (Fe2+-TPTZ). To conduct this assay, a 1.0 mL aliquot of a FRAP reagent, a mixture of 0.3 M acetate buffer, 10 mM TPTZ in 40 mM HCl, and 20 mM ferric chloride (10:1:1 v/v/v) were combined with 0.05 mL of 1 mM MG. The absorbance was then measured at wavelength of 593 nm using a spectrophotometer. To determine the antioxidant capacity of MG, absorbance values were compared to those obtained from a standard curve of FeSO4 (0–5 mM). Antioxidant capacity values were expressed as mM FeSO4 equivalent in 1 mM compound (mM FeSO4 equivalents/1 mM compound).
Lipid peroxidation inhibition assay in linoleic acid system
Ferric thiocyanate (FTC) method
FTC method described by Chang et al. (2002) was used in this study to determine the amount of peroxide at the initial stage of lipid peroxidation with minor modifications. Briefly, a reaction solution containing MG (4.0 mL, 1 mM), 2.51% linoleic acid emulsion (4.1 mL), phosphate buffer (8.0 mL, 0.05 M, pH 7.0), and D.W (3.9 mL) was placed in a snap tube and mixed using a vortex mixer. The reaction mixture was then incubated at 40 °C in the dark and the degree of oxidation was measured according to the thiocyanate method. Peroxide value was determined by recording the absorbance value at wavelength of 500 nm using a spectrophotometer every 2 days until the absorbance of the control reached a maximum.
Thiobarbituric acid (TBA) method
Samples prepared for FTC method were also used for this assay. Briefly, 0.1 mL of the sample solution in a tube was added with 0.2 mL 20% aqueous trichloroacetic acid and 0.2 mL 0.67% aqueous thiobarbituric acid using the method described by kikuzaki and Nakatani (1993). The mixture was then placed in a boiling water bath for 10 min. After cooling, it was centrifuged at 3000 rpm for 20 min. Absorbance of the supernatant was measured at wavelength of 532 nm using a spectrophotometer.
Cell culture
Chang liver cells were purchased from American Type Culture Collection (ATCC CCL- 13™) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with heat-inactivated 10% fetal bovine serum (FBS), 100 U/mL of penicillin and 100 μg/mL of streptomycin at 37 °C in a humidified incubator containing 5% CO2 and 95% air mixture.
Cell viability assay
Cell viability was estimated using MTT assay. Briefly, Chang liver cells were seeded into 48-well cell culture plates at a density of 8.0 × 103 cells/well. After 12 h of incubation, cells were treated with different concentrations of MG and incubated in a humidified incubator at 37 °C for 1 h. After that, 150 μM (final concentration) of t-BHP was added and incubated for 6 h. Thereafter, 200 μL of MTT stock solution (0.5 mg/mL) was added and incubated for 2 h. The supernatant in each well was aspirated and formazan crystals formed in each well were dissolved in 200 μL of DMSO. Absorbance was measured at a wavelength of 540 nm using a spectrophotometer. Relative cell viability was determined by measuring the amount of MTT converted to insoluble formazan salt. The optical density of formazan formed in control cells was taken as 100% viability.
Intracellular ROS measurement
Intracellular formation of ROS was assessed using oxidation sensitive dye DCFH-DA as a substrate as described previously (Senevirathne et al., 2011). Briefly, Chang liver cells were seeded into 96-well black cell culture plates at a density of 1.5 × 104 cells/well. Cells were then treated with various concentrations of MG and incubated for 1 h. Then 200 μM (final concentration) of t-BHP was added and incubated for 30 min. Control cells and treated cells were incubated at 37 °C for 30 min in the presence of DCFH-DA (100 μL of 10 mM DCF-DA in 5 mL of media) in the dark. After 30 min of incubation, the formation of DCF due to oxidation of DCFH in the presence of ROS was measured at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a spectrofluorometer.
Cell cycle analysis by flow cytometry
A total of 4 × 105 Chang liver cells were seeded into 100-mm cell culture dishes with 10 mL culture medium for 12 h, pretreated with MG for 1 h, and then exposed to 150 μM of t-BHP for 6 h. For flow cytometry cell cycle analysis, cells were harvested and washed with PBS buffer (pH 7.4). After fixing with 80% ethanol for overnight, cells were washed and re-suspended in PBS buffer containing PI (propidium iodide) and ribonuclease A for DNA staining (1000:10:1 v/v/v). These cells were then analyzed with a FACS Calibur flow cytometer (Becton & Dickinson Co., USA).
Annexin V/PI staining
Protective effect of MG on t-BHP-induced apoptosis was determined with Annexin V-FITC/PI staining by Annexin V FITC Apoptosis Detection Kit (BD, Franklin Lakes, NJ, USA). Cells were harvested and then 5 μL of FITC-Annexin V and 5 μL of propidium iodide (PI) were added for 15 min of staining, the cells were subjected to flow cytometry analysis with a FACS Calibur flow cytometer. Data were analyzed using Cell Quest Pro software. Cells were classified as live cells (Annexin V−, PI−), early apoptotic cells (Annexin V+, PI−), late apoptotic cells (Annexin V+, PI+), and necrotic cells (Annexin V−, PI+).
Western blotting
Cells were lysed for 20 min at 4 °C with RIPA buffer containing protease and phosphatase inhibitors. Insoluble material was removed by centrifugation at 13,000 rpm for 15 min. Equal amounts of lysates (20–30 μg of protein) were subjected to 10–15% SDS–polyacrylamide gel electrophoresis and transferred to PVDF membranes (Amersham Hybond, GE Healthcare Life science, Germany). After blocking with 5% non-fat dry milk, membranes were subsequently incubated with primary antibodies at 4 °C overnight. After three washing steps with TBS-T, these membranes were incubated with secondary antibodies (horseradish peroxidase-conjugated goat anti-mouse IgG and anti-rabbit IgG, 1:3000 in TBS-T solution) at RT for 1 h. Resulting protein bands were visualized using chemiluminescent HRP detection reagent with a Davinch-K detector (Davinch-Western™, Youngwha scientific co., Seoul, South Korea). Immunoblotting was performed for actin as an internal control.
Statistical analysis
Data are expressed as mean ± standard deviation from triplicate determination. Analysis of variance (ANOVA) accompanied with Bonferroni tests (GraphPad Prism 5) were conducted to identify significant difference between samples. Statistical significance was considered at p < 0.05.
Results and discussion
Antioxidant activity and lipid peroxidation inhibitory effect of MG
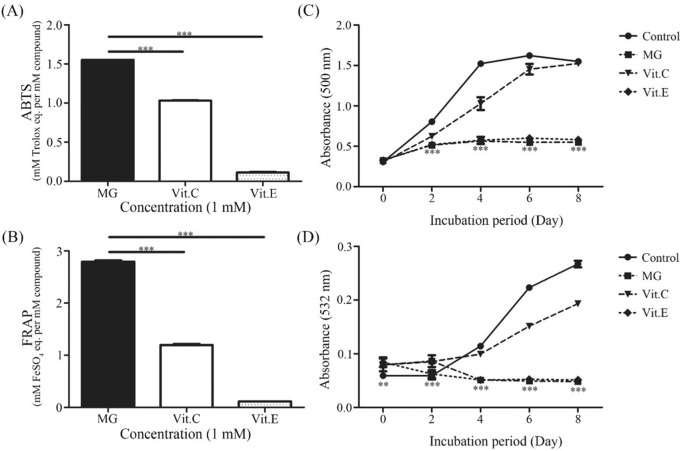
Antioxidant activity was evaluated using ABTS radical scavenging activity and FRAP assay. In antioxidant assays, well-known antioxidants vitamin C and vitamin E were employed as positive controls. ABTS results revealed that MG (1 mM) possessed a stronger antioxidant capacity (1.552 ± 0.001 mM Trolox equivalent) than standards (1 mM) vitamin C (1.030 ± 0.003 mM Trolox equivalent) and vitamin E (0.112 ± 0.007 mM Trolox equivalent) [Fig. 1(A)]. Moreover, FRAP assay confirmed that MG had stronger antioxidant capacity (2.796 ± 0.021 mM FeSO4 equivalent) at concentration of 1 mM than 1 mM vitamin C and 1 mM vitamin E (1.200 ± 0.017 mM and 0.116 ± 0.000 mM FeSO4 equivalent, respectively) [Fig. 1(B)].
Fig. 1.
Antioxidant activity of MG and its inhibition effect on lipid peroxidation. Antioxidant activities of MG, vit.C, and vit.E based on ABTS radical scavenging activity assay (A) and FRAP assay (B). Data are expressed as mean ± SD and analyzed using one-way ANOVA followed by Bonferroni tests. **p < 0.01, ***p < 0.001 compared to vitamin C or vitamin E treatment. Lipid peroxidation activity in the presence of MG, vit.C and vit. E measured though FTC assay (C) and TBA assay (D). **p < 0.01, ***p < 0.001 compared to the Control group
Lipid peroxidation has been found to be an important event in cellular damage process caused by oxidative stress (Kučera et al., 2014). Lipid peroxidation of polyunsaturated fatty acids in membranes is initiated by free radicals. It leads to membrane disruption, formation of reactive aldehydes, and depletion of cellular storage (Kučera et al., 2014). Therefore, lipid peroxidation inhibitory effect and antioxidant capacity of MG were evaluated by FTC and TBA lipid peroxidation assays. MG has inhibitory effect on both early and latter stages of lipid peroxidation. FTC assay was performed in a total of 8 days. Results revealed that MG had the highest inhibitory effect on lipid peroxidation, it was higher than vitamin E and vitamin C with the following order: MG > Vitamin E > Vitamin C [Fig. 1(C)]. TBA assay was also conducted for 8 days. Results affirmed that MG possessed the greatest inhibitory effect on lipid peroxidation among the three tested compounds [Fig. 1D)].
Previous studies have also reported that oxidative stress can potentially act as a catalyst for progression of fatty liver disease to nonalcoholic steatohepatitis (NASH) (Harrison et al., 2003). It has been reported that well-known antioxidant vitamin E (300 mg/day) can improve inflammation and fibrosis on liver biopsy in majority of NASH patients over a 12-month period through inhibiting oxidative stress (Harrison et al., 2003). In this study, we found that MG contained a stronger antioxidant activity and lipid peroxidation inhibitory activity than vitamin C and vitamin E.
Effect of MG on cell viability, t-BHP induced ROS production
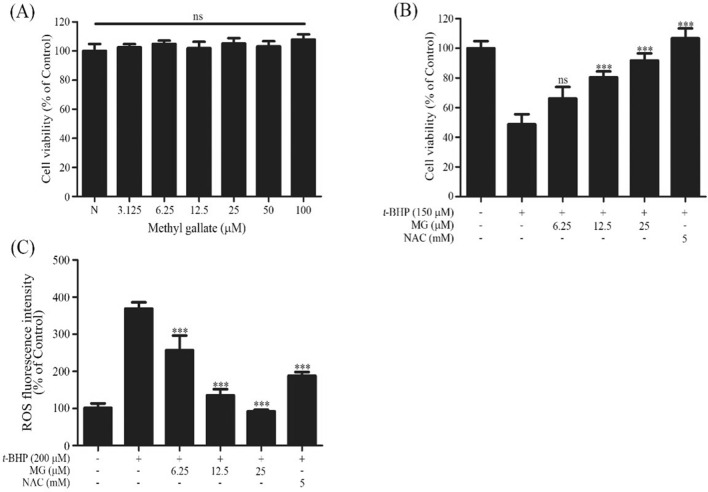
The effect of MG on cell viability was evaluated by MTT assay. Cells were treated with six different concentrations of MG ranging from 3.125 to 100 μM (Chaudhuri et al., 2015). As shown in Fig. 2(A), no significant cytotoxic effect was found for MG for these tested concentrations. Therefore, 6.25, 12.5, and 25 μM MG concentrations were selected for subsequent analyses. Next, the effect of MG on t-BHP induced cell toxicity was determined. As shown in Fig. 2(B) shows that exposure to 150 µM t-BHP for 24 h induced about 52% increase in cell death. However, pretreatment with MG (6.25, 12.5, 25 µg/mL) remarkably decreased cell death in a dose-dependent manner (36%, 20%, 11%), indicating that MG-pretreated cells were protected against the oxidative damage. Recently, polyphenols have been found to be beneficial in decreasing oxidative stress and increasing overall health. Epigallocatechin-3-gallate (EGCG) has been reported to prevent oxidative damage in healthy cells (Ning et al., 2016).
Fig. 2.
Effect of MG on cell viability and protective effects against t-BHP induced Chang cells. (A) Cytotoxic effect of MG on Chang cells was measured by MTT assay. (B) The protective effect of MG on t-BHP induced change to Chang cells based on MTT assay. (C) The protective effect of MG on t-BHP induced ROS production in Chang cell measured by a spectrofluorometer. Data are expressed as mean ± SD and analyzed using a one-way ANOVA followed by Bonferroni test. ***p < 0.001 compared to t-BHP treatment group
ROS accumulation damages intracellular proteins and DNA, causing dysfunction of cells and oxidative stress damage, leading to various liver diseases (Valko et al., 2007). Therefore, effects of MG on t-BHP induced ROS generation were evaluated. In this study, NAC was employed as a positive control. t-BHP treatment induced ROS generation in Chang cells by 3.6-fold compared to untreated cells. However, treatment with MG attenuated t-BHP induced ROS production in a dose-dependent manner. In addition, MG exhibited a ROS inhibition efficacy as strong as positive control (NAC) [Fig. 2(C)]. In previous report, t-BHP treatment induced cell cytotoxicity, ROS production and increased sub-G1 phase cell percentage, an indicator of cell damage in Chang cells (Kim et al., 2014).
Effect of MG on cell cycle analysis
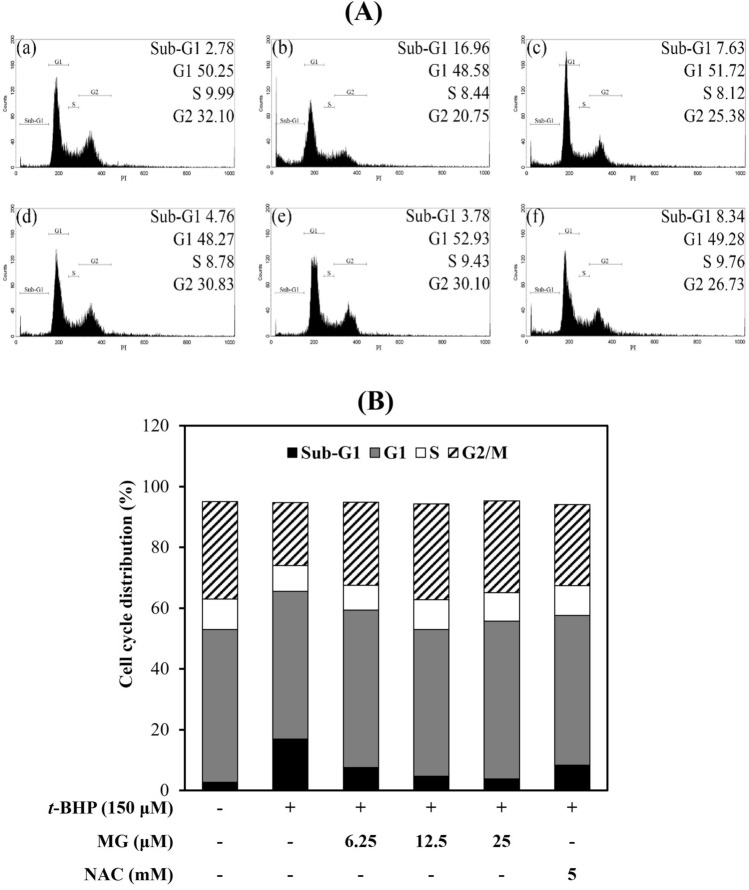
The principle of widely used detection methods using flow cytometry is DNA content differentiation in varies of phases. Sub-G1 phase means apoptotic cells with fractional DNA content. As shown in Fig. 3, treatment of t-BHP cells showed that increased sub-G1 phase cell (16.96%), and it mean caused DNA damage in the sub-G1 phase by oxidative stress. However, pretreated MG at 6.25, 12.5, and 25 μM decreased sub-G1 phase (from 16.96 to 7.63%, 4.76%, 3.78% respectively) in dose-dependent manner. NAC (8.34%) also reduced sub-G1 phase compared with the t-BHP-induced oxidative stress cells. Our results showed that both MG and NAC affected the cell cycle and inhibited apoptosis. MG might be a potential drug candidate with hepatoprotective effects by inhibiting cytotoxicity, ROS generation and sub-G1 phase cell percentage.
Fig. 3.
Effects of MG on cell cycle distribution of Chang cells. (A) Cell cycle phase distribution was assessed using flow cytometry, PI labeling of total DNA content. (B) The histogram shows the percentage of cells in each phase. Cells were treated with (a) Contol, (b) 150 μM t-BHP, (c) 6.25 μM MG + 150 μM t-BHP, (d) 12.5 μM MG + 150 μM t-BHP, (e) 25 μM MG + 150 μM t-BHP and (f) 5 mM NAC + 150 μM t-BHP
Effect of MG on Annexin V/PI staining
To examine whether the MG could efficiently protect against oxidative damage, the relative amounts of Annexin V and/or PI-stained cells were measured by flow cytometry analysis. Using Annexin V/PI double staining, it was possible to separate normal cells (Annexin V−/PI−), early apoptotic cells (Annexin V+/PI−), late apoptotic (Annexin V+/PI+) and necrotic cell (Annexin V−/PI+). As shown in Fig. 4, t-BHP treatment increased the levels of early apoptotic cells (3.77%), late apoptotic cells (12.08%), and necrotic cells (12.09%) compare with control group. However, pre-treatment with MG decreased the numbers of early, late apoptotic cells and necrotic cells dose-dependently. These data showed a similar trend to the results of cell cycle analysis, in which apoptosis was increased with t-BHP treatment, and decreased apoptosis by pretreatment of MG.
Fig. 4.
Effect of MG on Annexin V/PI staining (A, B). Chang cells were pretreated with different concentrations of MG, and stained with Annexin V and PI, and analyzed using flow cytometry. Proportion of live cells, early apoptotic cells, late apoptotic cells, and necrotic cells. (a) Control, (b) 150 μM t-BHP, (c) 6.25 μM MG + 150 μM t-BHP, (d) 12.5 μM MG + 150 μM t-BHP, (e) 25 μM MG + 150 μM t-BHP and (f) 5 mM NAC + 150 μM t-BHP
Effect of MG on signaling pathway against t-BHP induced oxidative stress in Chang cells
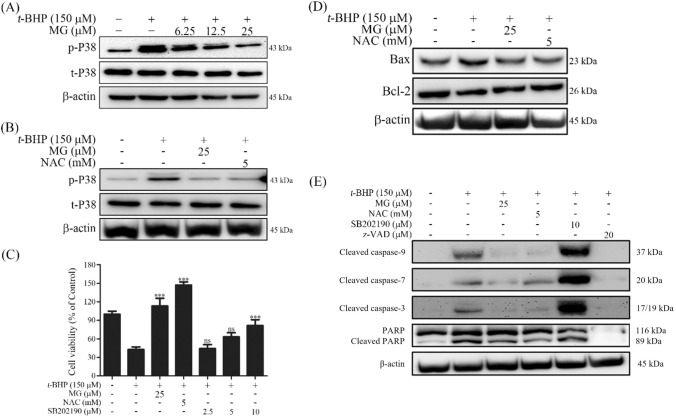
According to our previous results, MG showed protective effect against t-BHP induced cytotoxicity in Chang cells. Molecular mechanisms associated with this protective effect were investigated in this study using western blot experiments. To understand the molecular mechanisms involved in the hepatoprotective effect of MG, its effects on some downstream cell death related signaling molecules were investigated. p38, one of stress-activated protein kinases (SAPKs), is widely expressed in most tissues. It participates in several different stress signaling pathways that control a spectrum of cellular processes, including cell proliferation, differentiation, and apoptosis (Tan et al., 2013). t-BHP treatment increased the phosphorylation level of p38. However, MG pre-treatment decreased t-BHP induced p38 phosphorylation levels in a dose-dependent manner [Fig. 5(A)]. NAC is commonly used as an antioxidant agent in clinical practice to treat some diseases associated with ROS generation, including cancer, cardiovascular diseases, human immunodeficiency virus infections, acetaminophen-induced liver toxicity, and metal toxicity (Shimamoto et al., 2011). NAC pre-treatment also reduced t-BHP induced p38 phosphorylation levels [Fig. 5(B)]. When cells were pretreated with a p38 inhibitor SB202190, results showed that SB202190 decreased the t-BHP induced cytotoxicity by increasing cell survival in a dose-dependent manner [Fig. 5(C)]. It has been reported that t-BHP induces oxidative stress, causing mitochondria dependent apoptosis (Bhattacharya et al., 2011). Hence, we investigated the effect of MG on t-BHP induced mitochondria dependent apoptosis signaling pathway. Our results showed that Bcl-2 family protein, an upstream regulator of MMP with critical roles in mitochondrial-mediated cell death, was affected by t-BHP [Fig. 5(D)]. Then, we measured the effect of MG on t-BHP-induced pro-apoptotic protein Bax, anti-apoptotic protein Bcl-2, various caspase, and PARP cascades. t-BHP treatment induced Bax protein expression level but reduced Bcl-2 protein expression level. However, MG pre-treatment reversed the effect of t-BHP by reducing Bax protein expression and increasing Bcl-2 protein expression [Fig. 5(D)].
Fig. 5.
Effects of MG on cell death signaling pathways involved in t-BHP induced oxidative stress in Chang cells. Cells were pretreated with MG at indicated concentrations for 1 h (30 min for SB202190) and then treated with 150 μM t-BHP. (A) MG inhibited t-BHP induced p38 phosphorylation. Phosphorylation levels of p38 were analyzed after 3 h of treatment with t-BHP by western blot analysis. (B) NAC inhibited t-BHP induced p38 phosphorylation. Phosphorylation levels of p38 were analyzed after 3 h of treatment with t-BHP by western blot analysis. (C) p38 inhibitor SB202190 rescued cells from t-BHP induced cell death. Cell viability was assessed after for 6 h of t-BHP treatment by MTT assay. All data are expressed as mean ± SD and analyzed using one-way ANOVA followed by Bonferroni tests. ***p < 0.001 compared to t-BHP treatment group. (D) MG increased anti-apoptotic protein Bcl-2 expression but inhibited pro-apoptotic protein Bax expression. (E) Effects of MG on expression levels of caspases and PARP in Chang cells
As shown in Fig. 5(E), t-BHP increased the cleavage activity of caspase 9, caspase 7, and caspase 3. However, such t-BHP induced caspase cleavage could be attenuated by MG pre-treatment. Cleavage of poly (ADP-ribose) polymerase (PARP), the last apoptotic signaling protein, was enhanced by t-BHP. However, pre-treatment of MG could be suppressed on t-BHP induced cleavage of PARP. These results demonstrate the molecular mechanisms associated with the effect of MG on t-BHP induced oxidative stress. z-VAD-FMK (z-VAD) is an irreversible a pan-caspase inhibitor that prevents apoptosis in many different cell types (Li et al., 2019). Our results showed that z-VAD inhibit t-BHP induced apoptosis by inhibiting the caspase-3, -7, -9. In previous reports, it has been shown that SB202190 can induce cell death through stimulating the activity of CPP32-like caspases with typical apoptotic features such as nucleus condensation and DNA fragmentation (Nemoto et al., 1998). During this process, expression of p38 can attenuate the apoptotic effect of SB202190 (Nemoto et al., 1998). Our results showed that synergy effect of t-BHP and SB202190 occur hyper activation of caspase [Fig. 5(E)]. The expression of cleaved PARP in treatment with SB202190 and t-BHP was not different from treatment of single t-BHP. The result show that synergy effect of t-BHP and SB202190 more affect initiate apoptosis than late apoptosis.
In conclusion, we found that MG possessed strong antioxidant activity and inhibitory effect on lipid peroxidation. In addition, we confirmed that MG could modify mitochondria dependent apoptosis signaling pathway to rescue liver cells through inhibiting ROS production due to t-BHP induced oxidative stress. These results indicate that MG could be potentially useful as a hepatoprotective agent in therapy for non-alcoholic fatty liver disease by modulating oxidative stress.
Acknowledgements
This paper was supported by the Semyung University Research Grant 2021.
Declarations
Conflict of interest
The authors have no conflict of interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bo-Im Ryu, Email: qhdla1234@daum.net.
Ki-Tae Kim, Email: onehorn@hanmail.net.
References
- Acharyya S, Sarkar P, Saha DR, Patra A, Ramamurthy T, Bag PK. Intracellular and membrane-damaging activities of methyl gallate isolated from Terminalia chebula against multidrug-resistant Shigella spp. Journal of Medical Microbiology. 2015;64:901–909. doi: 10.1099/jmm.0.000107. [DOI] [PubMed] [Google Scholar]
- Alía M, Horcajo C, Bravo L, Goya L. Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutrition Research. 2003;23:1251–1267. doi: 10.1016/S0271-5317(03)00131-3. [DOI] [Google Scholar]
- Babu BH, Shylesh BS, Padikkala J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia. 2001;72:272–277. doi: 10.1016/S0367-326X(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Baek JM, Kim JY, Lee CH, Yoon KH, Lee MS. Methyl gallate inhibits osteoclast formation and function by suppressing Akt and Btk-PLCγ2-Ca2+ signaling and prevents lipopolysaccharide-induced bone loss. International Journal of Molecular Sciences. 2017;18:581. doi: 10.3390/ijms18030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Gachhui R, Sil PC. Hepatoprotective properties of kombucha tea against TBHP-induced oxidative stress via suppression of mitochondria dependent apoptosis. Pathophysiology. 2011;18:221–234. doi: 10.1016/j.pathophys.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Chang LW, Yen WJ, Huang SC, Duh PD. Antioxidant activity of sesame coat. Food Chemistry. 2002;78:347–354. doi: 10.1016/S0308-8146(02)00119-X. [DOI] [Google Scholar]
- Chaudhuri D, Ghate NB, Singh SS, Mandal N. Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacognosy Magazine. 2015;11:269. doi: 10.4103/0973-1296.153078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EJ, Yokozawa T, Kim HY, Shibahara N, Park JC. Rosa rugosa attenuates diabetic oxidative stress in rats with streptozotocin-induced diabetes. The American Journal of Chinese Medicine. 2004;32:487–496. doi: 10.1142/S0192415X04002132. [DOI] [PubMed] [Google Scholar]
- Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chemistry. 2008;110(1):76–82. doi: 10.1016/j.foodchem.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Gambino R, Musso G, Cassader M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanisms and therapeutic opportunities. Antioxidant & Redox Signaling. 2011;15:1325–1365. doi: 10.1089/ars.2009.3058. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. The American Journal of Gastroenterology. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- Hwang JM, Wang CJ, Chou FP, Tseng TH, Hsieh YS, Lin WL, Chu CY. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Archives of Toxicology. 2002;76:664–670. doi: 10.1007/s00204-002-0351-9. [DOI] [PubMed] [Google Scholar]
- Kamatham S, Kumar N, Gudipalli P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicology Reports. 2015;2:520–529. doi: 10.1016/j.toxrep.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuzaki H, Nakatani N. Antioxidant effects of some ginger constituents. Journal of Food Science. 1993;58:1407–1410. doi: 10.1111/j.1365-2621.1993.tb06194.x. [DOI] [Google Scholar]
- Kim YS, Hwang JW, Han YK, Kwon HJ, Hong H, Kim EH, Moon SH, Jeon BT, Park PJ. Antioxidant activity and protective effects of Trapa japonica pericarp extracts against tert-butylhydroperoxide-induced oxidative damage in Chang cells. Food and Chemical Toxicology. 2014;64:49–56. doi: 10.1016/j.fct.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Kim YS, Hwang JW, Sung SH, Jeon YJ, Jeong JH, Jeon BT, Moon SH, Park PJ. Antioxidant activity and protective effect of extract of Celosia cristata L. flower on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Food Chemistry. 2015;168:572–579. doi: 10.1016/j.foodchem.2014.07.106. [DOI] [PubMed] [Google Scholar]
- Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clinica Chimica Acta. 2011;412:1297–1305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Kučera O, Endlicher R, Roušar T, Lotková H, Garnol T, Drahota Z, Cervinková Z. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxidative Medicine and Cell Longevity. 2014 doi: 10.1155/2014/752506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Kim KS, Ko W, Li B, Jeong GS, Jang JH, Oh H, Kim YC. The cytoprotective effect of sulfuretin against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2/ARE and JNK/ERK MAPK-mediated heme oxygenase-1 expression. International Journal of Molecular Sciences. 2014;15:8863–8877. doi: 10.3390/ijms15058863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yao X, Zhu Y, Zhang H, Wang H, Ma Q, Yan F, Yang Y, Zhang J, Shi H, Ning Z, Dai J, Li Z, Li C, Su F, Xue Y, Meng X, Dong G, Xiong H. The caspase inhibitor Z-VAD-FMK alleviates endotoxic shock via inducing macrophages necroptosis and promoting MDSCs-mediated inhibition of macrophages activation. Frontiers in Immumology. 2019;10:1824. doi: 10.3389/fimmu.2019.01824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Gokulakrishnan K, Sampathkumar R, Farooq S, Ravikumar R, Mohan V, Balasubramanyam M. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clinical Biochemistry. 2010;43:815–821. doi: 10.1016/j.clinbiochem.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Xiang J, Huang S, Lin A. Induction of apoptosis by SB202190 through inhibition of p38β mitogen-activated protein kinase. Journal of Biological Chemistry. 1998;273:16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- Ning W, Wang S, Liu D, Fu L, Jin R, Xu A. Potent effects of peracetylated (−)-epigallocatechin-3-gallate against hydrogen peroxide-induced damage in human epidermal melanocytes via attenuation of oxidative stress and apoptosis. Clinical and Experimental Dermatology. 2016;41:616–624. doi: 10.1111/ced.12855. [DOI] [PubMed] [Google Scholar]
- Park DJ, Jung HJ, Park CH, Yokozawa T, Jeong JC. Root bark of Paeonia suffruticosa extract and its component methyl gallate possess peroxynitrite scavenging activity and anti-inflammatory properties through NF-κB inhibition in LPS-treated mice. Molecules. 2019;24:3483. doi: 10.3390/molecules24193483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman N, Jeon M, Kim YS. Methyl gallate, a potent antioxidant inhibits mouse and human adipocyte differentiation and oxidative stress in adipocytes through impairment of mitotic clonal expansion. BioFactors. 2016;42:716–726. doi: 10.1002/biof.1310. [DOI] [PubMed] [Google Scholar]
- Rosas EC, Correa LB, de Almeida Pádua T, Costa TEMM, Mazzei JL, Heringer AP, Bizarro CA, Kaplan MAC, Figueiredo MR, Henriques MG. Anti-inflammatory effect of Schinus terebinthifolius Raddi hydroalcoholic extract on neutrophil migration in zymosan-induced arthritis. Journal of Ethnopharmacology. 2015;175:490–498. doi: 10.1016/j.jep.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Sharanya CS, Arun KG, Vijaytha V, Sabu A, Haridas M. Designing of enzyme inhibitors based on active site specificity: lessons from methyl gallate and its lipoxygenase inhibitory profile. Journal of Receptors and Signal Transduction. 2018;38:256–265. doi: 10.1080/10799893.2018.1478856. [DOI] [PubMed] [Google Scholar]
- Senevirathne M, Ahn CB, Je JY. Hepatoprotective effect of chitooligosaccharides against tert-butylhydroperoxide-induced damage in Chang liver cells. Carbohydrate Polymers. 2011;83:995–1000. doi: 10.1016/j.carbpol.2010.09.016. [DOI] [Google Scholar]
- Shimamoto K, Hayashi H, Taniai E, Morita R, Imaoka M, Ishii Y, Suzuki K, Shibutani M, Mitsumori K. Antioxidant N-acetyl-L-cysteine (NAC) supplementation reduces reactive oxygen species (ROS)-mediated hepatocellular tumor promotion of indole-3-carbinol (I3C) in rats. The Journal of Toxicological Sciences. 2011;36:775–786. doi: 10.2131/jts.36.775. [DOI] [PubMed] [Google Scholar]
- Spahis S, Delvin E, Borys JM, Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxidant & Redox Signaling. 2016;26:519–541. doi: 10.1089/ars.2016.6776. [DOI] [PubMed] [Google Scholar]
- Tan C, Qian X, Jia R, Wu M, Liang Z. Matrine induction of reactive oxygen species activates p38 leading to caspase-dependent cell apoptosis in non-small cell lung cancer cells. Oncology Reports. 2013;30:2529–2535. doi: 10.3892/or.2013.2727. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]